Abstract
Background/Purpose
The role of process measures used to predict quality in pediatric colorectal surgery enhanced recovery protocols has not been described. The purpose of this study was to demonstrate the feasibility of abstracting and monitoring process measures over protocol improvement iteration.
Methods
Patients enrolled in the Pediatric Colorectal Enhanced Recovery After Surgery pathway at our institution were grouped by stage of implementation. We used a quality improvement database to compare multi-stage enhanced recovery process measures and 30-day patient outcomes.
Results
We identified 58 surgical patients with 28(48%) cases enrolled in the pathway. There was increased use of regional anesthesia techniques in pathway patients (83% versus 20%, p<0.001). All preoperative process measures clinically improved between early and full implementation. Improvements included a dramatic increase in formal preoperative education (56% versus 0%, p=0.004) and administration of preoperative medication (p=0.025). Overall, 12 (21%) patients experienced postoperative complications, which were similarly distributed between implementation groups. Readmissions were highest during the early implementation phase (40%,p=0.029). Children in the late implementation group experienced fewer complications, which clinically correlated with process measure adherence.
Conclusions
Process measures complement outcome measures in assessing quality and effectiveness of a pediatric colorectal recovery protocol. Adherence to processes may reduce complications.
Level of evidence:
Treatment study, Level III
Keywords: enhanced recovery after surgery, colorectal surgery, child, process assessment, outcome assessment, quality improvement
1. INTRODUCTION
Enhanced recovery protocols for post-surgical care have led to a dramatic improvement in surgical outcomes over the last two decades. In adult patients, where these standardized perioperative care programs originated, implementing evidence-based protocols has resulted in decreased length of stay without increasing readmissions, decreased postoperative complications, faster return of bowel function, decreased provider costs, and decreased payer liabilities.[1-4] For pediatric surgical populations, the evidence is limited; however, early single-arm trials and physiologic evidence suggest that similar reductions in length of stay, faster return of bowel function, and decreased opioid consumption are observed.[5-9]
Although early studies evaluating enhanced recovery protocols focused largely on outcome measures, there has been more recent attention to measuring adherence to specific processes as a correlate to improved outcomes. Multiple high-quality studies in adult populations have demonstrated enhanced recovery protocols yield previously reported benefits in proportion to adherence with individual bundled components.[10,11] Thus, enhanced recovery protocols behave in a manner similar to many quality improvement interventions, in that process adherence is critical to achieve improvement in outcomes.[12] Early reports of pediatric enhanced recovery implementation have reflected some emphasis on process adherence, but their relative importance remains understudied.[13]
Given the known importance of process measures for enhanced recovery protocol implementation in other populations and the current innovation in such protocols for pediatric surgery, there exists an essential need for further defining specific process measures contributing to protocol success in pediatric populations. The purpose of this study was to demonstrate the feasibility of monitoring process measures and demonstrate their evolution over time during ERAS program iteration using data from our institution’s implementation of an enhanced recovery protocol in pediatric colorectal surgery.
2. MATERIALS AND METHODS
2.1. Study Population
This study was granted expedited review by the Johns Hopkins Medicine Institutional Review Board (IRB 00088002). We collected data on four groups of children at a single United States-based academic children’s hospital. First, we identified all children enrolled in the Pediatric Colorectal Enhanced Recovery After Surgery (ERAS) pathway from the program’s inception on January 15, 2016 to November 30, 2017. Care components and quality measures were introduced in a staged fashion based on team resource availability and scheduling (e.g., staffing acute pain team; in-service for advanced practice providers; quality improvement database construction) over six months. Thus, cases prior to August 1, 2016 (“early implementation” stage) were retrospectively added to a newly designed prospective quality measures database. The remaining (“full implementation” stage) cases were added prospectively within 30 days of surgery. Then, as a baseline comparison group, we also identified gastrointestinal tract surgeries performed by the same group of eight pediatric surgeons from January 1, 2014 to November 30, 2015 in our institution’s American College of Surgeons National Surgical Quality Improvement Program (NSQIP) Pediatric database. Finally, we identified NSQIP Pediatric cases performed by the same group after ERAS pathway implementation but not formally enrolled in the pathway as a post-implementation control group. These non-enrolled patients resulted from patients being scheduled for surgery in a clinic not yet having the personnel to enroll for ERAS but still staffed by the same group of eight surgeons. Controls were matched to enrolled patients by age (+/− 5 years) and gender.
