Abstract
Nkx2.5 and Nkx2.6 are murine homologs of Drosophila tinman. Their genes are expressed in the ventral region of the pharynx at early stages of embryogenesis. However, no abnormalities in the pharynges of embryos with mutations in either Nkx2.5 or Nkx2.6 have been reported. To examine the function of Nkx2.5 and Nkx2.6 in the formation of the pharynx, we generated and analyzed Nkx2.5 and Nkx2.6 double-mutant mice. Interestingly, in the double-mutant embryos, the pharynx did not form properly. Pharyngeal endodermal cells were largely missing, and the mutant pharynx was markedly dilated. Moreover, we observed enhanced apoptosis and reduced proliferation in pharyngeal endodermal cells of the double-mutant embryos. These results demonstrated a critical role of the NK-2 homeobox genes in the differentiation, proliferation, and survival of pharyngeal endodermal cells. Furthermore, the development of the atrium was less advanced in the double-mutant embryos, indicating that these two genes are essential for both pharyngeal and cardiac development.
The Nkx2.5 and Nkx2.6 genes are members of the NK-2 homeobox gene family and are closely related to the Drosophila tinman gene (2, 5, 21). At early stages of embryogenesis, Nkx2.5 is expressed in myocardium and pharyngeal endoderm (6, 7), while Nkx2.6 expression can be observed in pharyngeal endoderm, sinus venosa, and myocardium of the outflow tract (1, 12). In the pharynx, both Nkx2.5 and Nkx2.6 are expressed in the ventral region between embryonic day 8 (E8.0) and E8.5 (1, 7). However, by E9.0, Nkx2.6 expression becomes restricted to the lateral side of the pharynx, the pharyngeal pouches, while Nkx2.5 is still expressed on the ventral side of the pharynx, the pharyngeal floor (1, 7, 22). In the heart, redundant expression of Nkx2.5 and Nkx2.6 has been observed in the sinus venosa at E8.5 and in the outflow tract at E9.5 (1, 7). Inactivation of Nkx2.5 arrested heart formation at the looping stage, revealing a critical role of this gene at early stages of cardiac development (9, 20). However, no abnormalities in the pharynges of Nkx2.5−/− embryos were reported (9, 20). Moreover, targeted disruption of Nkx2.6 did not cause any abnormalities either in the pharynx or in the heart (22). However, interestingly, in Nkx2.6−/− embryos, expression of Nkx2.5 extended to the lateral side of the pharynx, suggesting a compensatory function of Nkx2.5 in the forming pharynx (22).
To investigate the function of Nkx2.5 and Nkx2.6 in the formation of the pharynx, we generated and analyzed Nkx2.5 and Nkx2.6 double-mutant mice. Interestingly, in the double-mutant embryos, the numbers of pharyngeal endodermal cells were severely reduced and the pharynges were markedly dilated, suggesting critical roles of Nkx2.5 and Nkx2.6 in pharyngeal development.
MATERIALS AND METHODS
Generation of Nkx2.5−/− Nkx2.6−/− mice.
The generation and viability of Nkx2.5 and Nkx2.6 knockout mice have been described previously (20, 22). The bacterial LacZ gene was inserted into the Nkx2.5 locus (20). Homozygous mutations of Nkx2.5 (Nkx2.5−/− mice) were embryonically lethal, while heterozygous Nkx2.5 mutant mice (Nkx2.5+/− mice) and homozygous Nkx2.6 mutant mice (Nkx2.6−/− mice) were viable and fertile (20, 22). We first interbred Nkx2.5+/− mice and Nkx2.6−/− mice to generate Nkx2.5+/− Nkx2.6+/− mice mice, and these in turn were crossed to generate Nkx2.5+/− Nkx2.6−/− mice. Nkx2.5+/− Nkx2.6−/− mice were viable and fertile. Nkx2.5+/− Nkx2.6−/− mice were then crossed, and the offspring were genotyped by PCR as previously described (20). All the mutant mice were maintained in a mixed 129 and C57BL background.
