Abstract
The molecular machinery underlying peroxisomal membrane biogenesis is not well understood. The observation that cells deficient in the peroxins Pex3p, Pex16p, and Pex19p lack peroxisomal membrane structures suggests that these molecules are involved in the initial stages of peroxisomal membrane formation. Pex19p, a predominantly cytosolic protein that can be farnesylated, binds multiple peroxisomal integral membrane proteins, and it has been suggested that it functions as a soluble receptor for the targeting of peroxisomal membrane proteins (PMPs) to the peroxisome. An alternative view proposes that Pex19p functions as a chaperone at the peroxisomal membrane. Here, we show that the peroxisomal sorting determinants and the Pex19p-binding domains of a number of PMPs are distinct entities. In addition, we extend the list of peroxins with which human Pex19p interacts to include the PMP Pex16p and show that Pex19p's CaaX prenylation motif is an important determinant in the affinity of Pex19p for Pex10p, Pex11pβ, Pex12p, and Pex13p.
In the prevailing model of peroxisome biogenesis, peroxisomes arise by the budding and fission of preexisting peroxisomes (22). How nascent peroxisomes acquire the capacity to import appropriate membrane and matrix proteins is a topical subject. Matrix protein import is a sequential process that begins with the recognition of peroxisome targeting sequences (PTSs) in substrate proteins by specific cytosolic receptors (22). After the docking of the receptor-substrate complexes at the cytoplasmic face of the peroxisomal membrane, the transport substrates are translocated into the peroxisomal matrix. Whether the receptors are imported along with the transport substrates is still a matter of debate. To date, the soluble receptors (Pex5p and Pex7p) as well as the docking proteins (Pex13p and Pex14p) have been identified in many organisms, including mammals (12, 32, 33). Other mammalian peroxins that are implicated in peroxisomal matrix protein translocation are the RING finger proteins Pex2p, Pex10p, and Pex12p and the AAA-ATPases Pex1p and Pex6p (6, 17).
Compared with matrix protein import, our knowledge about peroxisomal membrane protein (PMP) import remains extremely limited. The vast majority of PMPs appear to be synthesized on cytosolic ribosomes and posttranslationally inserted into the peroxisomal membrane (20). The targeting signals (designated mPTSs) of only a few integral PMPs have been defined, and at first glance, these mPTSs do not possess a readily identifiable conserved primary amino acid sequence. However, the mPTSs of Candida boidinii PMP47 (CbPMP47) (7, 34), fungal and human Pex3p (2, 21, 31, 35), Saccharomyces cerevisiae Pex15p (ScPex15p) (8), Pichia pastoris Pex22p (PpPex22p) (22), and human PMP34 (19) all contain patches of positively charged amino acids and are, with the exception of human PMP34, thought to be localized to the matrix side of the peroxisome membrane. The mPTSs of these PMPs all require a transmembrane domain (TMD) to be functional. In rat PMP22, the mPTS is located at the N-terminal cytoplasmic tail of PMP22 and, in addition, requires two TMDs to be functional (27). In the case of human PMP34, the loop region between transmembrane segments 4 and 5 plus three additional transmembrane segments function as a peroxisomal targeting and topogenic signal (19).
Experiments conducted with PMP22 in an in vitro transcription and translation system revealed that, in the postribosomal supernatant, PMP22 is present in two polypeptide complexes (26). In complex I, PMP22 is associated with the cytosolic chaperonin TCP1 ring complex (TRiC). In complex II, PMP22 is bound to a single 40-kDa polypeptide (P40). TriC may maintain PMP22 in a transport-compatible conformation (26). Based on the observation that PMP22 is predominantly inserted into the peroxisomal membrane when present in complex II, it is tempting to speculate that P40 may function as a cytosolic PMP import receptor. However, attempts to identify P40 at the molecular level have not been successful (26). Another PMP import receptor candidate is Pex19p, a predominantly cytosolic peroxin that contains a C-terminal CaaX box, which represents a site for farnesylation (29). Pex19p binds a broad spectrum of PMPs, and cells with a deficiency of this peroxin lack peroxisomal membrane structures (18, 25, 28). These data, combined with the observations that Pex19p interacts with the mPTSs of some PMPs (28) and that a small but significant amount of Pex19p is also associated with the outer surface of peroxisomes, make Pex19p a prime candidate for a cycling PMP receptor protein (17). However, Snyder et al. (29) recently suggested that, at least in the yeast P. pastoris, Pex19p does not function as a general PMP protein import receptor but rather acts as a type of molecular chaperone to facilitate the insertion and orientation of PMPs in the peroxisome membrane. Currently, the role of Pex19p farnesylation in peroxisome biogenesis is not clear. In S. cerevisiae, prenylation of Pex19p is essential for its proper biological activity, and the primary role of the farnesyl moiety is to trigger the binding properties of Pex19p (16). In humans, farnesylation of Pex19p is required for peroxisomal localization (24). However, whether or not farnesylation of human Pex19p (HsPex19p) is essential for peroxisome biogenesis (24) or has an ancillary function (28) is not clear. In P. pastoris, the farnesylation consensus sequence of Pex19p is dispensable for its function (30).
The observation that pretreating peroxisomes with proteases significantly reduces PMP binding and completely abolishes PMP insertion (20) indicated the involvement of PMPs in the PMP docking and membrane insertion process. To date, only the integral membrane proteins Pex3p and Pex16p have been directly implicated in PMP protein import. Cells deficient in these peroxins mislocalize PMPs and have no peroxisomal remnants (17). However, the exact function of these peroxins in the PMP assembly process is not known.
In this study, we investigated whether human Pex19p functions as the mPTS receptor. We have carefully defined the Pex19p-binding domains and the sorting sequences of a number of peroxisomal integral membrane proteins. Our results provide evidence that human Pex19p binds PMPs at regions distinct from their sorting sequences and, as a result, does not function as the mPTS receptor. Furthermore, we describe a novel interaction of Pex19p and investigate the role of Pex19p's prenylation signal.
MATERIALS AND METHODS
Plasmid constructions and mutagenesis.
The cDNA fragments encoding the analyzed proteins were amplified by PCR with the appropriate primers and cloned into the yeast two-hybrid vectors pGBT9 or pGAD424 (Clontech), the mammalian expression vectors pEGFP-N1, pEGFP-C1, or pDsRed-N1 (Clontech), or the bacterial expression vectors pQE32 (Qiagen) or pBADHisA,B (InVitrogen). PCRs were routinely performed using Pfx DNA polymerase (Life Technologies). Error-prone PCR mutagenesis was performed exactly as described by Cadwell and Joyce (4) by using cloned Taq DNA polymerase (Amersham Pharmacia Biotech). Missense mutations were separately introduced into the full-length BD-Pex13p and Pex13p-green fluorescent protein (GFP) fusion proteins by sequential PCR steps using primers designed to incorporate the desired point mutations. The identities of all essential constructs were confirmed by DNA sequencing. Bacterial manipulations were carried out in the Escherichia coli strain Top10F′ (InVitrogen). The detailed cloning procedures (including the list of oligonucleotides) of the extensive number of constructs can be obtained from the corresponding authors.
Cell culture, transfections, and fluorescence microscopy.
Chinese hamster ovary (CHO) cells were cultured in alpha minimal essential medium supplemented with 10% (vol/vol) fetal calf serum, 100 μg of penicillin G/ml, 100 μg of streptomycin sulfate/ml, and 0.25 μg of amphotericin B/ml in a humidified 37°C, 5% CO2 incubator. The cells were transiently transfected by the polyethylenimine transfection method (3). After transfer to coverslips, the cells were processed for direct or indirect (immuno)fluorescence as described (10). The peroxisomal localization of GFP-fusion proteins was confirmed by colocalization studies with the peroxisomal membrane marker protein Pex14p or the peroxisome-targeted DsRed-KSKL reporter protein. Fluorescence was observed under a Leica DMR microscope equipped with standard fluorescein isothiocyanate and rhodamine isothiocyanate filters.
Fractionation of CHO cells.