2.2. Johns Hopkins Pediatric Colorectal Enhanced Recovery After Surgery Pathway
Our institutional pediatric colorectal ERAS pathway has been previously described and was originally based on the institution’s successful adult colorectal ERAS pathway.[5,14] Relevant features of the pathway design included the multidisciplinary team that developed the original standardized care processes, process measures (see Appendix), and monitored outcomes.[15] The lack of prior process measure reporting in pediatric enhanced recovery protocols led to a design process that was intentionally iterative and allowed to continue beyond initial implementation. Important modifications to the original pathway protocol since its implementation included: 1) a broadening in the age of patients to include any patient greater than 2 years of age; and 2) a broadening of the anatomic eligibility to include any surgical intervention that incises any gastrointestinal lumen distal to the ligament of Treitz to the anus.
2.3. Data Collection
Data collection was performed in stages with the implementation of a prospective quality monitoring database. Upon enrollment in the Pediatric Colorectal ERAS pathway during the patient’s preoperative surgical clinic visit, we entered each patient into the database recording preoperative risk factors, comorbidities, and demographic information. Patients were followed prospectively. Documentation of process measure compliance was performed as part of the standard ERAS pathway workflow using preformatted medical record notes in the preoperative and postoperative periods and checklist-based paper documentation on the day of surgery; both practices were consistent with the usual workflow in our institution. At 30 days following surgery, the database record was updated with chart review of these prospectively designed data collection instruments to record individual process measure adherence. Chart review was also performed of all in-person and phone-based medical record encounters to ascertain index admission lengths of stay, readmissions, and 30-day postoperative complications. Complications were reported in a manner consistent with definitions used by the NSQIP Pediatric program.[16]
For the comparison groups, preoperative risk factors, comorbidities, and clinical outcomes were obtained via chart review and our internal NSQIP Pediatric database. Differences between data sources were reconciled through review and discussion with three authors (I.L., M.L., and E.J.).
2.4. Statistical Analysis
Analyses were performed using Stata® 15.1 IC (College Station, TX). The primary unit of analysis was the aggregate process measure performance of the program with patient clinical outcomes reported as a secondary outcome measure. Bivariate analysis of preoperative risk factors, comorbidities, and outcomes was performed between the four patient groups of interest described above using χ2 and Wilcoxon rank-sum tests as appropriate. Select process measures were compared in a similar fashion based on those found to be clinically most relevant to current adherence priorities for effective clinical care (reviewed by I.L., M.L., and E.J.). Although this study was not powered to address the relationship between outcomes and process measure adherence, we also performed a univariate pre-planned exploratory analysis comparing outcomes to specific process measures of interest. A simple logistic regression was performed of outcomes by adherence proportion to 6 pre-selected process measures that the quality improvement team felt most closely aligned with ERAS goals (enrollment prior to day of surgery, provided ERAS manual, antibiotic bowel prep, postoperative ketorolac, postoperative acetaminophen, and early diet advancement). For interpretation of this exploratory analysis, clinical significance was favored over statistical significance and multivariable methods due to the small sample size and early implementation assessment.
3. RESULTS
We reviewed 30 cases prior to introduction of our institution’s Pediatric ERAS pathway versus 10 cases during an early implementation phase, 18 cases during a full implementation phase, and 8 matched non-enrolled control patients. When comparing differences in population characteristics across implementation stages (Table 1), there was an increased used of regional anesthesia techniques (e.g., epidural and transverse abdominis plane block) in the ERAS groups (83% versus 20%, p < 0.001). More patients underwent surgery for functional or anatomic indications in the pre-implementation group (57%) while Crohn’s disease was more common in the ERAS implementation stages (50%). In the early implementation stage, there were less patients on immunosuppressive agents at the time of surgery than in the full implementation stage (20% versus 67%, p = 0.029).
Table 1.
