Abstract
The Nkx homeobox genes are expressed in a variety of developing tissues and have been implicated in controlling tissue patterning and cell differentiation. Expression of Nkx6.2 (Gtx) was previously observed in the embryonic neural tube, testis, and differentiating oligodendrocytes. To investigate the role of Nkx6.2 in the control of cell specification and differentiation, we generated mice with null mutations in Nkx6.2 using the standard gene targeting approach. Null mutant mice were viable and fertile without apparent histological and immunohistochemical changes in the central nervous systems and testis. The absence of detectable phenotypes suggests a redundant function of Nkx6.2 in mouse development.
Nkx6.2 was originally identified as a novel homeobox gene that was specifically expressed in brain glial cells and testis germ cells (Gtx) (8). Subsequent studies by Awatramani et al. (1) had demonstrated that Gtx expression in glial cells was restricted in differentiated, postmitotic oligodendrocytes but not in oligodendrocyte precursor cells or astrocytes. The expression of Gtx in maturing oligodendrocytes had been suggested to regulate oligodendrocyte myelination. Recently, we renamed Gtx as Nkx6.2 based on its high degree of sequence homology to the Nkx6.1 homeobox gene, including a nearly identical homeodomain and a characteristic Nkx domain (11). Gtx is related to Nkx6.1 not only in its sequence but also in its expression pattern in the developing mouse and chicken neural tube (4). At the early stage of neural development, both Nkx6.1 and Nkx6.2 are induced in the ventral neural tube by the sonic hedgehog midline signaling molecule secreted from the notochord and floor plate (4, 11). In the midbrain and hindbrain regions, Nkx6.1 and -6.2 are expressed in similar domains of the ventrolateral neuroepithelium flanking the floor plate. From the Nkx6.2+ Nkx6.1+ ventral neuroepithelium arise the cranial motor neurons and serotonergic neurons in the hindbrain (3, 7) and dopaminergic neurons in the midbrain (6). Thus, Nkx6.2 transcription factor may play an important role in the control of neuronal specification and differentiation.
To investigate the function of Nkx6.2 in the specification and differentiation of neuronal cells, oligodendrocytes, and testis germ cells, we inactivated the mouse Nkx6.2 homeobox gene by the standard gene targeting approach. The mutant mice were born and grew normally like their littermates. The lack of detectable phenotypes in the development of tissues that express Nkx6.2 suggests a possible functional compensation from other related homeobox genes, such as Nkx6.1.
MATERIALS AND METHODS
Gene targeting.
Genomic DNA including the Nkx6.2 gene was isolated by screening a mouse 129Sv genomic library using the full-length Nkx6.2 cDNA as a probe. The genomic organization was determined by restrictive digestions and extensive sequencing. The replacement targeting vector was constructed by replacing the entire coding region with a neomycin resistance gene. Targeting vector was subsequently linearized and introduced into embryonic stem (ES) cells by electroporation. Genomic DNA isolated from ES clones was digested with ClaI and SpeI prior to hybridization with the flanking sequence probe 1, as indicated in Fig. 1A. The wild-type allele gave a band of 10 kb, whereas the targeted allele produced a band of 6.0 kb. Mutant ES clones were characterized and injected into C57BL blastocysts using the methods described previously (10).
FIG. 1.
Gene targeting of Nkx6.2. (A) Genomic organization of the Nkx6.2 locus and the structure of the targeting vector. Abbreviations for restriction sites: B, BamHI; C, ClaI; E, EcoRI; Sp, SpeI; X, XhoI. (B) Southern blot analysis of BamHI-digested genomic DNA from representative F2 pups with probe 2. A weak nonspecific band was also seen below the wild-type allele. (C) PCR genotyping of representative pups using two sets of primers simultaneously. The UP1 and DP1 primers amplified a 657-bp fragment from the wild-type allele, whereas the UP2 and DP2 primers amplified a 760-bp band from the mutant allele. MW, molecular weight (numbers on the left are base pairs).
Genotyping the Nkx6.2 mutants.
