Summary
Background
The long-term consequences of human umbilical cord-derived mesenchymal stem cell (UC-MSC) treatment for COVID-19 patients are yet to be reported. This study assessed the 1-year outcomes in patients with severe COVID-19, who were recruited in our previous UC-MSC clinical trial.
Methods
In this prospective, longitudinal, cohort study, 100 patients enrolled in our phase 2 trial were prospectively followed up at 3-month intervals for 1 year to evaluate the long-term safety and effectiveness of UC-MSC treatment. The primary endpoint was an altered proportion of whole-lung lesion volumes measured by high-resolution CT. Other imaging outcomes, 6 min walking distance (6-MWD), lung function, plasma biomarkers, and adverse events were also recorded and analyzed. This trial was registered with ClinicalTrials.gov (NCT04288102).
Findings
MSC administration improved in whole-lung lesion volume compared with the placebo with a difference of −10.8% (95% CI: −20.7%, −1.5%, p = 0.030) on day 10. MSC also reduced the proportion of solid component lesion volume compared with the placebo at each follow-up point. More interestingly, 17.9% (10/56) of patients in the MSC group had normal CT images at month 12, but none in the placebo group (p = 0.013). The incidence of symptoms was lower in the MSC group than in the placebo group at each follow-up time. Neutralizing antibodies were all positive, with a similar median inhibition rate (61.6% vs. 67.6%) in both groups at month 12. No difference in adverse events at the 1-year follow-up and tumor markers at month 12 were observed between the two groups.
Interpretation
UC-MSC administration achieves a long-term benefit in the recovery of lung lesions and symptoms in COVID-19 patients.
Funding
The National Key R&D Program of China, the Innovation Groups of the National Natural Science Foundation of China, and the National Science and Technology Major Project.
Keywords: COVID-19, Mesenchymal stem cells treatment, RCT, 1-year follow-up results
Research in context.
Evidence before this study
We performed a PubMed search for studies published, up to July 20, 2021, evaluating the effect of mesenchymal stem cells (MSCs) in patients with COVID-19. The search terms used were “COVID-19″ or “SARS-CoV-2″ and “mesenchymal stem cells” and (“clinical trial” or “randomized controlled trial”). 9 study reports were found, and preliminary data indicated MSCs treatment benefit clinical outcome in the disease. However, no 1-year follow-up results of clinical trial of MSCs treatment in patients with COVID-19 has been reported.
Added value of this study
This study is the first randomised, double-blind, and placebo-controlled clinical trial to further evaluate the long-term safety and efficacy of intravenous infusions of human UC-MSCs in severe COVID-19 patients. MSC medication showed numerically improvement in lung lesion volume compared with the placebo. MSC also contributed to higher proportion of normal CT images, lower incidence of symptoms in the 1-year follow-up. MSC treatment did not affect the production and maintenance of neutralizing antibodies in COVID-19 patients after 1 year. The incidence of adverse events was similar in the two groups.
Implications of all the available evidence
1-year follow-up results indicate that human UC-MSC administration achieves a long-term benefit in the recovery of lung lesions and symptoms in COVID-19 patients with good tolerance.
Alt-text: Unlabelled box
Introduction
Coronavirus disease 2019 (COVID-19) has become a global pandemic and has caused diverse clinical statuses ranging from asymptomatic carriers and mild upper respiratory tract symptoms, to severe acute respiratory distress syndrome.1, 2, 3 As of October 12, 2021, COVID-19 has affected more than 200 countries, resulting in more than 237.5 million identified cases with 4847,462 confirmed deaths.4 Although most people recover from COVID-19 at around 2 to 3 weeks, approximately 10% of patients still have symptoms after 3 weeks and up to several months. Long-term follow-up studies of discharged severe COVID-19 patients have been reported.5,6 Improvement in exercise capacity and pulmonary physiology was found in most patients; however, 76% of patients still experienced at least one symptom 6 months after symptom onset when the patients had more severe illness. Even 12 months after discharge, persistent physiological and radiographic abnormalities remained in some patients with COVID-19. These data indicate that discharged COVID-19 patients, especially those with severe or critical disease, still need suitable intervention to improve their long-term recovery.
Mesenchymal stem cells (MSCs) derived from bone marrow, umbilical cord tissue, adipose tissue, and dental pulp have been widely studied in basic research and clinical applications.7 MSC treatment reduced the pathological changes of the lung and inhibited the abnormal immune-mediated inflammatory response induced by the influenza virus infection in animal models and clinical trials.8, 9, 10 In patients with acute respiratory distress syndrome (ARDS), the number of ventilator-free and organ-failure-free days was numerically fewer in the MSC group than in the placebo group.11,12 Since the outbreak of the COVID-19 pandemic, a series of stem cell therapy clinical trials have been launched and the results showed that MSCs not only lead to remarkable decrease of lung damage and time to recovery, but also improved patient survival with good tolerance in the early phase.2,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 However, it remains unknown how the long-term effectiveness and safety of MSC treatment are in severe COVID-19.
In our previous double-blind, randomized, placebo-controlled trial (ClinicalTrials.gov: NCT04288102), we have reported the short-term safety and effectiveness of UC-MCS treatment, from baseline to day 28 after treatment, and found that UC-MCS administration significantly reduced the proportions of solid component lesion volume in the lungs and numerically increased the 6 min walking distance (6-MWD) compared with the placebo control.13 Herein, we report the 1-year follow-up results of UC-MSCs treatment for severe COVID-19 patients in the double-blind, randomized, placebo-controlled trial.
Methods
Study design and participants
Our previous study was a phase 2, double-blind, randomized, placebo-controlled trial of UC-MSC treatment. A total of 100 patients were finally enrolled and received either UC-MSCs (n = 65) or placebo (n = 35) in addition to standard care.13After the 28-day follow-up, a 3-month follow-up was completed according to the previous protocol.13 Subsequently, the follow-up visit was conducted for all the patients at month 6, month 9, and month 12 in the outpatient clinic of General Hospital of Central Theater Command in Wuhan, Hubei, China to evaluate the safety and effectiveness of the treatment. Written informed consent was obtained from all participants. This study was approved by the Ethics Committee of the Fifth Medical Center, Chinese PLA General Hospital (2020-013-D). This trial was registered with ClinicalTrials.gov (NCT04288102).
