Abstract
Background:
The study on lymphatic filariasis (LF) in Igbo-Eze North was conducted to determine the prevalence and predisposing factors to LF among its residents between May and October 2018.
Methods:
A total of 201 residents who have lived in the area for at least one year were recruited. They were stratified according to age, gender and occupation, and were clinically examined firstly by rapid assessment method for any lymphoedema and hydrocele. At recruitment, blood samples were collected from all volunteered participants for LF test. In addition, demographic information and risk factors of the respondents were captured using a structured questionnaire by oral interview.
Results:
The overall prevalence for LF was 84 (41.8%). Furthermore, LF prevalence was significant (P < 0.05) in all the studied communities: 61.5% in Umuogbuagu, 48.1% in Aguibege, 32.7% in Umuagama and 21.7% in Umuopu. The sex-related prevalence of LF was higher among females than males, with slight significant difference (P = 0.046). In relation to age and occupation, higher prevalences (P < 0.0001) were recorded among older (≥ 50 years) subjects (49, 61.2%) and traders (55, 57.9%) respectively. The risk associated with LF implicated lack of knowledge, non-use of mosquito nets, as well as visit and proximity to water bodies as major predispositions (P < 0.05).
Conclusion:
The prevalence of LF in this study was high. Higher prevalence was among females, older people and traders. Notable risks to the disease outcome are environmental, attitudinal and occupational with chances of scaled up prevalence and burden overtime.
Keywords: Lymphatic Filariasis, Nigeria, Knowledge, Epidemiology, Wuchereria bancrofti
Introduction
Lymphatic filariasis (LF) is among the parasitic diseases that cause the most socioeconomic burden on infected people in endemic communities (1). It is caused by Wuchereria bancrofti and Brugia spp. (2). The arthropod vectors that transmit LF are Culex, Anopheles, Aedes mosquitoes (3).
Nigeria has a significant burden of LF ranging from social, psychological to economic burden (4). It is one of the most debilitating diseases related with the lymphatic systems, which affects and manifests itself in a variety of severe clinical pathologies (5). The disease causes widespread chronic suffering and social stigma resulting from ignorance and incorrect beliefs (6). Some individuals believed that elephantiasis is an abominable disease (7), and very few believed mosquitoes were associated with elephantiasis (8). Infection of LF microfilariae may be acquired during childhood, with its visible manifestations occurring later in life, causing temporary or permanent disability (9).
For proper understanding of the geographical distribution, prevalence and degree of risk of LF, there is need for continuous surveillance to help suggest additional strategies to complement mass drug administration needed to accelerate LF control and elimination programme. We aimed to assess LF prevalence and risk in Igbo-Eze North.
Materials and Methods
Study area
The study was carried out between May and Oct 2018 in Igbo-Eze North Local Government Area located in the northern part of Enugu State, Nigeria (Fig. 1).
Fig. 1:
Map of Igbo-Eze North Local Government Area showing the selected communities
Source: GIS Unit, Department of Geography, University of Nigeria Nsukka
It is located between Latitude 6° 57′ 36″ and 7° 2′ 24″ N and Longitude 7° 22′ 48″ and 7° 30′ 0″ E. It shares borders in the north with Benue State, in the south with Ovoko and Iheakpu-Awka in Igbo Eze South Local Government Area; in the east Amala and Obollo in Udenu Local Government Area, and Kogi State in the West and partly in the north. The Local Government has an area of 293 km² and a population of 259,431 at the 2006 census (10). In Igbo-Eze North Local Government Area, the people are predominantly traders, farmers and palm wine tappers. The area is renowned for her palm wine production and African Traditional Religion (ATR). Overwhelming majority of the people live in rural settlements; where they mainly engage in subsistence agriculture and related activities.
Ethical clearance
Ethical approval (MH/MSD/REC18/007) was procured from the State Ministry of Health, Enugu State. Also, permission from the Health Department, Igbo-Eze North Local Government Council was sought. Informed consent of the heads, medical personnel of health facilities, and study participants were solicited to enable prompt recruitment.