Histological analysis.
Mouse embryos were fixed, dehydrated, and embedded in paraffin, and in situ hybridization was performed as previously described (20). Plasmids containing full-length cDNA for HNF3β and Shh were kindly provided by B. Hogan (Vanderbilt University Medical School, Nashville, Tenn.). The entire coding region of Pax9 was a kind gift from H. Peters (Brigham and Women's Hospital, Boston, Mass.). The plasmids for atrial natriuretic factor (ANF), B-type natriuretic peptide (BNP), myosin light chain 2A (MLC2A), and MLC2V were described previously (20). Whole-mount β-galactosidase staining was performed according to the method of Schlaeger et al. (17). TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining and immunohistochemistry were performed as previously described (20).
RESULTS
Embryonic lethality of the Nkx2.5−/− Nkx2.6−/− mutation.
We generated Nkx2.5+/− Nkx2.6−/− mice by crossing compound heterozygotes (Nkx2.5+/− Nkx2.6+/−; see (Materials and Methods). Nkx2.5+/− Nkx2.6−/− mice were viable and fertile and exhibited no detectable abnormalities either in the pharynx or in the heart. Nkx2.5+/− Nkx2.6−/− mice were then intercrossed, and the offspring were genotyped. As expected, no viable Nkx2.5−/− Nkx2.6−/− mice were found.
Morphology and histology of Nkx2.5−/− Nkx2.6−/− embryos.
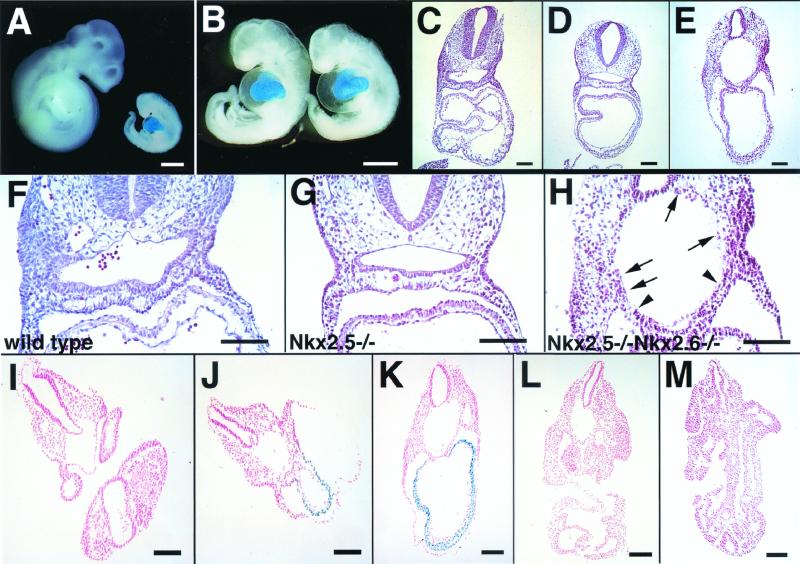
To determine the phenotype of Nkx2.5−/− Nkx2.6−/− embryos, offspring of Nkx2.5+/− Nkx2.6−/− mice were examined during development. By E9.5, the expected Mendelian frequency of homozygous mutants was recovered (Table 1). We first examined Nkx2.5−/− Nkx2.6−/− embryos at E9.5 and E10.5. Nkx2.5−/− Nkx2.6−/− embryos at E10.5 showed severe growth retardation and massive pericardial effusion (Fig. 1A, and B), looking similar to Nkx2.5−/− embryos (Fig. 1B) (9, 20). However, histological examination at E9.5 and E10.5 revealed disrupted formation of the pharynx in Nkx2.5−/− Nkx2.6−/− embryos (Fig. 1E and H). In wild-type embryos, the surface of the pharynx was covered with pharyngeal endodermal cells. A monolayer of endodermal cells covered the pharyngeal roof, and two or three layers of cuboidal endodermal cells were observed in the pharyngeal floor and pouches (Fig. 1F). In contrast, in Nkx2.5−/− Nkx2.6−/− embryos, the number of pharyngeal endodermal cells was severely reduced and a continuous endodermal cell layer was lost. Only a small number of endodermal cells were observed, mainly on the ventral side (Fig. 1H), and the mutant pharynx showed severe dilatation (Fig. 1E and H). The abnormal dilatation was detected only in the pharyngeal part of the foregut (Fig. 1I to M). Interestingly, Nkx2.5−/− embryos also showed a defect in the pharynx; there were fewer endodermal cells in the pharyngeal floor and pouches, although the surface of the pharynx was completely covered by endodermal cells (Fig. 1G).
