Abstract
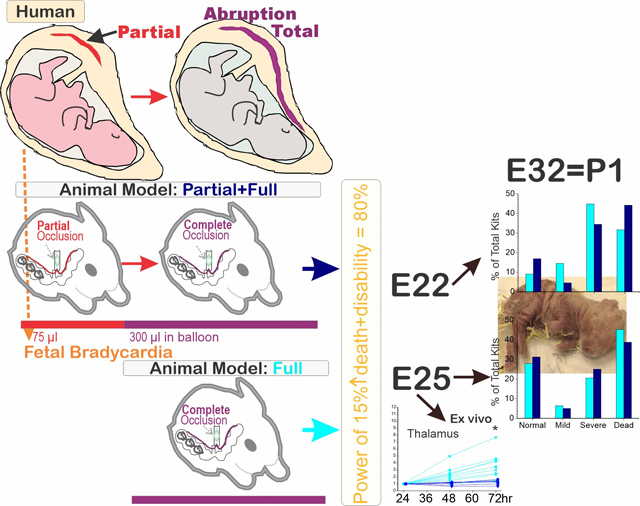
All placental abruptions begin as partial abruptions, which sometimes manifest as fetal bradycardia. The progression from partial to total abruption was mimicked by a new rabbit model of placental insufficiency, and we compared it, with sufficient statistical power, with the previous model mimicking total placental abruption. The previous model uses total uterine ischemia at E22 or E25 (70% or 79% term, respectively), in pregnant New Zealand white rabbits for 40 min (Full H-I). The new model, Partial+Full H-I, added a 30-min partial ischemia before the 40-min total ischemia. Fetuses were delivered either at E31.5 (full term) vaginally for neurobehavior testing, or by C-section at E25 for ex-vivo brain cell-viability evaluation. The onset of fetal bradycardia was within the first 2 minutes of either H-I protocol. There was no difference between Full H-I (n=442 for E22, 312 for E25) and Partial+Full H-I (n=154 and 80) groups in death or severely affected kits at E22 (76% vs 79%) or at E25 (66% vs 64%), or normal kits at E22 or E25, or any of the individual newborn neurobehavioral tests at any age. No sex differences were found. Partial+Full H-I (n=6) showed less cell viability than Full H-I (n=8) at 72-hr ex vivo in the brain regions studied. Partial+Full H-I insult produced similar cerebral palsy phenotype as our previous Full H-I model in a sufficiently powered study and may be more suitable for testing of potential neuroprotectants.
Keywords: fetus; hypoxia; infant, newborn; placental abruption
Graphical Abstract
Introduction
Hypertonia and movement disorders are characteristic of cerebral palsy (CP), which is the most common motor disability in childhood (Graham et al., 2016; Koman, Smith, & Shilt, 2004). There have been many animal models that claim to be translatable to clinical CP, employing different insults (hypoxia or inflammation), different timing of insults (prenatal or postnatal), and in different animals (rodents and higher mammals) (Cavarsan, Gorassini, & Quinlan, 2019; Saadani-Makki et al., 2008; Z. Shi et al., 2019). The translational rationale for developing rabbit models for perinatal brain injury is that rabbits are perinatal motor developers akin to humans (Harel et al., 1978), with similar time course in oligodendrocyte development and myelination (van Tilborg et al., 2018). Rodent models are most commonly utilized but suffer from the drawback that naïve rodent motor development starts only in the postnatal period, that they do not reflect the perinatal stage in humans (Fragopoulou, Qian, Heijtz, & Forssberg, 2019), and do not produce stable and reproducible hypertonia (Bona, Johansson, & Hagberg, 1997; Fan et al., 2005; McQuillen, Sheldon, Shatz, & Ferriero, 2003). Pig and non-human primates are also inappropriate for the study of perinatal origins of cerebral palsy (CP) because their motor development is prenatal and is almost complete at birth, unlike humans. The rabbit is the only animal model to manifest motor deficits of cerebral palsy and dystonia caused by perinatal hypoxia-ischemia (Shi, Luo, & Tan, 2019).
Fetal bradycardia and non-reassuring fetal heart rate patterns are often considered ominous signs of fetal injury leading to obstetric intervention because they are believed to reflect uteroplacental insufficiency. Placental abruption is one example of placental insufficiency affecting the fetus, and when observed in the context of fetal bradycardia (Matsuda, Ogawa, Konno, Mitani, & Matsui, 2013), is a known risk factor for further clinical problems such as CP (Hasegawa et al., 2016; Matsuda, Maeda, & Kouno, 2003).
The translational rationale for modeling placental abruption comes from the higher incidence of death and adverse neurodevelopmental outcomes (Ananth, Berkowitz, Savitz, & Lapinski, 1999; Gilbert, Jacoby, Xing, Danielsen, & Smith, 2010; Kayani, Walkinshaw, & Preston, 2003; Logitharajah, Rutherford, & Cowan, 2009; Matsuda et al., 2003). We had previously developed a rabbit uterine-ischemia model of CP phenotype after hypoxia ischemia (H-I) at preterm gestation, mimicking the clinical scenario of abruptio placentae (Derrick, Drobyshevsky, Ji, & Tan, 2007; Derrick et al., 2004; Tan et al., 2005). Despite this, there was still concern that we were not mimicking the actual clinical course and progression of abruptio placentae or of uteroplacental insufficiency. All available models of H-I in animals until now have used a single insult consist of a sudden onset of 100% anoxia to the fetal brain. Clinically, it is rare to have an insult that causes an immediate complete cessation of blood supply to the uterus such as massive clot, sudden cardiovascular collapse of mother. All cases of total placental abruption start off with partial abruption and change over time to total abruption. Placental abruption progresses in a relatively slower manner and in some cases develops incrementally into total abruption. Uteroplacental insufficiency states also may take time to develop. Thus, the rationale for developing a new model was to study the transition from partial to total abruption. It became imperative for preclinical investigators to come up with a suitable translational animal model. We wondered if a more translational prenatal H-I model would be more suitable for testing of neuroprotectants in addition to expanding its clinical relevance. We added a period of partial uterine-ischemia to the full uterine-ischemia and thus, we hypothesized that there would be increased brain injury with the additional partial insult.
In the present study, we demonstrated a modified H-I model based on our previous prenatal H-I model in rabbit, and compared the long-term postnatal death and neurobehavioral outcomes, as well as acute brain cell viability after the H-I insult. Due to the principle of conserving animals (Kendall et al., 2019), we compared the new model to our entire data set of experiments done since inception.
Materials and methods
All animal related procedures were performed under the permission of IACUCs of Wayne State University or NorthShore University HealthSystem Research Institute and all studies were conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. The data were derived from two time-epochs. From 2004 to 2016, the rabbit studies were conducted at NorthShore University HealthSystem, and from 2016 onwards, at Wayne State University. The physical condition for housing/husbandry were almost identical, based on the same IACUC protocol. Laboratory personnel conducting the experiments have been unchanged since 2013. We retrieved all available neurobehavioral data in surgical groups, sham groups, and naïve group. No data were excluded. The investigators performing the neurobehavioral battery of tests or cell viability analysis were blinded to the dams’ H-I group. Timed-pregnant New Zealand White dams were initially ordered from Myrtles (now defunct) but the later batches were ordered from Charles River, since 2015 following an identical protocol for breeding. Single housing was done for pregnant female rabbits in order to reduce stress during pregnancy, facilitate monitoring, and allow appropriate nesting behavior, and all rabbits were placed in a separate room. Dams were allowed 5–7 days to acclimate following arrival animal facility and prior to the initiation of experiments. After corresponding surgical procedures, dams and kits were euthanized after neurobehavioral tests of the kits.
In vivo animal models
Global H-I of fetuses was induced by uterine ischemia at E22 or E25 (70% or 79% gestation, respectively), in pregnant New Zealand White rabbits as previously described (Derrick et al., 2007; Derrick et al., 2004; Tan et al., 2005). A detailed description is provided in (J. Shi et al., 2019). Briefly, dams were anesthetized with an initial regimen of intravenous fentanyl (75 μg/kg/h) and droperidol (3.75 mg/kg/h), then maintenance of general anesthesia with lower doses for preparation of spinal/epidural anesthesia. Spinal or epidural anesthesia was with 0.75% bupivacaine. After spinal/epidural anesthesia, the dose of anesthesia was dropped so that the levels of fentanyl and droperidol maintained the animal in deep sedation. The dam was allowed to breathe spontaneously. A balloon catheter was introduced into the left femoral artery and advanced into the descending aorta between the uterine arteries and the renal arteries. Inflation of the balloon results in uterine ischemia that causes global fetal hypoxia and subsequently hypoxia-ischemia to fetal brain.
Experimental Groups: 1) Full H-I and 2) Partial+Full H-I.
In the Full H-I group, the aortic balloon was inflated for 40 min, which caused complete cessation of uterine blood flow and subsequent global fetal brain H-I. The cessation of blood supply to the uterus was confirmed by monitoring of blood pressure on the hind limb without catheter insertion in every dam. At the end of H-I, the balloon was deflated, and the reperfusion period started. The time of 40 min was chosen to give a balance of motor deficits, deaths and normal kits. There were no neurobehavioral deficits with naive, sham controls, or partial H-I for 30 min, or even full H-I for 30 min previously. Some of the Full H-I group of newborn kits have been reported for MRI diagnosis (Drobyshevsky, Derrick, Wyrwicz, et al., 2007; Drobyshevsky et al., 2014; Drobyshevsky, Yu, et al., 2012) and treatment of CP, including nNOS inhibitors (Ji et al., 2009; Yu et al., 2011) and stem cells (Drobyshevsky et al., 2015).
Inclusion criteria was simple as we were using the same model for these studies. Exclusion criteria was if there were no neurobehavioral studies performed at P1.