Study design
A cross-sectional survey involving a multistage sampling procedure was used for this study. The first stage involved a purposive selection and stratification of the Local Government. The second stage involved the random selection of communities within different strata. In the community level, individuals were demographically stratified. Selection of participants was by random sampling. Blood samples of 1 ml volume were collected from each participant, while their demographic information was recorded in a questionnaire.
Determination of sample size
The study population included residents of the study area who have lived for at least one year. A sample size of 201 individuals determined using the method devised by Sarmukaddam and Garad (11) was sampled from four locations endemic for LF in the area; all of them gave consent to participate in the study.
Rapid assessment and examination of blood samples
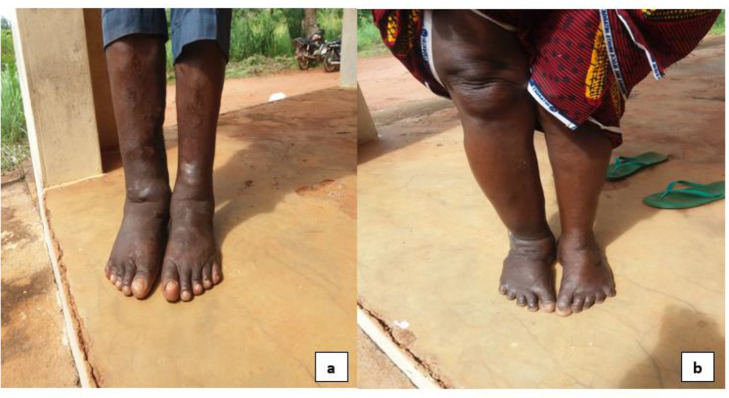
The subjects were examined for clinical manifestations by trained medical personnel. Examination for LF involved the search for lymphoedema (Fig. 2) and hydrocoele. Participants were clinically examined for hydrocoele, and was diagnosed based on the finding of a non-tender, soft, fluid-filled mass (12). For the examination of lymphoedema, participants were simply asked to lift up their clothing to expose their legs or swollen limbs with different degree of swelling (6). Peripheral blood specimens were collected from the participants using a 5 ml syringe into an EDTA tube at night to coincide with the appearance of the microfilariae from 22:00 to 24:00 (3). Prior to this, the site for blood collection was cleaned and disinfected with a ball of cotton wool soaked in 70% ethanol. The specimen bottles were transported and examined microscopically in the Parasitology and Public Health Laboratory, Department of Zoology and Environmental Biology, University of Nigeria, Nsukka. Blood smears were prepared and stained using Field staining technique (13). Presence of microfilariae was confirmed under the microscope. Identification of microfilariae was depended on the ability to discriminate sheath/tail structures.
Fig. 2:
Aguibege participants with lymphoedema on both legs (a) a man (b) a woman
Administration of questionnaire
A standardized close-ended questionnaire was self-administered to each participant by oral interview of the respondents (14). Information on demography, domestic and peridomestic environment, and personal activities outside of the peridomestic area that might be related to exposure to vector bites were included in the questionnaire. Parents and the guardians answered the questionnaire for their children or wards below 10 years of age.
Statistical analysis
All statistical analyses were carried out using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). The prevalence of LF among the study population was compared across the different study locations using Chi-square (χ2) test to determine differences in prevalences. Binary logistic regression was carried out to evaluate the risks associated with LF in the study area. Differences in values were statistically significant at p < 0.05.
Results
Prevalence of lymphatic filariasis in Igbo-Eze North LGA
The overall prevalence of LF in Igbo-Eze North LGA is presented on Table 1, while LF prevalence according to gender, age and occupation are presented on Tables 2–4 respectively.
