Introduction
New York City's (NYC) public health system, which predominantly serves lower-income communities, bore the burden of care and had to ramp up services to respond to the rapidly evolving COVID-19 pandemic. An increase in critical-care beds, staffing, and equipment was integral to the response, especially in our hospital in the South Bronx, where the number of intensive care unit (ICU) beds were augmented from 34 to 195 (Uppal et al., 2020). Hospitalized patients with COVID-19 usually have severe/critical infection with acute respiratory distress syndrome (ARDS), shock, coagulopathies, and multiorgan failure (Zaim et al., 2020). Multiple studies have confirmed that a high frequency of coinfections including bloodstream infections (BSI) have been observed in hospitalized patients with COVID-19 infection, like with other respiratory viruses (Lee et al., 2011; Chertow and Memoli, 2013; Bhatt et al., 2021). Our study aimed to compare the incidence of BSI, clinical and microbial characteristics of infection among patients with BSI before and during the surge of the COVID-19 pandemic.
Methods
This is a single institution, retrospective study of adult hospitalized patients with BSI admitted before (Jan to Feb 2020) and during the COVID-19 surge (March to May 2020). All adult patients hospitalized to Medicine including Medical Intensive Care from January to May 2020 were included in the study if they had a laboratory-confirmed bloodstream infection during their stay. CLABSI (central line-associated bloodstream infection), defined according to the NHSN (National Healthcare Safety Network) 2020 criteria, is a BSI in a patient that had a central line in place for 48 or more hours before the development of the BSI and is not related to an infection at another site. Primary BSI was determined if the patient did not have a clear source of infection whereas secondary BSI was defined by the identification of the same microorganism in blood culture and the suspected source of infection. BSI was classified as community-acquired if occurred within 48 hours of hospital admission and hospital-acquired if occurred after 48 hours of admission. Polymicrobial BSI encompasses the identification of more than one species of microorganisms from a single positive blood culture.
During the pandemic surge, all the blood cultures were collected by accessing the central line. Peripheral blood cultures were not collected. Hence, in the absence of an alternate source of infection, a positive blood culture was considered a CLABSI. Usual skin commensals were excluded from the analysis. Data regarding the incidence of BSI, clinical and microbial characteristics, various therapeutic interventions including central lines, days to positive blood cultures, DOT (days of antibiotic treatment) previous to BSI, length of stay, patient outcomes, etc. were obtained by retrospective chart review. Interventions associated with COVID-19 care including the use of steroids, mechanical ventilation, proning, inotropes, therapeutic anticoagulation were evaluated for correlation with BSI. Descriptive statistics and chi-square tests were used to compare the characteristics of infection. Univariate Cox regression models were used to evaluate the factors independently associated with the development of BSI during the COVID-19 period. Significant variables from this analysis were included in a multivariate model to determine risk associations for the most prevalent BSI.
Results
Of the 148 patients with BSI, 59 were admitted in the pre-COVID-19 period while 89 were admitted during the surge. Baseline characteristics and therapeutic interventions during hospitalization are shown in Table 1 . The incidence of BSI was 4.37 per 1000 patient days in the pre-COVID-19 period compared with 8.36 during the surge (p = 0.004). A significant majority of patients during the COVID period had ARDS (40.4%), required mechanical ventilation (57.3%), pressors (46.1%), therapeutic anticoagulation (31.5%), proning (22.5%), rectal tube (29.2%), and steroids (28.1%) in comparison to the pre-COVID-19 period. The median DOT previous to BSI during the COVID-19 surge was 3 days (0 to 20.5) whereas it was <1 day (0 to 6) before the COVID-19 period. Mortality was higher among patients with BSI during the surge (40.4% vs 15.3%, p = 0.001).
Table 1.
Baseline characteristics, comorbidities, risk factors for BSI & characteristics of BSI during the pre-COVID-19 period (Jan to Feb 2020) & COVID-19 period (March to May 2020).