Transfected CHO cells, grown in culture dishes to 90% confluency, were washed twice with phosphate-buffered saline and freed from the culture dishes by scraping. To isolate the total membrane fraction, the scraped cells were resuspended in buffer A (10 mM morpholinepropanesulfonic acid [MOPS]-NaOH buffer–1 mM EDTA–1 mM dithiothreitol–0.1% [vol/vol] ethanol; pH 8.0) and sonicated in ice with a Branson Sonifier B15 P Cell Disrupter equipped with a microtip (output 5, duty 50%; six times for 15 s each time). After centrifugation for 1 h at 100,000 × g, the pellet was resuspended in buffer A and the entire procedure was repeated. To isolate a membranous fraction containing only integral membrane proteins, the scraped cells were resuspended in 0.1 M Na2CO3 (pH 11), homogenized with a Teflon-glass Potter-Elvehjem homogenizer (20 strokes), and subjected to a 100,000 × g spin for 1 h (13).
Two-hybrid analysis and blot overlay assays.
The two-hybrid reporter strain SFY526 (Clontech) was used for all yeast two-hybrid experiments. The transformation of two-hybrid vectors into competent yeast cells, the colony lift β-galactosidase filter assay, and the liquid culture β-galactosidase assay with o-nitrophenyl-β-d-galactopyranoside as substrate were performed as described by the manufacturer (Clontech). However, for the liquid culture β-galactosidase assay, the yeast cells were grown for 72 h in minimal dropout medium without leucine and tryptophan. Blot overlay assays were performed as previously described (9, 11).
Antibodies.
Polyclonal antisera against His6-GFP (encoded by pEGFPH1, a plasmid kindly provided by Y. Sakai [Kyoto, Japan]), His6-HsPex13p/SH3 (10), His6-HsPex14p (10), His6-HsPex19p, and His6-HsPex3p(229-365) were raised in New Zealand White rabbits as previously described (1). The anti-Pex5p antiserum was kindly provided by M. Baes (Leuven, Belgium). Animal care approval was granted by the local institutional ethics committee.
RESULTS
Pex19p interacts with multiple peroxisomal integral membrane proteins.
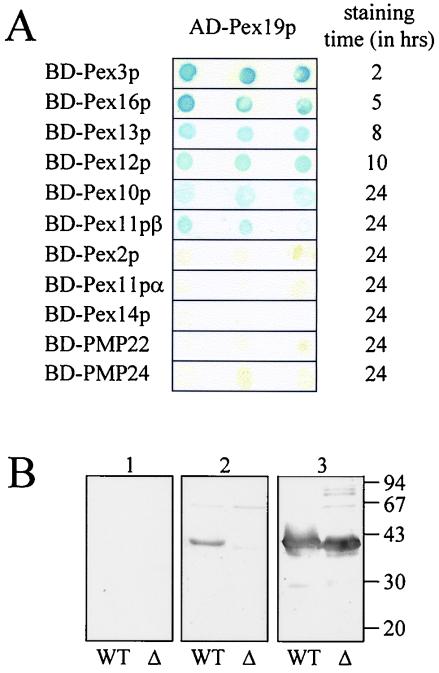
As part of our ongoing attempts to define a network of interacting mammalian peroxins, we used yeast two-hybrid assays to identify Pex19p-interacting proteins. Pex19p, fused to the Gal4p activation domain (AD), was screened against rat PMP22, human PMP24, and the presently identified mammalian integral membrane peroxins, which were all fused to the Gal4p DNA-binding domain (BD). When double transformants were selected, lysed, and incubated with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), SFY526 yeast cells expressing Pex19p and Pex3p, Pex10p, Pex11pβ, Pex12p, Pex13p, or Pex16p turned blue (Fig. 1A). While Pex19p interacted strongly with Pex3p and Pex16p, its interaction with Pex10p and Pex11pβ was rather weak. None of the Gal4p fusion proteins alone were able to autoactivate the transcription of the lacZ reporter gene (data not shown). However, since Pex19p autoactivates as a fusion with BD, we were not able to reverse the positions of the prey and bait vectors. No interaction of Pex19p could be observed with the integral membrane proteins Pex2p, Pex11pα, Pex14p, PMP22, and PMP24 (Fig. 1A).
FIG. 1.
Pex19p interacts with multiple integral PMPs. (A) Human Pex19p, fused to the Gal4p AD, was tested for interaction with all known mammalian integral membrane peroxins, as well as with PMP22 and PMP24 fused to the Gal4p DNA-BD in the yeast two-hybrid system. Double transformants were selected and tested for β-galactosidase expression by using a filter assay with X-Gal as the substrate. Three representative independent transformants are shown. (B) Two micrograms of purified His6-tagged Pex19p(1-299) (WT) and Pex19p(31-299) (Δ) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and incubated with a bacterial lysate containing no recombinant protein (panel 1), with His6-Pex3p(44-373) (panel 2), or with an anti-Pex19p antiserum (panel 3). Pex19p-Pex3p complexes were visualized by using an anti-Pex3p antiserum (1, 2). The migration of the molecular mass markers (in kilodaltons) is indicated.
In order to verify the two-hybrid data, we further examined the binding properties of HsPex19p in vitro. For this, we immobilized purified recombinant His6-Pex19p on nitrocellulose and examined its interaction with bacterially expressed PMPs. Using this approach, we could confirm the interaction of Pex19p with Pex3p (Fig. 1B). Unfortunately, as proteins containing two or more TMDs were poorly expressed and insoluble (data not shown), no conclusions could be drawn for the in vitro interactions of Pex19p with the other PMPs.
Mapping of the Pex19p-binding sites of Pex3p, Pex12p, Pex13p, and Pex16p.
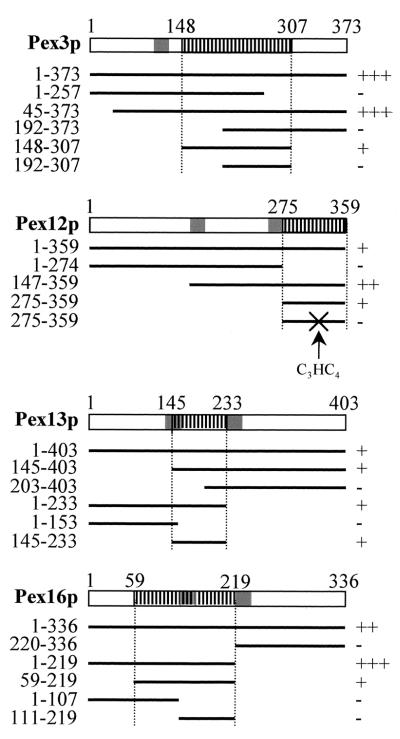
The yeast two-hybrid system was again employed to delineate the Pex19p-BDs of the Pex19p-interacting PMPs. Since the binding of Pex19p to Pex10p and Pex11pβ resulted in such a weak expression of the lacZ reporter gene (Fig. 1A), we narrowed down only the Pex19p-BDs of Pex3p, Pex12p, Pex13p, and Pex16p (Fig. 2). For Pex3p, the Pex19p-BD was located to the C-terminal portion (amino acids 148 to 307) of the protein, which is exposed to the cytosol (14, 21, 31). For Pex12p, the Pex19p-BD was also localized to the C-terminal part (amino acids 275 to 359) of the protein. This region of Pex12p contains a C3HC4 ring finger motif, which is reported to be essential for Pex12p function and its ability to interact with Pex5p and Pex10p (5, 25). That the Pex12p-Pex19p interaction is indeed mediated by the C3HC4 ring finger motif is further illustrated by the fact that mutating the C3HC4 ring residues C-1 to W at position 304 and C-2 to Q at position 307 abolished binding (Fig. 2). For Pex13p, the Pex19p-BD was localized to the central matrix loop of Pex13p. The Pex19p-binding site of Pex16p encompasses amino acids 59 to 219. Notice that the delineated Pex19p-BDs of Pex3p and Pex16p displayed a much weaker interaction than the corresponding full-length proteins. This may suggest that the affinity or folding of this region is influenced by the corresponding deletions.
FIG. 2.