Table 1:
Overall prevalence of LF in Igbo-Eze North LGA
| Communities | Number Examined | Number Infected | Prevalence (%) | χ2 | df | P-value |
|---|---|---|---|---|---|---|
| Aguibege | 54 | 26 | 48.1 | 43.227 | 1 | < 0.0001 |
| Umuopu | 46 | 10 | 21.7 | 36.204 | 1 | < 0.0001 |
| Umuogbuagu | 52 | 32 | 61.5 | 47.903 | 1 | < 0.0001 |
| Umuagama | 49 | 16 | 32.7 | 44.700 | 1 | < 0.0001 |
| Overall | 201 | 84 | 41.8 | 174.438 | 1 | < 0.0001 |
| χ2 = 18.518, df = 3, P< 0.0001 | ||||||
Table 2:
Prevalence of LF in Igbo-Eze North LGA according to gender of the participants in this study
| Communities | Gender | Total (%) | Infected (%) | Non-Infected (%) |
|---|---|---|---|---|
| Aguibege | Male | 17 (31.5) | 4 (23.5) | 13 (76.5) |
| Female | 37 (68.5) | 22 (59.5) | 15 (40.5) | |
| Total | 54 (100.0) | 26 (48.1) | 28 (51.9) | |
| χ 2 = 6.023, df = 1, p = 0.014 | ||||
| Umuopu | Male | 18 (39.1) | 2 (11.1) | 16 (88.9) |
| Female | 28 (60.9) | 8 (28.6) | 20 (71.4) | |
| Total | 46 (100.0) | 10 (21.7) | 36 (78.3) | |
| χ 2 = 1.963, df = 1, p = 0.161 | ||||
| Umuogbuagu | Male | 15 (28.8) | 10 (66.7) | 5 (33.3) |
| Female | 37 (71.2) | 22 (59.5) | 15 (40.5) | |
| Total | 52 (100.0) | 32 (61.5) | 20 (38.5) | |
| χ 2 = 0.234, df = 1, p = 0.628 | ||||
| Umuagama | Male | 21 (42.9) | 7 (33.3) | 14 (66.7) |
| Female | 28 (57.1) | 9 (32.1) | 19 (67.9) | |
| Total | 49 (100.0) | 16 (32.7) | 33 (67.3) | |
| χ 2 = 0.008, df = 1, p = 0.930 | ||||
| Total | Male | 71 (35.3) | 23 (32.4) | 48 (67.6) |
| Female | 130 (64.7) | 61 (46.9) | 69 (53.1) | |
| Total | 201 (100.0) | 84 (41.8) | 117 (58.2) | |
| χ 2 = 3.985, df = 1, p = 0.046 | ||||
Table 4:
Prevalence of LF in Igbo-Eze North LGA according to occupation of the participants in this study
| Communities | Total (%) | Occupation | Infected (%) | Non-Infected (%) |
|---|---|---|---|---|
| Aguibege | 1 (1.9) | None | 0 (0.0) | 1 (100.0) |
| 6 (11.1) | Student | 1 (16.7) | 5 (83.3) | |
| 8 (14.8) | Farmer/Fisherman* | 4 (50.0) | 4 (50.0) | |
| 5 (9.3) | Civil Servant | 1 (20.0) | 4 (80.0) | |
| 6 (11.1) | Artisan | 2 (33.3) | 4 (66.7) | |
| 28 (51.9) | Trader | 18 (64.3) | 10 (35.7) | |
| 54 (100.0) | Total | 26 (48.1) | 28 (51.9) | |
| χ 2 = 8.356, df = 5, p = 0.138 | ||||
| Umuopu | 4 (8.7) | None | 0 (0.0) | 4 (100.0) |
| 6 (13.0) | Student | 1 (16.7) | 5 (83.3) | |
| 6 (13.0) | Farmer/Fisherman * | 3 (50.0) | 3 (50.0) | |
| 6 (13.0) | Civil Servant | 1 (16.7) | 5 (83.3) | |
| 7 (15.2) | Artisan | 1 (14.3) | 6 (85.7) | |
| 17 (37.0) | Trader | 4 (23.5) | 13 (76.5) | |
| 46 (100.0) | Total | 10 (21.7) | 36 (78.3) | |
| χ 2 = 4.370, df = 5, p = 0.497 | ||||
| Umuogbuagu | 10 (19.2) | Student | 0 (0.0) | 10 (100.0) |
| 5 (9.6) | Farmer/Fisherman * | 3 (60.0) | 2 (40.0) | |
| 4 (7.7) | Civil Servant | 0 (0.0) | 4 (100.0) | |
| 5 (9.6) | Artisan | 3 (60.0) | 2 (40.0) | |
| 28 (53.8) | Trader | 26 (92.9) | 2 (7.1) | |
| 52 (100.0) | Total | 32 (61.5) | 20 (38.5) | |
| χ 2 = 34.014, df = 4, p < 0.0001 | ||||
| Umuagama | 3 (6.1) | None | 1 (33.3) | 2 (66.7) |
| 3 (6.1) | Student | 0 (0.0) | 3 (100.0) | |
| 10 (20.4) | Farmer/Fisherman * | 6 (60.0) | 4 (40.0) | |
