Abstract
Background:
The genus Abbreviata (Spirurida: Physalopteridea) currently contains 47 species. Physalopteridae nematodes infect a large number of vertebrates, including mammals, birds, reptiles and amphibians. The current study is a report of the first morphological and molecular identification of A. kazakhstanica (Spirurida: Physalopteridea) in Pseudopus apodus in Iran.
Methods:
Eleven road-killed P. apodus, were collected from, Iran during 2016–2018. The nematodes were isolated from stomach. After morphological study, the genomic DNA of the parasites was extracted using CTAB method. The DNA was used for PCR amplification of cytochrome c oxidase subunit I (cox1). The PCR products were sequenced, the sequence data were analyzed and multiple alignments were conducted using the Clustal Omega.
Results:
After detailed microscopic examination, the A. kazakhstanica was identified. The cox1 sequences confirmed the species of helminth. The new sequences of A. kazakhstanica were submitted to GenBank under the accession number MK578751-2.
Conclusion:
Regarding the limited data on parasitological status of Iranian reptiles, more specific and comprehensive investigations are needed to identify the parasitic fauna.
Keywords: Abbreviata kazakhstanica, Molecular identification, Pseudopus apodus, Physalopteridea, Iran
Introduction
A broad range of pathogens especially those with public health concerns are associated with reptiles (1). Until now, 125 species of lizards with 36 genera from 8 families have been reported in Iran that Pseudopus apodus (Anguidae) (Pallas, 1775) is one of them (2). This lizard species is distributed from southern Europe to Central Asia. They prey on rodents, lizards, and eggs and fledglings of ground-nesting birds (3). Despite that lizards are well-studied group in Iran (4), the data on their parasitic infections is limited. The reported parasites from lizard in Iran are 14 species as follows: 12 species of nematodes, Abbreviata baltazardi in Phrynocephalus heliosopus (Agamidae), and Skrjabinodon pigmentatus, Spauligodon lacertae, Thelandros baylisi, and Oswaldo filaria chlamydorauri in Laudakia caucasia (Agamidae), Parapharyngodon sp., Thelandros spp., and Thubunea mobedii from Large-scaled (Rock) Agama, Laudakia nupta nupta (Agamidae), and Spauligodon persiensis from Rough bent-toed gecko, Cyrtopodion scabrum (Gekkonidae), and two species of cestode, Nematotaenia dispar from the desert monitor, Varanus griseus (Varanidae), and Oochoristica tuberculata from the snake-eyed lizard, Ophisops elegans (Lacertidae) (5–12). There is just one report of parasitic infection in European legless lizard, P. apodus in Iran that was the nematode E. entomelas (8).
The nematodes of Physalopteridae family can infect mammals, birds, reptiles and amphibians (13). As well, there are several reports of infection in humans in different regions of the world highlighting the zoonotic importance of these parasites (14). The genus Abbreviata, bisected from the genus Physalopteris by Travassos, 1920, contains 47 species (15). They are characterized through one externo-lateral tooth, one interno-lateral tooth and two double submedian teeth on each pseudolabium. The number of uterus branches is the main factor for subgeneric classification (16).
Life cycles of Abbreviata species are poorly known and invertebrates as Coleopterans, Mantopterans, and Othopterans are probably suitable intermediate hosts (17), although the larval form has been observed in mammals, reptiles, and amphibians and it is difficult to determine whether they are definitive, intermediate, or paratenic hosts. Sometimes pseudoparasite may be found in an unusual host, which occurs when predator swallows definitive host as the prey with the helminthes inside its body (15). Nematodes in the genus Abbreviata spp. have a worldwide distribution and more than one nematode species may occur in more than one host species. Lizards are the most important hosts and among parasitic species for reptile, there are host-specific and euryxenous species (18).
Considering that no study in this regard has been done in Iran so far, this study was performed with the aim of molecular study on A. kazakhstanica isolated from Pseudopus apodus.
Materials and Methods
Sampling
Eleven road-killed P. apodus, were collected from Guilan Province in Iran during 2016–2018. At necropsy, the digestive tract, lungs, and body cavity of each specimen was examined for helminths using a dissecting microscope. All of the nematodes were isolated from stomach.
Parasitology test
Collected helminths were relaxed in water and preserved in 70% ethanol. Nematodes were placed on a glass slide in a drop of glycerol, a coverslip was added, and identification was made from these temporary wet mounts.
