Structured Abstract
Objective:
Expand Operative Stress Score (OSS) increasing procedural coverage and assessing OSS and frailty association with Preoperative Acute Serious Conditions (PASC), complications and mortality in females versus males.
Summary Background Data:
Veterans Affairs male-dominated study showed high mortality in frail veterans even after very low stress surgeries (OSS1).
Methods:
Retrospective cohort using NSQIP data (2013–2019) merged with 180-day postoperative mortality from multiple hospitals to evaluate PASC, 30-day complications and 30-, 90- and 180-day mortality.
Results:
OSS expansion resulted in 98.2% case coverage versus 87.0% using the original. Of 82,269 patients (43.8% male), 7.9% were frail/very frail. Males had higher odds of PASC (aOR=1.31, 95%CI=1.21–1.41, P<.001) and severe/life–threatening Clavien-Dindo IV (CDIV) complications (aOR=1.18, 95%CI=1.09–1.28, P<.001). While mortality rates were higher (all time points, P<.001) in males versus females, mortality was similar after adjusting for frailty, OSS, and case status primarily due to increased male frailty scores. Additional adjustments for PASC and CDIV resulted in a lower odds of mortality in males (30-day, aOR=0.81, CI=0.71–0.92, P=.002) that was most pronounced for males with PASC compared to females with PASC (30-day, aOR=0.75, CI=0.56–0.99, P=.04).
Conclusions:
Similar to the male-dominated Veteran population, private sector, frail patients have high likelihood of postoperative mortality, even after low stress surgeries. Preoperative frailty screening should be performed regardless of magnitude of the procedure. Despite males experiencing higher adjusted odds of PASC and CDIV complications, females with PASC had higher odds of mortality compared to males, suggesting differences in the aggressiveness of care provided to men and women.
Mini-Abstract
Cohort study of 82,269 patients comparing sex differences in Preoperative Acute Serious Conditions (PASC), complications and mortality adjusted for frailty, operative stress and case status. Despite having higher odds of PASC and Clavien-Dindo IV/life-threatening complications, males exhibited a survival advantage compared to females suggesting sex-related differences in care.
INTRODUCTION
Frailty defines the state of decreased physiological reserve contributing to adverse outcomes in the setting of stress, especially in older populations.1, 2 Frailty more accurately predicts adverse outcomes than age alone.3 Studies across multiple specialties associated frailty with increased postoperative complications, prolonged hospital stays, discharge to non-home facilities, and mortality.3–14 Frailty can be used to identify high-risk patients for postoperative complications to improve shared decision making and preoperative optimization.15
Surgeries of different complexity place variable amounts of stress on patients. Many surgical outcome studies used groups of high-risk procedures rather than broadly assessing risk across diverse surgeries.3, 4, 6–8, 11 One study defined high-risk surgeries as procedures having a 30-day mortality rate >1%.13 However, more surgeries have >1% mortality in patients ≥65 years of age compared to younger patients.16 A recent study by Shinall et al. introduced the Operative Stress Score (OSS) assigning a score from minimal (1) to maximal (5) operative stress17 to evaluate the intersection of frailty and OSS on postoperative mortality in Veterans Health Administration (VHA) patients. Frailty was measured using the Risk Analysis Index (RAI),18, 19 a validated instrument using variables in the Veterans Affairs Surgical Quality Improvement Program (VASQIP) and American College of Surgeons National Surgical Quality Improvement Program (NSQIP). Mortality increased as OSS increased at 30-, 90-, and 180-day time points after surgery in robust, normal and frail patients. The study found a high 30-day mortality of 10% for very frail patients after even very low stress surgeries. These patterns remained consistent when procedures were grouped by elective or emergent operations.20
The VHA study17 provided an important new method for comparing diverse procedure using a scale of surgical-induced physiologic stress, highlighting the negative impact of frailty, and applicable to control for procedure-related complexity in samples of diverse specialties.14 However, study generalizability to the overall United States population was limited by VHA patient demographics of 92.8% males, 69.3% White and 5.2% Hispanic.17 Although the OSS contains 565 Current Procedural Terminology (CPT) codes covering 90% of the cases in VASQIP, it may not be representative of the most common surgical procedures in the private sector, especially procedures more commonly performed among women.
NSQIP includes variables for acute conditions present at time of surgery (PATOS) to distinguish preoperative risk factors from postoperative complications. However, most studies using NSQIP data do not include PATOS variables. Urinary tract infection, pneumonia, or septic shock PATOS variables were associated with increased postoperative complications and mortality,21–24 and other PATOS variables were associated with higher risk of 30-day admissions.25 However, there is a paucity of data exploring the importance of PATOS variables in postoperative outcomes overall and specific to sex.
Accurate outcome assessment is crucial to informed decision making and guides preoperative health status optimization and multidisciplinary interventions. This study aims to 1) expand the number of CPT codes assigned an OSS value from the original VHA study,17 especially for female-specific procedures, 2) confirm whether the high postoperative mortality in frail Veterans17 was generalizable to a more diverse, female-majority population, and 3) assess the association of frailty, operative stress and PATOS conditions with complications and mortality. We hypothesized that the odds of postoperative mortality between males and females would be similar after adjusting for OSS, RAI, case status, preoperative acute conditions/PATOS and complications.
METHODS
Expansion of OSS
The original OSS contained 565 CPT codes assigned scores designating the level of physiological stress induced by each procedure using a modified Delphi consensus method by a panel of surgeons and anesthesiologists across specialty fields.17 The CPT codes in the original OSS were chosen to include 90% of the procedures in VASQIP that covered a population that was 92.8% male17 resulting in a predisposition to include male-specific procedures such as prostatectomy with minimal representation of female-specific procedures such as hysterectomy. The VASQIP dataset was originally chosen as long-term mortality was available from the VHA while only 30-day mortality was available in NSQIP. The OSS ranges from 1–5 with 1 and 5 representing surgeries inducing very low and very high physiological stress, respectively. Three surgeons leveraged hierarchies implicit within the Unified Medical Language System (UMLS) to arrange CPT scores according to anatomical location, extending the original OSS ratings to CPT codes located within the same level of UMLS hierarchy (see Supplemental Digital Content (SDC)).
Original and Expanded OSS Case Coverage
Case coverage, defined as the percent of records with an OSS-rated principal CPT code, for the original and expanded OSS was assessed in the NSQIP Participant Use Data File (PUF) from 2015–2018 and the study cohort derived from NSQIP data from multiple hospitals in 4 Academic Medical Centers (AMC).
Study Populations
Retrospective cohort study using patients in the 2013–2019 NSQIP registries at multiple hospitals in four AMC following STROBE reporting guidelines.26 NSQIP contains detailed data on surgical patients including medical and surgical history, CPT codes, and 30-day outcomes retrospectively collected by certified surgical clinical reviewers with high inter-rater reliability.27 The identified NSQIP data were merged with long-term mortality at each site using electronic health record (EHR) data augmented by state mortality and Social Security Death Master File data.28 De-identified datasets from each institution were merged for analysis with University of Texas Health San Antonio Institutional Review Board (IRB) serving as the single IRB. The University of Pittsburgh IRB determined exempt status for using the deidentified NSQIP PUF.
Frailty was assessed by the recalibrated and validated RAI18, 19, 29 defining frailty categories as robust (<20), normal (20–29), frail (30–39) and very frail (≥40). Cases missing any NSQIP variable needed to calculate the RAI were excluded from the analysis (see SDC). Case status determined using NSQIP variables for elective and emergency surgeries, urgent defined as “no” for elective and emergency variables.
OSS Assignment in AMC NSQIP Cohort
NSQIP allows up to 11 CPT codes for each case. After excluding cases without an expanded OSS assigned to the principal CPT code, OSS was assigned using the highest score for all available procedures within each case.
Preoperative Acute Serious Conditions (PASC)
NSQIP contains 8 PATOS variables including 6 serious conditions: on ventilator >48hrs, deep incisional surgical site infection, organ/space surgical site infection, pneumonia, sepsis and septic shock and 2 lower acuity conditions including superficial incisional surgical site infection and urinary tract infection. NSQIP also contains preoperative variables for acute renal failure and dialysis occurring within 24 hours and 2 weeks prior to surgery, respectively. We derived two additional preoperative conditions: 1) acute renal failure not requiring preoperative dialysis for patients with yes to acute renal failure and no to dialysis and 2) acute renal failure requiring preoperative dialysis for patients with yes for both acute renal failure and dialysis.
We defined PASC as including the 6 serious PATOS variables and the 2 renal failure variables. The lower acuity PATOS variables were not included in PASC. The distribution of individual PASC variables was assessed and PASC was used as a binary variable.
Study Outcomes
Primary outcomes were 30-, 90- and 180-day mortality grouped by OSS and RAI. Secondary outcomes were 1) occurrences of PASC, 2) any 30-day postoperative complication including reoperation using NSQIP variables 3) 30-day severe/life-threatening Clavien-Dindo level IV (CDIV) complications; CDIV complications included NSQIP variables of postoperative septic shock, postoperative dialysis, pulmonary embolus, myocardial infarction, cardiac arrest, prolonged ventilation, reintubation, coma or stroke18, 30 and 4) mortality adjusted odds ratios (aOR).
