Abstract
Background
Currently, only dexamethasone, tocilizumab, and sarilumab have conclusively been shown to reduce mortality of coronavirus disease 2019 (COVID-19). Safe and effective treatments will need to be both affordable and widely available globally to be used alongside vaccination programs. This analysis will estimate and compare potential generic minimum costs of a selection of approved COVID-19 drug candidates with available international list prices.
Methods
We searched for repurposed drugs that have been approved by at least one of the World Health Organization, US Food and Drug Administration, or the United Kingdom National Institute of Health and Care Excellence organizations or at least given emergency use authorization or recommended for off-label prescription. Drug prices were searched for dexamethasone, budesonide, baricitinib, tocilizumab, casirivimab, and imdevimab, and sarilumab, using active pharmaceutical ingredients (APIs) data extracted from global shipping records. This was compared with national pricing data from a range of low-, medium-, and high-income countries. Annual API export volumes from India were used to estimate the current availability of each drug.
Results
Repurposed therapies can be generically manufactured for some treatments at very low per-course costs, ranging from US $2.58 for intravenous (IV) dexamethasone (or US $0.19 orally) and US $4.34 for inhaled budesonide. No export price data were available for baricitinib, tocilizumab, casirivimab, and imdevimab, or sarilumab, but courses of these treatments have higher prices, ranging from US $6.67 for baricitinib to US $875.5 for sarilumab. When comparing international list prices, we found wide variations between countries.
Conclusions
Successful management of COVID-19 will require equitable access to treatment for all populations, not just those able to pay high prices. Dexamethasone and budesonide are widely available and affordable, whereas monoclonal antibodies and IV treatment courses are more expensive.
Keywords: access to medicine, COVID-19, drug availability, drug prices, drug repurposing
Repurposed drugs must be affordable worldwide to compliment COVID-19 vaccine programs. Estimated costs/course: dexamethasone (oral $0.22, IV $2.58), budesonide ($4.34), baricitinib ($6.67), tocilizumab ($410.59), sarilumab ($875.70). Data are not available for casirivimab and imdevimab. High drug prices will limit access.
Eighteen months from the initial coronavirus disease 2019 (COVID-19) outbreak, drug repurposing with intention to treat and prevent infections is still an important strategy alongside vaccine rollout. The uneven global vaccine deployment, “vaccine nationalism” [1], difficulties caused by vaccine hesitancy and emerging new variants of concern all mean that an excessively vaccine-dependent strategy may not be fully effective. Even in countries with high vaccine coverage, there are still symptomatic patients who may benefit from treatment options. For many developing countries, the mass vaccination required to reduce the significant healthcare burden of COVID-19 may remain unattainable for several years.
Treatments, when they emerge from the vast number of clinical trials currently underway, will need to overcome the same barriers of efficacy, safety, affordability, and availability as vaccines. In our 2020 analysis of potential costs of generic production for drug candidates under active investigation at the time [2], we proposed 4 key tests: (1) Efficacy - drugs should demonstrate therapeutic clinical efficacy and/or prophylactic properties; (2) Safety - drugs should be safe and well tolerated in all populations with minimal side effects or drug interactions; (3) Affordability -drugs should be widely accessible as low-cost generics so that all populations can equitably afford the cost of treatment; (3) Availability - drugs must be readily available or have the potential for rapid up-scaled production to meet global demand.
Some treatments have passed the first and second hurdle; dexamethasone, remdesivir, tocilizumab, and sarilumab are World Health Organization (WHO) approved, whereas the US Food and Drug Administration (FDA) has also granted an emergency use authorization (EUA) for baricitinib, and the United Kingdom National Institute of Health and Care Excellence (NICE) has given advise for off-label prescriptions of inhaled budesonide in certain patients with COVID-19. However, many previously investigated drugs have now been proven ineffective. Large-scale studies such as SOLIDARITY and RECOVERY have found previously leading candidates such as hydroxychloroquine [3], lopinavir/ritonavir [4, 5], subcutaneous interferon beta-1a [4], and azithromycin [6] to have no clinical benefit. In addition, no evidence of efficacy was found for colchicine in the RECOVERY trial [7] despite some preliminary evidence of efficacy from the published COLCORONA trial [8]. Furthermore, despite studies with fraudulent data attempting to portray ivermectin as a promising treatment, meta-analysis has demonstrated no clinical benefit for ivermectin [9, 10]. Despite FDA approval, remdesivir use has led to shorter hospitalization times, but with no significant mortality benefits [4].
