Abstract
Background
Venous thromboembolism (VTE) is a common complication in acute COVID-19 and those with hematologic malignancy (HM) may be at an even higher risk. We performed a retrospective analysis of patients with history of HM and acute COVID-19 to evaluate thrombotic and clinical outcomes.
Methods
Patients with COVID-19 were identified by positive SARS-CoV-2 PCR test. Our primary endpoints were rate of VTE and CVA in patients with HM compared to the general population (GP). Secondary outcomes included composite thrombotic events (CVA + VTE), COVID-19 fatality, respiratory support, ICU admission rates, and length of ICU stay
Results
A total of 833 patients were evaluated, 709 in the GP cohort, 124 patients in the HM cohort. CVA was more prevalent in the HM cohort (5.4% vs. 1.6%, P = .011). Rates of VTE were numerically higher for the HM cohort (8.0% vs. 3.6%, P = .069). The composite thrombotic rate was increased in the HM cohort (13.4% vs. 5.2%, P = .005). Patients with HM had a higher inpatient fatality rate (35.5% vs. 11.3%, P < .001), required more respiratory support (74.6% vs. 46.5%, P < .001) and had a higher rate of ICU admission (31.9% vs. 12.1%, P = .001).
Conclusion
Our data demonstrated an increased rate of composite thrombotic (CVA + VTE) outcomes, indicating HM patients with acute COVID-19 are at increased risk of thrombosis. Irrespective of disease status, HM patients also have significantly increased need for intensive care, respiratory support, and have higher fatality rates.
Keywords: Cerebrovascular accident (CVA), Outcomes research, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 or COVID-19), Venous thromboembolism (VTE), Hematologic malignancy (HM)
Micro-Abstract
Background: We performed a retrospective analysis of patients with history of HM and acute COVID-19 to evaluate thrombotic and clinical outcomes., Methods: We evaluated various clinical parameters in patients with HM (n = 124) compared to the general population (GP; n = 709)., Results: The composite thrombotic rate (VTE + CVA) was increased in the HM cohort (13.4% vs. 5.2%, P = .005)., Conclusion: HM patients with acute COVID-19 are at increased risk of thrombosis and have significantly increased need for intensive care, respiratory support, and have higher fatality rates.
Introduction
Individuals with COVID-19 can have complex coagulation abnormalities leading to a hypercoagulable state and thrombosis. Similarly, hematologic malignancy (HM) is a risk factor for venous thromboembolism (VTE).1 Patients with HM and COVID-19 have been shown to have increased mortality compared to the general population with acute COVID-19,2 but the driving pathophysiologic mechanism has yet to be elucidated.
The pathogenesis of hypercoagulability in COVID-19 is incompletely understood but has been postulated similar to Virchow's triad: Endothelial injury, stasis, and hypercoagulability. There has been evidence of direct invasion of endothelial cells by SARS-CoV-2, potentially leading to cell injury. Endothelial injury, endothelial exocytosis, and endothelitis are believed to play a central role in the pathogenesis of acute respiratory distress syndrome and organ failure in severe COVID-19.3, 4, 5 In vitro testing has shown the SARS-CoV-2 spike protein (subunit 1 and 2) activates the alternative pathway of complement, which may explain various clinical manifestations such as microangiopathy and thrombophilia.6 Stasis, a common trigger for all hospitalized or critically ill patients regardless of COVID-19, is a well-described contributor to the development of venous thromboembolism. Finally, various prothrombotic factors such as elevated factor VIII, elevated fibrinogen, circulating prothrombotic microparticles, neutrophil extracellular traps, and hyperviscosity have been reported or proposed in patients with severe COVID-19. Laboratory abnormalities show normal to slightly prolonged PT/aPTT, normal to increased platelet count, and elevated fibrinogen and D-dimer.7, 8, 9 This phenotypic hypercoagulable state has been termed “COVID-19 associated coagulopathy or thromboinflammation.”10
Patients with hematologic malignancy are a vulnerable group. Recent publications report inferior outcomes compared to the general population with acute COVID-19 and inferior vaccination responses in hematologic malignancy patients.11 , 12 As variant strains continue to drive hospitalization and death from COVID-19 across the globe,13 more information is needed to optimize care for these patients. The purpose of this study was to assess thrombotic complications and overall outcomes in patients with a history of HM and COVID-19. We hypothesized patients with HM and COVID-19 would have an increased incidence of thromboembolism, more severe infection, and overall inferior outcomes compared to the general population.
