Abstract
Background
The COVID-19 pandemic can affect the elderly population’s general health. This study aimed to compare the effects of a remote home-based exercise program to improve the mental state, balance, and physical function and to prevent falls in adults aged 65 years and older during the COVID-19 pandemic in Seoul, Korea.
Material/Methods
Seventy participants were randomly assigned to an experimental group of 35 participants who underwent a remote home-based fall prevention exercise program and a control group of 35 participants. The experimental group performed an exercise program twice weekly for 8 weeks from June 2 to July 21, 2021. The Geriatric Depression Scale, 5 times sit to stand test, grip strength, 10-m walk test, gait analysis, Timed Up and Go test, and static balance test were assessed before and after the 8-week program.
Results
The group-by-time interaction effect was statistically significant for the Geriatric Depression Scale, five times sit to stand test, grip strength, 10-meter walk, gait speed, step length, stride length, Timed Up and Go test, and static balance test (P<0.05). Compared with the control group, the experimental group showed a significant effect in all dependent variables except dynamic balance (P<0.05).
Conclusions
In this population, the remote home-based fall prevention exercise program resulted in a significant improvement in physical function, psychological factors, and balance during the COVID-19 pandemic. The findings may have implications for community public health measures to protect the vulnerable during future epidemics and pandemics of infectious disease.
Keywords: Accidental Falls, Aged, COVID-19, Postural Balance
Background
Recently, a new type of coronavirus emerged worldwide, which was declared a pandemic by the World Health Organization has affected more than 140 countries [1]. The COVID-19 pandemic has negatively affected not only the health of the world’s population but also that of the global economy [2]. As of April 11, 2020, approximately 1 746 607 individuals had been confirmed to have COVID-19, with approximately 107 327 deaths reported worldwide [3].
COVID-19 can be fatal, particularly in the elderly and in individuals with comorbidities as this disease can lead to death due to significant damage to the alveoli and massive failure of the respiratory system to perform functional gas exchange [4]. Notably, COVID-19-related mortality rates increase dramatically with age. The hospital mortality rate in the New York City area for March to April 2020 was less than 5% for patients younger than 40 years but was 35% for patients aged 70 to 79 years and 60% for patients aged 80 to 89 years [5].
Social distancing and quarantine protocols have been established to decelerate the spread of COVID-19. These strategies are important to prevent the spread of the coronavirus [6,7]. However, these recommendations can lead to physical and mental complications due to a lack of physical activity [8]. Social distancing as a result of the COVID-19 pandemic can have negative consequences on the physical health of older people [9]. Increased time at home has unintended negative consequences, resulting in sedentary behavior and a general lack of physical activity. Social distancing and quarantine protocols limit access to health- and welfare-related services as well as to opportunities for physical activity. Recently, Schuch et al [10] found that the time spent on moderate to vigorous physical activity decreased by 60% in young adults, while sitting time increased by 42% during the current pandemic. This is important because the impact of a sedentary lifestyle can be lower for children and young adults than for older people, in whom the impact can be serious.
A reduction to 1500 steps per day for 14 days can reduce the mass of the lower limb muscles in older adults by up to 4% [11]. There is also evidence that 10 days of bed rest reduces muscle mass by 2% and muscle strength by 12.5% in older adults [12]. Loss of muscle mass weakens the body’s resistance to disease and infection in older adults [13].
Esain et al [14] also found that without exercise for 3 months, the physical function, mental health, and quality of life, even for active older adults, declined. In addition, increased blood sugar levels, susceptibility to infection, and the prevalence of cardiovascular disease, cognitive impairment, chronic diseases, and musculoskeletal disorders can result from the lack of physical activity [15].
Consequently, the physical health of older adults is negatively affected during the period of confinement caused by this pandemic [16].
Falls affect 1 in 3 adults over the age of 65 annually and can have detrimental effects on older adults [17]. With advancing age, fall rates and severity of injuries increase significantly. Furthermore, fall-related injuries cause significant mortality, disability, loss of independence, quality of life, and early hospitalization, which result in an increase in healthcare expenditure for older adults [18,19]. Physical activity is not only an important indicator of healthy and active aging but is also a risk factors of falls among older adults [20,21]. Physical inactivity is associated with a decrease in physical functions, such as frailty and muscle weakness [22,23], which are well-known risk factors for falling.
Self-isolation can lead to an increase in anxiety and depression rates, particularly among older adults, who are most affected by this pandemic [24,25]. During quarantine, isolation affects older adults’ mental health and physical function. Several studies have found that psychological factors have effects on postural control and balance in older adults [26]. Stress, anxiety, fear, and depression are different fall risk factors that increase the risk and incidence of falls [27]. Higher scores on depression indicate greater vulnerability to falls and functional dependence [28].
Many fall prevention programs based on these risk factors have been established and evaluated. These include exercise programs designed to improve strength or balance, education programs through booklets and phone calls, and environmental modifications at home [29].
Among these fall prevention programs, exercise is an efficient and cost-effective way to prevent functional decline and depression, improve self-esteem and life expectancy, and reduce mortality and risk of falls in older adults [30]. Consequently, there may be plausible explanations for recommending an increase in exercise level to reduce several devastating outcomes, such as physical functional decline, psychological problems, and falls, in older adults during the COVID-19 pandemic. Although the fall prevention exercise program for older adults was provided at the older adult welfare centers or public health centers at the local and national levels, the offline group exercise program was limited due to social distancing.