Baricitinib is a Janus kinase (JAK) inhibitor being tested in 14 studies among 56 168 patients and is mostly being given together with remdesivir [11]. It has been granted an EUA by the FDA [12]. More recently, promising press reports have claimed a 50% reduction in the risk of hospitalization or death (from 14% to 7%) for the repurposed antiviral therapy molnupiravir, but full peer reviewed data are not yet available [13], and neither the FDA, NICE, nor WHO have given approval yet (as of October 10, 2021). Furthermore, the antibody cocktail casirivimab and imdevimab has been shown to reduce COVID-19-related hospitalization or all-cause death in small, randomized studies for seronegative patients [14, 15] and has received an EUA from the FDA and recent approval by NICE for seronegative patients [16].
However, questions of affordability and availability should be just as important as efficacy and safety. Treatments are of limited value if their supply is limited and the cost prohibitive, as in the case of tocilizumab and sarilumab. These monoclonal antibody interleukin-6 (IL-6) receptor inhibitors were found to improve outcomes in patients with severe COVID-19 [17], but they cost several hundred dollars per dose and require complex bioreactors to produce. Dexamethasone, the first treatment found to increase survival and is widely available as a low-cost generic, is only applicable for patients requiring oxygen supplementation [18]. An effective antiviral agent for asymptomatic, mild, or moderate patients that limits progression and treats COVID-19 is yet to be approved.
We analyzed their costs of production based on cost of active pharmaceutical ingredients (APIs) for drugs that have been approved by regulatory bodies, and we offer a snapshot of global API availability and current list prices in a range of countries where data were available. Given the limited transparency on drug costs and prices, this study aimed to contribute to the current evidence base to support public health decision makers if any of the analyzed drugs proved to be effective against COVID-19.
METHODS
Drug Selection
Clinical trial registries such as clinicaltrials.gov and covid-nma.com were screened alongside current published literature to identify promising repurposed drug candidates. Only those approved by the FDA, WHO, or NICE or at least given emergency use authorization or recommended for off-label use were included in the final analysis. The Panjiva database [19] and national drug price sources were searched for available export data (Appendix 1). Remdesivir was not reanalyzed in this paper because it was assessed in our previous publication [2].
Oral Medications
Methodologies for estimating minimum costs of oral medication production were previously published [2]. Costs of Indian API exports were analyzed (J.W. and J.L.) and independently reviewed (L.E.) using the online tracking database Panjiva, which showed API shipment details including quantities and costs per kilogram. All available API shipment data from Panjiva, between 2016 and June 2021, were extracted and processed. For all oral drugs, we excluded shipments <1 kg in volume and excluded the 15% highest and lowest priced shipments were removed to exclude outliers. For oral drugs, (1) 5% API loss during tableting process and (2) a conversion cost of US $0.01 per tablet were factored into calculations, alongside a multiplier based on API mass to account for the expense of excipients (additional substances needed to convert API into the finished pharmaceutical product). Profit margins of 10% and Indian taxation of 27% on profit were added. Indian production was assumed given the country’s leading role in global generic medicine manufacturing [20].
Intravenous Dexamethasone
For injectables, an adjusted published methodology was followed to account for the cost of ampules, additional transport costs, and projected 20% API loss during formulation and filling [2, 21].
Inhaled Budesonide
In the STOIC and PRINCIPLE studies, patients were asked to use 2 puffs of a 400-mcg inhaler twice daily (800 mcg twice per day [BD]) for 14 or 28 days, with a median duration of 7 days [22, 23]. Cost per kilogram of budesonide dry powder API was estimated using the same methodology described above, before accounting for the cost of inhaler devices (Pulmicort Turbohaler US $3), additional transport costs, and a projected 20% API loss during formulation and filling. The following alternative inhalers are also permitted for use by NICE: Pulmicort Turbohaler 200 mcg, Budelin Novolizer 200 mcg, Easyhaler Budesonide 400 mcg, and Easyhaler Budesonide 200 mcg.
Tocilizumab, Sarilumab, and Baricitinib
No data for the cost or volume of monoclonal antibodies production were available, because this depends heavily on the efficiency and size of bioreactors used [24]. In addition, no API data were available for baricitinib or casirivimab and imdevimab. Therefore, list prices in a range of countries, in particular developing economies, were tracked as a proxy, because it is assumed that the generic medicine could be reasonably produced at this level or less. It is unfortunate that national price data were not available for casirivimab and imdevimab. There are news reports of tocilizumab and sarilumab being sold in Indian black markets at approximately US $658.50 per 600-mg dose [25].
International List Price Comparison
Estimated minimum production costs were compared with published list prices in a range of countries across the economic development spectrum to give a representative sample of prices globally. Data sources by country are detailed in Appendix 2. Data were collected and reviewed independently by J.L. and L.E. and intended to offer a snapshot at time of writing.
