Abstract
Marine invertebrates are a significant source of biologically active compounds. Recent studies have highlighted the role of microbiota associated with marine invertebrates in the production of bioactive compounds. Corals and sponges are the main marine invertebrates producing bioactive substances, and Symbiodiniaceae dinoflagellates are well-recognized endosymbionts with corals and sponges playing vital functions. The biological properties of Symbiodiniaceae-derived compounds have garnered attention in the past decades owing to their ecological implications and potentiality for bioprospecting initiatives. This study aims to systematically review studies on bioactivities and potential biotechnological applications of Symbiodiniaceae-derived compounds. The PRISMA guidelines were followed. Our study showed that anti-inflammatory and vasoconstrictive activities of Symbiodiniaceae-derived compounds have been the most investigated. However, very few studies have been published, with in vitro culturing of Symbiodiniaceae being the most significant challenge. Therefore, we surveyed for the metabolites reported so far, analyzed their chemodiversity, and discussed approaches to overcome culturing-related limitations.
1. Introduction
Although marine invertebrates have significant bioprospecting potential, it is associated with several methodological, ecological, and logistical challenges owing to limited sampling and the risk of changes in population dynamics [1]. These challenges are owing to the prolonged time and poor reproducibility of the complex environmental conditions required for the cultivation of invertebrate biomass (e.g., sponges) [2, 3]. Consequently, although marine invertebrates produce bioactive compounds of biotechnological utility, their biological characteristics limit large-scale harvesting of secondary metabolites in vitro [4]. In addition, challenges associated with large-scale production of the target bioactive compounds from a marine organism, that is, low yield on animal extraction and high costs and practical limitations of chemical synthesis, further limit the use of these compounds [5]. The origin of marine natural products (MNP) on bioprospecting studies on sessile or nonsessile marine invertebrates remains uncertain, which could be the organisms themselves, the associated microbiota, or the interaction between them [6]. The specialized metabolism of the associated microbiota remains to be studied.
Marine invertebrates are hosts to a rich and dynamic microbiota [7, 8] that are closely linked to the metabolism and survival of the invertebrates [9]. Endosymbiotic relationships result in mechanisms that promote mutual nutrition and predator defense; for example, sponges and their symbionts can produce toxic compounds to prevent attacks by other marine organisms [10, 11]. Studies that have reported on the production of such active compounds by the symbiotic microbiota also highlighted the need for further research [6, 12–14]. For instance, the endosymbiotic Symbiodiniaceae dinoflagellates growing on Pseudopterogorgia elisabethae produce pseudopterosins that provide the host with oxidative stress tolerance [15]. In addition, unknown free-living marine bacteria, frequently associated with the tunicate Ecteinascidia turbinata, express groups of essential genes for the synthesis of bioactive metabolites (e.g., trabectedin) that are under research as potential oncologic treatment agents [16].
The dinoflagellate family Symbiodiniaceae (phylum Myzozoa, class Dinophyceae) is a symbiotic organism under active research [17]. These endosymbiotic microalgae provide hosts with nutrients through photosynthesis and receive protection and inorganic compounds [18–20]. Their ecology focuses on host compatibility, which depends on the type of the associated host, their distribution, and variations in abiotic factors such as irradiance, depth, pH, and temperature [21]. The systematics of the family Symbiodiniaceae (formerly, the Symbiodinium genus) were recently revised, with new genera replacing the earlier clades. Currently, the Symbiodinium genus only includes clade A (considered a living fossil) [22]. This taxon is known to produce secondary metabolites involving compounds with unique chemical structures and activities. Although several genera are known, several remain undiscovered [23]. The association between extensive genetic diversity and metabolic processes also remains unclear [24].
Although the importance of associated microbiota, particularly of Symbiodiniaceae endosymbionts, in marine invertebrate metabolism is understood, most literature reviews have focused on the biology of these dinoflagellates or their ecological implications. Two reviews (Gordon and Leggat [25] and Kita et al. [26]) highlighted some bioactive metabolites isolated from some Symbiodiniaceae species. However, to our knowledge, this review is the first to systematically collect, summarize, and analyze the data available in the literature regarding Symbiodiniaceae-derived bioactive metabolites. This review focuses mainly on the chemodiversity of the reported naturally occurring compounds and their potential biotechnological applicability.
2. Methods
2.1. Search and Eligibility Criteria
We conducted a systematic search for literature on naturally occurring compounds isolated from Symbiodiniaceae family members, their bioactivities, and biotechnological potential. Scopus, the Web of Science, and PubMed databases were used. The following search query was used: ((symbiodinium OR zooxanthellae) AND (metabolite OR compound OR agent OR substance OR molecule) AND (activity OR potential OR bioactivity OR bioactive OR effect OR extract OR isolated OR isolate OR derived OR isolation OR biotechnology)). Inclusion criteria regardless of the year of publication were (a) high-quality original articles, (b) describing the isolation of secondary metabolites from cultures of microalgae of the Symbiodiniaceae family (former genus Symbiodinium), and (c) written in English.
2.2. Study Selection and Data Collection
Duplicate articles were removed, and the title and abstract of each study were independently filtered according to the eligibility criteria by 2 of the authors using the Rayyan QCRI tool, with the classification categorized as included, excluded, or maybe [27]. Papers matching the classification criteria were studied. Articles classified as “maybe” were finally classified after discussion and consensus. After initial classification, the full texts of the articles were analyzed, and the information was captured using a form to ensure comprehensive data collection and bias elimination.
2.3. Analysis of Retrieved Compounds
All compounds identified in the systematic review were represented using the simplified molecular-input-line-entry system (SMILES) notation, and these were enlisted to obtain a custom-made library. The SMILES-annotated compounds were incorporated in the software Osiris DataWarrior (Idorsia Pharmaceuticals Ltd., Switzerland, version 5.2.1) [28], which was used to determine the physicochemical properties: molecular weight (MW), octanol/water partition coefficient (cLogP), aqueous solubility (cLogS), hydrogen bond acceptor (H-acceptor), hydrogen bond donors (H-donors), total surface area (TSA), polar surface area (PSA), relative polar surface area (rPSA), and druglikeness.