Mapping of the Pex19p-binding sites of Pex3p, Pex12p, Pex13p, and Pex16p. Deletion constructs of Pex3p, Pex12p, Pex13p, and Pex16p fused to Gal4p-BD were tested for interaction with Pex19p fused to the Gal4p-AD in the yeast two-hybrid system. Double transformants were selected and assayed for β-galactosidase activity by using a filter assay with X-Gal as the substrate. The colony staining times were less than 2 h (+++), less than 5 h (++), or less than 10 h (+) or the colonies did not stain at all (−). TMDs are shaded. The smallest delineated Pex19p-BDs are hatched with vertical lines. Residue numbers are on top and on the left. X, alterations of the Pex12p C3HC4 RING residues from C-1 to W at position 304 and C-2 to Q at position 307.
Mapping of the mPTSs of Pex3p, Pex12p, Pex13p, and Pex16p.
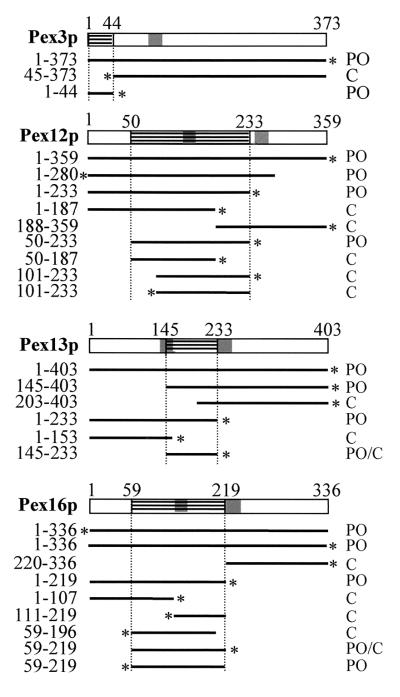
In order to delineate the mPTSs of Pex3p, Pex12p, Pex13p, and Pex16p, GFP-tagged deletion proteins were expressed in CHO cells and the localization of the fusion proteins was determined by direct fluorescence microscopy (Fig. 3). Representative pictures of transfected CHO cells that illustrate the observed staining patterns of the GFP-fusion proteins are shown in Fig. 4. Our results demonstrate that all the information for the sorting of Pex3p to the peroxisome membrane is contained within the amino-terminal 45 amino acids (Fig. 3). These observations confirm the results of Kammerer et al. (21) and Soukupova et al. (31), who determined that the first 40 and 33 amino acids of human Pex3p, respectively, are sufficient to target a reporter protein to the peroxisome membrane. The N-terminal 33 amino acids of Pex3p include a hydrophobic region (amino acids 18 to 33) that, in the holomolecule, is thought to be localized inside the peroxisome (21, 31).
FIG. 3.
Mapping of the mPTS of Pex3p, Pex12p, Pex13p, and Pex16p. CHO cells were transiently transfected with plasmids expressing deletion fragments of Pex3p, Pex12p, Pex13p, and Pex16p N terminally or C terminally fused to GFP (*). After 24 h, the cells were processed for direct fluorescence and the subcellular localization of the GFP-fusion proteins was determined: peroxisome (PO), cytosol (C), and peroxisome-cytosol (PO/C). TMDs are shaded. Residue numbers are on top and on the left. The smallest delineated domains sufficient to target the GFP reporter protein to the peroxisomes are hatched with horizontal lines.
FIG. 4.
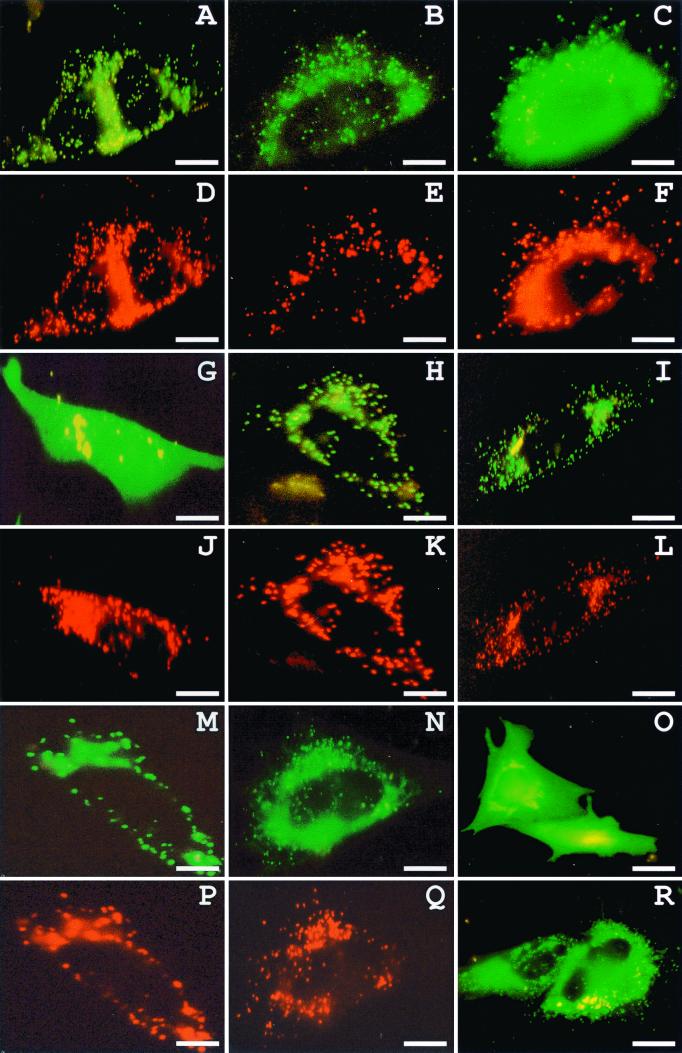
Targeting of Pex13p-GFP fusion proteins in CHO cells. CHO cells transiently transfected with plasmids expressing the peroxisomal marker protein DsRed-KSKL (D, E, F, J, K, L, P, Q) and Pex13p(1-403) (A), Pex13p(145-233) (B), Pex13p(155-233) (C), Pex13p(159-233) (G), Pex13p(1-403)V164E (H), Pex13p(1-403)L191P (I), Pex13p(136-233) (M), Pex13p(145-233)R186W, S214C (N), Pex13p(145-233)F158S, V164E (O), or Pex13p(116-197) (R) N terminally fused to GFP were examined for direct fluorescence 24 h after transfection. The subcellular localization of the GFP-fusion proteins was determined by the staining pattern: peroxisome (A, H, I, M), peroxisome-cytosol (B, N), cytosol-peroxisome (C), cytosol (G), endoplasmic reticulum-cytosol (O), and endoplasmic reticulum-cytosol-peroxisome (R). The punctate structures observed (A, B, C, H, I, M, N) are peroxisomes, as illustrated by their colocalization with DsRed-KSKL. Bar = 10 μm.
Sorting of Pex12p to the peroxisome membrane is mediated by the region present between the amino acid residues 50 and 233 (Fig. 3). Recently, it was reported that the N-terminal region of Pex12p, including the amino acids from position 17 to 76, was necessary, but not sufficient, for peroxisomal localization (25). In addition, internal deletions of 21 and 9 amino acids at positions 77 to 97 and 98 to 106, respectively, did not prevent the peroxisomal localization of the corresponding deletion proteins. These data, combined with our results, suggest that the topogenic information of Pex12p does not reside within a linear sequence but rather consists of two cooperatively acting subdomains. However, as amino acid residues 17 to 76 of Pex12p are also required for its stability in cells (25), the direct involvement of the N-terminal region of Pex12p in targeting still has to be demonstrated.
For Pex13p, we found that amino acid residues 145 to 233 drive the import of this PMP into the peroxisome membrane (Fig. 3). In transfected CHO cells expressing fusion proteins that still contain this domain, GFP fluorescence was observed in numerous punctate structures (Fig. 4A, B, and M) that could be identified as peroxisomes (Fig. 4D, E, and P). For Pex16p, all information required for sorting to the peroxisome membrane is provided by the amino acid residues present between positions 59 and 219 (Fig. 3). These amino acids were also found to be required for Pex19p binding (Fig. 2).