Southern analysis and/or PCR was used to genotype the offspring. For Southern analysis, the genomic DNA was digested with BamHI and hybridized with probe 2 indicated in Fig. 1A. The wild-type allele was detected as a 5.4-kb band, whereas the mutant was seen as a 4.2-kb band (Fig. 1B).
For genotyping by PCR, two sets of PCR primers were used simultaneously in the same reaction mixture containing 3% dimethyl sulfoxide. Primers UP1 (CTG AAG CTT GAC GCT AAC CGC CCG GGT GCG) and DP1 (CAC TTG GCT CTC GGT CAT) amplify part of the first exon (657 bp) in the wild-type allele (Fig. 1A). Primers UP2 (GGA TGT CTG CAG CCC TGC TTA) and DP2 (TAC CCG TGA TAT TGC TGA AGA GC) amplified a 0.76-kb fragment from Neo to the 3′ flanking sequence in the mutant allele (Fig. 1A). The PCR conditions are 95°C for 5 min and 35 cycles of 94°C for 40 s, 60°C for 50 s, and 72°C for 1 min.
In situ RNA hybridization.
Embryos from various stages of mouse development were fixed in 4% paraformaldehyde at 4°C overnight. Tissue preparation and in situ hybridization with digoxigenin- or fluorescein-labeled riboprobes were performed according to Schaeren-Wiemers and Gerfin-Moser (13) with minor modifications.
Immunohistochemistry.
Spinal cord and brain tissues were isolated from various stage of mouse embryos, fixed in 4% paraformaldehyde, cryosectioned, and immunostained with anti-Islet-1 (Developmental Studies Hybridoma Bank, Iowa City, Iowa) monoclonal supernatant or anti-tyrosine hydroxylase (TH) (Chemicon) or antiserotonin (anti-5-HT; DiaSorin) polyclonal antibodies. Immunohistochemistry was performed with the Vecstain ABC staining kit (Vector) with diaminobendidine substrate according to the manufacturer's protocol.
RESULTS
Targeted disruption of mouse Nkx6.2 gene.
To determine the role of Nkx6.2 in mouse development, we inactivated Nkx6.2 by gene targeting. The targeting vector was designed to replace the entire coding region with the neomycin gene. Following electroporation and drug selection, 100 independent ES clones were analyzed by genomic Southern blot with the flanking probe. Eight clones harboring homologous recombination were obtained. Two mutant ES clones with correct karyotypes were injected into blastocysts from C57BL mice, and both clones transmitted the mutant allele through the germ line. Homozygous mice derived from these two lines showed the same phenotype.
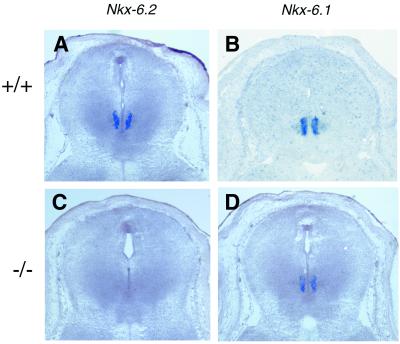
The deletion of Nkx6.2 was confirmed by the lack of amplification of the Nkx6.2 sequence from genomic DNA (Fig. 1C) or from the reverse-transcribed cDNA from the homozygous mutants (data not shown). The Nkx6.2 mutation was further verified by in situ RNA hybridization on midbrain from embryonic day 14.5 (E14.5) (Fig. 2). As reported previously (4), Nkx6.2 expression is detected mainly in the ventral region of the midbrain (Fig. 2A) and the hindbrain (4) in the wild-type embryos. Nkx6.1 is expressed at similar D-V positions (Fig 2B and D) in these regions. In the Nkx6.2 homozygous mutants, expression of Nkx6.2 was not detected (Fig. 2C), although Nkx6.1 expression was not compromised in the midbrain (Fig. 2D).
FIG. 2.
Lack of Nkx6.2 transcript in the homozygous mutants. Midbrain sections from E14.5 wild-type (A and B) and Nkx6.2 mutant (C and D) embryos were used for in situ RNA hybridization with Nkx6.2 and Nkx6.1 riboprobes. Nkx6.2 and Nkx6.1 are both expressed in the ventral midbrain. Expression of Nkx6.2, but not Nkx6.1, is absent in the mutants.