| 2 (4.1) | Civil Servant | 0 (0.0) | 2 (100.0) | |
| 9 (18.4) | Artisan | 2 (22.8) | 7 (77.2) | |
| 22 (44.9) | Trader | 7 (31.8) | 15 (68.2) | |
| 49 (100.0) | Total | 16 (32.7) | 33 (67.3) | |
| χ 2 = 6.278, df = 5, p = 0.280 | ||||
| Total | 8 (4.0) | None | 1 (12.5) | 7 (87.5) |
| 25 (12.4) | Student | 2 (8.0) | 23 (92.0) | |
| 29 (14.4) | Farmer/Fisherman * | 16 (55.2) | 13 (44.8) | |
| 17 (8.5) | Civil Servant | 2 (11.8) | 15 (88.2) | |
| 27 (13.4) | Artisan | 8 (29.6) | 19 (70.4) | |
| 95 (47.3) | Trader | 55 (57.9) | 40 (42.1) | |
| 201 (100.0) | Total | 84 (41.8) | 117 (58.2) | |
| χ 2 = 34.760, df = 5, P < 0.0001 |
Farmers plant agricultural crops
Table 3:
Prevalence of LF in Igbo-Eze North LGA according to age of the participants in this study
| Communities | Total (%) | Age (Years) | Infected (%) | Non-Infected (%) |
|---|---|---|---|---|
| Aguibege | 1 (1.9) | 0 – 9 | 0 (0.0) | 1 (100.0) |
| 4 (7.4) | 10 – 19 | 0 (0.0) | 4 (100.0) | |
| 7 (13.0) | 20 – 29 | 2 (28.6) | 5 (71.4) | |
| 11 (20.4) | 30 – 39 | 3 (27.3) | 8 (72.7) | |
| 10 (18.5) | 40 – 49 | 4 (40.0) | 6 (60.0) | |
| 21 (38.9) | ≥ 50 | 17 (81.0) | 4 (19.0) | |
| 54 (100.0) | Total | 26 (48.1) | 28 (51.9) | |
| χ 2 = 16.955, df = 5, p = 0.005 | ||||
| Umuopu | 4 (8.7) | 0 – 9 | 0 (0.0) | 4 (100.0) |
| 6 (13.0) | 10 – 19 | 0 (0.0) | 6 (100.0) | |
| 6 (13.0) | 20 – 29 | 0 (0.0) | 6 (100.0) | |
| 6 (13.0) | 30 – 39 | 1 (16.7) | 5 (83.3) | |
| 9 (19.6) | 40 – 49 | 1 (11.1) | 8 (88.9) | |
| 15 (32.6) | ≥ 50 | 8 (53.3) | 7 (46.7) | |
| 46 (100.0) | Total | 10 (21.7) | 36 (78.3) | |
| χ 2 = 13.933, df = 5, p = 0.016 | ||||
| Umuogbuagu | 3 (5.8) | 0 – 9 | 0 (0.0) | 3 (100.0) |
| 6 (11.5) | 10 – 19 | 0 (0.0) | 6 (100.0) | |
| 4 (7.7) | 20 – 29 | 2 (50.0) | 2 (50.0) | |
| 8 (15.4) | 30 – 39 | 4 (50.0) | 4 (50.0) | |
| 13 (25.0) | 40 – 49 | 10 (76.9) | 3 (23.1) | |
| 18 (34.6) | ≥ 50 | 16 (88.9) | 2 (11.1) | |
| 52 (100.0) | Total | 32 (61.5) | 20 (38.5) | |
| χ 2 = 22.064, df = 5, p = 0.001 | ||||
| Umuagama | 1 (2.0) | 0 – 9 | 0 (0.0) | 1 (100.0) |
| 3 (6.1) | 10 – 19 | 0 (0.0) | 3 (100.0) | |
| 4 (8.2) | 20 – 29 | 2 (50.0) | 2 (50.0) | |
| 4 (8.2) | 30 – 39 | 2 (50.0) | 2 (50.0) | |
| 11 (22.4) | 40 – 49 | 4 (36.4) | 7 (63.6) | |
| 26 (53.1) | ≥ 50 | 8 (30.8) | 18 (69.2) | |
| 49 (100.0) | Total | 16 (32.7) | 33 (67.3) | |
| χ 2 = 3.145, df = 5, p = 0.678 | ||||
| Total | 9 (4.5) | 0 – 9 | 0 (0.0) | 9 (100.0) |
| 19 (9.5) | 10 – 19 | 0 (0.0) | 19 (100.0) | |
| 21 (10.4) | 20 – 29 | 6 (28.6) | 15 (71.4) | |
| 29 (14.4) | 30 – 39 | 10 (34.5) | 19 (65.5) | |
| 43 (21.4) | 40 – 49 | 19 (44.2) | 24 (55.8) | |
| 80 (39.8) | ≥ 50 | 49 (61.2) | 31 (38.8) | |
| 201 (100.0) | Total | 84 (41.8) | 117 (58.2) | |
| χ 2 = 34.802, df = 5, p < 0.0001 |
Risk factors for lymphatic filariasis in Igbo-Eze North LGA
The risk factors associated with LF in the study area included non-use of mosquito nets, unawareness of LF, visit to water bodies, and proximity of water bodies to house.
Discussion