TABLE 1.
Viability of Nkx2.5−/− Nkx2.6−/− embryos
| Day of analysis | No. of embryos with phenotype
|
||
|---|---|---|---|
| Nkx2.5+/+ Nkx2.6−/− | Nkx2.5+/− Nkx2.6−/− | Nkx2.5−/− Nkx2.6−/− | |
| E8.5 | 14 | 32 | 16 |
| E9.5 | 14 | 31 | 15 |
| E10.5 | 11 | 19 | 7 |
| E11.5 | 6 | 9 | 0 |
FIG. 1.
Morphological and histological analysis of Nkx2.5−/− and Nkx2.5−/− Nkx2.6−/− embryos. (A) Whole-mount β-galactosidase staining of Nkx2.5+/+ Nkx2.6−/− and Nkx2.5−/− Nkx2.6−/− embryos at E10.5 (littermates) (B) Whole-mount β-galactosidase staining of Nkx2.5−/− Nkx2.6−/− (left) and Nkx2.5−/− (right) embryos at E10.5. (C to E) Hematoxylin-and-eosin-stained transverse sections of wild-type (C), Nkx2.5−/− (D), and Nkx2.5−/− Nkx2.6−/− (E) embryos at E9.5. Note disrupted formation of the pharynges in Nkx2.5−/− Nkx2.6−/− embryos. (F to H) Higher-magnification pictures of panels C to E. Note that a monolayer of endodermal cells remained only in a small region on the ventral side (between the arrowheads) in Nkx2.5−/− Nkx2.6−/− embryos. A small number of endodermal cells also survived in other parts of the pharynx (arrows). (I to M) Transverse sections of the double-mutant embryo shown in panel B. The disruption of pharyngeal formation was observed only in the pharyngeal part of the foregut. Bars, 200 μm.
To exclude the possibility that the observed defects might be due to general growth retardation, we analyzed Nkx2.5−/− and Nkx2.5−/− Nkx2.6−/− embryos at earlier stages. No growth retardation was detected in Nkx2.5−/− or Nkx2.5−/− Nkx2.6−/− embryos by E9.0 (Fig. 2A and B). Histological analysis showed that the foregut pocket of Nkx2.5−/− Nkx2.6−/− embryos at E8.0 was indistinguishable from that of wild-type littermates (Fig. 2C to E). However, at E8.5, pharyngeal endodermal cells were lost in some parts of the pharynx and the pharynx was abnormally dilated in Nkx2.5−/− Nkx2.6−/− embryos, indicating that the pharyngeal phenotype was not due to growth retardation (Fig. 2J). At this stage, no significant differences in the pharynx between Nkx2.5−/− and wild-type embryos were detected (Fig. 2F and H). However, at E9.0, the number of pharyngeal endodermal cells was significantly lower in Nkx2.5−/− embryos than in wild-type embryos, suggesting that proliferation or differentiation of these cells may be affected by inactivation of Nkx2.5 (Fig. 2G and L).
FIG. 2.