3. Results and Discussion
3.1. General Findings
The literature search identified 686 articles; 160 of the retrieved articles were identified as duplicates among the 3 databases, leaving 356 unique articles. Titles and abstracts of these 356 articles were screened and filtered according to eligibility and selection criteria. After screening, only 27 studies were selected for data extraction. Based on full-text assessment and data extraction, 20 articles were finally included in this systematic review (Figure 1).
Figure 1.

PRISMA flow diagram. Modified from Moher et al. [29]. Compliance with the items in the statement guideline is summarized in Table S1 in Supplementary Materials.
The included articles (n = 20) were published between 1993 and 2018 (Figure 2(a)). Most studies were published by authors in Japan (n = 16), followed by those in the USA and France (Figure 2(b)), which involved Symbiodiniaceae strains from 5 different countries (Figure 2(c)). To assess the publication behavior of bioprospecting studies on Symbiodiniaceae dinoflagellates, we compared all papers (that were screened) published within the same years. The number of studies increased during the same period, except for 2004 and 2005 (Figure 2(a)). However, a considerably low number of studies investigating Symbiodiniaceae-derived compounds and their bioactivity potential were published, with the highest of 4 studies published in 2004. This low academic productivity is associated with the small number of countries researching on this topic (Figure 2(b)), which is in contrast with the worldwide distribution of the Symbiodiniaceae species [30] and the metabolite diversity that marine invertebrates represent (e.g., corals and sponges) [1, 31–36], including their associated microbiota [12]. Japan had the highest contribution to research on this topic, with 45% of the included papers from Nagoya University. Focus on bioprospecting was minimal, with most studies focusing on topics such as ecology.
Figure 2.

The number of studies published on naturally occurring compounds isolated from Symbiodiniaceae. (a) The distribution of published studies included (left axis) and screened (right axis) in this systematic review per year, (b) the number of publications according to corresponding authors' country, and (c) the number of publications according to the country of sample origin.
With regard to the diversity of Symbiodiniaceae isolates, 12 Symbiodinium strains have been studied for the specialized metabolism-derived products from these dinoflagellates (Table 1). The most reported strain was Symbiodinium sp Y-6 (isolated from the flatworm Amphiscolops sp), from which 7 compounds were reported (i.e., 1, 2, 6, 8, 9, 21, and 22; see Table 2). Although Symbiodiniaceae dinoflagellates have been reported to be symbionts for various marine invertebrates [37], their symbiosis with cnidarians has been most investigated. Of interest, we found few studies (15.79%) on symbiosis with corals (i.e., Sarcophyton glaucum, Pseudopterogorgia elisabethae, and Eunicea fusca; see Table 1), which implies a subexploration of the Symbiodiniaceae family diversity.
Table 1.
Symbiodiniaceae strains and their hosts in the included articles.
| Strain code | Strain | Clade | Hosta | References |
|---|---|---|---|---|
| S1 | Symbiodinium sp 2012-7-4S | N/Ab | Amphiscolops sp | [38] |
| S2 | Symbiodinium sp HA3-5 | A1 | Free-living | [39–41] |
| S3 | Symbiodinium sp JCUCS-1 | B | Cassiopea xamachana | [42] |
| S4 | Symbiodinium sp JCUSG-1 | A | Sarcophyton glaucum | [42, 43] |
| S5 | Symbiodinium sp OTCL2A | N/A | Tridacna crocea | [44] |
| S6 | Symbiodinium sp P083-2 | A1 | Amphisorus hemprichii | [43] |
| S7 | Symbiodinium sp P-78 | D | N/A | [45] |
| S8 | Symbiodinium sp PL-TS-1 | A3 | Tridacna crocea | [43] |
| S9 | Symbiodinium sp Y-6 | A2 | Amphiscolops sp | [42, 43, 46–48] |
| S10 | Symbiodinium sp | N/A | Pseudopterogorgia elisabethae | [15, 49, 50] |
| S11 | Symbiodinium sp | N/A | Eunicea fusca | [50] |
| S12 | Symbiodinium sp | N/A | Amphiscolops sp | [51–54] |
aScientific name of the host from which the Symbiodiniaceae strain was isolated. bN/A: not available.
Table 2.
Symbiodiniaceae-derived metabolites with the reported bioactivity and microalgae strain source.
| Compound no. | Metabolite | Bioactivity | Symbiodiniaceae strain | References |
|---|---|---|---|---|
| 1 | Zooxanthellatoxin A | Vasoconstriction, platelet aggregation | S9 | [47, 55, 56] |
| 2 | Zooxanthellatoxin B | Vasoconstriction | S9 | [46, 47] |
| 3 | Zooxanthellamide A | N/Ab | S2 | [39] |
| 4 | Zooxanthellamide B | N/A | S2 | [40] |
| 5 | Zooxanthellamide C | Vasoconstriction | S2 | [41] |
| 6 | Zooxanthellamide D | Cytotoxicity | S3 | [42] |
| 7 | Symbiodinolide | N-type Ca2+ channel inhibition, COX-2 inhibition (anti-inflammatory) | S12 | [53] |
| 8 | Symbioramide-C16 | N/A | S9 | [48] |
| 9 | Zooxanthellactone | Cytotoxicity | S4 | [43] |
| 10 | Symbiospirol A | Protein kinase C inhibition (anti-inflammatory) | S12 | [54] |
| 11 | Symbiospirol B | N/A | S12 | [54] |
| 12 | Symbiospirol C | N/A | S12 | [54] |
| 13 | Symbiodinolactone A | N/A | S1 | [38] |
| 14 | Pseudopterosin A | N/A | S10 | [15, 49, 50] |
| 15 | Pseudopterosin B | N/A | S10 | [15, 49, 50] |
| 16 | Pseudopterosin C | N/A | S10 | [15, 49, 50] |
| 17 | Pseudopterosin D | N/A | S10 | [15, 49, 50] |
| 18 | Fuscol | N/A | S11 | [50] |
| 19 | Symbioimine | Osteoclastogenesis inhibition, COX-2 inhibition (anti-inflammatory) | S12 | [51, 52] |
| 20 | Neosymbioimine | N/A | S12 | [52] |
| 21 | Zooxanthellabetaine A | N/A | S9 | [48] |
| 22 | Zooxanthellamine | N/A | S9 | [48] |
| 23 | Peridinin | Delayed-type hypersensitivity inhibition (anti-inflammatory) | S5 | [44] |
aAssigned compound number. bN/A: not available.