For consistency, a single data source per country was used for all searches of drug prices, based on the organization of data and perceived reliability, except for the United States. For the United States, we used the commercial pharmacy (USA, Pharm) cost as well as public costs from the veterans’ affairs system (USA, Vets) listed separately. This is because the United States has a healthcare system with a wide range of insurance options, and the US veterans’ affairs program insures millions of Americans, whereas millions more Americans are uninsured and must pay out of pocket for most medications. Not all drugs analyzed were available in every country, and in some countries without official databases, online pharmacy sites were used instead. In countries where several prices were available in the same database, the lowest reliable price was selected.
Active Pharmaceutical Ingredients Availability
Panjiva data were used to analyze API export volumes per drug from India and estimate the maximal number of treatment courses potentially producible over a 12-month period.
Key Assumptions
For some drugs, such as dexamethasone and tocilizumab, which are dosed on a per kilogram basis, we used an average adult body mass of 70 kg. The costs of regulatory filings and approvals are often significant add-ons to the initial use of drugs in any specific country. All drugs analyzed in this study have been approved for treatment for some indications already. We have not included the cost associated with regulatory approvals for the use of these drugs. We assume that the WHO and other influential regulatory agencies will cooperate to define a pathway for use of these drugs that does not include additional financial outlays or filing for marketing approvals.
RESULTS
The results are summarized in Table 1.
Table 1.
Summary of Repurposed Drug Prices for COVID-19 and Estimated Availabilitya
| Drug, Duration, and Dose | Highest List Price | Lowest List Price | Estimated Production Cost (Course) | FDA Approval | NICE Approval | WHO Approval | Estimated Current Availabilityb |
|---|---|---|---|---|---|---|---|
| Dexamethasone | US $26.47 | US $0.98 | US $2.58 | Yes | Yes | Yes | 7.4 million treatment courses |
| IV 10 days | UK | Vietnam | |||||
|
6 mg OD (60 mg total) | |||||||
| Dexamethasone | US $30.79 | US $0.22 | US $0.19 | Yes | Yes | Yes | |
|
PO 10 days 6 mg OD (60 mg total) |
UK | Peru | |||||
| Baricitinib | US $2326.38 | US $6.67 | --- | No (EUA) [12] | No | No | NA |
|
PO 14 days 4 mg OD (56 mg total) |
USA Pharmacy |
Bangladesh | |||||
| Sarilumab | US $4850.90 | US $875.70 | --- | No | Yes | Yes | NA |
|
IV once only 400 mg single dose |
USA (Veterans) |
France | |||||
| Tocilizumab | US $3625.14 | US $410.59 | --- | No (EUA) [47] | Yes | Yes | NA |
|
IV once only 600 mg single dose |
USA (Pharmacy) | Australia | |||||
| Budesonide | US $45.74 | US $3.49 | US $4.34 per inhaler | No | No (off-label use advised) [48] | No | 80 million inhalers |
| INH (inhaler price) | Norway | India | |||||
| 20 mg device (100×200 mcg/dose) | |||||||
| Casirivimab and imdevimab (Ronapreve) | NA | NA | NA | No (EUA) | No (off-label use advice) [48] | No | NA |
|
IV once only 2400 mg/1200 mg |
Abbreviations: BD, twice per day; COVID-19, coronavirus disease 2019; EUA, emergency use authorization; FDA, US Food and Drug Administration; IV, intravenous; NA, not applicable; NICE, United Kingdom National Institute of Health and Care Excellence; OD, once daily; PO, by mouth; UK, United Kingdom; WHO, World Health Organization.
Summary of list prices, estimated production co
sts, and current availability of potential COVID-19 drugs selected for analysis. Baricitinib only has EUA to be given in combination with remdesivir.
In most recent 12-month period, based on India active pharmaceutical ingredients (API) export volumes only. Current availability may be an underestimation because it is not based on global API availability data.
Dexamethasone
The widely used corticosteroid dexamethasone has an economy of scale that supports low-cost production. Current national guidelines for dexamethasone treatment in COVID-19 most commonly recommend a 7- to 10-day courses of 6 mg oral or intravenous (IV) [26, 27]. Shorter courses are used if patients improve or are discharged early, and sometimes longer courses and higher doses are used in patients with ongoing fibrosis and hyperinflammation confirmed by high-resolution computed tomography imaging.