Statistical Analysis
Data reported as categorical (count and percentage) and continuous (means with standard deviation). Crude mortality rates calculated with 95% confidence interval (CI) for each RAI/OSS group. Chi-square test, Graham-Mengersen-Morton test,31 and two sample t-test assuming a binomial or normal distribution were used to compare categorical, binary, and continuous variables between males and females. Logistic regression analyses assessed PASC, complications, and mortality adjusting for sex, RAI, OSS, case status, PASC, and complications. Interaction terms of sex with CDIV and PASC were used to assess effect of sex on the associations of CDIV or PASC with mortality. Sensitivity analyses performed using PASC subgroups. Analyses performed using RStudio version 1.3.1056 (RStudio, Inc., Boston, MA).
RESULTS
Expansion of OSS and NSQIP Case Coverage of Original Versus Expanded OSS
The 565 CPT codes in the original OSS17 were increased to 2,343 CPT codes in the expanded OSS (SDCTable1). NSQIP 2015–2018 PUF contained 3,935,119 cases (43.2% male). Coverage of original versus expanded OSS increased from 87.0% to 98.2% of cases, and improvement was most pronounced in females, increasing by 13.6% in females (84.9% to 98.5%) compared to 8.2% in males (89.7% to 97.9%).
The AMC study population included 90,819 non-cardiac NSQIP cases from 2013–2019. The expanded OSS covered 98.6% of cases compared to 83.4 % using the original OSS (SDCTable2). Coverage varied across centers, ranging from 71.0%–91.5% for the original OSS and 96.5%–99.4% for the expanded OSS.
Cases were excluded due to missing 1) variables used to calculate the RAI (n=527), 2) expanded OSS assignment of principal CPT code (n=1,290) and 3) case status variables (n=667), resulting in 88,335 cases in 82,269 patients. Random selection of a single case/patient was used for patients with multiple cases to assess the mortality endpoint (SDCFigure1) resulting in 82,269 cases for analysis.
Population Demographics
The 82,269 unique patients were 43.8% male, 76.6% Caucasian, 13.8% Black and 17.7% Hispanic ethnicity (Table 1). The cohort was mostly robust (63.6%) and normal (28.5%), while 7.1% were frail and 0.8% were very frail. Most patients underwent OSS2 (37.3%) and OSS3 (47.2%) procedures. The principal CPT code had the highest OSS in 78,680 cases, while 3,589 cases (4.4%) were categorized to a higher OSS by additional procedures included in the case. For these 3,589 cases, using the highest OSS CPT code increased OSS by 1 point for 3,486 cases (97.1%), by 2 points for 97 cases (2.7%) and by 3 points for 6 cases (0.2%).
Table 1.
Demographic Characteristics of Patients from 4 Academic Medical Centers
| Total | Male | Female | P Value | |
|---|---|---|---|---|
| No. (%) | 82,269 | 36,022 (43.8) | 46,247 (56.2) | |
| Age, mean (SD), years | 54.4 (16.7) | 55.5 (16.6) | 53.6 (16.7) | <.001 |
| Race | <.001 | |||
| Caucasian, No. (%) | 62,990 (76.6) | 28,162 (78.2) | 34,828 (75.3) | |
| African American, No. (%) | 11,345 (13.8) | 4,361 (12.1) | 6,984 (15.1) | |
| Other, No. (%)a | 1,379 (1.7) | 554 (1.5) | 825 (1.8) | |
| Unknown, No. (%) | 6,555 (8.0) | 2,945 (8.2) | 3,610 (7.8) | |
| Ethnicity | <.001 | |||
| Non-Hispanic or Latino, No. (%) | 57,496 (69.9) | 25,471 (70.7) | 32,025 (69.2) | |
| Hispanic or Latino, No. (%) | 14,583 (17.7) | 5,943 (16.5) | 8,640 (18.7) | |
| Unknown, No. (%) | 10,190 (12.4) | 4,608 (12.8) | 5,582 (12.1) | |
| Expanded Operative Stress Score (OSS) | <.001 | |||
| OSS1, No. (%) | 2,516 (3.1) | 1,468 (4.1) | 1,048 (2.3) | |
| OSS2, No. (%) | 30,725 (37.3) | 13,894 (38.6) | 16,831 (36.4) | |
| OSS3, No. (%) | 38,820 (47.2) | 15,335 (42.6) | 23,485 (50.8) | |
| OSS4, No. (%) | 8,710 (10.6) | 4,455 (12.4) | 4,255 (9.2) | |
| OSS5, No. (%) | 1,498 (1.8) | 870 (2.4) | 628 (1.4) | |
| Risk Analysis Index | <.001 | |||
| Robust (≤20), No. (%) | 52,340 (63.6) | 17,418 (48.4) | 34,922 (75.5) | |
| Normal (21–29), No. (%) | 23,410 (28.5) | 14,938 (41.5) | 8,472 (18.3) | |
| Frail (30–39), No. (%) | 5,862 (7.1) | 3,262 (9.1) | 2,600 (5.6) | |
| Very Frail (≥40), No. (%) | 657 (0.8) | 404 (1.1) | 253 (0.5) | |
| PASC present, No. (%) | 3,492 (4.2) | 1,970 (5.5) | 1,522 (3.3) | <.001 |
| 30-day Complications | ||||
| No Complications, No. (%) | 69,213 (84.1) | 29,773 (82.7) | 39,440 (85.3) | <.001 |
| Any Complication, No. (%) | 13,056 (15.9) | 6,249 (17.3) | 6,807 (14.7) | <.001 |
| CDIV Complications, No. (%) | 2,689 (3.3) | 1,497 (4.2) | 1,192 (2.6) | <.001 |
| Case status | ||||
| Elective, No. (%) | 58,319 (70.9) | 24,355 (67.6) | 33,964 (73.4) | <.001 |
| Urgent/Emergent, No. (%) | 23,950 (29.1) | 11,667 (32.4) | 12,283 (26.6) | <.001 |
| Mortality | ||||
| 30-day Mortality, No. (%) | 1,267 (1.5) | 683 (1.9) | 584 (1.3) | <.001 |
| 90-day Mortality, No. (%) | 1,884 (2.3) | 1,026 (2.8) | 858 (1.9) | <.001 |
| 180-day Mortality, No. (%) | 2,483 (3.0) | 1,355 (3.8) | 1,128 (2.4) | <.001 |
Abbreviations: CDIV, Clavien-Dindo Level IV; SD, Standard deviation
Pearson’s Chi-square tests were used to calculate P values.
Other includes American Indian, Asian and Pacific Islander
Males had higher rates of PASC (P<.001), any complication (P<.001), CDIV complications (P<.001), urgent/emergent cases (P<.001), and mortality at all 3 time points (P<.001) compared to females (Table 1). Frailty (P<.001) and OSS distribution (P<.001) differed between males and females. A higher percentage of females were robust while more males were normal, frail, or very frail. Males underwent higher percents of OSS1, 2, 4 and 5 procedures while the majority of females (50.8%) underwent OSS3 procedures. The five most common procedure types per OSS level represented 62.7%, 47.9%, 41.8%, 74.2% and 92.6% of OSS1 to 5 categories, respectively (SDCTable3).
Mortality Grouped by Frailty and Expanded OSS
Mortality at 30, 90 and 180 days grouped by RAI generally increased with increasing expanded OSS (Table 2 and Figure 1A–C). Case numbers in each group were >100 except for the very frail patients undergoing OSS1 and OSS5 procedures which only contained 18 and 17 cases, respectively. Mortality patterns were similar comparing the expanded and original OSS (n=69,677 cases) groups (SDCFigure2). Mortality patterns across expanded OSS/frailty groups were also similar for male compared to female patients (Figure 1D–I).
Table 2.