Dams in the Partial+Full H-I group received an extra 30 min partial H-I immediately before the Full H-I; partial H-I confirmed by lowered blood pressure to about two thirds of that before balloon inflation on the hind limb without catheter insertion. Partial+Full H-I is the newer model. During the development of the newer model, we had conducted a block randomized comparison with Full H-I done at the same time in a ratio of 1:2. In the present study, we added historical controls of Full H-I kits to have sufficient power (Fig 1).
Figure 1: Same time and historical comparisons.
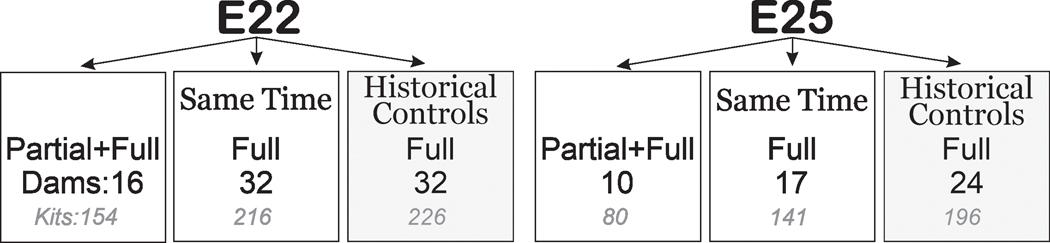
Number of dams (black) and kits (gray) are depicted. Block-randomization was done at the same time in the ratio of 1:2 for Partial+Full H-I vs. Full H-I dams. Also depicted are historical controls (dark gray). Each dam gave birth to only one litter.
Death and Disability
For primary endpoint experiments, the dams were allowed to recover and deliver at term gestation (E31.5). Neurobehavioral assessment was done on postnatal day 1 (P1) using a protocol published before (Derrick et al., 2004; Z. Shi et al., 2019), with minor revisions. All kits that underwent neurobehavioral tests were included in this study. Primary endpoints were death at delivery or neurobehavioral deficits based on locomotion, motor ability and muscle tone. For each animal, the tests were videotaped and scored on a scale of 0–4 (0=worst and 4=best of corresponding parameter, except for muscle tone where 0 is hypotonia and hypertonia is depending on fore- or hindlimbs, and dystonia where 0 is normal and higher scores are worst) by two blinded observers. Olfaction was tested by aversive response to a cotton swab soaked with peppermint, amyl acetate, or pure ethanol. Locomotion on a flat surface was assessed by grading the amount of spontaneous movement of head, trunk and limbs. Tone was assessed by active flexion and extension of the fore and hind limbs and scored (0–4) according to the Ashworth scale (Damiano et al., 2002). The righting reflex was assessed when the pups were placed on their backs. Suck and swallow were assessed, by introduction of infant milk formula into the pup’s mouth with a plastic pipette. Categorization of overall neurobehavioral status was as follows:
1) Severe: presence of a) moderate hypertonia (defined as tone≥3.0 in forelimb OR ≥2.0 in hind limb), OR combined hypertonia in both fore- and hind limbs (tone=2.5 in forelimb AND 1.5 in hind limb);
2) Mild: a) Mild hypertonia in one limb (defined as either tone=2.5 in forelimb OR 1.5 in hind limb) by itself or accompanied by minor locomotor deficit (locomotion 2.5–3.0); OR b) locomotor deficit (locomotion≤2.5) without hypertonia.
3) Normal: No locomotor deficit.
Sex assignment was done if we had the kits in our laboratory, after euthanasia, by visual inspection of abdominal gonads (Nielsen & Torday, 1983).
RealTime-Glo MT cell viability assay
Different fetal brain regions, including cortex, basal ganglia, thalamus, and cerebellum, were collected by C-section immediately after either Partial+Full H-I or Full H-I at E25. Cells were obtained from brain tissue by digestion in Neurobasal medium (ThermoFisher Scientific) containing 0.025% trypsin and incubated on a rotating shaker at 37°C for 45 min (Derrick, He, Brady, & Tan, 2001). Cells were washed with Neurobasal medium and passed through a sterile 70-μm filter to produce a single-cell suspension. Cells were plated at 5×104 per well in 96-well plates and grown in 100 μl Neurobasal medium supplied with 10% B-27 supplement (Gibco) and 1x RealTime-Glo MT Cell Viability Assay reagent (Promega, G9713) at 37 °C with 21% O2 and 5% CO2. Luminescent signals were obtained at 24hr, 48hr, and 72hr post H-I using a Synergy™ HTX multi-mode microplate reader (Biotek, Winooski, VT).
Statistics
Neurobehavioral data were depicted as box and whisker plots and each data point is shown. Analysis was done with SAS software ver 9.4 (SAS Institute Inc., Cary, NC, USA). We checked for normal distribution by examining the Q-Q plots (Proc Univariate, SAS). Primary outcome data were expressed as number of cases (percentages) and analyzed by Kendall tau b rank (Khamis, 2008) correlation coefficient and Cochran-Armitage Trend Test. Student’s t test was used to analyze continuous data between corresponding groups, and ordinal data were also analyzed using χ2-test or Fisher’s exact test. For rigor (Steward & Balice-Gordon, 2014), Bonferroni correction was used for multiple tests and power analysis for number of dams was done, assuming moderate effect for odorant response at P1, α error=0.05 and β error=0.2, power 80%. A two tailed p<0.05 was determined as significant difference. For overall outcome, the power for E22 comparisons for the distribution of kits and total number (596 kits) and an increase of 15% in number of severe kits and death was = 81% using a two-sample Wilcoxon-Mann-Whitney test (Proc Power, SAS). The bivariate Pearson Correlation was used to produce a correlation coefficient, r, which measured the strength and direction of linear relationships between pairs of neurobehavioral test variables (Proc Corr, SAS).
Results
Overall birth outcomes and neurobehavioral test
In the E22 H-I experiments, there were 16 dams with 154 newborns in the Partial+Full H-I group with an average of 9.6 per litter, and 64 dams with 442 newborns in the Full H-I group, average 6.9. There were no differences in the percentages of dead, severe, mild, or normal newborns between the two groups. There was no difference in sex or birth weight in each birth outcome between the two groups (Table 1). In the E25 H-I experiments, there were 10 dams with 80 newborns in the Partial+Full H-I group, and 41 dams with 312 newborns in the Full H-I group. The total number of kits was 988. There were no differences in the percentages of dead, severe, mild, or normal newborns between the two groups at either E22 or E25 (Fig 2). There were more male newborns as well as dead male newborns in the Full H-I group at E25 (Table 2). There was no difference found between E22 and E25 outcomes, in each category of H-I.
Table 1.
Summary of animals in different experimental groups of H-I at E22.
| Characteristics | Full H-I | Partial+Full H-I | P value |
|---|---|---|---|
| No. of Dams | 64 | 16 | - |
| No. of newborns | 435 | 122 | - |
| No. of Males (%) | 152/286 (53.1) | 35/72 (48.6) | 0.49* |
| Weight (Mean±SD) | 48.2±16.0 | 48.7±16.2 | 0.76# |
| No. of Dead (%) | 136 (31.3) | 37 (30.3) | 0.84* |
| No. of Males (%) | 37/73 (50.7) | 8/19 (42.1) | 0.51* |
| Weight (Mean±SD) | 31.5±17.7 | 30.2±18.0 | 0.74# |
| No. of Severe (%) | 208 (47.8) | 61 (50) | 0.67* |
| No. of Males (%) | 73/138 (52.9) | 17/36 (47.2) | 0.54* |
| Weight (Mean±SD) | 53.8±8.0 | 55.3±8.9 | 0.22# |
| No. of Mild (%) | 31 (7.1) | 10 (8.2) | 0.69* |
| No. of Male (%) | 12/20 (60) | 2/4 (50) | 1$ |
| Weight (Mean±SD) | 57.5±9.9 | 57.7±9.2 | 0.97# |
| No. of Normal (%) | 60 (13.8) | 14 (11.5) | 0.51* |
| No. of Male (%) | 20/37 (54.1) | 8/13 (61.5) | 0.64* |
| Weight (Mean±SD) | 58.0±8.3 | 54.3±8.0 | 0.11# |
| No. of Survival (%) | 299 (68.7) | 85 (69.7) | 0.84* |
| No. of Male (%) | 105/195 (53.8) | 27/53 (50.9) | 0.71* |
| Weight (Mean±SD) | 55.0±8.5 | 55.3±8.8 | 0.82# |
Survival is defined as (severe + mild+ normal).
x2 test,
t test,
Fisher exact test
Figure 2: Overall outcome.
