Abstract
Background.
Outcomes of liver transplantation (LT) from donation after circulatory death (DCD) have been improving; however, ischemic cholangiopathy (IC) continues to be a problem. In 2014, measures to minimize donor hepatectomy time (DHT) and cold ischemic time (CIT) have been adopted to improve DCD LT outcomes.
Methods.
Retrospective review of all patients who underwent DCD LT between 2005 and 2017 was performed. We compared outcomes of patients who were transplanted before 2014 (historic group) with those who were transplanted between 2014 and 2017 (modern group).
Results.
We identified 112 patients; 44 were in the historic group and 68 in the modern group. Donors in the historic group were younger (26.5 versus 33, P = 0.007) and had a lower body mass index (26.2 versus 28.2, P = 0.007). DHT (min) and CIT (h) were significantly longer in the historic group (21.5 versus 14, P < 0.001 and 5.3 versus 4.2, P < 0.001, respectively). Fourteen patients (12.5%) developed IC, with a significantly higher incidence in the historic group (23.3% versus 6.1%, P = 0.02). There was no difference in graft and patient survival between both groups.
Conclusion.
In appropriately selected recipients, minimization of DHT and CIT may decrease the incidence of IC. These changes can potentially expand the DCD donor pool.
INTRODUCTION
There has been significant improvement in recipient outcomes using liver allografts from donation after circulatory death (DCD) donors over the past decade. Recipient survival following DCD liver transplantation can now approach that which is seen with grafts from donation after brain death (DBD) donors in appropriately selected patients, resulting in increased national utilization of DCD liver allografts.1-7
Despite these advances, biliary complications following DCD liver transplantation continue to be more frequently encountered as compared to DBD liver transplantation. Ischemic cholangiopathy (IC) was historically reported to occur in as high as 34% of cases and is associated with significant morbidity, multiple readmissions and biliary interventions, retransplantation, and mortality8,9; however, more contemporary reports implementing strategies like using grafts from younger donors, minimization of cold ischemic time (CIT) and warm ischemic time (WIT), improved recipient selection, and the use of tissue plasminogen activator have lowered IC rates as low as 3%.10-14 The donor hepatectomy time (DHT), defined as the time from the initiation of aortic flush to liver extraction, has been associated with better graft and patient survival, as well as lower rates of IC in both DCD and DBD livers.15-18
To increase organ utilization while concomitantly maintaining acceptable patient and allograft survival, we implemented several changes in our approach to DCD liver transplantation in 2014. The primary focus was on the minimization of CIT and DHT. In this study, we reviewed the efficacy of these changes. We hypothesized that the clinical focus on operative timing would result in broader application of DCD liver transplantation with improved outcomes.
MATERIALS AND METHODS
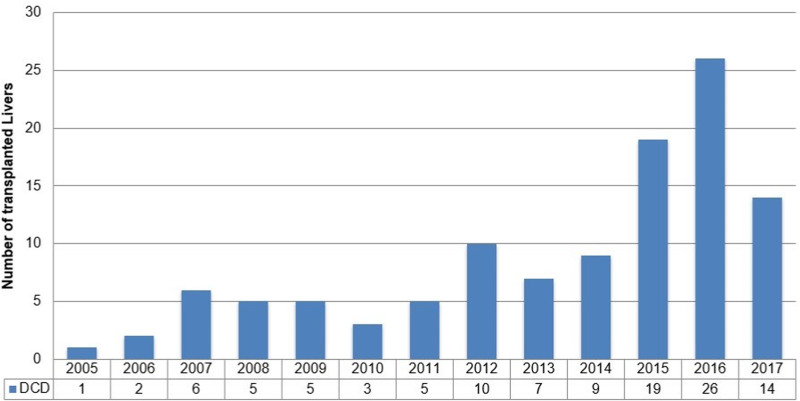
Institutional review board approval was obtained to perform a retrospective review of all DCD liver transplants performed at our institution between January 2005 and December 2017. Simultaneous liver and kidney (SLK) recipients were included in our cohort (n = 11). Our DCD liver transplant program was initiated in 2005 and witnessed sustained growth over the following years (Figure 1). In 2014, we modified our DCD liver transplant approach, placing a significant emphasis on rapid donor hepatectomy and minimization of CIT. We compared outcomes for patients who were transplanted before 2014 (historic group) and patients who were transplanted between 2014 and 2017 (modern group).
