Abstract
Objectives
Although clinical data have shown that the BNT162b2 vaccine, which is widely used in many countries, is safe and effective as a protection against the SARS-CoV-2 infection, extant research in adverse reactions using real-world data of various sociodemographic characteristics is scant.
Methods
We conducted a prospective cohort study to compare age differences in self-reported reactogenicity of BNT162b2 in Hong Kong. A total of 1,516 participants were intensively followed up for two weeks following both doses of BNT162b2 vaccination, during which their basic demographic, health conditions, and medication information were collected.
Results
Results from the generalized mixed model showed that compared with adults aged 18 to 59 years, older adults aged 60 years or above had a lower risk of adverse reactions and adolescents aged 12 to 17 years had a moderately higher risk.
Conclusions
Results of this study should be informative to parents considering BNT162b2 vaccination for their children in that moderately increased reactogenicity compared with adults is anticipated.
Keywords: COVID-19, vaccine safety, pharmacovigilance, epidemiology, pediatrics
The BNT162b2 messenger RNA COVID-19 vaccine, widely used in more than 100 countries worldwide, has been shown to be safe and effective in protecting populations from the infection of SARS-CoV-2 (Polack et al., 2020; Thomas et al., 2021; Walsh et al., 2020). According to clinical trial data, more than 80% of BNT162b2 recipients reported post-vaccination adverse reactions such as pain and tiredness, although an extremely small proportion of these reactions required medical interventions (Polack et al., 2020). Current research seldom examined such adverse reactions in sub-populations of various sociodemographic characteristics, however.
Since the emergency use of BNT162b2 in adolescents aged 12 years or above has been approved in an increasing number of jurisdictions worldwide with few substitutes (Frenck et al., 2021), its self-reported reactogenicity as compared with adult recipients in real-world settings should be examined to better inform parents’ decision to permit their children to receive the vaccine (Musa et al., 2021). This study aimed to assess the potential risk differences in the self-reported reactogenicity of BNT162b2 among adolescents, middle-aged adults, and older adults.
METHODS
A prospective cohort design with self-reported data was adopted for this study. Data were collected on the first-dose vaccination day as baseline. Participants were then followed up on the first, second, third, seventh, and fourteenth day following both doses of vaccination. Basic demographic, health conditions, and medication information were collected during baseline, and any self-reported adverse reactions were collected throughout the observation period*.
A generalized linear mixed model was performed to examine the association between age group and self-reported adverse reactions†, adjusting for person-level and measurement-level covariates‡. Listwise deletion was applied for missing data due to its relatively negligible proportion. Odds ratios and confidence intervals were obtained for comparisons of risks between the trichotomized age groups (adolescents, 12-17 years; middle-aged adults, 18-59 years; and older adults, 60 years or above) at .05 significant level by R Software (version 4.1.1).
RESULTS
As of August 12, 2021, we recruited 2,531 participants (1,016 aged 12-17 years, 759 aged 18-59 years, and 756 aged 60 years or above) who had received BNT162b2. The follow-up success rates of our study were 90.6%, 96.7%, and 72.0% for the adolescent, middle-aged adult, and older adult groups, respectively. Details of cohort characteristics are shown in Table 1 .
Table 1.
Cohort characteristics.