Randomization and masking
Eligible patients were randomly assigned in a 2:1 ratio to receive either UC-MSCs or the placebo using an interactive web response management system (IWRS). Patients, investigators, and outcome assessors were all blinded to the treatment allocation. Product Identification Authentication and Tracking System (PIATS) was introduced in this study to manage and track the study products logistics. The concealment of the randomization sequence could be ensured using PIATS and IWRS.13
Procedures
According to the method described in our previous study,13 UC-MSCs were supplied by VCANBIO Cell & Gene Engineering Corp, Tianjin, China (Accession number: VUM01). The UC-MSC product was an almost colourless suspension containing 4.0 × 107 MSC for each procedure in a volume of 100 ml/bag. The placebo had the same medium and appearance in packaging and suspension, but without the MSCs. Three procedures were carried out for each patient (4.0 × 107 cells for each procedure) on day 0, 3, and 6 after randomization. Cell viability was examined by using both 7-AAD/Annexin V staining and trypan blue in our study. The averages of cell viabilities were 94.4% ± 1.9% before shipment and 88.4% ± 4.8% before infusion, respectively. During the follow-up visit, patients were interviewed by trained physicians, underwent a physical examination, and were asked to complete a series of questionnaires regarding symptoms and quality of life (appetite, sleep difficulties, pain or discomfort, fatigue or muscle weakness, anxiety or depression, and usual activity). Other items included chest high-resolution CT (HRCT), a standardized 6-MWD test,26 pulmonary function tests, and blood sample tests, as previously described.
Lung lesions were evaluated by using the changes in high-resolution chest CT(HRCT) images and measured by centralized imaging interpretation based on both lung radiologist analyses and imaging software (LIAIS). The imaging data were derived from a software-assisted lung volumetry and densitometry procedure as described in our previous report.13 After month 3, the boundary of the ground glass lesion was blurred, it is difficult to conduct segmentation of the ground glass lesion area and distinguish from normal lung tissue along with lung repair, but it worked well in evaluation of segmentation of solid component lesions. For this reason, we only evaluated the difference in solid component lesions using LIAIS after 3 months. Additionally, image analysis was performed independently by three radiologists (JH.D, JZ.Z, and MM.Z, who had 22, 25, and 18 years of experience in radiology, respectively) to evaluate the outcomes of lung damage. All radiologists were blinded to the treatment allocation during analysis, and the final outcomes were determined by consensus.
The 6-MWD test was performed according to the ATS practical guidelines.26 We further calculated the predicted values of the same gender, age, and height in healthy adults according to equations for the 6-MWD.6,27(Appendix 1) Ultimately, the results were expressed as measured values, percentages, and differences in predicted values. Pulmonary function and peripheral blood tests were carried out as previously described.13 We also performed a surrogate virus neutralization assay (Appendix 2), which was performed according to the manufacturer's instructions at baseline, day 10, month 1, month 3, month 6, month 9, and month 12, respectively. Blood tumor markers at month 12 were simultaneously assessed.
Imaging and clinical outcomes
As previously reported,13 the primary outcome was a change in the total lesion proportion (%) of the whole lung volume from baseline to month 1, as measured by chest CT. The secondary imaging outcome was the change in the total or solid component lesion proportion (%) of the whole lung volume from baseline to follow-up. Clinical outcomes included the 6-MWD, lung function, plasma biomarkers, and adverse events.
Statistical analysis
This study was designed as a phase 2 clinical exploratory trial. The aim of this study was to describe the 1-year health consequences of patients with COVID-19 who were recruited in our previous UC-MSC clinical trial. There were no predefined hypotheses made in this study; therefore, we focused on description instead of inference for statistical analyses: all statistical tests, confidence intervals, and p-values were used for exploration, not for inference.
Continuous variables were expressed as median (IQR) or mean (SD) and compared between groups using the Wilcoxon rank sum test or t-test. The 95% confidence interval (CI) of the median differences was calculated using the Hodges–Lehmann estimation for the variables that were not normally distributed. Categorical variables were expressed as n/N (%) and compared between groups using the chi-square test or Fisher's exact test, if appropriate. A logistic regression model was used to estimate odds ratios (ORs). The mITT population was considered as the primary analysis population, and safety analysis was performed for all patients who started their assigned treatment. If the patient missed a chest CT scan, the last scan's results were carried forward to the missing visit for primary endpoints in the analysis. Other missing values were not imputed. Statistical analyses were performed using SAS software (version 9.4; Cary, NC, USA), while the figures were generated using the R software (version 4.1.0).
Role of the funding source
This study was supported by the National Key R&D Program of China (2020YFC0841900, 2020YFC0844000, 2020YFC08860900), the Innovation Groups of the National Natural Science Foundation of China (81,721,002), and the National Science and Technology Major Project (2017YFA0105703). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
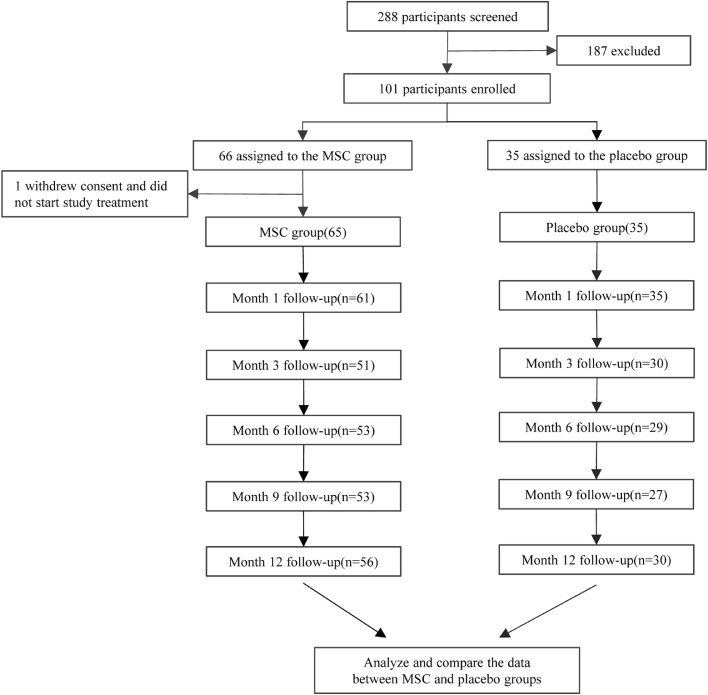
One hundred and one eligible patients were randomised in a 2:1 ratio (66 to the UC-MSC group and 35 to the placebo group). Finally, there were 65 and 35 patients in UC-MSC and placebo group, respectively 13, since a patient withdrew her informed consent after randomization. The baseline characteristics were highly consistent between the two groups of patients, including age, sex, body weight, time from symptom onset, distribution of comorbidities, concomitant medication, median time from symptoms onset to study baseline and lesion proportions assessment from chest CT, etc. (Table 1). From April 8, 2020, to March 31, 2021, patients received follow-up visit for a median of 32 days (IQR 30–35) at month 1, 89 days (88–90) at month 3, 172 days (171–173) at month 6, 266.5 days (265–268) at month 9, and 362.5 days (360–367) at month 12 (Figure 1). Of the 100 enrolled patients, 81 patients (51/65 in MSC group, 30/35 in placebo group) at month 3, 82 patients (53/65 in the MSC group, 29/35 in the placebo group) at month 6, 80 patients (53/65 in MSC group, 27/35 in placebo group) at month 9, and 86 patients (56/65 in MSC group, 30/35 in placebo group) at month 12 were assessed. One patient in the placebo group died of liver cancer 3 months after enrollment.
Table 1.