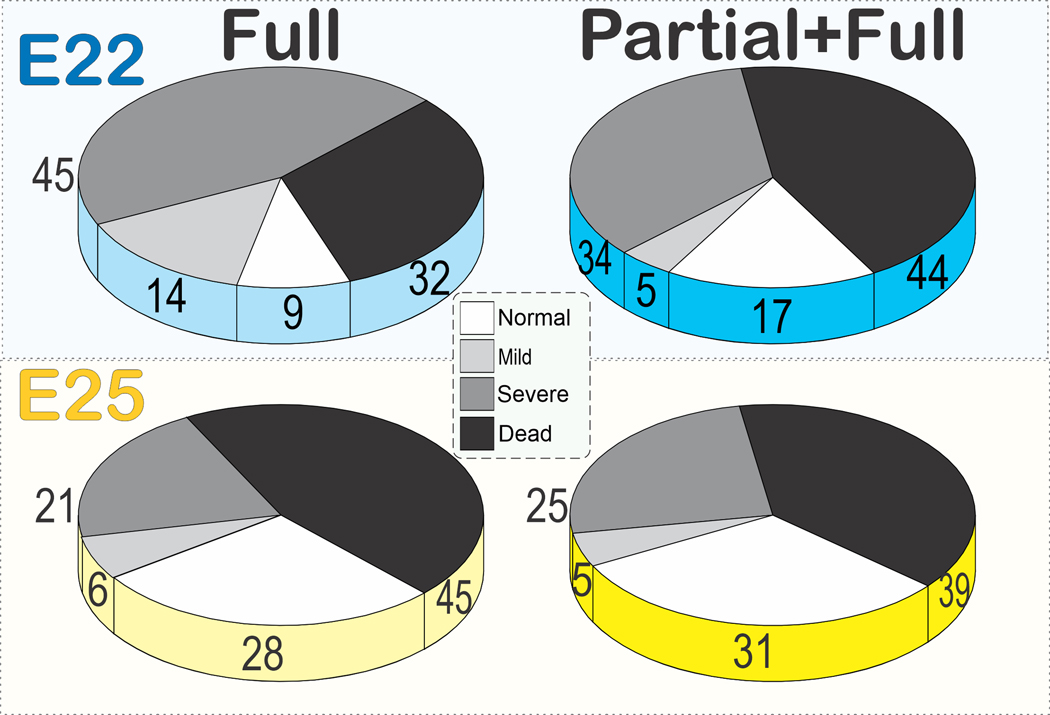
There were no differences between the normal, mild, severe deficits or death between Full (left column) and Partial+Full H-I groups (right) at E22 (blue) or E25 yellow). Percentages of total kits shown in each pie. At E22, no. of kits were 442 and 154 respectively; at E25, 312 and 80 kits respectively. Kendall tau b 95% confidence intervals: 1) Full vs Partial+Full at E22, −0.053 to 0.207, 2) at E25, −0.180 to 0.078, 3) Comparing E22 to E25 for Full, ¬0.137 to 0.129, and 4) Comparing E22 to E25 for Partial+Full, −0.241 to 0160. The actual power for no difference between the ordinal distribution of deficits and death, and relative number of patients at E22 was 58%.
Table 2.
Summary of animals in different experimental groups of H-I at E25.
| Characteristics | Full H-I | Partial+Full H-I | P value |
|---|---|---|---|
| No. of Dams | 41 | 10 | - |
| No. of newborns | 337 | 80 | - |
| Male (%) | 95/164 (57.9) | 22/53 (41.5) | 0.04 * |
| Weight (g, Mean±SD) | 43.7±14.7 | 45.8±16.1 | 0.31# |
| Dead (%) | 141 (41.8) | 31 (38.8) | 0.61* |
| Male (%) | 25/40 (62.5) | 6/18 (33.3) | 0.04 * |
| Weight (g, Mean±SD) | 32.2±13.8 | 27.3±14.6 | 0.17# |
| Severe (%) | 67 (19.9) | 20 (25) | 0.31* |
| Male (%) | 26/48 (54.2) | 7/13 (53.8) | 0.98* |
| Weight (g, Mean±SD) | 50.1±9.5 | 50.2±9.5 | 0.99# |
| Mild (%) | 13 (3.9) | 6 (7.5) | 0.16* |
| Male (%) | 6/8 (75) | 2/5 (40) | 0.29$ |
| Weight (g, Mean±SD) | 48±9.2 | 49±9.2 | 0.84# |
| Normal (%) | 91 (27.0) | 23 (28.8) | 0.75* |
| Male (%) | 32/57 (56.1) | 7/17 (41.2) | 0.28* |
| Weight (g, Mean±SD) | 51.4±11.0 | 56.4±8.9 | 0.05# |
| Survival (%) | 196 (58.2) | 49 (61.3) | 0.61* |
| Male (%) | 70/124 (56.5) | 16/34 (47.1) | 0.33* |
| Weight (g, Mean±SD) | 50.5±10.3 | 52.9±9.8 | 0.14# |
Survival is defined as (severe + mild+ normal).
x2 test,
t test,
Fisher exact test
No sex differences
In a subset of the total population, sex assignments were done. There was no difference in sex on the severity of outcomes in either Full or Partial+Full at either gestational age (Fig 3).
Figure 3: No sex differences.
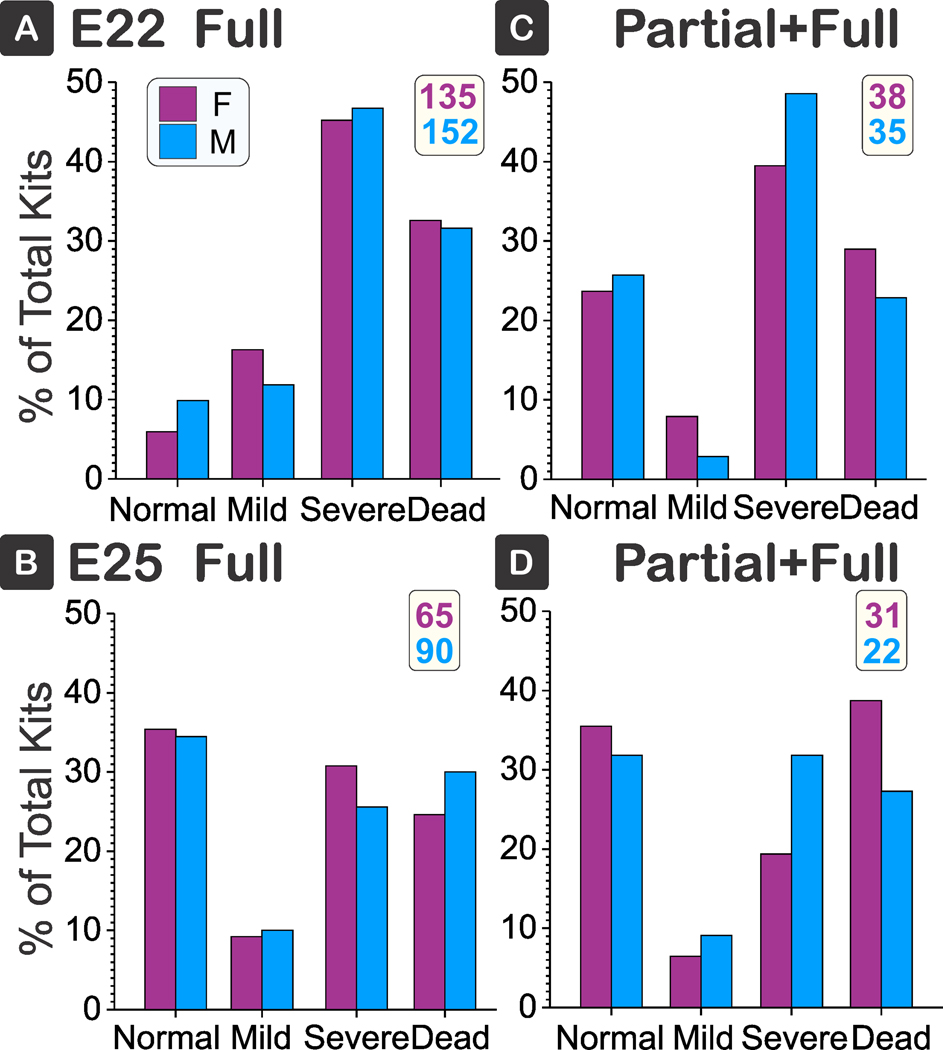
There were no differences between the normal, mild, severe deficits or death between sex in Full (A, B) or Partial+Full H-I (C, D) groups at E22 (A, C) or E25 (B, D). Female in pink and Male in blue, total number of kits given in insert on top right. Kendall tau b 95% confidence intervals: A) −0.141 to 0.118, B) −0.099 to 0.157, C) −0.159 to 0.100, D) −0.170 to 0.088. F = female, M = Male.
Itemized neurobehavioral tests
There were no differences in sensory deficits including olfactory tests (peppermint, alcohol, or amyl acetate), or movement of eyelids and upper face to stimulation (Fig 4A–D).
Figure 4. Sensory Deficits.
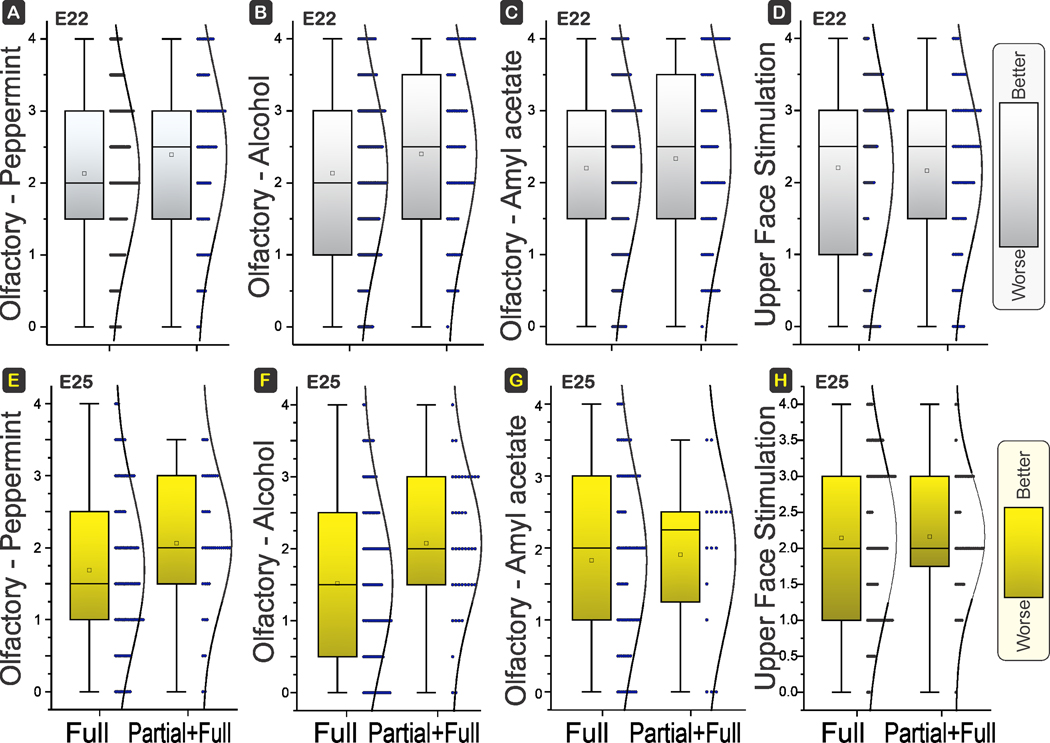
Box and whisker plots with distribution depicted. E22 H-I in top row and E25 H-I in bottom row. There were no differences between the olfactory response to peppermint (A, E), ethyl alcohol (B, F), and amyl acetate (C, G), between Full and Partial+Full H-I groups at E22 (power 75%, 72%, 32%; P=0.0568, 0.0650, 0.3711) or E25 (power 75%, 97%, 16%; P=0.0523, 0.0050, 0.8103). There were also no differences in the response of the upper face to stimulation of eyelid or upper face at E22 (power 2%; P=0.7944) or E25 (D, H) (power 1%; P=0.9183). Scoring of odor response: 0, no response; 0.5, mouth and nose twitch; 1, head moves <6 degrees; 2, head moves 6–30 degrees, 3, head moves 31–60 degrees; 4, head moves >60 degrees from midline. Shading to show which direction is worse.
