Abstract
Nicotine addiction is a leading avoidable brain disorder globally. Although nicotine induces a modest reinforcing effect, which is important for the initial drug use, the transition from nicotine use to nicotine addiction involves the mechanisms responsible for the negative consequences of drug abstinence. Recent study suggested that trace amine-associated receptor 1 (TAAR1) is a promising pharmacological target for the modulation of positive reinforcing effects of nicotine. However, whether TAAR1 plays a part in the negative reinforcement of nicotine withdrawal remains to be determined. Here, using a long-access (LA) self-administration model, we investigated whether LA rats show increased nicotine intake and withdrawal symptoms in comparison with saline and ShA rats and then tested the effect of TAAR1 partial agonist RO5263397 on nicotine withdrawal effects. We found that rats from long-access group showed significant abstinence-induced anxiety-like behaviour, mechanic hypersensitivity, increased number of precipitated withdrawal signs and higher motivation for the drug, while rats from short-access did not differ from saline group. TAAR1 partial agonist RO5263397 significantly reduced the physical and motivational withdrawal effects of nicotine in LA rats, as reflected by increased time spent on the open arm in the elevated plus maze (EPM) test, normalized paw withdrawal threshold, decreased withdrawal signs and motivation to self-administer nicotine. This study indicates that activation of TAAR1 attenuates the negative-reinforcing effects of nicotine withdrawal and further suggests TAAR1 as a promising target to treat nicotine addiction.
Keywords: long-access, negative reinforcement, nicotine withdrawal, stress, TAAR1
1 |. INTRODUCTION
Tobacco is one of the most widely used substances worldwide, with nearly 50 million people in the United States are addicted to tobacco products.1 Tobacco addiction results in more than 7 million deaths each year globally,2 making it a leading avoidable cause of disease and premature death in the world.3,4 The main psychoactive component responsible for tobacco addiction is nicotine.5 Nicotine produces a modest reinforcing effect partially through the stimulation of mesolimbic dopamine system.6–9 Although the acute reinforcing effect of nicotine is important for the initial drug use,10,11 the transition from nicotine use to nicotine dependence involves neuroadaptations in the brain that underlies continued drug use.12 Such neuroadaptations may involve the mechanisms responsible for the negative consequences of drug abstinence.13–15 Thus, continued drug use to avoid an unpleasant affective state induced by drug abstinence through negative reinforcement mechanisms may lead to nicotine addiction.12,16
Nicotine withdrawal can be elicited after the discontinuation of chronic nicotine exposure,17–19 which is characterized by both somatic and affective symptoms. Somatic symptoms include gastrointestinal discomfort, increased appetite and bradycardia while affective symptoms include depressed mood, irritability, anxiety and difficulty concentrating.4,20 These aversive aspects of nicotine withdrawal are thought to be important for the maintenance of nicotine use and contribute to nicotine relapse even after prolonged abstinence.21,22 Thus, strategies that can attenuate these aversive symptoms resulted from nicotine withdrawal may serve as an effective treatment for nicotine addiction.
Trace amine-associated receptors (TAARs) are a group of receptors of trace amines that exist at a very low level in the brains of vertebrates, of which TAAR1 is best characterized.23 TAAR1 is broadly expressed in the dopaminergic system and has emerged as an effective target for modulating dopamine activity.24,25 It is widely associated with cocaine, methamphetamine and other drug-related behaviours.26–29 Our recent study has shown that TAAR1 agonists attenuate nicotine-associated behaviours.30 For example, activation of TAAR1 significantly reduces nicotine-induced sensitization, nicotine intake and cue- or drug-induced nicotine reinstatement in short-access (ShA) self-administration rats.30 However, whether TAAR1 plays a part in nicotine withdrawal remains to be determined.
Studies have shown that extended-access (or long-access, LA), other than ShA to nicotine self-administration is associated with prolonged nicotine dependence.31,32 Rats with a history of LA to nicotine demonstrate significant withdrawal symptoms when nicotine exposure is terminated.33,34 However, rats with ShA to nicotine did not show any differences.35 These studies suggest that LA self-administration might be a more valid model for nicotine withdrawal study. Thus, in the present study, we aimed to verify whether rats with LA to nicotine show withdrawal symptoms after drug abstinence and whether TAAR1 activation could regulate these abnormal behaviours. For this purpose, we first examined whether LA rats show increased nicotine intake and withdrawal symptoms in comparison with saline and ShA rats and then tested the effect of TAAR1 partial agonist RO5263397 on nicotine withdrawal-induced anxiety-like behaviour, mechanical hypersensitivity, mecamylamine-precipitated withdrawal symptoms and nicotine intake under a progressive-ratio (PR) schedule.
2 |. MATERIALS AND METHODS
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and complied with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
2.1 |. Animals
Adult male Sprague–Dawley rats (initial weight 260–280 g; Harlan, Indianapolis, IN) were housed individually on a 12/12-h light/dark cycle with free access to water and food. To assure that there will be no difference among different groups of rats before treatment of TAAR1 agonist, we assigned rats into different groups based on the training of self-administration in all experiments. The groups of rats were then assigned into treatments of vehicle and RO5263397 randomly. Experimenters were blinded to the group assignment. All behavioural studies were performed during the light cycle (8:00–20:00) except for nicotine LA training, which was partially undertaken during the dark phase. A total of 66 adult male rats were used in this study. Six rats were excluded because of catheter failure. Eight rats were excluded because they did not acquire stable responses for nicotine after training (the variance of the total number of injections was <20% for two consecutive days).