30-, 90-, and 180-day Mortality Grouped by Frailty and Expanded Operative Stress Score
| Mortality | OSS1 | OSS2 | OSS3 | OSS4 | OSS5 |
|---|---|---|---|---|---|
| n=2,516 | n=30,725 | n= 38,820 | n=8,710 | n=1,498 | |
| 30-day Deaths, No. | 10 | 183 | 614 | 381 | 79 |
| 30-day Mortality by RAI % (95% CI) | |||||
| Robust (≤20) | 0.06 (0.00–0.31) | 0.14 (0.09–0.20) | 0.32 (0.25–0.40) | 1.83 (1.43–2.31) | 2.12 (1.02–3.87) |
| Normal (21–29) | 0.36 (0.04–1.28) | 0.96 (0.75–1.20) | 2.09 (1.83–2.38) | 5.10 (4.37–5.92) | 6.78 (5.19–8.68) |
| Frail (30–39) | 3.38 (1.11–7.71) | 5.10 (3.93–6.51) | 8.44 (7.45–9.53) | 6.95 (5.72–8.36) | 7.10 (3.60–12.34) |
| Very Frail (≥40) | 11.11 (1.38–34.71) | 14.63 (8.91–22.14) | 18.39 (14.46–22.87) | 27.15 (20.24–34.98) | 0.00 (0.00–19.51) |
| Overall Mortality | 0.40 (0.19–0.73) | 0.60 (0.51–0.69) | 1.58 (1.46–1.71) | 4.37 (3.95–4.83) | 5.27 (4.20–6.53) |
| 90-day Deaths, No. | 20 | 300 | 917 | 541 | 106 |
| Mortality by RAI, % (95% CI) | |||||
| Robust (≤20) | 0.11 (0.01–0.40) | 0.23 (0.17–0.30) | 0.48 (0.40–0.58) | 2.36 (1.90–2.89) | 2.97 (1.63–4.94) |
| Normal (21–29) | 0.36 (0.04–1.28) | 1.50 (1.24–1.80) | 3.12 (2.80–3.46) | 7.21 (6.34–8.15) | 8.77 (6.96–10.87) |
| Frail (30–39) | 9.46 (5.27–15.36) | 8.62 (7.09–10.36) | 12.79 (11.58–14.07) | 10.79 (9.27–12.47) | 10.97 (6.52–16.98) |
| Very Frail (≥40) | 11.11 (1.38–34.71) | 26.02 (18.52–34.70) | 25.57 (21.07–30.50) | 36.42 (28.75–44.64) | 0.00(0.00–19.51) |
| Overall Mortality | 0.79 (0.49–1.23) | 0.98 (0.87–1.09) | 2.36 (2.21–2.52) | 6.21 (5.71–6.74) | 7.08 (5.83–8.49) |
| 180-day Deaths, No. | 26 | 434 | 1186 | 677 | 160 |
| Mortality by RAI, % (95% CI) | |||||
| Robust (≤20) | 0.17 (0.03–0.49) | 0.35 (0.27–0.43) | 0.67 (0.57–0.78) | 2.83 (2.33–3.41) | 5.31 (3.46–7.74) |
| Normal (21–29) | 0.53 (0.11–1.55) | 2.15 (1.84–2.50) | 4.12 (3.76–4.51) | 8.78 (7.83–9.81) | 12.28 (10.16–14.67) |
| Frail (30–39) | 12.16 (7.37–18.54) | 12.55 (10.73–14.57) | 16.08 (14.75–17.48) | 14.64 (12.89–16.52) | 18.06 (12.35–25.04) |
| Very Frail (≥40) | 11.11 (1.38–34.71) | 34.96 (26.58–44.08) | 31.03 (26.21–36.19) | 42.38 (34.39–50.68) | 11.76 (1.46–36.44) |
| Overall Mortality | 1.03 (0.68–1.51) | 1.41 (1.28–1.55) | 3.06 (2.89–3.23) | 7.77 (7.22–8.35) | 10.68 (9.16–12.36) |
Abbreviations: CI, Confidence Interval; OSS, Operative Stress Score; RAI, Risk Analysis Index
Graham-Mengersen-Morton tests were used to calculate 95% confidence intervals.
Figure 1. Mortality Grouped by Expanded Operative Stress Score (OSS) and Frailty.
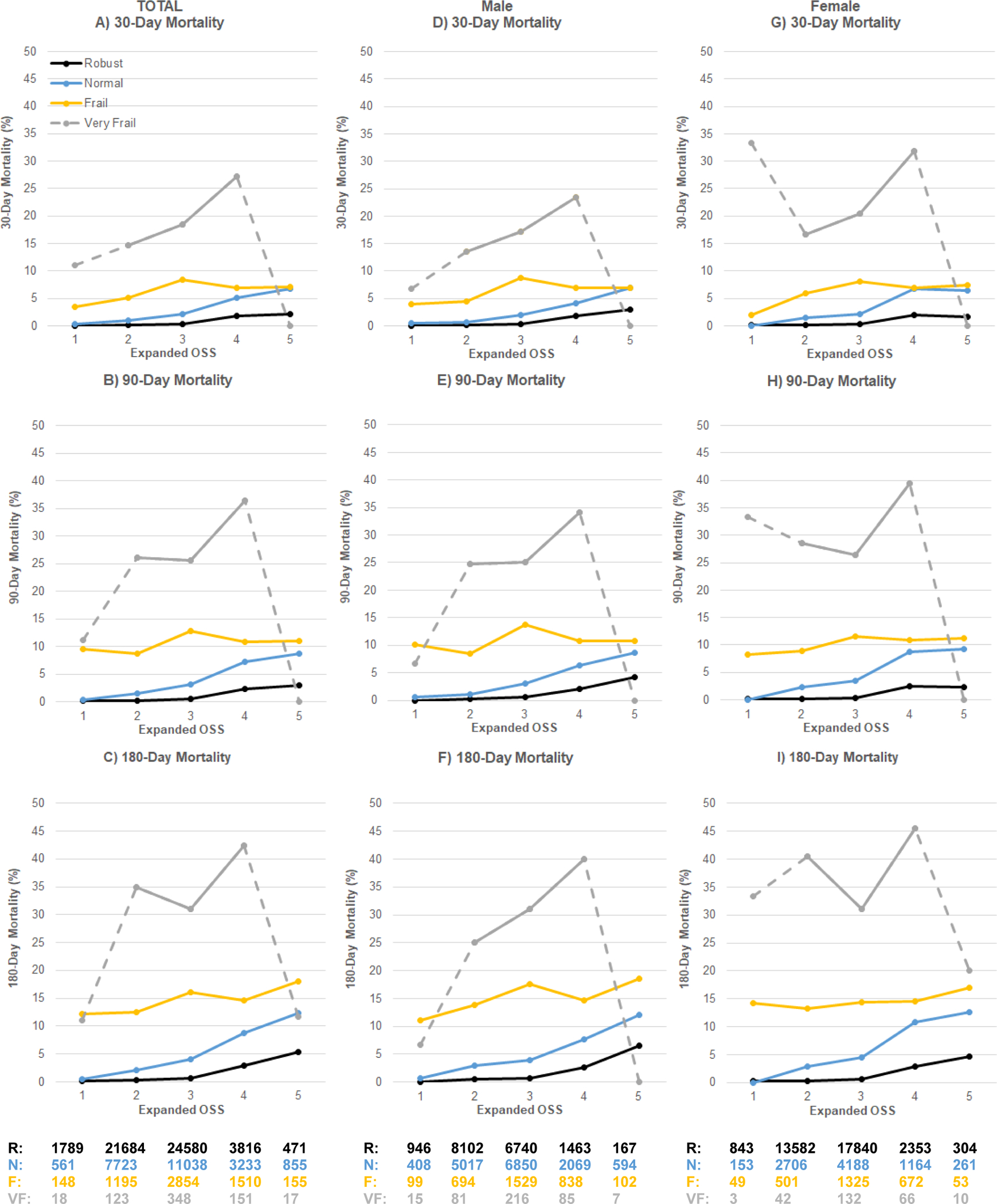
Total cases (n=82,269) A–C, cases performed in males (n=36,022) D–F and females (n=46,247) G–I. Expanded OSS defined physiologic stress induced by surgery as 1 (very low), 2 (low), 3 (moderate), 4 (high) and 5 (very high). Frailty assessed by Risk Analysis Index scores of ≤20 robust (R), 21–29 normal (N), 30–39 frail (F), and ≥40 very frail (VF). Small sample size for very frail patients undergoing OSS1 and OSS5 procedures shown with dashed gray line.
Increased PASC and 30-day Complication Rates in Males Compared to Females
PASC was present in 4.2% of patients and higher in males (P<.001) compared to females (Table1). PASC variable distribution was similar between males and females except males had increased preoperative acute renal failure (P=.005) and requiring acute dialysis (P=.03) with lower rates of septic shock (P=.04) compared to female patients (Table 3). The composite distribution of patients with multiple occurrences of PASC variables was similar between males and females. Patients with PASC predominately (87.9%) underwent urgent/emergent surgeries.
Table 3.