FIGURE 1.
Annual volume of DCD liver transplantation in our center. DCD, donation after circulatory death.
We reviewed recipient demographics, physiological model of end-stage liver disease-sodium score at the time of transplantation, and cause of liver disease. DonorNet was used to retrieve donor demographics, cause of death, ischemic times, the host organ procurement organization (OPO), and the preservation solution. For patients who underwent SLK transplantation, the kidney donor profile index was obtained.
Outcomes
The primary outcome of interest was the development of recipient biliary complications. Biliary complications were divided into anastomotic strictures, bile leak, and IC. IC was defined by the presence of multiple intrahepatic biliary strictures, nonanastomotic biliary strictures, or the presence of biliary casts in the absence of hepatic artery thrombosis (HAT). Biliary interventions including endoscopic retrograde cholangiogram (ERC) and percutaneous transhepatic cholangiogram were recorded. Secondary outcomes included patient and graft survival, the incidence of surgical technical complications such as HAT or portal vein thrombosis, and the occurrence of primary nonfunction (PNF) or early allograft dysfunction (EAD). Patient, liver, and kidney graft survivals were recorded on the basis of the last follow-up visit. Liver graft failure was defined as patient death or retransplantation. Kidney graft failure was defined as death, retransplantation, or initiation of dialysis. PNF was defined as failure of the liver allograft to function, resulting in retransplantation or patient death within 1 wk posttransplantation. EAD was defined by the presence of one of the following: peak aspartate aminotransferase or alanine aminotransferase >2000 in the first week, bilirubin >10 mg/dL, or international normalized ratio >1.6 at posttransplant day 7.19
Donor Operation
All liver allografts were procured from Maastricht III DCD donors. Withdrawal of care, initiation of comfort measures, and declaration of death were done in accordance with OPOs and hospital-specific policies. Withdrawal of support was performed either in the operating room or in a preoperative holding area. A heparin bolus of 300 IU/kg was given intravenously before withdrawal of support. After declaration of death by the local hospital physician, a mandatory 2 to 5 min (depending on the hospital policy) observation period was maintained, during which the donor was prepped and draped for organ recovery if not done previously. After the observation period, a super-rapid organ recovery was performed. The abdominal cavity was entered through a generous midline laparotomy, the small bowel was reflected toward the left upper quadrant, the infrarenal aorta was cannulated, and in situ flush with a cold preservation solution was initiated. The time from skin incision to aortic cannulation was typically <3 min. After initiation of the aortic flush, the thoracic cavity was entered through a median sternotomy, and the descending aorta was cross-clamped in the left chest. The venous system was decompressed in the chest. Topical cooling with slush ice was then initiated. Nonvascular dissection was performed throughout flushing. As soon as the aortic flush was complete, the vascular attachments were divided. The portal flush was performed on the back table with 1 to 2 L of preservation solution until the effluent from the suprahepatic cava cleared of blood. The biliary tree was irrigated retrograde through the common hepatic duct after incising the gallbladder.
In 2014, we implemented several modifications to our procurement technique to minimize DHT. All procurements were performed by an experienced liver transplant surgeon along with a transplant fellow. Heavy emphasis was placed on prompt liver mobilization as soon as the aorta is cross-clamped. All diaphragmatic and retroperitoneal attachments were divided while the abdominal cavity was full of slush ice, leaving the liver attached only by vascular structures. To shorten the duration of the in situ aortic flush, pressurized bags (130 mm Hg) were used to infuse the solution. Thrombolytic agents were not used in the donor or recipient operation in either group.
Recipient Selection and Operation
Beginning in 2014, deliberate effort was made to appropriately match donors and recipients. To facilitate a rapid hepatectomy, we avoided recipients with a hostile upper abdomen (patients with prior upper abdominal surgeries, portal vein thrombosis, or prior transplants). The recipient operation was started while the donor team was en route to the recipient hospital to minimize CIT. Efforts were made to select recipients with stronger metabolic and cardiopulmonary reserve that would be able to safely maintain hemodynamic stability following liver reperfusion, as well as during a perioperative period with the potential for EAD.