The need for a fall prevention exercise program among older adults raises questions about its application during the current isolation period. There is a need to help older adults incorporate simple and safe methods to participate in fall prevention exercise programs in confined spaces. Although group-based exercise programs are beneficial in enhancing functional performance in older adults, a home-based exercise program is as effective as a center- or group-based program for improving strength and physical function [31].
Currently, the geriatric health field needs to respond proactively rather than reactively and provide services that reduce the impact of social distancing and isolation on older adults by utilizing alternative strategies, such as remote interventions. A video was used as a medium to motivate older adults, increase participation rates, and increase effectiveness. This medium combined audiovisual information, such as sound, text, and pictures [32]. With the development and generalization of mobile devices, such as smartphones and tablet PCs, participants can engage in the program at any time and place they want.
We aimed to bridge this gap via a remote home-based fall prevention exercise program by employing technological advancements and providing older adults with the opportunity to exercise at home. It is necessary to cope with the current situation by devising and practically applying exercise experts in a home-based fall prevention exercise program from the conventional group fall prevention exercise program. Therefore, this study aimed to compare the effects of a remote home-based exercise program to improve the mental state, balance, and physical function and to prevent falls in adults aged 65 years and older during the COVID-19 pandemic in Seoul, Korea.
Material and Methods
Participants
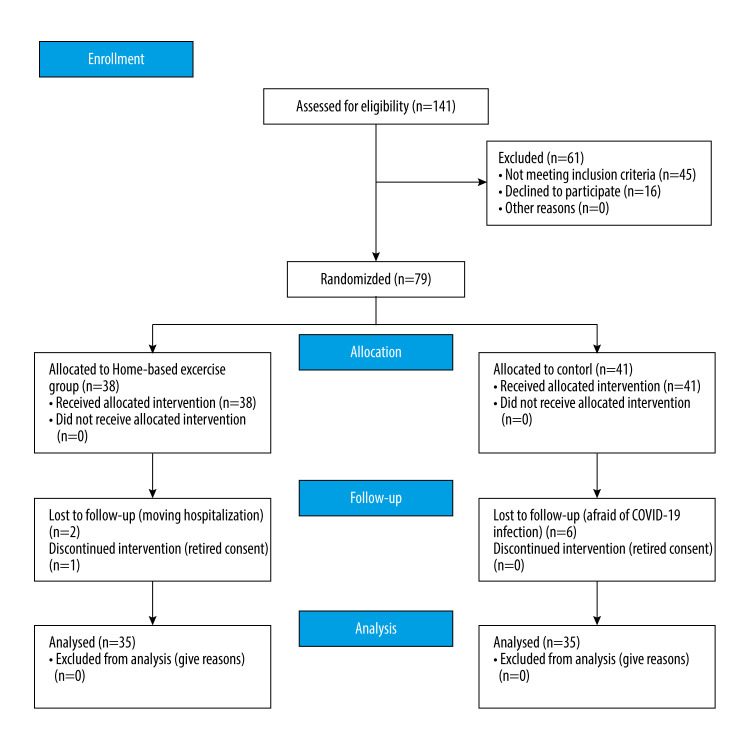
Seventy-nine community-dwelling older adults aged 65 years and older, who were enrolled at a welfare senior center in Seoul, Korea, were recruited for the study. The inclusion criteria were as follows: age 65 years and older; ability to walk without a walking aid; ability to communicate; and ability to provide written informed consent to take part in the study. The exclusion criteria were as follows: cardiopulmonary diseases, visual impairment, vestibular diseases, psychological disorders, unstable hypertension, and cognitive disorders. After evaluating inclusion and exclusion criteria, participants were randomly assigned to a remote home-based fall prevention exercise program group or a control group to have equal numbers in each group. A random number generated by a computer program (Microsoft Co, WA, USA) was used to perform the random assignment procedure. All participants provided written informed consent. Demographic data from the participants are presented in Table 1. The G*Power 3.1 program was used to calculate the sample size. A 2-tailed t test with statistical power (1-β)=0.8, significance level (α)=0.05, and effect size (d)=0.80 showed that each group required at least 26 participants. However, 79 participants (38 in the experimental group and 41 in the control group) were recruited in anticipation of dropouts. Two people in the experimental group were withdrawn from the study for personal reasons, and 1 person in the control group was excluded because of an incomplete questionnaire. A total of 70 participants (35 in the experimental group and 35 in the control group) were finally included in the study.
Table 1.
Contents of exercise program.
| Type | Position | |
|---|---|---|
| One paddle exercise | Sitting/Standing | The subject performed the exercise while holding one paddle with both hands. The exercise was comprised raising both hands and rotating the trunk horizontally while holding the paddle vertically, bending the body laterally to the left and right while holding the paddle vertically, and paddling to the left and right |
| Two paddle exercise | Sitting/Standing | The subject performed the exercise while holding a paddle in each hand. The exercise included forward paddling, backward paddling, alternately lifting the hands upward, raising the paddles by reaching with the arms diagonally, moving the paddles forward by reaching straight, raising both hands diagonally at the same time, and spreading both arms out with the elbows flexed to 90 degrees |
| Combined paddle exercise | Standing | The subject performed the exercise while holding one combined paddle in both hands including head rotation, lateral bending, and tilting. The exercise consisted of reaching horizontally to move the paddle forward, bending the body laterally to the left and right after raising both hands with the paddle held horizontally, rotating the trunk while holding the paddle horizontally, rotating the trunk while holding the paddle vertically with one hand, as well as squat, lunge, and stepping while holding the paddle |
Procedures
Physical function, psychological factors, and balance were evaluated in both groups before and after the 8-week program. Prior to the start of this study, pre-tests were executed on the dependent variables, which included physical function, psychological factors, and balance. The same dependent variables were examined after the intervention. All participants were administered a 10-m walk test (10MW), five times sit to stand test (FTSS), grip strength test, gait analysis, timed Up and Go (TUG) test, and static balance test for physical function. Depression was measured as a psychological factor. The evaluators who performed the assessments were unaware of the participants’ group assignment. After the pre-test, the experimental group participated in a remote home-based fall prevention exercise program. The post-test was performed after 8 weeks of the exercise program (Figure 1), which was conducted from June 2 to July 21, 2021. The study was approved by the Institutional Review Board of Sahmyook University (no. 2-1040781-A-N-012020112HR).
