Abstract
The intentional use of viruses for cancer therapy dates back over a century. As viruses are inherently immunogenic and naturally optimized delivery vehicles, repurposing viruses for drug delivery, tumor antigen presentation, or selective replication in cancer cells represents a simple and elegant approach to cancer treatment. While early virotherapy was fraught with harsh side effects and low response rates, virus-based therapies have recently seen a resurgence due to newfound abilities to engineer and tune oncolytic viruses, virus-like particles, and virus-mimicking nanoparticles for improved safety and efficacy. However, despite their great potential, very few virus-based therapies have made it through clinical trials. In this review, we present an overview of virus-inspired approaches for cancer therapy, discuss engineering strategies to enhance their mechanisms of action, and highlight their application for overcoming the challenges of traditional cancer therapies.
Keywords: Cancer, Oncolytic viruses, Virus-like particles, Virus-mimicking nanoparticles, Immunotherapy, Gene therapy, Nanomedicine, Drug delivery
1. Introduction
Cancer is the second leading cause of death in the United States, responsible for more than half a million deaths each year [1]. Despite significant progress in the fight against cancer, prognosis remains poor with an overall 5-year survival rate of only 60-70% [2]. Traditional cancer treatments, such as surgical operations, chemotherapy, radiation therapy, and hormonal therapy, deliver suboptimal outcomes due to issues of tumor-induced immunosuppression and multidrug resistance, which increase the chance of cancer metastasis or relapse [3–5]. Thus, next generation treatments with improved efficacy are desperately needed.
Recently, viruses and virus-inspired platforms have been drawn into the spotlight with the potential to overcome the limitations of traditional cancer therapy. The intentional use of pathogens for cancer therapy has a long history, dating back to at least the 1890s (Figure 1). After observing cancer regression in patients with severe bacterial infections, surgeon William Coley administered Streptococcus pyogenes bacteria to a patient with non-operative bone carcinoma, leading to tumor regression [6]. Over the next several decades, many cases of tumor regression were reported following not only microbial infection, but viral infection as well. For instance, in 1904, a cervical cancer patient was vaccinated with a live attenuated rabies virus to treat a dog bite wound [7,8]. To the doctors’ surprise, the tumor miraculously disappeared, and the patient remained cancer-free for the next eight years [7,9]. Soon, the rabies vaccine was administered to eight other cervical cancer patients, some of whom experienced a reduction in tumor size [7,9]. These viruses would eventually be known as oncolytic viruses (OVs) due to their ability to selectively target and kill tumor cells [10]. With early successes, virotherapy research gained momentum and reached a height between the 1940s and 1960s, when researchers treated cancer patients with a wide range of OVs, including flavivirus, Epstein-Barr virus (EBV), and hepatitis virus [11–16]. Among the clinical trials yielding remarkable outcomes were those conducted by Asada, who used the mumps virus to treat 18 different cancer types [17]. The treatment showed low toxicity and in more than a third of the patients, the tumor either completely disappeared or shrank to less than half the initial volume [17]. In the vast majority of cases, however, tumor regression was short-lived and the use of wild-type OVs often came with adverse side effects, occasionally even leading to death [18]. Due to safety concerns and limited efficacy, the field went nearly dormant by the 1970s and 1980s [19].
Figure 1:
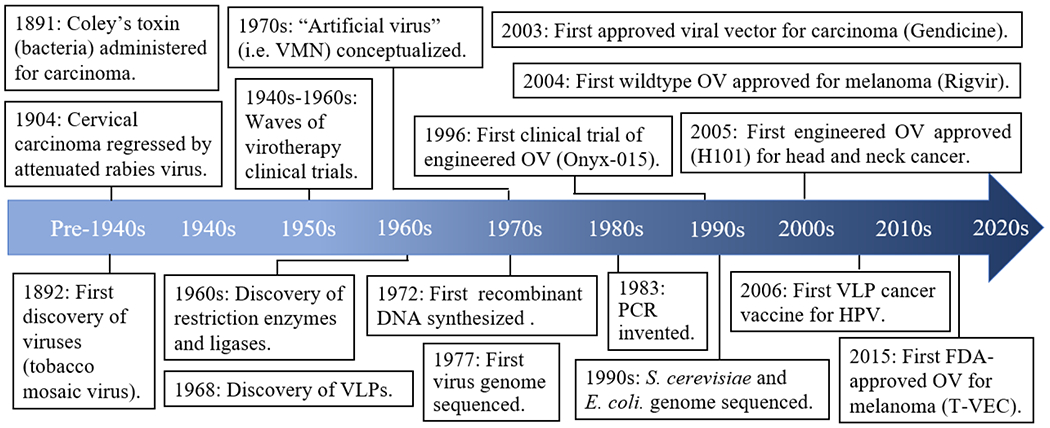
Milestones in virus-based cancer treatment
In recent decades, however, the rapid advancement of biotechnology tools has been met with the resurgence of virus-based cancer therapy (Figure 1). Most notably, the discovery of restriction enzymes [20] and ligases [21–25] led to the birth of recombinant technology [26,27], which was further revolutionized by the development of DNA sequencing [28,29] and polymerase chain reaction (PCR) [30]. In turn, recombinant technology has enabled the modification of wild-type viruses to produce safer and more effective virus strains by deleting virulence genes or introducing tumor-suppressive genes [31]. By 1996, the first engineered OV, Onyx-015, an adenovirus harboring an E1B deletion for selective replication in tumor cells, entered clinical trials for human head and neck carcinoma [32–34]. Onyx-015 demonstrated remarkable safety with flu-like symptoms as the main side effect, and patient biopsies showed selective replication and necrosis in tumor tissues, but not neighboring normal tissues [33,34]. Onyx-015 was later engineered with an additional E3 deletion to generate H101, which became the first genetically modified OV approved for head and neck cancer treatment [35,36].
The successful use of OVs for cancer treatment led to the emergence of two new branches of viral-based platforms: virus-like particles (VLPs) and synthetic virus-mimicking nanoparticles (VMNs). VLPs and VMNs are engineered nanostructures that emulate the advantageous features of OVs to evoke antitumor responses. At the same time, they open new opportunities in terms of safety, design, and functionalities, thus expanding the repertoire of viral-based strategies for cancer therapy. Compared to their microbial counterparts, oncolytic bacteria, viral-based strategies offer several distinct advantages: 1) small genome that allows easy gene manipulation, 2) physical size and shape that have been optimized for gene delivery, and 3) viral tropism that enables targeting to specific tissues [37–39], Thus, in this review, we discuss recent progress of using OVs, VLPs, and VMNs for overcoming pivotal challenges of cancer treatment, including solid tumor penetration, immunosuppressive and drug resistant tumors, and cancer stem cells.
2. The Virus-inspired Toolbox for Cancer Therapy
In the simplest sense, viruses are targeted delivery vehicles of genetic material that have been honed by eons of evolution. Viruses lend themselves incredibly well to the treatment of many diseases due to their distinct properties: (1) protein or lipid coats that package and protect the cargo (genetic material) from degradation, (2) a relatively small genome that enables easy gene manipulation, (3) tropism toward specific tissues, (4) ability to lyse cells, (5) capacity to reproduce in host cells to disseminate viral progenies, and (6) mechanisms to modulate the host immune system. By harnessing or adopting these features, viruses and nanoparticles can be repurposed to specifically kill tumor cells or deliver an antitumor therapeutic cargo for cancer treatment. Viral-based approaches to cancer treatment can be broadly divided into the three aforementioned platforms: OVs, VLPs, and VMNs. This section introduces the three viral-based platforms (VBPs) along with engineering strategies to functionalize them and highlights their distinct advantages and disadvantages (Figure 2).
Figure 2: Comparison of VBP strategies for cancer therapy.
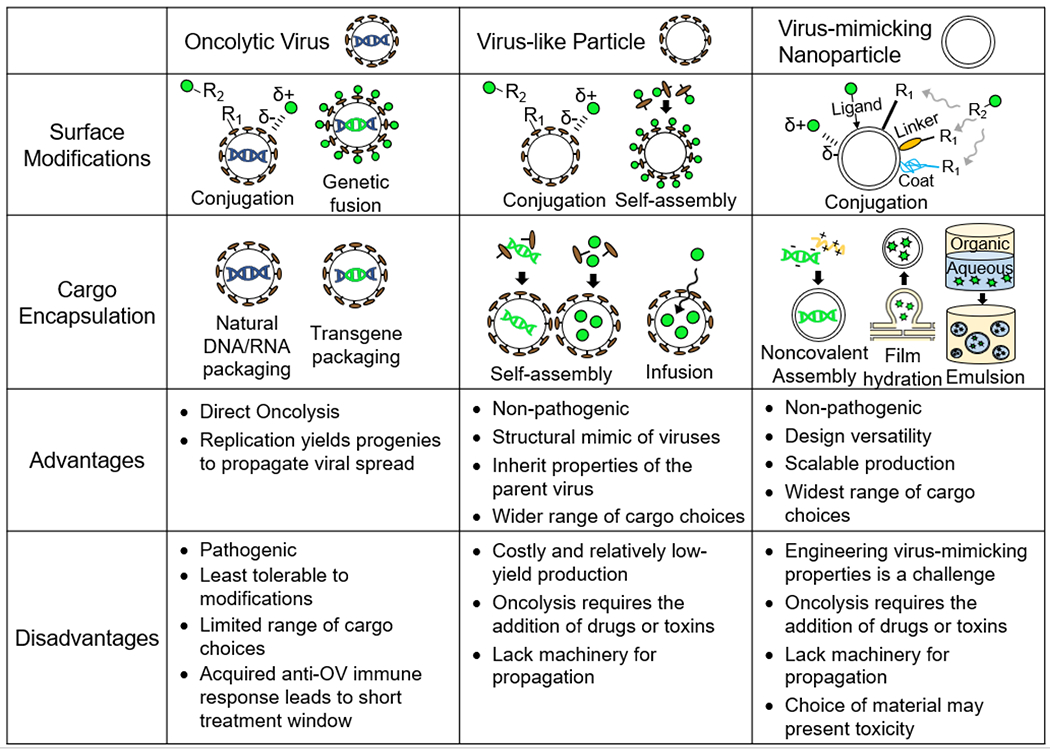
Engineering strategies for surface modifications and cargo encapsulation and the advantages and disadvantages of the three viral-based platforms are listed.
2.1. Oncolytic Viruses
OVs are replicating viruses that preferentially target and kill cancerous cells while sparing healthy cells. While there are several naturally occurring viruses with an innate propensity to infect tumor cells, the majority of OVs are engineered for tumor selectivity [40]. As early OV therapies in the mid 20th century had risks associated with off-site targeting [18], tumor selectivity has become an important engineering strategy to enhance OV safety. This combined with advances in recombinant technology and a greater understanding of the molecular mechanism of viruses has enabled OVs to be designed with improved safety and outcomes that are just beginning to reach the clinic. For example, T-VEC, an attenuated OV-based herpes simplex virus (HSV) encoding the transgene granulocyte-macrophage colony stimulating factor (GM-CSF), was approved by the FDA and EMA in 2015 for the treatment of unresectable melanoma [41]. Today, the field of OV therapies is rapidly expanding, with nearly 100 OVs being investigated in ongoing clinical trials [42]. A comprehensive list of recent clinical trials has been reviewed elsewhere [43,44].
2.1.1. Strategies to Functionalize OVs
Although OVs are naturally armed with tumor-killing abilities, they can be endowed with additional functionalities through surface modifications or cargo encapsulation [45] (Figure 2). In the former case, the OV surface can be decorated with a range of molecules, such as proteins to enhance selective targeting [46], drugs to induce tumor death [47], and polymers that facilitate immune evasion of the OV [48,49]. A common strategy of introducing these molecules is through covalent or noncovalent bioconjugations [50]. Covalent bioconjugations form stable and permanent bonds to the OV surface and are typically achieved by reacting the molecule of interest to amino acid residues in the viral coat proteins [51]. For example, the amine group in lysine and the thiol group in cysteine are amenable to condensation reactions, nucleophilic substitutions, and redox reactions [52]. On the other hand, noncovalent bioconjugations rely on electrostatic interactions between the OVs and the molecules of interest [51]. While noncovalent methods are simpler to implement, the interactions are weak in nature and prone to dissociation. Another strategy to functionalize the OV surface is by genetic fusion of the protein of interest to the viral capsid or envelope protein [53]. This approach offers the advantages of bypassing downstream conjugation processes and producing OVs with more homogeneous protein display. However, not all capsid and envelope proteins are tolerant to genetic fusions, which could interfere with proper protein folding [53].
OVs can also impart genetic or transcriptomic changes in the host cells through delivery of the encapsulated cargo, that is, their genetic material (Figure 2). Transgenes are typically inserted into the OV genome such that they can be expressed by the host cell after viral infection. For DNA OVs, the transgene may be either integrated into the host cell genome or maintained as an episome in the nucleus [54–57], where it is transcribed into interfering RNA (RNAi) or mRNA encoding proteins of interest [58,59]. On the other hand, most RNA OVs deliver their genetic material into the cytoplasm, where it is directly translated into protein. [60]. DNA viruses are generally more suitable for transgene delivery because they tend to have larger genomes with greater genome packaging capacity than RNA viruses [61].
2.1.2. Advantages and Disadvantages of OVs
The most notable advantage of OVs lies in their inherent nature as viruses to deliver, lyse, and reproduce without the need for additional genetic modifications (Figure 2). Their tumor selectivity and lytic activity make OVs exceptional tumor-killing agents. Tumor cell destruction is further amplified when OVs reproduce viral progenies that spread the infection to neighboring tumor cells. However, the infectious and lytic nature of OVs also comes with several disadvantages. Pathogenicity is of great concern for OVs due to the risk of uncontrollable viral replication. Even when attenuated through genetic modifications, safety issues are not completely eliminated. There is a possibility that the OVs could revert back to their pathogenic wildtype through mutation or recombination with wildtype virus strains [62,63]. Lastly, imparting new functionalities to OVs is more challenging than with VLPs or VMNs. As viruses evolved to have an optimized structure and genome to carry out their lifecycles, surface modifications and genetic fusions can negatively impact viral fitness [64]. Another disadvantage of OVs is that the immune system generates an anti-OV immune response within 1-2 weeks that clears the OV infection [65]. For this reason, it is critical to choose OVs that do not commonly circulate in the human population, as preexisting anti-OV antibodies drastically reduce treatment efficacy [66]. Strategies to increase the therapeutic window include administration of bispecific adapters that retarget anti-OV antibodies to tumor cells [67], delivery of OV genome via a separate vector [68], or the ‘Trojan horse’ technique where patient cells are infected with the OV ex vivo and readministered [69].
2.2. Virus-like Particles
VLPs are nanostructures derived from native viral proteins to structurally resemble viruses but are devoid of viral genetic material. The non-infectious nature of VLPs makes them comparably safer than OVs. However, without lytic activity and the ability to self-replicate and spread in host cells, VLP possess no inherent tumor-killing capabilities. Nevertheless, as essentially empty viral shells, VLPs are adept at eliciting strong immune responses due to the evolution of the immune system to recognize and react to the small, particulate morphology and dense antigen structure of viruses [70]. For these reasons, VLPs are typically formulated into prophylactic vaccines to protect against oncoviruses – such as hepatitis B virus (HBV), human papillomavirus (HPV), and EBV – that are estimated to cause 12% of cancers [71] or into therapeutic vaccines to sensitize the immune system towards existing tumor cells by decorating the VLP surface with cancer antigens. Beyond antigen delivery, VLPs are highly suitable for targeted delivery of therapeutic cargoes to tumors [72]. The empty VLP shell offers protection and enables drug encapsulation without steric hinderance of a viral genome. While the majority of approved VLPs are prophylactic vaccines, their uses as therapeutic vaccines or encapsulated cargo delivery vehicles are only very recent, with very few VLP therapies in clinical trials, which have been reviewed elsewhere [73,74].