(A) Wild-type (left), Nkx2.5−/− (middle), and Nkx2.5−/− Nkx2.6−/− (right) embryos at E8.5. bar, 1.0 mm. (B) Wild-type (left), Nkx2.5−/− (middle), and Nkx2.5−/− Nkx2.6−/− (right) embryos at E9.0. Bar, 1.0 mm. (C to E) Histology of the foregut pockets in hematoxylin-and-eosin-stained wild-type (C), Nkx2.5−/− (D), and Nkx2.5−/− Nkx2.6−/− (E) embryos at E8.0. Bar, 100 μm. (F to K) Histology of the pharynges in wild-type (F and G), Nkx2.5−/− (H and I), and Nkx2.5−/− Nkx2.6−/− (J and K) embryos at E8.5 (F, H, and J) and E9.0 (G, I, and K). (J and K) A monolayer of endodermal cells on the ventral side (between the arrowheads) and a few endodermal cells in other parts (arrows) were observed. Note that a continuous layer of endodermal cells was already lost and the pharynx was dilated in Nkx2.5−/− Nkx2.6−/− embryos at E8.5.
Enhanced apoptosis and reduced proliferation of pharyngeal endodermal cells.
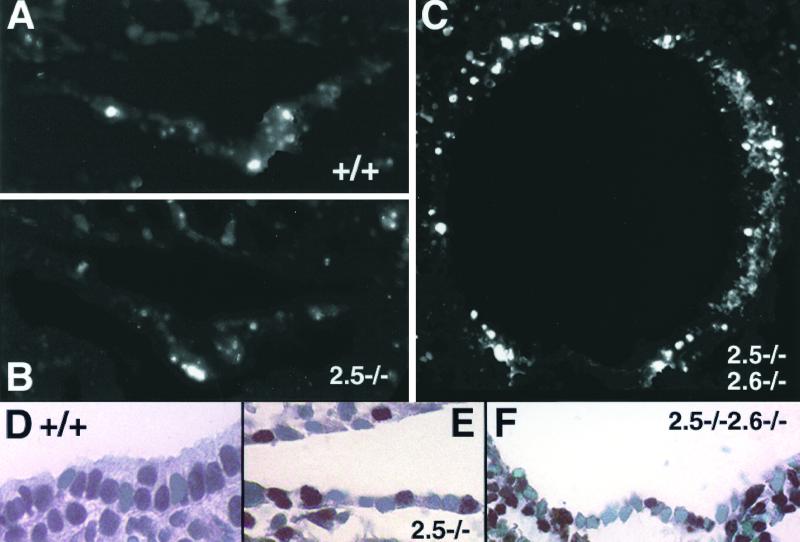
We then examined whether proliferation and/or apoptosis of pharyngeal endodermal cells was affected by inactivation of Nkx2.5 and Nkx2.6. TUNEL staining demonstrated that pharyngeal endodermal cells were largely apoptotic, except for a small number of cells on the ventral side in Nkx2.5−/− Nkx2.6−/− embryos (Fig. 3C). There were no differences in the frequency of apoptotic cells in the pharynx between wild-type and Nkx2.5−/− embryos (Fig. 3A and B), suggesting that disruption of both Nkx2.5 and Nkx2.6 enhanced apoptosis. We next performed immunohistochemistry using anti-PCNA (proliferating cell nuclear antigen) antibody to assess proliferation of pharyngeal endodermal cells. The rates (means ± standard deviations) of PCNA-positive cells were 0.71 ± 0.05, 0.43 ± 0.03, and 0.43 ± 0.02 in wild-type, Nkx2.5−/−, and Nkx2.5−/− Nkx2.6−/− embryos, respectively. (Six sections from three embryos of each group were analyzed. The differences between wild-type and Nkx2.5−/− or Nkx2.5−/− Nkx2.6−/− embryos were significant [P < 0.01].) This result indicated that proliferation of pharyngeal endodermal cells was affected by inactivation of Nkx2.5.
FIG. 3.
TUNEL staining and PCNA staining. (A to C) TUNEL staining of transverse sections of wild-type (A), Nkx2.5−/− (B), and Nkx2.5−/− Nkx2.6−/− (C) embryos at E8.75. Note enhanced apoptosis in the pharynx of Nkx2.5−/− Nkx2.6−/− embryos. (D to F) PCNA staining of transverse sections of wild-type (D), Nkx2.5−/− (E), and Nkx2.5−/− Nkx2.6−/− (F) embryos at E8.75.