Methods
Patients with confirmed COVID-19 were identified via the MedStar electronic health record at MedStar Georgetown University Hospital and/or Washington Hospital Center by positive SARS-CoV-2 PCR test, documented in an emergency department (ED) encounter or inpatient admission. From March 2020 to May 2020 patient characteristics and clinical course were abstracted and entered into a REDCap database, from which the initial HM cohort and general population (GP) cohort was extracted.14 , 15 Due to initial small sample size of HM patients, the cohort was expanded to include patients from any MedStar hospital that presented with COVID-19 from March 2020 to April 2021, using ICD-10 codes for any HM and acute COVID-19. Patients were excluded if the COVID-19 testing was deemed incidental and unrelated to their visit to the ED or hospitalization. If a patient had multiple encounters for a single COVID-19 infection (eg, multiple ED visits without admission) the first encounter or the encounter leading to the highest level of care was selected for chart review.
Variables collected for all patients included demographic information (age, race, sex, BMI, history of VTE prior to COVID-19) and clinical information from the encounter related to COVID-19 (admission and/or discharge dates, laboratory values, new VTE/CVA, inpatient respiratory support, ICU admission, ICU days, ventilator days, and death). For the HM cohort, additional information was collected regarding the type of HM, treatment and remission status of the cancer, date of last clinical follow-up or date of death.
The primary endpoints were the rate of VTE and cerebrovascular accident (CVA) in the HM cohort compared to the GP cohort. Secondary outcomes included composite thrombotic events (VTE + CVA), COVID-19 related mortality, Intensive Care Unit (ICU) admission rates, length of ICU stay and/or ventilator requirements, and complete blood count and/or LDH levels at presentation. Within the HM cohort, we sought to compare primary and secondary outcomes between subpopulations of patients dictated by treatment status (active vs. maintenance and/or surveillance) and remission status (complete remission vs. any other remission status documented).
Continuous variables without a normal distribution were log-transformed prior to analysis. Descriptive statistics were presented using mean and standard deviation for normally distributed continuous variables. Median and IQR were calculated for non-normal continuous variables. Categorical variables were presented using frequency and percentages. Comparison between groups for continuous variables was conducted using T-test for normally distributed variables, and Kruskal Wallis test for non-normal variables. Association between categorical variables was tested using Fisher's exact test.
Odds ratios (OR) and age-adjusted odds ratios comparing the HM cohort to the GP cohort were calculated and shown along with 95% confidence intervals and associated P values. Kaplan-Meier survival curves were plotted to make comparisons within subpopulations of the HM cohort. The hazard ratios and age-adjusted hazard ratios were calculated using the Cox Proportional Hazard model. The R statistical package was used for all data analysis. (R version 4.0.5).16
Results
A total of 833 patients with COVID-19 were evaluated, 709 in the GP cohort and 124 patients in the HM cohort. Demographic data for each cohort is depicted in Table 1 . Statistical differences between the groups were seen for age (69.4 vs. 53.95, P < .001), race (P < .001), and rate of historical VTE prior to COVID-19 (15.4% vs. 4.2%, P < .001).
Table 1.
Baseline Demographics
| General Population(n = 709) | Hematologic Malignancy (n = 124) | P value | |
|---|---|---|---|
| Age (mean [SD]) | 53.95 (16.81) | 69.40 (14.80) | <.001 |
| Sex, % (n) | .496 | ||
| Female | 51.2% (363) | 47.6% (59) | |
| Male | 48.8% (346) | 52.4% (65) | |
| Race, % (n) | <.001 | ||
| African American | 60.9% (432) | 59.7% (74) | |
| Hispanic/Latino | 29.3% (208) | 2.4% (3) | |
| White | 6.3% (45) | 26.6% (33) | |
| Unknown/Other | 3.4% (24) | 11.3% (14) | |
| BMI (median [IQR]) | 29.4 [26.1, 34.7] | 27.66 [24.84, 33.85] | .047 |
| History VTE | 4.2% (30) | 15.4% (19) | <.001 |
Abbreviations: IQR = interquartile range; SD = standard deviation; VTE = venous thromboembolism.