| 12 to 17 years | 18 to 59 years | 60+ years | P-value | |
|---|---|---|---|---|
| n | 1,016 | 759 | 756 | |
| Gender = Male (%) | 547 (53.8) | 383 (50.9) | 397 (52.5) | 0.464 |
| Educational attainment (%) | < 0.001 | |||
| Primary and below | 28 (2.8) | 4 (0.5) | 157 (20.8) | |
| Secondary | 985 (96.9) | 139 (18.3) | 327 (43.3) | |
| Post-secondary | 1 (0.1) | 88 (11.6) | 84 (11.1) | |
| University or above | 2 (0.2) | 527 (69.5) | 188 (24.9) | |
| Number of chronic medications (%) | < 0.001 | |||
| None | 991 (97.5) | 653 (87.0) | 297 (39.3) | |
| 1-2 | 23 (2.3) | 79 (10.5) | 294 (38.9) | |
| 3-4 | 1 (0.1) | 15 (2.0) | 117 (15.5) | |
| 5-9 | 0 (0.0) | 4 (0.5) | 45 (6.0) | |
| 10 or more | 1 (0.1) | 0 (0.0) | 3 (0.4) | |
| Chronic conditions (%) | ||||
| Asthma | 19 (1.9) | 16 (2.1) | 10 (1.3) | 0.492 |
| Inflammatory bowel disease | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0.309 |
| Psoriasis | 0 (0.0) | 1 (0.1) | 3 (0.4) | 0.112 |
| Rheumatoid arthritis | 1 (0.1) | 0 (0.0) | 10 (1.3) | < 0.001 |
| Cancer under treatment | 1 (0.1) | 4 (0.5) | 5 (0.7) | 0.137 |
| Cancer remission | 0 (0.0) | 3 (0.4) | 15 (2.0) | < 0.001 |
| Hypertension | 0 (0.0) | 36 (4.7) | 294 (38.9) | < 0.001 |
| Hypercholesterolemia | 0 (0.0) | 19 (2.5) | 239 (31.6) | < 0.001 |
| Heart disease | 2 (0.2) | 5 (0.7) | 48 (6.3) | < 0.001 |
| Diabetes | 1 (0.1) | 15 (2.0) | 91 (12.0) | < 0.001 |
| Stroke | 0 (0.0) | 1 (0.1) | 6 (0.8) | 0.005 |
| Neurologic disorder | 0 (0.0) | 3 (0.4) | 2 (0.3) | 0.158 |
| Mental health disorder | 5 (0.5) | 7 (0.9) | 19 (2.5) | < 0.001 |
| Liver problems | 0 (0.0) | 4 (0.5) | 24 (3.2) | < 0.001 |
| Kidney problems | 0 (0.0) | 3 (0.4) | 19 (2.5) | < 0.001 |
| Other chronic conditions | 9 (0.9) | 31 (4.1) | 125 (16.5) | < 0.001 |
Among all participants, 1,516 (59.90%) reported the experience of adverse reactions after the first dose, and 1,278 (50.49%) reported adverse reactions following the second. The rates of adverse reactions on the seventh follow-up day of the first dose were 4.94%, 5.86%, and 5.71% (the same day after the second dose: 7.12%, 8.29%, and 7.72%) for adolescents, middle-aged adults, and older adults, respectively. For all examined age groups, the proportion of participants reporting any type of adverse reactions peaked on the first follow-up day (first dose: 63.44%, second dose: 62.22%) after both doses of vaccination and gradually declined, with only very few reports of adverse reactions toward the fourteenth day of follow-up (first dose: 4.44%, second dose: 4.12%).
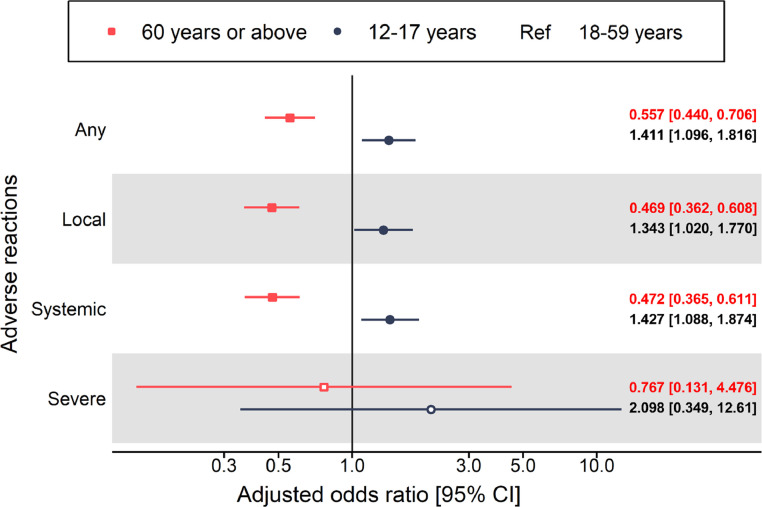
Results from generalized linear mixed model indicated that compared with middle-aged adults, older adults had reduced odds in any (adjusted odds ratio [aOR] = 0.557, 95% CI 0.440-0.706), local (aOR = 0.469, 95% CI 0.362-0.608), and systemic adverse reactions (aOR = 0.472, 95% CI 0.365-0.611). For the adolescent group, the results showed there were increased risks in any (aOR = 1.411, 95% CI 1.096-1.816), local (aOR = 1.343, 95% CI 1.020-1.770), and systemic (aOR = 1.427, 95% CI 1.088-1.874) adverse reactions. Estimates for severe adverse reactions were imprecise due to relatively low incidence for both adolescents and older adults. The model results are summarized in Figure 1 .
Figure 1.
Self-reported adverse reactions over 14-day post-vaccination of both doses for adolescents and older adults compared with middle-aged adults among BNT162b2 recipients.