3.2. Biologically Active Compounds
Among the 23 compounds identified from the selected publications (see chemical structures in Figures S1 and S2 in Supplementary Materials), biological activity evaluations were available only for 9 compounds (Table 2). We examined the vasoconstrictive and anti-inflammatory potency of these compounds. Vasoconstriction was the first bioactivity reported for Symbiodiniaceae-derived metabolites (i.e., 1 and 2), with activity at concentrations >0.70 μM [47]. Furthermore, metabolite 1 was also shown to induce platelet aggregation [55]. Metabolite 2 effects vasoconstriction through voltage-sensitive Ca2+ channels [46]. This finding is consistent with the calcium-dependent induction of platelet aggregation by metabolite 1 [56]. Compound 5 reportedly exhibits 3-fold higher vasoconstriction potency than compound 1 although its mechanism of action remains unclear [41]. To our knowledge, these compounds are yet being studied.
Metabolites 7, 10, 19, and 23 have been reported to have anti-inflammatory potential. Metabolites 7 and 8 showed 65% and 32% cyclooxygenase-2 inhibition at 2 and 10 μM concentrations, respectively [52, 53]. Compound 23 was evaluated basis its ability to suppress delayed-type hypersensitivity in mice [44]. Metabolite 10 has been reported to inhibit L-phosphatidylserine-stimulated protein kinase C activity [54]. Limited data availability makes estimating the biotechnological potential of these compounds (except for metabolite 23 [peridinin]) difficult.
Cytotoxicity of Symbiodiniaceae-derived metabolites has been studied. Two compounds, 6 and 9, have been reported to show antineoplastic potential against carcinomas [42, 43]. Both compounds were evaluated on A432 and Nakata cell lines and exhibited inhibitory activity at concentrations <10 μM. Compound 9 exhibited 16-fold higher potency [43]. Despite these encouraging results, we could not find further studies on the anticancer potential of these compounds.
The biological activities of compounds 3, 4, 8, 11–18, and 20–22 were not evaluated in the included papers; however, the bioactivity potential of some of these have been reported as they were isolated from other sources. In this regard, the phenolic diterpenes pseudopterosins (i.e., 14–17) have been shown to have potential anti-inflammatory, analgesic [57], neuroprotective [58], and antioxidant [15, 59] activities. Similarly, compound 18 has shown anti-inflammatory properties superior to those of indomethacin [60]. Fuscol (18) has also been reported to exhibit moderate inhibition activity on elastase release assays [61]. These findings present an opportunity in the bioprospecting potential of Symbiodiniaceae dinoflagellates.
3.3. Chemodiversity of the Symbiodiniaceae-Derived Compounds
We clustered the retrieved compounds by structural similarity to analyze chemodiversity. Four clusters were formed, including 15 compounds, whereas 8 compounds had unique structural fingerprints (Figure 3). Most of the compounds corresponded to amides (i.e., 1–8) followed by polyketides (i.e., 9–13), diterpenes (i.e., 14–18), and alkaloids (i.e., 19–22); one was an apocarotenoid (i.e., 23). These data are consistent with those from recent reports on the diversity and abundance of specialized metabolite biosynthesis genes in Symbiodiniaceae genomes, which show that polyketide synthase genes are the primary diversified gene clusters associated with specialized metabolism [62].
Figure 3.
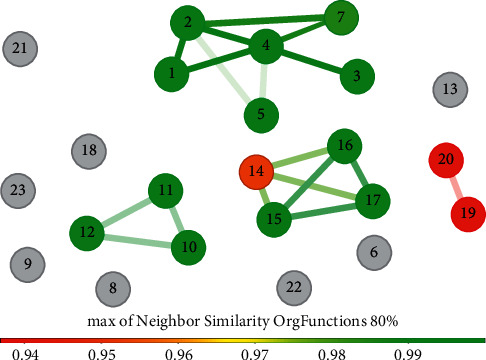
Similarity analysis of the Symbiodiniaceae-derived compounds. The similarity analysis was performed using the OrgFunctions descriptor. The color scale bar indicates the level of similarity from red (0.9) to green (1.0).
With regard to similarity analysis (Figure 3), compounds 3 and 4 in the larger group (i.e., 1, 2, 3, 4, 5, and 7) have been described as artifacts produced under chemically active conditions during the isolation process [41], leaving the number of natural compounds at 21. In addition, this group has compounds with vasoconstrictive bioactivity, possibly involving Ca2+ channels [46, 53, 55], reinforcing the role of the macrolactone moiety in this mechanism. With regard to the remaining compounds, except for 6, clustering was anticipated by the metabolite type and trivial names (Table 2, Figure 3).
Given the crucial role of physicochemical properties in the development of therapeutic agents [63], we examined the relevant properties of the selected compounds based on some descriptors (i.e., MW, cLogP, cLogS, H-acceptor, H-donors, and druglikeness; Figure 4). We identified 3 groups of compounds for MW, TSA, PSA, H-acceptors, and H-donors, whereas rPSA, cLogP, cLogS, and druglikeness showed a wider distribution. For instance, only 4 compounds had positive druglikeness values (i.e., 16, 17, 22, and 23), and the remaining compounds displayed values between −0.2 and −25.0 (Figure 4(h)). An implication of these results is the attrition associated with a high likelihood of failures in further research stages.
Figure 4.