Figure 1.
Flow diagram of the experimental procedure.
Remote Home-Based Fall Prevention Exercise Program
In this study, the remote home-based fall prevention exercise program enabled older adults to exercise indoors at home. The exercise program was modified and supplemented to suit the purpose of the study. For the program to improve the physical function and prevent falls in older adults, a total of 16 sessions were conducted for 40 min twice a week for 8 weeks. The participants in the experimental group underwent 40 min of the exercise program, including 10 min of warm-up exercise, 20 min of main exercise, and 10 min of cool-down exercises. The warm-up consisted of stretching and deep breathing exercises. After the main exercise, the cool-down exercise was performed in the same way as the warm-up exercise. The program consisted of movements using kayak paddles as a basis and consisted of stretching, vestibular rehabilitation, core and limb strengthening exercises, joint motion range exercises, and balance exercises. To adjust the difficulty level, the program was configured in stages to perform various movements from a sitting position to a standing position. As the exercise program progressed, faster tempo and dynamic movements were added. The detail of the exercises are shown in Table 1.
The older adults participated in real time by accessing the welfare center’s live streaming through their Wi-Fi internet connection. When it was time to exercise, the participants used their smartphones to perform the exercise program. The videos in the remote home-based fall prevention exercise program were efficient because they provided accurate visual guidelines on how to exercise and were motivating with music and verbal instructions. During the 8-week program, in the remote home-based fall prevention exercise group, the physical therapist did not visit the participant’s home. However, the physical therapist communicated with each participant by telephone twice a week to maintain their motivation. Participants in the control group were instructed to continue their usual daily activities for 8 weeks.
Outcome Measurements
Psychological Factor – Korean Form of Geriatric Depression Scale
The Geriatric Depression Scale (GDS) developed by Brink (1984) and Yesavage (1983) was used in the Korean form standardized by Jung In-Gwa (1997) [33]. The standardized reliability of this tool is a Cronbach’s coefficient of 0.88. This test is easy to perform and can be conducted in a relatively short time. It is a dichotomous scale in which the participant answers “yes” or “no” to questions. It consists of a total of 30 questions, comprising 16 negative and 14 positive questions. For the negative questions, 1 point was assigned for “yes” and 0 points for “no”, whereas 0 points were allocated for “yes” and 1 point for “no” for positive questions. Converted into a score, it can range from a minimum of 0 points to a maximum of 30 points. A score of 14 to 18 is classified as depression, suspicious, and mild depression; a score of 19 to 21 is moderate; and a score of 22 or more is classified as severe depression. The higher the score, the more severe the degree of depression.
Five Times Sit to Stand Test
The FTSS test was used to measure lower limb muscle strength and functional mobility in older adults. The FTSS test evaluates the ability to rise from a chair. The test was used to measure functional mobility and assess fall risk in older adults. At the start of this test, the participants were seated in a chair with a standardized seat height (46 cm) with their back in a self-selected degree of comfortable knee flexion. The participants were asked to sit with their feet on the ground and take a position with their arms crossed. The time taken to transition between the sitting and standing positions 5 times as quickly as possible was recorded. The time from the “go” command to when the participant’s buttocks touched the chair on the fifth repetition was recorded in seconds using a stopwatch [34]. The test has excellent relative and absolute reliability and reproducibility.
Handgrip Strength
Hand grip strength was measured with an adjustable Jamar dynamometer in the sitting position. While seated, the participants were instructed to grasp the dynamometer as tightly as possible. The left and right hands were evaluated alternately with a 1-min break between assessments. The mean score of the second trial was used in this study. The test–retest reliability was ICC=0.97 [35].
10-M Walk Test
To evaluate gait speed, the 10MW test was performed. To rule out the acceleration and deceleration periods, a 14-m total length included 2 m of approach, 10 m for the time measure, and 2 m beyond the measure to ensure a constant walking speed across 10-m distance. The participants were instructed to start at the “start” signal and walk as fast as possible. The walking time to 10 m was measured twice, and the average value for each participant was recorded.
Gait Analysis
Gait parameters were assessed using OptoGait (Microgate Co, Bolzano, Italy). OptoGait is a 3-m walkway designed for optical-sensitive gait analysis. The participants completed the third trial with the instruction to “walk at your normal speed”. The recess period was provided between the single trials undertaken. Specific software (OptoGait analysis software, version 1.6.4.0) was used to calculate average gait speed and average stride length.
Dynamic Balance
Time Up and Go Test
The TUG test was used to measure dynamic balance, lower extremity function, mobility, and fall risk. The time required until the participant got up from the seated position with the start signal, moved to the 3-m point marked in front of the chair, and returned to the chair was measured. The time was measured in seconds, begins at “go” and ends when the participant is seated. The time taken to complete the entire procedure was measured using a stopwatch. It was measured twice and an average value was obtained. The TUG test shows excellent test–retest reliability in older adults [36].
