Abstract
How neurons die in neurodegenerative diseases is still unknown. The distinction between apoptosis as a genetically controlled mechanism, and necrosis, which was viewed as an unregulated process, has blurred with the ever-increasing number of necrotic-like death subroutines underpinned by genetically defined pathways. It is therefore pertinent to ask whether any of them apply to neuronal cell death in tauopathies. Although Alzheimer's disease (AD) is the most prevalent tauopathy, tauopathies comprise an array of over 30 diseases in which the cytoplasmic protein tau aggregates in neurons, and also, in some diseases, in glia. Animal models have sought to distil the contribution of tau aggregation to the cell death process but despite intensive research, no one mechanism of cell death has been unequivocally defined. The process of tau aggregation, and the fibrillar structures that form, touch on so many cellular functions that there is unlikely to be a simple linear pathway of death; as one is blocked another is likely to take the lead. It is timely to ask how far we have advanced into defining whether any of the molecular players in the new death subroutines participate in the death process. Here we briefly review the currently known cell death routines and explore what is known about their participation in tau aggregation-related cell death. We highlight the involvement of cell autonomous and the more recent non-cell autonomous pathways that may enhance tau-aggregate toxicity, and discuss recent findings that implicate microglial phagocytosis of live neurons with tau aggregates as a mechanism of death.
Keywords: cell death, neurodegeneration, tau
Introduction
In 2022, it will be 50 years since the article by Kerr et al. [1] appeared that coined the phrase apoptosis. They described a mechanism of cell death that is characterised by cell condensation and fragmentation like the shedding of leaves or petals, and consequent phagocytic cell elimination, sometimes leaving an undigested residual body. The process was suggested to be ‘an active, inherently programmed phenomenon’ [2] as it was regulated by hormones and was observed to be prevalent in isolated cells during development. This was contrasted with necrosis, which was an indiscriminate process of cell lysis linked to inflammation. The remarkable conversion of apoptosis from an inherently morphological description into its detailed molecular underpinning has presented numerous potential therapeutic targets whose manipulation should allow either induction or inhibition of apoptosis and hence rescue of neuronal cell death in neurodegeneration. However, although occasional apoptotic footprints have been demonstrated in Alzheimer's disease (AD) and other tauopathies during neurodegeneration, or in animal models, it is clear that apoptosis is not the major mechanism of cell death in these diseases. On the other hand, interest in necrosis, previously considered an unregulated process, has blossomed and now encompasses several pathways underpinned by molecular mechanisms (all ending in ptosis although none seem to shed any parts of the cell) including: ferroptosis, pyropotsis, and necroptosis [3–5]. Additional necrotic types of death are mainly due to ATP depletion and include parthanatos, oncosis, autosis [4]. Intracellular digestion pathways have also been implicated, including lysosomal and autophagic cell death [3–5]. Finally, non-cell autonomous mechanisms include those instigated by phagocytosis of living neurons and possibly those instigated by immune cells that release granzyme B/perforin [6], the latter hardly explored in relation to neurodegenerative diseases. Another focus has been on degeneration of axons/synapses as a cause of retrograde degeneration. Table 1 summarises the features of the main cell death mechanisms sometimes also reported in tauopathies. Extensive discussions of the mechanisms of each pathway in relation to neuronal cell death and neurodegeneration can be found in recent reviews [4,5]. In this review, we reflect specifically on neuronal death in tauopathies.
Table 1. Summary of cell death mechanisms discussed in this review.
| Type of neuronal cell death | Initiators | Mediators | Executioners | Inhibitors | Outcome | References |
|---|---|---|---|---|---|---|
| Apoptosis | Death receptors, DNA damage, ROS, staurosporine | Intrinsic: pro-apoptotic Bcl2 members (Bax, Bim, Bak, Puma, Noxa), Bcl2 inhibitors (ABT737), extrinsic: upstream caspases 8/10 | Apoptosome (cytochrome c/apaf1 activation (dATP- > dADP exchange) to caspase 9 and downstream caspases mitochondrial Smac/diablo inhibits IAP/xIAPs | Natural: anti-apoptotic Bcl2 members (Bcl2, BclxL, McL1) IAP/XIAP family of caspase inhibitors, caspase activity inhibitors (only temporary) | DNA breaks, nuclear condensation, loss of MOMP, exposure of PtdSer, removal by phagocytosis Eventual ATP depletion and secondary necrosis | [4,5,81] |
| Ferroptosis (oxytosis) | Fe3+ entry via transferrin receptor and conversion into Fe2+ lack of cysteine supply via Xc− transporter | Oxygen/hydroxyl radicals (via the Fenton reaction), and/or chemical GPx4 inhibitors (1S, 3E-RSL3) | Loss of glutathione, Lipid reactive oxygen species (LOOH, L–O], preferential oxidation of polyunsaturated fatty acids (PUFAs) | Glutathione peroxidase 4 (GPx4; up-regulation by Nrf2), antioxidants (ferrostatin-1, liproxstatin-1, vitamin E) iron chelators (Deferoxamine) | Plasma membrane lipid fragmentation, mitochondrial shrinkage/deformation of cristae, loss of ATP and NAD+, lysosomal membrane permeabilisation | [82–85] |
| Pyroptosis | Pattern recognition receptors (PRRs)/other signals (bacteria/toxins/dsDNA breaks) | Inflammasome (NLRP3 or its homologues/apoptosis-associated speck-like protein containing CARD (ASC/procaspase‐1) but also other mechanisms | Gasdermins (GSDMs), especially GSDMD, activated by caspase-1 cleavage downstream of inflammasome formation | Disulfiram (via Cys191/192 human/mouse in GSDMD) | Large pores with electrostatic filtering (preference for positively charged/neutral molecules) but no notable cell swelling can mediate IL-1b export | [86–90] |
| Necroptosis | Death receptors (TNF), caspase-8 inhibition | RIPK1/TRIF/ZBP1 binding to RIPK3 and phosphorylation of MLKL | Phospho-MLKL oligomerisation and translocation to plasma membrane | Necrostatins (RIPK1 inhibitors) | Pores in plasma membrane (and mitochondria/lysosomes); Na+ permeability, water influx, and osmotic swelling morphology | [36,37,91–93] |
| Parthanatos | Poly(ADP-ribose) polymerase 1 (PARP-1) hyperactivation | Apoptosis inducing factor (AIF)-dependent and microphage migration inhibitory factor (MIF)-dependent | DNA degradation | PARP inhibitors | Shrunken and condensed nuclei, membranes disintegrate, and cells become propidium iodide-positive within a few hours after the onset | [94,95] |
| Autosis* | Hyper autophagy activation | Ions | Osmotic imbalance | Autosis - NaKATpase inhibitors, e.g. ouabain or autophagy inhibitors | Nuclear shrinkage; focal separation of inner and outer nuclear membranes with focal expansion of perinuclear space Extensive cytoplasmic vacuolisation increased adhesion | [96,97] |
| Primary phagocytosis† | Inflammation, stress LPS activation of microglia; ROS induced by tau aggregates | Phosphatidylserine/calreticulin exposure on the target cell | Opsonins (MFGE8, Gas6, protein S) made by phagocyte | CD47, excess AnnexinV or synaptotagmin C2 domain | Phagocytosis of live cell, inhibition of phagocytosis leaves behind a live cell | [50,57,98,99] |
| Perforin/Granzyme B | Cytotoxic T cells (CTLs) and natural killer (NK) cells | Ionic pores | Granzyme proteolysis and other activities | unknown | Stored as granules. Perforin creates ionic pores in the target cell, granzymes facilitate cell death by various mechanisms | [6,100,101] |
Forms of lysosome-dependent cell death, and autophagy-dependent cell death are not included because their molecular mechanisms are not proven except in invertebrates.