2.2 |. Drugs
Drugs used in this study included nicotine tartrate (MP Biomedicals, LLC, Solon, OH), RO5263397 (synthesized at Research Triangle Institute, purity > 98%) and mecamylamine hydrochloride (Sigma). Nicotine tartrate was dissolved in 0.9% physiological saline, and the pH was adjusted to 7.2–7.4 prior to injection. RO5263397 was dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia) and 18 parts physiologic saline.36 The dose of RO5263397 (intraperitoneally, i.p.; 5.6 mg/kg) used in these experiments was selected based on our previous findings in which there was no effect on general locomotor activity.30,37 Mecamylamine hydrochloride was dissolved in saline and administered i.p. (1.5 mg/kg).
2.3 |. Catheterization surgery
The rats (weighing 280–300 g at the beginning of the experiments) were anesthetized with ketamine and xylazine (75 and 5 mg/kg, respectively, i.p.). Rats were implanted with chronic indwelling jugular catheters as previously described.30 The rats were allowed to recover for at least 1 week after surgery. Catheters were flushed daily with 0.2-ml solution of enrofloxacin (4 mg/ml) mixed in a heparinized saline solution (50 IU/ml in 0.9% sterile saline) for 1 week after surgery to preserve catheter patency and prevent infection.
2.4 |. Nicotine self-administration
One week after surgery, rats began self-administration for nicotine (0.03 mg/kg per infusion) or saline. Training sessions were 1 h per day, during which responses to the active lever resulted in intravenous injections of nicotine or saline under a fixed ratio (FR) schedule of reinforcement followed by a 30-s time-out period. Infusions were accompanied by a 5-s illumination of the stimulus light above the active lever, and the house light was extinguished for the duration of the time-out period. The initial schedule of reinforcement was FR 1. The response requirement was gradually increased from FR 1 to FR 3 over 2 weeks. All rats maintained a stable self-administration behaviour for the last two sessions (variance of the number of injections <20% among the two sessions). Sessions were terminated after either a 1-h duration or 30 infusions had been earned, whichever occurred first. Then nicotine rats were divided into a ShA group that self-administered nicotine for 1 h per session and a LA group that self-administered nicotine for 21 h per session. Both groups self-administered nicotine for another 14 consecutive days.
2.5 |. Elevated plus maze
The EPM test was based on previous studies.38 Briefly, each rat was first placed in the central zone of the EPM. The rat was allowed to freely explore the maze for 5 min, and the entire test was conducted under dim light conditions. The illumination was 3 lx in the closed arms and 8 lx in the open arms.38 The time (in seconds) spent on the open arms were recorded by a camera and scored by two independent observers who were blind to the animal groups.
2.6 |. Von Frey assay
Mechanical hypersensitivity was measured using Von Frey filaments consisting of calibrated filaments (10–60 g; North Coast Medical, Morgan Hill, CA). Rats were placed in elevated plastic chambers with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA) and allowed to habituate prior to testing. Filaments were applied perpendicularly to the medial plantar surface of the same hind paw (right) from below the mesh floor in an ascending order of filament force, beginning with the lowest filament (10 g). A filament was applied until buckling occurred and maintained for approximately 2 s. Mechanical thresholds correspond to the lowest force that elicited a behavioural response (withdrawal of the hind paw) in at least two out of three applications.
2.7 |. Mecamylamine-precipitated withdrawal symptoms
Rats received an intraperitoneal injection of mecamylamine (1.5 mg/kg, i.p.) and were placed into an opaque plastic cylindrical container (30 × 29 cm) 30 min later for 10 min of somatic withdrawal sign observation. Somatic signs of nicotine withdrawal were rated according to the method developed by Malin et al. (1992).19 The rats were observed for eye blinks, body shakes, chews, escape attempts, foot licks, head shakes, teeth chatter, chews + teeth chatter, grooming and yawns. Multiple successive counts of any sign required a distinct pause between episodes. The total number of somatic signs during the 10-min observation period was defined as the sum of the number of occurrences of all of the aforementioned signs. The observer was blind to the animal's experimental condition.
2.8 |. PR test
Three days after the mecamylamine-precipitated withdrawal symptoms test, rats underwent a PR schedule of reinforcement. Rats were allowed to self-administer nicotine during a 3-h session. The first response of the session resulted in a drug infusion, after which the response requirements escalated by following the series 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, …, derived from the formula: response ratio (rounded to nearest integer) = [5e(injection numberX0.2)] – 5.30 Drug (5.6-mg/kg RO5263397, i.p.) or its vehicle was administered 20 min before the test sessions.
2.9 |. Statistics
All results were presented as mean ± standard error of the mean (SEM) and analysed by the Graphpad Prism 8 software (GraphPad Software, San Diego, CA). Data were tested for normal distribution using Shapiro–Wilk test and justified for the subsequent use of parametric methods. Two-way repeated-measures analysis of variance (ANOVA) was conducted for nicotine training experiment (Figure 1B). All other behavioural data were analysed by two-way ANOVA followed by post hoc Bonferroni's multiple comparisons test. Post hoc tests were conducted only if F in ANOVA achieved the necessary level of statistical significance (p < 0.05), and there was no significant variance in homogeneity (which precludes use of parametric statistics). Group size was selected based on our previous studies. The declared group size is the number of independent values, and that statistical analysis was done using these independent values. p < 0.05 was considered statistically significant.