Characteristics of patients by implementation stage for a Pediatric Intestinal Surgery Enhanced Recovery After Surgery pathway.
| Pre-Implementation n=30 |
Early Implementation n=10 |
Full Implementation n=18 |
Non-enrolled n=8 |
p | |
|---|---|---|---|---|---|
| Age (Median, IQR) | 14 (9 – 16) | 17 (16 – 17) | 14 (8 – 16) | 12.5 (9 – 16) | 0.063 |
| Female (%) | 50 | 30 | 39 | 63 | 0.490 |
| Non-white (%) | 40 | 30 | 28 | 38 | 0.959 |
| ASA Class (Median, IQR) | 2 (2 – 3) | 2 (2 – 3) | 2 (2 – 3) | 2 (2 – 2) | 0.103 |
| Comorbidities (%) | |||||
| Cardiac | 13 | 0 | 0 | 0 | 0.279 |
| Immunologic | 30 | 20 | 67 | 57 | 0.029 |
| Nutritional | 17 | 10 | 22 | 0 | 0.700 |
| Endocrine | 3 | 0 | 11 | 0 | 0.697 |
| Operative Indication (%) | 0.273 | ||||
| Crohn’s / Unspecified IBD | 33 | 50 | 50 | 50 | |
| Ulcerative colitis | 10 | 30 | 6 | 0 | |
| Motility and Other Anatomic Disorder (incl. ostomies) | 57 | 20 | 39 | 50 | |
| Neoplasia | 0 | 0 | 6 | 0 | |
| Operative Approach (%) | 0.666 | ||||
| Open | 40 | 50 | 50 | 25 | |
| Laparoscopic | 60 | 50 | 50 | 75 | |
| Regional Anesthesia (%) | 20 | 70 | 83 | 63 | <0.001 |
Immunologic includes steroids, immunosuppressives, and biologic therapies.
IBD – inflammatory bowel disease
All preoperative process measures clinically improved between the early and full implementation period (Figure 1A). Introduction of an ERAS educational manual led to a marked increase in documented education (56% versus 0%, p = 0.004). In the perioperative period, we identified a marked improvement in the administration of preoperative gabapentin, acetaminophen, and biscacodyl (Figure 1B, p = 0.002, p = 0.001, p = 0.025, respectively). No differences were observed in post-operative process measure compliance between the early and late implementation periods (Figure 1C). A complete list of current process measures and their rates of adherence are reported in the Appendix.
Fig. 1. Process measure adherence by implementation stage for a Pediatric Intestinal Surgery Enhanced Recovery after Surgery pathway.
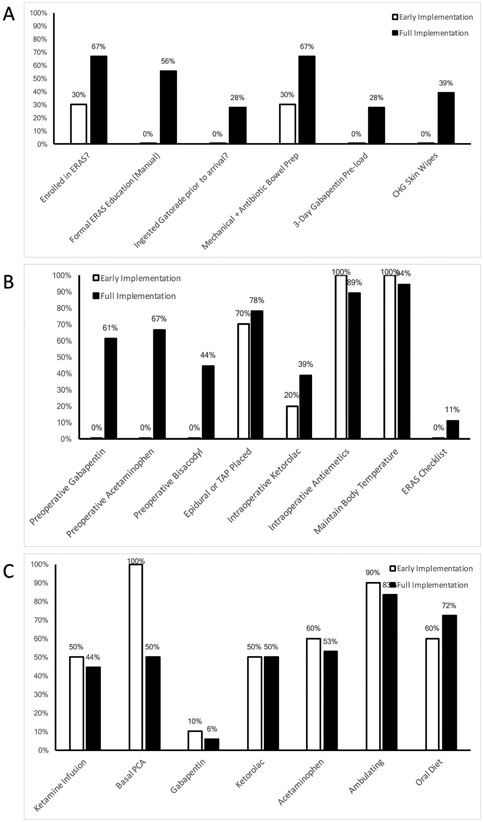
Panel A: preoperative process measures; Panel B: intraoperative process measures; Panel C: postoperative process measures.
We identified complications of surgery in 21% of all cases and readmissions in 18% (Table 2). No statistical differences were observed in complications between implementation stages. Readmissions were highest during the early implementation phase (40%, p = 0.029). Although not statistically significant, a consistent trend of reduced complications correlated with process measure adherence; complication rates in patients who met a process measure versus those who did not were lower those formally enrolled in ERAS in the electronic medical record (13% versus 31%), receiving formalized ERAS education (10% versus 28%), performing a home antibiotic bowel prep (15% versus 23%), and receiving at least one prescribed preoperative medication (9% versus 29%). Using six preselected high-value process measures, there was no statistical difference in complications by proportion adherent (OR = 0.09, 95%CI: 0.00-4.90, p = 0.240).
Table 2.