Static Balance
Postural Sway Test
To evaluate the static balance, the Good Balance system (Good Balance, Metitur Oy, Finland) was used. The measuring system consisted of a safety bar and an equilateral triangle-shaped force plate and was connected to a laptop based on Bluetooth. The sampling frequency was 50 Hz. For the trajectory of the center of pressure, the following 3 variables were calculated: medio-lateral sway velocity, anterio-posterior sway velocity, and velocity moment [37]. After the participant was placed barefoot on a force plate, measurements were taken with both feet together. The measurements entailed standing for 20 s with the eyes closed and standing on foam (Airex, Sins, Switzerland) for 20 s with the eyes closed. Data collection was performed twice, and the average values of postural sway distance and speed were obtained. The test–retest reliability measurements with eyes open and eyes closed were both 0.83 [38].
Statistical Analyses
SPSS 25.0 software for Windows 10 (IBM Corp, Armonk, NY, USA) was used for analyzing the data. Data were presented as mean and standard deviation (SD). Normality verification was performed using the Kolmogorov-Smirnov test. The chi-squared test was used to analyze the general characteristics of participants. To determine the group effect on outcome measures, 2×2 (time-by-group) repeated measures ANOVAs were performed with time (pre-test and post-test) as the repeated factor and group (remote home-based fall prevention exercise program group and control group) as the between-participants factor. Significant main or interaction effects were followed by appropriate post hoc analyses via the Bonferroni correction method. Statistical significance was set at α=0.05.
Results
Participant Characteristics
Seventy participants participated in this study. The participants’ demographic characteristics are shown in Table 2.
Table 2.
Physical characteristics of the participants (n=70). Data represented as mean±standard deviation.
| Home-based exercise group (n=35) | Control group (n=35) | |
|---|---|---|
| Age | 76.11±6.31 | 77.31±5.57 |
| Gender (male/female) | 8/27 | 4/31 |
| Height (cm) | 157.22±6.16 | 154.41±6.31 |
| Weight (kg) | 60.17±7.07 | 58.75±10.43 |
| BMI | 24.31±2.18 | 24.55±3.46 |
Mean±SD.
Psychological Factors
Changes in the GDS
There was a statistically significant group-by-time interaction effect for GDS (F[1,68]=4.025, P=0.049). The experimental group had significantly decreased GDS compared with the control group (P<0.05) (Table 3).
Table 3.
Changes in the Geriatric depression scale (n=70). Data represented as mean±standard deviation.
| Variables | Home-based exercise group (n=35) | Control group (n=35) | Group×time | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | ||
| GDS (score) | 8.20 ± 5.77 | 6.91 ± 5.20* | 10.83 ± 5.61 | 11.09 ± 6.79 | 4.025 (0.049) |
Mean±SD. GDS – Geriatric depression scale.
Physical Function
Changes in the FTSS and handgrip strength
The group-by-time interaction effect was statistically significant for the FTSS (F[1,68]=11.579, P=0.001). Also, there were significant differences in the group-by-time interaction for right and left grip strength (F[1,68]=8.764, P=0.004), (F[1,68]=4.942, P=0.030), respectively. The experimental group had significantly increased handgrip strength and FTSS compared with the control group (P<0.05) (Table 4).
Table 4.
Changes in grip and lower limb muscle strength (n=70).
| Variables | Home-based exercise group (n=35) | Control group (n=35) | Group×time | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | ||
| FTSS (s) | 8.91±2.13 | 8.12±1.82* | 8.62±2.03 | 8.90±2.13 | 11.579 (0.001) |
| R Grip (kg) | 25.31±6.30 | 26.37±5.86* | 23.46±6.52 | 22.63±6.08 | 8.764 (0.004) |
| L Grip (kg) | 24.06±5.79 | 24.94±5.98* | 23.37±5.36 | 23.03±5.18 | 4.942 (0.030) |
Data represented as mean±standard deviation.
Indicates was significantly different within the group (P<0.05).
FTSS – 5 times sit to stand test; R grip – right grip strength; L grip – left grip strength.
Changes in Gait Ability
The group-by-time interaction effect showed statistical significance in the 10MW test, gait speed, step length, and stride length (F[1,68]=9.612, P=0.003), (F[1,68]=4.979, P=0.029), (F[1,68]=9.055, P=0.004), (F[1,68]=9.423, P=0.003), respectively. The experimental group had significantly improved gait ability compared with the control group (P<0.05) (Table 5).
Table 5.
Changes in gait abilities (n=70).
| Variables | Home-based exercise group (n=35) | Control group (n=35) | Group×time | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | ||
| 10MW (s) | 8.33±1.28 | 7.50±1.30* | 8.00±1.38 | 7.81±1.30 | 9.612 (0.003) |
| Gait speed (m/s) | 1.30±0.21 | 1.37±0.20* | 1.38±0.22 | 1.36±0.22 | 4.979 (0.029) |
| Step length (cm) | 61.02±6.67 | 64.19±9.32* | 63.21±8.02 | 61.27±7.20 | 9.055 (0.004) |
| Stride length (cm) | 123.28±12.74 | 128.75±17.10* | 127.24±17.04 | 124.56±14.85 | 9.423 (0.003) |
Data represented as mean±standard deviation.
Indicates was significantly different within the group (P<0.05).
10MW – 10-m walk test.
Changes in Dynamic Balance
A statistically significant group-by-group interaction effect was found in the TUG test (F[1,68]=7.289, P=0.009). However, there were no significant differences between the 2 groups in the TUG test (P>0.05) (Table 6).
Table 6.