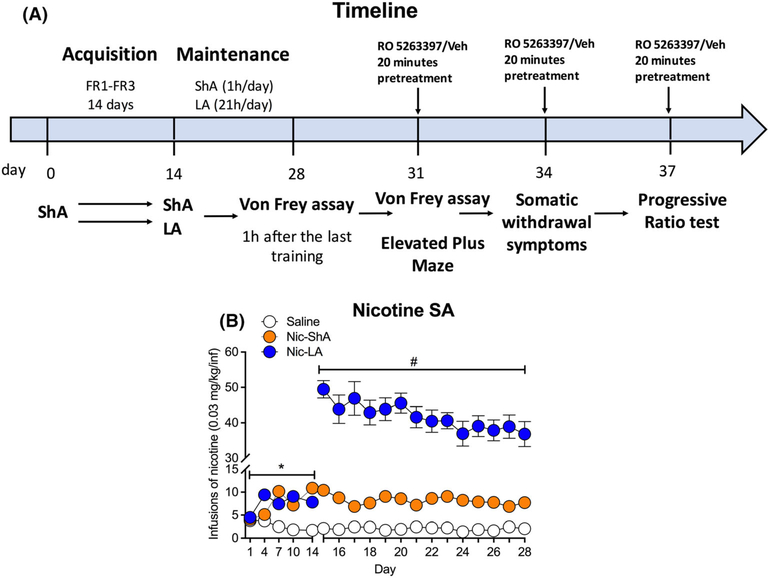
FIGURE 1.
Nicotine self-administration in rats under different access conditions. (A) Experimental timeline. Two groups (saline/nicotine) of rats underwent self-administration training for 2 weeks (acquisition phase, 1 h/day). Then, nicotine group was divided into two subgroups, that is, short-access group (Nic-ShA, 1 h/day) and long-access group (Nic-LA, 21 h/day). (B) Nicotine groups showed increased infusions compared with saline group during the acquisition phase. There was no difference between Nic-ShA group and Nic-LA group during this period. During the maintain phase, Nic-LA group showed increased total infusions compared with Nic-ShA group. Data are expressed as mean ± standard error of the mean (SEM); *p < 0.05, compared with saline; #p < 0.05, compared with Nic-ShA group. Saline: n = 18, Nic-ShA: n = 16, Nic-LA: n = 18
3 |. RESULTS
3.1 |. Nicotine self-administration in rats under different access conditions
Two groups (saline/nicotine) of rats underwent self-administration training for 2 weeks (acquisition phase, 1 h/day, Figure 1A). Nicotine groups showed increased infusions compared with saline group, as shown by a significant effect for the group (two-way repeated ANOVA, F (2,49) = 52.59, p < 0.05, post hoc: Nic-ShA and Nic-LA vs. saline: p < 0.05), time (F (4,179) = 8.89, p < 0.05) and group × time interaction (F (8,195) = 13.09, p < 0.05, Figure 1B), indicating a reinforcing effect of nicotine. Then, nicotine group was divided into two subgroups, that is, ShA group (1 h/day) and LA group (21 h/day), when they were given differential access to nicotine (Figure 1A). There was no difference between Nic-ShA group and Nic-LA group during the acquisition phase.
These three groups (saline, Nic-ShA and Nic-LA) continued self-administration training for additional 2 weeks (maintenance phase). During this phase, Nic-LA group showed increased total infusions compared with Nic-ShA group (two-way repeated ANOVA, group: F (2,49) = 374.4, p < 0.05; time: F (4,179) = 2.30, p < 0.05; group × time interaction: F (8,195) = 1.643, p < 0.05; post hoc: Nic-LA vs. Nic-ShA: p < 0.05, Figure 1B). These results confirmed a higher total nicotine intake in LA rats compared with ShA group.
3.2 |. Effects of RO5263397 on the abstinence-induced anxiety-like behaviour and mechanical hypersensitivity
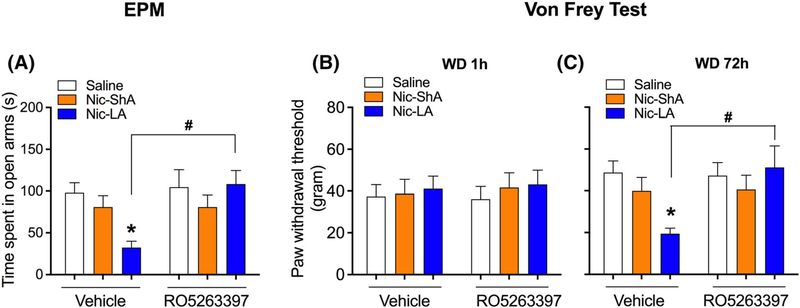
To examine the effect of RO5263397 on the anxiety-like behaviour and mechanical hypersensitivity induced by abstinence, EPM and Von Frey assay were tested 3 days after the last training. Moreover, Von Frey assay was also tested 1 h after the last session to observe any acute mechanical hyperalgesia. RO5263397 or vehicle was injected 20 min prior to the tests. For EPM, we found that Nic-LA group showed an increased anxiety-like behaviour compared with saline group, as evidenced by decreased time spent on the open arms (two-way ANOVA, group × treatment interaction: F (2,39) = 3.52, p < 0.05; post hoc: Nic-LA vs. saline: p < 0.05, Figure 2A), while Nic-ShA group showed no differences. Moreover, RO5263397 significantly attenuated the anxiety-like behaviour in the Nic-LA group, as we observed an increased time spent on the open arms in this group (treatment: F (1,39) = 4.56, p < 0.05; post hoc: RO 526 vs. Veh: p < 0.05, Figure 2A). For the Von Frey assay, we did not observe any differences among all groups at 1 h after the last session (Figure 2B). However, 3 days after the last training, Nic-LA group showed a decrease in paw withdrawal threshold compared with saline group (interaction: F (2,43) = 4.08, p < 0.05; post hoc: Nic-LA vs. saline: p < 0.05, Figure 2C), while Nic-ShA group showed no differences. Furthermore, this abnormal behaviour can be normalized by RO5263397 (treatment: F (2,43) = 1.98, p = 0.05; post hoc: RO 526 vs. Veh: p < 0.05, Figure 2C). These results showed that RO5263397 reduced the abstinence-induced anxiety-like behaviour and mechanical hypersensitivity in the Nic-LA group.
FIGURE 2.