Expression of molecular markers in Nkx2.5−/− Nkx2.6−/− embryos.
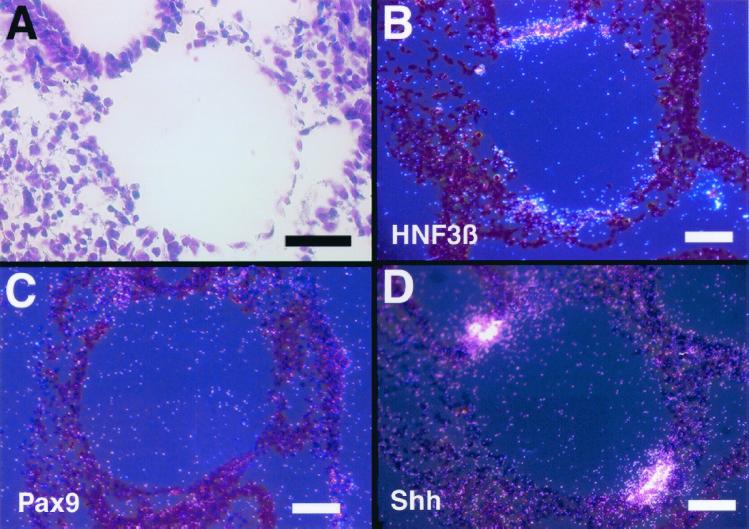
To determine whether transcription of pharyngeal endoderm-specific genes was affected in Nkx2.5−/− Nkx2.6−/− embryos, we performed in situ hybridization. Hepatocyte nuclear factor 3β (HNF3β) is a winged-helix transcription factor and is expressed in the node, notochord, floor plate, and embryonic endoderm (23). Expression of HNF3β was detectable around the pharynx but was significantly down-regulated in Nkx2.5−/− embryos (Fig. 4A and B). Moreover, in the pharynges of Nkx2.5−/− Nkx2.6−/− embryos, expression of HNF3β was lost except for endodermal cells on the ventral side and a small number of endodermal cells remaining in other parts (Fig. 4C). We next examined expression of Pax9, which is expressed in the endoderm of the pharyngeal pouches (15). In wild-type and Nkx2.5−/− embryos, Pax9 expression was observed in the epithelium of the pharyngeal pouches (Fig. 4D and E). However, in the pharynges of Nkx2.5−/− Nkx2.6−/− embryos, transcripts for Pax9 were not detected (Fig. 4F), indicating that pharyngeal pouches did not form. Sonic hedgehog (Shh) is a secreted signaling molecule and is involved in axial patterning, foregut formation, and distal limb formation (3, 8). In wild-type embryos, Shh expression was observed in the floor plate, notochord, and ventral region of the pharyngeal endoderm (Fig. 4G). In Nkx2.5−/− and Nkx2.5−/− Nkx2.6−/− embryos, expression of Shh in the pharynx was more restricted but was still detectable (Fig. 4I).
FIG. 4.
Expression of pharyngeal endoderm-specific genes. In situ hybridization of transverse sections of wild-type and mutant embryos at E8.75 using probes for the indicated proteins. Bars, 100 μm.
Phenotype of Nkx2.5−/− Nkx2.6+/− embryos.
We crossed Nkx2.5+/− Nkx2.6−/− mice and Nkx2.5+/− Nkx2.6+/+ mice to examine the phenotype of Nkx2.5−/− Nkx2.6+/− mice. Expectedly, the Nkx2.5−/− Nkx2.6+/− mutation was embryonically lethal. However, interestingly, Nkx2.5−/− Nkx2.6+/− embryos at E8.5 and E9.5 exhibited the same phenotype in the pharynx as Nkx2.5−/− Nkx2.6−/− embryos (Fig. 5A). Expression patterns of HNF3β, Shh, and Pax9 were same as those in Nkx2.5−/− Nkx2.6−/− embryos (Fig. 5B to D). This result indicated that one copy of Nkx2.6 in the absence of Nkx2.5 was not sufficient for the proper formation of the pharynx.
