Abstract
Objective
The cytokine storm presented in the hyperimmune response is related to poor prognosis in people with COVID-19. Interleukin-6 (IL-6) is one of the most prominent cytokines, especially on mucosal surfaces during infection, causing the cytokine storm. Polyunsaturated fatty acids (PUFAs) are the precursors of eicosanoids, which play critical roles in immune regulation and inflammation. The balance between ω-3 and ω-6 levels in the cell membrane has a critical role in regulating the equilibrium between proinflammatory and antiinflammatory processes and inducing IL-6 production. The present study focused on inflammatory and antiinflammatory mechanisms in COVID-19 over PUFAs and on relating their levels with disease prognosis and severity.
Methods
A total of 106 participants were included in the study. They were divided into three groups according to IL-6 level— 1: <35 pg/mL, 2: between 35 and 300 pg/mL, and 3: >300 pg/mL. Erythrocyte membrane PUFA compositions were analyzed by group.
Results
Levels of γ-linolenic acid and ω-6/ω-3 ratios were significantly increased in all comparison groups (P < 0.05). Total ω-6 and the ratio of arachidonic acid to eicosopentaenoic acid showed a statistically significant difference only between groups 1 and 3 (P < 0.05). There was a moderately negative correlation between total ω-3 and IL-6 and procalcitonin. There were positive correlations with ω-6/ω-3 ratio inflammatory markers, and the total ω-6 index also showed a moderately positive correlation with IL-6, procalcitonin, and D-dimer levels.
Conclusions
The ratio of arachidonic acid to eicosopentaenoic acid, and ω-3 PUFAs, can be systemic signs of poor prognosis, increased lung damage, and high mortality in COVID-19, together with IL-6.
Keywords: COVID-19, Cytokine storm, IL-6, PUFAs, ω-6/ω-3 ratio, AA/EPA ratio
Introduction
Coronaviruses are a family of zoonotic viruses that can cause lower respiratory tract infections and fatal respiratory failure characterized by hyperinflammation [1]. The SARS-CoV-2 virus, which caused a global pandemic beginning in 2019, triggers a variety of symptoms including dry cough, dyspnea, tremors, myalgia, headache, loss of taste and smell, and gastrointestinal discomfort. It causes more severe disease especially in individuals who are obese, have diabetes, or are immunocompromised, and in older people with comorbidities [2]. Although the virus affects multiple organ systems, the cardinal feature of COVID-19, the disease it causes, is respiratory failure. The inflammatory response plays an important role in the prognosis of COVID-19. The aggressive inflammatory response triggered by the disease and the high amounts of proinflammatory cytokines released into the circulation cause a situation called the cytokine storm. The cytokine storm presented in the hyperimmune response is related to severe lung damage, multiple organ failure, and poor prognosis in people with COVID-19 [3,4].
Interleukin-6 (IL-6) is a proinflammatory and multifactorial cytokine, alongside its functions of triggering antibody production causing secondary immune response and increasing the synthesis of acute-phase proteins such as C-reactive protein (CRP) and fibrinogen, making it one of the most prominent cytokines, especially on mucosal surfaces during infection [5,6]. In addition, a relationship between IL-6 and respiratory failure has been detected, especially in people with severe lung damage. And as indicated recently, the heavy viral burden triggers IL-6 production in people with severe COVID-19, which in turn causes the cytokine storm, with high mortality [7].
Although linoleic acid (LA), γ-linolenic acid (GLA), dihomo-γ-linolenic acid (DGLA), arachidonic acid (AA), α-linolenic acid (ALA), eicosopentaenoic acid (EPA), and docosahexaenoic acid (DHA) are all polyunsaturated fatty acids (PUFAs), only LA and ALA are essential fatty acids; the remaining ones are all synthesized from these two molecules through desaturation and elongation reactions [8]. The most important role of these PUFAs is as the precursor of eicosanoids, which are known to play critical roles in immune regulation and inflammation. They also induce the formation of proinflammatory molecules that cause the release of inflammatory mediator cytokines like IL-1β, IL-6, IL-8, and tumor necrosis factor-α [9,10]. The ω-3 PUFAs (EPA and DHA) are proclaimed to be the basis of the antiinflammatory effects, whereas ω-6 PUFAs (particularly AA) are potent mediators of inflammation and cell proliferation [11], [12], [13]. These antiinflammatory effects of ω-3 PUFAs can be competitive with the effects of ω-6 PUFAs, because ω-3 PUFAs are competitive substrates in the metabolism of ω-6 PUFAs [14]. Hence, the balance between ω-3 and ω-6 levels in the bloodstream and especially in the cell membrane has a critical role in regulating the equilibrium between proinflammatory and inflammatory processes.
In the light of this information, we aimed to evaluate the ω-3 and ω-6 PUFAs levels, and role of these fatty acids together with IL-6, in the development of the cytokine storm in people with COVID-19. By understanding the proinflammatory and antiinflammatory mechanisms in COVID-19 over PUFAs, it will be possible to obtain data at the cellular level to identify possible pathways that are important in the disease prognosis.
Methods
This study was approved by the local ethics committee of Gulhane School of Medicine (2021-13) and conducted according to the Helsinki Declaration.
Study population
There were 106 participants included, divided into the following three groups:
-
1.