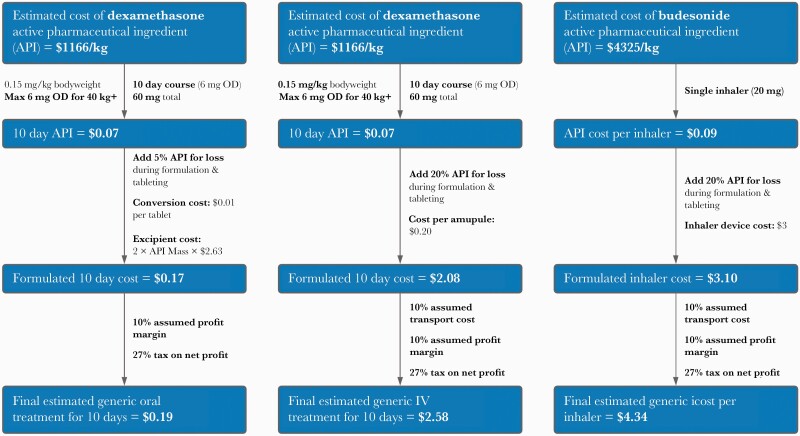
In this analysis, we used a 10-day, 6 mg/day course. The weighted-mean API cost between 2016 and 2020 was estimated to be US $1116/kg; after factoring in the costs of excipients, formulation, tax, and profit, a 10-day IV course could be reasonably producible for approximately US $2.58 and approximately US $0.19 as tablets (Figure 1a and b). The higher cost of injectables reflect the potential for greater loss during production, cost of vials, and increased cost of transport. Furthermore, significant nondrug costs associated with IV administration, including staff time, expendables required such as sterile water/syringes for reconstitution, and saline/IV lines for infusion were not included.
Figure 1.
Flowcharts showing cost estimations for dexamethasone, oral (a) and intravenous (b), and budesonide (c). API, active pharmaceutical ingredients; BD, twice per day; IV, intravenous; Max, maximum; OD, once daily.
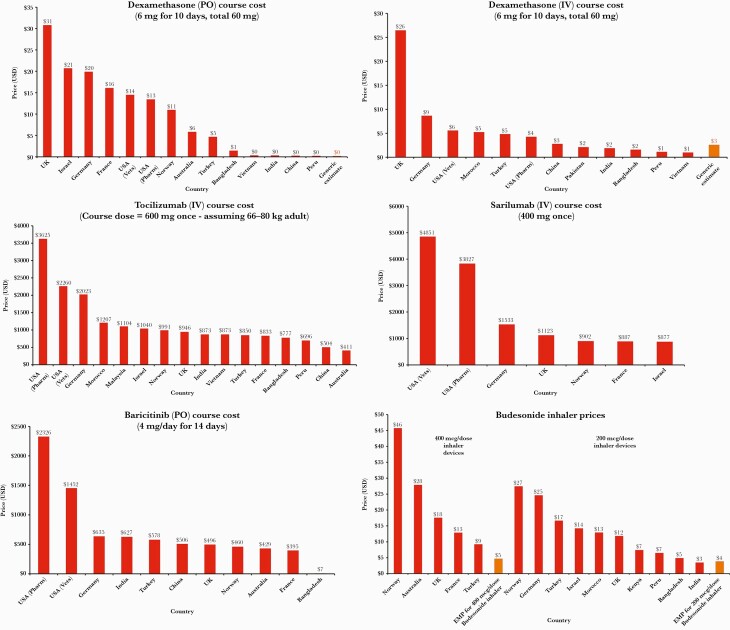
Comparatively, current list prices for oral dexamethasone were found to range from approximately US $30.79 and US $20.69 per course in the UK and Israel, respectively, to only $US 0.26 and US $0.22 in China and Peru. For IV dexamethasone, course costs ranged between US $26.47 (UK) and US $0.98 (Vietnam) (Figure 2a and b). Sufficient API export shipments were identified in the most recent 12-month period to form 7.4 million courses, a substantial decrease on the 4-year average between 2016 and 2019 at a theoretical 34.5 million 10-day courses per annum.
Figure 2.
(a–f) Graphs of national treatment course prices compared with generic estimates. (a) Dexamethasone (by mouth [PO]); (b) dexamethasone (intravenous [IV]); (c) tocilizumab (IV); (d) sarilumab (IV); (e) baricitinib (PO); (f) budesonide. API, active pharmaceutical ingredients; USD, US dollars.
Tocilizumab and Sarilumab
Dosages for both monoclonal antibodies are based on current UK guidelines [28]. Both are given as single-dose IV infusions: sarilumab at 400 mg and tocilizumab at 8 mg/kg (800 mg maximum). In rare circumstances, a second tocilizumab dose can be given 12–24 hours later, depending on response to first dose. This analysis is based a single infusion of sarilumab 400 mg and tocilizumab 600 mg, which are guideline dose-banding suggestions for bodyweights 66–80 kg inclusive. International list prices per single dose of sarilumab ranged between US $4850.90 (US) and US $875.70 (France); however, for tocilizumab, single dose costs ranged between US $3625.14 (US) and US $410.59 (Australia) (Figure 2c and d).