2.2.1. Strategies to Functionalize VLPs
As structural mimics of viruses, VLPs share many of the same functionalization strategies as OVs (Figure 2). VLP surface modifications can be implemented through covalent or noncovalent bioconjugation of the molecules of interest or genetic fusion of proteins of interest [75]. However, unlike OVs which are limited to encapsulating genetic material, VLPs have a hollow interior, enabling a greater flexibility in terms of the cargo they carry. VLPs have been engineered to package not only DNA and RNA [76,77] but also drugs and toxins [78,79]. These molecules can be encapsulated during the VLP assembly process or by molecular infusion through the VLP membrane or capsid pores [80,81]. VLPs also have natural binding affinities that have been exploited for cargo encapsulation. For example, the positively charged interior of the VLP capsid allows the encapsulation of nucleic acids and other negatively charged molecules through electrostatic interactions [82]. To expand the variety of the loaded cargo, sites for other noncovalent interactions can be introduced to capsid proteins. For example, engineering strategies to incorporate hydrophobic pockets, polyhistidine tags, or complimentary coiled coil sequences have enabled the encapsulation of nonpolar drugs, metal-containing compounds, and proteins, respectively [78,83,84].
2.2.2. Advantages and Disadvantages
Compared to OVs, VLPs have the advantage of encapsulating a wider range of cargoes (Figure 2). The cargoes receive greater protection by being packaged inside the VLPs rather than attached to the viral surface. VLPs are also more tolerant to modifications, as they are not limited by design changes that may interfere with viral replication. However, VLPs are generally more costly and difficult to produce, hindering their widespread use for cancer treatment. Compared to OVs, which can be easily propagated in producer cell lines through their natural reproduction capabilities [85], the production of VLPs is less straightforward and requires the expression and self-assembly of viral proteins in recombinant host cells. This is a challenge because the viral components must be noncytopathic and properly folded and glycosylated to enable correct self-assembly and authentic antigen presentation [86,87]. Eukaryotic systems are often used for VLP production due to their ability to perform complex post-translational modifications, but they suffer from slow growth, low yields, and costly purification [88,89]. Recently, cell-free systems have been developed for VLP production, which enable a faster and more facile manufacturing process [90]. With these advances, VLPs will see more widespread use in the clinics.
2.3. Virus-mimicking nanoparticles
VMNs are non-infectious nanostructures conferred with selected viral properties, but unlike VLPs, the VMN shell and core are derived from nonviral materials. VMNs are engineered to emulate viruses in terms of size, shape, surface properties, and/or function [91,92]. In this way, VMNs stand out from conventional nanoparticles in their enhanced ability to selectively target and enter cells while evading immune clearance [93]. In the context of cancer treatment, VMNs serve similar functions as VLPs in their ability to deliver cancer antigens and therapeutic cargoes. However, as a newly developing field, VMN usage has mainly been limited to research settings. Liposomes, which are the most prevalent nanoparticles used in clinical applications, are virus-mimicking in their ability to fuse with the plasma membrane for cargo release. However, the incorporation of other favorable viral features into conventional nanoparticles to generate VMNs has yet to reach the clinic.
2.3.1. Strategies to Functionalize VMNs
Surface functionalization is particularly critical for VMNs since it is these surface molecules that confer VMNs with virus-like properties. For example, decoration of VMNs with silica or synthetic spikes imparts surface roughness that aids in cell internalization, and glycosylation of VMNs mimics the ability of viruses to hide from the immune system [92], Although surface functionalization of VMNs also uses covalent and noncovalent strategies, there are differences to those used by OVs and VLPs, as VMNs can be made from a wide range of materials (Figure 2). These include organic materials, such as polymers, polypeptides, polysaccharides, lipids, and nucleic acids, or inorganic materials, such as carbon nanotubes, metals, and silica [94–98], Therefore, the conjugation strategy is largely material dependent. Noncovalent methods exploit intrinsic electrostatic interactions, hydrophobic interactions, and hydrogen bonding between the material of choice and the molecule of interest. Covalent methods take advantage of functional groups naturally present on the VMN surface, such as amino and carbonyl groups of organic materials [99,100]. For inorganic materials, functional groups can be introduced using polymer coats, such as poly(isobutylene-alt-maleic anhydride) (PIMA), or through cross linkers, such as aminosilane for silica-based nanoparticles and thiocarboxylic acid for metals [99–101], Metal oxides can be functionalized by using ligand exchange, which replaces the surface ligand with one of interest [102]. This is by no means an exhaustive list of surface functionalization strategies, which have been reviewed elsewhere [52,103–105].
Cargo encapsulation is also material-dependent and is typically achieved through self-assembly via electrostatic interactions or chemical crosslinking (Figure 2). For example, cationic polymers and polypeptides bind electrostatically with the negatively charged nucleic acids to form a VMN core containing DNA and RNA [106]. Amphiphilic polymers and lipids can assemble into drug-loaded polymersomes and liposomes by film hydration, a process in which an aqueous solution is added to a dried film containing the amphiphiles and drugs, inducing drug-encapsulated nanoparticles to bud off [107,108]. In addition, the diversity of materials from which VMNs can be made offers opportunities to incorporate a greater variety of substances. For example, VMNs can exploit double emulsion techniques, such as water-in-oil-in-water or oil-in-water-in-oil, to simultaneously encapsulate hydrophobic and hydrophilic compounds [109]. A more comprehensive list of strategies can be found in other review articles [110,111].
2.3.2. Advantages and Disadvantages
Of the three VBPs, VMNs are the most versatile engineering platform, offering vast design possibilities (Figure 2). VMNs are synthesized from a wide range of materials, and thus, by careful selection of the materials, VMNs can be produced with the desired size, shape, surface characteristics and functionalities. The possibility of a cell-free synthesis and the use of inexpensive design materials enable both a cost-effective and large-scale manufacture of VMNs. However, a major challenge of VMNs is the incorporation of virus-mimicking properties. Unlike VLPs, which take on the properties of the virus from which the viral proteins are derived, VMNs typically require extensive engineering efforts to acquire desirable viral features. For example, engineering the VMN shell entails emulating the surface charge, roughness, and glycosyl patterns of viruses. In addition, although VMNs can be synthesized from various materials, biocompatibility and toxicity may limit the available choices.
3. Engineering VBPs for Cancer Therapy
The potential of VBP therapies for cancer treatment has been demonstrated by the approval of Gendicine [112], Rigvir [113,114], H101 [35], and T-VEC [115] VBPs for the treatment of skin cancers. However, aside from these examples, no other VBP-based therapies have successfully completed clinical trials. The major challenge facing current and future VBP therapies is to enhance their potency while maintaining their tumor selectivity. As a result, recent engineering efforts have focused on two key areas: 1) enhancing selective targeting of the VBP to tumor cells and 2) enhancing tumor-specific therapeutic mechanisms.
3.1. Tumor Targeting of VBPs
Targeting of VBPs to cancerous cells can be controlled on multiple levels to maximize therapeutic potential while minimizing off-site targeting. OVs take advantage of the increased susceptibility of cancer cells to viral replication due to their high metabolic activity, dysregulated state, and immunosuppressive nature [116]. For example, reovirus and vaccinia viruses aggressively replicate in cells with an activated Ras pathway, a pro-survival pathway commonly upregulated in cancerous cells [117,118]. A complimentary mechanism of tumor tropism for many OVs is their recognition of tumor-associated antigens (TAAs) overexpressed on the surface of cancer cells. For instance, subtypes of HSV and adenoviruses target the TAA CD46 [119], an inhibitory complement receptor that aids in immune evasion [120]. As a result of their natural selectivity toward tumor cells, adenovirus, HSV, reovirus, and vaccinia virus make up the majority of OVs in clinical trials [42].
Unlike OVs, VLPs and VMNs cannot take advantage of selective replication in malignant cells to promote tumor tropism and therefore require alternative targeting strategies. One approach is to exploit mechanisms of natural viral tropism to direct VLP and VMN to specific tissues or tumor cells. As VLPs are assembled from native viral proteins, they typically inherit tropism of the virus from which they originate [74,81,121]. For example, JC polyomavirus VLPs have been shown to target tumor nodules in the bladder while HPV VLPs preferentially target tumors in a variety of tissues [122,123]. In the case of VMNs, tissue tropism is imparted by conjugating their surface with ligands that target specific tissue receptors or virus-derived peptides. For example, N-acetylgalactosamine, a sugar derivative that binds specifically to asialoglycoprotein receptors on hepatocytes, is a common attachment on VMNs to target liver tissues [124,125]. Lee and colleagues engineered VMNs to mimic the unique ability of rabies virus to cross the blood brain barrier by surface conjugation of a peptide derived from rabies virus glycoprotein, which binds to receptors on neuronal cells to facilitate entry to the central nervous system [97]. Similarly, researchers took inspiration from the HBV by conjugating VMNs with a myristoylated hepatitis-derived peptide to enable specific targeting of the liver [126,127].
Aside from harnessing natural viral tropisms, tumor targeting can also be engineered by taking advantage of cellular receptors and molecules upregulated on malignant cells. For instance, researchers have recently discovered cNGQ peptides that bind with high affinity and specificity to α3β1 integrins, which are upregulated on many forms of cancerous cells [128,129]. VMNs conjugated with cNGQ peptides demonstrated efficient uptake by α3β1 integrin-overexpressing lung cancer cells [130]. Similarly, VLPs and VMNs have been functionalized with TAA ligands, such as folate [131], epidermal growth factor [132,133], transferrin [134], and phosphorylcholine [135] to enhance tumor targeting. In another strategy, antibodies, which demonstrate even greater affinities than the aforementioned ligands, have been conjugated to VLPs and VMNs to enable tumor-specific targeting. For example, Rous sarcoma virus (RSV) VLPs specifically targeted colon adenocarcinoma cell line LS174T in vitro after being functionalized with a single chain variable fragment (scFv) specific for tumor-associated glycoprotein-72 (TAG 72) [136].
3.2. Antitumor Functions of VBPs
Following the targeting of VBPs to cancerous cells, VBPs must carry out their therapeutic function. Antitumor activity can be categorized into three distinct mechanisms (Figure 3): 1) oncolysis, 2) immune modulation, and 3) gene repair/regulation. These mechanisms are not mutually exclusive, with successful OVs, VLPs, and VMNs often incorporating multiple therapeutic modalities to enhance their efficacy.
Figure 3: Antitumor mechanisms of VBP.
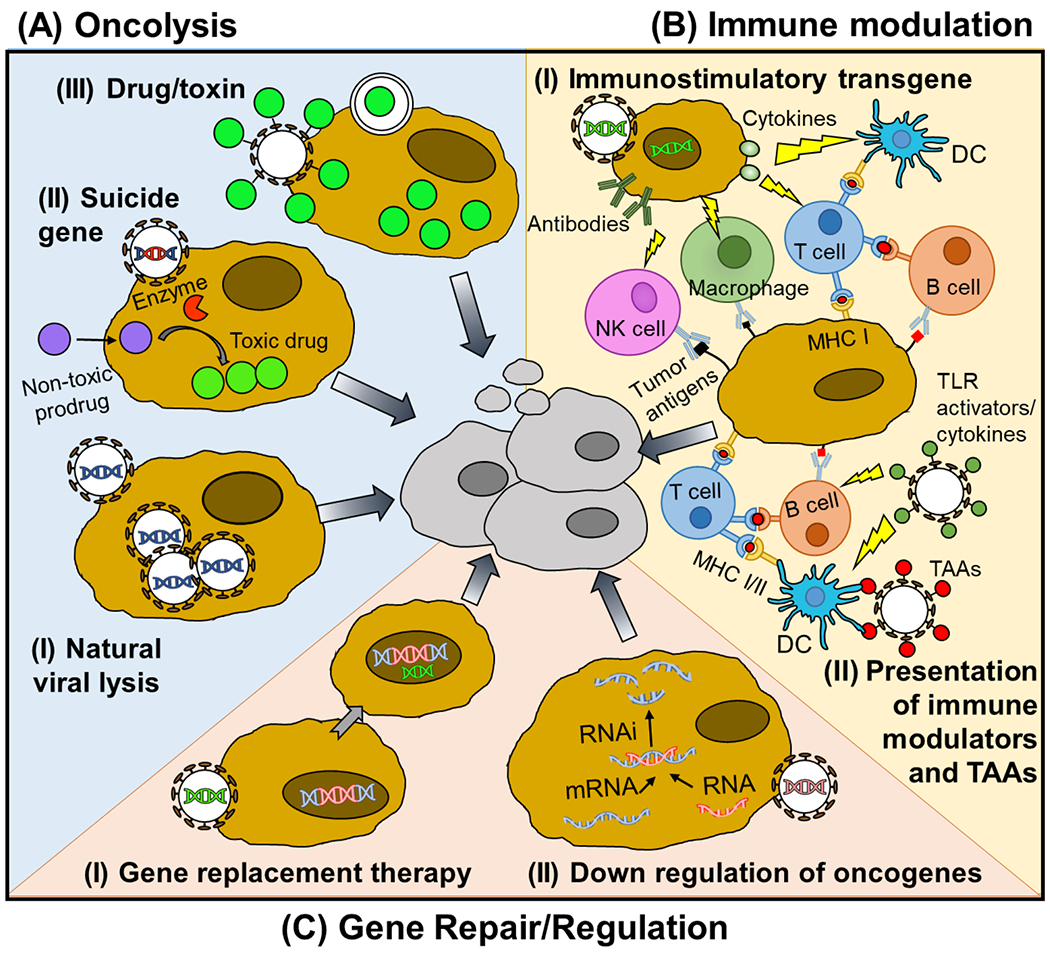
Antitumor mechanism of VBP can be divided into three groups. (A) VBPs can promote oncolysis through (I) natural viral lytic mechanisms, (II) delivery of a suicide gene, or (III) delivery of a drug or toxin. (B) VBPs can also relieve the immunosuppressive environment of the TME and promote a tumor-specific adaptive immune response through either (I) delivery of immune stimulating transgenes to tumor cells, encoding for example cytokines or antibodies, or (II) delivery of cancer antigens and immunostimulatory molecules to APCs. (C) Finally, VBPs can restore cellular antitumor functions through delivery of (I) tumor suppressive transgenes or (II) interfering RNA that degrades oncogenic miRNA or mRNA.
3.2.1. Oncolysis
While OVs induce tumor cell lysis as a result of their natural viral replication (Figure 3A(I)), the oncolytic potential of OVs has been further enhanced through their packaging of suicidal transgenes. Suicide genes generally encode enzymes that convert harmless prodrugs into toxic substances, providing OVs with another oncolytic mechanism (Figure 3A(II)). In addition to killing infected cells, suicide gene therapy can have significant bystander effects, where toxic drugs can disseminate to nearby tumor cells by diffusion or through gap junctions and enhance oncolysis [137]. The most studied suicide gene encodes the HSV thymidine kinase (TK). HSV TK phosphorylates a guanosine analogue prodrug, leading to single stranded breaks upon incorporation into DNA [138]. In another strategy, cytosine deaminase (CD) converts the prodrug 5-fluorocytosine to the chemotherapeutic drug 5-fluorouracil, leading to cell death. Together, HSV TK and CD make up the vast majority of suicide transgenes of OVs in past and present clinical trials [42,139].
Despite their inability to replicate, VLPs and VMNs can also induce oncolysis through delivery of therapeutic cargoes. Like OVs, VLPs and VMNs are capable of delivering suicide genes. For example, JC polyomavirus VLPs successfully delivered the HSV TK suicide gene to a human B cell lymphoma cell line, resulting in cell lysis [140]. Whereas OVs are generally limited to delivery of nucleic acids (with few exceptions [47]), VLPs and VMNs can deliver a wide variety of therapeutic cargoes (Figure 3A(III)). Doxorubicin is a common small molecule drug delivered by VLPs and VMNs [136,141–145], which intercalates with DNA and inhibits topoisomerases, inducing DNA damage [146]. Unable to repair the DNA damage, the cell initiates apoptosis [147]. Aside from doxorubicin, VLPs and VMNs have incorporated a variety of toxic drugs including paclitaxel [148], bleomysin [149], ricin toxin [150], Asiatic acid [151,152], and saporin [153,154] to trigger tumor cell death (Table 1). Compared to the delivery of suicide genes with a prodrug, the delivery of drug molecules is a more direct approach of bringing therapeutics to the tumor site, however, it provides less control over drug toxicity. Regardless of the choice of VBP or drug, an important consideration for drug delivery vehicles is how the VBP will gain entry into the tumor cell and how the therapeutic cargo will be released. Whereas OVs and VLPs can take advantage of their innate viral mechanisms for cellular entry, VMNs require functionalization with membrane fusion proteins or cell penetrating peptides (CPPs) to facilitate cell uptake [155–157]. The release of non-nucleotide drugs presents another challenge. Taking advantage of electrostatic interactions or conjugation chemistries, drug release from VLPs and VMNs has been activated through the reducing environment of the cytoplasm [158,159], endosomal proteases [141], and acidic endosomal pH [148]. In one approach, JC polyomavirus VLPs were internally modified with cyclodextrins though disulfide bond chemistry, enabling binding of the hydrophobic drug paclitaxel. Upon cellular entry, paclitaxel is released in the reducing environment of the cytoplasm [158]. In another approach, doxorubicin was encapsulated in RSV VLPs using electroporation and released into cells via the natural membrane fusion ability of RSV [136]. VMNs have the benefit of being designed from stimuli-responsive materials that trigger cargo release. For example, poly(L-histidine) has a pH-dependent solubility and is unstable at pH lower than 7.0, thus liberating the drug in the acidic tumor microenvironment (TME) [160].