According to information provided by the Beckman Coulter Access IL-6 Assay kit, which we used to measure serum IL-6 levels, IL-6 ˃ 35 pg/mL was used to identify 85.4% of participants who had a ratio of partial pressure arterial oxygen to fraction of inspired oxygen < 150 mm Hg, which is also indicative of the risk of mechanical ventilation (https://www.beckmancoulter.com/products/immunoassay/access-il-6-assay). On the other hand, there are publications in the literature that use IL-6 > 35 pg/mL as the cutoff for risk of mortality and admission to the intensive care unit (ICU) [15,16]. Therefore, our first participant group consisted of people with PCR-confirmed COVID-19 and IL-6 ≤ 35 pg/mL (n = 32).
-
2.
A retrospective study that aimed to assess IL-6 levels in critically ill patients with COVID-19 admitted to the ICU and to evaluate their relationship with the disease severity and outcome suggests a threshold value of 300 pg/mL [17]. This threshold is also indicated by other studies for use in admission to the ICU discrimination of critical and severe illness [18,19]. In light of the recent literature, we chose an IL-6 value of 300 pg/mL to discriminate critical cases from others (n = 45).
-
3.
Our third group of participants consisted of those with PCR-confirmed COVID-19 and IL-6 ˃ 300 pg/mL (n = 29).
Sample collection and preparation
Venous blood samples were collected in tubes containing dipotassium ethylenediaminetetraacetic acid. Hematocrit levels were determined with the Beckman Coulter UniCel DxH 800 Coulter Cellular Analysis System (Indianapolis, IN, USA) for all samples. Afterwards, the erythrocytes were separated from the plasma by centrifugation (3000 rpm, 1500g, for 10 min) and washed with an equal volume of saline. After the removal of the saline, the cells were resuspended with saline to a hematocrit of 45%. These erythrocyte suspensions and plasma were stored at −80°C in Eppendorf vials freshly treated with butylated hydroxytoluene, as previously described [20].
Erythrocyte suspensions were thawed before sample preparation, and 200 μL of erythrocyte suspension and 2 mL of 3N methanolic hydrochloric acid including internal standard—heptadecanoic acid (C17:0) at 0.1-μM concentration—were added to the 13 mL screw-cap glass tubes. Transmethylation was performed at 90°C for 3 h. After the tubes were cooled to room temperature, 2 mL of hexane was added and the tubes were closed again and vortexed for 10 s. The upper phase was transferred to a clean glass tube and evaporated in the SpeedVac (scan speed 32, 1900 rpm, 25°C). Finally, the residue was resuspended with 100 μL of hexane and the sample was transferred to a gas chromatography injection vial with a screw cap [9,20].
Analysis of samples
Levels of IL-6 were measured with a Beckman Coulter Access autoanalyzer and the chemiluminescent immunoassay method. The analytical coefficient of variation (CV) was < 12% and analytical sensitivity was 0.5 pg/mL. The linearity of the assay was 2 to 1500 pg/mL, with automatic twofold dilution for samples with IL-6 > 1500 pg/mL. Procalcitonin (PCT) levels were also detected with the Beckman Coulter Access autoanalyzer and the chemiluminescent immunoassay method. The limit of quantitation was 0.02 ng/mL and the CV was ≤ 8% at concentrations ≥ 0.150 ng/mL. The analytical measuring range was 0.05 to 100 ng/mL. Levels of CRP were detected with a Beckman Coulter AU5800 autoanalyzer and the immune-turbidimetric method, with an assay range of 0.2 to 160 mg/L. D-dimer was measured with a Sysmex CS-2500 autoanalyzer with particle-enhanced immunoturbidimetric assay. The analytical CV was 8.4% with a diagnostic sensitivity of 99.4% at a cutoff of 0.50 mg/L of fibrinogen equivalent units. Whole-blood analysis was carried out with a Beckman Coulter DxH 900 analyzer using the volume-conductivity-scatter principle.
An Agilent gas chromatography/mass spectrometry system was used for analysis, composed of a 7890B GC System coupled with a 5977E mass spectrometer (Santa Clara, CA, USA). The sample (1 μL) was injected in split mode at 240°C with a ratio of 1:50. Helium was used as the carrier gas and set at 1 mL/min. Samples were separated on a capillary gas chromatography column (Zebron ZB-Frame; 60 m, internal diameter = 250 μm, film thickness = 0.20 μm). The oven temperature was set at 50°C for 1.5 min, ramped to 190°C at 30°C/min and held for 5 min, then ramped to 230°C at 8°C/min and held for 5 min. (The total analysis time was approximately 21 min.) The front inlet was set to 240°C and the AUX temperature to 280°C. Linearity was obtained between 3 and 750 μmol/L, the recovery rate was 89% to 112%, and the analytical CV was < 5% for all PUFAs.
We evaluated levels of saturated fatty acids (palmitic acid, C16:0; stearic acid, C18:0), an ω-9 monounsaturated fatty acid (oleic acid, C18:1), ω-3 PUFAs (ALA, C18:3; EPA, C20:5; DHA, C22:6), and ω-6 PUFAs (LA, C18:2; AA, C20:4; DGLA, C20:3; GLA, C18:3), as well as EPA/AA and ω-6/ω-3 ratios, in the erythrocyte membrane (EM).
Participant complications
Sepsis is defined as a dysregulated and deleterious host response to infection leading to organ dysfunction. For COVID-19, in addition to participants with lung injury, impaired liver and kidney function, and microcirculatory dysfunction, those who met the criteria from the Sepsis-3 International Consensus were defined as experiencing sepsis. According to these criteria, organ dysfunction can be identified as an acute change in total Sequential Organ Failure Assessment score ≥ 2 points consequent to the infection [21,22]. Renal functioning of participants was also evaluated. Acute kidney injury (AKI) is defined as an abrupt (within hours) decrease in kidney function which encompasses both injury (structural damage) and impairment (loss of function). Overall, AKI was defined according to the Kidney Disease Improving Global Outcomes criteria [22].