There were no differences in locomotion of forelimbs or hind limbs. Both the range of motion (movement) and time of activity (activity) were not significantly different between Full and Partial+Full H-I groups at E22 or E25 gestational ages (Fig 5). The neck movement and trunk movement were also similar between the two groups at both ages (Fig 6).
Figure 5. Forelimb and Hind locomotion.
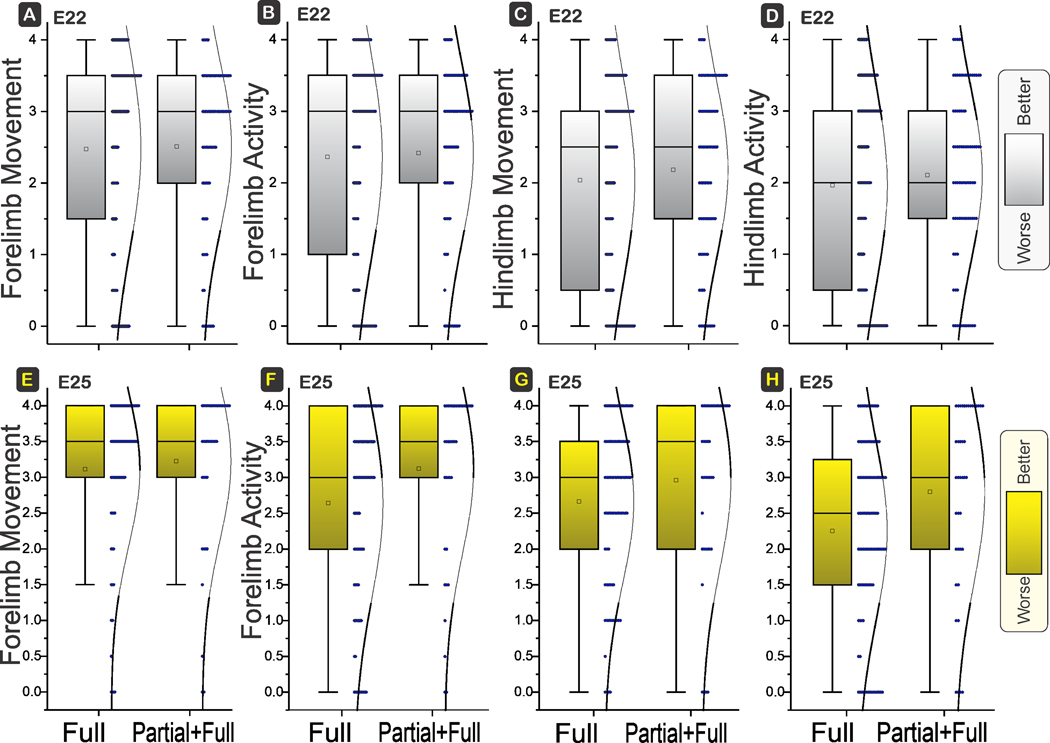
Box and whisker plots with distribution depicted. E22 H-I in top row and E25 H-I in bottom row. No differences in movement (range of motion, A,C,E,G) or activity (time spent B,D,F,H) in forelimbs or hind limbs between Full and Partial+Full H-I groups at E22 (power 6%, 9%, 26%, 26%; P=0.8327, 0.7556, 0.3975, 0.3960) or E25 (power 10%, 77%, 41%, 84%; P=0.6107, 0.0461, 0.1898, 0.0229). Shading to show which direction is worse.
Figure 6. Neck and Trunk Movement.
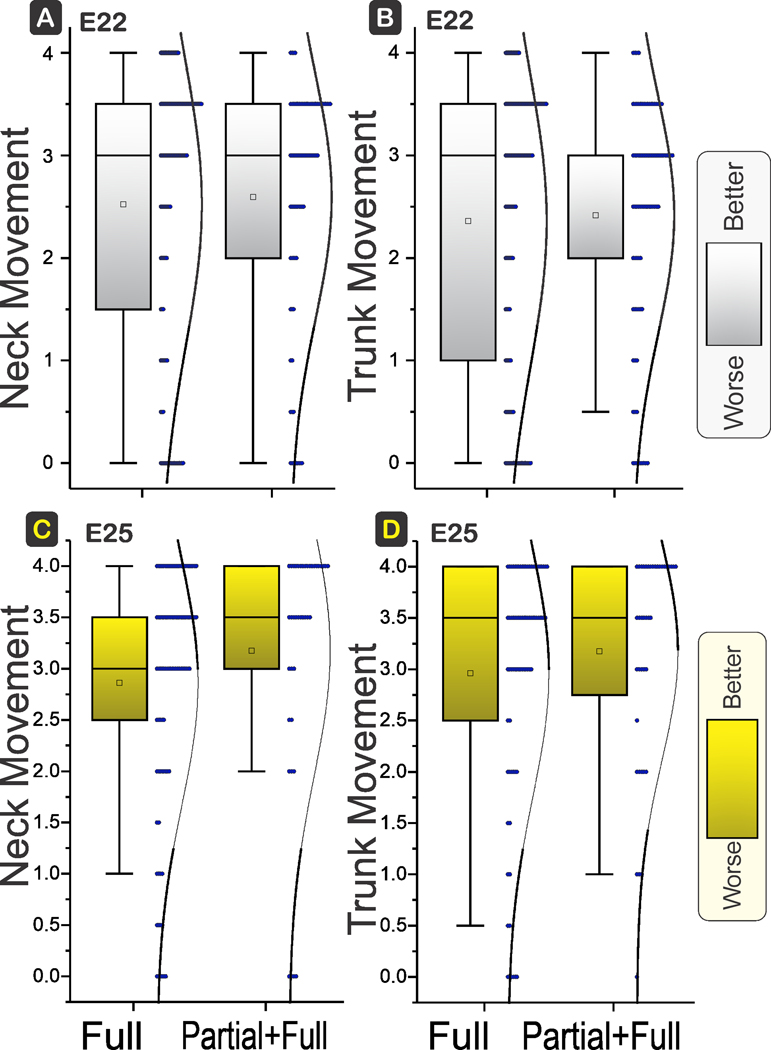
Box and whisker plots with distribution depicted. E22 H-I in top row and E25 H-I in bottom row. There were no differences between the neck (A, C) and trunk movements (B, D) between Full and Partial+Full H-I groups at E22 (power 12%, 9%; P=0.6657, 0.7385) or E25 (power 46%, 17%; P=0.1672, 0.3479). Shading to show which direction is worse.
There were no differences in suck and swallow, or muscle tone in fore limb (shoulder, elbow, carpal, or SEC) or hind limb (hip, knee, tarsal, or HKT), or dystonia score between the E22 Partial+Full H-I group and the Full H-I group (Figure 7).
Figure 7. Comparison of Suck swallow, Tone in fore- and hind limbs and Dystonia.
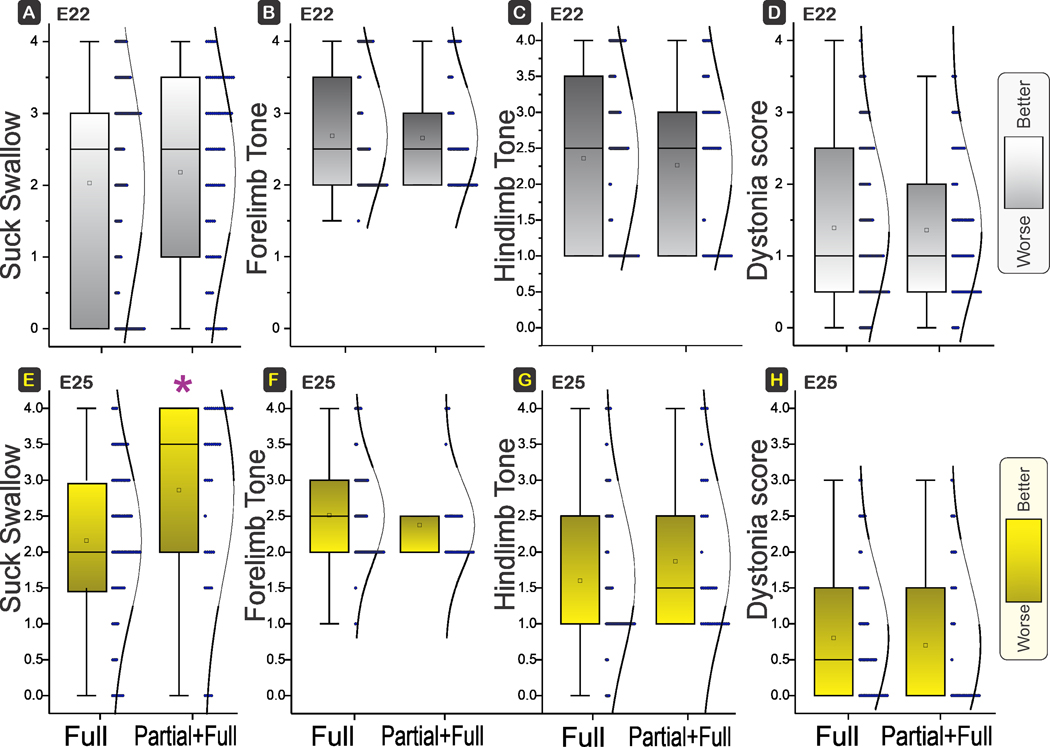
Box and whisker plots with distribution depicted. E22 H-I in top row and E25 H-I in bottom row. There were no differences between the suck swallow at E22 between Full and Partial+Full H-I groups. No differences also in forelimb tone or hind limb tone and dystonia between Full and Partial+Full H-I groups at E22 (power 28%, 2%, 0.8%; P=0.3869, 0.7553, 0.4953) or E25 (power 0.1%, 51%, 0.3%; P=*0.0013, 0.2664, 0.1642). Shading to show which direction is worse. E. Only suck swallow was different between Full and Partial+Full H-I groups at E25, P = 0.0013 (α<0.0029, with Bonferroni correction for multiple comparisons).