DISCUSSIONS
Although previous clinical trials have reported the reactogenicity of BNT162b2 (Frenck et al., 2021), there has been little existing research reporting the reactogenicity of BNT162b2 in adolescents in the literature. A plausible explanation of the observed age differences is that, consistent with previous research evidence (Woudenberg et al., 2021), the immune response triggered by a viral infection or vaccines in individuals of a younger age is typically stronger than in those of an older age.
Study limitations that need to be taken into consideration include the design of a serial self-reported online survey, which entails a risk of omitting the follow-up survey of who had more serious adverse reactions and required medical interventions or were even hospitalized, as evidenced by the imperfect response rates. Self-reported bias may also exist in the reactogenicity data, which might partially be induced by the educational gap across the age groups.
The results of this study should be informative to parents considering BNT162b2 vaccination for their children in that moderately increased reactogenicity compared with adults is anticipated and that severe adverse reactions are rare. Considering the entirety of the existing body of knowledge about the reactogenicity and adverse events following the use of BNT162b2, we believe that the benefits of receiving the vaccine still far outweigh the associated risks. The findings should inform the choice of vaccine uptake for parents of eligible adolescents.
Footnote:
*Participants aged 18 or above receiving the first dose of BNT162b2 at government-operated community vaccination centers were recruited since vaccine distribution was commenced on February 23, 2021. For adolescent participants aged 12 to 17 years, recruitment commenced upon vaccine distribution on June 24, 2021. The data collection period for all age groups ended on August 22, 2021. We supplemented the active in-person recruitment with flyers including a quick-response (QR) link to the online survey distributed at health care facilities. The link to follow-up surveys was sent to participants via instant text messages and surveys were conducted online using Qualtrics, an online data collection platform. Only those participants who were scheduled to complete the fourteenth-day follow-up survey for the second dose according to the recommended interval between the two doses were included in the analysis. Participants could withdraw from the study anytime.
†Self-reported adverse reactions include local (numbness, soreness, pain, swelling, redness, and itch), systemic (sore throat, tiredness, fever, chills, sweating, cough, headache, muscle pain, joint pain, pain in limbs, abdominal pain, diarrhea, nausea, vomiting, poor appetite, insomnia, feeling unwell, enlarged lymph nodes, rash, and temporary one-sided facial drooping), and severe allergic reactions (hypotension, dizziness, itchy skin rash, swelling of face or tongue, and wheezing/shortness of breath).
‡Person-level covariates include gender, educational attainment, number of chronic medications, and a range of specified chronic conditions, namely autoimmune diseases (ankylosing spondylitis, psoriasis, rheumatoid arthritis, systemic lupus erythematosus), cancer (both remission and under treatment), respiratory diseases (asthma, chronic obstructive pulmonary disease, and others), and other common chronic illnesses, including hypertension, hypercholesterolemia, heart disease, diabetes, stroke, neurologic disorders, mental health disorders, liver problems, and kidney problems. At the measurement level, specific follow-up days (vaccination day, first-, second-, third-, seventh-, and fourteenth-day after vaccination) of both doses were also adjusted for the given anticipated day-dependent reactogenicity.
FUNDING
This work was supported by the Food and Health Bureau of the Hong Kong Special Administration Region Government, Hong Kong, China [Ref: COVID19F01].
COMPETING INTERESTS
F.T.T.L. has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council. X.L. has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong, and consultancy fee from Merck Sharp & Dohme, unrelated to this work. C.S.L.C. has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fee from Primevigilance Ltd., outside of the submitted work. E.Y.F.W. has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR and the Hong Kong Research Grants Council, outside of the submitted work. I.C.K.W. reports research funding outside of the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia and also received speaker fees from Janssen and Medice in the previous 3 years. E.W.Y.C. reports honorarium from Hospital Authority, grants from Research Grants Council (RGC, Hong Kong), the Research Fund Secretariat of the Food and Health Bureau, the National Natural Science Fund of China, the Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and the Narcotics Division of the Security Bureau of HKSAR, outside of the submitted work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Our study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW-21-090) and the Department of Health Ethics Committee (LM 21/2021).
REFERENCES
- Frenck R.W., Jr., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Gruber W.C. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa S., Dergaa I., Abdulmalik M.A., Ammar A., Chamari K., Saad H.B. BNT162b2 COVID-19 Vaccine Hesitancy among Parents of 4023 Young Adolescents (12–15 Years) in Qatar. Vaccines. 2021;9(9) doi: 10.3390/vaccines9090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Jansen K.U. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. New England Journal of Medicine. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Gruber W.C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg T., Pelleau S., Anna F., Attia M., Donnadieu F., Gravet A., White M. Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103495. [DOI] [PMC free article] [PubMed] [Google Scholar]