Also named ‘phagoptosis’ although there is no ‘ptosis’ element in this form of death.
Tauopathies
Tau (an acronym of ‘tubule-associated unit’) in adult human brain comprises six isoforms that result from alternative splicing of the MAPT gene; splicing of exons 2 and 3 lead to the absence or presence of one or two N-terminal repeats (labelled 0N, 1N, or 2N), and splicing of exon 10 results in tau containing 3-repeat or 4-repeat units (3R, 4R) that are responsible for binding and stabilising microtubules besides other functions [7]. In disease, tau undergoes numerous post-translational modifications beginning with hyperphosphorylations that repel tau from the microtubules and contribute to its misfolding, eventually leading to tau aggregation and fibrillisation [8]. A recent study of post-translational modifications of tau from AD patients using mass spectrometry has proposed that following hyperphosphorylation, tau undergoes acetylations, methylations, and ubquitinations that facilitate the formation of fibrils [9]. This study also showed a great diversity in the spectrum of post-translational modifications in different patients, although all were diagnosed with AD.
Aggregation of the protein tau from a soluble unfolded state to an insoluble, β-sheet-rich filamentous structure underlies numerous human neurodegenerative diseases known as tauopathies [10,11]. These include AD, frontotemporal dementias, Pick's disease, progressive supranuclear palsy (PSP), corticobasal degeneration, chronic traumatic encephalopathy, and argyrophilic brain disease. The presence of intraneuronal aggregates of tau best correlate with the neuronal cell death that is associated with the clinical signs and symptoms of diseases such as AD, PSP, and Pick's disease [12]. The mechanism of cell death induced by tau is not well understood. Tau misfolds into distinct fibrillar forms depending on the specific tauopathy [13]. Different filamentous forms of tau can template specific pathological conformations on to naïve monomers through a mechanism of prion-like spreading [14]. Analysis of disease progression suggests that misfolded tau is released as seeds through synaptic connections, because pathology proceeds progressively via anatomically connected regions [15,16]. Since cryo-EM studies have shown that tau fibril structures differ between each type of tauopathy [17], it may not be surprising if the mechanisms of cell death will also be diverse. The exact structure of tau as well as the millieu of the cell may dictate which death pathway is followed. Tau toxicity studies in vitro have mainly relied on tau fibrils formed by co-incubation with heparin but given the recent report that heparin-aggregated tau does not resemble fibrils extracted from tauopathies [18], it is difficult to conclude that similar mechanisms occur in human disease. Indeed, non-neuronal cells containing tau aggregates formed by seeding can be amplified into clones, demonstrating that such tau aggregates in these models are not inherently toxic [19].
It has been hypothesised that oligomeric tau is the toxic species whereas filamentous tau is benign or is even formed in an attempt to sequester tau away from toxic substrates. A recent study of transgenic mice overexpressing the human mutant P301S 0N4R isoform of tau in neurons [20], where neuronal cell death in the cortex and motor neurons in the brain and spinal cord is prevalent, suggests that filamentous aggregated tau is the toxic species [21], although this does not preclude the co-presence of oligomers in the same neurons. Antibodies that detect oligomers [22] in our hands failed to find such oligomers in neurons expressing P301S tau unless they also contained insoluble tau aggregates (unpublished data). Indeed it has been hypothesised from a kinetic analysis that the formation of sarkosyl-insoluble tau species must also include a fragmentation process [23], which could give rise to secondary oligomers. A recent study using a tau-proximity ligation assay has suggested that tau–tau interactions occur before detection of formation of tau aggregates in AD (at Braak stage I/II ), with positive structures occuring in both the neuropile and in neuronal cell bodies [24] but the sizes of the multimers and their purity remains to be determined.
Tau aggregation or pathology and apoptosis
Apoptosis was studied extensively as a possible mode of death in AD, but less so in pure tauopathies. Most studies of apoptosis were directed at detecting elevated levels of executioner caspases (mainly caspase 3) but caspases have numerous non-apoptotic roles in the nervous system [25]. Of interest is the observation that caspases play a key role in synapse pruning by microglia, by promoting the exposure of phosphatidylserine (PtdSer) and recruitment of C1q [26–28]. Apoptosis is a rapid cell degradation process with clear molecular and morphological correlates, but because apoptotic cells are rapidly cleared, it is difficult to confirm apoptotic death in neurons containing tau aggregates/filaments and in diseases that last for decades. Nevertheless, it has been suggested that executioner caspases play a role in generating more aggregation-prone tau after tau cleavage at Aspartyl 421 (D421) residue. However, in a study on rTg4510 tau transgenic mice, caspase activation preceded tau tangle formation by hours to days yet neurons remained alive after a new tangle formed within the same neurons [29], thus dissociating caspase activity from apoptosis, which is normally executed within 24–48 h of caspase activation. Moreover, in the P301S tau mouse model, hardly any active caspase 3 or its substrate, cleaved fodrin (an enodgenous intracellular reporter used to demonstrate productive caspase activity), was detected in regions where neuronal death exceeds 50% [20]. Truncated D421 tau was also very low in dispersed filaments from AD and FTDP-17 brains and appeared late in the progression of the disease. Although the possibility that there are undetectable small amounts of D421-truncated tau cannot be excluded, these data suggest that truncation at D421 is not necessary for the assembly of tau into filaments [30]. Interestingly, phosphorylation of tau at Ser422 (a site modified specifically in tauopathies [9]), appears to prevent caspase cleavage [31], indicating that caspase activation deduced from staining by antibodies against the active form does not necessarily result in tau cleavage to a more aggregation-prone form of tau or cell death.