The overall prevalence of LF was 41.8% in the present study. This is quite high unlike areas with low prevalence such as 6.1% among Yakurr people of Cross River State (15). Earlier works (16, 17) reported 6.5% and 5.5% in Benue State and in some rural communities of the Lower Cross River Basin respectively.
In addition, other studies of LF in Nigeria observed 18.8% prevalence in Aguata, Anambra State (18), 12.9% prevalence from central states of Plateau and Nassarawa States (19), 16.9% prevalence among the Ezza people of Ebonyi State (20), and 15.5% prevalence among Mbembe people of Cross River State (21). According to earlier reports, the populations at high risk for contracting LF infection are usually those that are poor, and concentrated mainly in rural areas (22,23). Also, significant variation in prevalence between communities could be attributed to differences on the socio-economic status, local environmental and ecological conditions that favor the breeding of the vectors (20,21). These factors explain probably the high prevalence recorded among the studied population who are mainly traders (95, 47.3%) and farmers (29, 14.4%) with low economic status (Table 4).
Sex-related prevalence in the present study implicated LF infection to be higher among females than males (Table 2). This is not consistent with earlier reports (15,24, 25) that there exists no statistically significant difference between both sexes. However, our study corroborates with other studies (4, 26, 27) which showed that females had more LF than the males. Sex-related differences are usually attributed to occupational differences between males and females. Majority of the participants were widowed women who are either traders or farmers. In relation to age, the present study found that prevalence of infection increased with age in both sexes. It is understandable that older individuals have been exposed throughout their lives and as such are more exposed to the vectors because of their occupations, mostly as farmers in the fields, and other adult occupations as most probable. Traders had highest prevalence (P < 0.05) of LF. The high prevalence among traders could be attributed to the fact that trading in the study area involves a complex process of purchasing of agricultural crops from farmers mostly in their farms at hinterlands, conveyance of these crops to some other villages with high demand for them, and prolonged outdoor stay due to market sales as well as possible late night return.
The risk associated with LF in the study area implicated lack of knowledge about LF, nonuse of mosquito nets and visit to water bodies as the major predispositions to LF. Others include close proximity of water body to houses and whether seen LF patient. It was observed that people with LF awareness are less likely to present with the disease. Likewise, those that do not visit water bodies. Partly compliance to bed net ownership and usage were associated with high LF prevalence, as against its adherence as an effective vector control tool, as shown in other studies (28–32). It is foreseeable that the scale-up of universal bed net coverage for malaria may lead to a wider reduction in LF transmission in the future.