Distribution of Preoperative Acute Serious Conditions (PASC) and Clavien-Dindo IV (CDIV) Complications
| Total | Male | Female | P value | |
|---|---|---|---|---|
| PASC a | 3,492 | 1,970 | 1,522 | |
| Deep incision surgical site infection (DSSIPATOS), No. (%) | 128 (3.7) | 71 (3.6) | 57 (3.7) | .90 |
| Organ/space surgical site infection (OSSIPATOS), No. (%) | 447 (12.8) | 254 (12.9) | 193 (12.7) | .89 |
| Pneumonia (PNAPATOS), No. (%) | 298 (8.5) | 178 (9.0) | 120 (7.9) | .25 |
| On ventilator >48hrs (VENTPATOS), No. (%) | 406 (11.6) | 216 (11.0) | 190 (12.5) | .18 |
| Sepsis (SEPSISPATOS), No. (%) | 1551 (44.4) | 876 (44.5) | 675 (44.3) | .97 |
| Septic shock (SEPSHOCKPATOS), No. (%) | 849 (24.3) | 453 (23.0) | 396 (26.0) | .04 |
| Acute renal failure not requiring dialysisb, No. (%) | 334 (9.6) | 213 (10.8) | 121 (8.0) | .005 |
| Acute renal failure requiring dialysisc, No. (%) | 247 (7.1) | 156 (7.9) | 91 (6.0) | .03 |
| Composite Distribution of PASC | .59 | |||
| Patients with 1 PASC, No. (%) | 2,857 (81.8) | 1,603 (81.4) | 1,254 (82.4) | |
| Patients with 2 PASC, No. (%) | 524 (15.0) | 302 (15.3) | 222 (14.6) | |
| Patients with 3 PASC, No. (%) | 91 (2.6) | 51 (2.6) | 40 (2.6) | |
| Patients with 4–5 PASC, No. (%) | 20 (0.6) | 14 (0.7) | 6 (0.4) | |
| Urgent/Emergent case status | 3069 (87.9) | 1729 (87.8) | 1340 (88.0) | .85 |
| CDIV complications | 2689 | 1497 | 1192 | |
| Reintubation (REINTUB), No. (%) | 1,014 (37.7) | 583 (38.9) | 431 (36.2) | .15 |
| Pulmonary embolism (PULEMBOL), No. (%) | 348 (12.9) | 192 (12.8) | 156 (13.1) | .89 |
| Failure to wean (ventilator >48hrs) (FAILWEAN), No. (%) | 1,120 (41.7) | 622 (41.5) | 498 (41.8) | .94 |
| Acute renal failure (require dialysis) (OPRENAFL), No. (%) | 355 (13.2) | 222 (14.8) | 133 (11.2) | .006 |
| Cerebral vascular event/stroke (CNSCVA), No. (%) | 210 (7.8) | 103 (6.9) | 107 (9.0) | .052 |
| Cardiac arrest (CDARREST), No. (%) | 343 (12.8) | 208 (13.9) | 135 (11.3) | .054 |
| Myocardial infarction (CDMI), No. (%) | 324 (12.0) | 196 (13.1) | 128 (10.7) | .07 |
| Septic shock (OTHSESHOCK), No. (%) | 450 (16.7) | 256 (17.1) | 194 (16.3) | .61 |
| Composite Distribution of CDIV complications | .03 | |||
| Patients with 1 CDIV | 1,700 (63.2) | 915 (61.1) | 785 (65.9) | |
| Patients with 2 CDIV | 629 (23.4) | 359 (24.0) | 270 (22.7) | |
| Patients with 3 CDIV | 265 (9.9) | 163 (10.9) | 102 (8.6) | |
| Patients with ≥4 CDIV | 95 (3.5) | 60 (4.0) | 35 (2.9) | |
| Urgent/Emergent case status | 1739 (64.7) | 990 (66.1) | 749 (62.8) | .08 |
| Patients with PASC and CDIV | 848 | 475 | 373 | |
| Urgent/Emergent case status, No. (%) | 802 (94.6) | 448 (94.3) | 354 (94.9) | .82 |
Abbreviations: NSQIP, National Surgical Quality Improvement Program, NSQIP variable names included in parentheses
Pearson’s Chi-square test was used to calculate P values.
144 patients with only preoperative urinary tract infection or superficial surgical site infection were not included in PASC.
defined as a positive response to RENAFAIL and negative response to DIALYSIS NSQIP variables
defined as a positive response to both RENAFAIL and DIALYSIS NSQIP variables
Any complication and CDIV complications occurred in 15.9% and 3.3% of patients, respectively (Table 1) with males exhibiting higher rates than females. The types of CDIV complications were similar between males and females except males had increased postoperative acute renal failure requiring dialysis (P=.006, Table 3). Distribution of multiple occurrences of CDIV complications showed that males were more likely than females to have higher numbers of CDIV complications (P=.03). Patients experiencing CDIV complications predominately (64.7%) underwent urgent/emergent surgeries. Patients having both PASC and CDIV complications (1.0% of AMC cohort) predominately (94.6%) underwent urgent/emergent surgeries.
PASC and 30-Day Complication Logistic Regression Models; Males had Increased Odds of PASC and CDIV Complications Compared to Females
Males were more likely to have PASC (aOR=1.31, CI=1.21–1.41, P<.001, Table 4). Increasing RAI categories and urgent/emergent cases were associated with higher odds of PASC. Patients undergoing OSS4 procedures were the most likely to have PASC.
Table 4.
PASC and 30-day Complications Odds Ratios Adjusted for Sex, RAI, OSS and Case Status
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| PASC | ||
| Male (Ref = Female) | 1.31 (1.21–1.41) | <.001 |
| RAI (Ref = Normal 21–29) | ||
| Robust (≤20) | 0.60 (0.55–0.66) | <.001 |
| Frail (30–39) | 1.46 (1.31–1.63) | <.001 |
| Very frail (≥40) | 2.41 (1.97–2.95) | <.001 |
| Expanded OSS (Ref = OSS1) | ||
| OSS2 | 0.71 (0.59–0.87) | .001 |
| OSS3 | 0.71 (0.58–0.86) | .001 |
| OSS4 | 2.24 (1.83–2.74) | <.001 |
| OSS5 | 0.57 (0.39–0.85) | .005 |
| Urgent/Emergent (Ref = elective) | 19.08 (17.19–21.16) | <.001 |
| Model C-Statistic | 0.87 | |
| No complications | ||
| Male (Ref = Female) | 1.07 (1.02–1.11) | .003 |
| RAI (Ref = Normal 21–29) | ||
| Robust (≤20) | 1.75 (1.68–1.84) | <.001 |
| Frail (30–39) | 0.64 (0.60–0.68) | <.001 |
| Very frail (≥40) | 0.39 (0.33–0.46) | <.001 |
| Expanded OSS (Ref = OSS1) | ||
| OSS2 | 0.87 (0.74–1.03) | .11 |
| OSS3 | 0.37 (0.32–0.43) | <.001 |
| OSS4 | 0.12 (0.11–0.15) | <.001 |
| OSS5 | 0.06 (0.05–0.07) | <.001 |
| Urgent/Emergent (Ref = elective) | 0.40 (0.38–0.42) | <.001 |
| Model C-Statistic | 0.75 | |
| Any complication | ||
| Male (Ref = Female) | 0.94 (0.90–0.98) | .003 |
| RAI (Ref = Normal 21–29) | ||
| Robust (≤20) | 0.57 (0.54–0.60) | <.001 |
| Frail (30–39) | 1.56 (1.46–1.67) | <.001 |
| Very frail (≥40) | 2.56 (2.16–3.02) | <.001 |
| Expanded OSS (Ref = OSS1) | ||
| OSS2 | 1.14 (0.97–1.35) | .11 |
| OSS3 | 2.71 (2.31–3.18) | <.001 |
| OSS4 | 8.10 (6.88–9.55) | <.001 |
| OSS5 | 16.70 (13.82–20.19) | <.001 |
| Urgent/Emergent (Ref = elective) | 2.51 (2.41–2.62) | <.001 |
| Model C-Statistic | 0.75 | |
| Clavien-Dindo IV complications | ||
| Male (Ref = Female) | 1.18 (1.09–1.28) | <.001 |
| RAI (Ref = Normal 21–29) | ||
| Robust (≤20) | 0.40 (0.36–0.44) | <.001 |
| Frail (30–39) | 1.44 (1.29–1.61) | <.001 |
| Very frail (≥40) | 2.00 (1.61–2.50) | <.001 |
| Expanded OSS (Ref = OSS1) | ||
| OSS2 | 1.21 (0.81–1.80) | 0.35 |
| OSS3 | 3.17 (2.16–4.67) | <.001 |
| OSS4 | 7.26 (4.92–10.72) | <.001 |
| OSS5 | 13.78 (9.10–20.86) | <.001 |
| Urgent/Emergent (Ref = elective) | 4.50 (4.14–4.89) | <.001 |
| Model C-Statistic | 0.81 | |
Abbreviations: OR, Odds Ratio; RAI, Risk Analysis Index; OSS, Operative Stress Score; PASC, Preoperative Acute Serious Conditions; Ref, reference
Logistic regression analyses
Increasing OSS or RAI categories and urgent/emergent cases were associated with increased aOR for complications (Table 4). Males had lower odds of experiencing any complication (aOR=0.94, CI=0.90–0.98, P=.003) compared to females. However, males had higher odds of experiencing CDIV complications (aOR=1.18, CI=1.09–1.28, P<.001).
Mortality Logistic Regression Models; Similar aOR between Males and Females after Adjusting for RAI, OSS and Case Status
Increasing RAI and OSS categories were associated with increased mortality rates (Table 2). Males had higher percent mortality (Table 1) at all 3 time points compared to females. Unadjusted mortality for males was higher at all time points (30-day: OR=1.51, CI=1.35–1.69, 90-day: OR=1.55, CI=1.41–1.70, 180-day: OR=1.56, CI=1.44–1.69). However, mortality aOR were similar between males and females after RAI adjustment (30-day: aOR=0.90, CI=0.80–1.01, 90-day: aOR=0.92, CI=0.83–1.01, 180-day: aOR=0.93, CI=0.85–1.01) and remained similar after additional OSS and case status adjustment (30-day: aOR=0.95, CI=0.84–1.06, 90-day: aOR=0.96, CI=0.87–1.06, 180-day: aOR=0.96, CI=0.88–1.05), suggesting that the increased, unadjusted mortality rates in males were primarily due to higher RAI scores (Table 1).
Males with PASC Exhibit Lower Odds of Mortality Compared to Females with PASC
PASC was associated with higher odds of mortality after adjustment for sex, RAI, OSS and case status (Table 5). Subgroup analyses suggested that males with PASC had lower odds of mortality/survival advantage compared to females at all time points. Interaction term analysis examining sex and PASC showed that the increased odds of mortality associated with PASC at 90 (P=.02) and 180 days (P=.004) were more pronounced for women than men.