Effects of RO5263397 on the abstinence-induced anxiety-like behaviour and mechanical hypersensitivity. (A) Nic-LA group showed a decreased time spent on the open arms, while Nic-ShA group showed no differences. RO5263397 significantly increased the time spent on the open arms in LA rats without affecting Nic-ShA group. (B) No differences were observed among all groups 1 h after the last session for the Von Frey assay. (C) Three days after the last training, Nic-LA group showed a decrease in paw withdrawal threshold compared with saline group, while Nic-ShA group showed no differences. RO5263397 reversed the mechanical hypersensitivity in LA rats. Data are expressed as mean ± standard error of the mean (SEM); *p < 0.05, compared with saline-vehicle; #p < 0.05, compared with Nic-LA-vehicle group. n = 8–9 for all groups
3.3 |. Effect of RO5263397 on the mecamylamine-precipitated withdrawal symptoms
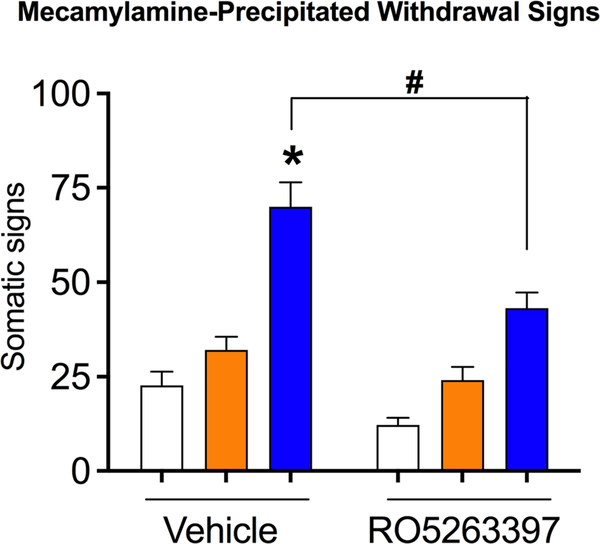
To test whether RO5263397 could reduce the precipitated withdrawal symptoms, we tested the mecamylamine-precipitated withdrawal signs 3 days after the EPM test. RO5263397 or vehicle was injected 20 min prior to the test. We found a significant effect for the group (two-way ANOVA, F (2,50) = 52.39, p < 0.05, Figure 3), treatment (F (1,50) = 21.51, p < 0.05) and interaction (F (2,50) = 3.22, p < 0.05). Post hoc analysis showed that Nic-LA rats had more withdrawal signs compared with saline group (Nic-LA-veh: 70.0 ± 19.3; saline-veh: 22.7 ± 6.0, p < 0.05, Table 1). However, Nic-ShA rats did not differ from saline group (34.3 ± 4.9, Table 1) in the total withdrawal signs. More importantly, systemic administration of RO5263397 significantly reduced the precipitated withdrawal symptoms in the Nic-LA group (Nic-LA-RO: 43.1 ± 12.5; Nic-LA-Veh: 22.7 ± 6.0, p < 0.05, Table 1), without affecting the Nic-ShA rats (Nic-ShA-RO: 22.6 ± 2.1; Nic-ShA-Veh: 34.3 ± 4.9, p > 0.05, Table 1).
FIGURE 3.
Effect of RO5263397 on the mecamylamine-precipitated withdrawal symptoms. Three days after the EPM test, mecamylamine-precipitated withdrawal signs were tested. Nic-LA rats had more withdrawal signs compared with saline group. Nic-ShA rats did not differ from saline group in the total withdrawal signs. Systemic administration of RO5263397 significantly reduced the precipitated withdrawal symptoms in the Nic-LA group without affecting the Nic-ShA rats. Data are expressed as mean ± standard error of the mean(SEM); *p < 0.05, compared with saline-vehicle; #p < 0.05, compared with Nic-LA-vehicle group. n = 8–9 for all groups
TABLE 1.
Mecamylamine-precipitated withdrawal symptoms
| Group | Symptoms | Saline | ShA | LA |
|---|---|---|---|---|
| Eye blinks | 2.9 ± 3.4 | 1.8 ± 1.6 | 24.4 ± 14.0 | |
| Body shakes | 0.1 ± 0.3 | 3.0 ± 2.9 | 4.0 ± 2.5 | |
| Chews | 0.7 ± 1.1 | 4.7 ± 2.6 | 4.3 ± 3.9 | |
| Escape attempts | 16.7 ± 9.0 | 15.3 ± 5.5 | 20.3 ± 7.1 | |
| Foot licks | 2.1 ± 1.5 | 5.4 ± 2.8 | 8.2 ± 4.6 | |
| Head shakes | 0 | 0 | 1.8 ± 1.2 | |
| Vehicle | Teeth chatter | 0 | 0 | 3.0 ± 2.5 |
| Chews + teeth chatter | 0 | 0 | 7.3 ± 4.6 | |
| Grooming | 0.1 ± 0.3 | 3.9 ± 3.2 | 3.6 ± 3.8 | |
| Yawn | 0.1 ± 0.3 | 0.2 ± 0.4 | 0.3 ± 1.0 | |
| Total | 22.7 ± 6.0 | 34.3 ± 4.9 | 70.0 ± 19.3* | |
| Eye blinks | 1.4 ± 1.3 | 2.3 ± 1.6 | 14.2 ± 8.0 | |
| Body shakes | 0 | 0.5 ± 0.7 | 1.2 ± 2.0 | |
| Chews | 1.0 ± 1.2 | 5.0 ± 3.3 | 4.6 ± 2.1 | |
| Escape attempts | 7.9 ± 4.3 | 6.5 ± 4.3 | 8.9 ± 3.4 | |
| Foot licks | 1.4 ± 1.7 | 4.3 ± 3.3 | 4.6 ± 2.7 | |
| RO5263397 | Head shakes | 0 | 0 | 3.8 ± 3.2 |
| Teeth chatter | 0 | 0 | 3.4 ± 2.2 | |
| Chews + teeth chatter | 0 | 0 | 8.0 ± 1.7 | |
| Grooming | 0.3 ± 0.6 | 2.0 ± 1.6 | 2.4 ± 1.9 | |
| Yawn | 0.3 ± 0.6 | 2.2 ± 3.5 | 0 | |
| Total | 12.2 ± 2.8 | 22.6 ± 2.1 | 43.1 ± 12.5# |
Note: n = 8–9 for all groups.
p < 0.05, compared with saline vehicle.
p < 0.05, compared with Nic-LA vehicle.