Patients most commonly underwent liver transplant using the piggyback method for caval reconstruction without venovenous bypass. After completion of the caval anastomosis, the allograft was flushed with 1 L of chilled lactated Ringer’s solution with albumin via the portal vein. Before systemic reperfusion, the allograft was flushed with portal blood that was vented through the donor infrahepatic cava (blood flush). The liver was then reperfused off the portal vein before arterial anastomosis. After completion of the arterial anastomosis, the biliary system was typically reconstructed via a primary duct-to-duct anastomosis without a stent regardless of the cause of liver disease. Roux-en-Y hepaticojejunostomy was performed as required by the condition of the recipient biliary system. A 5 French internal–external biliary stent was used in the first 21 cases. It was introduced through the native cystic duct, fixed with hemorrhoidal bands and a 5-0 Monocryl stitch, and then externalized through the abdominal wall. In such recipients, a tube cholangiogram was performed routinely within the first postoperative week and repeated as needed. The stent was removed in the outpatient setting after 6 wk.
Definition of Ischemic Times
Donor WIT was calculated from the withdrawal of support to the initiation of the aortic flush. Agonal WIT (aWIT) was calculated from the time when the systolic blood pressure dropped to <80 mm Hg or when oxygen saturations dropped to <80% to the initiation of the aortic flush. DHT was calculated from the initiation of aortic perfusion to the completion of liver extraction and placement of the graft on ice at the back table. CIT was defined as the time from aortic cross-clamp in the donor to portal reperfusion in the recipient.
Statistics
We compared donor and recipient demographics and clinical variables between patients in the historic and modern groups. Summary statistics for continuous variables are represented as median and interquartile ranges. All categorical variables are listed as frequencies and percentages. We used the Wilcoxon rank-sum test to determine differences in continuous variables between the 2 groups, and the Fisher exact test was used for the comparison of categorical data. The level of statistical significance was set at P < 0.05.
We created a multivariate logistic regression model to assess which variables were predictive to the development of IC. The complete model included the following features: recipient age, recipient gender, recipient race (dichotomized as Caucasian versus not), physiological model for end-stage liver disease (MELD), hepatitis C status, hepatocellular carcinoma status, year of transplant (dichotomized as before/after 2014), donor age, donor gender, donor race (dichotomized as Caucasian versus not), donor body mass index (BMI), cause of death, solution, location (local or import), CIT (h), WIT (min), aWIT (min), and DHT (min). DHT was analyzed both as a continuous variable and as a dichotomized variable using a sliding window to determine a cutoff time that significantly impacts the development of IC. Correlation among variables was assessed before analysis, and highly correlated variables were tested separately in the model. Variables were centered and scaled; then, multivariate logistic regression with backward feature selection was used to determine a subset of relevant variables to be included in the final model. The fitness of the different models was assessed using the C-statistic, where values closest to 1 indicate a model is strongly predictive. Odds ratios, 95% confidence intervals, and P values were calculated.
All statistical analyses were performed in R (version 4.0.2). Survival analysis was performed using the “survival” package, and Kaplan-Meier curves were generated using the “survminer” package in R.20
RESULTS
Over the 13-y period of the study, our center performed 112 DCD liver transplants, including 11 (9.8%) patients who underwent DCD SLK transplantation. Forty-four (39.2%) patients were transplanted before 2014 (historic group), and 68 (60.7%) were transplanted between 2014 and 2017 (modern group).
Recipient Characteristics
The median age of our overall cohort was 56 (50–61) y. Seventy-one (63.4%) recipients were males, and 80 (71.4%) were Caucasians. The most common cause of liver disease was hepatitis C virus infection (46 patients, 41.1%). The median physiological MELD score at transplantation was 22 (14–29). Five patients were transplanted after being listed as status 1A; 4 patients had fulminant hepatic failure (3 secondary to acetaminophen overdose and 1 from hepatitis B virus infection), and 1 patient developed HAT on postoperative day 7 after living donor liver transplantation using a left lobe graft. There were no significant demographic differences between patients in the historic and modern groups (Table 1).
TABLE 1.