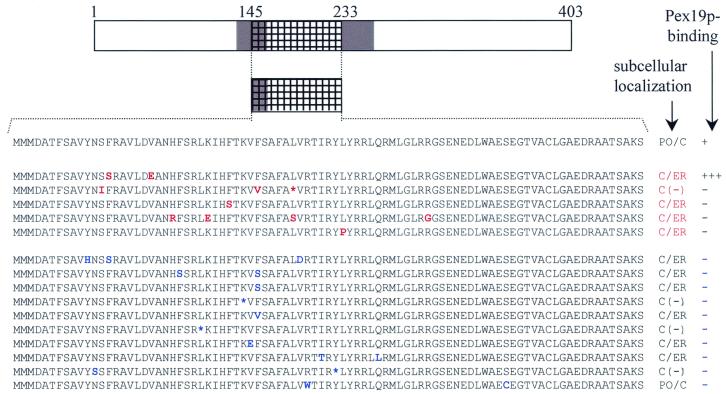
Pex19p-binding site and mPTS of Pex13p can be functionally separated.
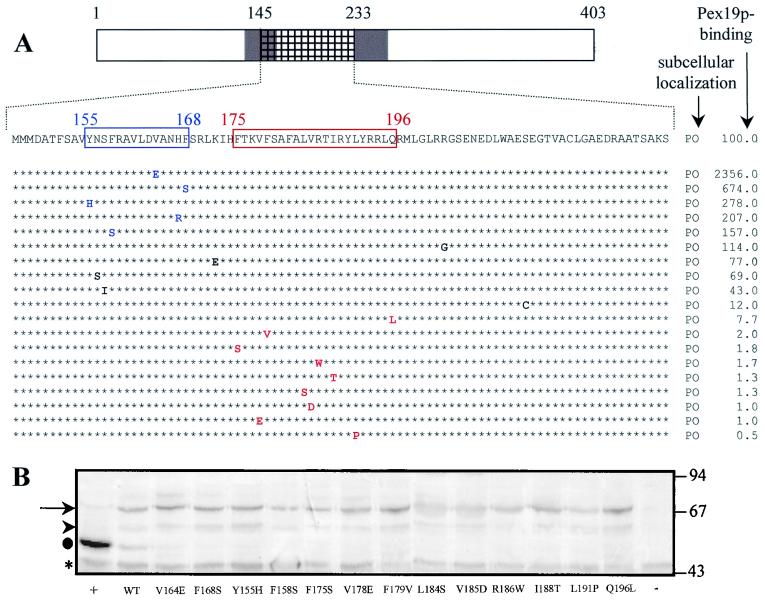
Recently, it was suggested that Pex19p may function as a general PMP import receptor (28). Our results clearly show that, in the case of Pex3p and Pex12p, the Pex19p-BD (Fig. 2) and the mPTS (Fig. 3) of these proteins do not physically overlap. The fact that Pex19p does not bind the mPTSs of Pex3p and Pex12p is difficult to reconcile with the hypothesis that Pex19p directly mediates the targeting of these PMPs to the peroxisome membrane. However, the Pex19p-BDs (Fig. 2) and the mPTSs (Fig. 3) of Pex13p and Pex16p do physically overlap. To investigate whether the Pex19p-binding site and the mPTS in Pex13p are also functionally linked, we subjected the cDNA fragment of Pex13p encoding the amino acids 145 to 233 to error-prone PCR mutagenesis. After cloning of the resulting PCR products into the yeast two-hybrid vector pGBT9 (82 clones) or the mammalian expression vector pEGFP-N1 (84 clones), the corresponding BD- and GFP-fusion proteins were screened for mutants displaying an altered Pex19p-binding affinity or a different subcellular localization pattern from Pex13p(145-233). Five mutants unable to target the GFP-reporter protein to the peroxisomes and 10 mutants displaying no Pex19p-binding affinity were isolated (Fig. 5). Exchanging the cDNA inserts from the selected clones between pGBT9 and pEGFP-N1, followed by an appropriate analysis of the corresponding fusion proteins, revealed that (i) one mutant which mislocalized the GFP-reporter protein (Fig. 4O) displayed a strong Pex19p-binding affinity (Fig. 5) and (ii) another mutant displayed no binding affinity for Pex19p (Fig. 5), yet carried peroxisomal targeting information (Fig. 4N). These data indicate that for Pex13p, Pex19p binding and peroxisomal sorting can be functionally separated. A cDNA sequence analysis of the 15 selected Pex13p (amino acids 145 to 233) mutants revealed the occurrence of 4 silent mutations (data not shown), 19 missense mutations (Fig. 5), and 4 nonsense mutations (Fig. 5). To determine the amino acids of Pex13p that are critical for Pex19p binding or peroxisomal targeting, we introduced the missense mutations individually into the full-length Pex13p molecule (Fig. 6). The resulting Pex13p(1-403) mutants were analyzed for their Pex19p-binding affinity as well as their ability to target the GFP-reporter protein to peroxisomes (Fig. 6A). Compared to wild-type Pex13p, five Pex13p(1-403) mutants displayed an enhanced Pex19p-binding affinity (Fig. 6A). In contrast, eight mutants lost Pex19p-binding affinity. That the altered Pex19p-binding affinities of the Pex13p(1-403) mutants are not indirectly the result of an enhanced or decreased stability of the corresponding BD-fusion proteins is illustrated by the fact that the expression levels of both the wild-type and mutant proteins are similar (Fig. 6B). Interestingly, all the amino acids that seem to be critical for Pex19p binding are clustered in the region flanked by amino acids 175 and 196 (Fig. 6A). Surprisingly, none of the 19 selected amino acid substitutions affected the peroxisomal targeting of the full-length Pex13p-GFP reporter protein (Fig. 6A). These data provide additional evidence that, at least for Pex13p, Pex19p binding and peroxisomal sorting are not functionally linked.
FIG. 5.
The mPTS and the Pex19p-binding site of Pex13p can be functionally separated. Pex13p cDNA encoding the amino acids from position 145 to 233 was subjected to error-prone PCR. The resulting PCR products were subcloned into pEGFP-N1 or pGBT9, and clones with a subcellular distribution pattern (red) or Pex19p-binding affinity (blue) different from the wild-type fragment were selected and sequenced. The corresponding amino acid mutations are, depending on the selection procedure, indicated in red or in blue. The cDNAs coding for proteins with an altered Pex19p-binding affinity (or subcellular distribution pattern) were transferred into the pEGFP-N1 (or pGBT9) vector and further analyzed for the subcellular localization (or Pex19p binding) of the corresponding GFP-fusion protein (or BD-fusion protein). Translational stops are indicated by an asterisk. The weak cytosolic staining pattern [C(−)] observed in CHO cells expressing mutants with premature stop codons is most likely the result of the presence of a functional weak start codon further downstream in the GFP-fusion protein. The other GFP-fusion proteins were bimodally distributed between the peroxisomes and the cytosol (PO/C) or the cytosol and the endoplasmic reticulum (C/ER). The TMDs are shaded. The fragment subjected to random mutagenesis is hatched.
FIG. 6.
The Pex13p amino acids from position 175 to 196 are essential for Pex19p binding but not for protein sorting. (A) The missense mutations obtained by random mutagenesis were separately introduced into the full-length BD-Pex13p and Pex13p-GFP molecules. The corresponding mutants were analyzed for their ability (i) to target the GFP reporter protein to the peroxisomes (PO) and (ii) to bind Pex19p in the two-hybrid system. To compare the binding affinities between the different mutants, the expression of the yeast two-hybrid lacZ reporter gene was quantitatively measured by using o-nitrophenyl-β-d-galactopyranoside as the substrate. The results (average of three independent clones), expressed as the percentage of the observed β-galactosidase activity of wild-type BD-Pex13p, are shown. Amino acids that, when mutated, enhance Pex19p binding are blue. Mutations resulting in a negative staining pattern when assayed for β-galactosidase activity using a filter assay with X-Gal as the substrate are red. TMDs are shaded, and the fragment that originally was subjected to error-prone PCR is hatched. (B) The mutants displaying an enhanced or reduced Pex19p-binding affinity were equally expressed in the yeast reporter strain SFY526. Double yeast transformants were selected and analyzed for the expression of the BD-fusion proteins by using an anti-Pex13p antiserum. →, full-length BD-Pex13p proteins; ▸, putative degradation products; ●, the C-terminal 269 amino acids of Pex13p, expressed as a BD-fusion protein (+); *, nonspecific anti-Pex13p-cross-reactive yeast proteins. In the yeast transformant (−), the BD-domain was fused to Pex14p. The migrations of the molecular mass markers (masses in kilodaltons) are indicated.