IQR = Interquartile range; COPD = chronic obstructive pulmonary disease; ARDS = acute respiratory distress syndrome; BSI = bloodstream infection; CLABSI = central line associated bloodstream infection; COVID-19 = coronavirus disease 2019.
| Overall n = 148 | Pre-COVID-19 Period n = 59 | COVID-19 Period n = 89 | P value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, median (IQR) | 60.0 (48.3 - 69.0) | 60.0 (45.0-69.0) | 60.0 (52.0-69.5) | 0.158 |
| Females, no (%) | 66 (44.6%) | 31 (52.5%) | 35 (39.3%) | 0.113 |
| Race | 0.094 | |||
| Hispanic | 85 (57.4%) | 30 (50.8%) | 55 (61.8%) | |
| Black | 32 (21.6%) | 10 (16.9%) | 22 (24.7%) | |
| White | 4 (2.7%) | 2 (3.4%) | 2 (2.2%) | |
| Asian | 3 (2.0%) | 2 (3.4%) | 1 (1.1%) | |
| Others | 24 (16.2%) | 15(25.4%) | 9 (10.1%) | |
| Body mass index | 0.158 | |||
| Underweight | 9 (6.1%) | 4 (6.8%) | 5 (5.6%) | |
| Normal | 55 (37.2%) | 28 (47.5%) | 27 (30.3%) | |
| Overweight | 34 (23.0%) | 12 (20.3%) | 22 (24.7%) | |
| Obese | 44 (28.4%) | 15 (25.4%) | 35 (39.3%) | |
| Comorbidities | ||||
| Charlson comorbidity index | 4.0 (2.0-6.0) | 3.0 (2.0-6.0) | 4.0 (2.0 -6.0) | 0.299 |
| Hypertension | 88 (59.5%) | 29 (49.2%) | 59 (66.3%) | 0.038 |
| Diabetes mellitus | 64 (43.2%) | 20 (33.9%) | 44 (49.4%) | 0.062 |
| Asthma/COPD | 29 (19.6%) | 13 (22.0%) | 16 (18.0%) | 0.543 |
| Autoimmune disease | 7 (3.9%) | 5 (8.5%) | 2 (2.2%) | 0.090 |
| Chronic kidney disease | 21 (14.2%) | 6 (10.2%) | 15 (16.9%) | 0.254 |
| Dementia | 7 (4.7%) | 1 (1.7%) | 6 (6.7%) | 0.157 |
| Previous history of cancer | 14 (9.5%) | 5 (8.5%) | 9 (13.0%) | 0.739 |
| HIV | 19 (12.8%) | 10 (16.9%) | 9 (10.1%) | 0.223 |
| Smoking history | 46 (31.1%) | 24 (40.7%) | 22 (24.7%) | 0.040 |
| Risk factors for BSI | ||||
| ARDS on admission, n (%) | 41 (27.7%) | 5 (8.5%) | 36 (40.4%) | 0.001 |
| Mechanical ventilation, n (%) | 67 (45.3%) | 16 (27.1%) | 51 (57.3%) | 0.001 |
| Days of mechanical ventilation, median (IQR) | 14.0 (7.0-31.0) | 12.0 (1.5-46.2) | 16.0 (8.0 -34.0) | 0.128 |
| Pressor Use during hospitalization | 54 (36.5%) | 13 (22.0%) | 41 (46.1%) | 0.003 |
| Days of pressors, median (IQR) | 6.5 (2.8-13.0) | 4.0 (1.5-12.5) | 7.0 (3.0-13.5) | 0.400 |
| Proning, n (%) | 21 (14.2%) | 1 (1.7%) | 20 (22.5%) | 0.001 |
| Rectal tube, n (%) | 31 (20.9%) | 5 (8.5%) | 26 (29.2%) | 0.002 |
| Anticoagulation use, n (%) | 32 (21.6%) | 4 (6.8%) | 28 (31.5%) | 0.001 |
| Steroid use, n (%) | 35 (23.6%) | 10 (16.9%) | 25 (28.1%) | 0.118 |
| Length of stay- days, median (IQR) | 13.5 (4.3-29.0) | 9.0 (4.0 -21.8) | 17.5 (9.4-36.2) | 0.136 |
| Death, n (%) | 45 (30.4%) | 9 (15.3%) | 36 (40.4%) | 0.001 |
| BSI characteristics | Overall n = 164 | Pre-COVID-19 Period n = 53 | COVID-19 Period n = 111 | P value |
| Primary BSI, n (%) | 87 (53.0%) | 42 (79.2%) | 45 (40.5%) | 0.001 |
| Secondary BSI, n(%) | 77 (47.0%) | 11 (20.8%) | 66 (59.5%) | |