30-day postoperative outcomes and complications by implementation stage for a Pediatric Intestinal Surgery Enhanced Recovery After Surgery pathway.
| Outcome (%) | Pre- Implementation |
Early Implementation |
Full Implementation |
Non- enrolled |
ERAS vs. non-ERAS p |
Early vs. Late p |
|---|---|---|---|---|---|---|
| n=30 | n=10 | n=18 | n=8 | |||
| Any complication | 17 | 40 | 11 | 25 | 0.277 | 0.147 |
| Surgical site infection | 10 | 10 | 6 | 13 | 0.932 | 1.000 |
| Pneumonia | 3 | 0 | 0 | 0 | 1.000 | 1.000 |
| Reintubation | 0 | 0 | 0 | 11 | 0.121 | 1.000 |
| Venothrombembolism | 3 | 0 | 0 | 0 | 1.000 | 1.000 |
| Urinary tract infection | 3 | 0 | 6 | 0 | 1.000 | 1.000 |
| Perioperative transfusion | 0 | 10 | 0 | 0 | 0.273 | 0.357 |
| Sepsis | 3 | 20 | 6 | 0 | 0.214 | 0.284 |
| Septic shock | 0 | 10 | 0 | 0 | 0.273 | 0.357 |
| Length of stay (Median, IQR) | 5 (4 – 8) | 6 (5 – 7) | 5 (4 – 8) | 5 (4 – 7) | 0.921 | 0.554 |
| Readmission | 7 | 40 | 17 | 38 | 0.029 | 0.207 |
Complications without events for any group excluded: anastomotic leak, acute renal failure, coma, cerebrovascular accident, peripheral nerve injury, cardiac arrest, and death.
4. DISCUSSION
Pediatric colorectal surgery presents a logical target for further expansion of enhanced recovery protocols and other bundled interventions. Early reports have supported their feasibility and potential for replicating the benefits seen in adult surgical populations.[5,6,9,13] However, two pediatric-specific barriers impede widespread uptake. First, children undergoing colorectal surgery have more variable disease presentation compared to adult colorectal patients. The variety of functional and anatomic surgical indications in pediatric colorectal surgery does not have an adult surgery comparison group. Second, the heterogeneity of patients themselves complicates standardization because the physiologic needs of very young children may differ from a teenager with the same diagnosis. Thus, efforts to promulgate pediatric-specific enhanced recovery protocols must reconcile local, successful adult protocols against the added complexity of pediatric physiology and surgical indications. Even when local consensus is achievable, there remain ongoing concerns by national experts that pathway heterogeneity remains a concern and evidence supporting pathway alternatives is limited. A recently described Delphi-model expert panel further demonstrated that while many surgeons, anesthesiologists, and other related clinicians converge on many enhanced recovery components, substantial variability in the acceptance of each persists. In addition, there remains debate about the appropriateness of some components entirely, such as volume restrictions, thromboembolism prophylaxis, and epidural use.[17,18]
The current state of enhanced recovery protocol adoption in pediatric colorectal surgery converges with adoption and implementation efforts of bundled interventions more broadly. The adult enhanced recovery literature emphasizes that these bundles yield benefits in direct proportion to adherence. Multiple studies have demonstrated that the improved outcomes following enhanced recovery protocol implementation are relative to the proportion of enhanced recovery components being utilized.[10,11] Early reports of pediatric enhanced recovery implementation have reflected some emphasis on process measures, but their relative importance remains understudied. In addition, these early studies have carefully selected participating surgeons and patients to assure a degree of uniformity appropriate for enrollment in a standardized pathway.[13]
The purpose of this study was to provide a demonstration of a pediatric colorectal surgery enhanced recovery protocol under pragmatic conditions. All colorectal pediatric patients of all pediatric surgeons at the institution were included. In addition, outcomes and process measure reporting were conducted through quality improvement workflows rather than an idealized research protocol infrastructure. Lapses in process measure data collection and limited control patients reflect the real-world quality improvement approach we used. This approach was purposeful to demonstrate the institutional learning curve of ERAS program implementation. Outcomes were also standardized to those of accepted quality reporting measures (i.e., NSQIP Pediatric) to allow comparability of these results to future studies.
We demonstrated a successful transition from non-standardized pediatric colorectal surgical care to a pediatric colorectal ERAS-guided pathway over a two-year period. By the end of the process, the majority of pediatric colorectal surgical patients entering our tertiary care institution received care dictated by standardized ERAS principles that have been previously described.[5] This experience included a diverse array of patient demographics and operative indications for elective colorectal surgery. We identified marked improvements in care practices including the increased and widespread use of multimodal analgesia to reduce opioid burdens, surgical site infection reduction measures, and increased patient education. Although the current series was not powered to demonstrate improvements in patient outcomes following use of an enhanced recovery protocol, we demonstrated complication rates and lengths of stay consistent with prior published studies.[13] Finally, further investigation with large pediatric populations in the future will be important to observe for benefits in outcomes and any potential heterogeneity by age, disease type, or degree of adherence.