Recipient characteristics
| Historic cohort(n = 44) | Modern cohort(n = 68) | P | |
|---|---|---|---|
| Age (y) | 55 (49.0–59.2) | 57 (50.8–62.2) | 0.18 |
| Gender (male) | 26 (59.1%) | 45 (66.2%) | 0.55 |
| Race | 0.49 | ||
| Caucasian | 32 (72.7%) | 48 (70.6%) | |
| African American | 10 (22.7%) | 16 (23.5%) | |
| Hispanic | 0 (0.0%) | 3 (4.4%) | |
| Asian | 2 (4.5%) | 1 (1.5%) | |
| Physiological MELD-Na at transplantation | 22.5 (14.2–29.8) | 20 (13.0–28.0) | 0.48 |
| Hepatocellular carcinoma | 11 (25.0%) | 21 (30.9%) | 0.53 |
| Hepatitis C virus infection | 21 (47.7%) | 25 (36.8%) | 0.33 |
| Cause of liver disease | 0.51 | ||
| Viral hepatitis | 21 (47.7%) | 27 (39.7%) | |
| Alcohol | 12 (27.3%) | 14 (20.6%) | |
| NASH | 4 (9.1%) | 14 (20.6%) | |
| Cholestatic | 2 (4.5%) | 3 (4.4%) | |
| Other | 5 (11.4%) | 10 (14.7%) |
Data are presented as median (interquartile range) and number (percentage).
MELD-Na, model of end-stage liver disease-sodium; NASH, nonalcoholic steatohepatitis.
Donor Characteristics
The median age of the donors was 30.5 (25–39.5) y. Seventy-five (67.0%) were male, and the median BMI was 26.96 (23.26–31.97) kg/m2. The most common cause of death was anoxia (52 donors, 56.4%). Sixty-one (54.5%) grafts were procured from our local OPO. The median kidney donor profile index for the 11 SLK grafts was 23 (18–44.5). Donors in the historic group were significantly younger (26.5 versus 33.0, P = 0.007) and had a lower BMI (26.2 versus 28.2, P = 0.03). Anoxia as a cause of death was more common in the modern group (31.8% versus 55.9%, P = 0.007). There were more organs imported from outside OPOs (20.5% versus 61.8%, P < 0.001) and a higher utilization of University of Wisconsin as the flush solution (63.6% versus 95.6%, P < 0.001) in the modern group (Table 2).
TABLE 2.
Donor characteristics
| Historic cohort(n = 44) | Modern cohort(n = 68) | P | |
|---|---|---|---|
| Age | 26.5 (23.0–34.2) | 33.0 (26.0–41.2) | 0.007 |
| Gender (male) | 34 (77.3%) | 41 (60.3%) | 0.07 |
| Race | 0.67 | ||
| Caucasian | 35 (79.5%) | 50 (73.5%) | |
| African American | 7 (15.9%) | 11 (16.2%) | |
| Hispanic | 2 (4.5%) | 4 (5.9%) | |
| Asian | 0 (0.0%) | 3 (4.4%) | |
| BMI | 26.2 (21.5–29.5) | 28.2 (23.8–33.0) | 0.03 |
| Cause of death | 0.007 | ||
| Anoxia | 14 (31.8%) | 38 (55.9%) | |
| Cerebrovascular accident | 5 (11.4%) | 11 (16.2%) | |
| Head trauma | 22 (50.0%) | 19 (27.9%) | |
| Other | 3 (6.8%) | 0 (0.0%) | |
| Graft origin (import) | 9 (20.5%) | 42 (61.8%) | <0.001 |
| Flush solution (UW) | 28 (63.6%) | 65 (95.6%) | <0.001 |
Data are presented as median (interquartile range) and number (percentage).
BMI, body mass index; UW, University of Wisconsin.
Ischemic Times
The overall median WIT, aWIT, and DHT were 21 (17–25) min, 18 (14 –22) min, and 16 (12–23.5) min, respectively. The median CIT was 4.5 (3.77–5.56) h. The DHT (21.5 versus 14 min, P < 0.001) and the CIT (5.3 versus 4.2 h, P < 0.001) were significantly longer in the historic group. There were no other significant differences when the groups were stratified by era (Table 3). DHT and CIT was significantly longer in patients that developed IC compared with patients who did not (24.5 [21.2–32.2] versus 15.5 [12.0–21.0] min, P < 0.001 and 5.8 [4.4–6.5] versus 4.5 [3.7–5.3] h, P = 0.02, respectively).
