Abstract
Background
Peripartum cardiomyopathy (PPCM) disproportionately affects women of African ancestry. Additionally, clinical outcomes are worse in this subpopulation compared to White women with PPCM. The extent to which socioeconomic parameters contribute to these racial disparities is not known.
Methods
We aimed to quantify the association between area-based proxies of socioeconomic status (SES) and clinical outcomes in PPCM, and to determine the potential contribution of these factors to racial disparities in outcomes. A retrospective cohort study was performed at the University of Pennsylvania Health System, a tertiary referral center serving a population with a high proportion of Black individuals. The cohort included 220 women with PPCM, 55% of whom were Black or African American. Available data included clinical and demographic characteristics as well as residential address georeferenced to US Census-derived block group measures of SES. Rates of sustained cardiac dysfunction (defined as persistent LVEF <50%, LVAD placement, transplant, or death) were compared by race and block group-level measures of SES, and a composite neighborhood concentrated disadvantage index (NDI). The contributions of area-based socioeconomic parameters to the association between race and sustained cardiac dysfunction were quantified.
Results
Black race and higher NDI were both independently associated with sustained cardiac dysfunction (relative risk [RR] 1.63, confidence interval [CI] 1.13–2.36; and RR 1.29, CI 1.08–1.53, respectively). Following multivariable adjustment, effect size for NDI remained statistically significant, but effect size for Black race did not. The impact of low neighborhood education on racial disparities in outcomes was stronger than that of low neighborhood income (explaining 45% and 0% of the association with black race, respectively). After multivariate adjustment, only low area-based education persisted as significantly correlating with sustained cardiac dysfunction (RR 1.49; CI 1.02–2.17).
Conclusions
Both Black race and NDI independently associate with adverse outcomes in women with PPCM in a single center study. Of the specific components of NDI, neighborhood low education was most strongly associated with clinical outcome and partially explained differences in race. These results suggest interventions targeting social determinants of health in disadvantaged communities may help to mitigate outcome disparities.
Peripartum cardiomyopathy (PPCM) is a sometimes severe form of cardiomyopathy occurring toward the end of pregnancy or months postpartum in women with previously normal hearts.1,2 The estimated incidence of PPCM is 1 in 2,000 pregnant women worldwide, with hotspots such as Haiti and Nigeria.1,3 In the U.S., the prevalence of PPCM is higher in Black women than White women.4,5 Clinical outcomes in PPCM vary widely, with most women ultimately recovering cardiac function, but a significant minority of women developing worsening function, need for LVAD or cardiac transplant, or death.1,3 Again, Black women fare worse than White women: they are generally diagnosed later in the postpartum period and, when diagnosed, are less likely to have recovery of left ventricular (LV) function and more likely to experience adverse events compared to White women.6,7 We previously described the outcomes of 220 women with PPCM diagnosed and treated within the University of Pennsylvania Health System (Penn) and demonstrated that Black women were half as likely to recover, and took twice as long to do so when they did recover.6
The reasons for differences in clinical outcomes between Black and White women with PPCM remain unclear, and may be related to underlying biology, genetics, or a complex interplay of individual and social factors. Social determinants of health impact the development of cardiovascular (CV) risk factors and disease, health behaviors, treatment patterns, and clinical outcomes in complex ways. Neighborhood disadvantage, for example, is associated with incident heart failure and risk of readmission, in addition to individual race/ethnicity and socioeconomic status.8–10 Neighborhood characteristics and associations with outcomes in PPCM have not previously been examined.
We therefore sought to determine: (1) if neighborhood level social determinants impact clinical outcomes in PPCM; and (2) to what extent the poor outcomes observed in Black women with PPCM may be attributed to neighborhood disadvantage. To do so, we leveraged our large, racially diverse cohort of PPCM patients, the majority of whom live in Philadelphia, a large city with noted differences in access to care and clinical outcomes based on race.11 We further integrated our cohort with a spatially enabled data set to understand how neighborhood disadvantage impacts clinical outcomes in PPCM, and interacts with racial background.