Baseline characteristics.13
| UC-MSC group (n = 65) | Placebo group (n = 35) | |
|---|---|---|
| Age, years | 60.72(9.14) | 59.94(7.79) |
| Sex – no. (%) | ||
| Men | 37 (56.92%) | 19 (54.29%) |
| Women | 28 (43.08%) | 16 (45.71%) |
| BMI (Body Mass Index), Kg/m2* | 24.71(3.19) | 25.01(3.12) |
| Time from symptom onset to baseline, days | 45.00(39.00,51.00) | 47.00(41.00,53.00) |
| Any comorbidities | 34 (52.31%) | 18 (51.43%) |
| Hypertension | 17 (26.15%) | 10 (28.57%) |
| Diabetes | 12 (18.46%) | 5 (14.29%) |
| Chronic bronchitis | 2(3.08%) | 3(8.57%) |
| Chronic obstructive pulmonary disease | 2(3.08%) | 0(0.00%) |
| Concomitant medication | ||
| Antiviral drugs | 32 (49.23%) | 20 (57.14%) |
| Antibiotics | 27 (41.54%) | 12 (34.29%) |
| Corticosteroids | 13 (20.00%) | 9 (25.71%) |
| Lesion proportion (%): total lesion volume (in cm3) / whole lung volume (in cm3) | 26.31(11.62,38.42) | 27.98(11.57,44.14) |
| Solid component lesion proportion (%): Solid component lesion volume (in cm3) / whole lung volume (in cm3) | 2.59(0.69,5.20) | 2.52(0.77,4.91) |
| Six-category scale | ||
| 2-Hospitalized, not requiring supplemental oxygen | 14 (21.54%) | 10 (28.57%) |
| 3-Hospitalized, requiring supplemental oxygen | 50 (76.92%) | 25 (71.43%) |
| 4-Hospitalized, on noninvasive ventilation or high flow oxygen devices | 1 (1.54%) | 0 (0.00%) |
| White blood cell count* (109/L) | 5.70(5.00,6.60) | 5.80(5.00,6.80) |
| Lymphocyte count (109/L) | 1.39(1.19,1.80) | 1.47(1.24,1.84) |
| CD4 T cells (/µl) † | 641.00(482.00,760.00) | 734.00(502.00,1031.00) |
| CD8 T cells (/µl) † | 371.00(275.00,520.00) | 401.00(307.00,593.00) |
| B cells (/µl) † | 148.50(99.60,251.00) | 148.50(94.70,248.00) |
| NK cells (/µl) † | 233.50(151.00,393.00) | 197.50(136.00,309.00) |
| Neutrophil count (109/L) | 3.48(2.91,4.32) | 3.83(2.85,4.48) |
| Platelet count (109/L) | 214.00(174.00,255.00) | 210.00(176.00,247.00) |
| Hemoglobin (g/L) | 122.68 (14.44) | 124.26 (11.83) |
| D-dimer (mg/L) ‡ | 0.58 (0.36,1.11) | 0.56 (0.31,1.12) |
| IL-6 (pg/ml) § | 7.86(5.63,9.84) | 8.76(6.54,11.77) |
| CRP(mg/L) ¶ | 1.95(0.84,3.53) | 1.38(0.68,2.26) |
| SARS-CoV-2 test result‖ | ||
| SARS-Cov-2 IgG positive | 63 (100.00%) | 34 (100.00%) |
| SARS-Cov-2 IgM positive | 58 (92.06%) | 32 (94.12%) |
| SARS-Cov-2 nucleic acid detection positive | 47(72.31%) | 20(57.14%) |
Data are median (interquartile range (IQR)), n (%), or mean (SD).
BMI values were available for 59 patients in the UC-MSC group and 33 patients in the placebo group.
CD4, CD8, CD19, and CD56 values were available for 62 patients in the UC-MSC group and 34 patients in the placebo group.
D-dimer values were available for 55 patients in the UC-MSC group and 29 patients in the placebo group.
IL-6 values were available for 64 patients in the UC-MSC group and 35 patients in the placebo group.
CRP values were available for 27 patients in the UC-MSC group and 14 patients in the placebo group.
The test results are summarized from hospitalization to the pre-random test. If there is any positive, it is defined as positive. The IgG and IgM values were available for 63 patients in the UC-MSCs group and 34 patients in the placebo group.
Figure 1.
Trial profile.
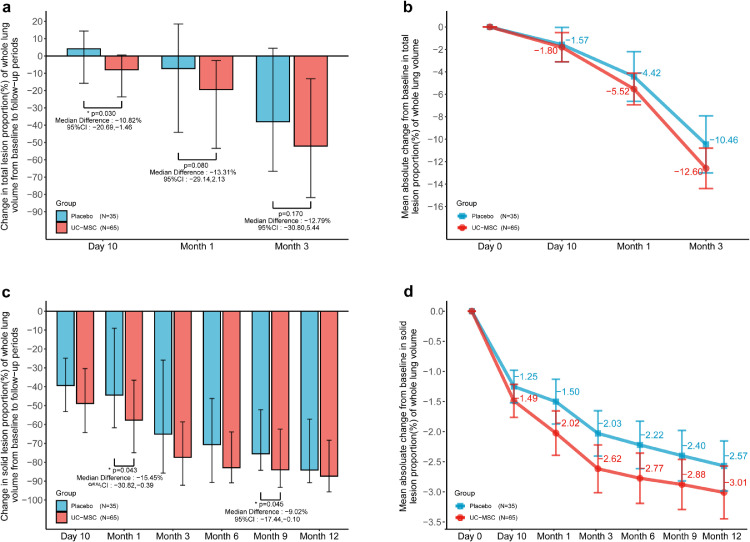
To evaluate the difference in lung lesions between the MSC and placebo groups, we measured the lesions by using centralized imaging interpretation based on the evaluation of both radiologist analyses and lung imaging artificial intelligence software. Through comparison of the Hodges–Lehmann estimator of the total lesion proportion (%) of the whole lung volume by LIAIS, the median change was −8.0 (−23.6, 0.5) in the MSC group and 4.1 (−16.6, 14.8) in the placebo group at day 10 with a difference of −10.8% (95% CI: −20.7%, −1.5%, Wilcoxon, p = 0.030); −19.4 (−53.4, −2.6) in the MSC group and −7.3 (−46.6, −19.1) in the placebo group at month 1 with a difference of −13.3% (95% CI, −29.1%, 2.1%, Wilcoxon, p = 0.080); −52.1% (95% CI, −81.8%,−13.1%) in the MSC group and −38.0% (95% CI, −71.6%, 6.8%) in the placebo group at month 3 with a difference of −12.8% (95% CI, −30.8%, 5.5%, Wilcoxon, p = 0.170) (Figure 2, Table 2). Interestingly, in the evaluation of the solid component lesions as a specific lesion type, we found that the median change was −77.4% (−92.2%,−58.6%) in the MSC group and −65.1% (−87.3%,−16.3%) in the placebo group at month 3; −82.89% (−90.9%, −63.9%) in the MSC group and −70.65% (−92.0%, −43.0%) in the placebo group at month 6; −84.0% (−93.3%,−62.5%) in the MSC group and −75.5% (−84.3%, −51.3%) in the placebo group at month 9; median change of −87.4% (−95.7%, −68.4%) in the MSC group and −84.1% (−92.0%,−54.0%) in the placebo group at month 12 from baseline, respectively. UC-MSC administration exerted a numerical improvement at each follow-up point (Figure 2, Table 2).