Tau aggregation or pathology and regulated types of necrosis – focus on necroptosis
While there is some indirect evidence implicating tau and activation of regulated forms of necrosis like pyroptosis [32], parthanatos [33] and ferroptosis [34,35], reviewed in [5], necroptosis has been more directly implicated in AD. Initial studies were based on the elevated expression of RIPK1 that correlated with Braak staging [36]. RIPK1 is activated by TNF-α and hence is also implicated in inflammatory processes in AD, this so far has been mainly associated with β-amyloid deposition and disposal [37]. Importantly, the execution of necroptosis is via pores formed by P-MLKL so it is necessary to demonstrate that P-MLML has ben recruited to the plasma membrane and not just demonstrate expression of the upstream kinases.
Recently, it was reported that necrosome components accumulate alongside tau inside granulovacuolar bodies (GvBs) in AD [38,39], correlating with tau pathology and neuronal loss, but not with amyloid plaques. The RIPK1 inhibitor, Nec-1, was also reported to reduce tau phosphorylation at Ser199 alongside cognitive functions in APP/PS1 mice that produce β-amyloid [40]. GvBs are associated with many classes of degenerating neurons that contain tau neurofibrillary tangles in AD and other tauopthies [41], hence the hypothesis that tau-associated cell death could occurs through granuolvacuolar degeneration (GvD) [42]. Phospho-tau has been reported to be contained within GvB, alongside an unusually large cohort of other phosphoproteins and kinases such as CK1∂ [43]. GvB vacuoles were suggested to be late-stage autophagic organelles [44] and recently GvBs have been identified as specific neuronal lysosomes that form as a direct result of tau aggregation (induced by seeding) due to impaired vesicle trafficking [45]. Since tau fibril formation is dependent on tau concentration, sequestering tau in vesicles might contribute to increased tau toxicity or reduce it, as hypothesised in [46]. Possible implication of lysosomal and autophagic mechanisms of cell death has been an area of debate, especially as these could theoretically lead to clearance of pathological forms of tau [47,48]. One problem is how to interpret a change in expression of markers that participate in these processes such as the autophagosomal marker LC3II, whose accumulation could either be due to bona fide activation of autophagic flux, or to a block in lysosomal proteolytic function, resulting in autophagosome accumulation but unproductive autophagy [49].
Studying the mechanism of death of dorsal root ganglion (DRG) neurons containing insoluble tau aggregates cultured from adult P301S tau mice, we found that neuronal loss was not ameliorated by incubation with Nec-1 nor did it differ from the rate of loss in the presence of the inactive isomer Nec-1i [50]. The implication of coexistence of tau aggregates and necrosome in relation to pathology and neuron death remain to be deciphered.
Tau aggregation or pathology and non-cell autonomous death mechanisms
Although the participation of necroptosis in tau-related neuronal cell death is still debated, an interesting avenue is the finding that necroptosis is a major mechanism that is involved in microglial-mediated cell death. Microglia and astrocytes have now been implicated in mediating non-cell autonomous neuronal cell death in several neurodegenerative disease models [51–53]. In vitro, inhibition of caspase 8 (which complexes with RIPK1) – but not caspase 3 – was sufficient to rescue live neurons that would otherwise have been killed by microglial phagocytosis, thus revealing a new mechanism of neuronal cell death called primary phagocytosis. In this example, primary phagocytosis was due to an interplay of oxidative stimuli that activated the microglia and caused neurons to expose the ‘eat-me’ signal PtdSer [54,55].
It has long been known that apoptotic cells display PtdSer and are thereby eliminated by phagocytosis without producing inflammation, unlike forms of unregulated necrosis. The cardinal sign of primary phagocytosis is that inhibition of microglia in this context rescues live neurons, whereas dead neurons accumulate in the case of apoptotic phagocytosis. Such mechanisms also contribute to synapse elimination in health and disease [26]. We have recently reported that neurons with insoluble tau aggregates that are cultured from P301S tau mice are eliminated by primary phagocytosis by microglia, and not by apoptosis or necroptosis. Tau aggregates caused the neurons to display PtdSer because of reactive oxygen species (ROS) induction [50], and contact with microglia caused them to release opsonins that enabled the phagocytic process. Interestingly, these microglia released tau aggregates they had ingested with the neurons, while releasing other senescence-associated paracrine factors that caused other microglia to become hypophagocytic, which could exacerbate the development of tauopathy, tau toxicity, and cell death [56,57]. Interestingly, microglial senescence has been associated with tau pathology and neurodegeneration in AD and other tauopathies [58,59] and tau aggregate-associated neuronal senescence has also been implicated in neurodegeneration [60], although the mechanism of neuronal death has not been defined. While aggregated tau alongside neuronal nuclei were found in the microglia in the brains of these mice, it is possible that not all the neurons with aggregated tau die by this process. Indeed, some motor neurons that contain tau aggregates in the brain and spinal cord of P301S tau mice and in tauopathies show signs of nuclear condensation and cytoplasmic swelling, hallmarks of late necrosis, but whether this is a stage en route to phagocytosis remains to be resolved. Studies in PS19 tau mice corroborate the active role of microglia in neurodegeneration and in synapse removal but how the neurons die in these models remains to be described [61–64].