FIG. 5.
Histological analysis of the pharynges of Nkx2.5−/− Nkx2.6+/− embryos. (A) Hematoxylin and eosin staining of a transverse section at E8.5. (B to D) In situ hybridization of transverse sections of Nkx2.5−/− Nkx2.6+/− embryos at E8.75 using probes for the indicated proteins. Bars, 100 μm.
Cardiac phenotype of Nkx2.5−/− Nkx2.6−/− embryos.
In the course of analyzing mutant embryos, we noticed a difference in heart morphology in Nkx2.5−/− Nkx2.6−/− embryos from Nkx2.5−/− embryos. As described previously, the heart of Nkx2.5−/− embryos lacked endocardial cushion formation but still had a cleft between the common atrium and ventricle (9, 20) (Fig. 1D). Notably, the distinction between the atrium and ventricle was less clear and the expansion of the common atrium was significantly less extensive in the heart of Nkx2.5−/− Nkx2.6−/− embryos (Fig. 1E). Nkx2.5−/− Nkx2.6+/− embryos showed the same cardiac phenotype (data not shown).
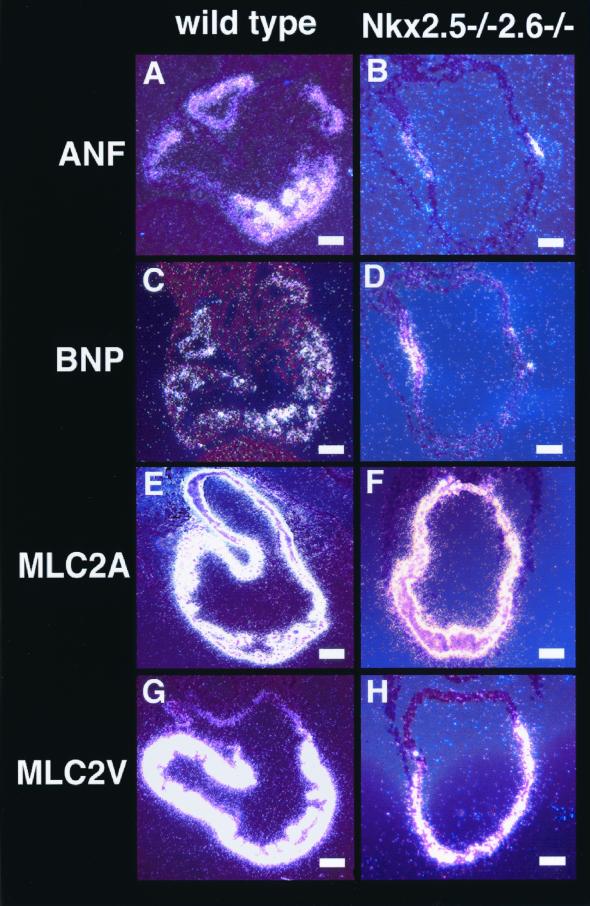
We next investigated alterations in cardiac gene expression in Nkx2.5−/− Nkx2.6−/− embryos. Expression of ANF and BNP was observed in both the atrium and ventricle of wild-type embryos (Fig. 6A and C). In Nkx2.5−/− embryos, although expression of these genes in the ventricle was abolished, the atrial expression of ANF and BNP was still maintained (20). Interestingly, in Nkx2.5−/− Nkx2.6−/− embryos, expression of ANF and BNP in the atrium was also down-regulated. These genes were expressed only in a restricted region close to the atrioventricular canal (Fig. 6B and D). To assess the specification of the atrium and ventricle in Nkx2.5−/− Nkx2.6−/− embryos, we examined expression of MLC2A and MLC2V. In Nkx2.5−/− Nkx2.6−/− embryos. MLC2A was expressed in the atrium and ventricle and MLC2V was expressed only in the ventricle, albeit at a lower level, similar to results for Nkx2.5−/− embryos (20) (Fig. 6E to H). This result indicated that the differentiation of atrial myocytes was less advanced but that the specification of the atrium and ventricle occurred normally in Nkx2.5−/− Nkx2.6−/− embryos.