Structure-based physicochemical properties of the Symbiodiniaceae-derived compounds. Properties were estimated using the DataWarrior software. Box plots showing the distributions for (a) molecular weight (MW), (b) total surface area (TSA), (c) polar surface area (PSA), (d) relative polar surface area (rPSA), (e) octanol/water partition coefficient (cLogP), (f) aqueous solubility (cLogS), (g) hydrogen bond acceptor (H-acceptor) and hydrogen bond donors (H-donors), and (h) druglikeness.
The compounds were classified as low (approximately 500 Da), medium (approximately 1200 Da), or large (approximately 2700 Da) MW compounds. Most compounds (61.90%, excluding 3 and 4) were in the low MW group, which is desirable in drug development. However, several compounds in this group remain unevaluated for bioactivity potential (i.e., 8, 13, 20, 21, and 22). These compounds, except 22, were isolated in quantities <5 mg (Table 3), which could have limited their biological screening. In contrast, the compounds from dinoflagellates have been recognized to have long carbon-chain backbones (also known as super carbon-chain compounds) [64]. Ten Symbiodiniaceae-derived compounds have been reported (i.e., 1–7 and 10–12) to be closely related structurally to polyketide-like compounds with an amide moiety (see Figure 3).
Table 3.
Summary of the culture conditions and isolation yield data reported by some included articles regarding the biomass production of the compounds isolated from Symbiodiniaceae strains.
| Compound no. | Culture time (days) | Culture volume (L) | Wet weight (g) | Compound amount (mg)b | Yield × 10−2 (%)c | References |
|---|---|---|---|---|---|---|
| 1 | 30–40 | 250 | 164.0 | 35.8 | 2.18 | [47] |
| 2 | 30–40 | 250 | 164.0 | 19.6 | 1.20 | [47] |
| 3 | 42 | 198 | 130.3 | 4.6 | 0.35 | [39] |
| 4 | 40 | 132 | 103.9 | 5.5 | 0.53 | [40] |
| 5 | 40 | 132 | 103.9 | 21.8 | 2.10 | [41] |
| 6 | 43 | 140 | 98.5 | 2.3 | 0.23 | [42] |
| 7 | 60 | 78 | 88.0 | 9.3 | 1.06 | [53] |
| 8 | 63 | 160 | 192.0 | 2.7 | 0.14 | [48] |
| 9 | 42 | 156 | 138.7 | 1.0 | 0.07 | [43] |
| 10 | 60 | 145 | 129.0 | 117.0 | 9.07 | [54] |
| 11 | 60 | 145 | 129.0 | 9.4 | 0.73 | [54] |
| 12 | 60 | 145 | 129.0 | 3.4 | 0.26 | [54] |
| 13 | 42 | 100 | N/A | 0.3 | N/A | [38] |
| 19 | 20 | 80 | 36.0 | 5.7 | 1.58 | [51, 52] |
| 20 | N/Aa | N/A | 112.0 | 4.2 | 0.38 | [52] |
| 21 | 63 | 160 | 192.0 | 0.8 | 0.04 | [48] |
| 22 | 63 | 160 | 192.0 | 37.6 | 1.96 | [48] |
| 23 | 40 | 132 | 82.0 | 24.7 | 3.01 | [44] |
aN/A: not available. bReported amount by authors for the isolated compound. cCalculated/reported yield for the isolated compound.
3.4. Challenges Related to the Biotechnological Application of Symbiodiniaceae-Derived Compounds
Studies published from 1962 have described Symbiodiniaceae dinoflagellates as slow-growing microorganisms under culture conditions requiring several weeks to achieve appreciable new colonies [65]. Studies have also reported poor yield of metabolites of interest in culture conditions [66]. These limitations pose challenges in the bioprospecting of Symbiodiniaceae microalgae. Although the yields of several of the isolated compounds are within the expected values (Table 3), significantly high culture volume and time are required to obtain sufficient biomass (an average of 150 L and 47 days; see Table 3). These challenges preclude pharmaceutical applicability which requires large-scale production [64, 67].
Establishing axenic microalgae cultures is a remarkable challenge [68]. Long-term culturing of endosymbionts such as Symbiodiniaceae dinoflagellates can be resource intensive. For instance, a study attempting to achieve a long-term Cladocopium (former clade C) culture reported the importance of host conditions for the survival of the Symbiodiniaceae microalgae. However, sustaining a long-term culture was technically unfeasible [69]. Dinoflagellates are sensitive to the hydrodynamic forces in culture media [64, 70–72]. Although immobilized culturing has overcome this limitation [73], it may not be applicable to culturing all Symbiodiniaceae strains, given some strains are obligate endosymbionts. Furthermore, discrepancies between endosymbiotic and free-living states remain incompletely understood [74–76], limiting the respective bioprospecting applications. In addition, the super carbon-chain compounds can also be considered a significant challenge from the chemistry perspective, given the high number of chiral carbons, functional groups, and aliphatic carbons in these metabolites may hinder their structural elucidation. These limitations restrict adequate identification, which in turn limits chemistry-driven studies.
As opposed to primarily establishing axenic cultures, microbial consortia have recently been proven effective in the targeted production of metabolites [77, 78]. In fact, except for studies by Fukatsu et al. [42] and Nakamura et al. [47], the included articles omit describing the axenic condition of the Symbiodiniaceae cultures; only 1 study [42] reported the use of an axenic culture. This implies a putative role of other co-occurring species in the synthesis of the specialized metabolites reported by the included papers.
Among the metabolites reviewed, peridinin (23) has been studied the most. This carotenoid pigment forms a molecular complex of significance as a light-harvesting agent for photosynthesis in dinoflagellates (reviewed in detail by Carbonera et al. [79]). Owing to this property, it is currently used as a fluorescent dye in flow cytometry [80]. The study reported producing this compound in a 132 L culture for more than 40 days [44], with a yield of 24.7 mg (Table 3). However, using the immobilized approach and after 28 days of culture, approximately 1 g of peridinin was produced from the S. voratum CCAC 0047 strain [81]. This improvement can possibly aid large-scale production and allow exploring other bioactivity potentials of peridinin and other Symbiodiniaceae-derived compounds.