Methods
The University of Pennsylvania Institutional Review Board approved the study and determined that informed consent was not required. We assembled a retrospective cohort of patients at Penn with a diagnosis of PPCM from January 1, 1986, through December 31, 2016 based on diagnostic codes (International Classification of Diseases, Ninth Revision codes 674.50–54) or echocardiographic evaluations obtained within 6 months of delivery.6 Patients with a history of congenital heart disease, valvular disease predating their PPCM diagnosis, history of radiation or cardiotoxic chemotherapy, or another explanation for their heart failure were excluded.
We abstracted demographic details, residential addresses, diagnosis, and clinical outcome information from the patient electronic medical records. We used ArcGIS Pro (Esri, Redlands, CA) to geocode addresses and identify corresponding Census block groups. A Census block group is the smallest geographic unit (average 1,500 people) in which sample data from decennial census and the American Community Survey are available. Using block groups as a proxy for neighborhoods, we spatiotemporally integrated a select set of variables from the 1990, 2000, and 2010 Census as well as the 2009 through the 2016 American Community Surveys.12 A composite neighborhood concentrated disadvantage index (NDI) was computed for each patient as the sum of the proportions of unemployment, households receiving public assistance, persons below the federal poverty line, adults without a high school diploma, female-headed households, renter-occupied residences, and residential tenure shorter than 1 year.13 Each of the components of the NDI were dichotomized at the threshold of their highest quartile for statistical analyses. Sustained cardiac dysfunction was defined as persistent LVEF <50%, cardiac transplant, LVAD placement, or death. Myocardial recovery was defined as at least one occasion of LVEF >50% on echocardiography.
Distributions of patient characteristics were compared by quartiles of block group level factors using χ2 or Fisher exact tests. We examined the potential for a non-linear association between NDI and persistent cardiomyopathy with restricted cubic splines.14 We examined the cardiac outcomes (sustained cardiac dysfunction, and myocardial recovery) relative to NDI, and then separately to each component informing the NDI. Crude and multivariate log binomial regression models were used to estimate risk ratios (RR) and corresponding 95% confidence intervals (95% CI). Multivariable models were adjusted first for patient characteristics that were associated with the area-based exposures, or race. The proportional contribution of each area-based socioeconomic parameters to the effect of race on cardiac outcomes was quantified.15