The only differences we saw at E25 was a significantly better outcome in suck and swallow (Fig 7E) in the Partial+Full H-I compared to the Full H-I group.
Correlation Matrix of Individual Behavioral Tests
We next examined whether there were differences in two-variable correlations between individual neurobehavioral tests. The strength of the correlation has been given a color code for easy comparisons in Tables 3 for E22 and Table 4 for E25. Most of the strengths of correlation were similar between Full and Partial+Full H-I groups. The variables reflective of the sensory system were the olfactory tests and response of upper face to touch. The olfactory tests did not seem to correlate with locomotor or tone variables in any group at either age. However, the upper face response correlated with locomotor and tone variables. Intriguingly, upper face response had weaker correlations after Full H-I at E25 compared to all other groups. There was also a suggestion that hind limb tone correlations with other locomotor activities is less in Partial+Full compared to Full for both E22 and E25.
Table 3:
Strength of Two-Variable Correlations at E22
| NAME | Smell_M | Smell_A | Smell_AA | Upper_face | Trunk_move | Neck_move | Fore_move | Fore_activity | Hind_move | Hind_activity | Suck_Swallow | Tone_SEC | Tone_HKT | Dystonia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E22 Full | ||||||||||||||
| Smell_M | 0.773 | 0.796 | 0.517 | 0.529 | 0.519 | 0.533 | 0.499 | 0.498 | 0.493 | 0.485 | −0.489 | −0.452 | −0.448 | |
| Smell_A | 0.867 | 0.561 | 0.570 | 0.562 | 0.580 | 0.546 | 0.527 | 0.524 | 0.525 | −0.557 | −0.482 | −0.508 | ||
| Smell_AA | 0.601 | 0.625 | 0.607 | 0.626 | 0.602 | 0.584 | 0.582 | 0.572 | −0.609 | −0.533 | −0.532 | |||
| Upper_face | 0.950 | 0.931 | 0.934 | 0.922 | 0.899 | 0.885 | 0.896 | −0.884 | −0.791 | −0.795 | ||||
| Trunk_move | 0.967 | 0.973 | 0.965 | 0.931 | 0.923 | 0.930 | −0.930 | −0.806 | −0.815 | |||||
| Neck_move | 0.961 | 0.944 | 0.882 | 0.872 | 0.892 | −0.898 | −0.756 | −0.791 | ||||||
| Fore_move | 0.977 | 0.922 | 0.912 | 0.920 | −0.945 | −0.792 | −0.811 | |||||||
| Fore_activity | 0.916 | 0.915 | 0.912 | −0.932 | −0.772 | −0.806 | ||||||||
| Hind_move | 0.985 | 0.926 | −0.885 | −0.851 | −0.815 | |||||||||
| Hind_activity | 0.917 | −0.875 | −0.851 | −0.816 | ||||||||||
| Suck_Swallow | −0.876 | −0.823 | −0.810 | |||||||||||
| Tone_SEC | 0.770 | 0.801 | ||||||||||||
| Tone_HKT | 0.765 | |||||||||||||
| E22 Partial+Full | ||||||||||||||
| Smell_M | 0.715 | 0.727 | 0.615 | 0.598 | 0.608 | 0.595 | 0.592 | 0.561 | 0.514 | 0.613 | −0.572 | −0.576 | −0.566 | |
| Smell_A | 0.754 | 0.488 | 0.524 | 0.524 | 0.522 | 0.500 | 0.479 | 0.414 | 0.562 | −0.552 | −0.541 | −0.507 | ||
| Smell_AA | 0.560 | 0.573 | 0.595 | 0.577 | 0.578 | 0.487 | 0.444 | 0.566 | −0.581 | −0.511 | −0.538 | |||
| Upper_face | 0.914 | 0.880 | 0.887 | 0.877 | 0.892 | 0.830 | 0.907 | −0.770 | −0.651 | −0.726 | ||||
| Trunk_move | 0.973 | 0.958 | 0.952 | 0.913 | 0.853 | 0.903 | −0.879 | −0.667 | −0.797 | |||||
| Neck_move | 0.964 | 0.955 | 0.862 | 0.801 | 0.876 | −0.890 | −0.590 | −0.773 | ||||||
| Fore_move | 0.965 | 0.857 | 0.787 | 0.889 | −0.902 | −0.628 | −0.796 | |||||||
| Fore_activity | 0.858 | 0.828 | 0.879 | −0.906 | −0.651 | −0.813 | ||||||||
| Hind_move | 0.935 | 0.890 | −0.755 | −0.714 | −0.715 | |||||||||
| Hind_activity | 0.836 | −0.716 | −0.690 | −0.679 | ||||||||||
| Suck_Swallow | −0.825 | −0.689 | −0.793 | |||||||||||
| Tone_SEC | 0.660 | 0.820 | ||||||||||||
| Tone_HKT | 0.703 | |||||||||||||
E22 correlations in Full and Partial+Full H-I groups. Significance was set as α<0.005 and colored. Strength of Pearson correlation coefficient was color coded as follows: 1) Red, 0.9 to 1.0, 2) Dark yellow, 0.8 to 0.9, 3) Light yellow, 0.7 to 0.8. Smell_M is olfactory test with peppermint, Smell_A with alcohol, Smell_AA with amyl acetate. Upper face is response of upper face to tactile stimulation. The locomotor variables are _Move being extent of movement from neutral position, and _Activity is based on quartiles of time of activity to maximum of 100%. Tone_SEC is fore limb tone reflecting testing of movement around shoulder, elbow and carpal joints, Tone_HKT is hind limb tone reflecting hip, knee and tarsal joints. Negative sign implies negative correlation.
Table 4:
Strength of Two-Variable Correlations at E25
| NAME | Smell_M | Smell_A | Smell_AA | Upper_face | Trunk_move | Neck_move | Fore_move | Fore_activity | Hind_move | Hind_activity | Suck_Swallow | Tone_SEC | Tone_HKT | Dystonia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E25 Full | ||||||||||||||
| Smell_M | 0.565 | 0.844 | 0.504 | 0.333 | 0.314 | 0.315 | 0.317 | 0.337 | 0.345 | 0.440 | −0.206 | −0.090 | −0.110 | |
| Smell_A | 0.696 | 0.617 | 0.274 | 0.311 | 0.240 | 0.204 | 0.255 | 0.263 | 0.307 | −0.109 | 0.044 | −0.003 | ||
| Smell_AA | 0.520 | 0.416 | 0.427 | 0.408 | 0.397 | 0.379 | 0.403 | 0.493 | −0.268 | −0.110 | −0.169 | |||
| Upper_face | 0.391 | 0.464 | 0.388 | 0.344 | 0.328 | 0.378 | 0.449 | −0.237 | −0.041 | −0.146 | ||||
| Trunk_move | 0.972 | 0.981 | 0.971 | 0.954 | 0.937 | 0.900 | −0.874 | −0.842 | −0.872 | |||||
| Neck_move | 0.968 | 0.954 | 0.930 | 0.924 | 0.888 | −0.833 | −0.795 | −0.815 | ||||||
| Fore_move | 0.971 | 0.930 | 0.922 | 0.887 | −0.882 | −0.819 | −0.869 | |||||||
| Fore_activity | 0.931 | 0.951 | 0.912 | −0.890 | −0.859 | −0.881 | ||||||||
| Hind_move | 0.965 | 0.896 | −0.809 | −0.825 | −0.804 | |||||||||
| Hind_activity | 0.922 | −0.784 | −0.790 | −0.799 | ||||||||||
| Suck_Swallow | −0.784 | −0.753 | −0.774 | |||||||||||
| Tone_SEC | 0.912 | 0.882 | ||||||||||||
| Tone_HKT | 0.920 | |||||||||||||
| E25 Partial+Full | ||||||||||||||
| Smell_M | 0.741 | 0.890 | 0.715 | 0.794 | 0.794 | 0.743 | 0.646 | 0.859 | 0.837 | 0.773 | −0.880 | −0.832 | −0.146 | |
| Smell_A | 0.869 | 0.618 | 0.649 | 0.591 | 0.626 | 0.575 | 0.612 | 0.583 | 0.597 | −0.768 | −0.833 | 0.223 | ||
| Smell_AA | 0.695 | 0.771 | 0.768 | 0.700 | 0.618 | 0.832 | 0.752 | 0.751 | −0.856 | −0.899 | −0.038 | |||
| Upper_face | 0.942 | 0.877 | 0.818 | 0.835 | 0.840 | 0.846 | 0.894 | −0.830 | −0.507 | −0.018 | ||||
| Trunk_move | 0.945 | 0.929 | 0.892 | 0.896 | 0.849 | 0.912 | −0.866 | −0.527 | −0.211 | |||||
| Neck_move | 0.936 | 0.917 | 0.952 | 0.924 | 0.974 | −0.913 | −0.516 | −0.362 | ||||||
| Fore_move | 0.964 | 0.879 | 0.852 | 0.908 | −0.882 | −0.430 | −0.424 | |||||||
| Fore_activity | 0.829 | 0.855 | 0.923 | −0.824 | −0.329 | −0.384 | ||||||||
| Hind_move | 0.949 | 0.961 | −0.932 | −0.656 | −0.386 | |||||||||
| Hind_activity | 0.960 | −0.909 | −0.572 | −0.344 | ||||||||||
| Suck_Swallow | −0.907 | −0.531 | −0.350 | |||||||||||
| Tone_SEC | 0.724 | 0.197 | ||||||||||||
| Tone_HKT | −0.197 | |||||||||||||
E25 correlations in Full and Partial+Full H-I groups. Significance was set as α<0.005 and colored. Strength of Pearson correlation coefficient was color coded as follows: 1) Red, 0.9 to 1.0, 2) Dark yellow, 0.8 to 0.9, 3) Light yellow, 0.7 to 0.8. See explanation of variables in Table 3.