Nkx6.2 null mutants have normal growth and reproductive function.
To determine the effects of Nkx6.2 mutation on mouse development, heterozygous mice were crossed to obtain homozygous mutant mice. Genotype analysis of the offspring from the heterozygous matings showed Mendelian segregation ratios (27:64:34), indicating that the Nkx6.2 mutation is not lethal. Homozygous mice were morphologically indistinguishable from their heterozygous and wild-type littermates and can reproduce normally, with a typical litter size of 8 to 12 pups.
Nkx6.2 mutation does not affect neuronal differentiation in the ventral central nervous system.
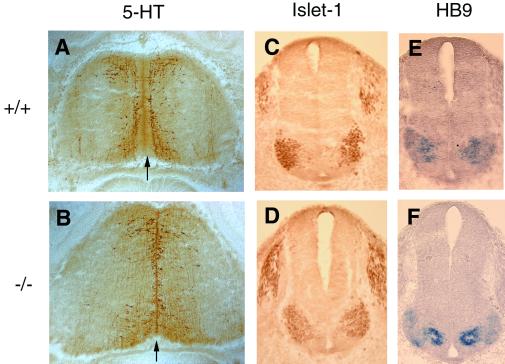
During the early stage of neural development, Nkx6.2 is selectively expressed in the ventral midbrain and hindbrain in close proximity to the floor plate (4, 11). The ventral neuroepithelial cells flanking the floor plate give rise to dopaminergic neurons in the midbrain (6, 7) and serotonergic neurons and cranial motor neurons in the hindbrain (3, 5, 18). To examine the dopaminergic neuron phenotype, we stained E12.5 to E18.5 midbrain sections with anti-TH antibody. No apparent differences in staining pattern or staining intensities between wild-type and mutant embryos were observed (Fig. 3). Development of serotonergic neurons in the E12.5 ventral hindbrain was examined with anti-5-HT antibody (6). The 5-HT-immunoreactive neurons were observed adjacent to the ventral midline or floor plate (Fig. 4A and B) in both wild-type and mutant embryos. For motor neuron development, we performed in situ RNA hybridization with HB-9, a motor neuron-specific gene (16) (Fig. 4C and D), and immunostaining with anti-Islet-1 antibody which labels motor neuron population (Fig. 4E and F) on E10.5 caudal hindbrain sections. Both studies revealed no significant difference in the production and distribution of motor neurons in the wild-type and mutant embryos.
FIG. 3.
Normal differentiation of dopaminergic neurons in the ventral midbrain. Cross sections of the midbrain tissues from wild-type (A, C, and E) and mutant (B, D, and F) animals were immunostained with anti-TH antibody. Similar numbers and patterns of dopaminergic neurons are observed in the wild-type and mutants.
FIG. 4.
Normal differentiation of serotonergic neurons and motor neurons in the ventral hindbrain in the Nkx6.2 mutants. (A and B) Detection of serontonergic neurons by immunostaining with anti-5-HT on horizontal sections of the rostral hindbrain from E12.5 wild-type (A) and mutant (B) embryos. The midline is indicated by the arrow. (C to F) Detection of cranial motor neurons on cross sections of the caudal hindbrain from E10.5 wild-type (C and E) and mutant (D and F) embryos by immunostaining with anti-Islet-1 (C and D) or in situ hybridization with HB-9 (E and F).
Nkx6.2 mutation has no apparent effects on oligodendrocyte development.