3.4 |. Effect of RO5263397 on the motivational properties of nicotine withdrawal
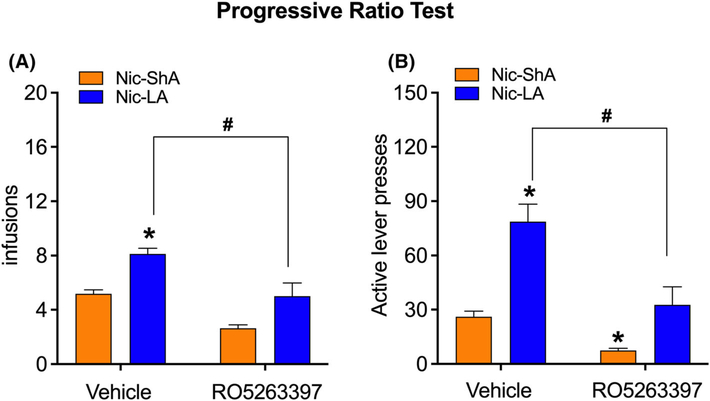
To determine whether RO5263397 could affect the motivational properties of nicotine withdrawal, a PR test was given 3 days after the precipitated withdrawal symptoms test. Here, only Nic-ShA and Nic-LA groups were tested given the fact that rats have much less motivation pressing for saline even after abstinence. Two-way ANOVA analysis showed a significant effect for treatment (F (1,33) = 36.27, p < 0.05) and group (F (1,33) = 31.77, p < 0.05, Figure 4A); however, there was no significant effect for the interaction (F (1,33) = 0.38, p > 0.05). Nic-LA rats showed higher motivation for nicotine after the abstinence compared with Nic-ShA rats, reflected by elevated infusions under a PR schedule (p < 0.05, Figure 4A). Moreover, administration of RO5263397 significantly reduced the infusions in the Nic-LA group (p < 0.05, Figure 4A). Here, RO5263397 had no effect on the infusions in Nic-ShA group possibly because the total number of infusions in the Nic-ShA group was too low.
FIGURE 4.
Effect of RO5263397 on the motivational properties of nicotine withdrawal. (A) Nic-LA rats showed higher motivation for nicotine after the abstinence compared with Nic-ShA rats, reflected by elevated infusions under a progressive-ratio schedule. Administration of RO5263397 significantly reduced the infusions in the Nic-LA group. B. Nic-LA rats showed increased active lever presses at the progressive-ratio test compared with Nic-ShA rats, while RO5263397 attenuated the increased active lever responses in the Nic-LA group. RO5263397 also decreased the active lever responses in the Nic-ShA group. Data are expressed as mean ± standard error of the mean (SEM); *p < 0.05, compared with Nic-ShA-vehicle; #p < 0.05, compared with Nic-LA-vehicle group. n = 8–9 for all groups
Consistently, Nic-LA rats showed increased active lever presses at the PR test compared with Nic-ShA rats (group: F (1,33) = 41.47, p < 0.05; interaction: F (1,33) = 5.12, p < 0.05; post hoc: p < 0.05), while RO5263397 attenuated the increased active lever responses in the Nic-LA group (treatment: F (1,33) = 28.63, p < 0.05; post hoc: p < 0.05, Figure 4B). Meanwhile, R05263397 also decreased the active lever presses in the Nic-ShA group (p < 0.05) in the PR test, which is consistent with our previous finding that R05263397 (5.6 mg/kg) increased the elasticity of nicotine demand curve in ShA rats.30
4 |. DISCUSSION
The present results demonstrate that rats with LA (21 h/day) to nicotine have higher drug intake than rats in ShA (1 h/day) group. Rats from LA group showed significant abstinence-induced anxiety-like behaviour, mechanic hypersensitivity, increased number of precipitated withdrawal signs and higher motivation for the drug, while rats from ShA did not differ from saline group. TAAR1 partial agonist RO5263397 significantly reduced the physical and motivational withdrawal effects of nicotine in LA rats, as reflected by increased time spent on the open arm in the EPM test, normalized paw withdrawal threshold, decreased withdrawal signs and motivation to self-administer nicotine. Meanwhile, RO5263397 also decreased the active lever presses in ShA rats in PR test. This study provides new information that activation of TAAR1 attenuates the negative-reinforcing effects of nicotine withdrawal, in addition to its critical role in nicotine's acute positive rewarding effects.
We found that rats from ShA group had more infusions than rats from ShA group, which is consistent with previous studies that demonstrate higher drug intake in extended-access models.31,32 Moreover, we found that rats with LA of nicotine did not show an escalation of drug intake as compared with studies that utilized extended-access of cocaine self-administration,39–42 as shown by relatively stable nicotine infusions during the maintenance phase in LA rats. Consistently, studies have shown that extended-access of nicotine did not result in increased self-administration.31,32 This disparity can be explained by the different pharmacological properties of drug actions. Although the effects of cocaine can be increased by extended self-administration,42 nicotine acetylcholine receptors (nAChRs) desensitize fast to nicotine exposure in both humans and rats.43,44 On the other hand, both clinical and preclinical studies show that nicotine dependent subjects tend to maintain a stable daily nicotine intake, which may involve the possible aversion induced by high dose nicotine.45–47 However, intermittent-access to extended nicotine self-administration induced a robust escalation in nicotine intake, which might be caused by nicotine withdrawal effects during the acute abstinence.34,48 This result highlights the importance of negative reinforcement to nicotine dependence.