Statistical analysis
All statistical analyses were done with SPSS 22.0. Tests of normality were carried out with visual (histograms, possibility graphs) and analytical methods (Kolmogorov–Smirnov and Shapiro–Wilk tests). The results are expressed as mean ± SD for parametric variables and median (interquartile range) for non-parametric variables. Comparisons of parametric variables between groups were analyzed with independent-samples t tests, and comparisons of non-parametric variables with Mann–Whitney U tests. Correlation analyses for parametric variables were done with the Pearson test and those for non-parametric variables with the Spearman test. A value of P < 0.05 was accepted as the limit of statistical significance.
Results
A total of 106 participants were divided into three groups according to IL-6 level. Distribution and comparison results of demographic data like age and gender, and laboratory parameters including IL-6, CRP, D-dimer, PCT, neutrophils, lymphocytes, neutrophil-to-lymphocyte ratio, with mortality rates, are presented in Table 1 . Mortality rate was the lowest in group 1, increased in group 2, and was highest in group 3 (P < 0.001 for all).
Table 1.
Distribution and comparison of demographic data and other laboratory parameters of participants by group
| Parameter | Group |
P |
|||||
|---|---|---|---|---|---|---|---|
| 1 (n = 32) | 2 (n = 45) | 3 (n = 29) | Group 1 to 2 | Group 1 to 3 | Group 2 to 3 | Groups 1, 2, and 3 | |
| Age, y | 55.9 ± 9.23 | 65.8 ± 15.9 | 66.7 ± 13.8 | 0.007 | 0.008 | 0.963 | 0.003 |
| Gender (M/F) | 19/13 | 30/15 | 20/9 | 0.515 | 0.440 | 0.838 | 0.707 |
| Mortality, % | 3.13 | 48.9 | 89.7 | <0.001 | <0.001 | <0.001 | <0.001 |
| IL-6, pg/mL | 5.23 (3.31–5.23) | 107 ± 71.5 | 908 ± 536 | <0.001 | <0.001 | <0.001 | <0.001 |
| CRP, mg/L | 20.7 (8.03–64.3) | 86.3 (37.9–143) | 166 (86.6–390) | <0.001 | <0.001 | 0.001 | <0.001 |
| D-dimer, mg/L | 0.55 (0.35–0.95) | 1.90 (0.63–5.78) | 3.80 (2.07–10.1) | <0.001 | <0.001 | 0.009 | <0.001 |
| Procalcitonin, ng/mL | 0.08 (0.05–0.15) | 0.35 (0.10–1.28) | 5.40 (1.29–15.2) | <0.001 | <0.001 | <0.001 | <0.001 |
| Neutrophils, × 103/µL | 7.21 (4.63–11.6) | 7.90 (5.10–12.2) | 12.7 (7.30–17.4) | 0.522 | 0.003 | 0.005 | 0.005 |
| Lymphocytes, × 103/µL | 0.75 (0.50–1.35) | 0.70 (0.40–1.00) | 0.50 (0.30–0.90) | 0.392 | 0.018 | 0.123 | 0.067 |
| NLR | 7.63 (3.92–20.6) | 11 (5.66–19.8) | 26.3 (9.95–52.2) | 0.301 | 0.001 | 0.009 | 0.002 |
CRP, C-reactive protein; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio
Values are expressed as mean ± SD, n, or median (interquartile range)
When the laboratory parameters were evaluated, a statistically significant difference was seen across all three groups in IL-6, CRP, PCT, and D-dimer. There was not a statistically significant difference between groups 1 and 2 on neutrophil count or neutrophil-to-lymphocyte ratio, but a statistically significant difference was observed between groups 1 and 3 and between groups 2 and 3.
Two participants from group 1, 25 from group 2, and 27 from group 3 were treated in the ICU. Although not presented in Table 1, the mean hospitalization durations for the three groups were, respectively, 13.6, 17.6, and 19.6 d, and lengths of stay in the ICU were 13.5, 12.64, and 13.81 d. No statistically significant difference between groups was found for hospital-stay duration. The proportion of participants treated in the ICU was 6.25%, 55.6%, and 93.1% for groups 1, 2, and 3, respectively, and the mortality rates in the ICU were 50%, 80%, and 96.3%. When ICU participants were evaluated in detail according to group, pneumonia was detected in one of the two participants from group 1 and sepsis was detected in the other. Among ICU participants from group 2, 23 had pneumonia, 11 had sepsis, and 6 had AKI. And finally, in group 3 all ICU participants had pneumonia, 13 had sepsis, and 14 had AKI. In addition, a total of eight participants required invasive mechanical ventilation (two from group 1 and six from group 2).