Table 1:
VBP modifications to enhance antitumor mechanisms
| Therapy | Class | Modification | Mechanism | Representative References |
|---|---|---|---|---|
| Oncolysis | Drug/toxin | Doxorubicin | Intercalates into DNA causing strand breakage and inhibits topoisomerase II, inducing apoptosis | [136,141–145] |
| Saporin | Ribosome-inactivating protein, inducing necrosis and apoptosis | [153] | ||
| Bleomycin | Induces double and single stranded DNA break, leading to apoptosis | [149] | ||
| Ricin toxin | Ribosome-inactivating protein, inducing necrosis and apoptosis | [150] | ||
| Suicide genes | Tyrosine kinase (TK) | Converts prodrug to toxic molecules, leading to apoptosis of infected and bystander cells | [138] | |
| Cytosine deaminase (CD) | [275],[276] | |||
| Immune Modulation | Delivery of cancer antigens and immune stimulators | TAAs and neoantigens | Deliver tumor antigens to APCs, initiating a tumor-specific adaptive immune response | [169–173] |
| Immunostimulatory cytokines | Enhance APC activation and antigen presentation, increase tumor-specific T cells and B cells | IL-2 [165], IL-12 [277], IL-18 [278], GM-CSF [279] | ||
| TLR activators | CpGDNA [173,174,280], Flagellin [176,177], E8Pam2Cys[175,176] | |||
| Transgene relieving immuno-suppressive TME | GM-CSF | Stimulates proliferation of granulocytes and monocytes, recruitment and maturation of DCs | [161,162] | |
| sFlt-1 | Sequester VEGF, inhibiting angiogenesis | [281] | ||
| Anti-PD-1 | Blocks PD-1 mediated inhibition of T cell activation | [173,282] | ||
| Anti-CTLA-4 | Blocks CTLA-4 mediated inhibition of T cell activation | [167,168] | ||
| Gene Regulation | Gene replacement therapy | p53 transgene | Restore p53 function, inducing cell cycle arrest and apoptosis | [180,188,283] |
| Gene modulation | siRNA | Knock down of oncogenic genes, inhibiting growth or inducing apoptosis | [284] | |
| Non-coding RNA complementary to oncogenic miRNA | Modulates miRNA environment to promote expression of tumor suppressor genes | [195] |
3.2.2. Modulating Immune Response
In addition to the direct antitumor effects of oncolysis, VBPs can directly or indirectly stimulate the immune system to destroy malignant cells. A natural consequence of the cellular destruction caused by drug-induced necrosis or viral lytic activity is the release of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) that stimulate local immune cells and relieve the immunosuppressive nature of the TME. To further enhance local activation of immune cells, OVs have been engineered to encode immunostimulatory molecules in their genome that are transcribed and released upon viral infection (Figure 3B(I), Table 1). The most common transgene of OVs in clinical trials is GM-CSF [42], an inflammatory cytokine that leads to the recruitment of monocytes and their maturation into macrophages and dendritic cells. The GM-CSF transgene has demonstrated therapeutic benefits in several studies [161,162] and is also present in T-VEC, the only FDA approved OV. Aside from GM-CSF, OVs have encoded immunostimulatory molecules B7.1 [163], CD40L [164], IL-2 [165] 4-BBL [164], and IL-12 [166] to enhance the antitumor immune response. Some OVs have also been endowed with genes encoding immunomodulating antibodies. For example, adenovirus encoding the immune checkpoint inhibitor (ICI) anti-CTLA-4 antibody was found to improve antitumor immunity in mouse models [167,168].
Aside from stimulation of immune cells in the local environment through transgene expression, VBPs can train the immune system to mount an adaptive response against cancer antigens displayed on the tumor cell surface. However, a significant challenge with TAAs is associated with their presence on both cancerous and healthy tissues. As a result, TAA-specific B and T cells are highly attenuated due to mechanisms of self-tolerance. Fortunately, the highly immunogenic presentation of TAAs on viral particles has been shown to break B cell self-tolerance [169–172], enabling the generation of anti-TAA antibodies that mark cancer cells for destruction by natural killer (NK) cells and macrophages [173]. In addition to B cells, successful antitumor response requires activation of self-reactive T cells. VBPs presenting TAAs and neoantigens are taken up by antigen presenting cells (APCs), leading to their presentation to T cells [73]. The activation of T cells can be further enhanced through inclusion of immunostimulatory oligonucleotides (CpG DNA, ssRNA) [174,175], lipopeptides (E8Pam2Cys) [176], and peptides (flagellin) that directly activate toll-like receptors (TLRs) (Figure 3B(II), Table 1) [177,178]. For example, CpG-loaded VLPs displaying a TAA were able to elicit a high fraction of tumor-specific central memory CD8+ T cells in melanoma patients in a phase I/II clinical trial [179]. Compared to delivery of transgene encoding immunostimulatory molecules, direct conjugation of antigens or immune modulators to the VBP surface allows a greater variety of molecules delivered to the tumor site because some of these molecules, such as CpG DNA, cannot be encoded by a transgene.
An alternative approach to enhancing TAA-specific immune response is to identify antigens that are tumor-specific, namely neoantigens [180]. While their identification is not yet straightforward, neoantigens incorporated into VLPs have successfully elicited antitumor immune responses [173].
Similar to VLPs, VMNs have also been engineered to display TLR ligands, such as CpG DNA, monophosphoryl lipid A, and R837 [181,182] to enhance the immune response against antigens. However, whereas OVs and VLPs are easily internalized by APCs, VMNs have to be engineered to increase uptake. It was recently found that the E2 subunit of pyruvate dehydrogenase, a self-assembling nanocage, has a high uptake by dendritic cells [183]. Using E2 to form the VMN shell, the surface was conjugated with the melanoma-associated gp100 TAA and CpG DNA, leading to dendritic cell uptake and a significant increase in CD8+ T cell proliferation and IFN-γ secretion [184].
3.2.3. Reversing Malignant Phenotype with Gene Replacement Therapy and RNA Interference
The onset of oncogenesis is a result of defective cellular processes that enable unchecked cellular mutation and proliferation. Gene replacement therapy seeks to permanently or transiently repair these regulatory cellular pathways to abolish or attenuate tumor growth (Figure 3C(I)). Compared to the delivery of toxic genes or drugs, gene replacement therapy has the advantage of minimally impacting healthy cells. The most commonly mutated protein in cancers is transcription factor p53 [185,186], the so-called “guardian of the genome,” which initiates DNA repair and inhibits the growth of cells with damaged DNA [187]. As a result, p53 gene replacement therapies have garnered significant attention. Gendicine, a non-replicative adenovirus vector that delivers wild-type p53 gene, was approved in China in 2003 to treat head and neck squamous cell carcinoma, becoming the world’s first commercial gene therapy (Figure 1). In combination with other traditional cancer therapies, Gendicine has repeatedly demonstrated significantly higher response rates compared to the traditional therapies alone [188]. Despite the positive results, Gendicine exhibits low transduction rates, limiting its therapeutic potential. The use of replicating adenoviral vectors has been shown to increase p53 expression and has stronger antitumor effects [189], However, a major challenge of gene replacement therapy remains, which is that individual tumors have unique and diversely mutated proteomes. Indeed, aside from p53, there are very few universally mutated tumor suppressor genes prevalent across cancers [190]. Nevertheless, several candidates have been explored for gene replacement therapy, such as PTEN [191]. BRAC1 [192], and RTVP1 [193].
In addition to a mutated genome, the onset of cancer is associated with a dysregulated transcriptome. Therefore, viral-based therapies that seek to restore the natural RNA environment in the cell are being investigated (Figure 3C(II)). Although transcriptomic modifications are transient, this approach is generally more common than gene replacement therapy because it is safer and avoids the risk of unintended mutations to the genome. An attractive RNA target is micro RNA (miRNA), which are endogenous non-coding RNAs that have the ability to degrade mRNA through RNA interference (RNAi). Cancerous cells have been shown to upregulate oncogenic miRNAs (oncomiRs) that silence tumor suppressor genes while upregulating genes that promote angiogenesis, survival, and metastasis [194]. In one study, an adenoviral vector was modified to express a specific long noncoding RNA (lncRNA), which has the ability to degrade multiple oncomiRs through RNAi. When administered to xenografted tumors in a mouse model, the modified adenoviral vector was able to significantly reduce the expression level of target oncomiRs, resulting in reduced tumor growth [195]. In addition to modifying endogenous miRNA, small interfering RNAs (siRNAs) can be delivered to directly modulate oncogene mRNA expression. In a human lung cancer mouse model, siRNA was delivered by virus-mimicking polymersomes to silence the PLK1 gene, which is responsible for regulation in mitosis [130,196]. The siPLK1 treated mice had a longer median survival (54 days) than that of the control mice treated with scrambled siRNA (22 days) and showed no signs of liver metastasis that was observed in the control mice [130]. While miRNA and siRNA approaches demonstrate promise, the field is still in its infancy with very few therapies in clinical trials [197,198].
4. Viral Strategies to Overcome Key Challenges in Traditional Cancer Treatment
The majority of cancer cases are treated by traditional means, such as surgery, chemotherapy, radiotherapy, immunotherapy or a combination thereof. While these approaches have resulted in a steady decline in cancer death rates – 1.8% per year for men and 1.4% per year for women from 2000-2014 [199] – traditional cancer therapies are not without limitations. They are not a feasible treatment option for many cancer patients, as in the case of tumors that are inoperable, multidrug resistant, immune resistant, or metastatic. In this section, strategies employed by VBPs to address these shortcomings are discussed.
4.1. Inoperable Solid Tumors
Solid tumors, which are characterized as an abnormal mass of tissues, represent almost 90% of all cancer cases in adults [200]. The most common treatment for solid tumors is surgical removal. However, surgical operations are not feasible for metastatic tumors or tumors that are close to critical structures, such as the brain, spinal cord, or major blood vessels. In these cases, systemic delivery is typically used to bring therapeutics to the tumor sites and is especially advantageous when the tumors are nonlocalized. However, therapeutics delivered systemically face a number of physical and biological barriers en route to their destination (Figure 4A, left). The first obstacle is the intravascular barrier consisting of proteases, nucleases, and immune cells that can degrade or eliminate the therapeutics [201,202]. Next, endothelial cells that line blood and lymphatic vessels prevent therapeutics from escaping circulation [203]. After crossing the endothelial barrier, the therapeutics must further migrate through the tumor extracellular matrix (ECM), which is significantly altered in the TME [204]. In particular, the abnormal vasculature causes a buildup of interstitial fluid pressure that restricts the infiltration of molecules [205,206]. Although therapeutics could be administered in the form of free drugs, the use of carriers helps to prolong the blood circulation time by protecting the therapeutics from enzyme degradation and immune clearance [207]. While VBPs work like other carriers to circumvent the intravascular barrier [208,209], VBPs offer several distinct advantages for overcoming the endothelial and ECM barrier (Figure 4A, right). In particular, OVs have cell penetrating peptides (CPPs), which mediate their entry into endothelial cells [210]. After infection and lysis of endothelial cells, viral progenies are released into the ECM where the tumor cells are embedded [211]. In the ECM, most carriers rely on diffusion to reach tumor cells, which is highly ineffective due to the densely packed ECM structure and high interstitial pressure. In contrast, OVs have mechanisms to bypass the ECM barrier [212], such as fusogenic glycoproteins that can induce the fusion of host cells to form a syncytia [213] and actin-related proteins that mediate the formation of membrane protrusions to reach neighboring cells [214]. These favorable viral features have subsequently inspired researchers to engineer CPPs and fusogenic glycoproteins into VLPs and VMNs [215–218], leading to their increased penetration into the solid tumors [210,215,218].
Figure 4: VBPs to Overcome Traditional Cancer Therapy Challenges.
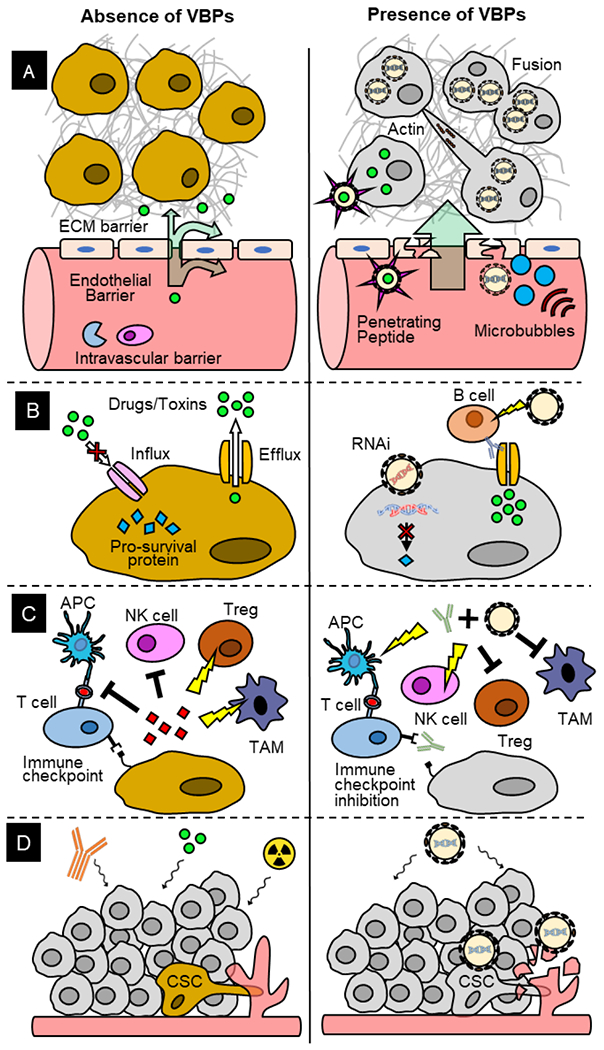
(A) Left. Intravascular barrier, endothelial barrier and extracellular matrix (ECM) barrier limit the bioavailability of therapeutics. (A) Right. VBPs protect cargoes from intravascular barrier. Cell-penetrating peptides and ultrasonic stimulation of microbubbles facilitate VBP penetration through endothelial barrier. VBPs induce cell fusion and actin polymerization to overcome ECM barrier. (B) Left. Abnormal transporter expression keeps therapeutic drugs or toxins out of the tumor cells and pro-survival mediate tumor cell survival. (B) Right. Retention of toxins and RNAi of pro-survival proteins in the tumor cells promote cell death. (C) Left. Cytokines secreted by tumor cells inhibit cytotoxic T cells and natural killer (NK) cells and activate tumor associated macrophages (TAM) and regulatory T cells (Treg). Immune checkpoint proteins on tumor cells inhibit T cells. (C) Right. Coadministration of VBP and checkpoint inhibitors activate immune cells to kill tumor cells. (D) Left. Cancer stem cells (CSCs) are resistant to chemotherapy, radiotherapy, and immunotherapy and promote angiogenesis to leave primary tumor site for metastasis. (D) Right. CSCs and their vasculatures are targeted by VBPs.