Table 5.
Mortality Odds Ratios Adjusted for Sex, RAI, OSS, Case Status, and PASC with Sex*PASC Interaction Term or PASC Subgroup Analyses
| 30-day | 90-day | 180-day | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) |
P value | Adjusted OR (95% CI) |
P value | Adjusted OR (95% CI) |
P value | |
| Sex*PASC Interaction Term Analysis (n=82,269) | ||||||
| Male (Ref = Female) | 0.98 (0.85–1.14) | .82 | 1.00 (0.89–1.13) | .99 | 1.00 (0.90–1.11) | .997 |
| RAI (Ref = Normal 21–29) | ||||||
| Robust (≤20) | 0.20 (0.17–0.24) | <.001 | 0.19 (0.17–0.22) | <.001 | 0.19 (0.17–0.22) | <.001 |
| Frail (30–39) | 2.39 (2.08–2.75) | <.001 | 2.80 (2.50–3.15) | <.001 | 3.05 (2.75–3.37) | <.001 |
| Very frail (≥40) | 4.02 (3.17–5.10) | <.001 | 4.88 (3.96–6.01) | <.001 | 5.52 (4.55–6.69) | <.001 |
| Expanded OSS (Ref = OSS1) | ||||||
| OSS2 | 1.90 (0.99–3.64) | .053 | 1.55 (0.97–2.47) | .07 | 1.72 (1.14–2.60) | .009 |
| OSS3 | 3.53 (1.87–6.69) | <.001 | 2.64 (1.67–4.18) | <.001 | 2.67 (1.78–4.00) | <.001 |
| OSS4 | 5.48 (2.88–10.43) | <.001 | 4.18 (2.63–6.65) | <.001 | 4.27 (2.83–6.42) | <.001 |
| OSS5 | 15.11 (7.65–29.81) | <.001 | 9.57 (5.79–15.83) | <.001 | 11.16 (7.20–17.31) | <.001 |
| Urgent/Emergent (Ref = elective) |
5.56 (4.78–6.48) | <.001 | 4.80 (4.27–5.41) | <.001 | 3.90 (3.54–4.30) | <.001 |
| PASC | 6.31 (5.19–7.67) | <.001 | 5.42 (4.56–6.45) | <.001 | 4.80 (4.08–5.65) | <.001 |
| Males*PASC | 0.78 (0.61–1.01) | .055 | 0.76 (0.60–0.95) | .02 | 0.73 (0.59–0.91) | .004 |
| Model C-Statistic | 0.91 | 0.90 | 0.88 | |||
| Subgroup Analyses | ||||||
| PASC subgroup (n=3,492) | ||||||
| Male (Ref = Female) | 0.80 (0.65–0.98) | .03 | 0.80 (0.66–0.96) | .02 | 0.78 (0.65–0.94) | .007 |
| RAI (Ref = Normal 21–29) | ||||||
| Robust (≤20) | 0.28 (0.21–0.36) | <.001 | 0.27 (0.21–0.34) | <.001 | 0.27 (0.22–0.35) | <.001 |
| Frail (30–39) | 1.87 (1.47–2.38) | <.001 | 2.29 (1.83–2.87) | <.001 | 2.32 (1.87–2.89) | <.001 |
| Very frail (≥40) | 2.69 (1.88–3.85) | <.001 | 3.06 (2.16–4.33) | <.001 | 3.38 (2.39–4.77) | <.001 |
| Expanded OSS (Ref = OSS1) | ||||||
| OSS2 | 2.89 (0.88–9.44) | .08 | 2.38 (0.93–6.09) | .07 | 2.98 (1.17–7.58) | .02 |
| OSS3 | 5.90 (1.84–18.94) | .003 | 4.51 (1.80–11.34) | .001 | 5.36 (2.14–13.45) | <.001 |
| OSS4 | 7.85 (2.44–25.26) | .001 | 6.73 (2.67–16.95) | <.001 | 7.76 (3.08–19.53) | <.001 |
| OSS5 | 7.70 (1.75–33.84) | .007 | 11.11 (3.38–36.57) | <.001 | 12.62 (3.89–40.99) | <.001 |
| Urgent/Emergent (Ref = elective) |
4.02 (2.48–6.50) | <.001 | 3.21 (2.18–4.74) | <.001 | 2.64 (1.87–3.73) | <.001 |
| Model C-Statistic | 0.76 | 0.77 | 0.77 | |||
| No PASC subgroup (n=78,777) | ||||||
| Male (Ref = Female) | 0.95 (0.82–1.11) | .53 | 0.98 (0.87–1.10) | .72 | 0.98 (0.89–1.09) | .69 |
| RAI (Ref = Normal 21–29) | ||||||
| Robust (≤20) | 0.17 (0.14–0.22) | <.001 | 0.17 (0.14–0.20) | <.001 | 0.17 (0.15–0.20) | <.001 |
| Frail (30–39) | 2.67 (2.24–3.17) | <.001 | 2.99 (2.61–3.43) | <.001 | 3.26 (2.90–3.66) | <.001 |
| Very frail (≥40) | 5.37 (3.99–7.24) | <.001 | 6.17 (4.83–7.88) | <.001 | 6.71 (5.38–8.37) | <.001 |
| Expanded OSS (Ref = OSS1) | ||||||
| OSS2 | 1.54 (0.72–3.33) | .27 | 1.31 (0.77–2.24) | .32 | 1.46 (0.93–2.30) | .10 |
| OSS3 | 2.70 (1.27–5.74) | .01 | 2.14 (1.27–3.60) | .004 | 2.15 (1.40–3.35) | .001 |
| OSS4 | 4.52 (2.10–9.70) | <.001 | 3.39 (1.99–5.77) | <.001 | 3.47 (2.21–5.46) | <.001 |
| OSS5 | 13.22 (6.01–29.10) | <.001 | 8.01 (4.57–14.06) | <.001 | 9.39 (5.83–15.11) | <.001 |
| Urgent/Emergent (Ref = elective) |
5.61 (4.77–6.59) | <.001 | 4.86 (4.29–5.51) | <.001 | 3.95 (3.56–4.37) | <.001 |
| Model C-Statistic | 0.88 | 0.87 | 0.86 | |||
Abbreviations: OR, Odds Ratio; OSS, Operative Stress Score; PASC, Preoperative Acute Serious Conditions; RAI, Risk Analysis Index; Ref, reference
Logistic regression analyses
Males Exhibit Lower Odds of Mortality in Logistic Regression Models Adjusted for CDIV Complications
Addition of any complication to mortality logistic regression models showed similar aOR for mortality between males and females (SDCTable4). Adjusting for CDIV complications resulted in lower odds of mortality for males at all time points (SDCTable5). However, the sex-CDIV interaction term was not statistically significant.
Males with PASC Exhibit Lower Odds of Mortality in Logistic Regression Models after adjusting for CDIV
PASC and CDIV were associated with higher odds of mortality after adjustment for sex, RAI, OSS, and case status (Table 6). Adjusting for PASC and CDIV complications resulted in lower odds of mortality for males at all time points (30-day aOR=0.81, CI=0.71–0.92, P=.002, SDCTable6). Subgroup analyses suggested that males with PASC had a survival advantage compared to females at all time points (Table 6). The sex-PASC interaction term again demonstrated the increased odds of mortality associated with PASC were more pronounced for women than men (Table 6).
Table 6.