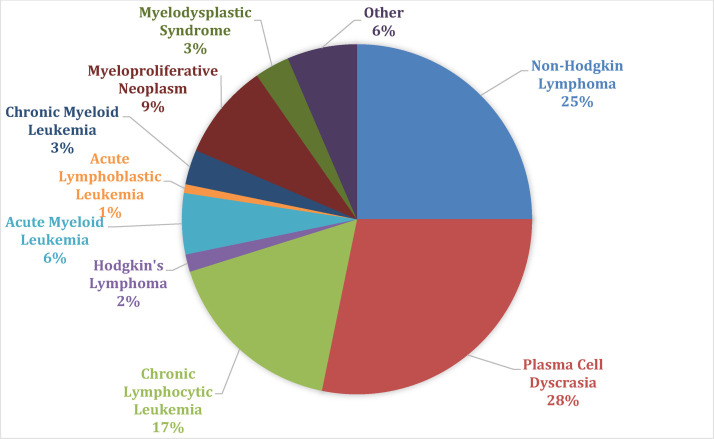
The most common HMs in the cohort were plasma cell dyscrasia (Figure 1: 28.2%, n = 35), non-hodgkin lymphoma (25%, n = 31), and chronic lymphocytic leukemia (16.9%, n = 21). Of those who had an anti-neoplastic treatment status documented (89.5%, n = 111), 60.4% of the patients were in surveillance (n = 67), 35.1% (n = 39) on active treatment, and 4.5% (n = 5) on maintenance therapy at time of COVID-19. Within the HM cohort, 57.3% (n = 71) had a remission status documented. Of that group, 25.4% of patients had a complete remission (CR; n = 18) documented at the time of COVID-19, 18.3% with partial remission (n = 13), 43.7% with stable disease (n = 31) and 12.7% with progressive disease (n = 9). 31.5% (n = 39) of patients either did not yet have a remission status evaluated or were treatment naïve. The remaining patients’ remission (n = 14) and treatment status (n = 13) were unknown.
Figure 1.
Hematologic malignancy cohort classification.
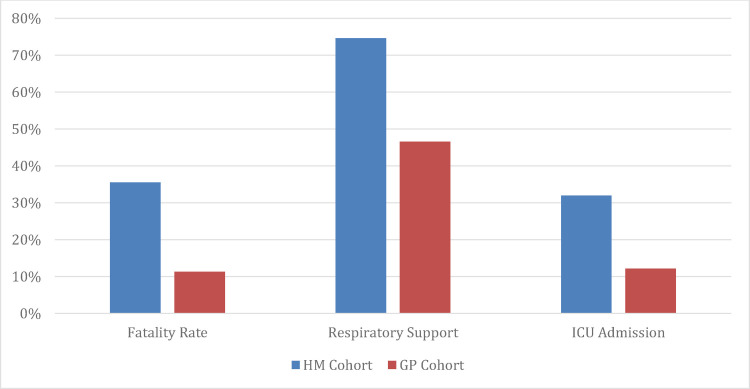
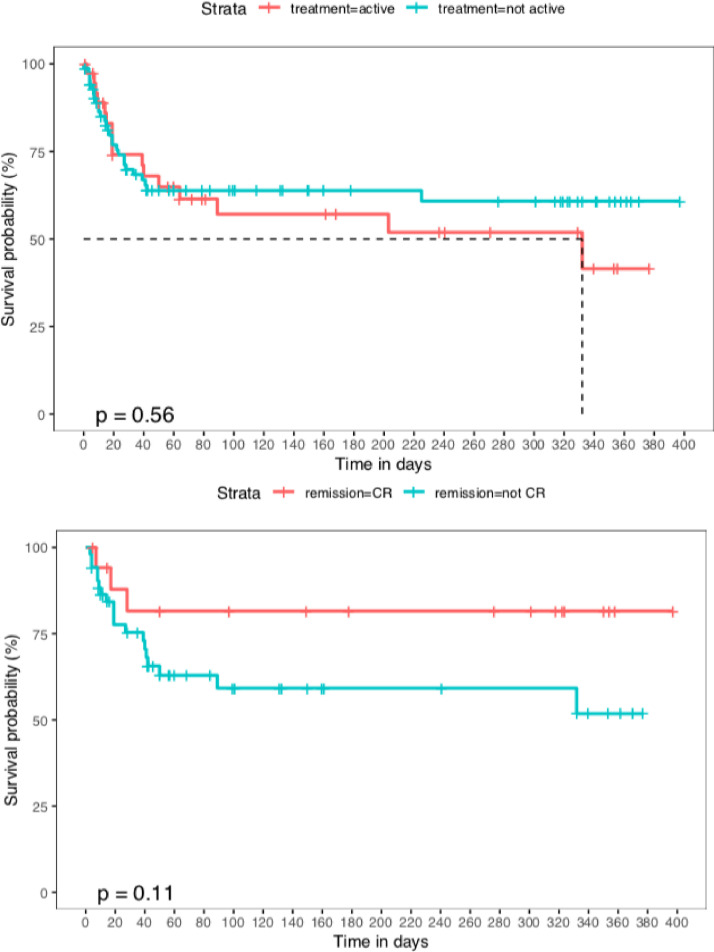
Primary and secondary outcome data is shown in Table 2 . CVA occurred in 5.4% of the HM cohort, compared to 1.6% of the GP cohort (P = .011). VTE incidence was 8.0% in the HM cohort vs. 3.6% in the GP cohort (P = .069). A composite thrombotic rate combining CVA and VTE was 13.4% in the HM cohort, compared to 5.2% for the GP cohort (P = .005). Patients with HM had an inpatient fatality rate of 35.5% (n = 44) compared to 11.3% (n = 80) in the GP cohort, P < .001 (Figure 2 ). A total of 74.6% of the HM cohort required respiratory support (GP cohort: 46.5%, P < .001), and 31.9% of this group required ICU admission (GP cohort: 12.1%, P = .001). Patients had a median stay in the Intensive