Astrocytes have also been suggested to promote neuronal death, for example [65,66]). In many pure tauopathies, tau aggregates appear in astrocytes, leading to the hypothesis that astrocytes are sources of tau spreading and loss of neuronal support, thus contributing to tau pathology [67]. In ALS, astrocytes were suggested to kill neurons by necroptosis although recent work suggests that other mechanisms prevail (for example [68]). In the P301S tau mice, we found that neuron death is reduced by transplantation of neural precursor-derived astrocytes from wildtype (wt) mice [69]. Moreover, astrocytes cultured from P301S or P301L tau mice were deficient in providing neuroprotection and synaptogenesis compared with wt astrocytes [70], and supplementation of P301S astrocyte-derived conditioned medium was sufficient to rescue both neuron survival and synaptogenesis. That P301S tau neurons can cause astrocytes to lose their neuroprotective functions was suggested by the loss of several important functional markers induced in astrocytes by their interaction with healthy wt neurons [71]. Considering possible therapeutic interventions, it is important to consider not only how neurons die but also what factors might be used to boost their survival. The question has arisen regarding why only some neurons are vulnerable to tau-related diseases, and why these populations differ in different tauopathies. Propagation of tau between connected neurons might explain part of this selectivity but recent reports suggest that vulnerable neurons that die are more sensitive to development of tau pathology, possibly linked to loss of neuroprotective functions by astrocytes [72]. In P301S tau mice, injection of α-B crystallin (HspB5), a member of a small chaperone family of proteins, reduced neuronal cell death while boosting production of neurotrophic factors from astrocytes in cooperation with factors released by stimulation of microglia with HspB5 [73].
With the increase in identifying inflammatory mechanisms in long-term diseases like AD and other tauopathies, it may be pertinent to ask whether cytotoxic T cells are involved in some cell death events in tauopathies. Cytotoxic T cells are cells that recognise antigens presented usually due to viral infections, and were recently reported to invade the CNS during inflammation caused by COVID-19 [74]. Some vulnerable neurons were also reported to increase antigen-presenting MHC-I on their surface and this was related to exacerbation of tau pathology [75]. While perforin/granzyme B in tauopathies has not been reported, we thought it is important to include this mechanism of cell death in Table 1, especially if secondary infections occur that escape treatment.
Are the mechanisms uncovered in mouse models matched by those in human disease?
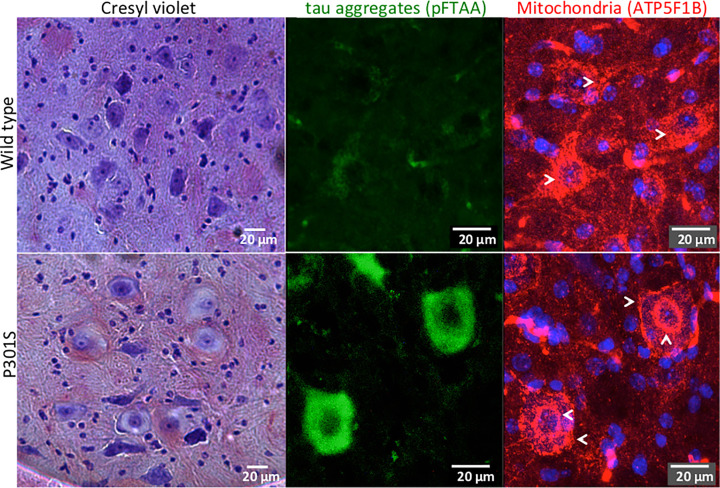
When symptoms appear in tauopathies the neurodegenerative process has possibly gone on for decades and it is very difficult in postmortem human brain to clearly identify how the neurons have died. Both mouse models and cellular models have been developed reproducing tau aggregation to identify possible mechanisms of neuronal death related to tau aggregation. However transgenic models for example offer a speeded up process in that a mouse lives only for a few years so it is important, when possible, to validate the finding in mouse in the human brain before any conclusions can be reached. Neuropathological features of postmortem human brain can also provide suggestions for mechanisms of neuronal death, for example, ‘ballooned neurons’, observed in corticobasal degeneration, PSP, Pick's disease, and argyrophilic brain diseases [76], have been associated with neuronal death due to axon degeneration [77]. This still enigmatic form of death is characterised by enlargement of the neuronal cell body with cytoplasmic dilation, acentric nuclei and accumulation of neurofilament and staining for aβ-crystallin; in some cases, neurons also contain tau aggregates [78,79]. In the P301S tau mice, similar neurons are found in areas with neuronal death such as the spinal cord, where either swollen or pyknotic neurons were reported [20]. In these P301S tau mice, we correlated the presence of aggregated tau with the morphological features of ballooned neurons and found that the swollen cytoplasm and nuclei were poorly stained with the nucleic acid dye Cresyl Violet. A rearrangement of mitochondria around the cell periphery and the nuclear membrane was also present (unpublished, Figure 1). It will be interesting to determine how the redistribution of mitochondria and lack of nucleic acid staining is related to the neuronal death process.
Figure 1. Neurons from P301S tau mice containing aggregated tau showing possible morphological features of ballooned neurons.
Brain stem sections from the facial nucleus region of 5-month-old wt C57 or P301S tau mice. Left column, stained with Cresyl Violet (note swollen and acentric nuclei in the sample from P301S tau mice). Middle and right columns, confocal Z stacks of the same region stained with pFTAA (green), a dye that detects tau aggregates with high affinity [102] and an antibody against the mitochondrial inner membrane protein ATP synthase β (ATP5F1B, red). Nuclei are in blue. Note the loss and rearrangement of remaining mitochondria around the nuclear and plasma membranes in neurons with tau aggregates (indicated by arrows).
Remaining questions abound. Is it naïve to expect that the mechanisms of neuronal cell death in tauopathies follow an exclusive pathway? Different mechanisms of cell death overlap in many of their biochemical pathways (in particular those affecting the integrity of the membranes in mitochondria, lysosomes, the endoplasmic reticulum (ER), and the plasma membranes). Organelle dysfunction can be triggered sequentially by one system going awry causing a step that is not part of a specific subroutine to be mobilised. In fixed postmortem tissue, we cannot measure flux events like calcium, which can activate proteases and cause intracellular dyshomoeostasis of all the internal organelles (mitochondria, ER, lysosomes, and nuclei), causing them not only to lose function but also to release further toxic factors. A second question is whether it is possible to define the cell death routines that indicate possible points of interventions. In this process, it may be useful to consider the concept of death commitment point, the point beyond which an intervention will not improve the prospect of survival. In apoptosis, it is clear that blocking caspases downstream of mitochondrial permeability transition is unlikely to rescue the cells unless mitochondria can be repaired, for example, by refilling them with cytochrome c [80]. However, preventing a dysfunctional neuron from dying without addressing the toxic cause may not rescue the dysfunction in the disease.