Mortality Odds Ratios Adjusted for Sex, RAI, OSS, Case Status, CDIV and PASC with Sex*PASC Interaction Term or PASC Subgroup Analyses
| 30-day | 90-day | 180-day | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Sex*PASC Interaction Term Analysis (n=82,269) | ||||||
| Male (Ref = Female) | 0.88 (0.75–1.04) | .13 | 0.92 (0.81–1.05) | .21 | 0.94 (0.85–1.05) | .27 |
| RAI (Ref = Normal 21–29) | ||||||
| Robust (≤20) | 0.25 (0.21–0.30) | <.001 | 0.22 (0.19–0.26) | <.001 | 0.21 (0.19–0.24) | <.001 |
| Frail (30–39) | 2.45 (2.10–2.86) | <.001 | 2.96 (2.61–3.36) | <.001 | 3.20 (2.87–3.57) | <.001 |
| Very frail (≥40) | 4.60 (3.51–6.03) | <.001 | 5.64 (4.50–7.09) | <.001 | 6.17 (5.03–7.57) | <.001 |
| Expanded OSS (Ref = OSS1) | ||||||
| OSS2 | 1.74 (0.89–3.40) | .11 | 1.43 (0.89–2.32) | .14 | 1.63 (1.07–2.48) | .02 |
| OSS3 | 2.52 (1.30–4.86) | .006 | 2.01 (1.26–3.23) | .004 | 2.16 (1.43–3.26) | <.001 |
| OSS4 | 3.18 (1.64–6.18) | .001 | 2.70 (1.67–4.35) | <.001 | 3.03 (1.99–4.59) | <.001 |
| OSS5 | 6.51 (3.21–13.21) | <.001 | 4.75 (2.82–8.01) | <.001 | 6.57 (4.19–10.31) | <.001 |
| Urgent/Emergent (Ref = elective) | 4.08 (3.48–4.79) | <.001 | 3.79 (3.35–4.29) | <.001 | 3.22 (2.90–3.56) | <.001 |
| CDIV | 15.50 (13.49–17.81) | <.001 | 12.13 (10.74–13.69) | <.001 | 9.30 (8.31–10.41) | <.001 |
| PASC | 3.50 (2.82–4.35) | <.001 | 3.28 (2.71–3.96) | <.001 | 3.08 (2.58–3.67) | <.001 |
| Males*PASC | 0.75 (0.56–0.989) | .04 | 0.73 (0.57–0.93) | .01 | 0.71 (0.56–0.89) | .003 |
| Model C-statistic | 0.95 | 0.93 | 0.95 | |||
| Subgroup Analyses | ||||||
| PASC subgroup (n=3,492) | ||||||
| Male (Ref = Female) | 0.76 (0.61–0.93) | .009 | 0.76 (0.62–0.92) | .006 | 0.74 (0.62–0.90) | .002 |
| RAI (Ref = Normal 21–29) | ||||||
| Robust (≤20) | 0.31 (0.23–0.41) | <.001 | 0.30 (0.23–0.39) | <.001 | 0.30 (0.24–0.39) | <.001 |
| Frail (30–39) | 1.89 (1.47–2.44) | <.001 | 2.40 (1.89–3.05) | <.001 | 2.42 (1.92–3.05) | <.001 |
| Very frail (≥40) | 2.64 (1.80–3.88) | <.001 | 3.10 (2.14–4.50) | <.001 | 3.46 (2.39–4.99) | <.001 |
| Expanded OSS (Ref = OSS1) | ||||||
| OSS2 | 2.75 (0.83–9.12) | .10 | 2.27 (0.87–5.88) | .09 | 2.87 (1.11–7.39) | .03 |
| OSS3 | 4.45 (1.37–14.47) | .01 | 3.43 (1.34–8.74) | .01 | 4.18 (1.65–10.63) | .003 |
| OSS4 | 5.43 (1.66–17.72) | .005 | 4.78 (1.87–12.24) | .001 | 5.69 (2.23–14.50) | <.001 |
| OSS5 | 3.56 (0.79–15.97) | .10 | 5.26 (1.55–17.79) | .008 | 6.19 (1.86–20.60) | .003 |
| Urgent/Emergent (Ref = elective) | 3.07 (1.88–5.01) | <.001 | 2.49 (1.67–3.71) | <.001 | 2.07 (1.45–2.95) | <.001 |
| CDIV | 4.73 (3.82–5.84) | <.001 | 4.66 (3.82–5.70) | <.001 | 4.37 (3.60–5.31) | <.001 |
| Model C-Statistic | 0.82 | 0.82 | 0.82 | |||
| No PASC subgroup (n=78,777) | ||||||
| Male (Ref = Female) | 0.82 (0.70–0.97) | .02 | 0.88 (0.78–1.01) | .06 | 0.91 (0.82–1.02) | .10 |
| RAI (Ref = Normal 21–29) | ||||||
| Robust (≤20) | 0.24 (0.19–0.30) | <.001 | 0.21 (0.17–0.25) | <.001 | 0.20 (0.17–0.23) | <.001 |
| Frail (30–39) | 2.61 (2.15–3.16) | <.001 | 3.08 (2.65–3.57) | <.001 | 3.37 (2.98–3.81) | <.001 |
| Very frail (≥40) | 6.56 (4.63–9.29) | <.001 | 7.38 (5.63–9.68) | <.001 | 7.56 (5.97–9.57) | <.001 |
| Expanded OSS (Ref = OSS1) | ||||||
| OSS2 | 1.38 (0.62–3.05) | .43 | 1.22 (0.71–2.11) | .48 | 1.40 (0.88–2.21) | .15 |
| OSS3 | 1.88 (0.86–4.10) | .11 | 1.66 (0.97–2.84) | .06 | 1.80 (1.15–2.83) | .01 |
| OSS4 | 2.26 (1.02–5.00) | .04 | 2.09 (1.21–3.62) | .008 | 2.47 (1.56–3.92) | <.001 |
| OSS5 | 4.98 (2.18–11.36) | <.001 | 3.75 (2.09–6.75) | <.001 | 5.51 (3.38–9.00) | <.001 |
| Urgent/Emergent (Ref = elective) | 3.87 (3.25–4.59) | <.001 | 3.76 (3.29–4.29) | <.001 | 3.22 (2.89–3.59) | <.001 |
| CDIV | 29.54 (24.90–35.04) | <.001 | 18.73 (16.22–21.63) | <.001 | 12.64 (11.07–14.43) | <.001 |
| Model C-Statistic | 0.94 | 0.92 | 0.89 | |||
Abbreviations: CDIV, Clavien-Dindo IV complications; OR, Odds Ratio; OSS, Operative Stress Score; PASC, Preoperative Acute Serious Conditions; RAI, Risk Analysis Index; Ref, reference.
Logistic regression analyses
DISCUSSION
OSS scores reflect the estimated physiologic stress induced by a procedure. We expanded the original OSS from 565 to 2,343 CPT codes. Expanded OSS improved procedural coverage at four AMC and within the NSQIP PUF. Importantly, coverage in females improved, compensating for the original OSS using a male-dominated veteran population. Our private sector cohort with a more racially and ethnically diverse, female-majority population demonstrated similar patterns as the VHA study with high mortality in frail patients across the OSS spectrum.17 We confirmed the VHA study’s findings in longer-term outcomes, an analysis that is not possible using NSQIP PUF limited to 30-day outcomes. Importantly, our findings of 14.6%, 18.4%, 27.2% 30-day mortality in the very frail group were similar to the VHA study of 10.1%, 18.7%, 22.3% for OSS2, 3 and 4 procedures, respectively.
Studies on frailty and postoperative outcomes have focused heavily on emergent and major elective procedures that are considered either intermediate or high risk.3, 32, 33 Our data in a female-majority, private-sector population confirmed the VHA study17 finding that even low risk procedures carry elevated mortality risk for frail patients. High-risk procedures have been defined as those with 30-day mortality exceeding 1%.13 However, even for the low stress, OSS2 procedures, the VHA study demonstrated a 30-day mortality of 10.1% in very frail patients compared to 14.6% in our study, well exceeding the preset level for high-risk procedures.17 A recent study using VASQIP and NSQIP demonstrated that the association of frailty and postoperative mortality was consistent across non-cardiac surgical specialties regardless of case-mix.14 A study using Medicare inpatient data also found high mortality even after low risk emergency surgeries emphasizing the importance of preoperative frailty screening for all.34 Frailty screening used by a multidisciplinary team in preoperative planning reduced 30-day mortality in frail patients from 12.2% to 3.8%; possibly due to better preoperative planning, intraoperative management and postoperative rescue.35 Additionally, rehabilitation through exercise and nutrition supplementation are promising interventions to improve function and decrease perioperative complications and mortality.36–40 These observations suggest that frailty screening is important even for surgeries associated with low physiologic stress and may improve risk assessment accuracy for shared decision making in elective and emergency settings.
Although a variety of tools exist for measuring frailty, none has emerged as a gold standard.41 Frailty indices based on deficit accumulation are widely available,42 but typically deployed for post-hoc applications in registries. Measures of physical frailty are often used in research settings9 but are often too intensive for routine screening of robust populations. A recent consensus panel emphasized the need for a pragmatic approach to frailty screening that emphasizes feasible tests that can be implemented systematically.41 One such feasible tool is the Edmonton Frail Scale that measures frailty in 10 domains with mostly patient reported items and two performance-based items.43, 44 Alternatively, the RAI can be calculated from registry variables as done in this study or can be implemented as a 14-item survey taking less than a minute for patients to complete. Both versions of the RAI have been thoroughly validated in surgical patients,19, 45, 46 and the survey version is the only screening tool proven feasible for system-wide implementation in both the VHA35 and private sector45–47 and was associated with improved postoperative survival.47 Based on these data, the RAI survey will soon be released as a “clinical program” embedded in the Epic electronic record system, and work is also under way to make the RAI survey available within the VHA’s electronic record, both Computerized Patient Record System (CPRS) and Cerner.
The most common procedures within the OSS categories that differed in the VHA study versus our cohort were most notably sex-specific procedures.17 Overlap between the VHA and our study in the 5 most common procedure types was 20%, 60%, 60%, 40%, and 60% for OSS1 to OSS5, respectively. In the VHA study, male-specific procedures of hydrocele, transurethral prostate procedures, and prostatectomy ranked 2nd, 4th, and 2nd in terms of frequency for OSS1, OSS2 and OSS4 categories, respectively. Our most common procedures included mastectomy and hysterectomy ranking 4th in OSS2 and OSS3 categories, respectively, but no male-specific procedures were present. As such, expanded OSS provides improved CPT code coverage and is more appropriate than original OSS for use in NSQIP and non-male-majority populations.
Males had lower odds of any 30-day complications than females after adjusting for RAI, OSS, and case status but higher odds of CDIV complications. Other studies adjusting for frailty showed that males had complication risks compared to females that were higher after pancreatectomy,6 lower after ambulatory general surgeries48 and similar after paraoesophageal hernia repair49. Complication risk may be different for males versus females in surgeries of different stress levels and needs further evaluation.