4. Conclusions
Although studies on Symbiodiniaceae dinoflagellates are increasing, those on bioprospecting remain considerably low. In addition to a limited number of institutions conducting research on this topic, the challenges in culturing Symbiodiniaceae microalgae, such as slow growth, shear sensitivity, and indeterminate nutrient requirements, contribute to the lack of bioprospecting studies. However, using more adaptable strains (e.g., free-living forms) and culture approaches that prevent hydrodynamic stress (e.g., immobilized growing) could address culturing limitations. These considerations could facilitate more compelling studies by neophyte groups interested in the bioprospecting of dinoflagellates.
This comprehensive literature survey shows that the specialized metabolism of Symbiodiniaceae remains largely unexplored. Future studies should explore new sampling zones, including new hosts (e.g., sponges). Studies are also required to determine genera with free-living stages and in vitro conditions that affect their growth for improved biomass production and better isolation yields.
Acknowledgments
This work was funded by Minciencias (Ministerio de Ciencia, Tecnología e Innovación), Colombia (project code 123080864187, contract 80740-168-2019) and by Universidad de La Sabana (General Research Directorate, project ING-175-2016).
Data Availability
The data used to support the findings of this study are provided within this article. However, any required further information can be provided by the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
Table S1: PRISMA checklist. Table S2: list of SMILES-annotated compounds retrieved from the included papers. Figure S1: chemical structure of Symbiodiniaceae-derived compounds 1–5. Figure S2: chemical structure of Symbiodiniaceae-derived compounds 6–23.
References
- 1.Leal M. C., Puga J., Serôdio J., Gomes N. C. M., Calado R. Trends in the discovery of new marine natural products from invertebrates over the last two decades-where and what are we bioprospecting? PLoS One . 2012;7(1) doi: 10.1371/journal.pone.0030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur N., Singh A. Marine Sponges: Chemicobiological and Biomedical Applications . India: Springer; 2016. Chemical ecology of marine sponge. [Google Scholar]
- 3.Pereira R. C., Costa-Lotufo L. V. Bioprospecting for bioactives from seaweeds: potential, obstacles and alternatives. Revista Brasileira de Farmacognosia . 2012;22(4):894–905. doi: 10.1590/s0102-695x2012005000077. [DOI] [Google Scholar]
- 4.König G. M., Kehraus S., Seibert S. F., Abdel-Lateff A., Müller D. Natural products from marine organisms and their associated microbes. ChemBioChem . 2006;7(2):229–238. doi: 10.1002/cbic.200500087. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt E. W. From chemical structure to environmental biosynthetic pathways: navigating marine invertebrate-bacteria associations. Trends in Biotechnology . 2005;23(9):437–440. doi: 10.1016/j.tibtech.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Leal M., Sheridan C., Osinga R., et al. Marine microorganism-invertebrate assemblages: perspectives to solve the “supply problem” in the initial steps of drug discovery. Marine Drugs . 2014;12(7):3929–3952. doi: 10.3390/md12073929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tambutté S., Holcomb M., Ferrier-Pagès C., et al. Coral biomineralization: from the gene to the environment. Journal of Experimental Marine Biology and Ecology . 2011;408(1-2):58–78. [Google Scholar]
- 8.Whitehead L. F., Douglas A. E. Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to an animal host. Journal of Experimental Biology . 2003;206(18):3149–3157. doi: 10.1242/jeb.00539. [DOI] [PubMed] [Google Scholar]
- 9.Yellowlees D., Rees T. A. V., Leggat W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant, Cell and Environment . 2008;31(5):679–694. doi: 10.1111/j.1365-3040.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 10.Proksch P. Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs . 1994 doi: 10.1016/0041-0101(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 11.Thacker R. W., Freeman C. J. Advances in Marine Biology . Cambridge, MA, USA: Academic Press; 2012. Sponge-microbe symbioses. [DOI] [PubMed] [Google Scholar]
- 12.Raimundo I., Silva S. G., Costa R., Keller-Costa T. Bioactive secondary metabolites from octocoral-associated microbes—new chances for blue growth. Marine Drugs . 2018;16(12) doi: 10.3390/md16120485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radjasa O. K., Vaske Y. M., Navarro G., et al. Highlights of marine invertebrate-derived biosynthetic products: their biomedical potential and possible production by microbial associants. Bioorganic and Medicinal Chemistry . 2011;19(22):6658–6674. doi: 10.1016/j.bmc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman D. J. Predominately uncultured microbes as sources of bioactive agents. Frontiers in Microbiology . 2016;7:1–15. doi: 10.3389/fmicb.2016.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mydlarz L., Jacobs R. Comparison of an inducible oxidative burst in free-living and symbiotic dinoflagellates reveals properties of the pseudopterosins. Phytochemistry . 2004;65(24):3231–3241. doi: 10.1016/j.phytochem.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Calle F. Marine microbiome as source of natural products. Microbial Biotechnology . 2017;10(6):1293–1296. doi: 10.1111/1751-7915.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernando I. P. S., Nah J.-W., Jeon Y.-J. Potential anti-inflammatory natural products from marine algae. Environmental Toxicology and Pharmacology . 2016;48:22–30. doi: 10.1016/j.etap.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Davy S. K., Allemand D., Weis V. M. Cell biology of Cnidarian-dinoflagellate symbiosis. Microbiology and Molecular Biology Reviews . 2012;76(2):229–261. doi: 10.1128/mmbr.05014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fransolet D., Roberty S., Plumier J.-C. Establishment of endosymbiosis: the case of cnidarians and Symbiodinium. Journal of Experimental Marine Biology and Ecology . 2012;420-421:1–7. doi: 10.1016/j.jembe.2012.03.015. [DOI] [Google Scholar]