This experience also highlighted the continuous quality improvement aspects of maturing dissemination and implementation.[19] The multidisciplinary implementation group as well as the entire pediatric surgery care team met on a regular basis to review process measure adherence. We used these opportunities to clarify misconceptions of team members, introduce new workflows, and highlight observed benefits to further standardized care. Implementation was heterogeneous across different process measures and team efforts were redeployed as needed. For example, we were much more successful at quickly deploying a multimodal analgesia approach to pain management than we were to standardizing preoperative prehospital workflows (e.g., prehospital ERAS education, prehospital gabapentin loading). The lack of improvement in Figure 1C between early and full implementation periods is largely due to the acute pain management components of our pediatric ERAS pathway meeting adherence goals nearly immediately. In contrast, it was only through the iterative development and implementation of process measures throughout the early implementation phase that we clearly identified the deficits in prehospital care components.
We also observed a nonsignificant worsening of complications and readmissions in the early implementation phase which may also be an indicator of learning curve effects or adjustments to existing processes of care. We hypothesize that the lack of comprehensive preoperative education (i.e., family education manual) and front-line providers adjusting to telephone calls from patients with more recent surgery may have contributed to the increase in readmissions. Similarly, the introduction of formalized preoperative education and ongoing front-line provider meetings may have facilitated a better understanding of minor, immediate postoperative issues no longer requiring readmission.
These iterative steps align well with best-practices for implementation and dissemination efforts.[20,21] Maintaining an adaptive quality ecosystem, selectively emphasizing process measures (Appendix A), and reconciling differences in measurement versus differences in care has allowed the evolution of our pediatric ERAS program to meet current institutional quality goals. The process measures developed in this study were critical for highlighting further areas of improvement and mapping institutional learning curve progress. Importantly, we view the process measure results reported here as yet one more waypoint on an ongoing effort to standardize evidence-based care in pediatric colorectal surgery. We describe this evolution over time to highlight the importance of process measures to affirm whether an operationalized clinical program is achieving the individual components of care required.
This study had several limitations. By emphasizing implementation stages, consensus-building, and continuous quality improvement within our institution, methodologic elements – such as the enhanced recovery principles explicitly emphasized and those tracked by process measures – evolved over time. This variability has been noted above where relevant, and we believe these efforts better model a real-world stepwise implementation of bundled interventions as their widespread dissemination becomes a priority for current surgical practice. Second, although our results are consistent with existing pediatric enhanced recovery literature,[5,13] direct comparison is limited by the lack of standardization of protocols and methodology. Pediatric surgical enhanced recovery protocol efforts remain non-standardized and directly comparing two programs can be difficult. The debate about which components of enhanced recovery protocols are fundamental extends well beyond pediatric surgery with ample opportunity for subspecialty standardization as further research emerges.[7,10] Finally, in addition to the components selected for enhanced recovery protocols, how we measure ultimate clinical outcomes remains under-developed. Future studies of pediatric populations need to broaden observed outcomes to include patient satisfaction, quality of life measures, and other factors acknowledged to benefit from ERAS in adult populations.[22]
5. CONCLUSIONS
Pediatric colorectal surgery enhanced recovery protocols continue to evolve. Process measures represent an important and necessary element for successfully implementing these protocols in real-world pediatric surgical settings. Process measures should be used in real-time to indicate underperforming components and provide early warnings for quality risks.
Financial support:
I.L.L. received salary support during the preparation of this manuscript from a National Cancer Institute T32 Institutional Training Grant (5T32CA126607) and a Research Foundation of the American Society of Colon and Rectal Surgeons Resident Research Initiation Grant (GSRRIG-031). M.R.L. received salary support during preparation of this manuscript from a National Institute of Diabetes and Digestive and Kidney Diseases T32 Institutional Training grant (5T32DK007713).