Figure 2.
The effect of human umbilical cord-mesenchymal stem cells (UC‑MSCs) on the lung damage in patients with severe COVID-19. (a, b: total lesion; c, d: solid component lesion).
(a) shows the between-group median difference in the change in total lesion proportion (%) of the whole lung volume from baseline to month 3. I bars indicate the Q1(the first quartile),Q3(the third quartile).
(b) shows the mean absolute change from baseline to month 3 in the total lesion proportion (%) of the whole lung volume. I bars indicate the standard error.
(c) shows the between-group median difference in the change in solid component lesion proportion (%) of the whole lung volume from baseline to month 12. I bars indicate the Q1(the first quartile),Q3(the third quartile).
(d) shows the mean absolute change from baseline to month 12 in solid component lesion proportion (%) of the whole lung volume. I bars indicate the standard error.
Group difference assessed by Wilcoxon rank sum test. The 95% CI calculated by Hodges–Lehmann estimation.
Table 2.
Comparison of lung lesion imaging and 6 min walking distance (6-MWD) between MSC and placebo groups throughout 1-year follow-up visit.
| MSC group | Placebo group | Difference/OR (95% CI) | p value | |
|---|---|---|---|---|
| Change in the total lesion proportion (%) of the whole lung volume from baseline ‡ | ||||
| Day 10 | −7.99(−23.63, 0.51) | 4.13 (−16.58, 14.83) | −10.82(−20.69, −1.46)† | 0.030§ |
| Month 1 | −19.40(−53.40, −2.62) | −7.30(−46.59, −19.12) | −13.31(−29.14,2.13) † | 0.080§ |
| Month 3 | −52.06(−81.82, −13.13) | −38.00(−71.58,6.83) | −12.79(−30.80,5.44) † | 0.170§ |
| Change in solid component lesion proportion (%) of whole lung volume from baseline ‡ | ||||
| Month 1 | −57.70(−74.95, −36.57) | −44.45(−62.24, −8.82) | −15.45(−30.82,−0.39) † | 0.043§ |
| Month 3 | −77.37(−92.23, −58.63) | −65.12(−87.32, −16.26) | −9.77(−24.40,1.86) † | 0.099§ |
| Month 6 | −82.89(−90.88, −63.95) | −70.65(−91.95, −42.98) | −6.97(−19.10,2.98) † | 0.174§ |
| Month 9 | −84.00(−93.29, −62.49) | −75.51(−84.32, −51.32) | −9.02(−17.44,−0.10) † | 0.045§ |
| Month 12 | −87.35(−95.69, −68.39) | −84.13(−91.98, −53.92) | −4.65(−11.05,2.02) † | 0.161§ |
| Number of normal chest CT images | ||||
| Month 1 | 0/58 (0.00) | 0/34 (0.00) | NA | NA |
| Month 3 | 3/50 (6.00) | 0/30 (0.00) | 2.38(0.35,+∞)‖ | 0.239 |
| Month 6 | 6/51 (11.76) | 0/27 (0.00) | 4.72(−0.86,+∞)‖ | 0.070 |
| Month 9 | 6/53 (11.32) | 0/27 (0.00) | 4.52(0.82,+∞)‖ | 0.076 |
| Month 12 | 10/56 (17.86) | 0/30 (0.00) | 8.75(1.72,+∞)‖ | 0.010 |
| 6-MWD (meters) & | ||||
| Month 1* | 420.00(392.00,465.00) | 403.00(352.00,447.00) | 24.00(0.00,57.00) † | 0.057§ |
| Month 3 | 440.00(412.00,471.00) | 420.00(390.00,460.00) | 18.00(−8.00,45.00) † | 0.196§ |
| Month 6 | 447.00(426.00,489.00) | 450.00(420.00,480.00) | 6.00(−15.00,27.00) † | 0.561§ |
| Month 9 | 477.00(436.50,490.50) | 456.00(420.00,495.00) | 12.00(−9.00,35.00) † | 0.359§ |
| Month 12 | 478.50(432.00,492.00) | 441.00(424.00,501.00) | 12.00(−9.00,39.00) † | 0.214§ |
| 6-MWD (% of predicted value) & | ||||
| Month 1* | 81.24(72.78,89.32) | 77.06(65.44,84.67) | 3.83(−2.41,9.90) † | 0.236§ |
| Month 3 | 83.42(74.66,90.22) | 80.69(72.23,84.83) | 3.55(−1.75,8.70) † | 0.209§ |
| Month 6 | 86.79(81.56,97.26) | 84.74(79.06,88.77) | 3.27(−1.58,8.52) † | 0.197§ |
| Month 9 | 90.35(81.28,99.35) | 85.19(81.82,90.58) | 3.17(−1.85,8.40) † | 0.241§ |
| Month 12 | 90.28(83.67,98.77) | 85.72(82.21,91.53) | 4.41(−0.06,8.89) † | 0.054§ |
Data are n/N (%) or median (IQR), unless otherwise specified. The differing denominators used indicate missing data.
Differences are expressed as Hodges–Lehmann estimator and 95% confidence interval (CI).
In the 6-MWD, there were three cases who could not complete the test because of cardiopulmonary function problems. The data were calculated as 0 m at Month 1.
NA=not applicable.
AThe available values of lung lesion imaging were 65 in the MSC group and 35 in the placebo group.
& The available values of 6 min walking distance (6-MWD) refer to the Figure 4.
Group difference assessed by Wilcoxon rank sum test.
Calculated by the exact logistic regression model. OR= odds ratio.
These p values are provided for descriptive purposes only.
Three radiologists independently assessed lung damage at baseline, months 1, 3, 6, 9, and 12. Unexpectedly, abnormal CT images, which presented as ground-glass opacity (GGO), interlobular septal thickening, reticular opacity, fibrous stripes, air bronchogram sign, crazy-paving pattern, and honeycomb pattern were found in up to 92.3% (72/78) of patients at month 6 and 88.4% (76/86) patients at month 12 (Appendix 3, Appendix 4). Of note, 6 (6/51, 11.8%) patients had normal CT images in the MSC group, but none of the patients in the placebo group exhibited normal CT findings at month 6 (Fisher, p = 0.087). Moreover, normal CT images were found in 10 (10/56, 17.9%) patients in the MSC group but not in the placebo group at month 12(Fisher, p = 0.013, Table 2).