Conclusion
The prevalence of LF in Igbo-Eze North Local Government Area was high with chances of scaled up prevalence and burden overtime. Prevalence differences were implicated to be location, sex, age and occupation-dependent. Concerted awareness campaigns on the cause of LF, mode of transmission, the relationship between infection and clinical signs/symptoms should be intensified to enable increased acceptance and support of its control programme in the area.
Acknowledgement
The supports of other research colleagues and ethical authorities during the course of this work is deeply acknowledged.
Footnotes
Conflict of Interest
The authors declare no conflict of interest whatsoever.
References
- 1.WHO . Meeting of the international task force for disease eradication—October 2010. Weekly Epidem Rec. 2011; 86: 53–59. [PubMed] [Google Scholar]
- 2.Sabesan S, Vanamail P, Raju KHK, et al. Lymphatic filariasis in India: epidemiology and control measures. J Postgrad Med. 2010; 56(3): 232–8. [DOI] [PubMed] [Google Scholar]
- 3.Arora DR, Arora BB. Medical Parasitology. Third Edition. CBS Publishers and Distributors, New Delhi, India, 2014. [Google Scholar]
- 4.Omudu EA Okafor FC. Rapid epidemiological and socio-cultural appraisal of lymphatic filariasis amongst the Igede ethnic group in Benue State, Nigeria. Nigerian J Para. 2007; 28(2): 118 – 124. [Google Scholar]
- 5.Krentel A, Fischer P, Manoempil P, et al. Using knowledge, attitudes and practice (KAP) surveys on lymphatic filariasis to prepare a health promotion campaign for mass drug administration in Alor District, Indonesia. Trop Med Int Health . 2006; 11(11): 1731 – 1740. [DOI] [PubMed] [Google Scholar]
- 6.Omudu EA. Sero-Epidemiological and Socio-Cultural Studies on Lymphatic Filariasis in Benue State, Nigeria. A Ph.D. Unpublished Thesis submitted to the Department of Zoology, Faculty of Biological Sciences, University of Nigeria, Nsukka, 2008. [Google Scholar]
- 7.Norões J, Addiss D, Amaral F, et al. Occurrence of living adult Wuchereria bancrofti in the scrotal area of men with microfilaraemia. Trans Roy Soc Trop Med Hyg. 1996; 90(1): 55–56. [DOI] [PubMed] [Google Scholar]
- 8.Chandrakala G, Zulfeen M. Filariasis in pregnancy: prevalent yet less-known global health burden. J Basic Clin Rep Sci. 2016; 5(2): 107 – 109. [Google Scholar]
- 9.Das N, Das N. Treatment of tropical diseases. World Sci News. 2016; 45(1): 61 – 62. [Google Scholar]
- 10.National Population Commission (2007). 2006 national population and housing census. Federal Republic of Nigeria Official Gazelle, Lagos, 15th May, volume 94, number 24. [Google Scholar]
- 11.Sarmukaddam S, Garad S. On validity of assumptions while determining sample size. Indian J Comm Med. 2004; 29: 87 – 91. [Google Scholar]
- 12.Nwoke BEB, Dozie INS, Jiya J, et al. The prevalence of hydrocoele in Nigeria and its implication on mapping of lymphatic filariasis. Nigerian J Para. 2006; 27: 29 – 35. [Google Scholar]
- 13.Cheesbrough M. District Laboratory Practice in Tropical Countries. Second Edition. Cambridge University Press, Cape Town, South Africa, 2006. [Google Scholar]
- 14.Nwosu CG, Ivoke N, Okoye IC, et al. Assessment of the obstetric, demographic and economic burden on falciparum-malaria parasitaemia among women of child-bearing age receiving antenatal services in Nsukka, Nigeria. Intl J Sci Tech. 2018; 6(2): 193 – 200. [Google Scholar]