Previous studies had demonstrated that Nkx6.2 is expressed in both the spinal cord and the brain (1, 8) and in isolated brain oligodendrocytes (1). The expression of Nkx6.2 (Gtx) parallels that of myelin basic protein (MBP) and proteolipid protein (PLP), suggesting that Nkx6.2 may regulate the expression of myelin-specific genes during oligodendrocyte differentiation (1, 2). To investigate the role of Nkx6.2 in oligodendrocyte differentiation, we examined the expression of MBP and PLP genes in the mutant spinal cord and brain tissues at various developmental stages by in situ RNA hybridization. MBP expression can be detected as early as E18.5 (Fig. 5A and B) and persists in adults (Fig. 5C and D). Comparison of MBP expression in the wild-type and mutant littermates did not reveal significant differences in the onset and intensities of MBP expression. The normal expression of MBP was further confirmed by immunohistochemical staining with anti-MBP polyclonal antibody (data not shown). Another myelin-specific gene, the PLP gene, is also similarly expressed in the wild-type and mutant spinal cords (Fig. 5E and F).
FIG. 5.
Expression of myelin genes in the spinal cord is not compromised in the Nkx6.2 mutants. Spinal cord sections from wild-type (A, C, and E) and mutant (B, D, and F) animals were subjected to in situ hybridization with MBP and PLP genes.
To examine oligodendrocyte differentiation in the brain tissues, we examined MBP expression in the white matter of the adult cortex and cerebellum, where oligodendrocytes are highly enriched. In the coronal sections of the forebrain regions, MBP expression can be observed in the corpus callosum of both wild-type and mutant animals (Fig. 6A and B). In the cerebellum region, MBP expression is detected mainly in the white matter tissues (Fig. 6C and D) and appears to be unaffected by the Nkx6.2 mutation.
FIG. 6.
Normal expression of myelin genes in the Nkx6.2 mutant brain. Cross sections from adult forebrain (A and B) and cerebellum and brain stem (C and D) were subjected to in situ hybridization with an MBP riboprobe. Similar MBP expression was observed in the corpus callosum (arrows in panels A and B) and cerebellum white matter (arrowheads in panels C and D) in the wild type and mutants.
DISCUSSION
This study reports that mice harboring a null mutation in the Nkx6.2 homeobox gene did not display any apparent developmental and reproductive defects. To date, we have observed Nkx6.2 mutant mice for more than 1.5 years and did not detect any obvious phenotypes, including those related to body movement and life span.
The lack of phenotypes in the development of the ventral midbrain and hindbrain may reflect functional compensation from related members of the Nkx family, such as Nkx6.1. Previous studies demonstrated that Nkx6.1 and Nkx6.2 are expressed in similar and overlapping regions in ventrolateral domains flanking the floor plate (4) (Fig. 2). In addition, several other distantly related Nkx homeobox genes, e.g., Nkx2.2, Nkx2.9, Nkx5.1, and Nkx5.2, are also expressed in the ventral neural tube, including the midbrain and hindbrain at a similar time window (9, 12, 14). These Nkx homeobox genes may have redundant functions in the control of regional patterning and differentiation of the developing central nervous system. Thus, it would be interesting to examine the effects of compound mutations of these Nkx homeobox genes in the future.
It had been demonstrated that expression of Nkx6.2 in oligodendrocytes was regulated in parallel with MBP and PLP mRNAs (1). Promoters from both MBP and PLP genes contain multiple Nkx6.2 (Gtx) binding sites (1, 2). Based on these observations, Nkx6.2 was suggested to be involved in the regulation of myelin-specific gene expression. However, the normal expression of MBP and PLP mRNAs in the Nkx6.2 mutants indicates that Nkx6.2 is not required for expression of these two major myelin proteins. Again the lack of oligodendrocyte phenotype in the mutants may be due to a functional redundancy from other members of the Nkx family, such as Nkx2.2, which is selectively expressed in oligodendrocyte progenitor cells (17).
It was also reported that Nkx6.2 (Gtx) was specifically expressed in germ cells of the testis and thus may regulates spermatogenesis (8). Interestingly, the testes of the mutant males are histologically normal (data not shown) and the homozygous mutants have normal reproductive function, suggesting that Nkx6.2 may have a redundant function in the development of testis germ cells.
ACKNOWLEDGMENTS
We thank Seigo Izumo for the full-length Nkx6.2 (Gtx) cDNA probe.
This work was supported by NSF (IBN 9808126), NIH (R01 NS37717), and National Multiple Sclerosis Society.
J.C. and Y.Q. contributed equally to this work.