Summary
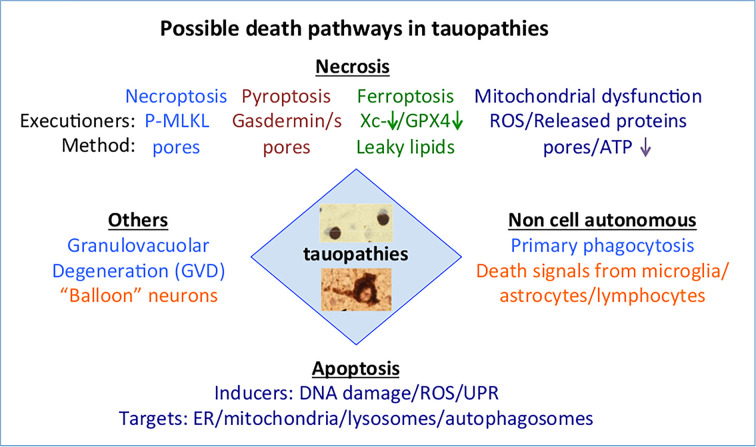
A scheme showing how tauopathies relate to the various forms of death under discussion is shown in Figure 2.
Figure 2. Scheme outlining the possible relationship between tauopathies and the various forms of death described in this review.
Different forms of tau aggregates/fibrils are found in different tauopathies but whether these are associated with specific neuronal cell death mechanisms remains to be determined. Two forms are illustrated in the central diamond-labelled tauopathies, the upper image shows two Pick bodies, while the lower image shows a neuronal tau tangle from an AD brain. Staining is with the anti-phospho-Ser202/205 antibody AT8 followed by immunohistochemistry with diaminobenzidine (DAB). The different colours under the various headings indicate separate mechanisms. Abbreviations: GPX4, glutathione peroxidase 4; ER, endoplasmic reticulum; UPR, unfolded protein response (induced by ER stress); Xc−, the glutamate/cystine transporter encoded by the SLC7A11 gene.
Neuronal cell death in tauopathies is not a simple linear process via one exclusive mechanism.
Some forms of neuronal death involve other types of cells besides neurons.
Identifying toxic species of tau will help unravel the downstream events that lead to death.
Rescue of neurons requires identifying the most upstream step that defines death commitment point; this step can then be targeted for treatment.
Acknowledgements
We thank Dr Ellie Tedford and Matthew Mason in our lab for critically reviewing the manuscript, and the other members of our lab for their perceptive comments when discussing cell death mechanisms during group meetings.
Abbreviations
- AD
Alzheimer's disease
- ALS
Amyotrophic Lateral Sclerosis
- CNS
central nervous system
- Cryo-EM
cryogenic electron microscopy
- ER
endoplasmic reticulum
- GvB
granulovacuolar body
- PSP
progressive supranuclear palsy
- PtdSer
phosphatidylserine
- tau
tubule-associated unit
- wt
wildtype
Contributor Information
Aviva M. Tolkovsky, Email: amt1004@cam.ac.uk.
Maria Grazia Spillantini, Email: mgs11@cam.ac.uk.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Alzheimer's Society [grant number ARUK-EXT2015B-2]; the Alzheimer's Research U.K. [grant numberAS-PG-17-026]; the European Union (EU/EFPIA/Innovative Medicines Initiative 2, Joint Undertaking n116060, IMPRIND) [grant number n116060]; and the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre [grant number BRC-1215-20014].
Open Access
Open access for this article was enabled by the participation of University of Cambridge in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contribution
A.M.T. and M.G.S. conceived and wrote the article.
References
- 1.Kerr J.F., Wyllie A.H. and Currie A.R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerr J.F. (2002) History of the events leading to the formulation of the apoptosis concept. Toxicology 181–182, 471–474 10.1016/S0300-483X(02)00457-2 [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P.et al. (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fricker M., Tolkovsky A.M., Borutaite V., Coleman M. and Brown G.C. (2018) Neuronal cell death. Physiol. Rev. 98, 813–880 10.1152/physrev.00011.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moujalled D., Strasser A. and Liddell J.R. (2021) Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 28, 2029–2044 10.1038/s41418-021-00814-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart S.E., Kondos S.C., Matthews A.Y., D’Angelo M.E., Dunstone M.A., Whisstock J.C.et al. (2014) The perforin pore facilitates the delivery of cationic cargos. J. Biol. Chem. 289, 9172–9181 10.1074/jbc.M113.544890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt R., Trushina N.I. and Bakota L. (2020) Much more than a cytoskeletal protein: physiological and pathological functions of the non-microtubule binding region of tau. Front. Neurol. 11, 590059 10.3389/fneur.2020.590059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alquezar C., Arya S. and Kao A.W. (2020) Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front. Neurol. 11, 595532 10.3389/fneur.2020.595532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesseling H., Mair W., Kumar M., Schlaffner C.N., Tang S., Beerepoot P.et al. (2020) Tau PTM profiles identify patient heterogeneity and stages of Alzheimer's disease. Cell 183, 1699.e13–713.e13 10.1016/j.cell.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L., Wells E.A. and Robinson A.S. (2021) Critical molecular and cellular contributors to tau pathology. Biomedicines 9, 10.3390/biomedicines9020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spillantini M.G. and Goedert M. (2013) Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622 10.1016/S1474-4422(13)70090-5 [DOI] [PubMed] [Google Scholar]
- 12.Braak H. and Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82, 239–259 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 13.Scheres S.H., Zhang W., Falcon B. and Goedert M. (2020) Cryo-EM structures of tau filaments. Curr. Opin. Struct. Biol. 64, 17–25 10.1016/j.sbi.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 14.Goedert M. (2020) Tau proteinopathies and the prion concept. Prog. Mol. Biol. Transl. Sci. 175, 239–259 10.1016/bs.pmbts.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 15.Vogel J.W., Iturria-Medina Y., Strandberg O.T., Smith R., Levitis E., Evans A.C.et al. (2020) Spread of pathological tau proteins through communicating neurons in human Alzheimer's disease. Nat. Commun. 11, 2612 10.1038/s41467-020-15701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H. and Del Tredici K. (2018) Spreading of tau pathology in sporadic Alzheimer's disease along cortico-cortical top-down connections. Cereb. Cortex 28, 3372–3384 10.1093/cercor/bhy152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Zhang W., Yang Y., Murzin A.G., Falcon B., Kotecha A.et al. (2021) Structure-based classification of tauopathies. Nature 598, 359–363 10.1038/s41586-021-03911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Falcon B., Murzin A.G., Fan J., Crowther R.A., Goedert M.et al. (2019) Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer's and Pick's diseases. eLife 8, e43584, 10.7554/eLife.43584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders D.W., Kaufman S.K., DeVos S.L., Sharma A.M., Mirbaha H., Li A.et al. (2014) Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 10.1016/j.neuron.2014.