Solid tumor penetration can be further enhanced by accompanying VBPs with the application of mechanical forces [219] (Figure 4A, right). The co-delivery of VBPs with microbubbles (MBs) is an attractive strategy. Under ultrasonic stimulation, the MBs expand and collapse to generate inertial cavitation that creates transient pores (up to several micrometers) in biological membranes, which promotes extravasation of the VBPs and can significantly increase their intratumoral biodistribution [220,221]. For example, MBs and adeno-OVs were intravenously injected into mice bearing human carcinoma xenograft and the spread of the OVs was tracked by measuring luciferase expression [222]. After 3 days, the tumors demonstrated 30-fold higher luciferase expression in the ultrasound-treated mice than non-ultrasound-treated mice [222]. This elegant experiment demonstrates the synergy between OVs and ‘sonoporation’ to promote solid tumor penetration: MBs propel the OVs deep into the tumor enabling OVs to further spread the infection through oncolysis.
4.2. Multidrug Resistant Cancer
A major obstacle for chemotherapy – the use of cytotoxic drugs to treat cancer – is the generation of tumor cells that become resistant to multiple structurally diverse chemotherapeutic agents over the course of treatment [223,224]. A major cause of multidrug resistance (MDR) is aberrant membrane transporter expression: overexpression of efflux transporters that pump therapeutic drugs or toxins out of the cell, such as ATP-binding cassette (ABC) proteins [4], and the downregulation of influx transporters that facilitate drug uptake, such as the solute carrier family (SCF) [225]. As a result, intracellular drug concentrations are significantly depleted, reducing treatment efficacy (Figure 4B, left).
Due to their regulatory role in drug transport, membrane transporters are promising targets to overcome MDR in cancer cells. The highly ordered and repetitive structure of VLPs is well suited for eliciting antibody responses against the undesirable efflux transporters (Figure 4B, right). In a triple negative breast cancer (TNBC) study, the cystine-glutamic acid transporter (xCT) was found to promote cell detoxification and inhibit apoptosis by pumping reactive oxygen species (ROS) out of the cell [226]. The extracellular domain 6 (ECD6) from the xCT protein was genetically fused to the AB loop of bacteriophage MS2 VLP to generate a prophylactic and therapeutic vaccine against xCT [227]. Vaccination with the ECD6-MS2 VLP led to an increase in ROS concentration in TNBC tumorspheres and a decrease in tumor growth and in the number of pulmonary metastases in the TNBC mouse model [227].
Another contributing factor to MDR is the expression of defective apoptotic pathways or enhanced DNA repair pathways that render pro-apoptotic drugs and DNA-damaging agents ineffective [228,229]. As discussed in Section 2 and Section 3.2.3, VBPs are adept at nucleic acid delivery for RNAi. Thus, VBPs could be leveraged to overcome MDR through the downregulation of pro-survival and antiapoptotic proteins (Figure 4B, right). For example, antiapoptotic BCL2 family proteins, which promote cell survival by inactivating mitochondrial permeabilization, and Survivin, which inhibits caspase activation required for programmed cell death, are commonly found to be upregulated in many MDR cancers [230,231]. A team of researchers engineered an HSV type 1 to simultaneously silence BCL-2 and Survivin using RNAi [232]. Dual knockdown of these genes led to 70% cell death in the MCF-7 human breast cancer cell line, whereas single knockdown of BCL-2 or Survivin only led to 10% or 30% cell death, respectively [232].
4.3. Immunotherapy Resistant Tumors
An equally challenging issue to traditional cancer therapies is immunotherapy resistance, the ability of tumor cells to evade immune surveillance. Immunotherapy resistance occurs by a number of mechanisms: (1) downregulation or loss of TAAs on tumor cells, which disrupts APCs from their proper recognition and presentation to T cells, (2) overexpression of immune checkpoints, such as PDL-1, which bind to the PD-1 receptors on T cells to inhibit immune response [233], (3) secretion of chemokines and cytokines, such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β), that suppress cytotoxic T cells and NK cells [234,235], and (4) recruitment of immunosuppressive cells, such as regulatory T (Treg) cells, suppressive myeloid cells, and tumor-associated macrophages (TAM), to the tumor site [236]. The combination of these factors contributes to a highly immunosuppressive TME (Figure 4C, left). As discussed in Section 3.2.2, VBPs, in general, are naturally adept at activating the immune system and can be further modified to enhance their immune response. For this reason, VBPs are often co-administered with immunotherapies when patients do not respond to immunotherapies alone (Figure 4C, right) [237], Immune checkpoint inhibitors (ICIs) were a paramount discovery in cancer immunotherapy, but a large portion of patients either have an innate or develop an acquired resistance to ICIs [238,239]. In a colon carcinoma mouse model, sensitization of tumors resistant to ICI was accomplished using a vaccinia OV and ICI (anti-CTLA4 and anti-PD-1 antibodies) combinational therapy [240]. Similarly, reversal of ICI resistance has been observed in an ongoing clinical trial, in which a bacteriophage Qβ-VLP encapsulating a TLR-9 agonist was co-administered with anti-PD-1 to melanoma patients [73].
4.4. Cancer Stem Cells
Metastasis is an advanced stage of cancer that is characterized by the spread of tumor cells to distant tissues. Following metastasis, treatment options are very limited and prognosis is poor with 5-year survival rates as low as 5% [200]. While the mechanism is still unclear, an increasing number of studies have pointed to cancer stem cells (CSCs) as the origin of metastases. CSCs are a rare subpopulation of tumor cells with the ability of self-renewal and differentiation into heterogeneous cancer lineages [241,242]. Because CSCs are resistant to radiotherapy, immunotherapy, and chemotherapy, it is believed that they survive cancer treatment and eventually migrate to distant tissues to seed new tumor cells [243–245] (Figure 4D, left).
VBPs have recently attracted attention for CSC targeting because the mechanisms of resistance for conventional therapies described in section 4.2 and 4.3 do not affect VBPs. In fact, some of these mechanisms play to the benefit of VBPs, particularly OVs. First, CSCs have enhanced DNA repair pathways that confer resistance to radiation and drugs. As a part of the DNA repair process, CSCs activate ATM kinase and histone phosphorylation, which is conducive to OV replication [246]. Second, CSCs tend to dwell in hypoxic regions of the tumor, which help to preserve their stem cell properties [247]. In the hypoxic TME, CSC are typically quiescent with low metabolic rates and thus unresponsive to chemotherapy drugs that target fast-dividing cells [248]. Interestingly, studies have demonstrated that OVs are not only unaffected by hypoxia but also show increased replication in hypoxic cells [249,250]. Third, CSCs undergo epithelial-mesenchymal transition (EMT), during which they acquire the ability to migrate to distant tissues [251]. It has been found that EMT-induced tumor cells have increased susceptibility to HSV infection [252]. In addition, CSCs secrete angiogenic factors that contribute to the formation of tumor vasculatures used for migration, proliferation, and metastasis [253]. Multiple studies have found that vaccinia OVs can infect endothelial cells to selectively destroy tumor vasculatures without harming normal vasculatures [254–256] (Figure 4D, right).
Although the use of VLPs and VMNs for CSC targeting has not been well-studied, they do have great potential in this area. For example, CSCs have self-renewal and differentiation surface markers, nine of which are FDA-approved drug targets [257]. With an organized and repetitive antigen presentation, VLPs make ideal vaccines against CSC markers. However, because CSCs share many markers with normal stem cells, the identification of markers unique to CSCs would be needed before this technology could reach its full potential. For VMNs, they have the benefit of being made from anti-CSC materials. Graphene oxide as a material has been found to induce CSC differentiation, thus reducing the “stemness” of CSCs [258]. Self-assembled DNA nanomaterials can reverse drug resistant phenotypes that are characteristic of CSCs [259].
Moreover, a new class of smart nanomaterials that is hypoxia-responsive has been identified, which can selectively target the hypoxic regions where CSCs localize [260,261]. By harnessing the synergy between these material properties and engineered viral properties, VMNs could serve as a powerful weapon against CSCs.
5. New Technologies for Engineering VBPs
While viral-based approaches continue to demonstrate great potential for cancer treatment, emerging technologies promise to improve their safety and efficacy and further accelerate their development. One such technology, heteromultivalent display on VBP, has the potential to greatly increase VBP avidity for cancer cells through the display of multiple targeting moieties [262] or enhance immune cell activation through the display of multiple costimulatory molecules [263]. For example, a study demonstrated that the display of four distinct ligands – αvβ3 integrin, P-selectin, EGFR and fibronectin – on liposomes chosen to specifically target metastatic cells, including CSCs, resulted in nearly 3-fold greater accumulation in metastases than each ligand displayed alone in a TNBC mouse model [262]. Despite the advantages of heteromultivalent display, it remains challenging to precisely pattern multiple molecules on VBP. However, recent progress in the implementation of unnatural amino acids (uAA) into the genetic code promises to make the heteromultivalent display much more feasible, enabling site-specific and bio-orthogonal conjugation of peptide and non-peptide targeting ligands, drug molecules, or adjuvants using mild chemical reactions like click chemistry [264–266]. For example, porphyrins were selectively delivered to Jurkat leukemia T cells using MS2 VLPs decorated with nucleic acid aptamers conjugated through uAAs [267].
Another persistent challenge of cancer treatment is that patients with tumors of the same type and stage differentially respond to the same treatment due to tumor heterogeneity. Thus, there is an urgent need to transition from one-size-fits-all approaches to precision medicine. Next generation sequencing has emerged as a powerful high-throughput technology that can rapidly identify patient-specific TAAs and neoantigens as targets for cancer immunotherapy [268]. However, such technology must be coupled with rapid, highly customizable therapeutic platforms that can incorporate the identified epitope targets. To this end, MY-NEOVAX has recently been developed as a personalized adeno-OV that can carry up to 50 neoantigen transgenes determined by next generation sequencing [269]. When administered to two patients with end-stage metastatic cancer, MY-NEOVAX prolonged the expected survival time of less than 6 months to over a year [269]. Another recent technology, mRNA- and DNA-based vaccines, offers an even more streamlined approach for personalized cancer antigen delivery [270]. Rather than manufacturing and purifying viral particles, nucleotide-based vaccines transfer the burden of manufacturing therapeutic proteins to the patient’s cells, greatly simplifying and accelerating the development pipeline. In addition, the success of SARS-CoV2 mRNA vaccines has clearly demonstrated that nucleotide-based vaccines can be safe, stable, immunogenic, and rapidly produced. Indeed, several therapeutic mRNA-based cancer vaccines encoding TAAs or neoantigens have shown promising results in clinical trials [270]. Aside from encoding short epitopes, nucleotide-based vaccines can also encode proteins that self-assemble into VLPs [271] or OVs [Hadac 2011], streamlining their manufacture and delivery.
Despite the clear progress that has been made in repurposing natural viruses for cancer therapeutics, the modification of viruses is still not straightforward due to their minimalistic nature that has been honed over billions of years of evolution. Aside from this “top-down” approach, advances in computational protein engineering in the past decade have made it possible to take a fully de novo strategy to designing virus-like capsids for encapsulating therapeutic molecules [272,273]. A recent study further demonstrated that in silico designed viral capsids can incorporate their own genetic material, enabling them to be evolved for improved genome packaging, stability, and circulation time [274]. While still in its infancy, such studies demonstrate the vast potential of this bottom-up approach to create designer artificial viruses that can be customized to overcome the challenges of cancer therapy.
6. Concluding Remarks and Future Perspectives
Viral-based strategies for cancer therapy have come a long way since the accidental discovery of viral infections leading to tumor regression in the early 1900s. While the use of wildtype OVs in early clinical trials was limited by transient therapeutic outcomes and adverse side effects, the advent of emerging engineering technologies has not only produced OVs with increased safety and efficacy, but also opened up two virus-inspired platforms, VLPs and VMNs, greatly expanding design possibilities and antitumor functionalities. The three VBPs form a powerful trio with their ability to kill tumor cells through oncolysis, immune modulation, and gene regulation. Viral-based strategies is at an exciting point of its development, as there is increasing evidence demonstrating its potential to overcome the limitations of traditional cancer therapy. [42,73] As such, the use of VBPs for refractory cancers that are resistant to conventional therapies is expected to greatly expand in the future. Since ideal therapeutic outcomes are difficult to achieve with a single treatment method, a multimodality approach combining VBPs with conventional therapies may hold the greatest promise for improving treatment efficacy in a wider range of cancers. However, VBPs are still an emerging field, requiring further research investigations before their full potential is to be realized. Areas of research that warrant exploration include the identification of novel surface conjugated molecules, therapeutic cargoes, smart nanomaterials, and functionalization strategies that can increase targeting specificity, therapeutic window, potency and safety. A greater understanding of the in vivo effects of VBPs as a monotherapy and as a combination therapy is also needed to determine the best treatment regimen. In addition, owing to tumor heterogeneity, tools to guide VBP design (choice of platform, material, cargo, etc) specific to individual patients are also of great interest for personalized medicine. Through continuous research efforts in these areas, along with advances in material science, chemistry, and biotechnology, VBPs can truly emerge as an effective new generation of cancer therapy.