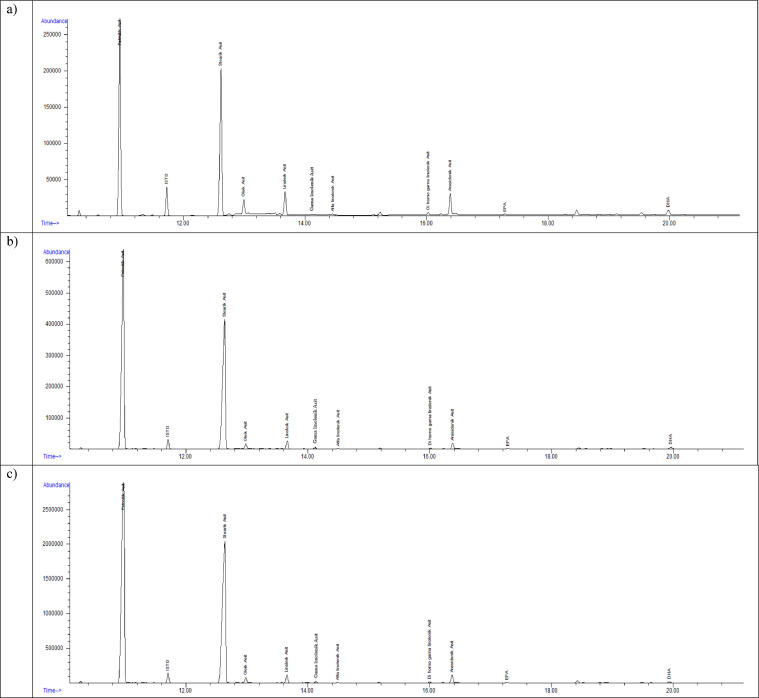
We evaluated 6 different EM PUFAs and one MUFA (OA) in the present study. Six different index data obtained by summing and dividing the values of these PUFAs with the current PUFAs values and the statistical results obtained from their comparison are presented in Table 2. It is noteworthy here that the GLA values and the ω-6/ω-3 ratio were significantly different among all three groups, compared individually and together. Four of these seven PUFAs—oleic acid, EPA, ALA, and DHA—as well as the ω-3 index, total ω-3, total ω-6, LA/ALA ratio, and AA/EPA ratio also showed statistically significant differences in the overall comparison of all groups (Fig. 1 ).
Table 2.
Distribution and comparison of polyunsaturated fatty-acid results in erythrocyte membranes by group
| Fatty acid, µmol/L | Group |
P |
|||||
|---|---|---|---|---|---|---|---|
| 1 (n = 32) | 2 (n = 45) | 3 (n = 29) | |||||
| Group 1 to 2 | Group 1 to 3 | Group 2 to 3 | Groups 1, 2, and 3 | ||||
| OA (C18:1, ω-9) | 632 (549–856) | 797 (685–1002) | 833 (599–1160) | 0.005 | 0.024 | 0.943 | 0.013 |
| ω-3 acids | |||||||
| ALA (C18:3) | 21.4 (10.1–43.0) | 8.17 (5.11–12.5) | 7.22 (2.07–13.0) | <0.001 | <0.001 | 0.261 | <0.001 |
| EPA (C20:5) | 4.42 (2.32–7.70) | 2.54 (1.14–4.57) | 2.02 (0.75–3.73) | 0.016 | 0.002 | 0.283 | 0.006 |
| DHA (C22:6) | 171 (131–210) | 136 (113–179) | 134 (76.9–188) | 0.047 | 0.030 | 0.406 | 0.046 |
| ω-3 index* | 186 (150–224) | 142 (117–178) | 137 (77.4–194) | 0.005 | 0.007 | 0.372 | 0.006 |
| Total ω-3 | 215 (177–299) | 152 (124–187) | 126 (78.4–198) | <0.001 | 0.001 | 0.196 | <0.001 |
| ω-6 acids | |||||||
| LA (C18:2) | 504 (441–655) | 569 (461–643) | 613 (449–911) | 0.168 | 0.100 | 0.450 | 0.188 |
| GLA (C18:3) | 10.9 (9.67–61.8) | 98.9 (10.8–163) | 132 (45.2–233) | 0.034 | <0.001 | 0.016 | <0.001 |
| DGLA (C20:3) | 74.8 (60.3–92.5) | 69.3 (49.8–86.2) | 78.9 (52.6–117) | 0.122 | 0.830 | 0.139 | 0.193 |
| AA (C20:4) | 329 (287–397) | 337 (268–427) | 366 (234–511) | 0.984 | 0.337 | 0.506 | 0.654 |
| Total ω-6 | 964 (861–1276) | 1110 (931–1277) | 1217 (956–1829) | 0.181 | 0.016 | 0.111 | 0.038 |
| ω-6/ω-3 | 5 (4–6) | 6 (6–7) | 8 (7–9) | 0.001 | <0.001 | 0.030 | <0.001 |
| LA/ALA | 25 (12–59) | 74 (47–115) | 118 (51–276) | <0.001 | <0.001 | 0.064 | <0.001 |
| AA/EPA | 78 (50–135) | 117 (62–265) | 173 (89–683) | 0.137 | 0.005 | 0.133 | 0.020 |
AA, arachidonic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosopentaenoic acid; GLA, γ-linolenic acid;; LA, linoleic acid OA, oleic acid
All values are expressed as median (interquartile range)
*EPA+DHA.
Fig. 1.
Chromatogram samples of participants in (a) group 1, (b) group 2, and (c) group 3.