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen B., Ingram E., Takao M., Smith M.J., Jakes R., Virdee K.et al. (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22, 9340–9351 10.1523/JNEUROSCI.22-21-09340.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald J.A., Bronner I.F., Drynan L., Fan J., Curry A., Fraser G.et al. (2019) Assembly of transgenic human P301S Tau is necessary for neurodegeneration in murine spinal cord. Acta Neuropathol. Commun. 7, 44 10.1186/s40478-019-0695-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lasagna-Reeves C.A., Castillo-Carranza D.L., Jackson G.R. and Kayed R. (2011) Tau oligomers as potential targets for immunotherapy for Alzheimer's disease and tauopathies. Curr. Alzheimer Res. 8, 659–665 10.2174/156720511796717177 [DOI] [PubMed] [Google Scholar]
- 23.Kundel F., Hong L., Falcon B., McEwan W.A., Michaels T.C.T., Meisl G.et al. (2018) Measurement of tau filament fragmentation provides insights into prion-like spreading. ACS Chem. Neurosci. 9, 1276–1282 10.1021/acschemneuro.8b00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bengoa-Vergniory N., Velentza-Almpani E., Silva A.M., Scott C., Vargas-Caballero M., Sastre M.et al. (2021) Tau-proximity ligation assay reveals extensive previously undetected pathology prior to neurofibrillary tangles in preclinical Alzheimer's disease. Acta Neuropathol. Commun. 9, 18 10.1186/s40478-020-01117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinosa-Oliva A.M., García-Revilla J., Alonso-Bellido I.M. and Burguillos M.A. (2019) Brainiac caspases: beyond the wall of apoptosis. Front. Cell. Neurosci. 13, 500 10.3389/fncel.2019.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokolova D., Childs T. and Hong S. (2021) Insight into the role of phosphatidylserine in complement-mediated synapse loss in Alzheimer's disease. Faculty Rev. 10, 19 10.12703/r/10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nonaka S. and Nakanishi H. (2019) Microglial clearance of focal apoptotic synapses. Neurosci. Lett. 707, 134317 10.1016/j.neulet.2019.134317 [DOI] [PubMed] [Google Scholar]
- 28.Dejanovic B., Huntley M.A., De Mazière A., Meilandt W.J., Wu T., Srinivasan K.et al. (2018) Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron 100, 1322.e7–1336.e7 10.1016/j.neuron.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 29.de Calignon A., Fox L.M., Pitstick R., Carlson G.A., Bacskai B.J., Spires-Jones T.L.et al. (2010) Caspase activation precedes and leads to tangles. Nature 464, 1201–1204 10.1038/nature08890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delobel P., Lavenir I., Fraser G., Ingram E., Holzer M., Ghetti B.et al. (2008) Analysis of tau phosphorylation and truncation in a mouse model of human tauopathy. Am. J. Pathol. 172, 123–131 10.2353/ajpath.2008.070627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandhu P., Naeem M.M., Lu C., Kumarathasan P., Gomes J. and Basak A. (2017) Ser (422) phosphorylation blocks human Tau cleavage by caspase-3: biochemical implications to Alzheimer's disease. Bioorg. Med. Chem. Lett. 27, 642–652 10.1016/j.bmcl.2016.11.087 [DOI] [PubMed] [Google Scholar]
- 32.Shen H., Han C., Yang Y., Guo L., Sheng Y., Wang J.et al. (2021) Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer's disease. Brain Behav. 11, e02063 10.1002/brb3.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salech F., Ponce D.P., Paula-Lima A.C., SanMartin C.D. and Behrens M.I. (2020) Nicotinamide, a poly [ADP-ribose] polymerase 1 (PARP-1) inhibitor, as an adjunctive therapy for the treatment of Alzheimer's Disease. Front. Aging Neurosci. 12, 255 10.3389/fnagi.2020.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuo Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L.et al. (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 22, 1520–1530 10.1038/mp.2017.171 [DOI] [PubMed] [Google Scholar]
- 35.Ayton S., Portbury S., Kalinowski P., Agarwal P., Diouf I., Schneider J.A.et al. (2021) Regional brain iron associated with deterioration in Alzheimer's disease: A large cohort study and theoretical significance. Alzheimers Dement. 17, 1244–1256 10.1002/alz.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caccamo A., Branca C., Piras I.S., Ferreira E., Huentelman M.J., Liang W.S.et al. (2017) Necroptosis activation in Alzheimer's disease. Nat. Neurosci. 20, 1236–1246 10.1038/nn.4608 [DOI] [PubMed] [Google Scholar]
- 37.Yuan J., Amin P. and Ofengeim D. (2019) Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 10.1038/s41583-018-0093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koper M.J., Van Schoor E., Ospitalieri S., Vandenberghe R., Vandenbulcke M., von Arnim C.A.F.et al. (2020) Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer's disease. Acta Neuropathol. (Berl.) 139, 463–484 10.1007/s00401-019-02103-y [DOI] [PubMed] [Google Scholar]
- 39.Bondareff W., Wischik C.M., Novak M. and Roth M. (1991) Sequestration of tau by granulovacuolar degeneration in Alzheimer's disease. Am. J. Pathol. 139, 641–647 [PMC free article] [PubMed] [Google Scholar]
- 40.Yang S.H., Lee D.K., Shin J., Lee S., Baek S., Kim J.et al. (2017) Nec-1 alleviates cognitive impairment with reduction of Aβ and tau abnormalities in APP/PS1 mice. EMBO Mol. Med. 9, 61–77 10.15252/emmm.201606566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spillantini M.G., Goedert M., Crowther R.A., Murrell J.R., Farlow M.R. and Ghetti B. (1997) Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc. Natl. Acad. Sci. U.S.A. 94, 4113–4118 10.1073/pnas.94.8.4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puladi B., Dinekov M., Arzberger T., Taubert M. and Köhler C. (2021) The relation between tau pathology and granulovacuolar degeneration of neurons. Neurobiol. Dis. 147, 105138 10.1016/j.nbd.2020.105138 [DOI] [PubMed] [Google Scholar]
- 43.Köhler C. (2016) Granulovacuolar degeneration: a neurodegenerative change that accompanies tau pathology. Acta Neuropathol. (Berl.) 132, 339–359 10.1007/s00401-016-1562-0 [DOI] [PubMed] [Google Scholar]