Acknowledgments
The authors would like to thank all members of the Fei Wen lab for helpful feedback and comments on the manuscript. We would also like to acknowledge the financial support provided by National Science Foundation (NSF) CAREER Award 1653611, National Institutes of Health (NIH) grants S100D020053 and P30CA046592, and the A. Alfred Taubman Medical Research Institute’s Taubman Institute Innovation Projects program at the University of Michigan. XYM was additionally supported by the NSF Graduate Research Fellowship DGE 1841052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- [1].Centers for Disease Control and Prevention., An Update on Cancer Deaths in the United States | CDC, Atlanta, GA: US Dep. Heal. Hum. Serv. Centers Dis. Control Prev. Div. Cancer Prev. Control. (2021). https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm. [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA. Cancer J. Clin 69 (2019) 7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- [3].Chakraborty S, Rahman T, The difficulties in cancer treatment., Ecancermedicalscience. 6 (2012) ed16. 10.3332/ecancer.2012.ed16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang X, Zhang H, Chen X, Drug resistance and combating drug resistance in cancer, Cancer Drug Resist. 2 (2019) 141–60. 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gujar S, Pol JG, Kroemer G, Heating it up: Oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies, Oncoimmunology. 7 (2018). 10.1080/2162402X.2018.1442169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coley WB, Contribution to the knowledge of sarcoma, Ann. Surg 14 (1891) 199–220. 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DePace N, Sulla Scomparsa di un enorme cancro vegetante del callo delTutero senza cura chirurgica., Ginecologia. 9 (1912) 82–9. https://www.sid.ir/en/journal/ViewPaper.aspx?ID=161583. [Google Scholar]
- [8].Power AT, Bell JC, Cell-based delivery of oncolytic viruses: A new strategic alliance for a biological strike against cancer, Mol. Ther 15 (2007) 660–5. 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- [9].Altinoz MA, Guloksuz S, Elmaci I, Rabies virus vaccine as an immune adjuvant against cancers and glioblastoma: new studies may resurrect a neglected potential, Clin. Transl. Oncol 19 (2017) 785–92. 10.1007/sl2094-017-1613-6. [DOI] [PubMed] [Google Scholar]
- [10].Love R, Sharpless GR, Studies on a Transplantable Chicken Tumor (RPL-12 Lymphoma), 1954. [Google Scholar]
- [11].Hosier HA, Zanes RP, von Haam E, Studies in Hodgkin’s Syndrome, Cancer Res. 9 (1949). [PubMed] [Google Scholar]
- [12].Higgins GK, Pack GT, The effects of virus therapy on the microscopic structure of human melanomas., Am. J. Pathol 27 (1951) 728–9. https://pubmed.ncbi.nlm.nih.gov/14846955/. [PubMed] [Google Scholar]
- [13].Southam CM, Moore AE, Clinical studies of viruses as antineoplastic agents, with particular reference to egypt 101 virus, Cancer. 5 (1952) 1025–34. . [DOI] [PubMed] [Google Scholar]
- [14].Taylor AW, Effects of glandular fever infection in acute leukaemia, Br. Med. J 1 (1953) 589–93. 10.1136/bmj.1.4810.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Georgiades J, Zielinski T, Cicholska A, Jordan E, Research on the oncolytic effect of APC viruses in cancer of the cervix uteri; preliminary report., Biul. Inst. Med. Morsk. Gdansk 10 (1959) 49–57. https://pubmed.ncbi.nlm.nih.gov/13827367/. [PubMed] [Google Scholar]
- [16].Zieliński T, Jordan E, Pozne wyniki obserwacji klinicznych nad dzialaniem onkolitycznym adenowirusow w raku szyjki macicy., Nowotwory. 19 (1969) 217–21. https://pubmed.ncbi.nlm.nih.gov/4313135/. [PubMed] [Google Scholar]
- [17].Asada T, Treatment of human cancer with mumps virus, Cancer. 34 (1974) 1907–28. . [DOI] [PubMed] [Google Scholar]
- [18].Kelly E, Russell SJ, History of oncolytic viruses: Genesis to genetic engineering, Mol. Ther 15 (2007) 651–9. 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- [19].Hemminki O, Dos Santos JM, Hemminki A, Oncolytic viruses for cancer immunotherapy, J. Hematol. Oncol 13 (2020) 84. 10.1186/s13045-020-00922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Linn S, Arber W, Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form., Proc. Natl. Acad. Sci. U. S. A 59 (1968) 1300–6. 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cozzarelli NR, Melechen NE, Jovin TM, Komberg A, Polynucleotide cellulose as a substrate for a polynucleotide ligase induced by phage T4, Biochem. Biophys. Res. Commun 28 (1967) 578–86. 10.1016/0006-291X(67)90353-1. [DOI] [PubMed] [Google Scholar]
- [22].Gefter ML, Becker A, Hurwitz J, The enzymatic repair of DNA. I. Formation of circular lambda-DNA., Proc. Natl. Acad. Sci. U. S. A 58 (1967) 240–7. 10.1073/pnas.58.l.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Olivera BM, Lehman IR, Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli., Proc. Natl. Acad. Sci. U. S. A 57 (1967) 1426–33. 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weiss B, Richardson CC, Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage., Proc. Natl. Acad. Sci. U. S. A 57 (1967) 1021–8. 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gellert M, Formation of covalent circles of lambda DNA by E. coli extracts., Proc. Natl. Acad. Sci. U. S. A 57 (1967) 148–55. 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cohen SN, Chang AC, Hsu L, Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA., Proc. Natl. Acad. Sci. U. S. A 69 (1972) 2110–4. 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cohen SN, Chang ACY, Boyer HW, Helling RB, Construction of biologically functional bacterial plasmids in vitro, Proc. Natl. Acad. Sci. U. S. A 70 (1973) 3240–4. 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu R, Nucleotide sequence analysis of DNA. I. Partial sequence of the cohesive ends of bacteriophage λ and 186 DNA, J. Mol. Biol 51 (1970) 501–21. 10.1016/0022-2836(70)90004-5. [DOI] [PubMed] [Google Scholar]
- [29].Sanger F, Nicklen S, Coulson AR, DNA sequencing with chain-terminating inhibitors., Proc. Natl. Acad. Sci. U. S. A 74 (1977) 5463–7. 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mullis KB, Faloona FA, Specific Synthesis of DNA in Vitro via a Polymerase-Catalyzed Chain Reaction, Methods Enzymol. 155 (1987) 335–50. 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- [31].Kirn DH, McCormick F, Replicating viruses as selective cancer therapeutics, Mol. Med. Today 2 (1996) 519–27. 10.1016/S1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- [32].Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. , An adenovirus mutant that replicates selectively in p53-deficient human tumor cells, Science (80-. ). 274 (1996) 373–6. 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- [33].Ganly I, Kim D, Eckhardt SG, Rodriguez GI, Soutar DS, Otto R, et al. , A Phase I Study of Onyx-015, an E1B Attenuated Adenovirus, Administered Intratumorally to Patients with Recurrent Head and Neck Cancer, Clin. Cancer Res. 6 (2000). [PubMed] [Google Scholar]
- [34].Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, et al. , Selective Replication and Oncolysis in p53 Mutant Tumors with ONYX-015, an E1B-55kD Gene-deleted Adenovirus, in Patients with Advanced Head and Neck Cancer: A Phase II Trial, Cancer Res. 60 (2000). [PubMed] [Google Scholar]
- [35].Lu W, Zheng S, Li XF, Huang JJ, Zheng X, Li Z, Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial, World J. Gastroenterol. 10 (2004) 3634–8. 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garber K, China Approves World’s First Oncolytic Virus Therapy For Cancer Treatment, JNCI J. Natl. Cancer Inst 98 (2006) 298–300. 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- [37].Saeed M, Shoaib A, Kandimalla R, Javed S, Almatroudi A, Gupta R, et al. , Microbe-based therapies for colorectal cancer: Advantages and limitations, Semin. Cancer Biol (2021). 10.1016/j.semcancer.2021.05.018. [DOI] [PubMed] [Google Scholar]
- [38].Diwan D, Cheng L, Usmani Z, Sharma M, Holden N, Willoughby N, et al. , Microbial cancer therapeutics: A promising approach, Semin. Cancer Biol (2021). 10.1016/j.semcancer.2021.05.003. [DOI] [PubMed] [Google Scholar]
- [39].Forbes NS, Coffin RS, Deng L, Evgin L, Fiering S, Giacalone M, et al. , White paper on microbial anti-cancer therapy and prevention, J. Immunother. Cancer 6 (2018) 1–24. 10.1186/s40425-018-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Roberts MS, Groene WS, Lorence RM, Bamat MK, Naturally Occurring Viruses for the Treatment of Cancer, Discov. Med 6 (2009) 217–22. [PubMed] [Google Scholar]
- [41].Andtbacka RHI, Collichio F, Harrington KJ, Middleton MR, Downey G, Ohrling K, et al. , Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma., J. Immunother. Cancer 7 (2019) 145. 10.1186/s40425-019-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Macedo N, Miller DM, Haq R, Kaufman HL, Clinical landscape of oncolytic virus research in 2020., J. Immunother. Cancer 8 (2020). 10.1136/jitc-2020-001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mondal M, Guo J, He P, Zhou D, Recent advances of oncolytic virus in cancer therapy, Hum. Vaccines Immunother 16 (2020) 2389–402. 10.1080/21645515.2020.1723363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cook M, Chauhan A, Clinical application of oncolytic viruses: A systematic review, Int. J. Mol. Sci 21 (2020) 1–36. 10.3390/ijms21207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mateu MG, Virus engineering: Functionalization and stabilization, Protein Eng. Des. Sel 24 (2011) 53–63. 10.1093/protein/gzq069. [DOI] [PubMed] [Google Scholar]
- [46].Ammayappan A, Peng K-W, Russell SJ, Characteristics of Oncolytic Vesicular Stomatitis Virus Displaying Tumor-Targeting Ligands, J. Virol 87 (2013) 13543–55. 10.1128/jvi.02240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Berry JTL, Munoz LE, Rodriguez Stewart RM, Selvaraj P, Mainou BA, Doxorubicin Conjugation to Reovirus Improves Oncolytic Efficacy in Triple-Negative Breast Cancer, Mol. Ther. - Oncolytics 18 (2020) 556–72. 10.1016/j.omto.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Choi JW, Lee YS, Yun CO, Kim SW, Polymeric oncolytic adenovirus for cancer gene therapy, J. Control. Release 219 (2015) 181–91. 10.1016/jjconrel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yokoda R, Nagalo B, Vernon B, Oklu R, Albadawi H, DeLeon T, et al. , Oncolytic virus delivery: from nano-pharmacodynamics to enhanced oncolytic effect, Oncolytic Virotherapy. Volume 6 (2017) 39–49. 10.2147/ov.s145262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hill C, Carlisle R, Achieving systemic delivery of oncolytic viruses, Expert Opin. Drug Deliv 16 (2019) 607–20. 10.1080/17425247.2019.1617269. [DOI] [PubMed] [Google Scholar]
- [51].Kaygisiz K, Synatschke CV, Materials promoting viral gene delivery, Biomater. Sci 8 (2020) 6113–56. 10.1039/d0bm01367f. [DOI] [PubMed] [Google Scholar]
- [52].Sunasee R, Narain R, Covalent and Noncovalent Bioconjugation Strategies, in: Chem. Bioconjugates, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2014: pp. 1–75. 10.1002/9781118775882.ch1. [DOI] [Google Scholar]
- [53].Verheije MH, Rottier PJM, Retargeting of viruses to generate oncolytic agents, Adv. Virol 2012 (2012) 15. 10.1155/2012/798526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cohen JI, Herpesvirus latency J Clin. Invest 130 (2020) 3361–9. 10.1172/JCI136225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Moquin SA, Thomas S, Whalen S, Warburton A, Fernanadez SG, McBride AA, et al. , The Epstein-Barr virus episome maneuvers between nuclear chromatin compartments during reactivation, BioRxiv. (2017) 177345. 10.1101/177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Singh S, Kumar R, Agrawal B, Adenoviral Vector-Based Vaccines and Gene Therapies: Current Status and Future Prospects, in: Adenoviruses, IntechOpen, 2019. 10.5772/intechopen.79697. [DOI] [Google Scholar]
- [57].Goswami R, Subramanian G, Silayeva L, Newkirk I, Doctor D, Chawla K, et al. , Gene therapy leaves a vicious cycle, Front. Oncol 9 (2019) 297. 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang YA, Nemunaitis J, Samuel SK, Chen P, Shen Y, Tong AW, Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA, Cancer Res. 66 (2006) 9736–43. 10.1158/0008-5472.CAN-06-1617. [DOI] [PubMed] [Google Scholar]
- [59].Anesti AM, Simpson GR, Price T, Pandha HS, Coffin RS, Expression of RNA interference triggers from an oncolytic herpes simplex virus results in specific silencing in tumour cells in vitro and tumours in vivo, BMC Cancer. 10 (2010) 486. 10.1186/1471-2407-10-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Boersma S, Rabouw HH, Bruurs LJM, Pavlovič T, van Vliet ALW, Beumer J, et al. , Translation and Replication Dynamics of Single RNA Viruses, Cell. 183 (2020) 1930–1945.e23. 10.1016/j.cell.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chaitanya KV, Structure and Organization of Virus Genomes, in: Genome and Genomics, Springer; Singapore, 2019: pp. 1–30. 10.1007/978-981-15-0702-1_1. [DOI] [Google Scholar]
- [62].Buijs PRA, Verhagen JHE, van Eijck CHJ, van den Hoogen BG, Oncolytic viruses: From bench to bedside with a focus on safety, Hum. Vaccines Immunother 11 (2015) 1573–84. 10.1080/21645515.2015.1037058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li L, Liu S, Han D, Tang B, Ma J, Delivery and Biosafety of Oncolytic Virotherapy, Front. Oncol 10 (2020). 10.3389/fonc.2020.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Willemsen A, Zwart MP, On the stability of sequences inserted into viral genomes, Virus Evol. 5 (2019). 10.1093/ve/vez045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lemos de Matos A, Franco LS, McFadden G, Oncolytic Viruses and the Immune System: The Dynamic Duo., Mol. Ther. Methods Clin. Dev 17 (2020) 349–58. 10.1016/j.omtm.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Harrington K, Freeman DJ, Kelly B, Harper J, Soria J-C, Optimizing oncolytic virotherapy in cancer treatment, Nat. Rev. Drug Discov 18 (2019) 689–706. 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- [67].Niemann J, Woller N, Brooks J, Fleischmann-Mundt B, Martin NT, Kloos A, et al. , Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy, Nat. Commun 10 (2019) 3236. 10.1038/s41467-019-11137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hadac EM, Kelly EJ, Russell SJ, Myeloma xenograft destruction by a nonviral vector delivering oncolytic infectious nucleic acid., Mol. Ther 19 (2011) 1041–7. 10.1038/mt.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ferguson MS, Lemoine NR, Wang Y, Systemic delivery of oncolytic viruses: hopes and hurdles., Adv. Virol 2012 (2012) 805629. 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hill BD, Zak A, Khera E, Wen F, Engineering Virus-like Particles for Antigen and Drug Delivery, Curr. Protein Pept. Sci 19 (2017) 112–27. 10.2174/1389203718666161122113041. [DOI] [PubMed] [Google Scholar]
- [71].Parkin DM, The global health burden of infection-associated cancers in the year 2002., Int. J. Cancer 118 (2006) 3030–44. 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- [72].Wang Z, Zhi K, Ding Z, Sun Y, Li S, Li M, et al. , Emergence in protein derived nanomedicine as anticancer therapeutics: More than a tour de force, Semin. Cancer Biol 69 (2021) 77–90. 10.1016/j.semcancer.2019.ll.012. [DOI] [PubMed] [Google Scholar]
- [73].Mohsen MO, Speiser DE, Knuth A, Bachmann MF, Virus-like particles for vaccination against cancer, WIREs Nanomedicine and Nanobiotechnology. 12 (2020) e1579. 10.1002/wnan.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, et al. , Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers, J. Nanobiotechnology 19 (2021) 59. 10.1186/sl2951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen W, Hartzell EJ, Lieser RM, Sullivan MO, Modular hepatitis b virus-like particle platform for biosensing and drug delivery, ACS Nano. 14 (2020) 12642–51. 10.1021/acsnano.9b08756. [DOI] [PubMed] [Google Scholar]
- [76].Cashion L, Ast O, Citkowicz A, Harvey S, Mitrovic B, Masikat MR, et al. , 172. In Vitro DNA Packaging and Gene Delivery Using JC Virus-Like Particles, Mol. Ther 11 (2005) S68. 10.1016/j.ymthe.2005.06.175. [DOI] [Google Scholar]