FIG. 6.
Expression of cardiac tissue-specific genes. In situ hybridization of transverse sections of wild-type and Nkx2.5−/− Nkx2.6−/− embryos at E8.75 using probes for the indicated proteins. Bars, 100 μm.
DISCUSSION
In this study, we demonstrated critical roles of the NK-2 homeobox genes Nkx2.5 and Nkx2.6 in pharyngeal development using a genetic approach. At the beginning of the embryonic period, cephalocaudal and lateral folding of the embryo incorporates the dorsal part of the endoderm-lined yolk sac into the embryo to form the primitive gut. The pharyngeal gut, or the pharynx, is the cephalic region of the primitive gut, extending from the buccopharyngeal membrane to the tracheobronchial diverticulum. The internal aspect of the pharynx is lined with epithelial endodermal cells, and a number of diverticula called pharyngeal pouches appear along the lateral wall of the pharynx (10). Although hoxa3 and Pax9 have been shown to be required for the normal development of pharyngeal pouch derivatives (4, 15), little is known about molecular mechanisms controlling pharyngeal development.
The ectopic expression of Nkx2.5 in pharyngeal pouches of Nkx2.6−/− embryos suggested a compensatory function of Nkx2.5 (22). Interestingly, in the double-mutant embryos, pharyngeal pouches did not form. Therefore, it is possible that inactivation of both Nkx2.5 and Nkx2.6 has eliminated overlapping functions of these genes in pharyngeal pouches. In the pharyngeal floors of Nkx2.5−/− embryos, the numbers of endodermal cells were reduced and significantly fewer PCNA-positive cells were observed, indicating a critical role of Nkx2.5 in proliferation of pharyngeal endodermal cells. Moreover, there were only a few endodermal cells remaining in the pharynges of Nkx2.5−/− Nkx2.6−/− embryos. Notably, we found markedly enhanced apoptosis in the pharynges of the double mutant embryos, revealing overlapping functions of Nkx2.5 and Nkx2.6 in survival of pharyngeal endodermal cells. However, neither of these genes is expressed in the pharyngeal roof. Endodermal cells expressing Nkx2.5 and Nkx2.6 on the ventral side of the pharynx may secrete a signal molecule(s) essential for the maintenance of pharyngeal endodermal cells. Taken together, the data show that pharyngeal endodermal cells were induced and specified but could not proliferate and survive in the absence of Nkx2.5 and Nkx2.6. The abnormal dilatation of the pharynx could be due to the absence of the epithelial lining in the mutant pharynx. A small number of nonapoptotic cells expressing Shh in the ventral regions of Nkx2.5−/− Nkx2.6−/− embryos remain to be characterized. These cells may derive from a different cell lineage from that of other pharyngeal epithelial cells.
The severe down-regulation of ANF and BNP expression in the atrium suggested that the cardiac program in the atrium is less advanced in Nkx2.5−/− Nkx2.6−/− embryos, although the specification of the atrium and ventricle appeared to be normal. Since Nkx2.6 is not expressed in the atrium, it is possible that the absence or down-regulation of a secreted factor(s) from the pharyngeal endoderm might have caused this cardiac abnormality. This hypothesis is in line with previous studies showing that signals from the pharyngeal endoderm were required for differentiation of cardiac myocytes as well as specification of the cardiac cell lineage (11, 18, 19).