- 20.Ramsby B. D., Hill M. S., Thornhill D. J., et al. Sibling species of mutualistic Symbiodinium clade G from bioeroding sponges in the western Pacific and western Atlantic oceans. Journal of Phycology . 2017;53(5):951–960. doi: 10.1111/jpy.12576. [DOI] [PubMed] [Google Scholar]
- 21.Finney J. C., Pettay D. T., Sampayo E. M., Warner M. E., Oxenford H. A., LaJeunesse T. C. The relative significance of host-habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microbial Ecology . 2010;60(1):250–263. doi: 10.1007/s00248-010-9681-y. [DOI] [PubMed] [Google Scholar]
- 22.LaJeunesse T. C., Parkinson J. E., Gabrielson P. W., et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology . 2018;28(16):2570–2580.e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi J. i., Kubota T. Comprehensive Natural Products II: Chemistry and Biology . Amsterdam, Netherlands: Elsevier; 2010. Bioactive metabolites from marine dinoflagellates. [DOI] [Google Scholar]
- 24.Baker A. C. Coral Health and Disease . Berlin, Heidelberg, Germany: Springer; 2004. Symbiont diversity on coral reefs and its relationship to bleaching resistance and resilience. [DOI] [Google Scholar]
- 25.Gordon B. R., Leggat W. Symbiodinium-invertebrate symbioses and the role of metabolomics. Marine Drugs . 2010;8(10):2546–2568. doi: 10.3390/md8102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kita M., Ohno O., Han C., Uemura D. Bioactive secondary metabolites from symbiotic marine dinoflagellates: symbiodinolide and durinskiols. The Chemical Record . 2010;10(2):57–69. doi: 10.1002/tcr.200900007. [DOI] [PubMed] [Google Scholar]
- 27.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Systematic Reviews . 2016;5(1):p. 210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander T., Freyss J., Von Korff M., Rufener C. DataWarrior: an open-source program for chemistry aware data visualization and analysis. Journal of Chemical Information and Modeling . 2015;55(2):460–473. doi: 10.1021/ci500588j. [DOI] [PubMed] [Google Scholar]
- 29.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine . 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Oppen M. J. H., Baker A. C., Coffroth M. A., Willis B. L. Coral Bleaching . Springer, Berlin, Germany: 2009. Bleaching resistance and the role of algal endosymbionts; pp. 83–102. [DOI] [Google Scholar]
- 31.Omarsdottir S., Einarsdottir E., Ögmundsdottir H. M., et al. Biodiversity of benthic invertebrates and bioprospecting in Icelandic waters. Phytochemistry Reviews . 2013;12(3):517–529. doi: 10.1007/s11101-012-9243-7. [DOI] [Google Scholar]
- 32.Lindequist U. Marine-derived pharmaceuticals - challenges and opportunities. Biomolecules and Therapeutics . 2016;24(6):561–571. doi: 10.4062/biomolther.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belarbi E. Producing drugs from marine sponges. Biotechnology Advances . 2003;21(7):585–598. doi: 10.1016/s0734-9750(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 34.Sipkema D., Franssen M. C. R., Osinga R., Tramper J., Wijffels R. H. Marine sponges as pharmacy. Marine Biotechnology . 2005;7(3):142–162. doi: 10.1007/s10126-004-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehbub M., Lei J., Franco C., Zhang W. Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Marine Drugs . 2014;12(8):4539–4577. doi: 10.3390/md12084539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjum K., Abbas S. Q., Shah S. A. A., Akhter N., Batool S., Hassan S. S. u. Marine sponges as a drug treasure. Biomolecules and Therapeutics . 2016;24(4):347–362. doi: 10.4062/biomolther.2016.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goulet T. L., Lucas M. Q., Schizas N. V. Symbiodiniaceae genetic diversity and symbioses with hosts from shallow to mesophotic coral ecosystems. Coral Reefs of the World . 2019:537–551. doi: 10.1007/978-3-319-92735-0_30. [DOI] [Google Scholar]
- 38.Kurimoto S.-i., Iinuma Y., Kobayashi J. i., Kubota T. Symbiodinolactone A, a new 12-membered macrolide from symbiotic marine dinoflagellate Symbiodinium sp. Tetrahedron Letters . 2018;59(51):4496–4499. doi: 10.1016/j.tetlet.2018.11.018. [DOI] [Google Scholar]
- 39.Onodera K.-i., Nakamura H., Oba Y., Ojika M. Zooxanthellamide A, a novel polyhydroxy metabolite from a marine dinoflagellate of Symbiodinium sp. Tetrahedron . 2003;59(7):1067–1071. doi: 10.1016/s0040-4020(02)01630-7. [DOI] [PubMed] [Google Scholar]
- 40.Onodera K.-i., Nakamura H., Oba Y., Ojika M. Zooxanthellamide B, a novel large polyhydroxy metabolite from a marine dinoflagellate ofSymbiodiniumsp. Bioscience, Biotechnology, and Biochemistry . 2004;68(4):955–958. doi: 10.1271/bbb.68.955. [DOI] [PubMed] [Google Scholar]
- 41.Onodera K.-i., Nakamura H., Oba Y., Ohizumi Y., Ojika M. Zooxanthellamide Cs: vasoconstrictive polyhydroxylated macrolides with the largest lactone ring size from a marine dinoflagellate of Symbiodinium sp. Journal of the American Chemical Society . 2005;127(29):10406–10411. doi: 10.1021/ja050810g. [DOI] [PubMed] [Google Scholar]