TABLE 3.
Ischemic times
| Historic cohort(n = 44) | Modern cohort(n = 68) | P | |
|---|---|---|---|
| Warm ischemic time (min) | 20.0 (17.0–26.0) | 21.5 (17.0–25.0) | 0.81 |
| Agonal warm ischemic time (min) | 18.0 (14.0–21.0) | 17.0 (13.8–22.0) | 0.76 |
| Donor hepatectomy time (min) | 21.5 (16.0–30.0) | 14.0 (11.0–19.0) | <0.001 |
| Cold ischemic time (h) | 5.3 (4.2–6.3) | 4.2 (3.5–5.0) | <0.001 |
Data are presented as median (interquartile range).
Outcomes
Biliary Complications
Anastomotic Strictures and Biliary Leaks
Thirteen patients developed localized anastomotic strictures, 11 of whom were managed successfully with ERC. One patient required percutaneous biliary drainage in addition to ERC, and the second patient underwent Roux-en-Y hepaticojejunostomy after inability to successfully manage the stricture endoscopically because of a prior Roux-en-Y gastric bypass. Three patients developed minor anastomotic bile leaks that were managed with endoscopic stenting. There was no statistical difference in the incidence of bile leak and anastomotic strictures between the historic and modern groups (Table 4).
TABLE 4.
Recipient perioperative complications and ischemic cholangiopathy
| Historic cohort(n = 44) | Modern cohort(n = 68) | P | |
|---|---|---|---|
| Bile leak | 2 (4.7%) | 1 (1.5%) | 0.56 |
| Anastomotic biliary strictures | 2 (4.7%) | 11 (16.7%) | 0.07 |
| Ischemic cholangiopathy | 10 (23.3%) | 4 (6.1%) | 0.02 |
| Patients undergoing ERC | 17 (38.6%) | 19 (27.9%) | 0.30 |
| Reoperation | 7 (20.0%) | 21 (31.3%) | 0.25 |
| Hepatic artery thrombosis | 1 (2.3%) | 2 (3.0%) | 1.00 |
| Portal vein thrombosis | 0 (0.0%) | 1 (1.5%) | 1.00 |
| Early allograft dysfunction | 32 (74.4%) | 45 (68.2%) | 0.53 |
| Primary nonfunction | 0 (0.0%) | 2 (3.0%) | 0.52 |
| Required retransplantation | 5 (11.4%) | 5 (7.4%) | 0.51 |
Data are presented as number (percentage).
ERC, endoscopic retrograde cholangiogram.
IC
During the study period, 14 patients (12.5%) developed IC. There was a higher incidence of IC in the historic group (23.3% versus 6.1%, P = 0.02). The average time from transplant to diagnosis was 73 d (range, 20–142 d). Patients required an average of 3 (range, 0–7) ERCs and 0.7 (range, 0–6) percutaneous transhepatic cholangiograms. Eight patients were managed successfully with endoscopic and percutaneous interventions alone; 5 are still alive, and 3 died from unrelated issues after 72, 1280, and 2569 d from transplant, respectively. Six patients developed severe forms of IC that were refractory to endoscopic and percutaneous interventions. These patients underwent retransplantation after an average of 440 d (range, 30–1970 d). Four patients are alive, and 2 died from septic complications 2954 and 73 d after retransplantation (Table 5).
TABLE 5.