Why single amino acid substitutions like V178E and L191P affect the peroxisomal localization of Pex13p(145-233) (Fig. 5), but not that of Pex13p(1-403) (Fig. 4H and I and 6A), is not clear. One possibility is that regions flanking the peroxisomal sorting signal cooperate with this signal to enhance the overall rate or efficiency of insertion. Such an effect may be more pronounced in cases where the mutated sorting signal is less efficient. Another possibility is that Pex13p may contain more than one peroxisomal sorting determinant.
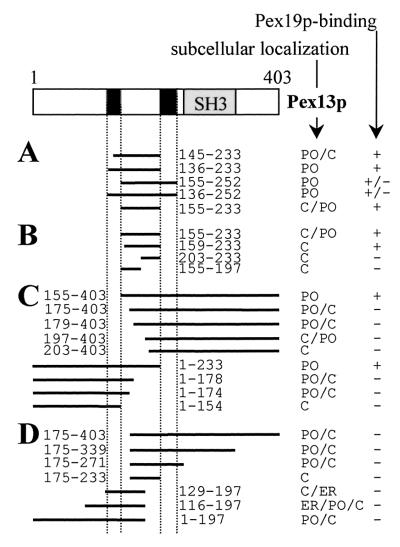
Pex13p contains multiple partially functional mPTSs that cooperate.
Although deletion analysis studies of Pex13p suggested that the import of this protein into the peroxisome membrane is driven by the region spanning the amino acid residues 145 to 233 (Fig. 3), error-prone mutagenesis studies indicated that Pex13p may contain more than one peroxisomal sorting determinant or that regions flanking the peroxisomal sorting determinant may cooperate with the mPTS (Fig. 5 and 6). In order to clarify these results, we conducted an additional series of Pex13p deletion analysis studies (Fig. 7).
FIG. 7.
Pex13p contains multiple partially functional sorting signals. (A to D) CHO cells were transiently transfected with plasmids expressing Pex13p deletion proteins N-terminally fused to GFP. After 24 h, the cells were processed for direct fluorescence and the subcellular localization of the fusion proteins was determined by staining pattern: peroxisome (PO), cytosol (C), peroxisome-cytosol (PO/C), cytosol-peroxisome (C/PO), cytosol-endoplasmic reticulum (C/ER), and endoplasmic reticulum-cytosol-peroxisome (ER/PO/C). The TMDs and the SH3-domain are shaded in black and in gray, respectively. The Pex19p-binding affinities of the corresponding BD-fusion proteins, analyzed in the yeast two-hybrid system, are also indicated: the BD-fusion protein either interacted with Pex19p (+), interacted weakly with Pex19p (+/−), or did not interact with Pex19p (−).
We first investigated the effect of the TMDs flanking the central matrix loop on Pex13p protein sorting (Fig. 7A). Adding nine additional amino acids to the N terminus of Pex13p(145-233)-GFP, a protein which contains only the 10 C-terminal amino acids of TMD1 and displays a combined peroxisomal and cytosolic distribution pattern (Fig. 4B), resulted in a fusion protein, Pex13p(136-233)-GFP, that is exclusively targeted to peroxisomes (Fig. 4M). Similar results were obtained for fusion proteins where TMD2 alone, or together with TMD1, was flanking the central matrix loop. Deleting the residual 10 C-terminal amino acids of TMD1 at the N terminus of Pex13p(145-233) resulted in a predominantly cytosolic fusion protein, Pex13p(155-233)-GFP (Fig. 4C). However, a small part of this fusion protein was also associated with peroxisomes (Fig. 4C and F). These results suggest that the central luminal domain of Pex13p contains the targeting information and that the TMDs function as an “anchor” sequence to drive bound Pex13p into the peroxisome membrane.
In order to narrow down the mPTS of Pex13p, three novel constructs were generated (Fig. 7B). However, all the corresponding Pex13p-GFP fusion proteins displayed an exclusively cytosolic staining pattern (Fig. 4G). Although these results did not yield a shorter targeting motif, they underscore the importance of the tetrapeptide YNSF (position 155 to 158) for targeting. Notice that this tetrapeptide is not essential for Pex19p binding (Fig. 7B), illustrating once again that Pex19p-binding and Pex13p sorting can be functionally uncoupled.
Since (i) we have indirect evidence that regions flanking the central matrix loop of Pex13p may cooperate in the peroxisomal sorting process (Fig. 5 to 6) and (ii) the targeting motif in the central matrix loop cannot be shortened in the absence of flanking sequences (Fig. 7B), we investigated whether the “minimal targeting motif” could be further narrowed down in the presence of flanking sequences. Indeed, additional deletion analysis studies (Fig. 7C) demonstrated that, under these conditions, the minimal targeting motif in the central matrix loop could be shortened from both sides. Moreover, we identified two nonoverlapping Pex13p-fusion proteins, Pex13p(1-178)-GFP and Pex13p(179-403)-GFP, which were bimodally distributed between the cytoplasm and peroxisomes (Fig. 7C and D). These results show that Pex13p contains multiple peroxisomal sorting signals that can function independently. However, none of these sorting signals alone was able to sort the GFP-reporter protein efficiently to the peroxisomes. From this, we conclude that these multiple sorting signals act cooperatively to ensure peroxisomal localization of the full-length Pex13p molecule. Notice that the flanking information required for proper sorting is not provided by the SH3-domain (Fig. 7D), which is reported to be essential for Pex14p binding (11).
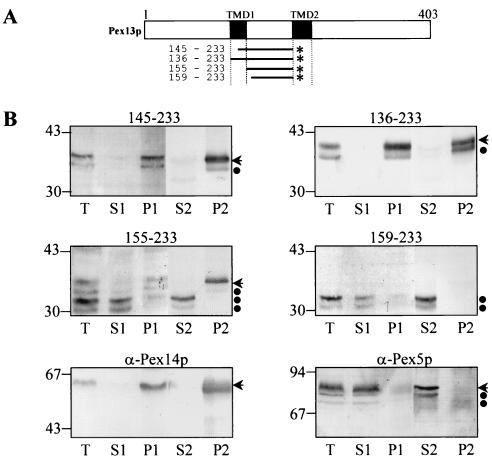
Central matrix loop of Pex13p interacts tightly with peroxisome membrane.
The preceding results suggest that the central luminal domain of Pex13p contains sufficient information to target a reporter protein to the peroxisome membrane and that the flanking TMDs enhance this sorting process, probably by driving bound Pex13p into the lipid bilayer. As expected for proteins that contain a hydrophobic domain, Pex13p(145-233)-GFP and Pex13p(136-233)-GFP were almost exclusively recovered in the membrane fraction, even after carbonate extraction (Fig. 8B). The expression of these Pex13p deletion proteins resulted in two protein species that were detected by Western blotting using an antiserum raised against GFP. Surprisingly, about half of the total amount of Pex13p(155-233)-GFP, a hydrophilic protein containing the central luminal domain of Pex13p (Fig. 8A), could not be removed from the peroxisome membrane (Fig. 8B). This result suggests that Pex13p(155-233)-GFP tightly interacts with another, presently unidentified integral PMP. The observation that Pex13p(159-233)-GFP is completely soluble, even after carbonate extraction (Fig. 8B), further demonstrates that the tetrapeptide YNSF (amino acids 155 to 158) plays a key role in this putative interaction. By comparing the molecular masses of the GFP-fusion proteins, it appears that the soluble forms of Pex13p(155-233)-GFP and Pex13p(159-233)-GFP are proteolytically degraded. Whether or not the partial cytosolic localization of Pex13p(155-233)-GFP is due to its inefficient targeting or to the absence of a hydrophobic domain that drives this protein into the membrane is currently not known. In the latter situation, bound Pex13p(155-233)-GFP can again dissociate from the membrane or, once the Pex13p(155-233)-GFP binding sites are saturated, prevent the association of other Pex13p(155-233)-GFP molecules with the peroxisome membrane. Notice that, by fluorescence microscopy studies, Pex13p(155-233)-GFP was classified as a predominantly cytosolic protein (Fig. 4C). One explanation for this discrepancy is that the fluorescence of GFP, when integrated in a membrane, can be partially quenched (7).