While males had higher unadjusted mortality compared to females, adjustment for only frailty and in conjunction with OSS and case status resulted in similar odds of postoperative mortality for males and females at all time points. OSS did not exhibit a substantial effect for sex on mortality possibly related to the bimodal distribution of OSS with males having more OSS1, 2, 4, and 5 procedures while females had a higher percentage of OSS3 procedures. Two studies found similar mortality rates between both sexes after adjusting for frailty, other patient characteristics and surgery types.3, 6 However, female sex was associated with higher mortality after vascular procedures8, 50 but lower mortality after emergency general surgery.34 Sex-related mortality differences may be related to different procedure types and requires further investigation.
NSQIP PATOS variables were associated with increased complications and mortality.21–24 However, the literature on preoperative acute conditions is limited with only select conditions studied for targeted surgeries. We found that while males had higher rates and aOR of PASC, males with PASC had lower odds of mortality compared to females with PASC using both subgroup and interaction term analyses across a broad array of surgical types. We speculate that the lower mortality odds in males could be related to differences in aggressiveness of treatment and intensive care unit (ICU) admission.51, 52 Studies have shown sex differences in hospitalization and mortality for acute diverticulitis53 and coronary artery disease54 where females were less likely to receive surgery or cardiac protective medication. Additionally, females had lower ICU admission rates and shorter ICU stays after adjusting for diagnosis and disease severity.51, 52 Surveys of physicians found that sex did not affect their willingness to admit patients to the ICU.55, 56 However, surveying elderly outpatients found females less frequently desired life-supporting medical therapies, suggesting male predominance in ICU admissions could be related to patient preference.57 These differences could affect other management decisions possibly leading to mortality differences in severely ill patients. Further investigation is needed to determine whether disparities in female patient care contribute to the survival advantage for males with PASC.
This was a retrospective observational study that did not allow for determination of causal relationships. Due to the small number of very frail patients that underwent OSS1 and OSS5 procedures, we also cannot draw conclusions for these two subgroups. There are other important outcome measures that were not evaluated in our study such as length of stay, discharge location, disability, and quality of life that are may be more meaningful, especially for frail patients. NSQIP does not distinguish deaths directly related to surgical procedures from all-cause mortality, nor does NSQIP specifically identify procedures that may have been performed with a goal of palliation rather than extending life. It is also likely that the RAI survey was assessed preoperatively for some of the patients included in our sample, but only from one of the participating AMC and only on cases after July 1, 2016. Although such RAI screening was associated with increased survival at 1year after surgery,47 this benefit was not apparent before 1 year (unpublished data), and thus not likely to impact the findings here limited to survival up to 180 days after surgery. Furthermore, the response to frailty identified by the RAI was left to the discretion of the treating surgeon without formal prehabilitation or decision support interventions beyond the identification of frailty.
In conclusion, frailty is associated with high mortality even after low-stress surgeries in both sexes and OSS expansion improved procedural coverage in a female-majority population. Our study provides further support14, 17 that preoperative frailty screening should be performed regardless of the magnitude of the planned procedure to improve shared decision making and preoperative optimization. Importantly, including PASC variables in analyses showed sex-specific differences in mortality. While males have higher rates and adjusted odds of PASC and CDIV complications, females with PASC had higher odds of mortality. The extent to which more aggressive treatment for males, patient care preferences and/or disparities in the care of female patients account for the survival differences is unknown and deserves further study.
Supplementary Material
SDCFigure 1. Flow Diagram of Study Cohort. National Surgery Quality Improvement Program cases from 2013–2019 from multiple hospitals at four academic medical centers. Cases were excluded for missing variables used to calculate the Risk Analysis Index (RAI), lack of an expanded Operative Stress Score (OSS) assignment for the principal CPT code, and erroneous or missing case status. Cases lacking an expanded OSS assignment for the principal CPT code were excluded to avoid erroneously assigning a lower stress OSS based upon additional procedures that were performed. For example, a principal CPT code for a highly stressful procedure not assigned an expanded OSS could be assigned an OSS1 if the additional CPT codes contained any procedure with an expanded OSS rating. A single case per patient was selected at random for patients with multiple cases.
SDCFigure 2. Mortality (%) Grouped by Original Operative Stress Score (OSS) and Frailty. Mortality at A) 30, B) 90 and C) 180 days after the date of surgery. The 82,269 cases using the expanded OSS contained the subset of 69,677 cases assigned original OSS values. OSS defined physiologic stress induced by surgery as 1 (very low), 2 (low), 3 (moderate), 4 (high) and 5 (very high). Frailty assessed by Risk Analysis Index scores of ≤20 robust (R), 21–29 normal (N), 30–39 frail (F), and ≥40 very frail (VF). Small sample size for very frail patients undergoing OSS1 and OSS5 procedures shown with dashed gray line.
Acknowledgements:
We would like to thank our informatics and data analyst teams on U01TR002393 Zaheer Sarwar, MD, PhD, Laura Manuel, BS, Deeksha Sharma, MS, Alejandro Araya, BS, Bill Ross, BS, Emily Pfaff, PhD, and Howard Su, BS.
Funding/Support:
This research was supported by grant U01TR002393 (Kim, Hall, Stitzenberg, Kao, Wang, Silverstein, Bernstam and Shireman), from the National Center for Advancing Translational Sciences and the Office of the Director, NIH; grant K12CA090625 (Shinall) from the National Cancer Institute, NIH; grant K76AG068436 (Shinall) and L30AG064730 (Reitz) from the National Institute on Aging, NIH; grant 5T32HL0098036 (Reitz) from the National Heart, Lung, and Blood Institute, NIH and Clinical Translational Science Awards UL1TR002489 (University of North Caroline), UL1TR001857 (University of Pittsburgh), UL1TR003167 (University of Texas Health Science Center Houston) and UL1TR002645 (University of Texas Health San Antonio) from the National Center for Advancing Translational Sciences, NIH. Funds were also provided by the Veterans Affairs Center for Innovation to Implementation research fellowship funding (George) from Health Services Research and Development.
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The opinions expressed here are those of the authors and do not necessarily reflect the position of the National Institutes of Health, Department of Veterans Affairs or the United States government.
Footnotes
Presented at the virtual Clinical Congress 2020 of the American College of Surgeons, October 3–7, 2020
Conflict of Interest Disclosures: Drs. Yan, Kim, Youk, Silverstein and Wang report no conflict of interest disclosures and Ms. Sharma report no conflict of interest disclosures. Dr. Hall reported receiving grants from the National Institutes of Health and Veterans administration during the conduct of this study; he also reported a consulting relationship with FutureAssure, LLC. Dr Shinall reported receiving grants from the National Cancer Institute and the National Institute on Aging during the conduct of the study. Dr. Reitz reported receiving grant funding from the National Heart, Lung, and Blood Institute and National Institute on Aging. Dr. Stitzenberg reported receiving grant funding from the National Institutes of Health. Dr. Kao reported receiving royalties from Springer, Wolters-Klower, and McGraw-Hill. Dr George reported receiving salary support from the Palo Alto Veterans Health Care system as part of a Veterans Affairs Center for Innovation to Implementation research fellowship during the conduct of the study. Dr. Bernstam reported receiving grants from the National Institutes of Health, Patient-Centered Outcomes Research Institute, Substance Abuse and Mental Health Services Administration, and Cullen Trust for Health Care. Dr. Shireman reported receiving grants from the National Institutes of Health and Veterans Health Administration and salary support from South Texas Veterans Health Care System and the University of Texas Health San Antonio during the conduct of the study. No other disclosures were reported.