Fetal brain cell viability
To investigate if there was any worsening effect of an extra partial H-I on the fetal brain, we performed Realtime-Glo MT cell viability assay in cells extracted from different regions of the brain including cortex, cerebellum, basal ganglia and thalamus (total number of fetuses was 16). Cell cultures from regional brain cell suspensions were maintained and cell viability monitored at various time points immediately after E25 Full H-I or Partial+Full H-I. There were no differences between Full H-I and Partial+Full H-I group in any brain regions at 24hr after the insult: cortex, 4,569±1,360 vs 6,389±1,444; cerebellum, 6,837±1,474 vs 7,472+1,992; basal ganglia, 13,754±2,483 vs. 16,289+2,494; thalamus, 11,190+4,483 vs 7,472+1,992 units, respectively (unpaired t test, n=6–9 fetuses per group). However, continued cell viability showed that Partial+Full had less significant viability of cells with time compared to Full H-I group (Fig 8).
Fig 8. Decrease of cell viability after 24 hr.
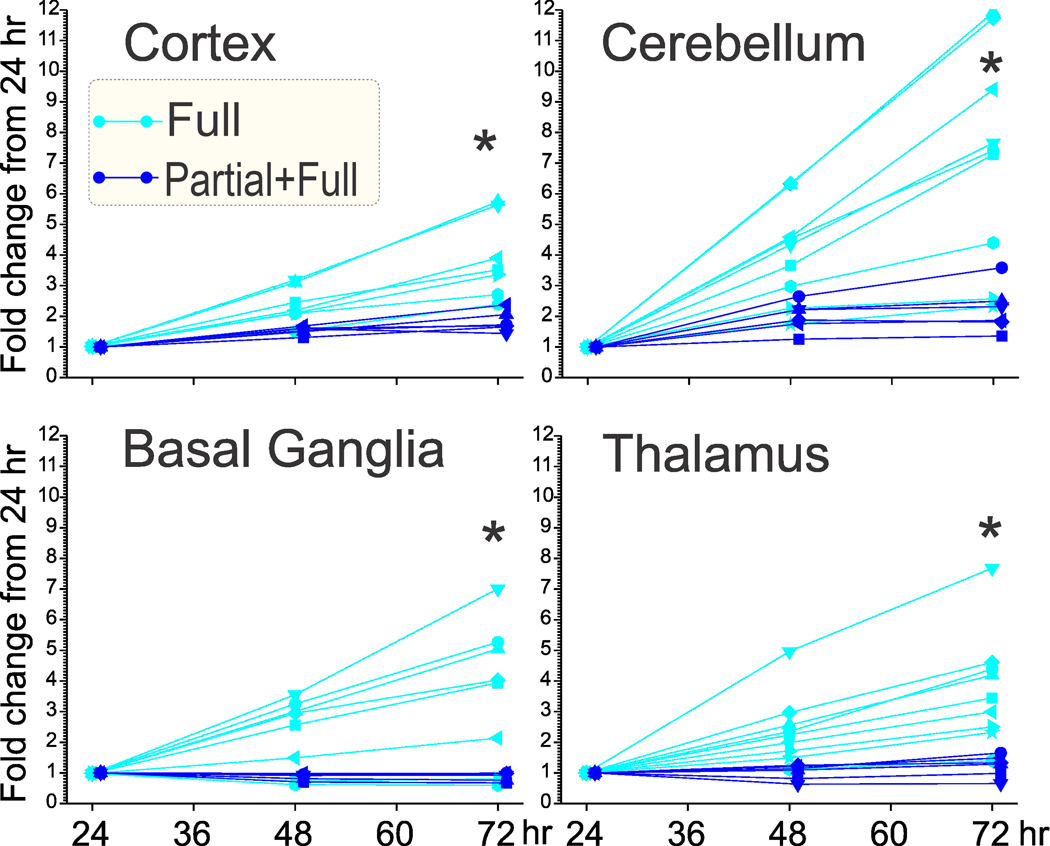
Partial+Full H-I (dark blue, offset by 1 hr for clarity) showed less cell viability after 24 hours (24 hr value normalized to 1) compared to Full H-I (light blue). *α<0.0125 with Bonferroni correction for multiple comparisons, repeated measures ANOVA. Actual F, df, and P values: Cortex – 12.99, 2, 0.0030; Cerebellum – 10.95, 2, 0.0052; Basal ganglia −8.42, 2, 0.0121; Thalamus – 10.36, 2, 0.0061.
Discussion
This is the first animal model to mimic the pathophysiological events from partial to total uteroplacental insufficiency. No significant differences in neurobehavioral outcomes were found in the premature rabbit at 70% and 79% gestation (E22, E25) between Full H-I and Partial+Full H-I insults. Sex differences made no contribution to outcomes. Two interesting points are noteworthy. Firstly, the study was powered for detecting a 15% increase in the ordinal distribution, with increase in E22 severe and dead kits, at 81% power or β error = 0.19. With the actual outcome-difference, our power was 58%, and we would need more than 1000 kits to detect a significant increase. Nevertheless, we may have shown that there is no biological difference between Full and Partial+Full H-I with respect to neurobehavioral outcomes. Secondly, to get these number of kits takes many years, making such a study expensive to complete and replicate. This also points to the expense of getting adequate numbers while performing neuroprotectant trials in the rabbit especially if the improvement is mild. In addition, individual neurobehavioral variables were not significantly different between Full and Partial+Full H-I insults at any gestational age. Since we used a more stringent cutoff for α error (0.0029) due to Bonferroni correction, a type II error could be a possibility. Surprisingly, there was a significant improvement, not worsening, in suck and swallow (Fig 7E) in the Partial+Full H-I group compared to the Full H-I group. We also showed in E25 that cell viability at 24 hr after the insult was not different in any of the four brain regions between the two groups. Again, due to small sample size, a Type II error is possible. As anticipated, at 72 hr, we found all regions showed less cell viability in the Partial+Full H-I group compared to the Full H-I group. To explain this discrepancy in the context of the better suck and swallow test results, it is possible that the medulla and brain stem regions controlling swallowing (Ludlow, 2015) were comparatively spared in Partial+Full H-I group; however, this is worthy of further investigation. Also, the cortex results of cell viability may not reflect the portions of the cortex involved in swallowing (Ludlow, 2015), or more likely, the cortical control of swallowing develops later than E25 in rabbits.
This is also the first study to comprehensively investigate the correlations between two individual neurobehavioral tests, made possible by the large number of kits studied. Specifically, we were interested in strength of correlation (Pearson coefficient) between sensory and locomotor or tone variables and also the strength of correlation between locomotion and tone or dystonia. Marked dystonia was rare on the whole, making correlations with dystonia less likely. There was expectedly strong correlation among various locomotor variables in all groups. The strong association of fore limb tone with locomotion was not surprising, since the newborn rabbit does most of its locomotion using fore limbs and less using the hind limbs. The weaker correlation between hind limb tone and locomotor variables in Partial+Full H-I compared to Full H-I at both ages, and the stronger correlation between odor response and tone in Partial+Full at E25, would suggest that the injury may affect different regions of the brain ultimately. The question that possibly different motor pathways or a different pattern of brain injury may be involved in Partial+Full H-I would require further investigation to be answered.
Cerebral palsy is a disease entity that involve survivors. Not much is known of the primary, secondary, tertiary phases of brain cell injury in these patients. The brain histopathology most probably recovers by the time of manifestation of hypertonia and postural deficits. This thesis is confirmed by numerous neuroimaging studies at older ages. In our animal model, histopathological changes are most obvious at the time of secondary and tertiary cell phases but taking brains 1–3 days after the insult has a) not shown any difference between sham controls and H-I, and b) we are unable to differentiate between fetuses that will ultimately become hypertonic (or Severe or Mild) from those fetuses that become normal-looking kits. Since there were no distinguishing histological lesions between Full H-I and sham controls, we did not attempt to look at histological differences between Full and Partial+Full H-I. We have developed a fetal MRI biomarker that reliably predicts postnatal hypertonia (Drobyshevsky, Derrick, Prasad, et al., 2007; Drobyshevsky, Luo, et al., 2012), but only for the E25 fetus and for Full H-I. Studies are ongoing to extend this MRI biomarker to the Partial+Full H-I and then to investigate cell death and histopathological changes.
Pre-acclimatization vs preconditioning
Since the only difference between the two H-I groups is the extra partial H-I period of 30 min, three possible mechanisms could be involved: 1) Additive or synergistic effect, 2) pre-acclimatization or 3) preconditioning. We were expecting an additive or synergistic effect where the injury of Partial+Full H-I would be more than that of Full H-I group. The fetus already operates at an oxygen level akin to being at 6000–8000 meters altitude (Singer, 1999). Addition of an H-I insult would be expected to deplete the physiological reserves and thus cause more injury. This mechanism explains the cell viability after 24 hr ex vivo where the cells fail to recover as well in Partial+Full H-I group. For neurobehavioral outcome, increased death at E25 compared to E22 was expected, as was previously published (Derrick et al., 2012); in this study, the higher percentages can be explained by the smaller denominator of kits from the previous study as the early perinatal deaths were not counted.