Baricitinib
Baricitinib is an antirheumatic drug, which in clinical trials for COVID-19 [11] is dosed at 4 mg/day for 14 days orally. No API data are currently available. International list prices for a 14-day treatment course were found to range between US $2326 (US) and US $6.67 (Bangladesh) (Figure 2e).
Budesonide
The STOIC study used a protocol of 800 mcg BD up to 28 days, with a median duration of 7 days [22, 23]. Clinically, patients generally receive individual inhaler devices containing 60–200 doses of 400 mcg/dose, 200 mcg/dose, or 100 mcg/dose, which is more than sufficient for the proposed treatment duration.
Therefore, production cost of dry power budesonide inhalers containing 20 mg of API was estimated to be US $4.34 (100 doses of 200-mcg puffs) or US $4.48 if containing 40 mg of API (100 doses of 400-mcg puffs), based on a weighted-mean API cost of US $4325/kg. This was after accounting for the cost of the inhaler device (US $3), a 20% potential API loss during production, transport costs, profit, and tax (Figure 1c). International list prices vary by country depending on the availability of the 200 mcg/dose or 400 mcg/dose inhaler devices and are shown in Figure 2f.
The API export from India in the most recent 12-month period was estimated to be sufficient for 80 million inhalers, whereas the 5-year average was estimated to be approximately 88 million inhaler equivalents, should the entire API production volume be used for inhalers.
DISCUSSION
Repurposed drugs with available API data could all be produced generically at very low cost. Oral tablets such as dexamethasone and baricitinib can potentially all be sold for US $7 to less than US $1 per course, whereas inhaled budesonide could cost US <$4. Intravenous drugs were more expensive, ranging from US $1/course of IV dexamethasone to US $411 for tocilizumab and US $875 for sarilumab. Indeed, list prices in developing economies such as Bangladesh, Kenya, and India are already sometimes less than our estimated costs. The API costs were previously found to be a key determinant of generic medicine prices—in a 2015 study of sofosbuvir/daclatasvir, an antiviral combination for hepatitis C, API costs accounted for 65%–95% of final drug prices in competitive markets [29]. No data were available for casirivimab and imdevimab, but we anticipate this neutralizing monoclonal antibody cocktail is likely to have a high price when it becomes available.
Our analysis of list prices, although it is only a snapshot, demonstrated large variations between countries, especially for monoclonal antibodies, and it again highlights the importance of international global health coordination to ensure accessibility. Public-private partnerships such as the Global Fund showed that it is possible to offer drug access at near-cost price through voluntary mechanisms. Strategies such as pooled procurement, differential pricing, and voluntary licenses should be utilized more widely, because over time these have become more exclusionary and available only to a shrinking list of low- and middle-income countries (LMICs). Studies of differential or tiered pricing have shown that they can result in higher prices for middle-income countries due to arbitrary market segmentation [30].
Other tools pertinent to expanding access to medicines in the current pandemic includes the use of Trade-Related Aspects of Intellectual Property Rights (TRIPS) flexibilities by national governments and civil society, particularly of compulsory licensing and patent oppositions. On multiple occasions, this proved to be effective in addressing high prices on medicines protected by intellectual property (IP) monopolies and triggering radical price reductions [31]. Given the pandemic situation, the so-called TRIPS waiver proposal at the World Trade Organization would be a crucial initiative for removing IP barriers to access generic and/or biosimilar medicines based on the cost of manufacturing [32].
In terms of availability, dexamethasone, ivermectin, budesonide, and fluvoxamine and are already available as generic medicines with a large volume of API being produced already. If promising results are demonstrated for any of these drugs in large studies, they could become an option earlier on in the disease process and production must be rapidly upscaled to meet demand. Regulators and decision makers should plan for this possibility and achieve accelerated deployment, especially given that the safety profiles of these drugs are already established. An important aspect will be to protect supplies for existing patients treated for indicated conditions, for example, parasitic infections and rheumatoid arthritis.