- 44.Funk K.E., Mrak R.E. and Kuret J. (2011) Granulovacuolar degeneration (GVD) bodies of Alzheimer's disease (AD) resemble late-stage autophagic organelles. Neuropathol. Appl. Neurobiol. 37, 295–306 10.1111/j.1365-2990.2010.01135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiersma V.I., Hoozemans J.J.M. and Scheper W. (2020) Untangling the origin and function of granulovacuolar degeneration bodies in neurodegenerative proteinopathies. Acta Neuropathol. Commun. 8, 153 10.1186/s40478-020-00996-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiersma V.I. and Scheper W. (2020) Granulovacuolar degeneration bodies: red alert for neurons with MAPT/tau pathology. Autophagy 16, 173–175 10.1080/15548627.2019.1680217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piras A., Collin L., Grüninger F., Graff C. and Rönnbäck A. (2016) Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. Commun. 4, 22 10.1186/s40478-016-0292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malik B.R., Maddison D.C., Smith G.A. and Peters O.M. (2019) Autophagic and endo-lysosomal dysfunction in neurodegenerative disease. Mol. Brain 12, 100 10.1186/s13041-019-0504-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshii S.R. and Mizushima N. (2017) Monitoring and measuring autophagy. Int. J. Mol. Sci. 18, 1825 10.3390/ijms18091865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brelstaff J., Tolkovsky A.M., Ghetti B., Goedert M. and Spillantini M.G. (2018) Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 24, 1939.e4–1948.e4 10.1016/j.celrep.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edison P., Donat C.K. and Sastre M. (2018) In vivo imaging of glial activation in Alzheimer's disease. Front. Neurol. 9, 625 10.3389/fneur.2018.00625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Podleśny-Drabiniok A., Marcora E. and Goate A.M. (2020) Microglial phagocytosis: a disease-associated process emerging from Alzheimer's disease genetics. Trends Neurosci. 43, 965–979 10.1016/j.tins.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Harten A.C.M., Phatnani H. and Przedborski S. (2021) Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 44, 658–668 10.1016/j.tins.2021.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown G.C. and Vilalta A. (2015) How microglia kill neurons. Brain Res. 1628, 288–297 10.1016/j.brainres.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 55.Fricker M., Vilalta A., Tolkovsky A.M. and Brown G.C. (2013) Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J. Biol. Chem. 288, 9145–9152 10.1074/jbc.M112.427880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopp S.C., Lin Y., Oakley D., Roe A.D., DeVos S.L., Hanlon D.et al. (2018) The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer's disease. J. Neuroinflammation 15, 269 10.1186/s12974-018-1309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brelstaff J.H., Mason M., Katsinelos T., McEwan W.A., Ghetti B., Tolkovsky A.M.et al. (2021) Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci. Adv. 7, eabg4980 10.1126/sciadv.abg4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Streit W.J., Braak H., Xue Q.S. and Bechmann I. (2009) Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. (Berl.) 118, 475–485 10.1007/s00401-009-0556-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bussian T.J., Aziz A., Meyer C.F., Swenson B.L., van Deursen J.M. and Baker D.J. (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Musi N., Valentine J.M., Sickora K.R., Baeuerle E., Thompson C.S., Shen Q.et al. (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17, e12840 10.1111/acel.12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leyns C.E.G., Ulrich J.D., Finn M.B., Stewart F.R., Koscal L.J., Remolina Serrano J.et al. (2017) TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. U.S.A. 114, 11524–11529 10.1073/pnas.1710311114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litvinchuk A., Huynh T.V., Shi Y., Jackson R.J., Finn M.B., Manis M.et al. (2021) Apolipoprotein E4 reduction with antisense oligonucleotides decreases neurodegeneration in a tauopathy model. Ann. Neurol. 89, 952–966 10.1002/ana.26043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Y., Andhey P.S., Ising C., Wang K., Snipes L.L., Boyer K.et al. (2021) Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron 109, 2413.e7–2426.e7 10.1016/j.neuron.2021.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W.et al. (2017) ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bi F., Huang C., Tong J., Qiu G., Huang B., Wu Q.et al. (2013) Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl. Acad. Sci. U.S.A. 110, 4069–4074 10.1073/pnas.1218497110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L.et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reid M.J., Beltran-Lobo P., Johnson L., Perez-Nievas B.G. and Noble W. (2020) Astrocytes in tauopathies. Front. Neurol. 11, 572850 10.3389/fneur.2020.572850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang T., Perera N.D., Chiam M.D.F., Cuic B., Wanniarachchillage N., Tomas D.et al. (2020) Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ. 27, 1728–1739 10.1038/s41418-019-0457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hampton D.W., Webber D.J., Bilican B., Goedert M., Spillantini M.G. and Chandran S. (2010) Cell-mediated neuroprotection in a mouse model of human tauopathy. J. Neurosci. 30, 9973–9983 10.1523/JNEUROSCI.0834-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sidoryk-Wegrzynowicz M., Gerber Y.N., Ries M., Sastre M., Tolkovsky A.M. and Spillantini M.G. (2017) Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta Neuropathol. Commun. 5, 89 10.1186/s40478-017-0478-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasel P., Dando O., Jiwaji Z., Baxter P., Todd A.C., Heron S.et al. (2017) Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun. 8, 15132 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leng K., Li E., Eser R., Piergies A., Sit R., Tan M.et al. (2021) Molecular characterization of selectively vulnerable neurons in Alzheimer's disease. Nat. Neurosci. 24, 276–287 10.1038/s41593-020-00764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hampton D.W., Amor S., Story D., Torvell M., Bsibsi M., van Noort J.M.et al. (2020) HspB5 activates a neuroprotective glial cell response in experimental tauopathy. Front. Neurosci. 14, 574 10.3389/fnins.2020.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P.et al. (2021) Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595, 565–571 10.1038/s41586-021-03710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zalocusky K.A., Najm R., Taubes A.L., Hao Y., Yoon S.Y., Koutsodendris N.et al. (2021) Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer's disease. Nat. Neurosci. 24, 786–798 10.1038/s41593-021-00851-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Togo T. and Dickson D.W. (2002) Ballooned neurons in progressive supranuclear palsy are usually due to concurrent argyrophilic grain disease. Acta Neuropathol. (Berl.) 104, 53–56 10.1007/s00401-002-0520-1 [DOI] [PubMed] [Google Scholar]