| CLABSI, n (%) | 58 (35.4%) | 5 (9.4%) | 53 (47.7%) | |
| Number of central venous access per patient, median (IQR) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | |
| Community acquired BSI, n (%) | 83 (56.1%) | 43 (81.1%) | 40 (36.0%) | 0.001 |
| Hospital acquired BSI, n (%) | 81 (49.4%) | 10 (18.9%) | 71 (64.0%) | 0.001 |
| Time to first positive blood culture from admission – days, median (IQR) | 1.8 (1.5-8.8) | 1.7 (1.4-2.1) | 2.7 (1.5-14.2) | 0.003 |
| Days of antibiotic therapy previous to BSI (DOT), median (IQR) | 0 (0-15.0) | 0 (0-6.0) | 3.0 (0-20.5) | 0.017 |
| Polymicrobial BSI | 15 (9.1%) | 7 (13.2%) | 8 (7.2%) | 0.191 |
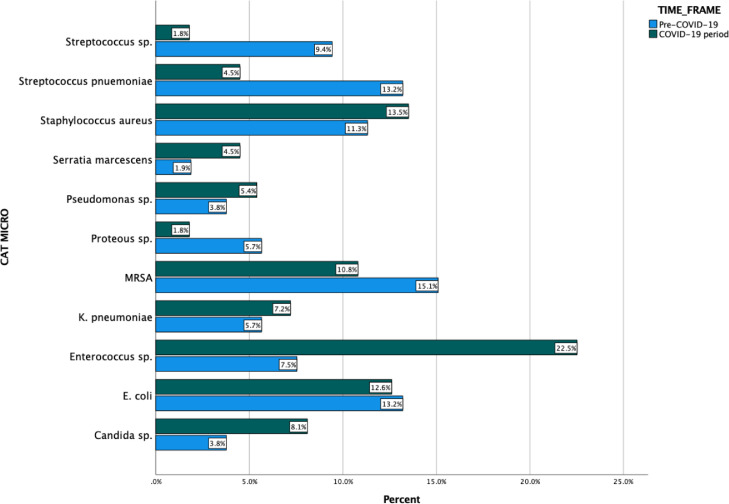
Of the 164 BSI events, 53 were pre-COVID-19 while 111 were during the surge. BSI during the COVID-19 period was predominantly monomicrobial (93%) and nosocomial (64%, p = 0.001). In the Pre-COVID-19 era, primary BSI was predominant compared with central line-associated secondary BSI seen during the COVID-19 surge. Enterococcus (22.5%), Staphylococcus aureus (13.5%) and Candida (8.1%) were more common BSI during the COVID-19 surge versus MRSA (15.1%), Escherichia coli (13.2%), and Streptococcus pneumoniae (13.2%) before COVID-19. On multivariate analysis, Enterococcal coinfection was strongly associated with SARS-CoV-2 positivity (odds ratio [OR] 2.685, p = 0.038), mechanical ventilation (OR 8.739, p = 0.002), and chronic obstructive pulmonary disease (COPD)/Asthma (OR 2.823, p = 0.035).
Discussion/Conclusion
Our results highlight the higher incidence of BSI during the COVID-19 surge compared with the pre-COVID-19 period at the NYC public hospital. The increased BSI during the COVID-19 surge in our hospital was higher than the pooled estimated BSI occurrence published in a systematic review (Ippolito et al., 2021), due to our higher burden of critically ill patients requiring mechanical ventilation, pressors, steroids, proning, etc. Empiric antibiotics were used in almost all patients due to a paucity of information about COVID-19 resulting in higher DOT. Data reported by the Centers for Disease Control and Prevention (CDC)/NHSN has confirmed a significant increase in CLABSI throughout the United States in the early months of the pandemic. Due to modification of the infection control practices to accommodate the surge in cases, shortages of personal protective equipment (PPE), staff and supplies occurred. Further adjustments reduced the frequency of contact with patients – decreasing compliance with central line care bundles and disrupting protocols, during proning sessions (Patel et al., 2021; McMullen et al., 2020). The increased mortality observed during the COVID-19 surge has been well described during previous influenza pandemics in patients with secondary BSI (Lee et al., 2011; Chertow and Memoli, 2013).