Changes in postural balance (n=70).
| Variables | Home-based exercise group (n=35) | Control group (n=35) | Group×time | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | ||
| Dynamic postural balance | |||||
| TUG (s) | 8.60±1.42 | 7.71±1.31* | 8.15±1.33 | 7.83±1.30* | 7.289 (0.009) |
| Static postural balance | |||||
| ECMLS (mm/s) | 8.05±2.96 | 6.63±3.25* | 6.62±2.31 | 6.30±2.21 | 4.145 (0.046) |
| ECAPS (mm/s) | 14.28±4.31 | 11.88±3.89* | 10.50±5.00 | 10.37±2.91 | 7.654 (0.007) |
| ECVMS (mm2/s) | 34.75±18.82 | 28.14±20.79* | 25.14±21.54 | 28.86±14.35 | 5.696 (0.020) |
| ECMLU (mm/s) | 22.85±7.92 | 15.69±6.31* | 19.65±9.27 | 18.41±7.38 | 8.800 (0.004) |
| ECAPU (mm/s) | 36.57±8.92 | 26.91±7.82* | 31.59±7.82 | 29.49±7.85 | 13.722 (0.000) |
| ECVMU (mm2/s) | 209.15±102.63 | 117.04±66.39* | 171.68±115.06 | 144.00±79.88 | 7.452 (0.008) |
Data represented as mean±standard deviation.
Indicates was significantly different within the group (P<0.05).
TUG – Timed Up and Go test; ECMLS – eye-closed mediolateral postural sway velocity on stable platform; ECAPS – eye-closed anteroposterior postural sway velocity on stable platform; ECVMS – eye-closed velocity moment on stable platform; ECMLU – eye-closed mediolateral postural sway velocity on unstable platform; ECAPU – eye-closed anteroposterior postural sway velocity on unstable platform; ECVMU – eye-closed velocity moment on unstable platform.
Changes in Static Balance
The group-by-time interaction effect was statistically significant for eye-closed mediolateral postural sway velocity on a stable platform, eye-closed anteroposterior postural sway velocity on a stable platform, and eye-closed velocity moment on a stable platform (F[1,68]=4.145, P=0.046), (F[1,68]=7.654, P=0.007), (F[1,68]=5.696, P=0.020), respectively. The statistical analysis revealed a significant group-by-time interaction effect for eye-closed mediolateral postural sway velocity on an unstable platform, eye-closed anteroposterior postural sway velocity on an unstable platform, and eye-closed velocity moment on an unstable platform (F[1,68]=8.800, P=0.004), (F[1,68]=10.032, P=0.002), (F[1,68]=7.452, P=0.008), respectively. Static balance was significantly improved in the experimental group compared with in the control group (P<0.05) (Table 6).
Discussion
The main finding in this study was that the remote home-based fall prevention exercise group had enhanced physical function, psychological factors, and balance after the program. The results of this study showed that 8 weeks of a remote home-based fall prevention exercise program significantly improved physical and psychological functions and balance, as reflected in improved GDS, TUG, FTSS, grip strength, gait, and balance tests in older adults.
Stress, anxiety, fear, and depression are different factors that increase the risk and incidence of falls [26]. Physical exercise is one of the solutions to relieve these negative outcomes in older adults. Exercise was recently shown as an effective non-pharmaceutical treatment for depression [39]. A meta-analysis found that exercise was moderately effective in reducing depressive symptoms [39].
In this study, after 8 weeks of the remote home-based fall prevention exercise program, the results showed that the GDS decreased significantly compared with the control group (P<0.05). Our findings are consistent with those of other home-based exercise interventions among older adults, in which a home-based exercise group had significantly greater reductions in depression than did the control group [40].
Increased self-efficacy, mastery, and alterations in self-concept appear to be involved in the therapeutic efficacy of exercise in depression [41]. Biological pathways have also been suggested. For example, neurotrophins cause lasting changes in brain structure, such as improved vasculature, and stimulate downstream cellular processes, such as angiogenesis, that improve brain function in areas associated with depression, such as the hippocampus [42]. Hence, a reduced GDS score through a remote home-based fall prevention exercise program is a significant result.
Notably, the decrease in the time to perform the FTSS test confirmed the gains in the exercise group in muscle strength after the exercise program. The present study clearly showed that the FTSS test outcomes decreased significantly upon comparing both groups after 8 weeks of the exercise program (P<0.05).
A previous study showed that the FTSS test is associated with lower extremity muscle strength and balance [43]. Our fall prevention exercises consisted of lower extremity exercises, such as squats, lunges, and various step movements. A small increase in muscle strength in older people leads to functional improvements, which are known to be one of the most crucial factors to physical functions in this population [44]. In addition, neural adaptations, such as increased activation of prime movers, improved coactivation of synergists, and reduced coactivation of antagonists, appear to be likely agents for the observed significant improvements in lower extremity strength [45].
These findings are consistent with those of Opdenacker et al [46], who reported an increase in the muscle strength of the hip flexors and extensors in home- and center-based exercises. Thus, it can be concluded that increases in muscle strength are directly related to improvements in physical function in older adults through a fall prevention exercise program.
Grip strength, a measure of body function, has been proposed as a biomarker of aging and is a useful indicator of overall health. Because of the practicality of hand grip dynamometers, grip strength measurement has been widely adopted as a single indicator of overall strength. Evidence of a predictive association between grip strength and fall incidence, all-cause and disease-specific mortality, bone density, fractures, cognition, and depression as well as problems related to hospitalization has also been provided [47,48].
In this study, the participants in the exercise group showed significant differences in grip strength after the program. In a previous study on an individualized home-based exercise, there was a significant difference in grip strength in the exercise group compared with the control group. Beneficial improvements in handgrip strength were observed in the exercise group (P<0.05) [49].