Care Unit (ICU) of 6 vs. 5 days (P = .192), and duration of ventilator requirement of 8 vs. 6 days (P = .326), for the HM cohort compared to the GP, respectively. Within the HM cohort, there were no differences noted in overall survival (Figure 3 ) or the primary and secondary outcome measures for patients in CR compared to another remission status (supplement, Table 1), or those on active treatment compared to maintenance treatment and/or active surveillance (supplement, Table 2). With regards to baseline presentation lab work, the median absolute lymphocyte count was 1.1 vs. 1.15 (P = .900) and median LDH was 318 vs. 358 (P = .116) in the 2two populations (supplement, Table 3 ).
Table 2.
Primary and Secondary Outcomes
| General Population (n = 709) | Hematologic Malignancy (n = 124) | P value | |
|---|---|---|---|
| Venous Thromboembolism (VTE) | .069 | ||
| Yes | 3.6% (16) | 8.0% (9) | |
| No | 96.4% (428) | 92% (103) | |
| Cerebrovascular Accident (CVA) | .011 | ||
| Yes | 1.6% (7) | 5.4% (6) | |
| No | 98.4% (437) | 93.8% (105) | |
| Composite VTE/CVA | .005 | ||
| Yes | 5.2% (23) | 13.4% (15) | |
| No | 94.8% (421) | 86.6% (97) | |
| Fatality Rate | 11.3% (80) | 35.5% (44) | <.001 |
| Respiratory Support | <.001 | ||
| None | 53.5% (379) | 25.4% (29) | |
| Nasal Cannula (NC)/ Non-rebreather (NRB) | 29.3% (208) | 36.0% (41) | |
| High Flow Nasal Cannula (HFNC) | 4.9% (35) | 20.2% (23) | |
| Non-invasive Positive Pressure Ventilation (NIPPV)/ Intubated | 12.3% (87) | 18.4% (21) | |
| ICU Admission | 12.1% (128) | 31.9% (36) | .001 |
| ICU Duration (days, median [IQR]) | 5 [3.0, 13.0] | 6 [4.0, 12.5] | .192 |
| Ventilator Duration (days, median [IQR]) | 6 [3.0, 12.5] | 8[4.0, 17.0] | .326 |
Abbreviations: IQR = interquartile range.