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. Sources of funding: ZA was supported by the Department of Defense (DOD W81XWH18–1-0503) and NIH (HL126797). KG was supported by the NIH (HL143153). JL was supported by the NIH (HL153667).
Results
The cohort of 220 women diagnosed with PPCM consisted of 121 Black women and 99 non-Black women. Demographic and clinical characteristics at presentation and clinical outcomes of this cohort have been described previously.6 Figures 1 and S1 present the relational geographic distribution of the patients to the greater Philadelphia Metropolitan Area. Neighborhoods with greater disadvantage are concentrated in Philadelphia, Camden, and Trenton.
Figure 1.
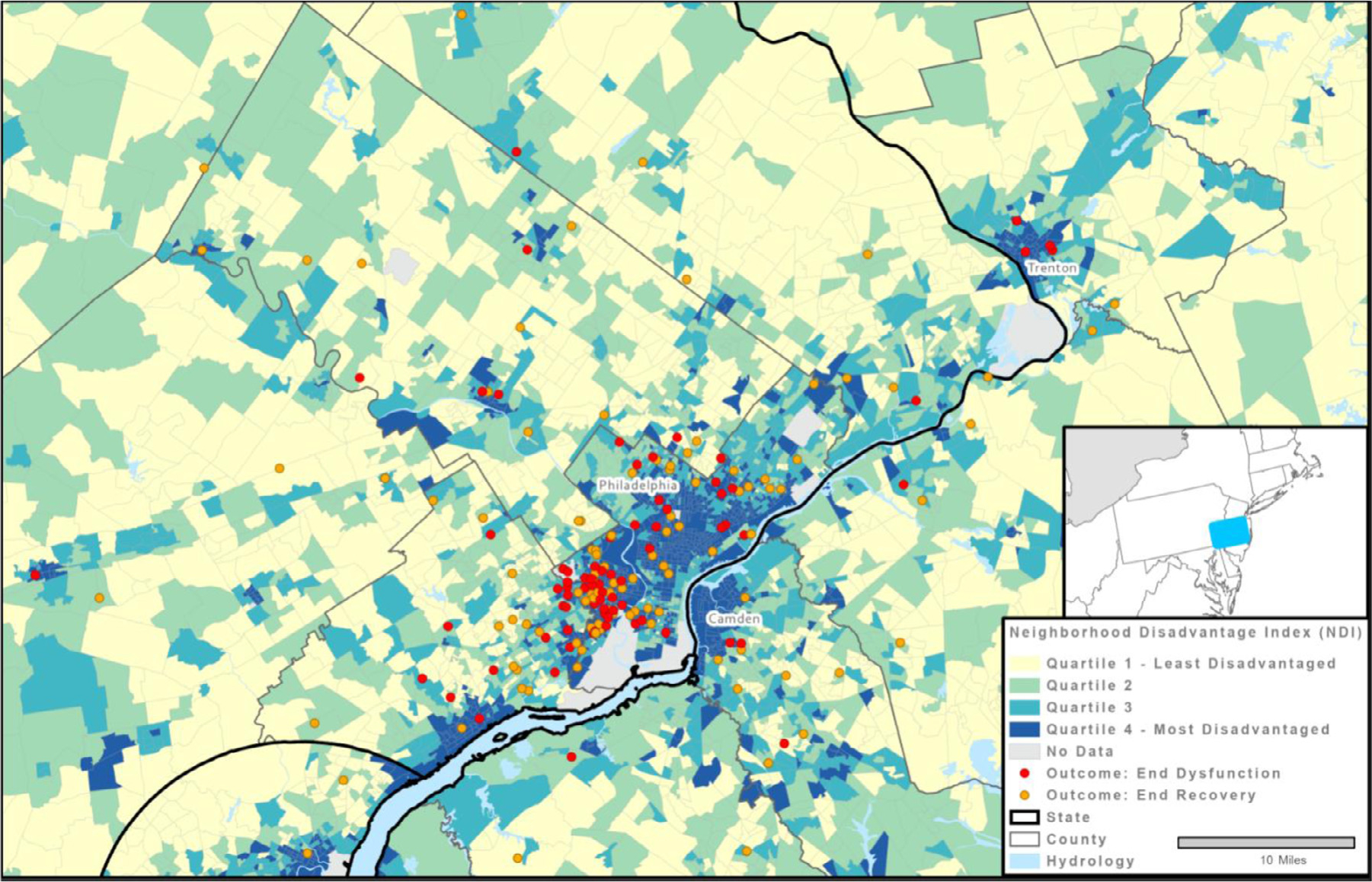
Relational geographic distribution of PPCM patients to the greater Philadelphia Metropolitan Area, distinguishing between patients who recovered (in yellow) versus those who did not (in red), and stratified by neighborhood disadvantage index (NDI). To protect the privacy of the patients, their locations were spatially anonymized using areal filters and random perturbation.
Table S1 provides the demographics and clinical characteristics for the study population overall, and subdivided by quartiles of NDI, median household income, and proportion of residents with less than high school education, according to block group. Patients in neighborhoods with greater disadvantage were younger (28 years in quartile 4 (Q4) vs. 32 years in quartile 1 (Q1), P = .002), and more likely to be Black (89% in Q4 vs 16% in Q1, P < .001). Patients from more disadvantaged neighborhoods tended to have a lower nadir LVEF compared to those from less disadvantaged neighborhoods.
Overall, 36.3% (n = 81) of patients experienced sustained dysfunction. Both Black race (44% vs 28%; RR 1.63, 95% CI 1.13–2.36) and higher NDI (RR 1.29, 95% CI 1.08–2.53) were independently associated with sustained cardiac dysfunction (Table I). Restricted cubic spline regression revealed that the association between NDI and sustained dysfunction was linear (P value for test for curvature: 0.440; overall significance of linear trend 0.004; Figure 2). Every unit increase in NDI was associated with a 29% higher likelihood of sustained cardiac dysfunction. Effect estimates for both exposures were attenuated following adjustment for age, twin pregnancy, number of children, and timing of diagnosis, but only NDI remained statistically significant (Table I, M1)
Table I.