- [77].Fang PY, Bowman JC, Gomez Ramos LM, Hsiao C, Williams LD, RNA: Packaged and protected by VLPs, RSC Adv. 8 (2018) 21399–406. 10.1039/c8ra02084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ren D, Dalmau M, Randall A, Shindel MM, Baldi P, Wang S-W, Biomimetic Design of Protein Nanomaterials for Hydrophobic Molecular Transport, Adv. Funct. Mater 22 (2012) 3170–80. 10.1002/adfm.201200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lizatović R, Assent M, Barendregt A, Dahlin J, Bille A, Satzinger K, et al. , A Protein-Based Encapsulation System with Calcium-Controlled Cargo Loading and Detachment, Angew. Chemie 130 (2018) 11504–8. 10.1002/ange.201806466. [DOI] [PubMed] [Google Scholar]
- [80].Chung YH, Cai H, Steinmetz NF, Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications, Adv. Drug Deliv. Rev 156 (2020) 214–35. 10.1016/j.addr.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yoo JW, Irvine DJ, Discher DE, Mitragotri S, Bio-inspired, bioengineered and biomimetic drug delivery carriers, Nat. Rev. Drug Discov 10 (2011) 521–35. 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- [82].Diaz D, Care A, Sunna A, Bioengineering strategies for protein-based nanoparticles, Genes (Basel). 9 (2018). 10.3390/genes9070370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shen L, Zhou J, Wang Y, Kang N, Ke X, Bi S, et al. , Efficient Encapsulation of Fe3O4 Nanoparticles into Genetically Engineered Hepatitis B Core Virus-Like Particles Through a Specific Interaction for Potential Bioapplications, Small. 11 (2015) 1190–6. 10.1002/smll.201401952. [DOI] [PubMed] [Google Scholar]
- [84].Minten IJ, Hendriks LJA, Nolte RJM, Cornelissen JJLM, Controlled encapsulation of multiple proteins in virus capsids, J. Am. Chem. Soc 131 (2009) 17771–3. 10.1021/ja907843s. [DOI] [PubMed] [Google Scholar]
- [85].Working PK, Lin A, Borellini F, Meeting product development challenges in manufacturing clinical grade oncolytic adenoviruses, Oncogene. 24 (2005) 7792–801. 10.1038/sj.onc.1209045. [DOI] [PubMed] [Google Scholar]
- [86].Yee CM, Zak AJ, Hill BD, Wen F, The Coming Age of Insect Cells for Manufacturing and Development of Protein Therapeutics, Ind. Eng. Chem. Res 57 (2018) 10061–70. 10.1021/acs.iecr.8b00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zak AJ, Hill BD, Rizvi SM, Smith MR, Yang M, Wen F, Enhancing the Yield and Quality of Influenza Virus-like Particles (VLPs) Produced in Insect Cells by Inhibiting Cytopathic Effects of Matrix Protein M2, ACS Synth. Biol 8 (2019) 2303–14. 10.1021/acssynbio.9b00111. [DOI] [PubMed] [Google Scholar]
- [88].Ong HK, Tan WS, Ho KL, Virus like particles as a platform for cancer vaccine development, PeerJ. 2017 (2017) e4053. 10.7717/peeij.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dai S, Wang H, Deng F, Minireview Open Access Advances and challenges in enveloped virus-like particle (VLP)-based vaccines, 2018. [Google Scholar]
- [90].Spice AJ, Aw R, Bracewell DG, Polizzi KM, Synthesis and Assembly of Hepatitis B Virus-Like Particles in a Pichia pastoris Cell-Free System, Front. Bioeng. Biotechnol 8 (2020) 72. 10.3389/fbioe.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Somiya M, Liu Q, Kuroda S, Current progress of virus-mimicking nanocarriers for drug delivery, Nanotheranostics. 1 (2017)415–29. 10.7150/ntno.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Maslanka Figueroa S, Fleischmann D, Goepferich A, Biomedical nanoparticle design: What we can learn from viruses, J. Control. Release (2020). 10.1016/j.jconrel.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Maslanka Figueroa S, Fleischmann D, Beck S, Tauber P, Witzgall R, Schweda F, et al. , Nanoparticles Mimicking Viral Cell Recognition Strategies Are Superior Transporters into Mesangial Cells, Adv. Sci 7 (2020) 1903204. 10.1002/advs.201903204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lou B, De Beuckelaer A, Boonstra E, Li D, De Geest BG, De Koker S, et al. , Modular core-shell polymeric nanoparticles mimicking viral structures for vaccination, J. Control. Release 293 (2019) 48–62. 10.1016/jjconrel.2018.11.006. [DOI] [PubMed] [Google Scholar]
- [95].Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D, et al. , Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants, Sci. Rep 1 (2011) 1–9. 10.1038/srep00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang W, Wang P, Tang X, Elzatahry AA, Wang S, Al-Dahyan D, et al. , Facile Synthesis of Uniform Virus-like Mesoporous Silica Nanoparticles for Enhanced Cellular Internalization, ACS Cent. Sci 3 (2017) 839–46. 10.1021/acscentsci.7b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lee C, Hwang HS, Lee S, Kim B, Kim JO, Oh KT, et al. , Rabies Virus-Inspired Silica-Coated Gold Nanorods as a Photothermal Therapeutic Platform for Treating Brain Tumors, Adv. Mater 29 (2017). 10.1002/adma.201605563. [DOI] [PubMed] [Google Scholar]
- [98].Fang J-H, Lee Y-T, Chiang W-H, Hu S-H, Magnetoresponsive Virus-Mimetic Nanocapsules with Dual Heat-Triggered Sequential-Infected Multiple Drug-Delivery Approach for Combinatorial Tumor Therapy, Small. 11 (2015) 2417–28. 10.1002/sm11.201402969. [DOI] [PubMed] [Google Scholar]
- [99].Thiruppathi R, Mishra S, Ganapathy M, Padmanabhan P, Gulyas B, Nanoparticle Functionalization and Its Potentials for Molecular Imaging, Adv. Sci 4 (2017) 1600279. 10.1002/advs.201600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sanità G, Carrese B, Lamberti A, Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization, Front. Mol. Biosci 7 (2020) 381. 10.3389/fmolb.2020.587012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Valdeperez D, Wang T, EuBner JP, Weinert B, Hao J, Parak WJ, et al. , Polymer-coated nanoparticles: Carrier platforms for hydrophobic water- and air-sensitive metallo-organic compounds, Pharmacol. Res 117 (2017) 261–6. 10.1016/j.phrs.2016.12.034. [DOI] [PubMed] [Google Scholar]
- [102].Korpany KV, Mottillo C, Bachelder J, Cross SN, Dong P, Trudel S, et al. , One-step ligand exchange and switching from hydrophobic to water-stable hydrophilic superparamagnetic iron oxide nanoparticles by mechanochemical milling, Chem. Commun 52 (2016) 3054–7. 10.1039/c5cc07107k. [DOI] [PubMed] [Google Scholar]
- [103].Sperling RA, Parak WJ, Surface modification, functionalization and bioconjugation of colloidal Inorganic nanoparticles, Philos. Trans. R. Soc. A Math. Phys. Eng. Sci 368 (2010) 1333–83. 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- [104].Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R, Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites - A review, Prog. Polym. Sci 38 (2013) 1232–61. 10.1016/j.progpolymsci.2013.02.003. [DOI] [Google Scholar]
- [105].Neouze M-A, Schubert U, Review Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands, Monatsh Chem. 139 (2008) 183–95. 10.1007/s00706-007-0775-2. [DOI] [Google Scholar]
- [106].Ni R, Zhou J, Hossain N, Chau Y, Virus-inspired nucleic acid delivery system: Linking virus and viral mimicry, Adv. Drug Deliv. Rev 106 (2016) 3–26. 10.1016/j.addr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- [107].Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. , Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer, ACS Nano. 10 (2016) 7738–48. 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- [108].Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K, Liposomes and polymersomes: a comparative review towards cell mimicking, Chem. Soc. Rev 47 (2018) 8572–610. 10.1039/c8cs00162f. [DOI] [PubMed] [Google Scholar]
- [109].Becker Peres L, Becker Peres L, de Araujo PHH, Sayer C, Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique, Colloids Surfaces B Biointerfaces. 140 (2016) 317–23. 10.1016/j.colsurfb.2015.12.033. [DOI] [PubMed] [Google Scholar]
- [110].Wang N, Cheng X, Li N, Wang H, Chen H, Nanocarriers and Their Loading Strategies, Adv. Healthc. Mater 8 (2019) 1801002. 10.1002/adhm.201801002. [DOI] [PubMed] [Google Scholar]
- [111].Deng S, Gigliobianco MR, Censi R, Di Martino P, Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities, Nanomaterials. 10 (2020) 847. 10.3390/nanol0050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhang WW, Li L, Li D, Liu J, Li X, Li W, et al. , The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic, Hum. Gene Ther 29 (2018) 160–79. 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- [113].Glinkina LS, Bruvere Zh R, Venskus DR, Garklava RR, Muceniece AJ, Indexes of cell-mediated immunity in patients with skin malignant melanoma following the use of immunomodulator rigvir, Vopr. Onkol 38 (1992) 540–7. https://europepmc.org/article/med/1300752. [PubMed] [Google Scholar]
- [114].Alberts P, Tilgase A, Rasa A, Bandere K, Venskus D, The advent of oncolytic Virotherapy in oncology: The Rigvir® story, Eur. J. Pharmacol 837 (2018) 117–26. 10.1016/j.ejphar.2018.08.042. [DOI] [PubMed] [Google Scholar]
- [115].Andtbacka RHI, Collichio F, Harrington KJ, Middleton MR, Downey G, Öhrling K, et al. , Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma., J. Immunother. Cancer 7 (2019) 145. 10.1186/s40425-019-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kazemi Shariat Panahi H, Dehhaghi M, Lam SS, Peng W, Aghbashlo M, Tabatabaei M, et al. , Oncolytic viruses as a promising therapeutic strategy against the detrimental health impacts of air pollution: The case of glioblastoma multiforme, Semin. Cancer Biol (2021). 10.1016/j.semcancer.2021.05.013. [DOI] [PubMed] [Google Scholar]
- [117].Chakrabarty R, Tran H, Selvaggi G, Hagerman A, Thompson B, Coffey M, The oncolytic virus, pelareorep, as a novel anticancer agent: a review., Invest. New Drugs 33 (2015) 761–74. 10.1007/sl0637-015-0216-8. [DOI] [PubMed] [Google Scholar]
- [118].Bonjardim CA, Viral exploitation of the MEK/ERK pathway - A tale of vaccinia virus and other viruses, 2017. https://www.sciencedirect.com/science/article/pii/S004268221630383X. [DOI] [PubMed] [Google Scholar]
- [119].Cattaneo R, Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet., J. Virol 78 (2004) 4385–8. 10.1128/jvi.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Elvington M, Liszewski MK, Atkinson JP, CD46 and Oncologic Interactions: Friendly Fire against Cancer., Antibodies (Basel, Switzerland). 9 (2020). 10.3390/antib9040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lee EB, Kim JH, Hur W, Choi JE, Kim SM, Park DJ, et al. , Liver-specific Gene Delivery Using Engineered Virus-Like Particles of Hepatitis E Virus, Sci. Rep 9 (2019) 1–10. 10.1038/s41598-019-38533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Fang CY, Tsai YD, Lin MC, Wang M, Chen PL, Chao CN, et al. , Inhibition of human bladder cancer growth by a suicide gene delivered by JC polyomavirus virus-like particles in a mouse model, J Urol. 193 (2015) 2100–6. 10.1016/j.juro.2015.01.084. [DOI] [PubMed] [Google Scholar]
- [123].Kines RC, Cerio RJ, Roberts JN, Thompson CD, de Los Pinos E, Lowy DR, et al. , Human papillomavirus capsids preferentially bind and infect tumor cells., Int. J. Cancer (2015). 10.1002/ijc.29823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sato Y, Matsui Η, Yamamoto Ν, Sato R, Munakata T, Kohara M, et al. , Highly specific delivery of siRNA to hepatocytes circumvents endothelial cell-mediated lipid nanoparticle-associated toxicity leading to the safe and efficacious decrease in the hepatitis B virus, J. Control. Release 266 (2017) 216–25. 10.1016/j.jconrel.2017.09.044. [DOI] [PubMed] [Google Scholar]
- [125].Mishra N, Yadav NP, Rai VK, Sinha P, Yadav KS, Jain S, et al. , Efficient hepatic delivery of drugs: Novel strategies and their significance, Biomed Res. Int. 2013 (2013). 10.1155/2013/382184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhang Q, Zhang X, Chen T, Wang X, Fu Y, Jin Y, et al. , A safe and efficient hepatocyte-selective carrier system based on myristoylated preS 1/21–47 domain of hepatitis B virus, Nanoscale. 7 (2015) 9298–310. 10.1039/c4nr04730c. [DOI] [PubMed] [Google Scholar]
- [127].Witzigmann D, Uhl P, Sieber S, Kaufman C, Einfalt T, Schoneweis K, et al. , Optimization-by-design of hepatotropic lipid nanoparticles targeting the sodium-taurocholate cotransporting polypeptide, Elife. 8 (2019). 10.7554/eLife.42276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Hamidi H, Ivaska J, Every step of the way: integrins in cancer progression and metastasis, Nat. Rev. Cancer 18 (2018) 1–16. 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sun H, Dong Y, Feijen J, Zhong Z, Peptide-decorated polymeric nanomedicines for precision cancer therapy, J. Control. Release 290 (2018) 11–27. 10.1016/j.jconrel.2018.09.029. [DOI] [PubMed] [Google Scholar]
- [130].Zou Y, Zheng M, Yang W, Meng F, Miyata K, Kim HJ, et al. , Virus-Mimicking Chimaeric Polymersomes Boost Targeted Cancer siRNA Therapy In Vivo, Adv. Mater 29 (2017) 1703285. 10.1002/adma.201703285. [DOI] [PubMed] [Google Scholar]
- [131].Destito G, Yeh R, Rae CS, Finn MG, Manchester M, Folic acid-mediated targeting of cowpea mosaic virus particles to tumor cells., Chem. Biol 14 (2007) 1152–62. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2293326&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kitai Y, Fukuda H, Enomoto T, Asakawa Y, Suzuki T, Inouye S, et al. , Cell selective targeting of a simian virus 40 virus-like particle conjugated to epidermal growth factor., J. Biotechnol 155 (2011)251–6. 10.1016/j.jbiotec.2011.06.030. [DOI] [PubMed] [Google Scholar]
- [133].Pokorski JK, Hovlid ML, Finn MG, Cell Targeting with Hybrid Qβ Virus-Like Particles Displaying Epidermal Growth Factor, ChemBioChem. 12 (2011) 2441–7. 10.1002/cbic.201100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Galaway FA, Stockley PG, MS2 viruslike particles: a robust, semisynthetic targeted drug delivery platform, Mol. Pharm 10 (2013) 59–68. 10.1021/mp3003368. [DOI] [PubMed] [Google Scholar]
- [135].Mable CJ, Canton I, Mykhaylyk OO, Ustbas Gul B, Chambon P, Themistou E, et al. , Targeting triple-negative breast cancer cells using Dengue virus-mimicking pH-responsive framboidal triblock copolymer vesicles, Chem. Sci 10 (2019) 4811–21. 10.1039/c8sc05589k. [DOI] [Google Scholar]
- [136].Kato T, Yui M, Deo VK, Park EY, Development of Rous sarcoma Virus-like Particles Displaying hCC49 scFv for Specific Targeted Drug Delivery to Human Colon Carcinoma Cells., Pharm. Res 32 (2015) 3699–707. 10.1007/s11095-015-1730-2. [DOI] [PubMed] [Google Scholar]
- [137].Zarogoulidis P, Darwiche K, Sakkas A, Yarmus L, Huang H, Li Q, et al. , Suicide Gene Therapy for Cancer - Current Strategies., J. Genet. Syndr. Gene Ther 4 (2013). 10.4172/2157-7412.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Balfour HH, Antiviral Drugs, N. Engl. J. Med 340 (1999) 1255–68. 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- [139].Karjoo Z, Chen X, Hatefi A, Progress and problems with the use of suicide genes for targeted cancer therapy., Adv. Drug Deliv. Rev 99 (2016) 113–28. 10.1016/j.addr.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chao C-N, Huang Y-L, Lin M-C, Fang C-Y, Shen C-H, Chen P-L, et al. , Inhibition of human diffuse large B-cell lymphoma growth by JC polyomavirus-like particles delivering a suicide gene., J. Transl. Med 13 (2015) 29. 10.1186/s12967-015-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Aljabali AAA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ, CPMV-DOX Delivers, Mol. Pharm 10 (2013) 3–10. 10.1021/mp3002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhao Q, Chen W, Chen Y, Zhang L, Zhang J, Zhang Z, Self-assembled virus-like particles from rotavirus structural protein VP6 for targeted drug delivery., Bioconjug. Chem 22 (2011) 346–52. 10.1021/bc1002532. [DOI] [PubMed] [Google Scholar]
- [143].Deo VK, Kato T, Park EY, Chimeric virus-like particles made using GAG and M1 capsid proteins providing dual drug delivery and vaccination platform., Mol. Pharm 12 (2015) 839–45. 10.1021/mp500860x. [DOI] [PubMed] [Google Scholar]