- 42.Fukatsu T., Onodera K.-I., Ohta Y., et al. Zooxanthellamide D, a polyhydroxy polyene amide from a marine dinoflagellate, and chemotaxonomic perspective of the symbiodinium polyols. Journal of Natural Products . 2007;70(3):407–411. doi: 10.1021/np060596p. [DOI] [PubMed] [Google Scholar]
- 43.Onodera K.-i., Fukatsu T., Kawai N., et al. Zooxanthellactone, a novel γ-Lactone-type oxylipine from dinoflagellates of Symbiodinium sp.: structure, distribution, and biological activity. Bioscience, Biotechnology, and Biochemistry . 2004;68(4):848–852. doi: 10.1271/bbb.68.848. [DOI] [PubMed] [Google Scholar]
- 44.Onodera K.-i., Konishi Y., Taguchi T., Kiyoto S., Tominaga A. Peridinin from the marine symbiotic dinoflagellate, Symbiodinium sp., regulates eosinophilia in mice. Marine Drugs . 2014;12(4):1773–1787. doi: 10.3390/md12041773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zea-Obando C., Tunin-Ley A., Turquet J., et al. Anti-bacterial adhesion activity of tropical microalgae extracts. Molecules . 2018;23(9) doi: 10.3390/molecules23092180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriya T., Ishida Y., Nakamura H., Asari T., Murai A., Ohizumi Y. Vasoconstriction induced by zooxanthellatoxin-B, a polyoxygenated long-chain product from a marine alga. European Journal of Pharmacology . 1998;350(1):59–65. doi: 10.1016/s0014-2999(98)00225-8. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura H., Asari T., Ohizumi Y., Kobayashi J. i., Yamasu T., Murai A. Isolation of zooxanthellatoxins, novel vasoconstrictive substances from the zooxanthella Symbiodinium sp. Toxicon . 1993;31(4):371–376. doi: 10.1016/0041-0101(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura H., Kawase Y., Maruyama K., Murai A. Studies on polyketide metabolites of a symbiotic dinoflagellate, Symbiodinium sp.: a new C30 marine alkaloid, zooxanthellamine, a plausible precursor for zoanthid alkaloids. Bulletin of the Chemical Society of Japan . 1998;71(4):781–787. doi: 10.1246/bcsj.71.781. [DOI] [Google Scholar]
- 49.Mydlarz L. D., Jacobs R. S., Boehnlein J., Kerr R. G. Pseudopterosin biosynthesis in Symbiodinium sp., the dinoflagellate symbiont of Pseudopterogorgia elisabethae. Chemistry and Biology . 2003;10(11):1051–1056. doi: 10.1016/j.chembiol.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Newberger N. C., Ranzer L. K., Boehnlein J. M., Kerr R. G. Induction of terpene biosynthesis in dinoflagellate symbionts of Caribbean gorgonians. Phytochemistry . 2006;67(19):2133–2139. doi: 10.1016/j.phytochem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Kita M., Kondo M., Koyama T., et al. Symbioimine exhibiting inhibitory effect of osteoclast differentiation, from the symbiotic marine dinoflagellate Symbiodinium sp. Journal of the American Chemical Society . 2004;126(15):4794–4795. doi: 10.1021/ja049277f. [DOI] [PubMed] [Google Scholar]
- 52.Kita M., Ohishi N., Washida K., et al. Symbioimine and neosymbioimine, amphoteric iminium metabolites from the symbiotic marine dinoflagellate Symbiodinium sp. Bioorganic and Medicinal Chemistry . 2005;13(17):5253–5258. doi: 10.1016/j.bmc.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 53.Kita M., Ohishi N., Konishi K., et al. Symbiodinolide, a novel polyol macrolide that activates N-type Ca2+ channel, from the symbiotic marine dinoflagellate Symbiodinium sp. Tetrahedron . 2007;63(27):6241–6251. doi: 10.1016/j.tet.2007.02.093. [DOI] [Google Scholar]
- 54.Tsunematsu Y., Ohno O., Konishi K., Yamada K., Suganuma M., Uemura D. Symbiospirols: novel long carbon-chain compounds isolated from symbiotic marine dinoflagellate symbiodinium sp. Organic Letters . 2009;11(10):2153–2156. doi: 10.1021/ol900299x. [DOI] [PubMed] [Google Scholar]
- 55.Rho M. C., Nakahata N., Nakamura H., Murai A., Ohizumi Y. Involvement of phospholipase C-γ2 in activation of mitogen-activated protein kinase and phospholipase A2 by zooxanthellatoxin-A in rabbit platelets. Journal of Pharmacology and Experimental Therapeutics . 1997;282(1):496–504. [PubMed] [Google Scholar]
- 56.Rho M.-C., Nakahata N., Nakamura H., Murai A., Ohizumi Y. Activation of rabbit platelets by Ca2+ influx and thromboxane A2 release in an external Ca2+-dependent manner by zooxanthellatoxin-A, a novel polyol. British Journal of Pharmacology . 1995;115(3):433–440. doi: 10.1111/j.1476-5381.1995.tb16352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Look S. A., Fenical W., Jacobs R. S., Clardy J. The pseudopterosins: anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proceedings of the National Academy of Sciences . 1986;83(17):6238–6240. doi: 10.1073/pnas.83.17.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niu X., Yu C., Jiang G., et al. Pseudopterosin A ameliorates ischaemia-induced brain injury by acting on Akt signalling pathway. Folia Neuropathologica . 2018;56(2):104–111. doi: 10.5114/fn.2018.76614. [DOI] [PubMed] [Google Scholar]
- 59.Caplan S. L., Zheng B., Dawson-Scully K., White C. A., West L. M., West L. M. Pseudopterosin A: protection of synaptic function and potential as a neuromodulatory agent. Marine Drugs . 2016;14(3) doi: 10.3390/md14030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchbank D. H., Berrue F., Kerr R. G. Eunicidiol, an anti-inflammatory dilophol diterpene from Eunicea fusca. Journal of Natural Products . 2012;75(7):1289–1293. doi: 10.1021/np300149y. [DOI] [PubMed] [Google Scholar]