Figure 2.
Clinical COVID-19 outcomes. Hematologic malignancy (HM) cohort (blue) compared to the general population (GP) cohort (orange). For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Figure 3.
Overall survival, hematologic malignancy (HM) subgroup analysis. (A) (top), compares the survival probability of the HM cohort who were on active treatment at the time of COVID-19 infection (red), compared to those who were not on active treatment (blue). (B) (bottom), compares the survival probability of the HM cohort who were in complete remission (CR) (red) compared to the HM patients not in CR (blue). Neither P value reaches statistical significance for a difference between these cohorts. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Table 3.
Primary and Secondary Outcomes Calculated Odds Ratio for Hematologic Malignancy (HM) Cohort vs General Population (GP) Cohort
| Odds Ratio(95% CI) | P value | Adjusted Odds Ratio (95% CI) | P value | |
|---|---|---|---|---|
| Venous Thromboembolism (VTE) | 2.34 (1.00 – 5.43) | 0.049 | 2.14 (0.87 – 5.22) | .094 |
| Cerebrovascular Accident | 3.53 (1.16 – 10.73) | 0.026 | 3.12 (0.96 – 10.15) | .058 |
| Composite VTE/CVA | 2.83 (1.42 – 5.63) | 0.003 | 2.55 (1.23 – 5.27) | .011 |
| Fatality Rate | 4.32 (2.80 – 6.68) | <0.001 | 2.14 (1.32 – 3.46) | .002 |
| Respiratory Support | 3.37 (2.15 – 5.26) | <0.001 | 1.89 (1.17 – 3.05) | .009 |
| ICU Admission | 2.12 (1.36 – 3.29) | 0.001 | 1.52 (0.95 – 2.43) | .079 |
| ICU Duration (days, median [IQR]) | 1.34 (0.93 – 1.93) | 0.113 | 1.53 (1.04 – 2.25) | .029 |
| Ventilator Duration (days, median [IQR]) | 1.20 (0.75 – 1.91) | 0.440 | 1.28 (0.78 – 2.08) | .317 |
Age adjusted odds ratios for HM cohort vs GP cohort.
Abbreviations: IQR = interquartile range.
To control for differences in age between the cohorts, odds ratio and adjusted odds ratio were calculated for primary and secondary outcomes (Table 3). The composite thrombotic outcome was noted to have an adjusted odds ratio of 2.55 (95% CI, 1.23 – 5.26, P = .011). Individual components of CVA and VTE were found to have adjusted odds ratio of 3.12 (95% CI 0.96 – 10.15, P = .058) and 2.14 (95% CI 0.87 – 5.22, P = .094), respectively.
Discussion
Our retrospective study of HM patients compared to the general population provides data evaluating thrombotic and overall clinical outcomes in acute COVID-19. Our primary endpoint showed that CVA occurred more frequently in the HM cohort. VTE was clinically higher in the HM cohort but did not reach statistical significance. The composite thrombotic outcome (CVA + VTE) showed significantly higher rates in the HM cohort. Age-adjusted odds ratio showed a significant increase in HM composite thrombotic events. Prior studies have demonstrated that VTE is common in acute COVID-19, with a range of 7.2%-43% in ICU patients17 , 18 and 3%-8.3%17 , 19 in non-ICU hospitalized patients. A study of 3334 patients with COVID-19 described VTE to be associated with an increased overall mortality rate and reported a 1.6% incidence of CVA.20 Additionally, rates of thrombotic complications in patients with HM and COVID-19 have been reported as high as 9% in a HM cohort.21 Our findings confirm thrombosis to be a common occurence amongst hospitalized patients with COVID-19 and suggest HM patients have an increased risk of thrombotic outcomes.