Despite the lack of escalation of nicotine intake in daily LA group, it represents a valid model for nicotine addiction as nicotine withdrawal effects are more pronounced in LA than ShA rats. This result is consistent with previous studies that rats with extended nicotine self-administration show persistent withdrawal signs.32,34 Withdrawal somatic signs are considered as a measure of ‘physical’ nicotine dependence, which has a significant validity as it is sensitive to clinical treatments.49 Moreover, studies have suggested that the severity of withdrawal signs is related to nicotine intake level.31 Because LA rats had higher level of nicotine intake, they might show more withdrawal signs when abstinent from the drug. However, studies also suggest that nicotine consumption level is not related to nicotine withdrawal both in rats and human smokers,32,50,51 which suggest that somatic signs may not be the driving force for nicotine intake in LA rats.
It is hypothesized that affective aspects of withdrawal are of more motivational significance to compulsive nicotine intake and relapse than the withdrawal signs.52 For example, both the smokers and rats after nicotine cessation showed elevated nociceptive sensitivity.53,54 Rats receiving mecamylamine demonstrate a higher brain stimulation reward threshold, a reliable measure of decreased reward and motivation related to withdrawal.52 Moreover, nicotine withdrawal mice showed a significant aversion to the withdrawal-paired place.55 All these studies suggest a remarkable motivational change during the nicotine abstinence. More importantly, increased levels of anxiety and nociceptive hypersensitivity during abstinence are shown to be predictive for the excessive nicotine intake in LA rats.33 Consistently, we found that LA rats showed increased anxiety-like behaviour, mechanic hypersensitivity with higher motivation to self-administer nicotine after the abstinence, indicating an altered emotional state during withdrawal, which might be a strong negative reinforcer for the development of nicotine addiction.
TAAR1 is the best characterized receptor of the TAARs family that has been known for modulating dopaminergic system.56,57 It plays a crucial role of negatively regulating dopamine transmission and thus is widely involved in drug addiction.58,59 In fact, previous studies have shown that activation of TAAR1 attenuates cocaine, methamphetamine, morphine and nicotine self-administration, cueand drug-induced reinstatement and ethanol- and nicotine-induced behavioural sensitization.26,29,30,60–62 Moreover, in our recent study, we showed that TAAR1 agonists were able to reduce the positive reinforcing effects of nicotine by extensive behavioural assay including nicotine self-administration, behavioural sensitization, nicotine discrimination and cue- and drug-induced reinstatement.30 Furthermore, TAAR1 knockout rats showed higher cue- and drug-induced reinstatement of nicotine-seeking compared with their wide-type littermates.30 More importantly, activation of TAAR1 blocked nicotine-induced dopamine release in the nucleus accumbens (NAc) in rats.30 In the present study, we showed that TAAR1 activation decreased nicotine withdrawal-induced anxiety-like behaviour, mechanic hypersensitivity, mecamylamine-precipitated withdrawal symptoms and the motivation to take the drug in LA rats. These results further expand the role of TAAR1 in nicotine addiction as it suggests that TAAR1 might be able to modulate the negative reinforcing effects of nicotine as well.
The TAAR1 activation-induced increased time spent on the open arm, reduced withdrawal symptoms and increased paw withdrawal threshold in LA rats during withdrawal cannot be explained by the possible sedative effect of TAAR1 partial agonist RO5263397. Although RO5263397 was shown to attenuate the hyperlocomotion induced by dopamine transporter inhibitor and NMDA receptor blockers (PCP and L-687,414),63 the dose used here (5.6 mg/kg) was shown not to affect the locomotor activity in naïve rats in previous studies.64–66 Meanwhile, the effect of TAAR1 activation on withdrawal might be nicotine-specific as we have shown that RO5263397 did not affect the naltrexone-induced jumping behaviour and conditioned place aversion (CPA) in morphine-dependent mice.60 In addition, the fact that RO5263397 alone did not affect the analgesic effect of morphine60 while remarkably reduced mechanic hypersensitivity in LA rats after abstinence suggest the effect of RO5263397 on nociception is dependent on the progressive neuroadaptations involved in nicotine withdrawal. Moreover, the effects of RO5263397 may not be due to pharmacokinetics factors. In rats, the half-life of RO5263397 (i.p. injection) is expected to be between those of intravenous (2.6 hr) and oral administration (4.3 hr).63,64 About 94%–97% of the drug will be eliminated after 4–5 half-lives; thus, the observed effect of RO5263397 was unlikely due to the drug accumulation from previous injections because we administrated RO5263397 on Days 3, 6 and 9, respectively after the last training.
It is not clear whether TAAR1-KO rats would self-administer more nicotine under an extended-access procedure and have more severe withdrawal symptoms after drug discontinuation than wild-type (WT) littermates. As our previous study reported that the knockout of TAAR1 increased the reinstatement of nicotine seeking,30 TAAR1 may negatively regulate nicotine-related behaviours. However, it is shown that high dose of nicotine induces aversion, which might contribute to a relatively stable nicotine intake,45–47 it is not known whether this effect can be generalized to TAAR1-KO rats. Therefore, further studies are needed to determine whether TAAR1-KO rats would differ from WT rats in terms of extended-access nicotine self-administration and nicotine withdrawal.