Patients that developed ischemic cholangiopathy
| Recipient age/gender | Cause of liver disease/MELD | Tx year | Donor age/BMI | Local vs import | Flush | WIT(min) | aWIT(min) | DHT(min) | CIT(h) | Disposition |
|---|---|---|---|---|---|---|---|---|---|---|
| 59 F | ALD/34 | 2006 | 49/26.3 | Import | HTK | 20 | 18 | 19 | 7.76 | Retransplanted. Expired. |
| 56 M | HBV/7 | 2007 | 44/28.6 | Local | HTK | 27 | 22 | 24 | 6.93 | Retransplanted. Alive. |
| 63 M | NASH/15 | 2008 | 34/20.5 | Local | HTK | 26 | 18 | 41 | 6.53 | Retransplanted. Alive. |
| 42 Ma | ALD/27 | 2009 | 40/36.5 | Local | UW | 17 | 11 | 26 | 5.93 | Biliary interventions. Expired. |
| 70 F | HCV/12 | 2010 | 29/27.0 | Local | UW | 25 | 24 | 33 | 6.00 | Biliary interventions. Alive. |
| 48 F | ALD/16 | 2011 | 31/21.6 | Local | UW | 18 | 15 | 22 | 5.57 | Biliary interventions. Alive. |
| 41 F | HBV/fulminant | 2012 | 25/32.0 | Local | UW | 19 | 18 | 24 | 4.05 | Biliary interventions. Expired. |
| 55 M | HCV/18 | 2012 | 29/28.8 | Import | UW | 26 | 25 | 36 | 6.28 | Retransplanted. Alive. |
| 51 M | NASH/20 | 2013 | 28/32.0 | Import | UW | 18 | 16 | 17 | 4.07 | Biliary interventions. Expired. |
| 53 F | NASH/22 | 2013 | 23/24.5 | Local | UW | 25 | 20 | 21 | 3.45 | Biliary interventions. Alive. |
| 44 F | ALD/35 | 2015 | 28/25.2 | Import | UW | 25 | 24 | 19 | 3.62 | Retransplanted. Expired. |
| 44 M | ALD/29 | 2016 | 29/46.4 | Import | HTK | 23 | 21 | 30 | 8.33 | Biliary interventions. Alive. |
| 65 M | ALD/26 | 2017 | 35/24.8 | Import | HTK | 40 | 10 | 37 | 5.73 | Biliary interventions. Alive. |
| 54 M | ALD/16 | 2017 | 50/32.3 | Import | UW | 23 | 22 | 25 | 5.35 | Retransplanted. Expired. |
aThis patient underwent a simultaneous kidney and liver transplant.
ALD, alcoholic liver disease; aWIT, agonal warm ischemic time; BMI, body mass index; CIT, cold ischemic time; DHT, donor hepatectomy time; F, female; HBV, hepatitis B virus; HCV, hepatitis C virus; HTK, histidine-tryptophan-ketoglutarate solution; M, male; MELD, model for end-stage liver disease; NASH, nonalcoholic steatohepatitis; Tx, transplant; UW, University of Wisconsin solution; WIT, donor warm ischemic time.
Vascular Complications and Early Graft Loss
There was no difference between historic and modern groups in regard to vascular complications, reoperation within 30 d, EAD, PNF, or retransplantation (Table 4). In the overall cohort, 7 patients (6.3%) lost their graft within the first 30 d after transplantation. Two patients died intraoperatively from cardiac arrest following reperfusion. One patient developed a portal vein thrombosis on postoperative day 3 that required multiple reoperations and a deceased donor iliac vein jump graft from the recipient superior mesenteric vein to the donor portal vein, but they eventually died on postoperative day 22 from liver failure and overwhelming sepsis. Two patients developed PNF and were successfully retransplanted; both are still alive. Two patients developed HAT and remain alive after successful retransplantation on postoperative day 7 and 23 (Table 6). A third patient developed HAT and underwent successful thrombectomy but unfortunately died of overwhelming sepsis on posttransplant day 63.
TABLE 6.
Patients with early graft loss
| Recipient age/gender | Cause of liver disease/MELD | Tx year | Donor age/BMI | Local vs import | Flush | WIT(min) | aWIT(min) | DHT(min) | CIT(h) | Disposition |
|---|---|---|---|---|---|---|---|---|---|---|
| 55 M | PSC/23 | 2007 | 53/28.9 | Local | HTK | 22 | 22 | 39 | 5.5 | HAT. Retransplanted. Alive. |
| 64 M | HCV/40 | 2011 | 25/26.1 | Local | UW | 13 | 11 | 21 | 3.78 | Intraoperative death. |
| 64 M | HCV/8 | 2015 | 35/35.9 | Import | UW | 23 | 22 | 11 | 4.28 | PVT. Death. |
| 49 M | Neuroendocrine tumor/18 | 2016 | 34/25.9 | Import | UW | 19 | 7 | 51 | 9.68 | HAT. Retransplanted. Alive. |
| 55 M | HCV/24 | 2016 | 21/18.9 | Import | UW | 17 | 14 | 11 | 5.33 | Intraoperative death. |
| 37 M | HCV and HBV/28 | 2016 | 48/23.8 | Import | UW | 31 | 24 | 20 | 5.12 | PNF. Retransplanted. Alive. |
| 32 F | Tylenol/fulminant | 2017 | 24/32.3 | Import | UW | 22 | 22 | 22 | 4.6 | PNF. Retransplanted. Alive. |
aWIT, agonal warm ischemic time; BMI, body mass index; CIT, cold ischemic time; DHT, donor hepatectomy time; F, female; HAT, hepatic artery thrombosis; HBV, hepatitis B virus; HCV, hepatitis C virus; HTK, histidine-tryptophan-ketoglutarate solution; M, male; MELD, model for end-stage liver disease; PNF, primary nonfunction; PSC, primary sclerosing cholangitis; PVT, portal vein thrombosis; Tx, transplant; UW, University of Wisconsin solution; WIT, donor warm ischemic time.