Secondary outcomes revealed higher case fatality rate, higher percentage of patients requiring respiratory support, and more HM patients admitted to the ICU when compared to the GP. Interestingly, there was no difference in length of ICU stay and ventilator duration. One plausible explanation for this finding could be a more rapid fatal course, or earlier transition to comfort or hospice care, particularly among older patients with HM. Case fatality rate, respiratory support, and days spent in the ICU were associated with inferior outcomes when adjusted for age. This aligns with other studies demonstrating patients with cancer have poor outcomes with COVID-19 in the pre-vaccination era.2 , 22 , 23 In particular, there is a higher risk of severe events (composite endpoint of admission to ICU with invasive ventilation or death) for patients with cancer compared to those without (39% vs. 8%).22 A systematic review and meta-analysis of 3377 patients found a 34% risk of death among adult, hospitalized patients with HM and COVID-19.23 What remains elusive is the driving mechanism behind the higher morbidity and mortality in HM patients.
A hypothesis for the poor outcomes witnessed in HM patients with COVID-19 is that those with active malignancy requiring anti-neoplastic therapy may have weakened immune systems from underlying disease or an impaired anti-viral response due to myelosuppressive chemotherapy or immunosuppressive targeted therapy. In HM subgroup analysis, we did not observe differences in mortality or thrombotic outcomes between patients on active treatment compared to active surveillance and/or maintenance treatment, or between complete responders and patients with any other documented response. While definitive conclusions are inhibited by the limited sample size of HM cohort, it is possible disease and treatment-related immunosuppression does not play a significant role in acute COVID19 infection.
Based on our results, we recommend patients with HM be treated with anticoagulation (AC) strategies that match the literature for the general population. Currently, the American Society of Hematology recommends prophylactic dose anticoagulant in absence of contraindication, over intermediate or therapeutic dose anticoagulant for hospitalized patients with COVID-19.24 The ATTACC, ACTIV-4a, and REMAP-CAP investigators reported an increased probability of survival after hospital discharge for non-critically ill patients on therapeutic dose AC, but no benefit for patients in ICU care.25 , 26 More data may be needed before definitive recommendations can be made.
There are several limitations of our study. First, this is a retrospective study and our cohorts were not age-matched. Age-adjusted statistical analysis was performed to adjust for this difference. Additionally, the HM cohort had a higher rate of historical VTE prior to COVID-19, with some patients being anticoagulated before and during hospitalization, thus potentially lowering thrombotic complications in this group. Limited sample size of the HM cohort may have led to underpowered statistical testing. Further, the longer duration of HM cohort recruitment may have led to outcome differences due to changes in supportive care measures that occurred later in the pandemic (eg, prone ventilation, steroid administration, use of targeted immunomodulation agents). Finally, remission status was only documented in 57.3% of the HM cohort, limiting our ability to detect differences amongst the remission-status cohorts.
Conclusion
In conclusion, our data suggest HM patients have a statistically significant increase rate of composite thrombotic (CVA + VTE) outcomes. Patients with a history of HM irrespective of disease status, have higher fatality rates and require more respiratory and intensive care support. Further investigation of this topic is necessary to understand the natural history of COVID-19 in patients with hematologic malignancies to better inform management of this population with impaired vaccination response, particularly as variant strains emerge.11, 12, 13
Clinical Practice Points
-
•
Patients with Hematologic Malignancy (HM) have inferior clinical outcomes during acute COVID-19, but what remains elusive is the driving mechanism behind the higher morbidity and mortality.
-
•
Our data suggests HM patients have a higher composite rate of venous thromboembolism and cerebrovascular accident during acute COVID-19 in comparison to the general population.
-
•
Irrespective of disease status, HM patients also have significantly increased need for intensive care, respiratory support, and have higher fatality rates.
-
•
Further investigation of thrombotic outcomes and the use of anticoagulant is needed for patients with HM and COVID-19, as they remain a particularly vulnerable population during the ongoing pandemic.