- [144].De Filette M, Min Jou W, Birkett A, Lyons K, Schultz B, Tonkyro A, et al. , Universal influenza A vaccine: optimization of M2-based constructs., Virology. 337 (2005) 149–61. 10.1016/j.virol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- [145].Jang HJ, Jeong EJ, Lee KY, Carbon Dioxide-Generating PLG Nanoparticles for Controlled Anti-Cancer Drug Delivery, Pharm. Res 35 (2018) 59. 10.1007/s11095-018-2359-8. [DOI] [PubMed] [Google Scholar]
- [146].Hilmer SN, Cogger VC, Muller M, Le Couteur DG, The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin, Drug Metab. Dispos 32 (2004) 794–9. 10.1124/dmd.32.8.794. [DOI] [PubMed] [Google Scholar]
- [147].Tacar O, Dass CR, Doxorubicin-induced death in tumour cells and cardiomyocytes: is autophagy the key to improving future clinical outcomes?, J. Pharm. Pharmacol 65 (2013) 1577–89. 10.1111/jphp.12144. [DOI] [PubMed] [Google Scholar]
- [148].Ohtake N, Niikura K, Suzuki T, Nagakawa K, Mikuni S, Matsuo Y, et al. , Low pH-triggered model drug molecule release from virus-like particles., Chembiochem. 11 (2010) 959–62. 10.1002/cbic.201000094. [DOI] [PubMed] [Google Scholar]
- [149].Zochowska M, Paca A, Schoehn G, Andrieu J-P, Chroboczek J, Dublet B, et al. , Adenovirus Dodecahedron, as a Drug Delivery Vector, PLoS One. 4 (2009) e5569. 10.1371/journal.pone.0005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino C. a., et al. , Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles, ACS Nano. 5 (2011) 5729–45. 10.1021/nn201397z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wannasarit S, Wang S, Figueiredo P, Trujillo C, Ebumea F, Simón- Gracia L, et al. , A Virus- Mimicking pH- Responsive Acetalated Dextran-Based Membrane- Active Polymeric Nanoparticle for Intracellular Delivery of Antitumor Therapeutics, Adv. Funct. Mater 29 (2019) 1905352. 10.1002/adfm.201905352. [DOI] [Google Scholar]
- [152].Lv J, Sharma A, Zhang T, Wu Y, Ding X, Pharmacological Review on Asiatic Acid and Its Derivatives: A Potential Compound, SLAS Technol. 23 (2018) 111–27. 10.1177/2472630317751840. [DOI] [PubMed] [Google Scholar]
- [153].Jiang Y, Yang W, Zhang J, Meng F, Zhong Z, Protein Toxin Chaperoned by LRP-1-Targeted Virus-Mimicking Vesicles Induces High-Efficiency Glioblastoma Therapy In Vivo, Adv. Mater 30 (2018). 10.1002/adma.201800316. [DOI] [PubMed] [Google Scholar]
- [154].Polito L, Bortolotti M, Mercatelli D, Battelli M, Bolognesi A, Saporin-S6: A Useful Tool in Cancer Therapy, Toxins (Basel). 5 (2013) 1698–722. 10.3390/toxins5101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Pan L, He Q, Liu J, Chen Y, Ma M, Zhang L, et al. , Nuclear-targeted drug delivery of tat peptide-conjugated monodisperse mesoporous silica nanoparticles, J. Am. Chem. Soc 134 (2012) 5722–5. 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- [156].Wang F, Liu P, Sun L, Li C, Petrenko VA, Liu A, Bio-mimetic nanostructure self-assembled from Au@Ag heterogeneous nanorods and phage fusion proteins for targeted tumor optical detection and photothermal therapy, Sci. Rep 4 (2014) 1–9. 10.1038/srep06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Adhikari S, Alahmadi TI, Gong Z, Karlsson AJ, Expression of Cell-Penetrating Peptides Fused to Protein Cargo, J. Mol. Microbiol. Biotechnol 28 (2018) 159–68. 10.1159/000494084. [DOI] [PubMed] [Google Scholar]
- [158].Niikura K, Sugimura N, Musashi Y, Mikuni S, Matsuo Y, Kobayashi S, et al. , Virus-like particles with removable cyclodextrins enable glutathione-triggered drug release in cells., Mol. Biosyst 9 (2013) 501–7. 10.1039/c2mb25420d. [DOI] [PubMed] [Google Scholar]
- [159].Koho T, Ihalainen TO, Stark M, Uusi-Kerttula H, Wieneke R, Rahikainen R, et al. , His-tagged norovirus-like particles: A versatile platform for cellular delivery and surface display., Eur. J. Pharm. Biopharm (2015). 10.1016/j.ejpb.2015.07.002. [DOI] [PubMed] [Google Scholar]
- [160].Shen Y, Tang H, Radosz M, Van Kirk E, Murdoch WJ, PH-responsive nanoparticles for cancer drug delivery, Methods Mol. Biol 437 (2008) 183–216. 10.1007/978-1-59745-210-6_10. [DOI] [PubMed] [Google Scholar]
- [161].Kemp V, van den Wollenberg DJM, Camps MGM, van Hall T, Kinderman P, Pronk-van Montfoort N, et al. , Arming oncolytic reovirus with GM-CSF gene to enhance immunity, Cancer Gene Ther. 26 (2019) 268–81. 10.1038/s41417-018-0063-9. [DOI] [PubMed] [Google Scholar]
- [162].Grossardt C, Engeland CE, Bossow S, Halama N, Zaoui K, Leber MF, et al. , Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine., Hum. Gene Ther 24 (2013) 644–54. 10.1089/hum.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ino Y, Saeki Y, Fukuhara H, Todo T, Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7–1 results in enhanced antitumor efficacy., Clin. Cancer Res 12 (2006) 643–52. 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- [164].Eriksson E, Milenova I, Wenthe J, Ståhle M, Leja-Jarblad J, Ullenhag G, et al. , Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4–1BB Signaling Induced by an Armed Oncolytic Virus., Clin. Cancer Res 23 (2017) 5846–57. 10.1158/1078-0432.CCR-17-0285. [DOI] [PubMed] [Google Scholar]
- [165].Choi IK, Li Y, Oh E, Kim J, Yun CO, Oncolytic Adenovirus Expressing IL-23 and p35 Elicits IFN-γ- and TNF-α-Co-Producing T Cell-Mediated Antitumor Immunity, PLoS One. 8 (2013). 10.1371/journal.pone.0067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Oh E, Choi I-K, Hong J, Yun C-O, Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model., Oncotarget 8 (2017) 4730–46. 10.18632/oncotarget.13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, et al. , Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4., Gene Ther. 19 (2012) 988–98. 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- [168].Du T, Shi G, Li YM, Zhang JF, Tian HW, Wei YQ, et al. , Tumor-specific oncolytic adenoviruses expressing granulocyte macrophage colony-stimulating factor or anti-CTLA4 antibody for the treatment of cancers, Cancer Gene Ther. 21 (2014) 340–8. 10.1038/cgt.2014.34. [DOI] [PubMed] [Google Scholar]
- [169].Ambiihl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, et al. , A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity., J. Hypertens 25 (2007) 63–72. 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- [170].Chackerian B, Lenz P, Lowy DR, Schiller JT, Determinants of Autoantibody Induction by Conjugated Papillomavirus Virus-Like Particles, J. Immunol 169 (2002) 6120–6. 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- [171].Röhn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, et al. , Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis., Eur. J. Immunol 36 (2006) 2857–67. 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- [172].Spohn G, Keller I, Beck M, Grest P, Jennings GT, Bachmann MF, Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis., Eur. J. Immunol 38 (2008) 877–87. 10.1002/eji.200737989. [DOI] [PubMed] [Google Scholar]
- [173].Mohsen MO, Speiser DE, Knuth A, Bachmann MF, Virus-like particles for vaccination against cancer, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 12 (2020). 10.1002/wnan.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W, et al. , Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients., J. Immunother 33 (2010) 848–58. 10.1097/CJI.0b013e3181f1d614. [DOI] [PubMed] [Google Scholar]
- [175].Jegerlehner A, Schmitz N, Storni T, Bachmann MF, Influenza A Vaccine Based on the Extracellular Domain of M2: Weak Protection Mediated via Antibody-Dependent NK Cell Activity, J. Immunol. 172 (2004) 5598–605. 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- [176].Chua BY, Johnson D, Tan A, Earnest-Silveira L, Sekiya T, Chin R, et al. , Hepatitis C VLPs Delivered to Dendritic Cells by a TLR2 Targeting Lipopeptide Results in Enhanced Antibody and Cell-Mediated Responses, PLoS One. 7 (2012) e47492. 10.1371/journal.pone.0047492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wang B-Z, Quan F-S, Kang S-M, Bozja J, Skountzou I, Compans RW, Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses., J. Virol 82 (2008) 11813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Qi Y, Kang H, Zheng X, Wang H, Gao Y, Yang S, et al. , Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs., Front. Microbiol 6 (2015) 169. 10.3389/fmicb.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W, et al. , Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients., J. Immunother. 33 (2010) 848–58. 10.1097/CJI.0b013e3181f1d614. [DOI] [PubMed] [Google Scholar]
- [180].Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ, Getting personal with neoantigen-based therapeutic cancer vaccines., Cancer Immunol. Res 1 (2013) 11–5. 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, F Nakaya H, et al. , Programming the magnitude and persistence of antibody responses with innate immunity, Nature. 470 (2011) 543–50. 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Lu Z, Zhang Y, Wang Y, Tan GH, Huang FY, Cao R, et al. , A biotin-avidin-system-based virus-mimicking nanovaccine for tumor immunotherapy, J. Control. Release 332 (2021) 245–59. 10.1016/j.jconrel.2021.02.029. [DOI] [PubMed] [Google Scholar]
- [183].Molino NM, Anderson AKL, Nelson EL, Wang SW, Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation, ACS Nano. 7 (2013) 9743–52. 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Molino NM, Neek M, Tucker JA, Nelson EL, Wang SW, Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses, Biomaterials. 86 (2016) 83–91. 10.1016/j.biomaterials.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. , Mutational landscape and significance across 12 major cancer types, Nature. 502 (2013) 333–9. 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Duffy MJ, Synnott NC, O’Grady S, Crown J, Targeting p53 for the treatment of cancer, Semin. Cancer Biol (2020). 10.1016/j.semcancer.2020.07.005. [DOI] [PubMed] [Google Scholar]
- [187].Toufektchan E, Toledo F, The Guardian of the Genome Revisited: p53 Downregulates Genes Required for Telomere Maintenance, DNA Repair, and Centromere Structure., Cancers (Basel). 10 (2018). 10.3390/cancers10050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zhang WW, Li L, Li D, Liu J, Li X, Li W, et al. , The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic, Hum. Gene Ther 29 (2018) 160–79. 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- [189].Chen G-X, Zhang S, He X-H, Liu S-Y, Ma C, Zou X-P, Clinical utility of recombinant adenoviral human p53 gene therapy: current perspectives., Onco. Targets. Ther 7 (2014) 1901–9. 10.2147/OTT.S50483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. , Mutational landscape and significance across 12 major cancer types, Nature. 502 (2013) 333–9. 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Li D, Zhang Y, Xie Y, Xiang J, Zhu Y, Yang J, Enhanced tumor suppression by adenoviral PTEN gene therapy combined with cisplatin chemotherapy in small-cell lung cancer, Cancer Gene Ther. 20 (2013) 251–9. 10.1038/cgt.2013.14. [DOI] [PubMed] [Google Scholar]
- [192].Tait DL, Obermiller PS, Holt JT, Preclinical studies of a new generation retroviral vector for ovarian cancer BRCA1 gene therapy., Gynecol. Oncol 79 (2000) 471–6. 10.1006/gyno.2000.5969. [DOI] [PubMed] [Google Scholar]
- [193].Naruishi K, Timme TL, Kusaka N, Fujita T, Yang G, Goltsov A, et al. , Adenoviral vector-mediated RTVP-1 gene-modified tumor cell-based vaccine suppresses the development of experimental prostate cancer, Cancer Gene Ther. 13 (2006) 658–63. 10.1038/sj.cgt.7700919. [DOI] [PubMed] [Google Scholar]
- [194].Peng Y, Croce CM, The role of MicroRNAs in human cancer, Signal Transduct. Target. Ther 1 (2016) 15004. 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Li X, Su Y, Sun B, Ji W, Peng Z, Xu Y, et al. , An Artificially Designed Interfering IncRNA Expressed by Oncolytic Adenovirus Competitively Consumes OncomiRs to Exert Antitumor Efficacy in Hepatocellular Carcinoma, Mol. Cancer Ther 15 (2016) 1436–51. 10.1158/1535-7163.MCT-16-0096. [DOI] [PubMed] [Google Scholar]
- [196].Liu Z, Sun Q, Wang X, PLK1, A potential target for cancer therapy, Transl. Oncol 10 (2017) 22–32. 10.1016/j.tranon.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, et al. , Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study., Lancet. Oncol 18 (2017) 1386–96. 10.1016/S1470-2045(17)30621-6. [DOI] [PubMed] [Google Scholar]
- [198].Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y-K, Stoudemire J, et al. , Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors, Invest. New Drugs 35 (2017) 180–8. 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB, et al. , Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival, JNCI J. Natl. Cancer Inst 109 (2017). 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].A.C. Society, American Cancer Society. Cancer Facts & Figures 2020, Am. Cancer Soc (2020) 1–52. http://www.cancer.org/acs/groups/content/@nho/documents/document/caff2007pwsecuredpdf.pdf. [Google Scholar]
- [201].Zhao Z, Ukidve A, Kim J, Mitragotri S, Paulson JA, Leading Edge Review Targeting Strategies for Tissue-Specific Drug Delivery, Cell. 181 (2020) 151–67. 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- [202].Barua S, Mitragotri S, Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects, Nano Today. 9 (2014) 223–43. 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Azzi S, Hebda JK, Gavard J, Vascular permeability and drug delivery in cancers, Front. Oncol 3 AUG (2013)211. 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Böckelmann LC, Schumacher U, Targeting tumor interstitial fluid pressure: will it yield novel successful therapies for solid tumors?, Expert Opin. Ther. Targets 23 (2019) 1005–14. 10.1080/14728222.2019.1702974. [DOI] [PubMed] [Google Scholar]
- [205].Minchinton AI, Tannock IF, Drug penetration in solid tumours, Nat. Rev. Cancer 6 (2006) 583–92. 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- [206].Piotrowski-Daspit AS, Tien J, Nelson CM, Interstitial fluid pressure regulates collective invasion in engineered human breast tumors via Snail, vimentin, and E-cadherin, Integr. Biol 8 (2016) 319–31. 10.1039/c5ib00282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Di J, Gao X, Du Y, Zhang H, Gao J, Zheng A, Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo, Asian J. Pharm. Sci (2020). 10.1016/j.ajps.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Cağdas M, Sezer AD, Bucak S, Liposomes as Potential Drug Carrier Systems for Drug Delivery, in: Appl. Nanotechnol. Drug Deliv, InTech, 2014. 10.5772/58459. [DOI] [Google Scholar]
- [209].Raucher D, Dragojevic S, Ryu J, Macromolecular Drug Carriers for Targeted Glioblastoma Therapy: Preclinical Studies, Challenges, and Future Perspectives, Front. Oncol 8 (2018) 624. 10.3389/fonc.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Váňová J, Hejtmánková A, Kalbáčová MH, španielová H, The utilization of cell-penetrating peptides in the intracellular delivery of viral nanoparticles, Materials (Basel). 12 (2019). 10.3390/ma12172671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Maroun J, Muñoz-Alía M, Ammayappan A, Schulze A, Peng KW, Russell S, Designing and building oncolytic viruses, Future Virol. 12 (2017) 193–213. 10.2217/fvl-2016-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Cifuentes-Muñoz N, Dutch RE, Cattaneo R, Direct cell-to-cell transmission of respiratory viruses: The fast lanes, PLOS Pathog. 14 (2018) e1007015. 10.1371/journal.ppat.1007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Guedan S, Grases D, Rojas JJ, Gros A, Vilardell F, Vile R, et al. , GALV expression enhances the therapeutic efficacy of an oncolytic adenovirus by inducing cell fusion and enhancing virus distribution, Gene Ther. 19 (2012) 1048–57. 10.1038/gt.2011.184. [DOI] [PubMed] [Google Scholar]
- [214].Zhong P, Agosto LM, Munro JB, Mothes W, Cell-to-cell transmission of viruses, Curr. Opin. Virol 3 (2013)44–50. 10.1016/j.coviro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Zhang X, Xu X, Li Y, Hu C, Zhang Z, Gu Z, Virion-Like Membrane-Breaking Nanoparticles with Tumor-Activated Cell-and-Tissue Dual-Penetration Conquer Impermeable Cancer, Adv. Mater 30 (2018) 1707240. 10.1002/adma.201707240. [DOI] [PubMed] [Google Scholar]