Molecular study
Genomic DNA was extracted using cetyl trimethylammonium bromide (CTAB) method (19). One ml of lysis buffer (8% Triton X-100, 0.25 M sucrose, 50mM Tris-HCl, 50mM EDTA, pH 7.5) and 50 µl of proteinase K (20 mg/ml) were added to 50 mg of homogenized tissue of worm. Then, it incubated at 65 °C for 1–2 h (until it becomes clear). DNA was precipitated by adding 1 ml of sterile 2% CTAB solution to the cleared lysate and centrifugation 1500×g for 5 min. Then, 0.5 ml of dissolving buffer 2.5 M NaCl, 10 mM EDTA, pH 7.7 and dilute with 1 ml of 40 mM Tris-HCl, 2 mM EDTA, pH: 7.7) added to pellet and then 1 ml of diluting buffer (40 mM Tris-HCl, 2 mM EDTA, pH: 7.7) was added to this solvent. Two volume of chloroform were add, mix gently and centrifuged at 12000 ×g for 5 min. The aqueous phase was transferred to a fresh tube, add 2 volume of ethanol (room temperature) and incubate the tube at −20 °C for overnight. Finally, pure DNA were precipitated by centrifugation at 12000 ×g for 10 min at room temperature.
The DNA was used for PCR amplification of cytochrome c oxidase subunit I (COX1), as is published (20). For PCR reaction mixtures were used Taq Polymerase master mix was used (AMPLIQON). The volumes of DNA and primer were set up based on concentration (50 ng DNA, 10 pM for each primers).
The PCR reaction was initiated by denaturation step at 94 °C for 5 min; and then the mixtures were subjected to 35 cycles of denaturation at 94 °C for 30 sec, annealing at 55 °C for 30 sec, and extension at 72 °C for 30 sec, with a final extension at 72 °C for 5 minutes. The PCR product was analyzed by electrophoresis in a 1.5% agarose gel and was visualized after DNA safe staining.
Nucleotide sequence analysis
The PCR products were sequenced in Macrogen Inc., South Korea. Sequence data were analyzed and multiple alignment were conducted using the Clustal Omega ( https://www.ebi.ac.uk/Tools/msa/clustalo/ ). Phylogenetic tree was created by MEGA7 software. The phylogenetic tree was drawn using sequences obtained in this study as well as published sequences available for Spirurida from GenBank. Thelazia callipaeda (AF182301) was applied as outgroup.
Results
Eleven adult P. apodus (8 male and 3 female) were collected during 2016–2018 in Guilan Province, Iran. After necropsy, 8 of 11 (72.7%) were infected by Abbreviata genus. Each of the eight hosts had a very severe infection (>10 worms in stomach). After detailed examination, we found genus Abbreviata and species A. kazakhstanica. In Abbreviata, oral opening surrounded by 2 large single-lobed lateral pseudolabia. Usually entire margin of pseudolabia dentate.
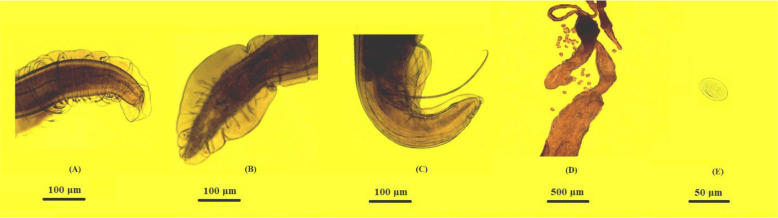
In males, tail with broad, lateral alae, which connect across the ventral surface in front of the cloac. Usually 4 pairs of pedunculated or stalked papillae supporting lateral alae, typically 2 pairs pre-cloacal and 2 pairs post cloacal. Ventral surface of tail also covered with longitudinal or broken rows of tubercle. Spicules equal, subequal, but generally unequal (Fig. 1 & Table 1).
Fig. 1:
Abbreviata kazakhstanica (A) Anterior part with pseudolabia dentat, (B) lateral alae in male with 4 pairs of pedunculated papillae, (C) unequal spicules, (D) uterus with 2 branches (didelphys), (E) embryonated egg
Table 1:
Body measurements of adult Abbreviata kazakhstanica isolated from Pseudopus apodus in Iran.
| Male | Range | Mean ± SE |
|---|---|---|
| Length (mm) | 15–17 | 16 ± 0.2 |
| Width (µm) | 386–403 | 394.5 ± 8.4 |
| Width (µm) | 386–403 | 394.5 ± 8.4 |
| Eggs (µm) | 50.1–51.4 x 30.2–32.0 | 50.3 x 31.2 |
| Female | ||
| Length (mm) | 10–12 | 10.5 ± 0.4 |
| Width (µm) | 403–691 | 494.5 ± 65.4 |
| Spicule Length (µm) | 1700–1900 | 1840 ± 95 |
In Females vulva in first half of body, usually in anterior third; uterus with 2 (didelphys), 4 (tetradelphys), or more than 4 (polydelphys) branches. Eggs usually oval, smooth, thick-shelled, containing embryos when deposited. For basic species affiliation, the new COI sequences were checked via BLAST ( https://blast.ncbi.nlm.nih.gov/Blast.cgi ).