Ethics approval statement
The authors confirm this analysis and manuscript were created in accordance with the Declaration of Helsinki and have been approved by the IRB at MedStar Health Research Institute at Georgetown University.
Table 4.
Primary and Secondary Outcomes, Hematologic Malignancy (HM) Cohort Subpopulations
| HM, Active Treatment (n = 39) | HM, Maintenance + Surveillance Tx Status (n = 72) | P value | |
|---|---|---|---|
| Venous Thromboembolism (VTE) | 8.1% (3) | 8.1% (5) | 1.000 |
| Cerebrovascular Accident (CVA) | 2.7% (1) | 8.1% (5) | .406 |
| Composite VTE/CVA | 10.8% (4) | 16.1% (10) | .560 |
| Fatality Rate | 41.0% (16) | 33.3% (24) | .535 |
| Respiratory Support (none) | 27.0% (10) | 26.6% (17) | .420 |
| ICU Admission | 40.5% (15) | 23.8% (15) | .113 |
| HM, CR cohort (n = 18) | HM, non-CR cohort (n = 53) | P value | |
| Venous Thromboembolism (VTE) | 7.7% (1) | 12.5% (6) | 1.00 |
| Cerebrovascular Accident | 15.4% (2) | 2.1% (1) | .112 |
| Composite VTE/CVA | 23.1% (3) | 14.6% (7) | .432 |
| Fatality Rate | 16.7% (3) | 35.8% (19) | .152 |
| Respiratory Support (none) | 40% (6) | 27.1% (13) | .238 |
| ICU Admission | 31.2% (5) | 25.5% (12) | .747 |
Note the subpopulations for the HM cohort do not make up the entire HM cohort. For the treatment status, 13 patients’ treatment status was unknown, therefore excluded from subgroup analysis. For the remission status, there were 53 patients whose status was unknown (n = 14) or not yet assessed (n = 39), which were also excluded from analysis. Respiratory support in this chart refers to patients who did not require any amount of supplemental oxygen therapy.
Disclosure
The authors declare no competing financial interests. There were no funding sources used for this analysis or creation of this manuscript. This article has not been published elsewhere, and is not currently under consideration for publication elsewhere.
Acknowledgments
Contribution: M.R.C, N.K.C and C.L designed study concept. M.R.C, K.D, K.W and N.K.C collected and organized data. S.D, R.A, S.F and X.H cleaned data and performed statistical analysis. MRC, K.D, N.K.C and C.L wrote and edited the manuscript. All listed authors have contributed to the manuscript substantially and have agreed to the final submitted version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clml.2021.12.011.
Appendix. Supplementary materials
References
- 1.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharafeldin N, Bates B, Song Q, et al. Outcomes of COVID-19 in Patients With Cancer: Report From the National COVID Cohort Collaborative (N3C). J of Clin Oncol.0(0):JCO.21.01074. [DOI] [PMC free article] [PubMed]
- 3.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowenstein CJ, Solomon SD. Severe COVID-19 Is a Microvascular Disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395:1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021 doi: 10.1101/2021.04.06.21254949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. New England Journal of Medicine. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foundation TR. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available at: https://www.r-project.org/. Published 2014. Accessed September 28, 2021.
- 17.Hill JB, Garcia D, Crowther M, et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4:5373–5377. doi: 10.1182/bloodadvances.2020003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. Jama. 2020;324 doi: 10.1001/jama.2020.13372. 799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulati S, Shah S, Kulkarni A, et al. Thrombotic complications with SARS-CoV-2 infection in patients with cancer on high-risk therapies: Data from the COVID-19 and Cancer Consortium (CCC19) J of Clin Oncol. 2021;39(15_suppl) e18788-e18788. [Google Scholar]
- 22.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: May 2021 update on the use of intermediate intensity anticoagulation in critically ill patients. Blood Advances. 2021 doi: 10.1182/bloodadvances.2021005945. PMID: 34727173; PMCID: PMC8566097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. New England Journal of Medicine. 2021;385:777–779. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. New England Journal of Medicine. 2021;385:790–792. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.