FIG. 8.
The central matrix loop of Pex13p interacts tightly with the peroxisome membrane. (A) Schematic presentation of the Pex13p-deletion mutants fused to the N terminus of GFP (*). The TMDs are shaded in black, and residue numbers are on the left. (B) CHO cells were transiently transfected with plasmids expressing one of the deletion proteins schematically presented in panel A. After 24 h, the cells were fractionated as described in Materials and Methods. Equivalent portions of the total (T), the buffer A-soluble (S1), the buffer A-insoluble (P1), the carbonate-soluble (S2), and the carbonate-insoluble (P2) material were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an antiserum raised against GFP. Similar fractions obtained from nontransfected CHO cells were probed with anti-Pex14p antiserum, an antiserum that specifically recognizes the integral PMP Pex14p (11), and anti-Pex5p antiserum, an antiserum that specifically recognizes the predominantly cytosolic PTS1-protein import receptor Pex5p. Arrows, the GFP fusion proteins Pex5p and Pex14p; ●, degradation products. The migrations of the molecular mass markers (masses shown in kilodaltons) are indicated.
CaaX farnesylation consensus sequence affects binding properties of Pex19p.
Human Pex19p can be farnesylated on the cysteine residue of its carboxy-terminal CaaX motif (24). Since yeast cells prenylate proteins in the same manner as mammalian cells (36), we performed a yeast two-hybrid analysis to investigate whether the farnesylation motif of Pex19p affected its ability to bind Pex3p, Pex10p, Pex11pβ, Pex12p, Pex13p, and Pex16p (Table 1). Deleting the CaaX prenylation motif from Pex19p (Pex19pΔCaaX) decreased its binding to Pex10p, Pex11pβ, Pex12p, and Pex13p nearly to background levels. That this reduced binding is not the result of a lower expression level of Pex19pΔCaaX is indirectly demonstrated by the fact that Pex19pΔCaaX displays a binding strength similar to Pex19p's for Pex3p and Pex16p. Unfortunately, as we presently lack antibodies of sufficient titer to Pex19p, we could not directly confirm the expression levels of Pex19p and Pex19pΔCaaX by immunoblot analysis. These experiments demonstrate that the CaaX farnesylation motif of Pex19p affects its binding properties for Pex10p, Pex11pβ, Pex12p, and Pex13p but not those for Pex3p and Pex16p.
TABLE 1.
CaaX farnesylation sequence affects binding properties of Pex19pa
| BD-hybrid | Optical density
|
|
|---|---|---|
| AD-Pex19p | AD-Pex19pΔCaaX | |
| Pex3p | 163 | 162 |
| Pex16p | 22.2 | 15.1 |
| Pex12p | 0.540 | 0.002 |
| Pex13p | 0.431 | 0.004 |
| Pex11pβ | 0.268 | 0.030 |
| Pex10p | 0.199 | 0.038 |
SFY526 yeast cells, transformed with plasmids encoding one of the indicated Ga14p DNA-BD fusion proteins (BD-hybrid) and the Ga14p AD fused to either Pex19p or Pex19pΔCaaX, were selected for leucine and tryptophan prototrophy. Double transformants were assayed for β-galactosidase using o-nitrophenyl-β-d-galactopyranoside as substrate. The optical densities were measured at 421 nm and normalized for culture densities (optical density at 600 nm = 10) and time (24 h). The values given are the means of three measurements performed on independent single colonies.
Mapping of the peroxin-BDs of Pex19p.
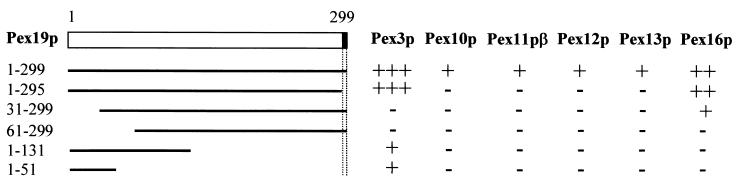
We have already demonstrated that human Pex19p can bind at least six different peroxins (Fig. 1). In addition, we showed that the CaaX farnesylation motif of Pex19p affects its binding to Pex10p, Pex11pβ, Pex12p, and Pex13p but not to Pex3p and Pex16p (Table 1). To further investigate the biological significance of these apparently different binding properties, we determined whether or not the peroxin-binding sites on Pex19p overlapped (Fig. 9). The results show that residues at both termini of Pex19p, including the farnesylation consensus sequence, were required for efficient binding to Pex10p, Pex11pβ, Pex12p, and Pex13p. On the other hand, the Pex3p-binding site on Pex19p could be narrowed down to a region of approximately 50 amino acids at the N terminus (Fig. 1B and 9), whereas the Pex16p-binding site required neither the 30 N-terminal amino acids nor the farnesylation consensus sequence (Fig. 9). That Pex19p has different binding sites for Pex3p and Pex16p suggests that Pex19p may bind both peroxins simultaneously. Since cells deficient in Pex3p, Pex16p, and Pex19p lack peroxisomal membrane structures, it is tempting to speculate that these peroxins may associate to form a functional PMP import complex.
FIG. 9.
Identification of the peroxin-binding sites on Pex19p. Deletion mutants of Pex19p fused to Gal4p-AD were tested for interaction with Pex3p, Pex10p, Pex11pβ, Pex12p, Pex13p, and Pex16p fused to Gal4p-BD in the yeast two-hybrid system. Double transformants were selected and assayed for β-galactosidase activity by using a filter assay with X-Gal as the substrate. The colony staining time was less than 2 h (+++), less than 5 h (++), or less than 10 h (+) or the colonies did not stain at all (−). The farnesylation consensus sequence CaaX is shaded in black.
DISCUSSION
The observation that mammalian cells deficient in Pex19p lack peroxisome membrane structures points towards a role for this peroxin in PMP biogenesis (24, 28). We found that Pex19p interacts with Pex3p, Pex10p, Pex11pβ, Pex12p, Pex13p, and Pex16p but not with Pex2p, Pex11pα, Pex14p, PMP22, and PMP24 in the yeast two-hybrid system (Fig. 1A). Similar results were recently reported by Sacksteder et al. (28). For unknown reasons, these authors failed to detect the binding of Pex19p to Pex3p and to Pex16p. That Pex19p can indeed interact with Pex3p in the yeast two-hybrid system was also recently reported by Ghaedi et al. (14). The fact that Pex10p, Pex11pβ, Pex12p, Pex13p, and Pex16p interact with Pex19p in the yeast two-hybrid system suggests that, in this system, other (noninteracting) PMPs with two transmembrane-spanning domains (e.g., Pex2p, Pex11pα, and PMP24) are also theoretically capable of being targeted to the nucleus. However, negative two-hybrid results do not necessarily prove a lack of interaction; for example, Sacksteder et al. (28) showed that Pex19p binds to PMP34 and Pex14p in blot overlay assays. Yet, the extent of binding is difficult to deduce from the provided data. Why Pex14p interacts with Pex19p in a blot overlay assay (28) but not in the yeast two-hybrid system (Fig. 1A) (28) is currently not clear. The fact that human Pex14p strongly interacts with Pex5p in the yeast two-hybrid system (M. Fransen and P. P. Van Veldhoven, unpublished data) eliminates the possibility of poor expression or failure to be targeted to the nucleus. Other mammalian Pex19p-binding proteins identified by using the yeast two-hybrid system are the ATP binding cassettes half-transporters ALDP, ALDRP, and PMP70 (15).