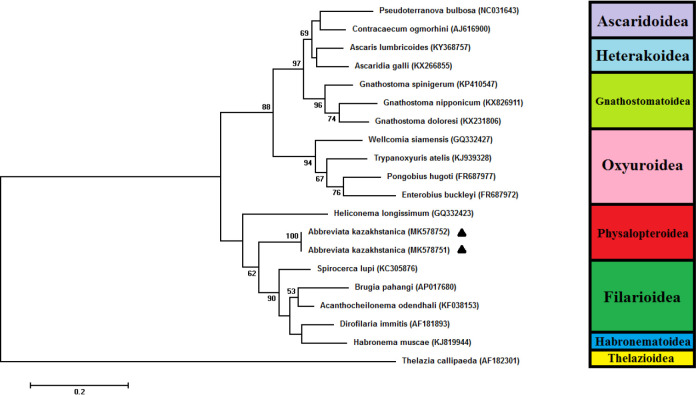
Up to now, the sequences of A. kazakhstanica were not presented in GenBank. The blast result revealed that the sequences of A. kazakhstanica have highest percentage of identity (77%) with Heliconema longissimum of Physalopteridae family. The new sequences of A. kazakhstanica were submitted to GenBank under the accession number MK578751-2. Based CO1 result of phylogenetic analysis revealed that two main cluster in Spirurida and 3 super-family Physalopteriodea, Filarioidea and Habronematoidea fall in cluster 2 and the rest subfamily placed in cluster 1 (Fig. 2).
Fig. 2:
Molecular phylogenetic analysis of Abbreviata kazakhstanica isolate from Pseudopus apodus by Maximum Likelihood method on partial mCOI 444 bp sequence. Sequences from Thelezia callipaeda (GenBank accession No.: AF182301) were employed as outgroup for phylogenetic analysis. The percentage of trees in which the associated taxa clustered together is shown next to the branches
Discussion
This is the first molecular identification of A. kazakhstanica in P. apodus in Iran. Glass lizard samples are rarely available for parasite examination, being often confined to dead carcasses in nature and road-killed animals. Previously, this genus from the Physalopteridae family has not been reported from any hosts in Iran.
In other study, the larvae and adult of A. kazakhstanica were reported from P. apodus (21–23). Until now, A. kazakhstanica was isolated from the Union of Soviet Socialist Republics (USSR), UzbSSR (21–24). Six species of tenebrionid beetles including Gryllus bimaculatus, Decticus verrucivorus, Platycleis intermedia, Tettigonra viridissima, Emenoviana tamerlana, Pimelia verrucosa and Stalagmoptera confuse play the role of intermediate hosts. Moreover, Dila laevicollis, Prosodes nitida, Prosodes baeri, and Hierodula kazachstanica were determined as intermediate hosts (25).
Conclusion
Considering the limited parasitological data on reptiles in Iran, identifying the parasite fauna precise and comprehensive studies with the aim of and particularly the helminthes diversity are required.
Acknowledgements
The authors would like to thank all staff of Department of Parasitology of Faculty of Medical Sciences, Tarbiat Modares University, Iran. This work was supported by the Slovak Research and Development Agency under the contract no. APVV-15-0147.
Footnotes
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Mendoza-Roldan JA, Modry D, Otranto D. Zoonotic parasites of reptiles: a crawling threat. Trends Parasitol. 2020; 36(8): 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rastegar-Pouyani N, Kami HG, Rajabzadeh M, et al. Annotated checklist of amphibians and reptiles of Iran. Iranian Journal of Animal Biosystematics (Ijab). 2008; 4(1): 7–30. [Google Scholar]
- 3.Çiçek K, Tok CV, Hayretdağ S, et al. Data on the food composition of European Glass Lizard, Pseudopus apodus (Pallas, 1775)(Squamata: Anguidae) from Çanakkale (Western Anatolia, Turkey). Acta Zool Bulg. 2014; 66(3):433–6. [Google Scholar]
- 4.Šmid J, Moravec J, Kodym P, et al. Annotated checklist and distribution of the lizards of Iran. Zootaxa. 2014; 3855:1–97. [DOI] [PubMed] [Google Scholar]
- 5.Chabaud AG. Un nouveau physaloptere parasite d’agame. Ann Parasitol Hum Comp 1953; 28(4:305–11. [PubMed] [Google Scholar]
- 6.Rezazadeh E, Tajbakhs F, Bursey CR, et al. Helminth parasites of the Caucasian agama, Laudakia caucasia (Squamata: Agamidae), from Iran. Comp. Parasitol. 2012; 79(1):160–163. [Google Scholar]