The neural mechanisms underlying the role of TAAR1 in nicotine withdrawal remains unclear. Although the positive reinforcing effects of the drug require the activation of mesolimbic dopaminergic system, the negative reinforcement during nicotine withdrawal recruits a major brain stress system, the corticotropin-releasing factor (CRF) system.49,67 Chronic nicotine exposure increases the level of CRF mRNA in the ventral tegmental area (VTA) and abolished GABAergic input on DA neurons induced by nicotine. Moreover, downregulation of CRF mRNA in the VTA rescued the GABAergic tone of DA neurons and blocked motivational effects of nicotine withdrawal.55 A further study demonstrated that inhibition of CRF reduced the abstinence-induced anxiety-like behaviour and nociceptive hypersensitivity in LA rats but not ShA rats.33 These findings suggest an important role of brain stress system in nicotine withdrawal. Interestingly, TAAR1 was shown to have anti-stress properties. For example, studies showed that TAAR1 agonists attenuated stress-induced hyperthermia.68,69 In our most recent study, TAAR1 activation was shown to abolish the reinstatement of cocaine seeking induced by yohimbine,42 a pharmacological stressor that can produce anxiety- and stress-like state by increasing the norepinephrine (NE) and cortisol levels.70 Thus, it is possible that TAAR1 activation may counteract with the stress-like state induced by nicotine withdrawal and attenuate negative reinforcement of nicotine addiction. However, multiple mechanisms including dysregulation of nAChRs, dopaminergic deficits and altered dynorphin system may also participated in nicotine withdrawal, further studies are needed to clarify the potential role of TAAR1 in interacting with these systems during withdrawal.71–73
In summary, we found that LA, but not ShA, rats showed increased nicotine intake and more pronounced withdrawal effects of nicotine after discontinuation of drug use. TAAR1 partial agonist RO5263397 reduced the abstinence-induced anxiety-like behaviour, mechanic hypersensitivity, mecamylamine-precipitated withdrawal signs and decreased the motivation to self-administer nicotine in a PR schedule. The present study supports the important role of TAAR1 in regulating negative reinforcement of nicotine and suggests that TAAR1 activation may serve as a promising pharmacological approach to treat nicotine addiction.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health National Institute on Drug Abuse (Grants R21DA040777 and R01DA047967 to J-X.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Institutes of Health National Institute on Drug Abuse, Grant/Award Numbers: R01DA047967, R21DA040777
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Creamer MLR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR and Morbidity and Mortality Weekly Report. 2019;68:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. WHO report on the global tobacco epidemic. https://www.who.int/tobacco/global_report/2017/en/. Published 2017. Accessed.
- 3.Wittenberg RE, Wolfman SL, De Biasi M, Dani JA. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology. 2020;177:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2(1):19–37. [DOI] [PubMed] [Google Scholar]
- 5.George O, Ghozland S, Azar MR, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104(43): 17198–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl). 1992;108(4):460–465. [DOI] [PubMed] [Google Scholar]
- 7.Grunberg NE. Overview: biological processes relevant to drugs of dependence. Addiction. 1994;89(11):1443–1446. [DOI] [PubMed] [Google Scholar]
- 8.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. [DOI] [PubMed] [Google Scholar]
- 9.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. [DOI] [PubMed] [Google Scholar]
- 10.Guillem K, Vouillac C, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically prolongs the duration of nicotine withdrawal-induced place aversion. Biol Psychiatry. 2008;63(2): 158–163. [DOI] [PubMed] [Google Scholar]
- 11.O'Dell LE, Koob GF. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86(2):346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. [DOI] [PubMed] [Google Scholar]
- 14.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89(11):1461–1470. [DOI] [PubMed] [Google Scholar]
- 16.Conklin CA, Clayton RR, Tiffany ST, Shiffman S. Introduction to concepts and measurement of the emergence of tobacco dependence: the Tobacco Etiology Research Network. Addiction. 2004;99(Suppl 1): 1–4. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl). 1976;50(1): 35–39. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH. Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology (Berl). 1997;129(4):348–356. [DOI] [PubMed] [Google Scholar]
- 19.Malin DH, Lake JR, Newlin-Maultsby P, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43(3): 779–784. [DOI] [PubMed] [Google Scholar]
- 20.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48(1):52–59. [DOI] [PubMed] [Google Scholar]
- 21.Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70(4):531–549. [DOI] [PubMed] [Google Scholar]
- 22.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. [DOI] [PubMed] [Google Scholar]
- 23.Grandy DK. Trace amine-associated receptor 1—family archetype or iconoclast? Pharmacol Ther. 2007;116(3):355–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainetdinov RR, Hoener MC, Berry MD. Trace amines and their receptors. Pharmacol Rev. 2018;70(3):549–620. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MD, Canales JJ, Zucchi R, Espinoza S, Sukhanov I, Gainetdinov RR. Trace amine-associated receptor 1: a multimodal therapeutic target for neuropsychiatric diseases. Expert Opin Ther Targets. 2018;22(6):513–526. [DOI] [PubMed] [Google Scholar]
- 26.Wu R, Liu J, Wang K, Huang Y, Zhang Y, Li JX. Effects of a trace amine-associated receptor 1 agonist RO 5263397 on ethanol-induced behavioral sensitization. Behav Brain Res. 2020;390: 112641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JF, Siemian JN, Seaman R Jr, Zhang Y, Li JX. Role of TAAR1 within the subregions of the mesocorticolimbic dopaminergic system in cocaine-seeking behavior. J Neurosci. 2017;37(4): 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei Y, Mortas P, Hoener MC, Canales JJ. Selective activation of the trace amine-associated receptor 1 decreases cocaine's reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63: 70–75. [DOI] [PubMed] [Google Scholar]