Pre-acclimatization is a mechanism where normoxic individuals undergo a hypoxic period of acclimatization prior to further exposure of greater hypoxia. The Partial+Full H-I scenario may be different because the fetus was already at relatively hypoxic conditions at baseline. Also, acclimatization ideally involves a sufficiently long period of time (Goyal, Van Wickle, Goyal, Matei, & Longo, 2013), and a stepwise increased exposure (Küpper & Schöffl, 2010). Nevertheless, compensatory mechanisms could be triggered (Viscor et al., 2018) by the short H-I period, which could cause amelioration of injury.
Preconditioning is a mechanism where normoxic individuals undergo a hypoxic period followed by 1–2 days of normoxia and then protects against a subsequent lethal insult. Studies in preconditioning suggest possible compensatory mechanisms that would ameliorate injury. In the piglet, another large animal, 3 hr preconditioning results in an increase of HIF-1α and VEGF, ameliorating the subsequent H-I injury (Ara et al., 2011). The Partial+Full scenario is different because of the baseline fetal hypoxic status, comparatively shorter time of initial hypoxia, and absence of the intermediary normoxic period sandwiched in preconditioning between the initial and final hypoxia. In mice, preconditioning of 60 min hypoxia was not different from 30 min (Sheldon, Aminoff, Lee, Christen, & Ferriero, 2007), which would suggest that changes in the initial 30-min-partial H-I in our study probably continued to evolve in the subsequent total H-I period.
The net effect of the three possible mechanisms could possibly cancel each other out resulting in the similarity of the incidence of death and disability. Somewhat unique to the perinatal field is the inverse relationship between the number of deaths and severely affected motor deficits. In the continuum of injury, first there is mild, then severe, then death. It is a well-known clinical fact that when we rescue babies with hypoxic-ischemic encephalopathy, the deaths can decrease resulting in greater numbers of severely affected survivors. With decreasing infant mortality, the incidence of CP in the survivors increases (Winter, Autry, Boyle, & Yeargin-Allsopp, 2002) or the prevalence of CP among survivors remains the same (Van Naarden Braun et al., 2016). Thus, most clinical studies use death and disability as a primary outcome variable. For neuroprotectant studies, it becomes important to consider the number of normal individuals as a decrease of death/severe individuals could trickle down to more severe/mild cases. This trickle-down effect and the inverse relationship between deaths and severe deficits are well illustrated in Figures 2 and 3, showing an increase of death but decrease in severely affected kits with increasing age. The comparisons between groups thus, becomes a bit more complex, necessitating the use of ordinal comparisons of a certain order.
Assessment of Sex
We had previously thought that female sex had more deaths but less severely affected kits in the survivors. With accumulation of enough numbers, we show no statistical difference in outcomes among males and females. What is intriguing is that in all groups there is slightly more deaths and slightly less severe cases in females except the E25 Full H-I group. Since the total number of females is surprisingly less than males in this group, it is possible that the natural selection tendency of rabbit dams to sometimes eat their newborns could have resulted in culling of dead females.
A potential platform to study mechanisms and treatment methods
Rabbit models of H-I injury (Derrick et al., 2001; Derrick et al., 2004) utilize reversible H-I unlike partial uteroplacental insufficiency models in rodents (Morton et al., 2019). A major advance has been the use of MRI biomarkers to identify which of the fetuses will become hypertonic during the H-I insult (Drobyshevsky, Derrick, et al., 2012; Drobyshevsky, Derrick, Prasad, et al., 2007; Drobyshevsky, Luo, et al., 2012). A future study is needed to show whether MRI would still be useful in this new model. The pure hypoxia-ischemia models are separate from the inflammatory models from our lab (Z. Shi et al., 2019) and others to investigate CP (Saadani-Makki et al., 2008), and the glycerol toxin-based models of intracranial hemorrhage (Georgiadis et al., 2008; Traudt, McPherson, Studholme, Millen, & Juul, 2014). Most importantly for neuroprotectant trials, this new model offers better clinical translation to the human setting as it avoids the drawback of total uterine ischemia preventing a neuroprotectant to cross the placenta to the fetus. Fetal bradycardia occurs in the first few minutes of both Full and Partial+Full H-I models. Since fetal bradycardia is the cardinal sign to intervene in pregnancy for obstetricians (Tan, 2014), we could use the onset of fetal bradycardia to start administering neuroprotectants in the prenatal period. For chronic administration of neuroprotectants, such as sepiapterin or tetrahydrobiopterin that has been shown to be a moderate neuroprotectant (Vasquez-Vivar et al., 2009), either of the models would suffice, especially as prior administration of antioxidants can sometimes change the fetal heart rate response (Tan et al., 1996). An ideal neuroprotectant would then be one which is able to not only cross the placenta but reach the fetal brain in enough quantities quickly as has been shown for neuronal nitric oxide synthase inhibitors (Ji et al., 2009; Yu et al., 2011).
In conclusion, this large 1,004-kits study introduces a new rabbit CP model that translates the human transition from partial to total uteroplacental insufficiency, which is more suitable for testing of neuroprotectants. We modified our existing preterm placental insufficiency rabbit model to better simulate the transition from partial to total abruption in human patients. A short period of partial-ischemia was added to full uterine-ischemia. The overall outcome of normal, mild, severe and death, and the neurobehavioral outcomes of olfactory deficits, response to upper face touch, neck, trunk and limb locomotion, fore and hind limb tone and dystonia, was similar to the total uteroplacental insufficiency model at either 70 or 79% gestation. Short-term fetal brain cell survival was not different at 1 day, but subsequent survival was worse in Partial+Full fetuses. The new model also exhibits fetal bradycardia immediately after onset of partial uterine ischemia, allowing for possible neuroprotectant administration that then will be able to enter the fetal brain.
Supplementary Material
Statement of significance.
We modified our existing preterm placental insufficiency rabbit model to better simulate the transition from partial to total abruption in human patients. A short period of partial-ischemia was added to full uterine-ischemia. Long-term postnatal death and disability in the newborn rabbits following Partial+Full uterine-ischemia were similar to the previous Full-uterine-ischemia model. Short-term fetal brain cell survival was not different at 1 day, but subsequent survival was worse in Partial+Full fetuses. The new model also exhibits fetal bradycardia immediately after onset of partial uterine ischemia, allowing for possible neuroprotectant administration that then will be able to enter the fetal brain.
Acknowledgements:
Funded by NIH for S.T. (NINDS NS051402, NS 043285, NS063141, NS081936, NS100088, 114972).
Footnotes
Data Accessibility
Data will be available from the corresponding upon reasonable contact.
Potential Conflicts of Interest
Nothing to report.
References
- Ananth CV, Berkowitz GS, Savitz DA, & Lapinski RH (1999). Placental abruption and adverse perinatal outcomes. JAMA, 282(17), 1646–1651. [DOI] [PubMed] [Google Scholar]
- Ara J, Fekete S, Frank M, Golden JA, Pleasure D, & Valencia I. (2011). Hypoxic-preconditioning induces neuroprotection against hypoxia-ischemia in newborn piglet brain. Neurobiol Dis, 43(2), 473–485. doi: 10.1016/j.nbd.2011.04.021 [DOI] [PubMed] [Google Scholar]
- Bona E, Johansson BB, & Hagberg H. (1997). Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatric Research, 42(5), 678–683. [DOI] [PubMed] [Google Scholar]
- Cavarsan CF, Gorassini MA, & Quinlan KA (2019). Animal models of developmental motor disorders: parallels to human motor dysfunction in cerebral palsy. J Neurophysiol, 122(3), 1238–1253. doi: 10.1152/jn.00233.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, & Abel MF (2002). What does the Ashworth scale really measure and are instrumented measures more valid and precise? Developmental Medicine & Child Neurology., 44(2), 112–118. [DOI] [PubMed] [Google Scholar]
- Derrick M, Drobyshevsky A, Ji X, & Tan S. (2007). A model of cerebral palsy from fetal hypoxia-ischemia. Stroke, 38(2 Suppl), 731–735. doi: 10.1161/01.STR.0000251445.94697.64 [DOI] [PubMed] [Google Scholar]