Neither steroids, monoclonal antibodies, nor IL-6 receptor antagonists will be a silver bullet against COVID-19. Dexamethasone has shown a small improvement in survival for treatment of oxygen-dependent, hospitalized patients (18%), with a larger benefit seen for the treatment of mechanically ventilated patients (36%) [18]. A recent meta-analysis found a small, improved survival benefit for tocilizumab in the treatment of hospitalized patients in high-income countries [33]. Conclusive benefits for other outcomes were inconclusive. It is unclear whether the same survival benefit would be seen in LMIC settings where infection control can be more challenging in immunocompromised patients. It is also unclear whether sufficient supplies of tocilizumab or sarilumab could be manufactured to meet worldwide demand.
A key barrier to regulatory decision making on repurposed drugs has been concerns regarding quality of evidence, highlighted by the latest WHO guidelines on ivermectin [34]. A recent meta-analysis of 23 randomised ivermectin clinical trials included far fewer within its main analysis due to high risks of bias associated with some included studies [9]. Funders and regulators should take a greater coproductive role with researchers to ensure clinical trials are well designed, adequately powered, and acceptable to the regulatory approval process when they are completed.
Overpriced treatments may harm already stretched healthcare systems. As is often the case, prevention is generally more cost effective that treatment, so excessive treatment costs may take up resources which can be better spent on other interventions. Vaccines remain very effective and relatively cheap per person. Despite this, the United States has spent more than US $1 billion preordering approximately 1.7 million courses of molnupiravir, which costs an estimated US $1200 per course [35]. Furthermore, the price of a 5-day remdesivir course in the United States is approximately US $2335.50, despite offering no mortality reduction and not currently being recommended by the WHO.
Limitations
This study presumed Indian generic production [20] with lower associated costs and no capital investment in new, dedicated facilities; however, after supply chain disruptions as seen earlier on in the pandemic, and currently with vaccines, countries may accept additional upfront costs and overheads for greater supply security in establishing domestic production. Fluctuating exchange rates and confidential in-country negotiated discounts for public healthcare providers means the prices we report are subject to change and are a snapshot of publicly available information at the time of writing [36]. The repurposed drugs landscape is also a rapidly evolving pipeline, and many of the drugs we initially analyzed were subsequently shown to be ineffective. Drug prices can evolve quite erratically as supply and demand, media hype, and drug approval processes exert significant pressure on pharmaceutical markets and supply chains.
The API production costs are subject to changes in supply and demand and optimization of manufacturing processes. Furthermore, analysis was dependent on Indian custom records from Panjiva. Data in other countries were unavailable so the real volume of global API production may be higher. Finally, regulatory approval costs were not considered, assuming that regulatory agencies such as WHO and FDA would coordinate rapid, emergency deployment of drugs without significant burden [37–39].
Our estimates do not include research and development costs. However, most of the investment for drug repurposing in COVID-19 has originated from public or philanthropic sources, with significant amounts of research funded directly by governments, universities, public institutions, and nongovernmental organizations. A recent estimate suggested that governments have spent almost US $100 billion in the last year solely on vaccine research [40].
Aspects for Further Research
As more information becomes available, it is possible that other promising drugs such as imatinib, infliximab, or molnupiravir may be approved by the WHO if initial clinical results from preprints are confirmed in larger peer-reviewed randomized, controlled trials. A recently published study (October 2021) has found that the 5-day course of the antiviral molnupiravir taken at 800 mg BD could profitably be sold for less than US $20 per person when calculated using Panjiva API export data [41], and using raw ingredients and a synthetic process with solvent capture this could drop to below US $5 per course [42]. When we ran our own preliminary analysis using API data available in June 2021 and calculated molnupiravir\'s estimated cost of production, we found a potential cost that was more than 10× higher than the later October study. This illustrates just how quickly small molecule nucleoside analogs can drop in price when production is upscaled. It is imperative that new and repurposed drugs are made available at fair, near-cost of production prices, to improve access and availability for all. When data are available for casirivimab and imdevimab, we plan to analyze their prices.
There are good global accessibility and availability for steroids and budesonide. High prices of JAK inhibitors and monoclonal antibodies are barriers for patient access in healthcare systems requiring out-of-pocket copayments or LMIC settings. The entry of biosimilars can lead to significant price reductions, even if not on the scale of chemical medications [43, 44]. One reason for higher biosimilar costs could be the overly cumbersome regulatory pathway [45] that many have argued for reform. If greater emphasis is placed on encouraging biosimilar entry, prices for tocilizumab and sarilumab could be much lower and allow for greater availability.
Historically, combination therapy proved most effective against human immunodeficiency virus/acquired immune deficiency syndrome, hepatitis C, and tuberculosis, and a similar approach might be needed for COVID-19 [46]. Combinations such as antivirals given concurrently with anti-inflammatory drugs or at different disease stages may prove more effective than monotherapy and warrants further investigation. Generic combination therapies can occasionally cost less than the sum of their parts due to increased efficiency in formulation, tableting, and packaging.