It can be said that this exercise contributed to the increase in grip strength because all movements were performed with the paddle during the exercise program. Because the participants hold paddles and row consecutively and repeatedly, grip strength increases [50]. It was concluded that the increased grip strength was associated with the movement of the exercise program performed with the paddle; therefore, the remote home-based fall prevention exercise program had a significant effect on increased grip strength.
Gait speed is expressed as a predictor of physical function and survival and is essentially to displacement speed. This is because older adults walking at speeds above 1.0 m/s are considered physically functional, and speeds above 1.2 m/s may be associated with increased survival of older adults [51]. Low walking speed has been shown to be a strong predictor of numerous adverse health outcomes, including falls, cognitive impairment, functional decline, and mortality in the elderly [52].
In the present study, gait speed and step length were significantly higher in the exercise group than in the control group after 8 weeks of the program. Previous studies have also observed improvements in gait speed after 12 weeks of lower limb muscle strengthening, indicating improvements in physical function [53]. Aging is associated with a general decline in walking speed and step length. Kinematic changes, such as decreased pelvic rotation, hip flexion/extension, and ankle plantar flexion, are reported to be associated with shorter step lengths [54]. Shorter stride length is an important factor directly related to the mechanisms of poor balance, which have been shown to be indicators of low survival rates, physical disability, and other adverse clinical events, such as falls [16]. Increased gait speed, step length, and stride length are associated with improved ankle dorsiflexion strength and hip extension strength. The increases in gait speed, step length, and stride length might be explained by the increased strength of the knee and hip extensors [55].
The present study demonstrated that an 8-week remote home-based fall prevention exercise program consisting of core exercise, proximal and distal muscle strengthening exercises, and cardiovascular or aerobic activities was safe and associated with an improvement in physical functions, including ankle and hip strength and mobility, as indicated by improvements in increased gait speed, step length, and stride length. Consistent improvements were also observed in the TUG test. TUG performance was highly dependent on gait speed and showed a comparable improvement.
A decrease in muscle strength and coordination of the lower extremities along with decreased gait and balance are the results of the physical deterioration of the body in older adults [56]. Inactivity refers to factors that negatively affect balance control and accelerate the decline of physical function with a negative impact on balance control [57,58].
The TUG test can comprehensively reflect the physical or functional abilities of the elderly [36]. In addition, an association between falls in the elderly and the TUG test has been reported [59]. The TUG test, a measure of dynamic balance, was significantly improved by a remote home-based fall prevention exercise program.
In the present study, the TUG test showed a statistically significant reduction in the exercise group compared with the control group after 8 weeks of the exercise program. According to a previous study, compared with the control group, the TUG test was significantly improved (P<0.05) in the home-based exercise group [60]. In addition, participants were randomized into an enhanced physical activity group and a standard of care group for the 8-week intervention. Both groups experienced significant improvements, albeit greater improvements in the enhanced physical activity group for the TUG test than in the standard of care group [61].
Turning, a commonly performed task in daily life [62], is a complex task that requires interaction between sensory and motor systems and can be involved in falls for older people [63]. In the present study’s program, we adopted turning movement and core exercises to improve balance. Because of the relationship between these various movements and core exercises in an exercise program, dynamic balance might improve.
In addition, a significant difference was found between the 2 groups with and without foam under the closed eyes condition. These conditions are used to confirm vestibular function, sight, and somatosensory function, among the 3 factors of balance. Postural control maintained by coordination between somatosensory, visual, and vestibular systems deteriorates with aging [64]. To maintain postural balance, the vestibular system uses the angular velocity of head movement, linear acceleration, and the position of the head relative to gravity [65]. Somatosensory and visual information are the most important factors for postural control, but if postural control is not maintained properly, the role of the vestibular system becomes even more important [66].
Previous studies have shown that postural control improves with gaze stabilization and head rotation exercises that can enhance vestibular function [65,67]. In addition, Gauchard et al [68] suggested that turning or moving the head over a wider range is a combined exercise that requires the sensory integration of the upper and lower limbs as well as the visual and somatosensory systems. It can be said that the results of the present study were derived by including gaze movements as well as those of the head and neck, which might improve vestibular function through the remote home-based fall prevention exercise program. We believe that the static postural balance of the participants improved significantly because the head and neck movements were included in the exercise program. This shows that decreased static balance, which might be a cause of vestibular hypofunction, was improved through the exercise program.
In the present study, the participation rate of the home-based exercise program without home visiting was 88.8%. Therefore, even if home visiting was not included during home-based exercise program, it was established that a high level of compliance was maintained. King et al [69] reported that telephone contact with participants plays a major role in maintaining compliance. Thus, in the present study, participants in the experimental group were contacted by phone regularly to maintain motivation. Even without home visiting, it was possible to maintain the exercise program at a high standard through regular contact.
This study had several limitations, including that the participants were recruited from 2 organizations on a volunteer basis, which might restrict the generalizability to the wider population. Feedback was also an important factor in this home exercise program, but an improved platform was needed in the limited situation caused by the COVID-19 pandemic. In future studies, it would be better to apply a long-term program.
Conclusions
In this elderly population, the remote home-based fall prevention exercise program resulted in a significant improvement in physical function, psychological factors, and balance during the COVID-19 pandemic. The findings may have implications for community public health measures to protect the vulnerable during future epidemics and pandemics of infectious disease.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (no. NRF-2020R1A2C1103035)
References
- 1.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–52. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Interim case reporting form for 2019 Novel Coronavirus (2019-nCoV) of confirmed and probable cases. 2020. Apr 11, Available from: https://www.who.int/docs/default-source/coronaviruse/20200121-2019-ncov-reporting-form.pdf.