The predominance of Enterococcus spp. BSI during the peak of the pandemic is consistent with observations from other parts of the world (Ippolito et al., 2021; Bonazzetti et al., 2021; Shukla et al., 2021; Bhatt et al., 2021). Some of the possible reasons for this preponderance could be due to the colonization of respiratory tract by Enterococcus spp. especially among those requiring prolonged intubation, increased risk of cross-transmission between mechanically ventilated patients leading to the BSI (Lund et al., 2002) and gut translocation of the enteric microorganisms facilitated by use of rectal tubes, proning, pressors, etc.
Limitations of our study include retrospective observational design, being single-center, and small sample size, which can all potentially restrict generalizability. We accept that the significant reduction in the frequency of sputum sampling and peripheral blood draws during the COVID-19 surge could have affected the diagnosis of secondary BSI. In addition, the retrospective design of the study prevented detailed genetic analysis of the predominant Enterococcus species to determine the role of patient cross-infection, especially during the surge of the COVID-19 infection.
A better understanding of the BSI trends, causative factors including re-evaluation of existing infection control measures and reinforcement of antimicrobial stewardship principles will be critical to mitigating future outbreaks Fig. 1 .
Figure 1.
Frequency of microorganisms detected in positive blood cultures in hospitalized patients during the pre-COVID-19 period (Jan to Feb 2020) & COVID-19 period (March to May 2020).
Ethical Approval Statement
The Study Protocol was approved by the Institutional Review Board (IRB). However, ethical approval for this retrospective study was not required.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bhatt PJ, Shiau S, Brunetti L, Xie Y, Solanki K, Khalid S, Mohayya S, Au PH, Pham C, Uprety P, Nahass R, Narayanan N. Risk Factors and Outcomes of Hospitalized Patients With Severe Coronavirus Disease 2019 (COVID-19) and Secondary Bloodstream Infections: A Multicenter Case-Control Study. Clin Infect Dis. 2021 Jun 15;72(12):e995–e1003. doi: 10.1093/cid/ciaa1748. PMID: 33216875PMCID: PMC7717183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzetti C, Morena V, Giacomelli A, et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit Care Med. 2021;49(1):e31–e40. doi: 10.1097/CCM.0000000000004748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow D, Memoli M. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309(3):275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- Ippolito M., Simone B., Filisina C., Catalanotto F.R., Catalisano G., Marino C., Misseri G., Giarratano A., Cortegiani A. Bloodstream Infections in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Microorganisms. 2021;9:2016. doi: 10.3390/microorganisms9102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Chan PKS, Lui GCY, et al. Complications and outcomes of pandemic 2009 influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis. 2011;203(12):1739–1747. doi: 10.1093/infdis/jir187. [DOI] [PubMed] [Google Scholar]
- Lund B, Agvald-Ohman C, Hultberg A, Edlund C. Frequent transmission of enterococcal strains between mechanically ventilated patients treated at an intensive care unit. J Clin Microbiol. 2002;40(6):2084–2088. doi: 10.1128/JCM.40.6.2084-2088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen KM, Smith BA, Rebmann T. Impact of SARS-CoV-2 on hospital-acquired infection rates in the United States: predictions and early results. Am J Infect Control. 2020;48:1409–1411. doi: 10.1016/j.ajic.2020.06.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PR, Weiner-Lastinger LM, Dudeck MA, et al. Impact of COVID-19 pandemic on central line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network [published online ahead of print, 2021 Mar 15] Infect Control Hosp Epidemiol. 2021:1–4. doi: 10.1017/ice.2021.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla BS, Warde PR, Knott E, et al. Bloodstream Infection Risk, Incidence, and Deaths for Hospitalized Patients during Coronavirus Disease Pandemic. Emerging Infectious Diseases. 2021;27(10):2588–2594. doi: 10.3201/eid2710.210538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal A, Silvestri DM, Siegler M, Natsui S, Boudourakis L, Salway RJ, Parikh M, Agoritsas K, Cho HJ, Gulati R, Nunez M, Hulbanni A, Flaherty C, Iavicoli L, Cineas N, Kanter M, Kessler S, Rhodes KV, Bouton M, Wei EK. Critical Care And Emergency Department Response At The Epicenter Of The COVID-19 Pandemic. Health Aff (Millwood) 2020;39(8):1443–1449. doi: 10.1377/hlthaff.2020.00901. AugEpub 2020 Jun 11PMID: 32525713. [DOI] [PubMed] [Google Scholar]
- Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]