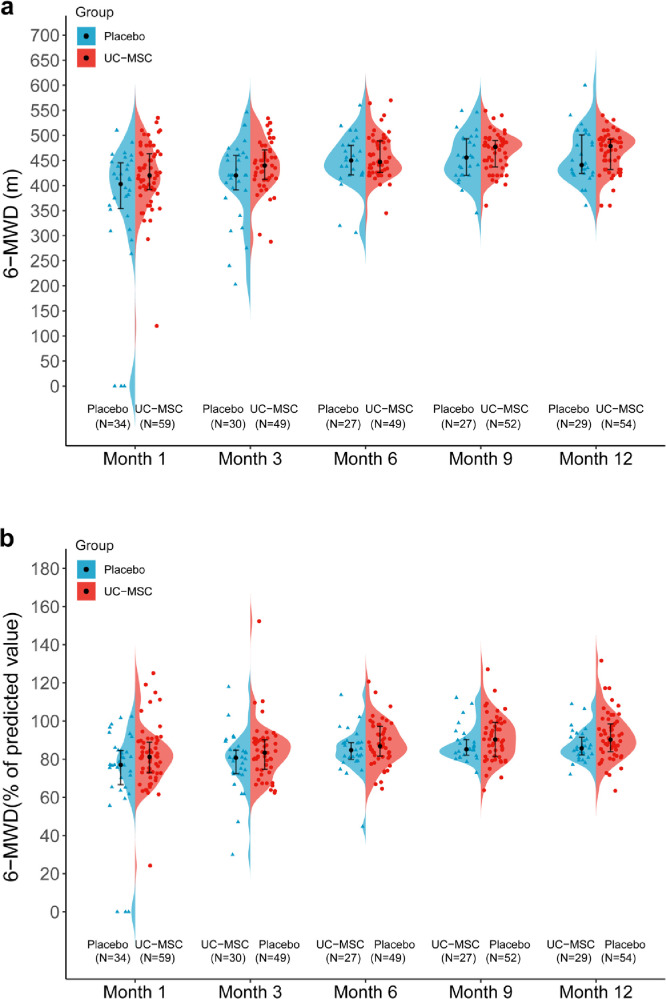
To compare the long-term restoration of lung function and integrated reserve capability between the two groups of patients, we continued to examine the 6-MWD at months 3, 6, 9, and 12 (Figure 3, Table 1). The median 6-MWD test gradually increased over time in both groups. In the MSC group, the results were from 440 m (IQR 412–471) at month 3 to 478 m (432–492) at month 12. In contrast, the results were from 420 m (IQR 390–460) at month 3 to 441 m (424–501) at month 12 in the placebo group. If normalized to the predicted values were calculated according to a previous method,6,27 the 6-MWD showed a numerically increased distance in the patients treated with UC-MSCs compared with the placebo group at each follow-up point. The median percentage was 83.4% (IQR, 74.7% and 90.2%) in the MSC group, and 80.7% (72.2%, 84.8%) in the placebo group at month 3; 86.8% (81.6%, 97.3%) in the MSC group and 84.7% (79.1%, 88.8%) in the placebo group at month 6; 90.4% (81.3%, 99.4%) in the MSC group and 85.2% (81.8% and 90.6%) in the placebo group at month 9; 90.3% (83.7%, 98.8%) in the MSC group and 85.7% (82.2%, 91.5%) in the placebo group at month 12. However, there were no statistically significant differences between the two groups (Figure 3, Table 2). We also calculated the deviation between the measured and predicted values, and a similar increasing trend was observed in the MSC group (Appendix 5).
Figure 3.
Comparison of UC‑MSCs on long-term follow-up 6MWD in patients with severe COVID-19.
(a) and (b) show temporal changes in 6-MWD in the MSC and placebo groups at 3, 6, 9, and 12 months after enrollment.
(a): absolute 6MWD. I bars indicate Q1(denotes the first quartile), Q3(the third quartile), and points indicate the median.
(b): normalized to predicted values(%). Data are presented as median (interquartile range). I bars indicate Q1(denotes the first quartile), Q3(the third quartile), and points indicate the median.
6MWD=6 min walking distance.
No significant differences in pulmonary function test parameters (including DLCO, functional residual capacity, VCmax, residual volume, total lung capacity, and vital capacity) were found between the MSC and placebo groups in the long-term follow-up (Appendix 6).
It has been reported that a substantial number of COVID-19 patients still experienced various symptoms 6 months after the onset of acute infection.5 Consistently, we found that 70.7% (58/82) of enrolled patients at month 6 and 68.6% (59/86) at month 12 reported at least one symptom since disease onset, and the proportions were much higher in the placebo group than in the MSC group at months 6 (64.2%, 34/53 in the MSC group; 82.8%, 24/29 in the placebo group, chi-square, p = 0.077) and at month 12 (62.5%, 35/56 in MSC group; 80.0%, 24/30 in placebo group, chi-square, p = 0.096). In addition, the incidence of fatigue or muscle weakness, sleep difficulties, pain, and usual activity was lower in the MSC group than in the placebo group (Table 3) at each follow-up time. The numerical rating scales (NRS) for pain was completed in 85 patients at month 12. The median score of NRS was 0 in the MSC group and 1 in the placebo group, with a difference of 0.00 (95% CI −1.00,0.00, Wilcoxon, p = 0.031). The percentages of patients with no pain (NRS=0), mild pain (1–3), moderate pain (4–6), and severe pain (7–10) were 65.5%, 32.7%, 1.8%, and 0.0% in the MSC group and 43.3%, 40.0%, 13.3%, and 3.3% in the placebo group, respectively, which indicates a difference of 0.34 (95% CI 0.14, 0.83, proportional odds model, p = 0.018, Appendix 7) between the two groups.
Table 3.
Comparison of symptoms and health-related quality of life between MSC and placebo groups throughout 1-year follow-up visit.