Interestingly, Nkx2.5−/− Nkx2.6+/− embryos showed the same phenotype as Nkx2.5−/− Nkx2.6−/− embryos. A similar dose-dependent requirement of transcription factors was shown in the developmental program of the skeletal muscle. Gene targeting experiments showed that Myf-5+/− MyoD−/− as well as Myf-5−/− MyoD−/− mutations were lethal, implying that two functional copies of Myf-5 were required to rescue MyoD−/− mice (16).
In Caenorhabditis elegans, there is no heart, but pharyngeal muscle is of mesodermal origin and contracts rhythmically like vertebrate cardiac muscle. Moreover, Ceh22, a homolog of tinman in C. elegans, is expressed in the pharyngeal muscle and transactivates several pharynx specific genes (13, 14). Thus, it has been suggested that functions and development of pharyngeal muscle in C. elegans may be similar to those of cardiac muscle in higher organisms. The result that the murine homologs of tinman were critical for both pharyngeal and cardiac development support the hypothesis that the vertebrate heart and pharynx may share a common developmental program.
ACKNOWLEDGMENTS
We thank B. Hogan and H. Peters for providing plasmids.
This work was supported by an NIH grant to S.I.
REFERENCES
- 1.Biben C, Hatzistavrou T, Harvey R P. Expression of NK-2 class homeobox gene Nkx2–6 in foregut endoderm and heart. Mech Dev. 1998;73:125–127. doi: 10.1016/s0925-4773(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 2.Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- 3.Chiang C, Litingtung Y, Lee E, Young K E, Corden J L, Westphal H, Beachy P A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 4.Chisaka O, Capecchi M R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- 5.Harvey R P. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- 6.Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci USA. 1993;90:8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lints T J, Parsons L M, Hartley L, Lyons I, Harvey R P. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- 8.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 9.Lyons I, Parsons L M, Hartley L, Li R, Andrews J E, Robb L, Harvey R P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 10.Moore K, Persaud T. The developing human, 215–256. W. B. Philadelphia, Pa: Saunders Company; 1998. [Google Scholar]
- 11.Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121:515–523. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- 12.Nikolova M, Chen X, Lufkin T. Nkx2.6 expression is transiently and specifically restricted to the branchial region of pharyngeal-stage mouse embryos. Mech Dev. 1997;69:215–218. doi: 10.1016/s0925-4773(97)00174-3. [DOI] [PubMed] [Google Scholar]
- 13.Okkema P G, Fire A. The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development. 1994;120:2175–2186. doi: 10.1242/dev.120.8.2175. [DOI] [PubMed] [Google Scholar]
- 14.Okkema P G, Ha E, Haun C, Chen W, Fire A. The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development. 1997;124:3965–3973. doi: 10.1242/dev.124.20.3965. [DOI] [PubMed] [Google Scholar]
- 15.Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudnicki M A, Schnegelsberg P N, Stead R H, Braun T, Arnold H H, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 17.Schlaeger T M, Qin Y, Fujiwara Y, Magram J, Sato T N. Vascular endothelial cell lineage-specific promoter in transgenic mice. Development. 1995;121:1089–1098. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- 18.Schultheiss T M, Xydas S, Lassar A B. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- 19.Sugi Y, Lough J. Anterior endoderm is a specific effector of terminal cardiac myocyte differentiation of cells from the embryonic heart forming region. Dev Dyn. 1994;200:155–162. doi: 10.1002/aja.1002000207. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Kasahara H, Bartunkova S, Schinke M, Komuro I, Inagaki H, Lee Y, Lyons G E, Izumo S. Vertebrate homologs of tinman and bagpipe: roles of the homeobox genes in cardiovascular development. Dev Genet. 1998;22:239–249. doi: 10.1002/(SICI)1520-6408(1998)22:3<239::AID-DVG6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Yamasaki N, Izumo S. Phenotypic characterization of the murine Nkx2.6 homeobox gene by gene targeting. Mol Cell Biol. 2000;20:2874–2879. doi: 10.1128/mcb.20.8.2874-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein D C, Ruiz i Altaba A, Chen W S, Hoodless P, Prezioso V R, Jessell T M, Darnell J E., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]