Abbreviations:
- ERAS
Enhanced Recovery After Surgery
- NSQIP
National Surgical Quality Improvement Program
APPENDIX
The following table has been reproduced from quality improvement documents used during the implementation of the pediatric ERAS protocol described in the accompanying manuscript. For the purposes of demonstrating “real-world” iterative implementation, we have intentionally included process measures that are yet to be operationalized (e.g., automated postoperative ordersets) and those that are being phased out due to revised prescribing protocols (e.g., disuse of basal PCA or ketamine as empiric standard of care). These are not provided for generalizable use but to demonstrate how we have used a variety of process measures at various points throughout the implementation process. Those process measures reported in the manuscript itself reflect those that were actively used by the implementation team to assess program success, acknowledge changing provider practices, and make protocol changes as necessary.
Table A1.
Adherence by Individual Process Measure Across Stages of Implementation
| Early Implementation |
Full Implementation |
|
|---|---|---|
| PRIOR TO DAY OF SURGERY | ||
| Enrolled in ERAS? | 30% | 67% |
| Provided ERAS manual? | 0% | 56% |
| Provided Gatorade instructions? | 0% | 56% |
| Provided mechanical bowel prep instructions? | 20% | 56% |
| Provided antibiotic bowel prep instructions? | 20% | 67% |
| Provided chlorhexidine washing instructions? | 0% | 50% |
| Ingested Gatorade prior to arrival? | 0% | 28% |
| Performed mechanical bowel prep over last 24 hours? | 30% | 67% |
| Performed antibiotic bowel prep over last 24 hours? | 30% | 67% |
| Completed three days of gabapentin preoperatively? | 0% | 28% |
| DAY OF SURGERY | ||
| Used CHG washcloths as instructed preoperatively? | 0% | 39% |
| ERAS pre-op orders written by Surgery? | 0% | 6% |
| APS Consult ordered? | 100% | 94% |
| Anesthesia ordered pre-op gabapentin? | 0% | 61% |
| Anesthesia ordered pre-op acetaminophen? | 0% | 67% |
| Anesthesia ordered pre-op bisacodyl? | 0% | 44% |
| Preoperative warming? | 0% | 0% |
| Medications ordered in preop given prior to OR? | 0% | 61% |
| Appropriate abx given less than 60 minutes prior to incision? | 100% | 100% |
| Anesthesia placed epidural? | 50% | 50% |
| Anesthesia placed TAP or other regional block? | 20% | 28% |
| Ketorolac given? | 20% | 39% |
| Antiemetics given? | 100% | 89% |
| Post-op temperature maintained > 36 degrees C? | 100% | 94% |
| Checklist placed in ERAS folder? | 0% | 11% |
| INPATIENT STAY FOLLOWING SURGERY | ||
| APS consulted at end of surgery? | 100% | 100% |
| Temperature confirmed greater than 36 C in PACU? | 100% | 94% |
| Ketamine infusion on POD1? | 50% | 44% |
| Basal PCA running on POD1? | 100% | 50% |
| Gabapentin ordered on POD1? | 10% | 6% |
| Ketorolac ordered on POD1? | 50% | 50% |
| Acetaminophen ordered on POD1 | 60% | 53% |
| GPS ordered ERAS post-op orderset? | 0% | 0% |
| Ambulating POD2? | 90% | 83% |
| Diet by POD1 | 60% | 72% |
| Foley out by POD2 | 100% | |
| Use of at least two non-narcotic pain adjuncts on POD2? | 60% | 78% |
| Chewing gum provided? | 0% | 0% |
Appendix abbreviations:
APS - Acute Pain Service
CHG - chlorhexidine gluconate
PACU - post-anesthesia care unit
PCA - patient controlled analgesia
POD - postoperative day
TAP - transverse abdominis plane
Footnotes
Conflicts of interest: none.
Prior presentation: Part of the data reported in this manuscript was previously presented at the American College of Surgeons Clinical Congress Scientific Forum in San Diego, California on October 24, 2017.