| MSC group | Placebo group | OR(95% CI) | p value | |
|---|---|---|---|---|
| Loss of appetite | ||||
| Month 1 | 7/60 (11.67) | 6/35 (17.14) | 0.64 (0.20,2.08) † | 0.450 |
| Month 3 | 5/51 (9.80) | 6/30 (20.00) | 0.43 (0.12,1.57) † | 0.313 |
| Month 6 | 3/53 (5.77) | 2/29 (6.90) | 0.81 (0.13,5.15) † | 1.000 |
| Month 9 | 5/53 (9.43) | 2/27 (7.41) | 1.30 (0.24,7.20) † | 1.000 |
| Month 12 | 5/56 (8.93) | 2/30 (6.67) | 1.37 (0.25,7.54) † | 1.000 |
| Sleep difficulties | ||||
| Month 1 | 35/60 (58.33) | 22/35 (62.86) | 0.83 (0.35,1.95) † | 0.664 |
| Month 3 | 14/51 (27.45) | 20/30 (66.67) | 0.19 (0.07,0.50) † | 0.001 |
| Month 6 | 17/53 (32.08) | 15/29 (51.72) | 0.44 (0.17,1.12) † | 0.081 |
| Month 9 | 11/53 (20.75) | 10/27 (37.04) | 0.45 (0.16,1.24) † | 0.118 |
| Month 12 | 12/56 (21.43) | 12/30 (40.00) | 0.41 (0.16,1.08) † | 0.067 |
| Pain or discomfort | ||||
| Month 1 | 10/60 (16.67) | 5/35 (14.29) | 1.20 (0.37,3.85) † | 0.759 |
| Month 3 | 7/51 (13.73) | 9/30 (30.00) | 0.37 (0.12,1.13) † | 0.076 |
| Month 6 | 12/50 (24.00) | 12/28 (42.86) | 0.41 (0.16,1.10) † | 0.083 |
| Month 9 | 12/53 (22.64) | 7/27 (25.93) | 0.84 (0.29,2.45) † | 0.744 |
| Month 12 | 20/56 (35.71) | 17/30 (56.67) | 0.42 (0.17,1.05) † | 0.061 |
| Fatigue or muscle weakness | ||||
| Month 1 | 27/60 (45.00) | 21/34 (61.76) | 0.55 (0.23,1.27) † | 0.118 |
| Month 3 | 11/51 (21.57) | 10/30 (33.33) | 0.55 (0.20,1.51) † | 0.243 |
| Month 6 | 12/53 (22.64) | 11/29 (37.93) | 0.48 (0.18,1.29) † | 0.141 |
| Month 9 | 13/53 (24.53) | 11/27 (40.74) | 0.47 (0.18,1.27) † | 0.135 |
| Month 12 | 20/56 (35.71) | 15/30 (50.00) | 0.56 (0.23,1.37) † | 0.199 |
| Decreased usual activity | ||||
| Month 1 | 34/60 (56.67) | 22/35 (62.86) | 0.77 (0.33,1.82) † | 0.554 |
| Month 3 | 28/51 (54.90) | 21/30 (70.00) | 0.52 (0.20,1.36) † | 0.180 |
| Month 6 | 5/51 (9.80) | 6/29 (20.69) | 0.40 (0.11,1.45) † | 0.194 |
| Month 9 | 1/53 (1.89) | 2/27 (7.41) | 0.24 (0.02,2.78) † | 0.262 |
| Month 12 | 2/55 (3.64) | 6/29 (20.69) | 0.15 (0.03,0.79) † | 0.018 |
| Anxiety or depression | ||||
| Month 1 | 4/60 (6.67) | 6/35 (17.14) | 0.35 (0.09,1.32) † | 0.120 |
| Month 3 | 1/50 (2.00) | 0/30 (0.00) | 0.60 (0.03,+∞) § | 0.625 |
| Month 6 | 1/52 (1.92) | 0/29 (0.00) | 0.59 (0.03,+∞) § | 0.642 |
| Month 9 | 1/52 (1.92) | 0/27 (0.00) | 0.52 (0.03,+∞) § | 0.658 |
| Month 12 | 5/56 (8.93) | 6/30 (20.00) | 0.39 (0.11,1.41) † | 0.152 |
Data are n/N (%), unless otherwise specified. The differing denominators used indicate missing data.
Group difference assessed by chi-square test or Fisher's exact test.
Calculated by the logistic regression model. OR = odds ratio.
Calculated by the exact logistic regression model. OR = odds ratio.
These p values are provided for descriptive purposes only.
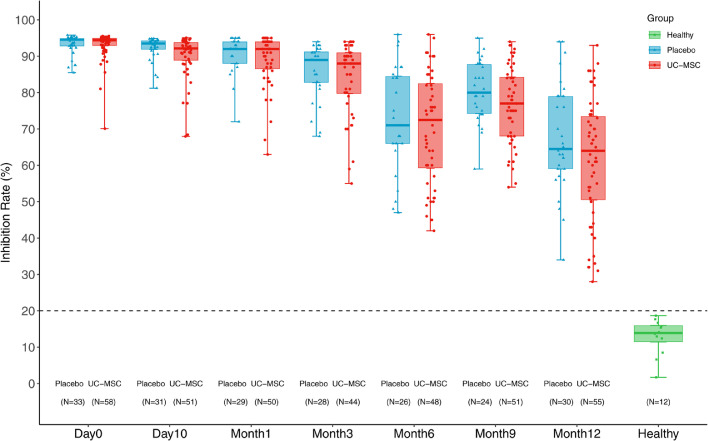
The inhibition rate (IR) of neutralizing antibodies gradually decreased from baseline to the 1-year follow-up in both groups. However, the IR were all positive (over 20%) with a similar median (61.6% vs. 67.6%) in the MSC group and placebo group at month 12, which was higher than that of healthy individuals (Figure 4). The subsets (naïve, central memory, effector memory, and terminally differentiated effector memory) and functional markers (PD-1, HLA-DR, and CD38) of peripheral blood T-cells were assessed using flow cytometric analyses at month 12. There was no significant difference in these parameters between CD4 T-cells and CD8 T-cells between the two groups (Appendix 8).
Figure 4.
Inhibition rate (IR) of neutralizing antibodies.
The inhibition rate (IR) of neutralizing antibodies decreased gradually from baseline to the 1-year follow-up. However, the IR were all positive (over 20%) with a similar median (61.6% vs. 67.55%) in either the MSC group or placebo group at 12 months, which was higher than that in healthy people. The bars indicate the minimum and maximum values.
The total incidence of adverse events reported during the 1-year follow-up was similar in the MSC group (83.1%) and the placebo group (74.3%) (Table 4). The most common adverse event in the MSC group was a 21.5% increase in lactic acid dehydrogenase, compared with 20% in the placebo group; a 13.9% elevation of serum alanine aminotransferase compared with 11.4% in the placebo group; a 13.9% increase in creatine phosphokinase compared with 14.3% in the placebo group; a 9.2% increase in aspartate aminotransferase compared with 11.4% in the placebo group; 9.2% increase in uric acid compared with 8.6% in the placebo group; and 9.2% increase in hypokalemia compared with 2.9% in the placebo group. There were a few other adverse events at grade 1 or 2 in both groups. After the 28-day follow-up, no grade 3–4 adverse events occurred in either group. All adverse events during the follow-up period were judged by the site investigators and found to be unrelated to the UC-MSC intervention. One patient in the placebo group died of liver cancer. To further clarify the long-term tumorigenicity of MSC treatment, we compared the tumor markers between the two groups of patients at month 12 (Appendix 9, Appendix 10). No significant differences were observed between the two groups.
Table 4.