CONCLUSIONS
More conclusive data on clinical efficacy are required for most repurposed drugs currently being used in clinical trials for COVID-19 treatment. This analysis shows that once efficacy is demonstrated, oral repurposed drugs can be manufactured at very low costs, whereas IV treatments are a little more costly, and the monoclonal antibody treatments are expensive. In the short term, until vaccination access is more widely available and uptake has improved, repurposed drugs continue to potentially offer affordable and accessible treatment options at all stages of disease, from pre-exposure prophylaxis to treatment of asymptomatic and mild infections and through critical care.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Appendix 1. Flow chart showing list of drugs screened for available data.
Appendix 2. List of drug price sources.
Acknowledgments
We would like to send sincere thanks to Kajal Bhardwaj and Sergey Kondratyuk for their expert insights, feedback and proofreading during the development of this manuscript. We also express gratitude to Professor Joseph Fortunak (Howard University, Washington DC) for expert advice and support regarding estimation of API costs for several drugs analyzed in this study. Finally, we thank Charlotte Mayne for technical support in the formatting of figures used in this article.
Financial support. This work was funded by the International Treatment Preparedness Coalition, Make Medicines Affordable Campaign.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Eaton L. Covid-19: WHO warns against “vaccine nationalism” or face further virus mutations. BMJ 2021; 372:n292. [DOI] [PubMed] [Google Scholar]
- 2. Hill A, Wang J, Levi J, et al. . Minimum costs to manufacture new treatments for COVID-19. J Virus Erad 2020; 6:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horby P, Mafham M, Linsell L, et al. ; RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020; 383:2030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med 2020. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020; 396:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. RECOVERY Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med 2021; 9:1419–26. doi: 10.1016/S2213-2600(21)00435-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tardif JC, Bouabdallaoui N, L’Allier PL, et al. . Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021. doi: 10.1016/S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hill A, Garratt A, Levi J, et al. . Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Open Forum Infect Dis 2021; 8:ofab358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Popp M, Stegemann M, Metzendorf M-I, et al. . Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2021. doi: 10.1002/14651858.CD015017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021; 384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes drug combination for treatment of COVID-19 [press release]. November 19, 2020. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19. Accessed 1 October 2021.
- 13. Mahase E. Covid-19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ 2021; 375:n2422. [DOI] [PubMed] [Google Scholar]
- 14. RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial [preprint]. medRxiv 2021. doi: 10.1101/2021.06.15.21258542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinreich DM, Sivapalasingam S, Norton T, et al. . REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients [preprint]. N Engl J Med 2021; 385:e81. doi: 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: managing COVID-19. Section 7.2: Casirivimab and imdevimab - hospital use. Available at: https://files.magicapp.org/guideline/ee9a60f9-6475-4f1c-9a31-d8046a513991/published_guideline_5679-13_2.pdf. Accessed 14 October 2021.
- 17. Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panjiva database. Available at: https://panjiva.com. Accessed 10 April 2021.
- 20. Love J. The production of generic drugs in India. BMJ 2011; 342:d1694. [DOI] [PubMed] [Google Scholar]
- 21. Gotham D, Barber MJ, Hill AM.. Estimation of cost-based prices for injectable medicines in the WHO essential medicines list. BMJ Open 2019; 9:e027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. . Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Resp Med 2021. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. PRINCIPLE Collaborative Group. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021; 398:843-855. doi: 10.1016/S0140-6736(21)01744-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klutz S, Holtmann L, Lobedann M, et al. . Cost evaluation of antibody production processes in different operation modes. Chem Eng Sci 2016; 141:63–74. [Google Scholar]
- 25. Bhuyan A. Scroll.in. In India, black markets for tocilizumab spring up as demand for the Covid-19 drug surges. Available at: https://scroll.in/article/966644/in-india-black-markets-for-tocilizumab-spring-up-as-demand-for-the-covid-19-drug-surges. Accessed 10 July 2021.
- 26. National Institute for Health and Care Excellence (NICE). COVID-19 prescribing briefing: corticosteroids. Available at: https://www.nice.org.uk/guidance/ng159/resources/covid19-prescribing-briefing-corticosteroids-pdf-8839913581. Accessed 10 February 2021.
- 27. Randomised Evaluation of COVID-19 Therapy (RECOVERY) Trial Protocol. Version 7.0. Available at: https://www.recoverytrial.net/files/recovery-protocol-v7-0-2020-06-18.pdf. Accessed 10 February 2021.