- [216].Theuerkauf SA, Michels A, Riechert V, Maier TJ, Flory E, Cichutek K, et al. , Quantitative assays reveal cell fusion at minimal levels of SARS-CoV-2 spike protein and fusion from without, ISCIENCE. 24 (2021) 102170. 10.1016/j.isci.2021.102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Sun Y, Sun Y, Zhao R, Establishment of MicroRNA delivery system by PP7 bacteriophage-like particles carrying cell-penetrating peptide, J. Biosci. Bioeng 124 (2017) 242–9. 10.1016/j.jbiosc.2017.03.012. [DOI] [PubMed] [Google Scholar]
- [218].Zhang W, Zhai Z, Huang S, Mao Z, Zhang Y, Gao C, Morphological and constituent viral-mimicking self-assembled nanoparticles promote cellular uptake and improve cancer therapeutic efficiency in vivo, Giant. 3 (2020) 100026. 10.1016/j.giant.2020.100026. [DOI] [Google Scholar]
- [219].Li Z, Shan X, Chen Z, Gao N, Zeng W, Zeng X, et al. , Applications of Surface Modification Technologies in Nanomedicine for Deep Tumor Penetration, Adv. Sci 8 (2021) 2002589. 10.1002/advs.202002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Fan Z, Kumon RE, Deng CX, Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery, Ther. Deliv 5 (2014) 467–86. 10.4155/tde.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Nande R, Howard CM, Claudio PP, Ultrasound-mediated oncolytic virus delivery and uptake for increased therapeutic efficacy: state of art., Oncolytic Virotherapy. 4 (2015) 193–205. 10.2147/OV.S66097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Carlisle R, Choi J, Bazan-Peregrino M, Laga R, Subr V, Kostka L, et al. , Enhanced Tumor Uptake and Penetration of Virotherapy Using Polymer Stealthing and Focused Ultrasound, JNCI J. Natl. Cancer Inst 105 (2013) 1701–10. 10.1093/jnci/djt305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Hamed AR, Abdel-Azim NS, Shams KA, Hammouda FM, Targeting multidrug resistance in cancer by natural chemosensitizers, Bull. Natl. Res. Cent 43 (2019) 1–14. 10.1186/s42269-019-0043-8. [DOI] [Google Scholar]
- [224].Lee HA, Chu KB, Moon EK, Kim SS, Quan FS, Sensitization to oxidative stress and G2/M cell cycle arrest by histone deacetylase inhibition in hepatocellular carcinoma cells, Free Radic. Biol. Med 147 (2020) 129–38. 10.1016/j.freeradbiomed.2019.12.021. [DOI] [PubMed] [Google Scholar]
- [225].Marin JJG, Monte MJ, Blazquez AG, F Macias R, Serrano MA, Briz O, The role of reduced intracellular concentrations of active drugs in the lack of response to anticancer chemotherapy, Acta Pharmacol. Sin 35 (2014) 1–10. 10.1038/aps.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Caldeira JC, Perrine M, Pericle F, Cavallo F, Virus-like particles as an immunogenic platform for cancer vaccines, Viruses. 12 (2020). 10.3390/v12050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Bolli E, O’Rourke JP, Conti L, Lanzardo S, Rolih V, Christen JM, et al. , A Virus-Like-Particle immunotherapy targeting Epitope-Specific anti-xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo, Oncoimmunology. 7 (2018) e1408746. 10.1080/2162402X.2017.1408746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Kwan BLY, Wai VWK, Autophagy in Multidrug-Resistant Cancers, in: Autophagy Curr. Trends Cell. Physiol. Pathol, In Tech, 2016. 10.5772/64274. [DOI] [Google Scholar]
- [229].Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA, DNA repair pathways as targets for cancer therapy, Nat. Rev. Cancer 8 (2008) 193–204. 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- [230].Liu F, Xie Z-H, Cai G-P, Jiang Y-Y, The Effect of Survivin on Multidrug Resistance Mediated by P-Glycoprotein in MCF-7 and Its Adriamycin Resistant Cells, Biol. Pharm. Bull 30 (2007) 2279–83. 10.1248/bpb.30.2279. [DOI] [PubMed] [Google Scholar]
- [231].Campbell KJ, Tait SWG, Targeting BCL-2 regulated apoptosis in cancer, Open Biol. 8 (2018). 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Chen X, Zhou Y, Wang J, Wang J, Yang J, Zhai Y, et al. , Dual silencing of Bel-2 and Survivin by HSV-1 vector shows better antitumor efficacy in higher PKR phosphorylation tumor cells in vitro and in vivo, Cancer Gene Ther. 22 (2015) 380–6. 10.1038/cgt.2015.30. [DOI] [PubMed] [Google Scholar]
- [233].Ju X, Zhang H, Zhou Z, Wang Q, Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy., Am. J. Cancer Res 10 (2020) 1–11. http://www.ncbi.nlm.nih.gov/pubmed/32064150. [PMC free article] [PubMed] [Google Scholar]
- [234].Zheng M, Huang J, Tong A, Yang H, Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances, Mol. Ther.-Oncolytics 15 (2019) 234–47. 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Ylösmäki E, Cerullo V, Design and application of oncolytic viruses for cancer immunotherapy, Curr. Opin. Biotechnol 65 (2020) 25–36. 10.1016/j.copbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- [236].Kitamura T, Qian BZ, Pollard JW, Immune cell promotion of metastasis, Nat. Rev. Immunol 15 (2015) 73–86. 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Nguyen H-M, Bommareddy PK, Silk AW, Saha D, Optimal timing of PD-1 blockade in combination with oncolytic virus therapy, Semin. Cancer Biol (2021). 10.1016/j.semcancer.2021.05.019. [DOI] [PubMed] [Google Scholar]
- [238].Jenkins RW, Barbie DA, Flaherty KT, Mechanisms of resistance to immune checkpoint inhibitors, Br. J. Cancer 118 (2018) 9–16. 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. , Association of pembrolizumab with tumor response and survival among patients with advanced melanoma, in: JAMA - J. Am. Med. Assoc, American Medical Association, 2016: pp. 1600–9. 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- [240].Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T, et al. , Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade, Sci. Transl. Med 12 (2020) 1–14. 10.1126/scitranslmed.aax7992. [DOI] [PubMed] [Google Scholar]
- [241].Yu Z, Pestell TG, Lisanti MP, Pestell RG, Cancer stem cells, Int. J. Biochem. Cell Biol 44 (2012) 2144–51. 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Ayob AZ, Ramasamy TS, Cancer stem cells as key drivers of tumour progression, J. Biomed. Sci 25 (2018) 1–18. 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Codony-Servat J, Rosell R, Cancer stem cells and immunoresistance: Clinical implications and solutions, Transl. Lung Cancer Res 4 (2015) 689–703. 10.3978/j.issn.2218-6751.2015.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Sultan M, Coyle KM, Vidovic D, Thomas ML, Gujar S, Marcato P, Hide-and-seek: The interplay between cancer stem cells and the immune system, Carcinogenesis. 38 (2017) 107–18. 10.1093/carcin/bgw115. [DOI] [PubMed] [Google Scholar]
- [245].Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, et al. , Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment, Stem Cells Int. 2018 (2018). 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Tarakanova VL, Leung-Pineda V, Hwang S, Yang CW, Matatall K, Basson M, et al. , γ-Herpesvirus Kinase Actively Initiates a DNA Damage Response by Inducing Phosphorylation of H2AX to Foster Viral Replication, Cell Host Microbe. 1 (2007) 275–86. 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Najafi M, Farhood B, Mortezaee K, Kharazinejad E, Majidpoor J, Ahadi R, Hypoxia in solid tumors: a key promoter of cancer stem cell (CSC) resistance, J. Cancer Res. Clin. Oncol 146 (2020) 19–31. 10.1007/s00432-019-03080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, et al. , The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment, Cancer Cell Int. 21 (2021) 1–26. 10.1186/s12935-020-01719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Fasullo M, Burch AD, Britton A, Hypoxia enhances the replication of oncolytic herpes simplex virus in p53- breast cancer cells, Cell Cycle. 8 (2009) 2194–7. 10.4161/cc.8.14.8934. [DOI] [PubMed] [Google Scholar]
- [250].Hiley CT, Yuan M, Lemoine NR, Wang Y, Lister strain vaccinia virus, a potential therapeutic vector targeting hypoxic tumours, Gene Ther. 17 (2010) 281–7. 10.1038/gt.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Ishiwata T, Cancer stem cells and epithelial-mesenchymal transition: Novel therapeutic targets for cancer, Pathol. Int 66 (2016) 601–8. 10.1111/pin.12447. [DOI] [PubMed] [Google Scholar]
- [252].Chen CH, Chen WY, Lin SF, Wong RJ, Epithelial-mesenchymal transition enhances response to oncolytic herpesviral therapy through nectin-1, Hum. Gene Ther 25 (2014) 539–51. 10.1089/hum.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Zhao Y, Bao Q, Renner A, Camaj P, Eichhorn M, Ischenko I, et al. , Cancer stem cells and angiogenesis, Int. J. Dev. Biol 55 (2011) 477–82. 10.1387/ijdb.103225yz. [DOI] [PubMed] [Google Scholar]
- [254].Breitbach CJ, Arulanandam R, De Silva N, Thome SH, Patt R, Daneshmand M, et al. , Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans, Cancer Res. 73 (2013) 1265–75. 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- [255].Arulanandam R, Batenchuk C, Angarita FA, Ottolino-Perry K, Cousineau S, Mottashed A, et al. , VEGF-Mediated Induction of PRD 1-BF1/Blimp 1 Expression Sensitizes Tumor Vasculature to Oncolytic Virus Infection, Cancer Cell. 28 (2015) 210–24. 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- [256].Hou W, Chen H, Rojas J, Sampath P, Thorne SH, Oncolytic vaccinia virus demonstrates anti angiogenic effects mediated by targeting of VEGF, Int. J. Cancer 135 (2014) 1238–46. 10.1002/ijc.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Kim WT, Ryu CJ, Cancer stem cell surface markers on normal stem cells, BMB Rep. 50 (2017) 285–98. 10.5483/BMBRep.2017.50.6.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Fiorillo M, Verre AF, Iliut M, Peiris-Pages M, Ozsvari B, Gandara R, et al. , Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”, Oncotarget. 6 (2015) 3553–62. 10.18632/oncotarget.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Jiang Q, Song C, Nangreave J, Liu X, Lin L, Qiu D, et al. , DNA origami as a carrier for circumvention of drug resistance, J. Am. Chem. Soc 134 (2012) 13396–403. 10.1021/ja304263n. [DOI] [PubMed] [Google Scholar]
- [260].Yang G, Phua SZF, Lim WQ, Zhang R, Feng L, Liu G, et al. , A Hypoxia-Responsive Albumin- Based Nanosystem for Deep Tumor Penetration and Excellent Therapeutic Efficacy, Adv. Mater 31 (2019) 1901513. 10.1002/adma.201901513. [DOI] [PubMed] [Google Scholar]
- [261].Liu H, Yao J, Guo H, Cai X, Jiang Y, Lin M, et al. , Tumor Microenvironment-Responsive Nanomaterials as Targeted Delivery Carriers for Photodynamic Anticancer Therapy, Front. Chem 8 (2020) 758. 10.3389/fchem.2020.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Peiris PM, He F, Covarrubias G, Raghunathan S, Turan O, Lorkowski M, et al. , Precise targeting of cancer metastasis using multi-ligand nanoparticles incorporating four different ligands, Nanoscale. 10 (2018) 6861–71. 10.1039/c8nr02513d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Derdak SV, Kueng HJ, Leb VM, Neunkirchner A, Schmetterer KG, Bielek E, et al. , Direct stimulation of T lymphocytes by immunosomes: Virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 13144–9. 10.1073/pnas.0602283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Strable E, Prasuhn DE, Udit AK, Brown S, Link AJ, Ngo JT, et al. , Unnatural amino acid incorporation into virus-like particles., Bioconjug. Chem 19 (2008) 866–75. 10.1021/bc700390r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Zheng Y, Yu F, Wu Y, Si L, Xu H, Zhang C, et al. , Broadening the versatility of lentiviral vectors as a tool in nucleic acid research via genetic code expansion., Nucleic Acids Res. 43 (2015) e73. 10.1093/nar/gkv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Lieser RM, Yur D, Sullivan MO, Chen W, Site-Specific Bioconjugation Approaches for Enhanced Delivery of Protein Therapeutics and Protein Drug Carriers, Bioconjug. Chem 31 (2020) 2272–82. 10.1021/acs.bioconjchem.0c00456. [DOI] [PubMed] [Google Scholar]
- [267].Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB, Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells., ACS Nano. 4 (2010) 6014–20. 10.1021/nn1014769. [DOI] [PubMed] [Google Scholar]
- [268].Lancaster EM, Jablons D, Kratz JR, Applications of next-generation sequencing in neoantigen prediction and cancer vaccine development, Genet. Test. Mol. Biomarkers 24 (2020) 59–66. 10.1089/gtmb.2018.0211. [DOI] [PubMed] [Google Scholar]
- [269].Bouvet M, Reid TR, Larson C, Oronsky B, Carter C, Morris JC, Extended treatment with MY-NEOVAX, personalized neoantigen-enhanced oncolytic viruses, for two end-stage cancer patients, Oxford Med. Case Reports 2019 (2019) 461–3. 10.1093/omcr/omz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Miao L, Zhang Y, Huang L, mRNA vaccine for cancer immunotherapy, Mol. Cancer 20 (2021)41. 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Szécsi J, Gabriel G, Edfeldt G, Michelet M, Klenk HD, Cosset F-L, DNA vaccination with a single-plasmid construct coding for viruslike particles protects mice against infection with a highly pathogenic avian influenza A virus., J. Infect. Dis 200 (2009) 181–90. 10.1086/599840. [DOI] [PubMed] [Google Scholar]
- [272].Céspedes MV, Unzueta U, Tatkiewicz W, Sánchez-Chardi A, Conchillo-Solé O, Álamo P, et al. , In vivo architectonic stability of fully de novo designed protein-only nanoparticles., ACS Nano. 8 (2014)4166–76. 10.1021/nn4055732. [DOI] [PubMed] [Google Scholar]
- [273].Hernandez-Garcia A, Kraft DJ, Janssen AFJ, Bomans PHH, Sommerdijk NAJM, Thies-Weesie DME, et al. , Design and self-assembly of simple coat proteins for artificial viruses., Nat. Nanotechnol 9 (2014) 698–702. 10.1038/nnano.2014.169. [DOI] [PubMed] [Google Scholar]
- [274].Butterfield GL, Lajoie MJ, Gustafson HH, Sellers DL, Nattermann U, Ellis D, et al. , Evolution of a designed protein assembly encapsulating its own RNA genome., Nature. 552 (2017) 415–20. 10.1038/nature25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Leveille S, Samuel S, Goulet ML, Hiscott J, Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy, Cancer Gene Ther. 18 (2011) 435–43. 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
- [276].Karjoo Z, Chen X, Hatefi A, Progress and problems with the use of suicide genes for targeted cancer therapy., Adv. Drug Deliv. Rev 99 (2016) 113–28. 10.1016/j.addr.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S, et al. , Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7–1 in an immunocompetent murine model, Clin. Cancer Res 12 (2006) 5859–68. 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- [278].Choi IK, Lee JS, Zhang SN, Park J, Lee KM, Sonn CH, et al. , Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12RB2 or IL-18Rα, Gene Ther. 18 (2011) 898–909. 10.1038/gt.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Wong RJ, Patel SG, Kim S-H, Dematteo RP, Malhotra S, Bennett JJ, et al. , Cytokine Gene Transfer Enhances Herpes Oncolytic Therapy in Murine Squamous Cell Carcinoma, Mary Ann Liebert, Inc, 2001. www.liebertpub.com. [DOI] [PubMed] [Google Scholar]
- [280].Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF, Nonmethylated CG Motifs Packaged into Virus-Like Particles Induce Protective Cytotoxic T Cell Responses in the Absence of Systemic Side Effects, J. Immunol 172 (2004) 1777–85. 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- [281].Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B, et al. , Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an anti angiogenic gene, Soluble Fit-1, Mol. Ther 11 (2005) 553–62. 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- [282].Passaro C, Alayo Q, De Laura I, McNulty J, Grauwet K, Ito H, et al. , Arming an oncolytic herpes simplex virus type 1 with a single-chain fragment variable antibody against PD-1 for experimental glioblastoma therapy, Clin. Cancer Res 25 (2019) 290–9. 10.1158/1078-0432.CCR-18-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, et al. , Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results, J. Clin. Oncol 21 (2003) 2508–18. 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- [284].Nascimento AV, Gattacceca F, Singh A, Bousbaa H, Ferreira D, Sarmento B, et al. , Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models, Nanomedicine. 11 (2016) 767–81. 10.2217/nnm.16.14. [DOI] [PMC free article] [PubMed] [Google Scholar]