Summary and comparison of adverse events that occurred between MSC and placebo groups throughout 1-year follow-up visit.
| Adverse Event Name | MSC group |
Placebo group |
||
|---|---|---|---|---|
| Grade 1 or 2 n (%) | Grade 3 or 4 n (%) | Grade 1 or 2 n (%) | Grade 3 or 4 n (%) | |
| Any adverse event | 53(81.54) | 1(1.54) | 26(74.29) | 0(0.00) |
| Blood lactate dehydrogenase increased | 14(21.54) | 0(0.00) | 7(20.00) | 0(0.00) |
| Alanine aminotransferase increased | 9(13.85) | 0(0.00) | 4(11.43) | 0(0.00) |
| Blood creatine phosphokinase increased | 9(13.85) | 0(0.00) | 5(14.29) | 0(0.00) |
| Aspartate aminotransferase increased | 6(9.23) | 0(0.00) | 4(11.43) | 0(0.00) |
| Blood uric acid increased | 6(9.23) | 0(0.00) | 3(8.57) | 0(0.00) |
| Hypokalaemia | 6(9.23) | 0(0.00) | 1(2.86) | 0(0.00) |
| Blood urea increased | 4(6.15) | 0(0.00) | 4(11.43) | 0(0.00) |
| Diarrhoea | 4(6.15) | 0(0.00) | 0(0.00) | 0(0.00) |
| Interleukin level increased | 4(6.15) | 0(0.00) | 2(5.71) | 0(0.00) |
| Anaemia | 3(4.62) | 0(0.00) | 0(0.00) | 0(0.00) |
| Palpitations | 3(4.62) | 0(0.00) | 0(0.00) | 0(0.00) |
| Ventricular extrasystoles | 3(4.62) | 0(0.00) | 2(5.71) | 0(0.00) |
| Abdominal distension | 2(3.08) | 0(0.00) | 0(0.00) | 0(0.00) |
| Cough | 2(3.08) | 0(0.00) | 1(2.86) | 0(0.00) |
| Dizziness | 2(3.08) | 0(0.00) | 0(0.00) | 0(0.00) |
| Gamma-glutamyltransferase increased | 2(3.08) | 0(0.00) | 1(2.86) | 0(0.00) |
| Abdominal pain | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Anxiety | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Atrioventricular block first degree | 1(1.54) | 0(0.00) | 1(2.86) | 0(0.00) |
| Bacterial infection | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Brain natriuretic peptide increased | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Bundle branch block left | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Bundle branch block right | 1(1.54) | 0(0.00) | 1(2.86) | 0(0.00) |
| C-reactive protein increased | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Cardiac failure | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Dysgeusia | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Dyspepsia | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Functional gastrointestinal disorder | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Gastrooesophageal reflux disease | 1(1.54) | 0(0.00) | 1(2.86) | 0(0.00) |
| Gingivitis | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Heart rate increased | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Metabolic alkalosis | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Nausea | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Neutrophil count increased | 1(1.54) | 0(0.00) | 1(2.86) | 0(0.00) |
| Pharyngeal disorder | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Pharyngitis | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Poor quality sleep | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Pulmonary oedema | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Rash | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Regurgitation | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Supraventricular extrasystoles | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Tension | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Thirst | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Toothache | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Urinary tract infection | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| Vomiting | 1(1.54) | 0(0.00) | 0(0.00) | 0(0.00) |
| White blood cell count increased | 1(1.54) | 0(0.00) | 1(2.86) | 0(0.00) |
| Blood creatine phosphokinase MB increased | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Blood creatinine increased | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Electrocardiogram Q wave abnormal | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Electrocardiogram ST-T segment abnormal | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Hepatic cyst | 0(0.00) | 0(0.00) | 2(5.71) | 0(0.00) |
| Hypocalcaemia | 0(0.00) | 0(0.00) | 2(5.71) | 0(0.00) |
| Initial insomnia | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Lymphocyte percentage decreased | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Pleural effusion | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
| Pneumothorax | 0(0.00) | 1(1.54) | 0(0.00) | 0(0.00) |
| Pruritus | 0(0.00) | 0(0.00) | 3(8.57) | 0(0.00) |
| Respiratory alkalosis | 0(0.00) | 0(0.00) | 1(2.86) | 0(0.00) |
Discussion
To our knowledge, this is the first and largest prospective long-term follow-up study of MSC therapy in patients with severe COVID-19. In our randomized placebo-controlled clinical trials,13 we verified the safety and preliminary efficacy of UC-MSCs as an adjunctive therapy for severe COVID-19 patients with lung damage at day 28. Consistently, this study showed that UC-MSC administration still exerted improvement in whole lung lesions in severe COVID-19 patients at month 3. Furthermore, we found that UC-MSC medication increased the resolution of lung solid component lesions compared with the placebo at each follow-up time. More interestingly, 17.9% of patients in the MSC group had normal CT images at month 12, while no patient recovered from lung damage in the placebo group at this point. In addition, MSC-related predefined adverse events were not observed throughout the 12-month follow-up period. The incidences of adverse events and evaluated tumor markers were similar between the MSC and placebo groups. These findings demonstrate that UC-MSC medication not only achieved a beneficial short-term effect but also exerted a long-term therapeutic benefit on lung lesions with good tolerance in severe COVID-19 patients.
The 6-MWD is an important parameter reflecting the integrated reserve capability of complex physiology, including the pulmonary and cardiovascular systems, and neuromuscular circulation.26 Our current study indicated that the 6-MWD was numerically increased in patients treated with UC-MSCs at each follow-up point compared with the control group. Considering the improvement of lung damage by UC-MSC delivery, we speculated that the increase in 6-MWD in UC-MSCs-treated patients may be partly ascribed to restoration of the lung reserve capability. Pulmonary function was also assessed for a comprehensive assessment in this study. It should be noted that residual abnormalities of lung diffusion capacity were observed in about half of the patients (33/63) at 12 months after disease onset in both groups. It is somewhat disappointing that significant differences in pulmonary function test parameters were not observed between the two groups. This may be attributed to the relatively small sample size, together with that some patients refused to be tested or were not suitable for pulmonary function examination during the follow-up visits. In this study, only patients in the placebo group died of liver cancer and no mortality difference was observed between two groups. In another randomized controlled trial,14 UC-MSC infusion significantly improved patient survival, time to recovery and serious adverse events in one month compared with the control, in which, 11 of 24 enrolled severe patients (46%) received invasive mechanical ventilation and 13 of them (54%) were with high flow oxygen therapy via noninvasive ventilation prior to initiation of MSC treatment. Therefore, the discrepancy between the above-mentioned studies may be due to the enrollment of different patients.
It has been reported that fatigue, muscle weakness, and sleep difficulties were the most common symptoms for COVID-19 patients 6 months after disease onset.5 Some COVID-19 patients still suffer from persistent pulmonary physiological abnormalities 12 months after discharge.6 Consistent with previous studies, 68.6% of COVID-19 enrolled patients in our cohort were still troubled with at least one symptom at the 12-month follow-up, although they recovered over time. Intriguingly, we observed a better alleviation of symptoms and quality of life in patients with UC-MSC treatment. The underlying mechanism of symptom alleviation in the UC-MSC group is likely to be multifactorial, but we believe that the improvement of lung damage at least partly contributed to this benefit. The potential mechanism for MSCs effect is not fully elucidated yet. It has been reported that MSCs have differentiational and regenerative properties and can secrete hepatocyte growth factor, vascular endothelial growth factor, and keratinocyte growth factor to promote the regeneration of type II alveolar epithelial cells. In addition, MSCs can be attracted to inflammatory sites through corresponding chemokines and then modulate the functions of various immunocytes through direct contact and paracrine effects.28,29 It is possible that the long-term effects of MSCs was derived from the initial biological effects after MSC therapy.