- 28. Department of Health and Social Care. COVID-19 therapeutic alert: Interleukin-6 inhibitors (tocilizumab or sarilumab) for patients admitted to ICU with COVID-19 pneumonia (adults)Reference CEM/CMO/2021/001. Available at: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103134. Accessed 10 March 2021.
- 29. Hill A, Simmons B, Gotham D, Fortunak J.. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad 2016; 2:28–31. [PMC free article] [PubMed] [Google Scholar]
- 30. Moon S, Jambert E, Childs M, et al. . A win-win solution?: A critical analysis of tiered pricing to improve access to medicines in developing countries. Glob Health 2011; 7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. United Nation Secretary General’s High-Level Panel on Access to Medicines. Report of the United Nation secretary general’s high-level panel on access to medicines: promoting innovation and access to health technologies. Available at: http://www.unsgaccessmeds.org/final-report. Accessed 10 April 2021.
- 32. World Trade Organization. Council for Trade-Related Aspects of Intellectual Property Rights. Waiver from certain provisions of the TRIPS agreement for the prevention, containment and treatment of COVID-19 - communication from India and South Africa. Available at: https://docs.wto.org/dol2fe/Pages/SS/directdoc.aspx?filename=q:/IP/C/W669.pdf&Open=True. Accessed 10 April 2021.
- 33. Khan FA, Stewart I, Fabbri L, et al. . Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021. doi: 10.1136/thoraxjnl-2020-215266. [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization. Therapeutics and COVID-19: living guideline. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1. Accessed 1 April 2021. [PubMed]
- 35. ThePharmaLetter. US govt procuring $1.2 billion of Merck’s COVID-19 candidate. Available at: https://www.thepharmaletter.com/article/us-govt-procuring-1-2-billion-of-merck-s-covid-19-candidate. Accessed 1 July 2021.
- 36. Hill AM, Barber MJ, Gotham D.. Estimated costs of production and potential prices for the WHO essential medicines list. BMJ Glob Health 2018; 3:e000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization. Commitment and call to action: Global collaboration to accelerate new COVID-19 health technologies. Available at: https://www.who.int/news-room/detail/24-04-2020-commitment-and-call-to-action-global-collaboration-to-accelerate-new-covid-19-health-technologies. Accessed 10 March 2021.
- 38. Mullard A. Flooded by the torrent: the COVID-19 drug pipeline. Lancet 2020; 395:1245–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Institute of Medicine (US) Forum on Medical and Public Health Preparedness for Catastrophic Events. Medical Countermeasures Dispensing: Emergency Use Authorization and the Postal Model, Workshop Summary. Washington DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 40. Business Wire. Governments spent at least €93bn on COVID-19 vaccines and therapeutics during the last 11 months. Available at: https://www.businesswire.com/news/home/20210110005098/en. Accessed 10 March 2021.
- 41. Barber M, Dzintars G.. Estimated cost-based generic prices for molnupiravir for the treatment of COVID-19 infection. Available at: https://scholar.harvard.edu/melissabarber/publications/estimated-cost-based-generic-prices-molnupiravir-treatment-covid-19. Accessed 10 October 2021.
- 42. Ahlqvist GP, McGeough CP, Senanayake C, et al. . Progress toward a large-scale synthesis of molnupiravir (MK-4482, EIDD-2801) from cytidine. ACS Omega 2021; 6:10396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simoens S, Vulto AG.. A health economic guide to market access of biosimilars. Expert Opin Biol Ther 2021; 21:9–17. [DOI] [PubMed] [Google Scholar]
- 44. Association for Accessible Medicines. 2020 generic drug & biosimilars access & savings in the U.S. report. Available at: https://accessiblemeds.org/sites/default/files/2020-09/AAM-2020-Generics-Biosimilars-Access-Savings-Report-US-Web.pdf. Accessed 10 April 2021.
- 45. New W. Revise biosimilar guidelines, scientists demand; WHO says not now. Health Policy Watch. Available at: https://healthpolicy-watch.news/revise-biosimilar-guidelines-scientists-demand-who-says-not-now. Accessed 10 April 2021. [Google Scholar]
- 46. Stebbing J, Phelan A, Griffin I, et al. . COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020; 20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. US Food and Drugs Administration. Emergency use authorization 099 [letter]. Available at: https://www.fda.gov/media/150319/download. Accessed 10 October 2021.
- 48. National Institute for Health and Care Excellence (NICE). Treatments being monitored by RAPID C-19. Available at: https://www.nice.org.uk/covid-19/rapid-c-19-treatments-currently-monitored. Accessed 10 October 2021.
- 49. US Food and Drugs Administration. Letter to Regeneron Inc. Available at: https://www.fda.gov/media/143891/download. Accessed 10 October 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.