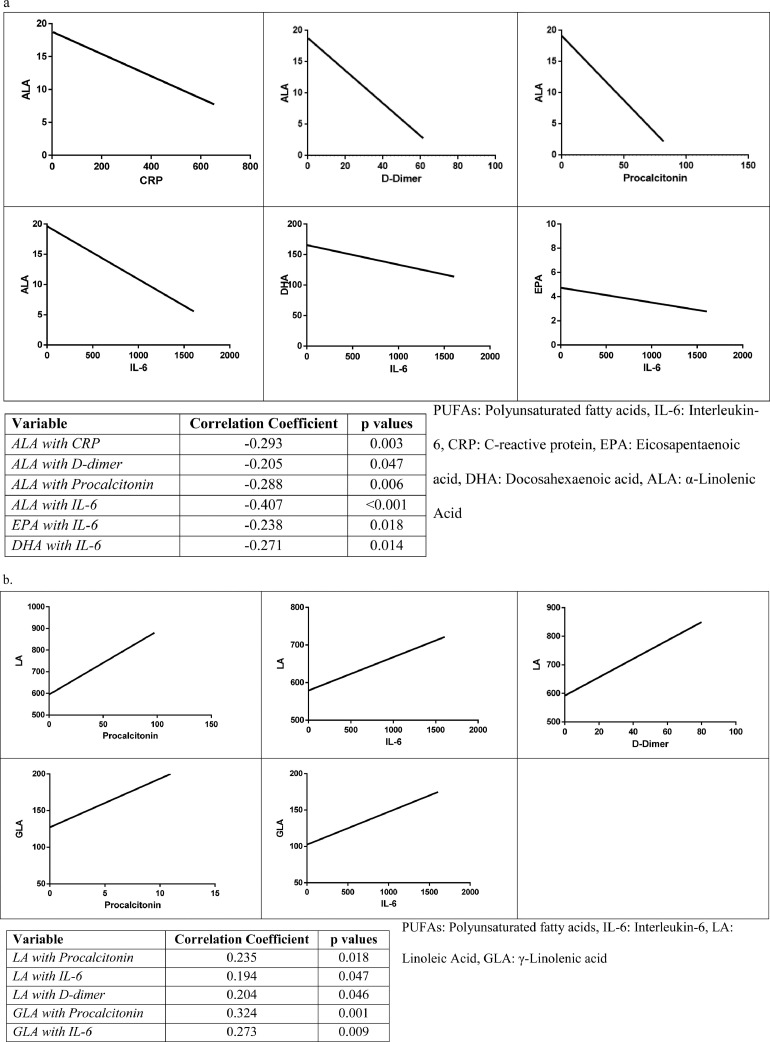
In the correlation analysis between inflammatory markers and ω-3 PUFA levels, weak to moderate negative correlations were found for ALA with IL-6, PCT, D-dimer, and CRP. The most significant correlation was observed between ALA and IL-6 (r = −0.407, P < 0.001). Among other PUFAs, EPA and DHA also showed weakly negative correlations with IL-6 (Fig. 2 a).
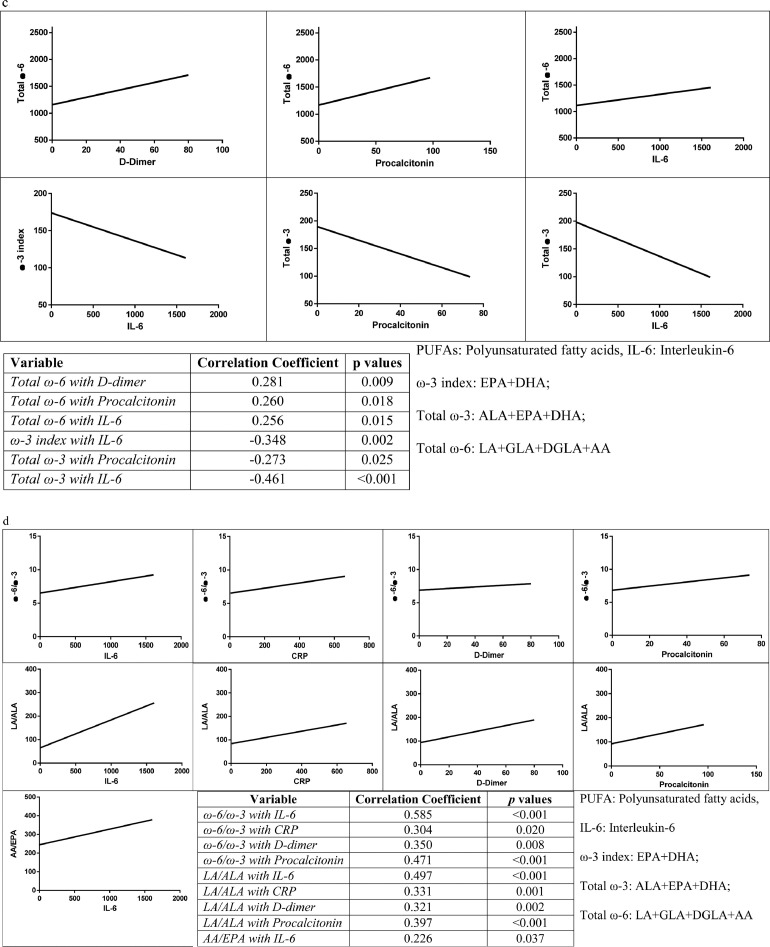
Fig. 2.