REFERENCES
- 1.Awatramani R, Scherer S, Grinspan J, Collarini E, Skoff R, O'Hagan D, Garbern J, Kamholz J. Evidence that the homeodomain protein Gtx is involved in the regulation of oligodendrocytes myelination. J Neurosci. 1997;17:6657–6668. doi: 10.1523/JNEUROSCI.17-17-06657.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awatramani R, Beesley J, Yang H, Jiang H, Cambi F, Grinspan J, Garbern J, Kamholz J. Gtx, an oligodendrocyte-specific homeodomain protein, has repressor activity. J Neurosci Res. 2000;61:376–387. doi: 10.1002/1097-4547(20000815)61:4<376::AID-JNR4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Briscoe J, Sussel L, Serup P, Hartigan-O'Conner D, Jessell T M, Rubenstein J L, Ericson J. Homeobox gene Nkx-2.2 and specification of neuronal identity by graded sonic hedgehog signaling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 4.Cai J, St Amand T, Yin H, Guo H, Li G, Zhang Y, Chen Y, Qiu M. Expression and regulation of the chicken Nkx6.2 homeobox gene suggest its possible involvement in the ventral neural patterning and cell fate specification. Dev Dyn. 1999;216:459–468. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<459::AID-DVDY14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell T, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 6.Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A. Control of neuronal diversity by the floor plate: contact-mediated induction of midbrain dopaminergic neurons. Cell. 1995;80:95–101. doi: 10.1016/0092-8674(95)90454-9. [DOI] [PubMed] [Google Scholar]
- 7.Hynes M, Rosenthal A. Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol. 1999;9:26–36. doi: 10.1016/s0959-4388(99)80004-x. [DOI] [PubMed] [Google Scholar]
- 8.Komuro I, Schalling M, Jahn L, Bodmer R, Jenkins N, Copeland N, Izumo S. Gtx: a novel murine homeobox-containing gene, expressed specifically in glial cells of the brain and germ cells of testis, has a transcriptional repressor activity in vitro for a serum-inducible promoter. EMBO J. 1993;12:1387–1401. doi: 10.1002/j.1460-2075.1993.tb05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pabst O, Herbrand H, Arnold H-H. Nkx-2.9 is a novel homeobox transcription factor which demarcates ventral domains in the developing mouse CNS. Mech Dev. 1998;73:85–93. doi: 10.1016/s0925-4773(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 10.Qiu M, Bulfone A, Martinez S, Meneses J, Shimamura K, Pedersen R, Rubenstein J. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- 11.Qiu M, Shimamura K, Sussel L, Chen S, Rubenstein J L. Control of anteroposterior and dorsoventral domains of Nkx-6.1 gene expression relative to other Nkx genes during vertebrate CNS development. Mech Dev. 1998;72:77–88. doi: 10.1016/s0925-4773(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 12.Rinkwitz-Brandt S, Justus M, Oldenettel I, Arnold H H, Bober E. Distinct temporal expression of mouse Nkx-5.1 and Nkx-5.2 homeobox genes during brain and ear development. Mech Dev. 1995;52:371–381. doi: 10.1016/0925-4773(95)00414-v. [DOI] [PubMed] [Google Scholar]
- 13.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labeled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 14.Shimamura K, Hartigan D J, Martinez S, Puelles L, Rubenstein J. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 15.Sim F, Hinks G, Franklin R J. The re-expression of the homeodomain transcription factor gtx during remyelination of experimentally induced demyelinating lesions in young and old rat brain. Neuroscience. 2000;100:131–139. doi: 10.1016/s0306-4522(00)00252-9. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe Y, William C, Jessell T. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Cai J, Hui F, Qi Y, Modderman G, Liu R, Qiu M. Selective expression of Nkx-2.2 transcription factor in the migratory chicken oligodendrocyte progenitor cells and implications for the embryonic origin of oligodendrocytes. Mol Cell Neurosci. 2000;16:740–753. doi: 10.1006/mcne.2000.0916. [DOI] [PubMed] [Google Scholar]
- 18.Ye W, Shimamura K, Rubenstein J, Hynes M, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]