| Crude RR (95% CI) | p-value | Adjusted RR (M1) | p-value | Adjusted RR (M2) | p-value | Adjusted RR (M3) | p-value | Adjusted RR (M4) | p-value | Adjusted RR (M5) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black race | 1.63 (1.13, 2.36) |
0.009 | 1.34 (0.88, 2.03) |
0.171 | 1.19 (0.74,1.92) |
0.474 | 1.17 (0.76, 1.81) |
0.419 | 1.18 (0.74,1.78) |
0.527 | 1.34 (0.87, 2.06) |
0.185 |
| Neighborhood concentrated disadvantage index | 1.29 (1.08,1.53) |
0.004 | 1.23 (1.00,1.51) |
0.021 | 1.17 (0.91,1.49) |
0.217 | ||||||
|
| ||||||||||||
| Assessments for the Individual Components of the Neighborhood Concentrated Disadvantage Indexa | ||||||||||||
| >22.3% adults with less than HS education | 1.73 (1.25, 2.39) |
<0.001 | 1.57 (1.10,2.24) |
0.014 | 1.49 (1.02,2.17) |
0.040 | ||||||
| >31.8% Renter Occupied Housing | 1.70 (1.20, 2.42) |
0.003 | 1.57 (1.07,2.30) |
0.020 | 1.50 (1.00, 2.25) |
0.049 | ||||||
| >28.5% HH w. annual income below poverty line | 1.23 (0.86,1.77) |
0.265 | 1.09 (0.75,1.60) |
0.647 | 1.00 (0.68,1.46) |
0.999 | ||||||
| >31.5% Female Headed Households | 1.23 (0.86,1.77) |
0.264 | 1.00 (0.68,1.47) |
0.991 | 0.95 (0.65,1.39) |
0.946 | ||||||
| >16.3% adults unemployed | 1.14 (0.78,1.67) |
0.482 | 0.98 (0.66,1.45) |
0.915 | 0.90 (0.60,1.33) |
0.585 | ||||||
| >10.2% adults on public assistance | 1.10 (0.75,1.62) |
0.621 | 0.96 (0.65,1.42) |
0.827 | 0.90 (0.60,1.34) |
0.900 | ||||||
| >28.4% with residential tenure less than 1 year | 1.11 (0.76,1.63) |
0.576 | 1.08 (0.74,1.59) |
0.682 | 1.10 (0.74,1.64) |
0.616 | ||||||
Each of the block group-level components of the neighborhood disadvantage index were dichotomized at highest quartile for statistical analyses (i.e. Q4 vs Q1-Q3). The numeric thresholds presented for each of the NDI components represent the cutoff value defining the highest quartile.
M1 = adjusted for age, twin pregnancy, number of children, and timing of diagnosis
M2 = race model adjusted for age, twin pregnancy, number of children, timing of diagnosis, and neighborhood concentrated disadvantage index (NDI); Models for individal NDI component area-based exposures adjusted for age, twin pregnancy, number of children, timining of diagnosis, and race
M3 = race model adjusted for age, twin pregnancy, number of children, timing of diagnosis, and neighborhood-based low education
M4 = race model adjusted for age, twin pregnancy, number of children, timing of diagnosis, and neighborhood-based high renter occupied housing
M5 = race model adjusted for age, twin pregnancy, number of children, timing of diagnosis, and neighborhood-based high poverty
Figure 2.