In order to verify whether or not the observed two-hybrid interactions are direct or bridged by an endogenous yeast protein, we further examined the binding properties of Pex19p in vitro. However, we encountered the problem that PMPs containing two or more TMDs were poorly expressed and insoluble; thus, only the Pex3p-Pex19p interaction could be confirmed in vitro. Yet, based on the fact that (i) our tests were performed on S. cerevisiae cells by using mammalian peroxins and (ii) human Pex19p fails to complement the corresponding yeast deletion mutant (16), it is likely that the interactions that we observed in the yeast two-hybrid system are direct. In conclusion, our results confirm that human Pex19p can bind multiple integral PMPs and extend the list of PMPs with which Pex19p interacts to include Pex16p.
To determine whether human Pex19p functions as a soluble receptor for the targeting of integral PMPs to the peroxisome, the Pex19p-BDs and the peroxisomal sorting signals of Pex3p, Pex12p, Pex13p, and Pex16p were delineated. Deletion analysis studies demonstrated that, for Pex3p and Pex12p, the Pex19p-BDs and the peroxisomal sorting signals are distinct (Fig. 2 and 3). For Pex13p and Pex16p, the domains essential for Pex19p binding were also necessary for protein sorting (Fig. 2 and 3). However, further random mutagenesis studies demonstrated that the Pex19p-BD and the peroxisomal sorting signal of Pex13p(145-233) could be functionally separated (Fig. 5). The separate introduction of all the missense mutations in the full-length Pex13p molecule showed that the amino acids from position 175 to 196 are essential for Pex19p binding but not for protein sorting (Fig. 6). Although similar experiments were not performed for Pex16p, these results indicate that human Pex19p does not function as a general soluble targeting receptor for integral PMPs. A similar conclusion was obtained for P. pastoris Pex19p (29). However, it has to be noted that the targeting elements of PMP70, ALDP, ALDPR, Pex11pβ, and Pex14p are bound by Pex19p (15, 28). But, as Sacksteder et al. (28) pointed out, these results do not indisputably establish that Pex19p binds to the PMP targeting signals in these elements. More specifically, as we report in this study that for Pex13p, the Pex19p-BDs and the peroxisomal targeting elements of these proteins might be functionally separated.
Since random mutagenesis studies revealed that single amino acid substitutions like V178E and L191P affect the peroxisomal localization of Pex13p(145-233) (Fig. 5) but not of the full-length Pex13p molecule (Fig. 6A), we conducted a refined Pex13p deletion analysis to investigate whether Pex13p contains more than one peroxisomal sorting determinant and/or whether regions flanking the peroxisomal sorting determinant cooperate with the mPTS (Fig. 7). We could demonstrate that the central matrix loop of Pex13p alone contains sufficient information to direct a reporter protein to the peroxisome (Fig. 7A). However, increasing the hydrophobicity of this loop by adding one of the flanking TMDs enhanced the overall sorting efficiency (Fig. 7A and 8). These observations suggest that the mPTS and the TMDs are separable entities that need to coexist for proper Pex13p biogenesis. Importantly, the portion of the central matrix loop of Pex13p (amino acids 155 to 233) that is bound to the peroxisome membrane cannot be removed from this membrane with 0.1 M Na2CO3, pH 11 (Fig. 8). Similar observations have been reported for the mPTSs of CbPMP47 and PpPex3p, and it has been suggested that these mPTSs are tightly anchored to the peroxisomal membrane via another integral PMP (7, 35). We could also show that, in the presence of flanking sequences, the minimal targeting motif in the central matrix loop of Pex13p can be further narrowed down (Fig. 7C and D). One explanation may be that these flanking sequences cooperate with the mPTS to enhance its overall sorting efficiency. However, further progressive truncation experiments yielded two nonoverlapping deletion proteins, each displaying a partial peroxisomal staining pattern. This result demonstrates that Pex13p possesses multiple, partially functional mPTSs. A cooperative recognition of these multiple sorting signals may be important for regulating the topology of Pex13p within the peroxisomal membrane.
Since it has been reported that the prenylation status of a protein can affect its binding properties (23), we investigated the impact of the CaaX farnesylation consensus sequence on the binding properties of Pex19p. We demonstrated that Pex19pΔCaaX has a strongly reduced binding affinity for Pex10p, Pex11pβ, Pex12p, and Pex13p but not for Pex3p and Pex16p (Table 1). These results suggest that prenylation of Pex19p is important for its association with Pex10p, Pex11pβ, Pex12p, and Pex13p but not for its association with Pex3p and Pex16p. However, in view of the recently published report that bacterially expressed Pex19p does bind Pex12p and Pex13p in a blot overlay assay (28), it seems that, under specific experimental conditions, nonfarnesylated Pex19p does display a certain affinity for these peroxins. Currently, we don't know whether these observed differences are the result of the different methodologies employed. In the context of the dilemma of whether or not farnesylation of Pex19p is absolutely required for its function, it is also interesting to mention the reported discrepancy that a C296S mutant of human Pex19p does (28) or does not (24) complement peroxisome biogenesis in pex19−/− fibroblasts. Although our experiments do not solve this dilemma, they demonstrate that the presence of the farnesylation motif of Pex19p strongly enhances its affinity for some PMPs. Similar conclusions were also drawn for S. cerevisiae Pex19p (16). In this organism, the interaction of Pex3p with Pex19p requires farnesylation of the latter molecule (16). Mapping the PMP-BDs of Pex19p further revealed that the prenylation-dependent interactions require not only the CaaX motif but also the N terminus (Fig. 9). This observation may indicate either that these PMPs bind to identical sites of Pex19p or that the deletions change the folding of Pex19p in such a manner that binding to distinct sites is affected. On the other hand, the prenyl-independent interactions are mediated by distinct domains of Pex19p (Fig. 9). These results suggest that the prenyl-dependent and the prenyl-independent interactions of Pex19p may serve another function in PMP biogenesis. It is tempting to speculate that Pex19p may bind to Pex3p and Pex16p to form a functional PMP import complex at the peroxisome membrane. What the function of Pex19p in this complex might be is not clear. Snyder et al. (29) recently suggested that, in P. pastoris cells, Pex19p might have a chaperone-like role at the peroxisome membrane. Nevertheless, since Pex19p is predominantly present in the cytosol, this molecule most likely also has other biological functions. However, the fact that human Pex19p binds integral PMPs at regions distinct from the mPTS indicates that this peroxin does not function as a general soluble targeting receptor for integral PMPs.
ACKNOWLEDGMENTS
We are grateful to Y. Sakai (Kyoto, Japan) and M. Baes (Leuven, Belgium) for the pEGFPH1 plasmid and the anti-Pex5p antiserum, respectively. The help of Jeroen Van Looy, Vanessa Brys, and Ilse Broekaert is highly appreciated.
M. Fransen is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO). This work was supported by a Geconcerteerde Onderzoeksacties grant (GOA/99/09) from the Flemish government.