References:
- 1.Fried LP, Ferrucci L, Darer J, et al. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J Gerontol A Biol Sci Med Sci 2004; 59(3):M255–M263. [DOI] [PubMed] [Google Scholar]
- 2.Kim SW, Han HS, Jung HW, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg 2014; 149(7):633–40. [DOI] [PubMed] [Google Scholar]
- 3.McIsaac DI, Taljaard M, Bryson GL, et al. Frailty as a Predictor of Death or New Disability After Surgery: A Prospective Cohort Study. Ann Surg 2018. [DOI] [PubMed]
- 4.Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013; 206(4):544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg 2011; 253(6):1223–9. [DOI] [PubMed] [Google Scholar]
- 6.Augustin T, Burstein MD, Schneider EB, et al. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery 2016; 160(4):987–996. [DOI] [PubMed] [Google Scholar]
- 7.Adams P, Ghanem T, Stachler R, et al. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg 2013; 139(8):783–9. [DOI] [PubMed] [Google Scholar]
- 8.Arya S, Kim SI, Duwayri Y, et al. Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J Vasc Surg 2015; 61(2):324–31. [DOI] [PubMed] [Google Scholar]
- 9.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210(6):901–8. [DOI] [PubMed] [Google Scholar]
- 10.Lin HS, Watts JN, Peel NM, et al. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 2016; 16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chattopadhyay S Modified Frailty Index (mFI) in major gynaecological surgery: does it predict outcome? BJOG 2016; 123(3):462. [DOI] [PubMed] [Google Scholar]
- 12.Suskind AM, Walter LC, Jin C, et al. Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU Int 2016; 117(5):836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah R, Attwood K, Arya S, et al. Association of Frailty With Failure to Rescue After Low-Risk and High-Risk Inpatient Surgery. JAMA Surg 2018; 153(5):e180214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George EL, Hall DE, Youk A, et al. Association Between Patient Frailty and Postoperative Mortality Across Multiple Noncardiac Surgical Specialties. JAMA Surg 2020:e205152. [DOI] [PMC free article] [PubMed]
- 15.Subramaniam A, Tiruvoipati R, Lodge M, et al. Frailty in the older person undergoing elective surgery: a trigger for enhanced multidisciplinary management - a narrative review. ANZ J Surg 2020; 90(3):222–229. [DOI] [PubMed] [Google Scholar]
- 16.Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg 2015; 150(4):325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinall MC Jr., Arya S, Youk A, et al. Association of Preoperative Patient Frailty and Operative Stress With Postoperative Mortality. JAMA Surg 2019:e194620. [DOI] [PMC free article] [PubMed]
- 18.Hall DE, Arya S, Schmid KK, et al. Development and Initial Validation of the Risk Analysis Index for Measuring Frailty in Surgical Populations. JAMA Surg 2017; 152(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arya S, Varley P, Youk A, et al. Recalibration and External Validation of the Risk Analysis Index: A Surgical Frailty Assessment Tool. Ann Surg 2020; 272(6):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinall MC Jr, Youk A, Massarweh NN, et al. Association of Preoperative Frailty and Operative Stress With Mortality After Elective vs Emergency Surgery. JAMA Netw Open 2020; 3(7):e2010358–e2010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pokrzywa CJ, Papageorge CM, Kennedy GD. Preoperative urinary tract infection increases postoperative morbidity. J Surg Res 2016; 205(1):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crouser N, Malik AT, Phieffer LS, et al. Urinary tract infection (UTI) at time of geriatric hip fracture surgery increases the risk of experiencing adverse 30-day outcomes. J Clin Orthop Trauma 2019; 10(4):774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamali S, Dagher M, Bilani N, et al. The Effect of Preoperative Pneumonia on Postsurgical Mortality and Morbidity: A NSQIP Analysis. World J Surg 2018; 42(9):2763–2772. [DOI] [PubMed] [Google Scholar]
- 24.Metzger G, Horwood C, Chen JC, et al. The Need for Accurate Risk Assessment in a High-Risk Patient Population: A NSQIP Study Evaluating Outcomes of Cholecystectomy in the Patient With Cancer. J Surg Res 2021; 257:519–528. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins BS, Yamaguchi JT, Garcia R, et al. Using machine learning to predict 30-day readmissions after posterior lumbar fusion: an NSQIP study involving 23,264 patients. J Neurosurg Spine 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 27.Shiloach M, Frencher SK Jr., Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2010; 210(1):6–16. [DOI] [PubMed] [Google Scholar]
- 28.Navar AM, Peterson ED, Steen DL, et al. Evaluation of Mortality Data From the Social Security Administration Death Master File for Clinical Research. JAMA Cardiol 2019; 4(4):375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothenberg KA, George EL, Trickey AW, et al. Assessment of the Risk Analysis Index for Prediction of Mortality, Major Complications, and Length of Stay in Patients who Underwent Vascular Surgery. Ann Vasc Surg 2020; 66:442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato TS. Confidence limits for the ratio of two rates based on likelihood scores: non-iterative methods by P. L. Graham, K. Mengersen and A. P. Morton, Statistics in Medicine 2003; 22:2071–2083. Stat Med 2005; 24(7):1135. [DOI] [PubMed] [Google Scholar]
- 32.McIsaac DI, Bryson GL, van Walraven C. Association of Frailty and 1-Year Postoperative Mortality Following Major Elective Noncardiac Surgery: A Population-Based Cohort Study. JAMA Surg 2016; 151(6):538–45. [DOI] [PubMed] [Google Scholar]
- 33.Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg 2012; 72(6):1526–30. [DOI] [PubMed] [Google Scholar]
- 34.Castillo-Angeles M, Cooper Z, Jarman MP, et al. Association of Frailty With Morbidity and Mortality in Emergency General Surgery By Procedural Risk Level. JAMA Surg 2020. [DOI] [PMC free article] [PubMed]
- 35.Hall DE, Arya S, Schmid KK, et al. Association of a Frailty Screening Initiative With Postoperative Survival at 30, 180, and 365 Days. JAMA Surg 2017; 152(3):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milder DA, Pillinger NL, Kam PCA. The role of prehabilitation in frail surgical patients: A systematic review. Acta Anaesthesiol Scand 2018; 62(10):1356–1366. [DOI] [PubMed] [Google Scholar]
- 37.Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg 2020; 155(3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humeidan ML, Reyes JC, Mavarez-Martinez A, et al. Effect of Cognitive Prehabilitation on the Incidence of Postoperative Delirium Among Older Adults Undergoing Major Noncardiac Surgery: The Neurobics Randomized Clinical Trial. JAMA Surg 2020. [DOI] [PMC free article] [PubMed]
- 39.Halloway S, Buchholz SW, Wilbur J, et al. Prehabilitation interventions for older adults: an integrative review. West J Nurs Res 2015; 37(1):103–23. [DOI] [PubMed] [Google Scholar]
- 40.Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014; 121(5):937–47. [DOI] [PubMed] [Google Scholar]
- 41.Walston J, Bandeen-Roche K, Buta B, et al. Moving Frailty Toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc 2019; 67(8):1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35(5):526–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmonton Frail Scale - Simple tool to assess frailty in older patients. Available at: https://qxmd.com/calculate/calculator_595/edmonton-frail-scale. Accessed May 27, 2021.
- 45.Shah R, Borrebach JD, Hodges JC, et al. Validation of the Risk Analysis Index for Evaluating Frailty in Ambulatory Patients. J Am Geriatr Soc 2020; 68(8):1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varley PR, Borrebach JD, Arya S, et al. Clinical Utility of the Risk Analysis Index as a Prospective Frailty Screening Tool within a Multi-practice, Multi-hospital Integrated Healthcare System. Ann Surg 2020. [DOI] [PubMed]
- 47.Varley PR, O’Halloran P, Su H-D, et al. System-Wide, Prospective Frailty Screening Is Associated with Reduction in the Rate of 1-year Mortality after Elective Operation. Journal of the American College of Surgeons 2020; 231(4):S149–S150. [Google Scholar]
- 48.Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of Patient Frailty With Increased Morbidity After Common Ambulatory General Surgery Operations. JAMA Surg 2018; 153(2):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chimukangara M, Frelich MJ, Bosler ME, et al. The impact of frailty on outcomes of paraesophageal hernia repair. J Surg Res 2016; 202(2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmbhatt R, Brewster LP, Shafii S, et al. Gender and frailty predict poor outcomes in infrainguinal vascular surgery. J Surg Res 2016; 201(1):156–65. [DOI] [PubMed] [Google Scholar]
- 51.Hill A, Ramsey C, Dodek P, et al. Examining mechanisms for gender differences in admission to intensive care units. Health Serv Res 2020; 55(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ 2007; 177(12):1513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sell NM, Perez NP, Stafford CE, et al. Are There Variations in Mortality From Diverticular Disease By Sex? Dis Colon Rectum 2020; 63(9):1285–1292. [DOI] [PubMed] [Google Scholar]
- 54.Merz CN. The Yentl syndrome is alive and well. Eur Heart J 2011; 32(11):1313–5. [DOI] [PubMed] [Google Scholar]
- 55.Zettersten E, Jäderling G, Larsson E, et al. The impact of patient sex on intensive care unit admission: a blinded randomized survey. Sci Rep 2019; 9(1):14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson E, Zettersten E, Jäderling G, et al. The influence of gender on ICU admittance. Scand J Trauma Resusc Emerg Med 2015; 23:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Philippart F, Vesin A, Bruel C, et al. The ETHICA study (part I): elderly’s thoughts about intensive care unit admission for life-sustaining treatments. Intensive Care Med 2013; 39(9):1565–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDCFigure 1. Flow Diagram of Study Cohort. National Surgery Quality Improvement Program cases from 2013–2019 from multiple hospitals at four academic medical centers. Cases were excluded for missing variables used to calculate the Risk Analysis Index (RAI), lack of an expanded Operative Stress Score (OSS) assignment for the principal CPT code, and erroneous or missing case status. Cases lacking an expanded OSS assignment for the principal CPT code were excluded to avoid erroneously assigning a lower stress OSS based upon additional procedures that were performed. For example, a principal CPT code for a highly stressful procedure not assigned an expanded OSS could be assigned an OSS1 if the additional CPT codes contained any procedure with an expanded OSS rating. A single case per patient was selected at random for patients with multiple cases.
SDCFigure 2. Mortality (%) Grouped by Original Operative Stress Score (OSS) and Frailty. Mortality at A) 30, B) 90 and C) 180 days after the date of surgery. The 82,269 cases using the expanded OSS contained the subset of 69,677 cases assigned original OSS values. OSS defined physiologic stress induced by surgery as 1 (very low), 2 (low), 3 (moderate), 4 (high) and 5 (very high). Frailty assessed by Risk Analysis Index scores of ≤20 robust (R), 21–29 normal (N), 30–39 frail (F), and ≥40 very frail (VF). Small sample size for very frail patients undergoing OSS1 and OSS5 procedures shown with dashed gray line.