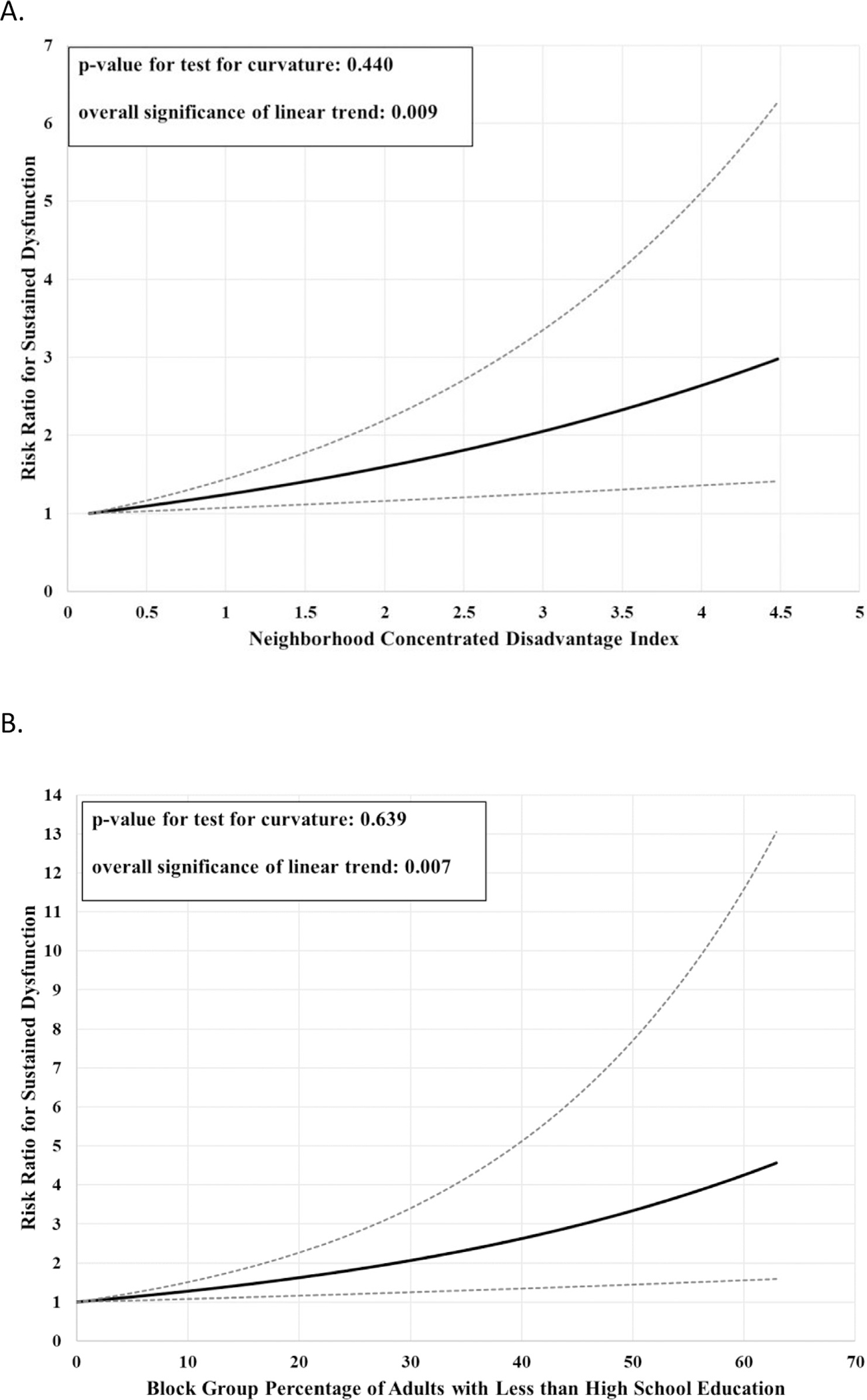
Linear relationships between (A) neighborhood-concentrated disadvantage index and occurrence of sustained cardiac dysfunction, and (B) proportion of adults in block group with less than a high school education and occurrence of sustained cardiac dysfunction.
Of the individual components of NDI, only area-based low education and high rental occupied housing were significantly associated with sustained cardiac dysfunction (Table I, first column), which persisted after adjusting for race (Table I, M2). Other components of NDI had adjusted risk ratios near null (Table I, M2). The effect estimate for race on sustained dysfunction was largely attenuated upon control for area-based low education: in fact, low education explained approximately 45% of the effect of race on sustained dysfunction (Table I, M3 vs M2). In contrast, control for area-based low income had no impact on the effect estimate for race on sustained dysfunction (Table I, M5 vs M1). Overall comparable results were observed for myocardial recovery (Supplemental Table S2).