- 4.Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–61. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. The Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–59. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cudjoe TK, Kotwal AA. “Social distancing” amid a crisis in social isolation and loneliness. J Am Geriatr Soc. 2020;68(6):E27–29. doi: 10.1111/jgs.16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph CW, Zacher H. “The COVID-19 generation”: A cautionary note. Work Aging Retire. 2020;6(3):139–45. doi: 10.1093/workar/waaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai H, Ouchi Y, Yokode M, et al. Toward the realization of a better aged society: Messages from gerontology and geriatrics. Geriatr Gerontol Int. 2012;12(1):16–22. doi: 10.1111/j.1447-0594.2011.00776.x. [DOI] [PubMed] [Google Scholar]
- 9.Roschel H, Artioli GG, Gualano B. Risk of increased physical inactivity during COVID-19 outbreak in older people: A call for actions. J Am Geriatr Soc. 2020;68(6):1126–28. doi: 10.1111/jgs.16550. [DOI] [PubMed] [Google Scholar]
- 10.Schuch F, Bulzing R, Meyer J, et al. Moderate to vigorous physical activity and sedentary behavior change in self-isolating adults during the COVID-19 pandemic in Brazil: A cross-sectional survey exploring correlates. medRxiv. 2020;2020:20154559. doi: 10.1007/s11332-021-00788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. Geriatr Gerontol Int. 2013;98(6):2604–12. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 12.Coker RH, Hays NP, Williams RH, et al. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. 2015;70(1):91–96. doi: 10.1093/gerona/glu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosqucéric G, Sebag A, Ducolombier C, et al. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96(5):895–901. doi: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 14.Esain I, Gil SM, Bidaurrazaga-Letona I, et al. Effects of 3 months of detraining on functional fitness and quality of life in older adults who regularly exercise. Aging Clin Exp Res. 2019;31(4):503–10. doi: 10.1007/s40520-018-0990-1. [DOI] [PubMed] [Google Scholar]
- 15.Guthold R, Stevens GA, Riley LM, et al. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Global Health. 2018;6(10):e1077–e86. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 16.Sepúlveda-Loyola W, Rodríguez-Sánchez I, Pérez-Rodríguez P, et al. Impact of social isolation due to COVID-19 on health in older people: Mental and physical effects and recommendations. J Nutr Health Aging. 2020;24(9):938–47. doi: 10.1007/s12603-020-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–56. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 18.Stevens JA, Corso PS, Finkelstein EA, et al. The costs of fatal and non-fatal falls among older adults. Injury Prevent. 2006;12(5):290–95. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: A prospective study. J Gerontol. 1991;46(5):M164–70. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 20.Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: A review of the epidemiologic evidence. J Am Geriatr Soc. 2000;48(8):883–93. doi: 10.1111/j.1532-5415.2000.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 21.Haskell WL, Lee IM, Pate RR, et al. ACSM/AHA recommendations. Circulation. 2007;116(9):1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 22.Puts M, Lips P, Deeg D. Static and dynamic measures of frailty predicted decline in performance-based and self-reported physical functioning. J Clin Epidemiol. 2005;58(11):1188–98. doi: 10.1016/j.jclinepi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Szulc P, Duboeuf F, Marchand F, et al. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80(2):496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 24.Pišot R, Marusic U, Biolo G, et al. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol. 2016;120(8):922–29. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 25.Purssell E, Gould D, Chudleigh J. Impact of isolation on hospitalised patients who are infectious: Systematic review with meta-analysis. BMJ Open. 2020;10(2):e030371. doi: 10.1136/bmjopen-2019-030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Chan JS, Yan JH. Neuropsychological mechanisms of falls in older adults. Front Aging Neurosc. 2014;6:64. doi: 10.3389/fnagi.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demanze Laurence B, Michel L. The fall in older adults: Physical and cognitive problems. Curr Aging Sci. 2017;10(3):185–200. doi: 10.2174/1874609809666160630124552. [DOI] [PubMed] [Google Scholar]
- 28.Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence: Unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–53. [PubMed] [Google Scholar]
- 29.Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;(4):CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- 30.Cvecka J, Tirpakova V, Sedliak M, et al. Physical activity in elderly. Eur J Transl Myol. 2015;25(4):249–52. doi: 10.4081/ejtm.2015.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia RNSdA, Costa SN, Garcia EDSdA, et al. Does home-based exercise improve the physical function of prefrail older women? Rejuvenation Res. 2021;24(1):6–13. doi: 10.1089/rej.2019.2292. [DOI] [PubMed] [Google Scholar]
- 32.Maniar N, Bennett E, Hand S, et al. The effect of mobile phone screen size on video based learning. J Softw. 2008;3(4):51–61. [Google Scholar]
- 33.Jung IK, Kwak DI, Joe SH, et al. a study of standardization of Korean Form of Geriatric Depression Scale (KGDS) J Korean Geriatr Psychiatry. 1997;1(1):61–72. [Google Scholar]
- 34.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 35.Wang CY, Chen LY. Grip strength in older adults: Test-retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91(11):1747–51. doi: 10.1016/j.apmr.2010.07.225. [DOI] [PubMed] [Google Scholar]