- 77.Armstrong R.A., Lantos P.L. and Cairns N.J. (2000) Laminar distribution of ballooned neurons and tau positive neurons with inclusions in patients with corticobasal degeneration. Neurosci. Res. Commun. 27, 85–93 [DOI] [Google Scholar]
- 78.Mori H., Oda M. and Mizuno Y. (1996) Cortical ballooned neurons in progressive supranuclear palsy. Neurosci. Lett. 209, 109–112 10.1016/0304-3940(96)12612-4 [DOI] [PubMed] [Google Scholar]
- 79.Sakurai A., Okamoto K., Fujita Y., Nakazato Y., Wakabayashi K., Takahashi H.et al. (2000) Fragmentation of the Golgi apparatus of the ballooned neurons in patients with corticobasal degeneration and Creutzfeldt-Jakob disease. Acta Neuropathol. (Berl.) 100, 270–274 10.1007/s004010000182 [DOI] [PubMed] [Google Scholar]
- 80.Fletcher G.C., Xue L., Passingham S.K. and Tolkovsky A.M. (2000) Death commitment point is advanced by axotomy in sympathetic neurons. J. Cell Biol. 150, 741–754 10.1083/jcb.150.4.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pemberton J.M., Pogmore J.P. and Andrews D.W. (2021) Neuronal cell life, death, and axonal degeneration as regulated by the BCL-2 family proteins. Cell Death Differ. 28, 108–122 10.1038/s41418-020-00654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang W.S. and Stockwell B.R. (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176 10.1016/j.tcb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J.et al. (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian R., Abarientos A., Hong J., Hashemi S.H., Yan R., Dräger N.et al. (2021) Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 24, 1020–1034 10.1038/s41593-021-00862-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masaldan S., Clatworthy S.A.S., Gamell C., Meggyesy P.M., Rigopoulos A.T., Haupt S.et al. (2018) Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 14, 100–115 10.1016/j.redox.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Voet S., Srinivasan S., Lamkanfi M. and van Loo G. (2019) Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 11, e10248 10.15252/emmm.201810248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi J., Gao W. and Shao F. (2017) Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 88.Hu J.J., Liu X., Xia S., Zhang Z., Zhang Y., Zhao J.et al. (2020) FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 10.1038/s41590-020-0669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia S., Hollingsworth L.R. and Wu H. (2020) Mechanism and regulation of Gasdermin-mediated cell death. Cold Spring Harb. Perspect. Biol. 12, a036400, 10.1101/cshperspect.a036400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Zeller M., Dias D., Sebastião A.M. and Valente C.A. (2021) NLRP3 inflammasome: a starring role in amyloid-β- and tau-driven pathological events in Alzheimer's disease. J. Alzheimers Dis. 83, 939–961 10.3233/JAD-210268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iannielli A., Bido S., Folladori L., Segnali A., Cancellieri C., Maresca A.et al. (2018) Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in Parkinson's disease models. Cell Rep. 22, 2066–2079 10.1016/j.celrep.2018.01.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ofengeim D., Mazzitelli S., Ito Y., DeWitt J.P., Mifflin L., Zou C.et al. (2017) RIPK1 mediates a disease-associated microglial response in Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 114, E8788–E8797 10.1073/pnas.1714175114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen X., He W.T., Hu L., Li J., Fang Y., Wang X.et al. (2016) Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 10.1038/cr.2016.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X. and Ge P. (2020) Parthanatos in the pathogenesis of nervous system diseases. Neuroscience 449, 241–250 10.1016/j.neuroscience.2020.09.049 [DOI] [PubMed] [Google Scholar]
- 95.Kam T.I., Mao X., Park H., Chou S.C., Karuppagounder S.S., Umanah G.E.et al. (2018) Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson's disease. Science 362, eaat8407 10.1126/science.aat8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y. and Levine B. (2015) Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 22, 367–376 10.1038/cdd.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernández Á F., Liu Y., Ginet V., Shi M., Nah J., Zou Z.et al. (2020) Interaction between the autophagy protein Beclin 1 and Na+,K+-ATPase during starvation, exercise, and ischemia. JCI Insight 5, e133282 10.1172/jci.insight.133282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown G.C. and Neher J.J. (2014) Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 15, 209–216 10.1038/nrn3710 [DOI] [PubMed] [Google Scholar]
- 99.Butler C.A., Popescu A.S., Kitchener E.J.A., Allendorf D.H., Puigdellívol M. and Brown G.C. (2021) Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 158, 621–639 10.1111/jnc.15327 [DOI] [PubMed] [Google Scholar]
- 100.Haque A., Samantaray S., Knaryan V.H., Capone M., Hossain A., Matzelle D.et al. (2020) Calpain mediated expansion of CD4+ cytotoxic T cells in rodent models of Parkinson's disease. Exp. Neurol. 330, 113315 10.1016/j.expneurol.2020.113315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voskoboinik I., Whisstock J.C. and Trapani J.A. (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 15, 388–400 10.1038/nri3839 [DOI] [PubMed] [Google Scholar]
- 102.Brelstaff J., Spillantini M.G. and Tolkovsky A.M. (2015) pFTAA: a high affinity oligothiophene probe that detects filamentous tau in vivo and in cultured neurons. Neural Regen. Res. 10, 1746–1747 10.4103/1673-5374.165298 [DOI] [PMC free article] [PubMed] [Google Scholar]