- Derrick M, Englof I, Drobyshevsky A, Luo K, Yu L, & Tan S. (2012). Intrauterine fetal demise can be remote from the inciting insult in an animal model of hypoxia-ischemia. Pediatr Res, 72(2), 154–160. doi: 10.1038/pr.2012.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick M, He J, Brady E, & Tan S. (2001). The in vitro fate of rabbit fetal brain cells after acute in vivo hypoxia. J Neurosci, 21(7), RC138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, . . . Tan S. (2004). Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci, 24(1), 24–34. doi: 10.1523/JNEUROSCI.2816-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Cotten CM, Shi Z, Luo K, Jiang R, Derrick M, Tan S(2015). Human Umbilical Cord Blood Cells Ameliorate Motor Deficits in Rabbits in a Cerebral Palsy Model. Dev Neurosci, 37(4–5), 349–362. doi: 10.1159/000374107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Luo K, Zhang LQ, Wu YN, Takada SH, . . . Tan S. (2012). Near-term fetal hypoxia-ischemia in rabbits: MRI can predict muscle tone abnormalities and deep brain injury. Stroke, 43(10), 2757–2763. doi: 10.1161/STROKEAHA.112.653857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Prasad PV, Ji X, Englof I, & Tan S. (2007). Fetal brain magnetic resonance imaging response acutely to hypoxia-ischemia predicts postnatal outcome. Ann Neurol, 61(4), 307–314. doi: 10.1002/ana.21095 [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Wyrwicz AM, Ji X, Englof I, Ullman LM, . . . Tan S. (2007). White matter injury correlates with hypertonia in an animal model of cerebral palsy. J Cereb Blood Flow Metab, 27(2), 270–281. doi: 10.1038/sj.jcbfm.9600333 [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Jiang R, Lin L, Derrick M, Luo K, Back SA, & Tan S. (2014). Unmyelinated axon loss with postnatal hypertonia after fetal hypoxia. Ann Neurol, 75(4), 533–541. doi: 10.1002/ana.24115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Luo K, Derrick M, Yu L, Du H, Prasad PV, . . . Tan S. (2012). Motor deficits are triggered by reperfusion-reoxygenation injury as diagnosed by MRI and by a mechanism involving oxidants. J Neurosci, 32(16), 5500–5509. doi: 10.1523/JNEUROSCI.5986-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Yu L, Yang Y, Khalid S, Luo K, Jiang R, . . . Tan S. (2012). Antenatal insults modify newborn olfactory function by nitric oxide produced from neuronal nitric oxide synthase. Exp Neurol, 237(2), 427–434. doi: 10.1016/j.expneurol.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, & Cai Z. (2005). Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behavioural Brain Research, 165(1), 80–90. [DOI] [PubMed] [Google Scholar]
- Fragopoulou AF, Qian Y, Heijtz RD, & Forssberg H. (2019). Can Neonatal Systemic Inflammation and Hypoxia Yield a Cerebral Palsy-Like Phenotype in Periadolescent Mice? Mol Neurobiol. doi: 10.1007/s12035-019-1548-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, . . . Ballabh P. (2008). Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke, 39(12), 3378–3388. doi: 10.1161/STROKEAHA.107.510883 [DOI] [PubMed] [Google Scholar]
- Gilbert WM, Jacoby BN, Xing G, Danielsen B, & Smith LH (2010). Adverse obstetric events are associated with significant risk of cerebral palsy. Am J Obstet Gynecol, 203(4), 328 e321–325. doi: 10.1016/j.ajog.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R, Van Wickle J, Goyal D, Matei N, & Longo LD (2013). Antenatal maternal long-term hypoxia: acclimatization responses with altered gene expression in ovine fetal carotid arteries. PLoS One, 8(12), e82200. doi: 10.1371/journal.pone.0082200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, . . . Lieber RL (2016). Cerebral palsy. Nat Rev Dis Primers, 2, 15082. doi: 10.1038/nrdp.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel S, Shapira Y, Hartzler J, Teng EL, Quiligan E, & Van Der Meulen JP (1978). Neuromotor development in relation to birth weight in rabbits. Biol.Neonate, 33(1–2), 1–7. [DOI] [PubMed] [Google Scholar]
- Hasegawa J, Toyokawa S, Ikenoue T, Asano Y, Satoh S, Ikeda T, . . . Ueda, S. (2016). Relevant Obstetric Factors for Cerebral Palsy: From the Nationwide Obstetric Compensation System in Japan. PLoS One, 11(1), e0148122. doi: 10.1371/journal.pone.0148122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Tan S, Igarashi J, Li H, Derrick M, Martasek P, . . . Silverman RB (2009). Selective neuronal nitric oxide synthase inhibitors and the prevention of cerebral palsy. Ann Neurol, 65(2), 209–217. doi: 10.1002/ana.21555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayani SI, Walkinshaw SA, & Preston C. (2003). Pregnancy outcome in severe placental abruption. BJOG., 110(7), 679–683. [PubMed] [Google Scholar]
- Kendall LV, Owiny JR, Dohm ED, Knapek KJ, Lee ES, Kopanke JH, . . . Ayers JD (2019). Replacement, Refinement, and Reduction in Animal Studies With Biohazardous Agents. ILAR J. doi: 10.1093/ilar/ily021 [DOI] [PubMed] [Google Scholar]
- Khamis H. (2008). Measures of Association: How to Choose? J Diagn Med Sonogr, 24, 155–162. doi:0.1177/8756479308317006 [Google Scholar]
- Koman LA, Smith BP, & Shilt JS (2004). Cerebral palsy. Lancet, 363(9421), 1619–1631. [DOI] [PubMed] [Google Scholar]
- Küpper TE, & Schöffl V. (2010). Preacclimatization in hypoxic chambers for high altitude sojourns. Sleep Breath, 14(3), 187–191. doi: 10.1007/s11325-009-0307-x [DOI] [PubMed] [Google Scholar]
- Logitharajah P, Rutherford MA, & Cowan FM (2009). Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging and outcome. Pediatr.Res [DOI] [PubMed] [Google Scholar]
- Ludlow CL (2015). Central Nervous System Control of Voice and Swallowing. J Clin Neurophysiol, 32(4), 294–303. doi: 10.1097/wnp.0000000000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Maeda T, & Kouno S. (2003). Comparison of neonatal outcome including cerebral palsy between abruptio placentae and placenta previa. Eur.J.Obstet.Gynecol.Reprod.Biol, 106(2), 125–129. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Ogawa M, Konno J, Mitani M, & Matsui H. (2013). Prediction of fetal acidemia in placental abruption. BMC Pregnancy Childbirth, 13, 156. doi: 10.1186/1471-2393-13-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, Sheldon RA, Shatz CJ, & Ferriero DM (2003). Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. Journal of Neuroscience., 23(8), 3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JS, Levasseur J, Ganguly E, Quon A, Kirschenman R, Dyck JRB, . . . Davidge ST (2019). Characterisation of the Selective Reduced Uteroplacental Perfusion (sRUPP) Model of Preeclampsia. Sci Rep, 9(1), 9565. doi: 10.1038/s41598-019-45959-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HC, & Torday JS (1983). Anatomy of fetal rabbit gonads and the sexing of fetal rabbits. Lab Anim, 17(2), 148–150. [DOI] [PubMed] [Google Scholar]
- Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, . . . Chugani D. (2008). Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. American Journal of Obstetrics & Gynecology, 199(6), 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RA, Aminoff A, Lee CL, Christen S, & Ferriero DM (2007). Hypoxic preconditioning reverses protection after neonatal hypoxia-ischemia in glutathione peroxidase transgenic murine brain. Pediatr Res, 61(6), 666–670. doi: 10.1203/pdr.0b013e318053664c [DOI] [PubMed] [Google Scholar]
- Shi J, Luo K, & Tan S. (2019). Cerebral Palsy Model of Uterine Ischemia in Pregnant Rabbits. In Chen J, Xu ZC, Xu XM, & Zhang JH (Eds.), Animal Models of Acute Neurological Injury (2nd ed., pp. 189–205): Springer International Publishing. [Google Scholar]
- Shi Z, Vasquez-Vivar J, Luo K, Yan Y, Northington F, Mehrmohammadi M, & Tan S. (2019). Ascending Lipopolysaccharide-Induced Intrauterine Inflammation in Near-Term Rabbits Leading to Newborn Neurobehavioral Deficits. Dev Neurosci, 1–13. doi: 10.1159/000499960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D. (1999). Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol, 123(3), 221–234. doi: 10.1016/s1095-6433(99)00057-4 [DOI] [PubMed] [Google Scholar]
- Steward O, & Balice-Gordon R. (2014). Rigor or mortis: best practices for preclinical research in neuroscience. Neuron, 84(3), 572–581. doi: 10.1016/j.neuron.2014.10.042 [DOI] [PubMed] [Google Scholar]
- Tan S. (2014). Fault and blame, insults to the perinatal brain may be remote from time of birth. Clin Perinatol, 41(1), 105–117. doi: 10.1016/j.clp.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Drobyshevsky A, Jilling T, Ji X, Ullman LM, Englof I, & Derrick M. (2005). Model of cerebral palsy in the perinatal rabbit. J Child Neurol, 20(12), 972–979. doi: 10.1177/08830738050200120801 [DOI] [PubMed] [Google Scholar]
- Tan S, Liu YY, Nielsen VG, Skinner K, Kirk KA, Baldwin ST, & Parks DA (1996). Maternal infusion of antioxidants (Trolox and ascorbic acid) protects the fetal heart in rabbit fetal hypoxia. Pediatr Res, 39(3), 499–503. doi: 10.1203/00006450-199603000-00019 [DOI] [PubMed] [Google Scholar]
- Traudt CM, McPherson RJ, Studholme C, Millen KJ, & Juul SE (2014). Systemic glycerol decreases neonatal rabbit brain and cerebellar growth independent of intraventricular hemorrhage. Pediatr Res, 75(3), 389–394. doi: 10.1038/pr.2013.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Naarden Braun K, Doernberg N, Schieve L, Christensen D, Goodman A, & Yeargin-Allsopp M. (2016). Birth Prevalence of Cerebral Palsy: A Population-Based Study. Pediatrics, 137(1), 1–9. doi: 10.1542/peds.2015-2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, Benders MJ, . . . Nijboer CH (2018). Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia, 66(2), 221–238. doi: 10.1002/glia.23256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Whitsett J, Derrick M, Ji X, Yu L, & Tan S. (2009). Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain. Ann Neurol, 66(3), 323–331. doi: 10.1002/ana.21738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscor G, Torrella JR, Corral L, Ricart A, Javierre C, Pages T, & Ventura JL (2018). Physiological and Biological Responses to Short-Term Intermittent Hypobaric Hypoxia Exposure: From Sports and Mountain Medicine to New Biomedical Applications. Front Physiol, 9, 814. doi: 10.3389/fphys.2018.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S, Autry A, Boyle C, & Yeargin-Allsopp M. (2002). Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics, 110(6), 1220–1225. doi: 10.1542/peds.110.6.1220 [DOI] [PubMed] [Google Scholar]
- Yu L, Derrick M, Ji H, Silverman RB, Whitsett J, Vasquez-Vivar J, & Tan S. (2011). Neuronal nitric oxide synthase inhibition prevents cerebral palsy following hypoxia-ischemia in fetal rabbits: comparison between JI-8 and 7-nitroindazole. Dev Neurosci, 33(3–4), 312–319. doi: 10.1159/000327244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