Discussion
We find here, in a large racially diverse cohort of women with PPCM, that the markedly worse average clinical outcome of Black women may be explained in large part by greater neighborhood disadvantage, specifically lower educational status at the block group level. The findings underscore the importance of social determinants of health as potential drivers of racial disparities in PPCM. Specifically, lower education status appears to impact PPCM outcomes independent of race, and may do so by creating barriers to timely access to high-quality medical care, communication with medical providers, understanding of symptoms, medication adherence, and self-advocacy, especially in the context of structural racism. Race and socioeconomic class are highly correlated in the U.S. Racial disparities in maternal health have been documented independent of education of the individual.16 The experiences of racism impact the quality of care delivered, patient care engagement, and communication between health care providers and patients. Women from lower SES groups report higher rates of perceived discrimination based on race and insurance status, which may further adversely impact maternal health outcomes.17, 18
Despite being the largest mixed-race PPCM cohort reported to date, limitations of our study include being single-center with variable duration of follow-up, which reduces generalizability. Due to relatively small numbers, the analyses do not include Latino or Asian women. Additionally, data were assessed only at the block group level, rather than individual level. Although area-based measures of socioeconomic status may serve as crude proxies to individual SES, they may also have distinct independent and synergistic impacts on PPCM outcomes. Nevertheless, the NDI and component area-based exposures examined do not account for psychological distress, food insecurity, or delays in medical care, all of which may directly impact outcomes for women with PPCM. Data on the use of in vitro fertilization, or on medical treatments for PPCM including type, intensity, or duration were not captured in the manual chart abstractions completed for the cohort and thus are not accounted for in the presented analyses.
In summary, our finding that low area-based education was associated with worse outcomes in women with PPCM suggests a potential role for strategies aimed at improving access to care, patient-provider communication, and in particular health education in mitigating such disparities.
Supplementary Material
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation 2016;133:1397–409. [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–241. [DOI] [PubMed] [Google Scholar]
- 3.Davis MB, Arany Z, McNamara DM, et al. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:207–21. [DOI] [PubMed] [Google Scholar]
- 4.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol 2012;120:1013–19. [DOI] [PubMed] [Google Scholar]
- 5.Brar SS, Khan SS, Sandhu GK, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 2007;100:302–4. [DOI] [PubMed] [Google Scholar]
- 6.Irizarry OC, Levine LD, Lewey J, et al. Comparison of Clinical Characteristics and Outcomes of Peripartum Cardiomyopathy Between African American and Non-African American Women. JAMA Cardiol. 2017;2:1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail 2013;19:214–18. [DOI] [PubMed] [Google Scholar]
- 8.Akwo EA, Kabagambe EK, Harrell FE, et al. Neighborhood deprivation predicts heart failure risk in a low-income population of Blacks and Whites in the Southeastern United States. Circ Cardiovasc Qual Outcomes 2018;11:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikdeli B, Wayda B, Bao H, et al. Place of residence and outcomes of patients with heart failure: analysis from the telemonitoring to improve heart failure outcomes trial. Circ Cardiovasc Qual Outcomes 2014;7:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foraker RE, Rose KM, Suchindran CM, et al. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: atherosclerosis risk in communities cohort (1987 to 2004). Circ Heart Fail 2011;4:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown EJ, Polsky D, Barbu CM, et al. Racial disparities in geographic access to primary care in Philadelphia. Health Aff (Millwood) 2016;35:1374–81. [DOI] [PubMed] [Google Scholar]
- 12.Bureau, U.C. American Community Survey. https://www.census.gov/programs-surveys/acs/news/data-releases/2017.html, 2017, Accessed March 8, 2021.
- 13.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000;126:309–37. [DOI] [PubMed] [Google Scholar]
- 14.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 15.MacKinnon DP. Introduction to statistical mediation analysis. New York, NY: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- 16.Petersen EE, Davis NL, Goodman D, et al. Racial/ethnic disparities in pregnancy-related deaths—United States, 2007–2016. MMWR Morb Mortal Wkly Rep 2019;68:762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- 18.Gadson A, Akpovi E, Mehta PK. Exploring the social determinants of racial/ethnic disparities in prenatal care utilization and maternal outcome. Semin Perinatol 2017;41:308–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