REFERENCES
- [1].Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667–78. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- [2].Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172–88. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- [3].Lee L, Li C, Landry T, Latimer E, Carli F, Fried GM, et al. A systematic review of economic evaluations of enhanced recovery pathways for colorectal surgery. Ann Surg 2014;259:670–6. [DOI] [PubMed] [Google Scholar]
- [4].Lee L, Mata J, Ghitulescu GA, Boutros M, Charlebois P, Stein B, et al. Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg 2015;262:1026–33. [DOI] [PubMed] [Google Scholar]
- [5].Leeds IL, Boss EF, George JA, Strockbine V, Wick EC, Jelin EB. Preparing enhanced recovery after surgery for implementation in pediatric populations. J Pediatr Surg 2016;51. doi: 10.1016/j.jpedsurg.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vrecenak JD, Mattei P. Fast-track management is safe and effective after bowel resection in children with Crohn’s disease. J Pediatr Surg 2014;49:99–102-3. doi: 10.1016/j.jpedsurg.2013.09.038. [DOI] [PubMed] [Google Scholar]
- [7].Shinnick JK, Short HL, Heiss KF, Santore MT, Blakely ML, Raval MV. Enhancing recovery in pediatric surgery: A review of the literature. J Surg Res 2016;202:165–76. doi: 10.1016/j.jss.2015.12.051. [DOI] [PubMed] [Google Scholar]
- [8].Schroeder VA, DiSessa T., Douglas WI. Postoperative fluid balance influences the need for antihypertensive therapy following coarctation repair. Pediatr Crit Care Med 2004;5:539–41. doi:01.PCC.0000144730.44552.E3 [pii]. [DOI] [PubMed] [Google Scholar]
- [9].Adibe OO, Iqbal CW, Sharp SW, Juang D, Snyder CL, Holcomb GW, et al. Protocol versus ad libitum feeds after laparoscopic pyloromyotomy: A prospective randomized trial. J Pediatr Surg 2014;49:129–32. doi: 10.1016/j.jpedsurg.2013.09.044. [DOI] [PubMed] [Google Scholar]
- [10].Ahmed J, Khan S, Lim M, Chandrasekaran TV., Macfie J. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Color Dis 2012;14:1045–51. doi: 10.1111/j.1463-1318.2011.02856.x. [DOI] [PubMed] [Google Scholar]
- [11].Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011;146:571–7. doi: 10.1001/archsurg.2010.309. [DOI] [PubMed] [Google Scholar]
- [12].Donabedian A Selecting approaches to assessing performance. In: Bashshur R, editor. An Introd. to Qual. Assur. Heal. Care, Oxford, England: Oxford University Press; 2003, p. 45–57. [Google Scholar]
- [13].Short HL, Heiss KF, Burch K, Travers C, Edney J, Venable C, et al. Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2017. doi: 10.1016/j.jpedsurg.2017.05.004. [DOI] [PubMed] [Google Scholar]
- [14].Wick EC, Galante DJ, Hobson DB, Benson AR, Lee KHK, Berenholtz SM, et al. Organizational Culture Changes Result in Improvement in Patient-Centered Outcomes: Implementation of an Integrated Recovery Pathway for Surgical Patients. J Am Coll Surg 2015;221:669–77. doi: 10.1016/j.jamcollsurg.2015.05.008. [DOI] [PubMed] [Google Scholar]
- [15].Weaver SJ, Benishek LE, Leeds I, Wick EC. The Relationship Between Teamwork and Patient Safety. Surg. Patient Care, Cham: Springer International Publishing; 2017, p. 51–66. doi: 10.1007/978-3-319-44010-1_5. [DOI] [Google Scholar]
- [16].American College of Surgeons. User Guide for the 2015 ACS NSQIP Pediatric Participant Use Data File. Revised version. Chicago, IL: 2016. [Google Scholar]
- [17].Short HL, Taylor N, Piper K, Raval MV. Appropriateness of a pediatric-specific enhanced recovery protocol using a modified Delphi process and multidisciplinary expert panel. J Pediatr Surg 2017. doi: 10.1016/j.jpedsurg.2017.09.008. [DOI] [PubMed] [Google Scholar]
- [18].Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg 2014;149:1224–30. doi: 10.1001/jamasurg.2014.210. [DOI] [PubMed] [Google Scholar]
- [19].Leeds IL, Wick EC. Local quality improvement. In: Wong S, Kelz R, editors. Surg. Qual. Improv, Geneva: Springer; 2016. [Google Scholar]
- [20].Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ 1998;317:465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Emmons KM, Weiner B, Fernandez ME, Tu S-P. Systems antecedents for dissemination and implementation: a review and analysis of measures. Health Educ Behav 2012;39:87–105. doi: 10.1177/1090198111409748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abola RE, Bennett-Guerrero E, Kent ML, Feldman LS, Fiore JF, Shaw AD, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Patient-Reported Outcomes in an Enhanced Recovery Pathway. Anesth Analg 2017:1. doi: 10.1213/ANE.0000000000002758. [DOI] [PubMed] [Google Scholar]