- 7.Hosseinzadeh AA, Lagzian A. First report of Oswaldo filaria chlamydosauri, Breinl, 1912 (Nematoda: Onchocercidae) from a new host Paralaudakia caucasia, Eichwald, 1831 (Squamata: Agamidae) and its prevalence and intensity in Mashhad, North-eastern Iran. JWB. 2018; 2(1):6–11. [Google Scholar]
- 8.Halajian A, Bursey CR, Goldberg SR, et al. Helminth parasites of the European glass lizard, Pseudopus apodus (Squamata: Anguidae), and European grass snake, Natrix natrix (Serpentes: Colubridae), from Iran. Comparative Parasitology. 2013; 80(1):151–156. [Google Scholar]
- 9.Rahimian H, Pazoki S, Habashi SA. Gastrointestinal nematodes of Laudakia nupta nupta (Sauria: Agamidae) from Iran with descriptions of two new species (Oxyuridea: Pharyngodonidae) and comments on the diagnostic features of Parapharyngodon and Thelandros. Zootaxa. 2014; 3852(1):51–82. [Google Scholar]
- 10.Pazoki S, Rahimian H. New species of Spauligodon Skrjabin, Schikhobalova & Lagodovskaja, 1960 and Thubunea Seurat, 1914 (Nematoda) from the gastro-intestinal tract of lizards in Iran. Syst Parasitol. 2014; 89(3):259–70. [DOI] [PubMed] [Google Scholar]
- 11.Dollfus R-P., Mission Yves-J., Golvan et Jean-A. Rioux en Iran-Cestodes de Carnivores, Rongeurs, Insectivores, Reptiles et Batraciens. Ann Parasitol Hum Comp. 1965; 40(1):61–86. [PubMed] [Google Scholar]
- 12.Goldberg S, Bursey C. Ophisops elegans (snake-eyed lizard): endoparasites. Herpetol Rev. 2010; 41:495. [Google Scholar]
- 13.Anderson RC. Nematode parasites of vertebrates: their development and transmission. CABI Publishing, Wallingford UK. 2000. [Google Scholar]
- 14.King C, Tay CY, Jones HI, Jowd J. Morphologic Observations and Novel 18S rRNA Sequences of Abbreviata hastaspicula and Abbreviata antarctica from Varanus spp. Lizards in Australia. J Wildl Dis. 2019; 55(1):149–152. [DOI] [PubMed] [Google Scholar]
- 15.Jablonski D, Vági B, Kardos G. Abbreviata abbreviata (Rudolphi, 1819) as a new nematode parasite for Malpolon insignitus (Geoffroy Saint-Hilaire, 1827) recorded in Albania. Ecologica Montenegrina. 2015;2(3):194–196. [Google Scholar]
- 16.Morgan BB. Host-parasite relationships and, geographical distribution of the Physalopterinae (Nematoda). Trans Wis Acad Sci Arts Lett. 1947; 38:273–92. [Google Scholar]
- 17.King C, Jones H, Tay CY. Arthropod intermediate hosts of Abbreviata antarctica (Nematoda: Physalopteridae) in Australia. J Parasitol. 2013; 99(4):708–11. [DOI] [PubMed] [Google Scholar]
- 18.Jones HI. Physalopterine nematodes in Australian reptiles: interactions and patterns of infection. Aust J Zool. 2014; 62(2):180–194. [Google Scholar]
- 19.Yap K, Thompson R. CTAB precipitation of cestode DNA. Parasitol Today. 1987; 3(7):220–2. [DOI] [PubMed] [Google Scholar]
- 20.Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54(2):165–73. [DOI] [PubMed] [Google Scholar]
- 21.Gafurov A, Lunkina E. A study of the developmental cycle of Abbreviata kazachstanica Markov & Paraskiv, 1956 (Nematoda: Spirurata). Izvestiya Akademii Nauk Tadzhikskoi SSR (Ahboroti Akademijai Fanhoi RSS Tociston) Otdelenie Biologicheskikh Nauk. 1970; 4(41)):100–104. [Google Scholar]
- 22.Kabilov T, Siddikov BK, editors. Identification of intermediate hosts of nematode Abbreviata kazakhstanica Markov et Paraskiv 1956. Dokl Akad Nauk Uzb SSR; 1978. [Google Scholar]
- 23.Vakker V, Brushko Z, Kolbintsev V. Parasite fauna of Ophisaurus apodus in the Kazakh SSR, USSR Izv. An Kazakhskoi SSR Biol. 1985; 4:36–9. [Google Scholar]
- 24.Markov G, Paraskiv K. The helminth fauna of reptiles in Kazakhstan. Trudy Instituta Zoologii Akademiya Nauk Kazakhskoi SSR. 1956; 5:120–8. [Google Scholar]
- 25.Kabilov T. [Life cycle of the nematode, Abbreviata kazachstanica]. Parazitologiia. 1980; 14(3):263–70. [PubMed] [Google Scholar]