Correlations between PUFAs and inflammation markers. (a) Correlations of ω-3 PUFAs with CRP, D-dimer, IL-6, and procalcitonin. (b) Correlations of ω-6 PUFAs with procalcitonin, IL-6, and D-dimer. (c) Correlations of PUFA indices with CRP, D-dimer, IL-6, and procalcitonin. (d) Correlations of PUFA ratios with CRP, D-dimer, IL-6, and procalcitonin. The ω-3 index is EPA + DHA; total ω-3 is ALA + EPA + DHA; total ω-6 is LA + GLA + DGLA+ AA. AA, arachidonic acid; ALA, α-linolenic acid; CRP, C-reactive protein; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosopentaenoic acid; GLA, γ-linolenic acid; IL-6, interleukin-6; LA, linoleic acid; PUFA, polyunsaturated fatty acid.
When the correlations between ω-6 PUFAs and inflammatory markers were analyzed weakly positive correlations were observed for LA with D-dimer, PCT, and IL-6, as well as for GLA with PCT and D-dimer (Fig. 2b).
For the correlation between PUFA indices and inflammatory markers, the total ω-6 index showed moderately positive correlations with IL-6, PCT, and D-dimer levels. On the other hand, the ω-3 index showed a moderately negative correlation with IL-6. There were also moderately negative correlations for total ω-3 with IL-6 and PCT (Fig. 2c).
Finally, for the correlations between PUFA ratios and inflammatory markers, there were moderately positive correlations for the ω-6/ω-3 ratio with IL-6, CRP, PCT, and D-dimer. Similarly, we observed moderately positive correlations for the LA/ALA ratio with IL-6, CRP, PCT, and D-dimer, and the AA/EPA ratio showed a moderately positive correlation with IL-6 (Fig. 2d).
Discussion
It has been discovered that IL-6 production is triggered by viral load, especially in severe COVID-19 [23]. During this infection, CD4+ T lymphocytes transform into pathogenic Th1 cells, which are known to stimulate the release of granulocyte-macrophage colony-stimulating factor and proinflammatory cytokines, and to activate monocytes with high IL-6 levels [24]. We designed our study by dividing participants into three different groups by predetermined IL-6 cutoff values.
The presence and ratios of ω-6 (AA, DGLA, GLA) and ω-3 (EPA and DHA) PUFAs in the cell membrane are known to depend on their conversion from nutrients and their precursors LA and ALA, which are essential [7]. The conversion of LA to AA is much more effective than the conversion of ALA to EPA or DHA. In addition to their competition for cyclooxygenase and lysyl oxidase enzymes, ω-6 and ω-3 fatty acids compete for elongation and desaturation enzymes and phospholipids. Therefore, in addition to the presence or absence of dietary intake of these fatty acids, inflammation may also affect their concentrations among cell membrane phospholipids. Consequently, the balance between the proinflammatory and antiinflammatory processes can be modulated [25]. In light of this information, and considering the superiority of membrane fatty acids over plasma fatty acids, thanks to their not being affected by short-term dietary intake, we evaluated EM fatty acids of people with COVID-19 [26]. Significant differences were observed between groups in four of the seven PUFAs. A significant decrease was observed from group 1 to group 3 in ω-3 PUFAs—ALA, EPA, and DHA—whereas GLA levels and ω-6/ω-3 ratios were significantly increased in all groups compared. All these changes are in line with changes in the severity of COVID-19, as expected. In addition, the LA/ALA ratio was significantly lower in group 1 than the other two groups, and the AA/EPA ratio was significantly lower only in group 1 compared to group 3.
The prominent finding of the study, which should be especially emphasized here, is the AA/EPA ratio. Taking into account the PUFAs, indices, and ratios with IL-6, CRP, D-dimer, and PCT levels, the AA/EPA ratio showed a significant positive correlation only with IL-6 (Fig. 2d). In addition, the AA/EPA ratio was significant only in the comparison of groups 1 and 3. This also means that this parameter comes to the forefront in the group with a mortality rate around 90%, and it is related to IL-6 levels independent of other parameters. Another point worth considering in calculating this ratio is that AA levels did not show a significant difference between groups. As is known, AA plays a crucial role in inflammation and cytokine production [27]. Spangelo et al. showed that the AA-induced increase in IL-6 release from macrophages was attenuated in the presence of a cyclooxygenase inhibitor [28]. On the other hand, there are also studies showing that alveolar macrophages release AA and other bioactive lipids (lipoxin A4, etc.) to inactivate pathogens and suppress microbial infections [29], [30], [31], [32]. This means there are conflicting results regarding AA levels in different clinical studies evaluating inflammatory processes. This reveals that the proinflammatory/antiinflammatory balance is not based on a single parameter, but is also affected by the intermediate PUFAs synthesized from LA and ALA and even their ratios during the process. Considering that AA and EPA are both synthesized by the same enzyme steps (Δ-5 desaturase and elongase/Δ-6 desaturase reactions, respectively) from LA and ALA, the AA/EPA ratio seems more important in the balance between proinflammatory and antiinflammatory pathways than AA and EPA individually. These results suggest that the AA/EPA ratio can be a marker of systemic inflammation like IL-6, and also an indicator of mortality, and it has a possible negative association with lung function [33].
The levels of LA (an ω-3 FA) did not differ between groups, whereas ALA (an ω-6 FA) was found to be low especially in participants considered to be at high risk for mechanical ventilation and intubation during their hospital stay (groups 2 and 3). An increasing trend in LA/ALA ratios was observed from group 1 to group 3 (Table 2). Moreover, although significant negative correlations were observed between ALA and evaluated inflammatory parameters (IL-6, CRP, D-dimer, and PCT), no correlation was observed between LA and any of these parameters. Also, the correlation values (P values) of the LA/ALA ratio with the other four parameters were lower than those for ALA individually. We think this finding is substantial because it shows that starting from the essential fatty acids LA and ALA, which are considered precursors for PUFAs, the ratios of fatty acids in the same step may be more valuable [8].