- 36.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–48. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 37.Salminen M, Vahlberg T, Sihvonen S, et al. Effects of risk-based multifactorial fall prevention on postural balance in the community-dwelling aged: A randomized controlled trial. Arch Gerontol Geriatr. 2009;48(1):22–27. doi: 10.1016/j.archger.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Ceria-Ulep CD, Grove J, Chen R, et al. Physical aspects of healthy aging: Assessments of three measures of balance for studies in middle-aged and older adults. Curr Gerontol Geriatr Res. 2010;2010:849761. doi: 10.1155/2010/849761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguiñaga S, Ehlers DK, Salerno EA, et al. Home-based physical activity program improves depression and anxiety in older adults. J Phys Act Health. 2018;15(9):692–96. doi: 10.1123/jpah.2017-0390. [DOI] [PubMed] [Google Scholar]
- 41.Ströhle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009;116(6):777–84. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- 42.Kandola A, Ashdown-Franks G, Hendrikse J, et al. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 2019;107:525–39. doi: 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 43.Lord SR, Murray SM, Chapman K, et al. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57(8):M539–43. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 44.Frontera WR. Physiologic changes of the musculoskeletal system with aging: A brief review. Phys Med Rehabil Clin N Am. 2017;28(4):705–11. doi: 10.1016/j.pmr.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Lacroix A, Kressig RW, Muehlbauer T, et al. Effects of a supervised versus an unsupervised combined balance and strength training program on balance and muscle power in healthy older adults: A randomized controlled trial. Gerontology. 2016;62(3):275–88. doi: 10.1159/000442087. [DOI] [PubMed] [Google Scholar]
- 46.Opdenacker J, Delecluse C, Boen F. A 2-year follow-up of a lifestyle physical activity versus a structured exercise intervention in older adults. J Am Geriatr Soc. 2011;59(9):1602–11. doi: 10.1111/j.1532-5415.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 47.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 48.Sayer AA, Kirkwood TB. Grip strength and mortality: A biomarker of ageing? Lancet (London, England) 2015;386(9990):226–27. doi: 10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh TJ, Su SC, Chen CW, et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1):1–15. doi: 10.1186/s12966-019-0855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park J, Yim J. A new approach to improve cognition, muscle strength, and postural balance in community-dwelling elderly with a 3-D virtual reality kayak program. Tohoku J Exp Med. 2016;238(1):1–8. doi: 10.1620/tjem.238.1. [DOI] [PubMed] [Google Scholar]
- 51.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Kan GA, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–89. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto H, Demura S, Nagasawa Y, et al. Changes in the physical functions of pre-frail elderly women after participation in a 1-year preventative exercise program. Geriatr Gerontol Int. 2014;14(4):975–82. doi: 10.1111/ggi.12198. [DOI] [PubMed] [Google Scholar]
- 54.JudgeRoy JO, Davis B, III, Õunpuu S. Step length reductions in advanced age: The role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci. 1996;51(6):M303–12. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- 55.Scarborough DM, Krebs DE, Harris BA. Quadriceps muscle strength and dynamic stability in elderly persons. Gait posture. 1999;10(1):10–20. doi: 10.1016/s0966-6362(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 56.Daley MJ, Spinks WL. Exercise, mobility and aging. Sports Med. 2000;29(1):1–12. doi: 10.2165/00007256-200029010-00001. [DOI] [PubMed] [Google Scholar]
- 57.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 58.Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–91. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 60.Kamide N, Shiba Y, Shibata H. Effects on balance, falls, and bone mineral density of a home-based exercise program without home visits in community-dwelling elderly women: A randomized controlled trial. J Physiol Anthropol. 2009;28(3):115–22. doi: 10.2114/jpa2.28.115. [DOI] [PubMed] [Google Scholar]
- 61.Dondzila CJ, Swartz AM, Keenan KG, et al. Translating exercise interventions to an in-home setting for seniors: Preliminary impact on physical activity and function. Aging Clin Exp Res. 2016;28(6):1227–35. doi: 10.1007/s40520-015-0518-x. [DOI] [PubMed] [Google Scholar]
- 62.Glaister BC, Bernatz GC, Klute GK, et al. Video task analysis of turning during activities of daily living. Gait posture. 2007;25(2):289–94. doi: 10.1016/j.gaitpost.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Wright RL, Peters DM, Robinson PD, et al. Differences in axial segment reorientation during standing turns predict multiple falls in older adults. Gait Posture. 2012;36(3):541–45. doi: 10.1016/j.gaitpost.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Cadore EL, Rodríguez-Mañas L, Sinclair A, et al. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013;16(2):105–14. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsugi A, Ueta Y, Oku K, et al. Effect of gaze-stabilization exercises on vestibular function during postural control. Neuroreport. 2017;28(8):439–43. doi: 10.1097/WNR.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 66.Srulijes K, Mack DJ, Klenk J, et al. Association between vestibulo-ocular reflex suppression, balance, gait, and fall risk in ageing and neurodegenerative disease: Protocol of a one-year prospective follow-up study. BMC Neurol. 2015;15(1):1–11. doi: 10.1186/s12883-015-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi W, Han C, Lee S. The effects of head rotation exercise on postural balance, muscle strength, and gait in older women. Women Health. 2020;60(4):426–39. doi: 10.1080/03630242.2019.1662870. [DOI] [PubMed] [Google Scholar]
- 68.Gauchard GC, Gangloff P, Jeandel C, et al. Physical activity improves gaze and posture control in the elderly. Neurosci Res. 2003;45(4):409–17. doi: 10.1016/s0168-0102(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 69.King AC, Haskell WL, Taylor CB, et al. Group-vs home-based exercise training in healthy older men and women: A community-based clinical trial. JAMA. 1991;266(11):1535–42. [PubMed] [Google Scholar]