REFERENCES
- 1.Amery L, Fransen M, De Nys K, Mannaerts G P, Van Veldhoven P P. Mitochondrial and peroxisomal targeting of 2-methylacyl-CoA racemase in human. J Lipid Res. 2000;41:1752–1759. [PubMed] [Google Scholar]
- 2.Baerends R J S, Faber K N, Kram A M, Kiel J A, van der Klei I J, Veenhuis M. A stretch of positively charged amino acids at the N-terminus of Hansenula polymorpha Pex3p is involved in incorporation of the protein into the peroxisomal membrane. J Biol Chem. 2000;275:9986–9995. doi: 10.1074/jbc.275.14.9986. [DOI] [PubMed] [Google Scholar]
- 3.Boussif O, Lezoualc'h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadwell R C, Joyce G F. Mutagenic PCR. In: Dieffenbach C W, Dveksler G S, editors. PCR primer: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 583–585. [Google Scholar]
- 5.Chang C C, Warren D S, Sacksteder K A, Gould S J. PEX12 interacts with PEX5 and PEX10 and acts downstream of receptor docking in peroxisomal matrix protein import. J Cell Biol. 1999;147:761–773. doi: 10.1083/jcb.147.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins C S, Kalish J E, Morrell J C, McCaffery J M, Gould S J. The peroxisome biogenesis factors Pex4p, Pex22p, Pex1p, and Pex6p act in the terminal steps of peroxisomal matrix protein import. Mol Cell Biol. 2000;20:7516–7526. doi: 10.1128/mcb.20.20.7516-7526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyer J M, McNew J A, Goodman J M. The sorting sequence of the peroxisomal integral membrane protein PMP47 is contained within a short hydrophilic loop. J Cell Biol. 1996;133:269–280. doi: 10.1083/jcb.133.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgersma Y, Kwast L, van den Berg M, Snyder W B, Distel B, Subramani S, Tabak H F. Overexpression of Pex15p, a phosphorylated peroxisomal integral membrane protein required for peroxisome assembly in S. cerevisiae, causes proliferation of the endoplasmic reticulum membrane. EMBO J. 1997;16:7326–7341. doi: 10.1093/emboj/16.24.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransen M, Brees C, Van Veldhoven P P, Mannaerts G P. The visualization of peroxisomal proteins containing a C-terminal targeting sequence on Western blot by using the biotinylated PTS1-receptor. Anal Biochem. 1996;242:26–30. doi: 10.1006/abio.1996.0423. [DOI] [PubMed] [Google Scholar]
- 10.Fransen M, Van Veldhoven P P, Subramani S. Identification of peroxisomal proteins by using M13 phage protein VI phage display: molecular evidence that mammalian peroxisomes contain a 2,4-dienoyl-CoA reductase. Biochem J. 1999;340:561–568. [PMC free article] [PubMed] [Google Scholar]
- 11.Fransen M, Terlecky S R, Subramani S. Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc Natl Acad Sci USA. 1998;95:8087–8092. doi: 10.1073/pnas.95.14.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiki Y. Peroxisome biogenesis and peroxisome biogenesis disorders. FEBS Lett. 2000;476:42–46. doi: 10.1016/s0014-5793(00)01667-7. [DOI] [PubMed] [Google Scholar]
- 13.Fujiki Y, Hubbard A L, Fowler S, Lazarow P B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaedi K, Tamura S, Okumoto K, Matsuzono Y, Fujiki Y. The peroxin Pex3p initiates membrane assembly in peroxisome biogenesis. Mol Biol Cell. 2000;11:2085–2102. doi: 10.1091/mbc.11.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gloeckner C J, Mayerhofer P U, Landgraf P, Muntau A C, Holzinger A, Gerber J K, Kammerer S, Adamski J, Roscher A A. Human adrenoleukodystrophy protein and related peroxisomal ABC transporters interact with the peroxisomal assembly protein Pex19p. Biochem Biophys Res Commun. 2000;271:144–150. doi: 10.1006/bbrc.2000.2572. [DOI] [PubMed] [Google Scholar]
- 16.Götte K, Girzalsky W, Linkert M, Baumgart E, Kammerer S, Kunau W H, Erdmann R. Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol Cell Biol. 1998;18:616–628. doi: 10.1128/mcb.18.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould S J, Valle D. Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet. 2000;16:340–345. doi: 10.1016/s0168-9525(00)02056-4. [DOI] [PubMed] [Google Scholar]
- 18.Hettema E H, Girzalsky W, van Den Berg M, Erdmann R, Distel B. Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 2000;19:223–233. doi: 10.1093/emboj/19.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honsho M, Fujiki Y. Topogenesis of peroxisomal membrane protein requires a short positively charged intervening-loop sequence and flanking hydrophobic segments: study using human membrane protein PMP34. J Biol Chem. 2001;276:9375–9382. doi: 10.1074/jbc.M003304200. [DOI] [PubMed] [Google Scholar]
- 20.Just W W, Diestelkötter P. Protein insertion into the peroxisomal membrane. Ann N Y Acad Sci. 1996;804:60–75. doi: 10.1111/j.1749-6632.1996.tb18608.x. [DOI] [PubMed] [Google Scholar]
- 21.Kammerer S, Holzinger A, Welsch U, Roscher A A. Cloning and characterization of the gene encoding the human peroxisomal assembly protein Pex3p. FEBS Lett. 1998;429:53–60. doi: 10.1016/s0014-5793(98)00557-2. [DOI] [PubMed] [Google Scholar]
- 22.Lazarow P B, Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- 23.Marshall C J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzono Y, Kinoshita N, Tamura S, Shimozawa N, Hamasaki M, Ghaedi K, Wanders R J A, Suzuki Y, Kondo N, Fujiki Y. Human PEX19: cDNA cloning by functional complementation, mutation analysis in a patient with Zellweger Syndrome, and potential role in peroxisomal membrane assembly. Proc Natl Acad Sci USA. 1999;96:2116–2121. doi: 10.1073/pnas.96.5.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okumoto K, Abe I, Fujiki Y. Molecular anatomy of the peroxin Pex12p. Ring finger domain is essential for Pex12p function and interacts with the peroxisome-targeting signal type 1-receptor Pex5p and a ring peroxin, Pex10p. J Biol Chem. 2000;275:25700–25710. doi: 10.1074/jbc.M003303200. [DOI] [PubMed] [Google Scholar]
- 26.Pause B, Diestelkötter P, Heid H, Just W W. Cytosolic factors mediate protein insertion into the peroxisomal membrane. FEBS Lett. 1997;414:95–98. doi: 10.1016/s0014-5793(97)00975-7. [DOI] [PubMed] [Google Scholar]
- 27.Pause B, Saffrich R, Hunziker A, Ansorge W, Just W W. Targeting of the 22 kDa integral peroxisomal membrane protein. FEBS Lett. 2000;471:23–28. doi: 10.1016/s0014-5793(00)01332-6. [DOI] [PubMed] [Google Scholar]
- 28.Sacksteder K A, Jones J M, South S T, Li X, Liu Y, Gould S J. PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J Cell Biol. 2000;148:931–944. doi: 10.1083/jcb.148.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder W B, Koller A, Choy A J, Subramani S. The peroxin Pex19p interacts with multiple, integral membrane proteins at the peroxisomal membrane. J Cell Biol. 2000;149:1171–1177. doi: 10.1083/jcb.149.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder W B, Faber K N, Wenzel T J, Koller A, Lüers G H, Rangell L, Keller G A, Subramani S. Pex19p interacts with Pex3p and Pex10p and is essential for peroxisome biogenesis in Pichia pastoris. Mol Biol Cell. 1999;10:1745–1761. doi: 10.1091/mbc.10.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soukupova M, Sprenger C, Gorgas K, Kunau W H, Dodt G. Identification and characterization of the human peroxin PEX3. Eur J Cell Biol. 1999;78:357–374. doi: 10.1016/S0171-9335(99)80078-8. [DOI] [PubMed] [Google Scholar]
- 32.Subramani S, Koller A, Snyder W B. Import of peroxisomal matrix and membrane proteins. Annu Rev Biochem. 2000;69:399–418. doi: 10.1146/annurev.biochem.69.1.399. [DOI] [PubMed] [Google Scholar]
- 33.Terlecky S R, Fransen M. How peroxisomes arise. Traffic. 2000;1:465–473. doi: 10.1034/j.1600-0854.2000.010604.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Unruh M J, Goodman J M. Discrete targeting signals direct PMP47 to oleate-induced peroxisomes in Saccharomyces cerevisiae. J Biol Chem. 2001;276:10897–10905. doi: 10.1074/jbc.M010883200. [DOI] [PubMed] [Google Scholar]
- 35.Wiemer E A C, Lüers G H, Faber K N, Wenzel T, Veenhuis M, Subramani S. Isolation and characterization of Pas2p, a peroxisomal membrane protein essential for peroxisome biogenesis in the methylotrophic yeast Pichia pastoris. J Biol Chem. 1996;271:18973–18980. doi: 10.1074/jbc.271.31.18973. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]