In the literature, there are studies showing that EPA and DHA can inhibit IL-6 production by venous endothelial cells [34]. Moreover, supplementation of ˃2 g EPA-DHA/d in the diet of healthy individuals has been shown to reduce IL-6 production by mononuclear cells [35,36]. The EPA, DHA, and ω-3 index values, which showed significant decreases from group 1 to group 3 in our study, and the total ω-3 values obtained by adding ALA to these, are compatible with these findings. Although our study did not aim to directly evaluate the effect of nutritional status or nutritional supplements on the development of pneumonia in COVID-19, it highlights the importance of revealing long-term data on ω-6/ω-3 ratios by evaluating EM fatty-acid levels and the subgroups of these PUFAs. As a matter of fact, an ω-6/ω-3 imbalance allows us to have an idea about the need for necessary reinforcement, especially in those at high risk. By the simplest definition, ω-3 PUFAs inhibit the conversion of LA to ALA, the precursor of prostaglandin E2, decreased production of which consequently leads to diminished production of inflammatory cytokines [26]. This is supported by our study in terms of the ω-3 index; the significant negative correlation between total ω-3 and IL-6; and the significant positive correlations between total ω-6 and IL-6, as well as between the ω-6/ω-3 ratio and all other parameters, including IL-6 (Fig. 2c, d). Also important are the changes in the ω-6/ω-3 ratio and LA/ALA levels among groups, and the relation of these data with the increase in the incidence of mortality (Table 1).
One of the other important findings of our study is that among ω-6 PUFAs, only GLA differed significantly in EM levels between groups. In addition, positive correlations were observed for GLA with PCT and IL-6. Levels of LA also showed positive correlations with D-dimer, PCT, and IL-6. Unfortunately, the literature evaluating plasma or EM PUFAs with systemic inflammation markers in people with COVID-19 is limited, although there are studies investigating the significance of these markers. In the study conducted by Liu et al. to evaluate the predictive values of IL-6, CRP, and PCT as to the severity of COVID-19 cases, it was shown that levels of these inflammatory markers were significantly elevated in the vast majority of severe cases compared to mild cases [37]. That study also indicated that these findings are consistent with the concept of the cytokine storm, as Cox proportional-hazard analysis supported the use of these factors as independent factors in predicting the severity of COVID-19. However, these findings still need to be studied further using a larger sample.
Our study is the first report in the literature showing significant positive correlations of ω-6 PUFAs, especially GLA and LA, with PCT, and negative correlations of ALA and ω-3 PUFAs. The difference between PCT levels among groups, and the correlations with GLA and total ω-3 fatty acids, can point to the fact that PCT can be assumed as an indicator of systemic response caused by infection rather than other causes, or to support activity of the proinflammatory process. The rate-limiting enzyme Δ-6 desaturase, which catalyzes the metabolic pathway from LA to GLA, is very slow. After synthesis, GLA is rapidly extended to DGLA by elongase, and DGLA is acetylated and incorporated into cell membrane phospholipids. A small amount is also converted to AA [38]. The reason that only GLA levels were found to be significantly different between groups may be that the level of Δ-6 desaturase is affected at different levels in different people. Although there are no current studies that directly evaluate Δ-6 desaturase in diseases like COVID-19 or other inflammatory diseases causing pneumonia, a number of cancer studies have shown knocked-down or inhibited Δ-6 desaturase to reduce expression of IL-6 and tumor necrosis factor-α and thereby cause a decrease in overall inflammation [39]. Of course, it should be kept in mind that this enzyme is also the enzyme that catalyzes the first step in the synthesis of EPA and DHA from ALA. This situation reveals that the activation of related enzymes may differ between the groups in our study depending on IL-6 level. This also suggests that our findings are new and valuable in this sense. The present study does have some limitations, as follows: The number of participants was relatively limited, levels of prostaglandins and leukotrienes were not assessed, and other possible mediators that could take part in the formation of the cytokine storm, such as lipoxin A4 and resolvin, were not evaluated. But as this is the first study in the literature to evaluate EM PUFAs in COVID-19, it can form a basis for more detailed studies in the future evaluating the membrane fatty-acid composition and their metabolism related to the cytokine storm in a larger perspective.
Conclusion
Detailed analysis of PUFAs can provide valuable information in the management of dietary intake or supplementation of PUFAs which are believed to be deficient in COVID-19 and considered responsible for its severity. In addition to individual measurements of PUFAs, evaluation of ratios like ω-6/ω-3, LA/ALA, and AA/EPA rises to prominence in the management of inflammation-related diseases. The ω-3 PUFAs and the AA/EPA ratio, like IL-6, are markers of systemic inflammation and indicators of mortality, suggesting that they may have a possible negative association with lung function in COVID-19.
Author Contributions
Designing research studies, conducting experiments, acquiring data, analyzing data, providing reagents, and writing the manuscript.
Erdim Sertoglu: Designing research studies, conducting experiments, acquiring data, analyzing data, providing reagents, writing the manuscript, and responding to reviewer(s) comments. Cigdem Yucel: Designing research studies, conducting experiments, acquiring data, and writing the manuscript. Ahmet Rifat Balik: conducting experiments, analyzing data, and writing the manuscript. Canturk Tasci: Patient selection and examination, acquiring data, critical review of the manuscript. Sedat Bilge: Patient selection and examination, acquiring data. Namik Kemal Nazaroglu: Conducting experiments Meryem Sebla Ertugrul: Conducting experiments Taner Ozgurtas: Designing research studies, critical review of the manuscript.