- 61.Ahmed A., Teng W.-T., Huang C.-Y., Dai C.-F., Hwang T.-L., Sheu J.-H. Anti-inflammatory lobane and prenyleudesmane diterpenoids from the soft coral lobophytum varium. Marine Drugs . 2017;15(10):p. 300. doi: 10.3390/md15100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beedessee G., Hisata K., Roy M. C., Van Dolah F. M., Satoh N., Shoguchi E. Diversified secondary metabolite biosynthesis gene repertoire revealed in symbiotic dinoflagellates. Scientific Reports . 2019;9(1):1204–1212. doi: 10.1038/s41598-018-37792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leeson P. D., Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nature Reviews Drug Discovery . 2007;6(11):881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 64.Assunção J., Guedes A. C., Malcata F. X. Biotechnological and pharmacological applications of biotoxins and other bioactive molecules from dinoflagellates. Marine Drugs . 2017;15(12):p. 393. doi: 10.3390/md15120393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freudenthal H. D. Symbiodiniumgen. Nov. andSymbiodinium microadriaticumsp. nov., a zooxanthella: taxonomy, life cycle, and morphology. Journal of Protozoology . 1962;9(1):45–52. doi: 10.1111/j.1550-7408.1962.tb02579.x. [DOI] [Google Scholar]
- 66.Sarker S. D., Nahar L. Natural Products Isolation . Totowa, NJ, USA: Humana Press; 2012. An introduction to natural products isolation. [DOI] [Google Scholar]
- 67.Sipkema D., Osinga R., Schatton W., Mendola D., Tramper J., Wijffels R. H. Large-scale production of pharmaceuticals by marine sponges: sea, cell, or synthesis? Biotechnology and Bioengineering . 2005;90(2):201–222. doi: 10.1002/bit.20404. [DOI] [PubMed] [Google Scholar]
- 68.Vu C. H. T., Lee H.-G., Chang Y. K., Oh H.-M. Axenic cultures for microalgal biotechnology: establishment, assessment, maintenance, and applications. Biotechnology Advances . 2018;36(2):380–396. doi: 10.1016/j.biotechadv.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 69.Krueger T., Gates R. D. Cultivating endosymbionts - host environmental mimics support the survival of Symbiodinium C15 ex hospite. Journal of Experimental Marine Biology and Ecology . 2012;413(2017):169–176. doi: 10.1016/j.jembe.2011.12.002. [DOI] [Google Scholar]
- 70.Sullivan J. M., Swift E., Donaghay P. L., Rines J. E. B. Small-scale turbulence affects the division rate and morphology of two red-tide dinoflagellates. Harmful Algae . 2003;2(3):183–199. doi: 10.1016/s1568-9883(03)00039-8. [DOI] [Google Scholar]
- 71.Hu W., Gladue R., Hansen J., Wojnar C., Chalmers J. J. Growth inhibition of dinoflagellate algae in shake flasks: not due to shear this time. Biotechnology Progress . 2010;26(1):79–87. doi: 10.1002/btpr.301. [DOI] [PubMed] [Google Scholar]
- 72.Camacho F. G., Rodríguez J. J. G., Mirón A. S., Belarbi E. H., Chisti Y., Grima E. M. Photobioreactor scale-up for a shear-sensitive dinoflagellate microalga. Process Biochemistry . 2011;46(4):936–944. doi: 10.1016/j.procbio.2011.01.005. [DOI] [Google Scholar]
- 73.Benstein R. M., Çebi Z., Podola B., Melkonian M. Immobilized growth of the peridinin-producing marine dinoflagellate symbiodinium in a simple biofilm photobioreactor. Marine Biotechnology . 2014;16(6):621–628. doi: 10.1007/s10126-014-9581-0. [DOI] [PubMed] [Google Scholar]
- 74.Huang H., Zhou G., Yang J., Liu S., You F., Lei X. Diversity of free-living and symbioticSymbiodiniumin the coral reefs of Sanya, South China Sea. Marine Biology Research . 2013;9(2):117–128. doi: 10.1080/17451000.2012.708045. [DOI] [Google Scholar]
- 75.Bellantuono A. J., Dougan K. E., Granados‐Cifuentes C., Rodriguez‐Lanetty M. Free‐living and symbiotic lifestyles of a thermotolerant coral endosymbiont display profoundly distinct transcriptomes under both stable and heat stress conditions. Molecular Ecology . 2019;28(24):5265–5281. doi: 10.1111/mec.15300. [DOI] [PubMed] [Google Scholar]
- 76.Maor‐Landaw K., Oppen M. J. H., McFadden G. I. Symbiotic lifestyle triggers drastic changes in the gene expression of the algal endosymbiont Breviolum minutum (Symbiodiniaceae) Ecology and Evolution . 2020;10(1):451–466. doi: 10.1002/ece3.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou K., Qiao K., Edgar S., Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nature Biotechnology . 2015;33(4):377–383. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang R., Zhao S., Wang Z., Koffas M. A. Recent advances in modular co-culture engineering for synthesis of natural products. Current Opinion in Biotechnology . 2020;62:65–71. doi: 10.1016/j.copbio.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Carbonera D., Valentin M., Spezia R., Mezzetti A. The unique photophysical properties of the peridinin-chlorophyll-a-protein. Current Protein and Peptide Science . 2014;15(4):332–350. doi: 10.2174/1389203715666140327111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Afar B., Merrill J., Clark E. A. Detection of lymphocyte subsets using three-color/single-laser flow cytometry and the fluorescent dye Peridinin chlorophyll-a protein. Journal of Clinical Immunology . 1991;11(5):254–261. doi: 10.1007/bf00918183. [DOI] [PubMed] [Google Scholar]
- 81.Langenbach D., Melkonian M. Optimising biomass and peridinin accumulation in the dinoflagellate Symbiodinium voratum using a twin-layer porous substrate bioreactor. Journal of Applied Phycology . 2019;31(1):21–28. doi: 10.1007/s10811-018-1513-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: PRISMA checklist. Table S2: list of SMILES-annotated compounds retrieved from the included papers. Figure S1: chemical structure of Symbiodiniaceae-derived compounds 1–5. Figure S2: chemical structure of Symbiodiniaceae-derived compounds 6–23.
Data Availability Statement
The data used to support the findings of this study are provided within this article. However, any required further information can be provided by the corresponding author upon request.