- 29.Cotter R, Pei Y, Mus L, et al. The trace amine-associated receptor 1 modulates methamphetamine's neurochemical and behavioral effects. Front Neurosci. 2015;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JF, Seaman R Jr, Siemian JN, et al. Role of trace amine-associated receptor 1 in nicotine's behavioral and neurochemical effects. Neuropsychopharmacology. 2018;43(12):2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Dell LE, Chen SA, Smith RT, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007; 320(1):180–193. [DOI] [PubMed] [Google Scholar]
- 32.Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl). 2004;173(1–2):64–72. [DOI] [PubMed] [Google Scholar]
- 33.Cohen A, Treweek J, Edwards S, et al. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol. 2015;20(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37(9): 2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abreu-Villaca Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhaes AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2006; 167(1):175–182. [DOI] [PubMed] [Google Scholar]
- 36.Liu JF, Thorn DA, Zhang Y, Li JX. Effects of trace amine-associated receptor 1 agonists on the expression, reconsolidation, and extinction of cocaine reward memory. Int J Neuropsychopharmacol. 2016;19(7): pyw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorn DA, Jing L, Qiu Y, et al. Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology. 2014;39(10):2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suo L, Zhao L, Si J, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013;38(8):1387–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387): 298–300. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl). 1999;146(3):303–312. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5(7):625–626. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Johnson B, Wu R, et al. TAAR1 agonists attenuate extended-access cocaine self-administration and yohimbine-induced reinstatement of cocaine-seeking. Br J Pharmacol. 2020;177(15): 3403–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16(5):905–908. [DOI] [PubMed] [Google Scholar]
- 44.Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4(beta)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21(6):1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl). 1995;117(1):2–10. discussion 14–20 [DOI] [PubMed] [Google Scholar]
- 46.McNeill AD, Jarvis MJ, Stapleton JA, West RJ, Bryant A. Nicotine intake in young smokers: longitudinal study of saliva cotinine concentrations. Am J Public Health. 1989;79(2):172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu Y, Matta SG, Brower VG, Sharp BM. Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: An in vivo microdialysis study. J Neurosci. 2001;21(22):8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66(3):553–558. [DOI] [PubMed] [Google Scholar]
- 49.McLaughlin I, Dani JA, De Biasi M. Nicotine withdrawal. Curr Top Behav Neurosci. 2015;24:99–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl). 2003; 168(3):280–292. [DOI] [PubMed] [Google Scholar]
- 51.Hughes JR, Gust SW, Keenan RM, Fenwick JW. Effect of dose on nicotine's reinforcing, withdrawal-suppression and self-reported effects. J Pharmacol Exp Ther. 1990;252(3):1175–1183. [PubMed] [Google Scholar]
- 52.Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292(3): 1053–1064. [PubMed] [Google Scholar]
- 53.John U, Meyer C, Rumpf HJ, Hapke U. Nicotine dependence criteria and nicotine withdrawal symptoms in relation to pain among an adult general population sample. Eur J Pain. 2009;13(1):82–88. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt BL, Tambeli CH, Gear RW, Levine JD. Nicotine withdrawal hyperalgesia and opioid-mediated analgesia depend on nicotine receptors in nucleus accumbens. Neuroscience. 2001;106(1): 129–136. [DOI] [PubMed] [Google Scholar]
- 55.Grieder TE, Herman MA, Contet C, et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci. 2014;17(12):1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26(5):274–281. [DOI] [PubMed] [Google Scholar]
- 57.Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindemann L, Meyer CA, Jeanneau K, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324(3):948–956. [DOI] [PubMed] [Google Scholar]
- 59.Asif-Malik A, Hoener MC, Canales JJ. interaction between the trace amine-associated receptor 1 and the dopamine D2 receptor controls cocaine's neurochemical actions. Sci Rep. 2017;7(1):13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Seaman R Jr, Johnson B, et al. Activation of trace amine-associated receptor 1 selectively attenuates the reinforcing effects of morphine. Br J Pharmacol. 2021;178(4):933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pei Y, Lee J, Leo D, Gainetdinov RR, Hoener MC, Canales JJ. Activation of the trace amine-associated receptor 1 prevents relapse to cocaine seeking. Neuropsychopharmacology. 2014;39(10):2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sukhanov I, Dorofeikova M, Dolgorukova A, Dorotenko A, Gainetdinov RR. Trace amine-associated receptor 1 modulates the locomotor and sensitization effects of nicotine. Front Pharmacol. 2018;9:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Revel FG, Moreau JL, Pouzet B, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18(5):543–556. [DOI] [PubMed] [Google Scholar]
- 64.Thorn DA, Zhang C, Zhang Y, Li JX. The trace amine associated receptor 1 agonist RO5263397 attenuates the induction of cocaine behavioral sensitization in rats. Neurosci Lett. 2014;566: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jing L, Zhang Y, Li JX. Effects of the trace amine associated receptor 1 agonist RO5263397 on abuse-related behavioral indices of methamphetamine in rats. Int J Neuropsychopharmacol. 2014;18(4): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sukhanov I, Dorotenko A, Dolgorukova A, Hoener MC, Gainetdinov RR, Bespalov AY. Activation of trace amine-associated receptor 1 attenuates schedule-induced polydipsia in rats. Neuropharmacology. 2019;144:184–192. [DOI] [PubMed] [Google Scholar]
- 67.Jackson KJ, Muldoon PP, De Biasi M, Damaj MI. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology. 2015;96 (Pt B):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Revel FG, Moreau JL, Gainetdinov RR, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A. 2011;108(20):8485–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Revel FG, Moreau JL, Gainetdinov RR, et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry. 2012;72(11):934–942. [DOI] [PubMed] [Google Scholar]
- 70.Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl). 2014;231(21): 4157–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30(27):9241–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chavkin C, Koob GF. Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology. 2016;41(1):373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]