With the continuous prevalence of COVID-19, the long-term consequences of survivors have raised a serious concern. Patients with more severe diseases had more abnormal chest CT images and impaired pulmonary diffusion capacities after 6 months of discharge.5 In this study, all enrolled patients were of the severe type, and only 10.5% (9/86) of patients had normal chest CT images at month 12, which was much lower than that in other studies. Thus, severe COVID-19 survivors are the main target population for intervention of long-term recovery. Our previous study revealed that UC-MSC medication remarkably accelerated the resolution of lung lesions in severe COVID-19 patients, including those in the convalescent stage.13 Of note, our current investigation showed that a complete resolution of lung lesions as detected by CT scan was only achieved in patients treated with UC-MSCs at 12-month follow-up. Thus, it may be worth evaluating the effect of UC-MSCs on pulmonary damage in convalescent patients in further clinical trials.
With mass vaccination worldwide, the generation and maintenance of neutralizing antibodies in COVID-19 were interesting issues.30, 31, 32 Herein, we found that the IR of neutralizing antibody remained positive at month 12 in both groups of patients, although it gradually decreased over time. Of note, there was no significant difference in neutralizing antibody titers between the two groups, indicating that MSC treatment did not affect the production and maintenance of neutralizing antibodies in COVID-19 patients.
This study has several limitations. First, the trial protocol was established in early February 2020 when the COVID-19 epidemic was still prevalent in China. The understanding of COVID-19 was still limited at that point. To ensure safety, the treatment dosage was 4.0 × 107 cells for each procedure, and three procedures were carried out for each patient on days 0, 3, and 6 after randomization. The optimal therapeutic regimen, including the dosage, interval duration, and number of cycles for MSC medication, remains to be clarified in future trials. Second, the sample size was not large enough. We recruited 100 patients in this study, and 14 patients were lost to follow-up at month 12. A larger sample size will improve efficacy analyses. Third, the baseline data of pulmonary function and 6-MWD were unavailable as it was not practical to conduct the test at baseline, which may underestimate the interpretation of these parameters.
In conclusion, our current study further demonstrates that UC-MSC administration exerted a long-term beneficial effect on lung lesions and symptom relief with good tolerance, indicating that the use of UC-MSCs as adjunctive therapy for severe COVID-19 patients is a feasible option. Since most severe COVID-19 patients still suffer from pulmonary disability 12 months after viral resolution, the therapeutic benefit of UC-MSCs deserves to be appraised in future trials for COVID-19-convalescent patients with severe pulmonary damage.
Declaration of interests
YZ is a current employee of Vcanbio Cell & Gene Engineering Co., Ltd. All authors declare no competing interests.
Acknowledgments
Contributors
FSW proposed initial proposal. FSW, WFX and LS designed the study and developed the protocol. LS, XY, WQY, ZX, LH and JLF were responsible for study enrolment. LS, XY, WQY, YGL, LH, ZX, JLF, MS, SYW and TTL were responsible for acquisition, analysis, and interpretation of data. CZ, JWS, YZ, YYL, RNX, GSL and YBX were responsible for biorepository management and biomarker analyses. FSW, WFX, LS, WQY, CZ, JWS and TTL verified the underlying data. JMC and JHD performed image analysis. LS, WQY and WFX drafted the manuscript. FSW and WFX critically revised the manuscript. All authors contributed to conducting the trial. All authors revised the report and read and approved the final version before submission.
Acknowledgement
We thank Xiaojing Jiang, Xuechun Lu, Fang Lian, Baoqiang Fu, Yan Zhang, Yingying Gao, Guanzhen Wang, Hongbin Pu, Junli He and Guiyue Shen for their excellent work in follow-up of all enrolled patients. We thank Chen Yao for statistical consultation. We also thank randomization system provider (Chengdu Cims-medtech), PIATS provider (Alibaba Health Technology), LIAIS provider (Shanghai united imaging intelligence Healthcare co. Ltd.), independent statisticians (Xiaoyan Yan, Yongpei Yu, Zhe Cao, Yuanfa Cai), radiologists (Jianzeng Zhang, Mengmeng Zhang) and academic secretaries (Lvshuai Huang, Yongbing Huang, Tengyun Dong, Haibo Dong, Lulu Zhao) for their services. This trial was supported by The National Key R&D Program of China (2020YFC0841900, 2020YFC0844000, 2020YFC08860900); The Innovation Groups of the National Natural Science Foundation of China (81721002); The National Science and Technology Major Project (2017YFA0105703).
Data sharing statement
After approval from the Human Genetic Resources Administration of China, this trial data can be shared with qualifying researchers who submit a proposal with a valuable research question. A contract should be signed.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103789.
Contributor Information
Wei-Fen Xie, Email: weifenxie@medmail.com.cn.
Fu-Sheng Wang, Email: fswang302@163.com.
Appendix. Supplementary materials
Reference
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang L., Jiang Y., Zhu M., et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Coronavirus disease (COVID-19) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 5.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X., Liu X., Zhou Y., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galipeau J., Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan M.C., Kuok D.I., Leung C.Y., et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113(13):3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Hu C., Chen L., et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza a (H7N9) infection: a hint for COVID-19 treatment. Engineering. 2020;6(10):1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e1e7. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthay M.A., Calfee C.S., Zhuo H., et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson J.G., Liu K.D., Zhuo H., et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi L., Huang H., Lu X., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzoni G., Linetsky E., Correa D., et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu L., Niu C., Li R., et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng F., Xu R., Wang S., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2(-) Mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashemian S.R., Aliannejad R., Zarrabi M., et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Shan Y., Wen Y., Sun J., Du H. Mesenchymal stem cell therapy in severe COVID-19: a retrospective study of short-term treatment efficacy and side effects. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H., Gong T., Huang X., et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther. 2020;11(1):291. doi: 10.1186/s13287-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang B., Chen J., Li T., et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31):e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haberle H., Magunia H., Lang P., et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med. 2021;36(6):681–688. doi: 10.1177/0885066621997365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X., Jiang W., Chen L., et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adas G., Cukurova Z., Yasar K.K., et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30 doi: 10.1177/09636897211024942. 9636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 28.Rogers C.J., Harman R.J., Bunnell B.A., et al. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18(1):203. doi: 10.1186/s12967-020-02380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi L., Wang L., Xu R., et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther. 2021;6(1):339. doi: 10.1038/s41392-021-00754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Muecksch F., Schaefer-Babajew D., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner J.S., Kim W., Kalaidina E., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 32.He Z., Ren L., Yang J., et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397(10279):1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.