- 15.Iboh CI, Okon OE, Opara KN, et al. Lymphatic filariasis among the Yakurr people of Cross River State, Nigeria. Parasites and Vectors. 2012; 5: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udoidung NI, Braide IE, Opara KN, et al. Current status of Bancroftian filariasis in rural communities of the Lower Cross River Basin, Nigeria: parasitological and clinical aspects. J Pub Health. 2008; 16: 383 – 388. [Google Scholar]
- 17.Targema CN, Onwuliri CO, Mafuya HB, et al. Mapping of lymphatic filariasis in Benue State, Nigeria. Nigerian J Para. 2008; 29(1): 55 – 60. [Google Scholar]
- 18.Mbah DC, Njoku OO. Prevalence of lymphatic filariasis in Oraeri, Aguata Local Government Area of Anambra State, Nigeria. Nigerian J Para. 2002; 21: 95 – 102. [Google Scholar]
- 19.Abel E, Frank OR, Blaney DD, et al. Rapid assessment for lymphatic filariasis in central Nigeria: a comparison of the immunochromatographic and test and hydrocele rates in an area of high endemicity. Am J Trop Med Hyg. 2003; 68(6): 643 – 646. [PubMed] [Google Scholar]
- 20.Anosike JC, Nwoke BEB, Ajayi EG, et al. Lymphatic filariasis among Ezza people of Ebonyi State, Eastern Nigeria. Ann Agric Environ Med. 2005; 12: 181 – 186. [PubMed] [Google Scholar]
- 21.Okon OE, Iboh CI, Opara KN. Bancroftian filariasis among the Mbembe people of Cross River State, Nigeria. J Vector Borne Dis. 2010; 47: 91 – 96. [PubMed] [Google Scholar]
- 22.Terranella A, Eigiege A, Gontor I, et al. Urban lymphatic filariasis in central Nigeria. Ann Trop Med Parasitol. 2006; 100: 163 – 172. [DOI] [PubMed] [Google Scholar]
- 23.Christiana O, Olajumoke M, Oyetunde S. Lymphatic filariasis and associated morbidities in rural communities of Ogun State, Southwestern Nigeria. Travel Med Infect Dis. 2014; 12(1): 95 – 101. [DOI] [PubMed] [Google Scholar]
- 24.Adekunle NO, Sam-Wobo SO, Adeleke MA, et al. Prevalence and distribution of Wucheria bancrofti in Osse Local Government Area, Ondo State. Nigerian J Para. 2016; 37(1): 96 – 100. [Google Scholar]
- 25.Elkanah OS, Elkanah DS, Wahedi JA, et al. Lymphatic filariasis in Muri Emirate: clinical and parasitological studies in Jalingo LGA, Taraba State, Nigeria. Asian J Med Health. 2017; 6(1): 1 – 7. [Google Scholar]
- 26.Dogara MM, Nock HI, Agbede RIS, et al. Prevalence of lymphatic filariasis in three villages in Kano State, Nigeria. Internet J Trop Med. 2012; 8: 1. [Google Scholar]
- 27.Okorie PN, Davies E, Ogunmola OO, et al. Lymphatic filariasis baseline survey in two sentinel sites of Ogun state, Nigeria. Pan Afr Med J. 2015; 20: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Berg H, Kelly-Hope LA, Lindsay SW. Malaria and lymphatic filariasis: the case for integrated vector management. Lancet Infect Dis. 2013; 13: 89 – 94. [DOI] [PubMed] [Google Scholar]
- 29.Richards FO, Emukah E, Graves PM, et al. Community-wide distribution of long-lasting insecticidal nets can halt transmission of lymphatic filariasis in southeastern Nigeria. Am J Trop Med Hyg. 2013; 89: 578 – 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebollo MP, Sambou SM, Thomas B, et al. Elimination of lymphatic filariasis in the Gambia. PLoS Negl Trop Dis. 2015; 9: e0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nsakashalo-Senkwe M, Mwase E, Chizema-Kawesha E, et al. Significant decline in lymphatic filariasis associated with nationwide scale-up of insecticide treated nets in Zambia. Parasite Epidemiol Control. 2017; 2(4): 7 – 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brant TA, Okorie PN, Ogunmola O, et al. Integrated risk mapping and landscape characterization of lymphatic filariasis and loaiasis in South West Nigeria. Parasite Epidemiol Control. 2017; 3: 21 – 35. [DOI] [PMC free article] [PubMed] [Google Scholar]