Consent for publication
All authors have consent to publication.
Availability of data and material
Data is available from the Gulhane Training and Research Hospital, Department of Clinical Biochemistry upon request.
Funding
None
Declaration of Competing Interest
Authors have no conflict of interest.
Acknowledgments
We would like to thank the laboratory technicians supporting us during the analysis steps at the Gulhane Training and Research Hospital and Synlab laboratories.
References
- 1.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blau N, Duran M, Blaskovics ME, Gibson KM, editors. Physician's guide to the laboratory diagnosis of metabolic diseases. Chapman and Hall; 1996. [Google Scholar]
- 9.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–897. doi: 10.1002/mnfr.200700289. [DOI] [PubMed] [Google Scholar]
- 11.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 13.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 14.Goldman DW, Pickett WC, Goetzl EJ. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983;117:282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- 15.Guirao JJ, Cabrera CM, Jiménez N, Rincón L, Urra JM. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol Immunol. 2020;128:64–68. doi: 10.1016/j.molimm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.05.008. 128–36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorham J, Moreau A, Corazza F, Peluso L, Ponthieux F, Talamonti M, et al. Interleukine-6 in critically ill COVID-19 patients: a retrospective analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Hu Y, Yu J, Ma T. Retrospective analysis of laboratory testing in 54 patients with severe- or critical-type 2019 novel coronavirus pneumonia. Lab Invest. 2020;100:794–800. doi: 10.1038/s41374-020-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Fei D, Li X, Zhao M, Yu K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020;46:1475–1476. doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37:85–98. [PMC free article] [PubMed] [Google Scholar]
- 23.Uciechowski P, Dempke WCM. Interleukin-6: a masterplayer in the cytokine network. Oncology. 2020;98:131–137. doi: 10.1159/000505099. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Huntington K, Zhang S, Carlsen L, So E-Y, Parker C, et al. MEK inhibitors reduce cellular expression of ACE2, pERK, pRb while stimulating NK-mediated cytotoxicity and attenuating inflammatory cytokines relevant to SARS-CoV-2 infection. Oncotarget. 2020;11:4201–4223. doi: 10.18632/oncotarget.27799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 26.Sertoglu E, Kurt I, Tapan S, Uyanik M, Serdar MA, Kayadibi H, et al. Comparison of plasma and erythrocyte membrane fatty acid compositions in patients with end-stage renal disease and type 2 diabetes mellitus. Chem Phys Lipids. 2014;178:11–17. doi: 10.1016/j.chemphyslip.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Tavakoli S, Cowan MJ, Benfield T, Logun C, Shelhamer JH. Prostaglandin E(2)-induced interleukin-6 release by a human airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2001;280:L127–L133. doi: 10.1152/ajplung.2001.280.1.L127. [DOI] [PubMed] [Google Scholar]
- 28.Spangelo BL, Jarvis WD, Judd AM, MacLeod RM. Induction of interleukin-6 release by interleukin-1 in rat anterior pituitary cells in vitro: evidence for an eicosanoid-dependent mechanism. Endocrinology. 1991;129:2886–2894. doi: 10.1210/endo-129-6-2886. [DOI] [PubMed] [Google Scholar]
- 29.Das UN. Bioactive lipids in COVID-19—further evidence. Arch Med Res. 2021;52:107–120. doi: 10.1016/j.arcmed.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer K, Schmidt R, Muhly-Reinholz M. In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFα impact of ω-3 versus ω-6 fatty acids. J Lipid Res. 2002;43:944–951. [PubMed] [Google Scholar]
- 31.Das UN. Bioactive lipids and coronavirus (COVID-19)—further discussion. Arch Med Res. 2020;51:445–449. doi: 10.1016/j.arcmed.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simões RL, De-Brito NM, Cunha-Costa H. Lipoxin A4 selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int J Cancer. 2017;140:346–357. doi: 10.1002/ijc.30424. [DOI] [PubMed] [Google Scholar]
- 33.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 34.Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Biol Med. 1997;400:589–597. [PubMed] [Google Scholar]
- 35.Abbate R, Gori AM, Martini F, Brunelli T, Filippini M, Francalanci I, et al. n-3 PUFA supplementation, monocyte PCA expression and interleukin-6 production. Prostaglandins Leukot Essent Fatty Acids. 1996;54:439–444. doi: 10.1016/s0952-3278(96)90028-9. [DOI] [PubMed] [Google Scholar]
- 36.Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, et al. Inhibition of tumour necrosis factor-α and interleukin-6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90:405–412. doi: 10.1079/bjn2003892. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezapour-Firouzi S. In: Nutrition and lifestyle in neurological autoimmune diseases: multiple sclerosis. Watson RR, Killgore WDS, editors. Academic Press; Amsterdam: 2017. Herbal oil supplement with hot-nature diet for multiple sclerosis; pp. 229–245. In: editors. [Google Scholar]
- 39.He C, Qu X, Wan J, Rong R, Huang L, Cai C, et al. Inhibiting delta-6 desaturase activity suppresses tumor growth in mice. PLoS One. 2012;7:e47567. doi: 10.1371/journal.pone.0047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the Gulhane Training and Research Hospital, Department of Clinical Biochemistry upon request.