Patient and Graft Survival
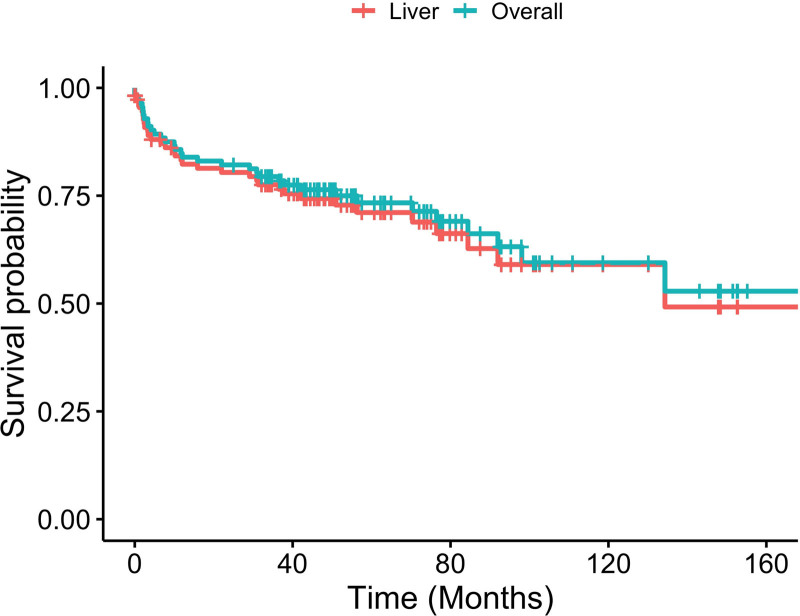
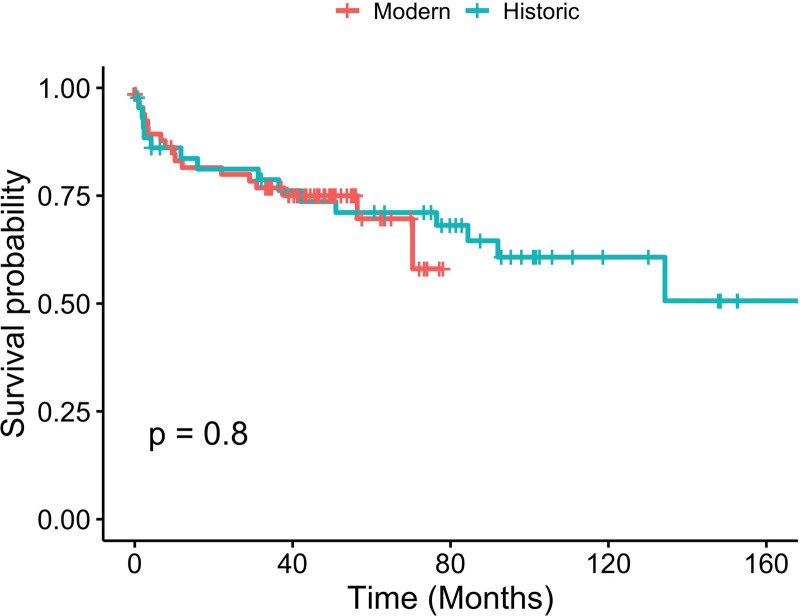
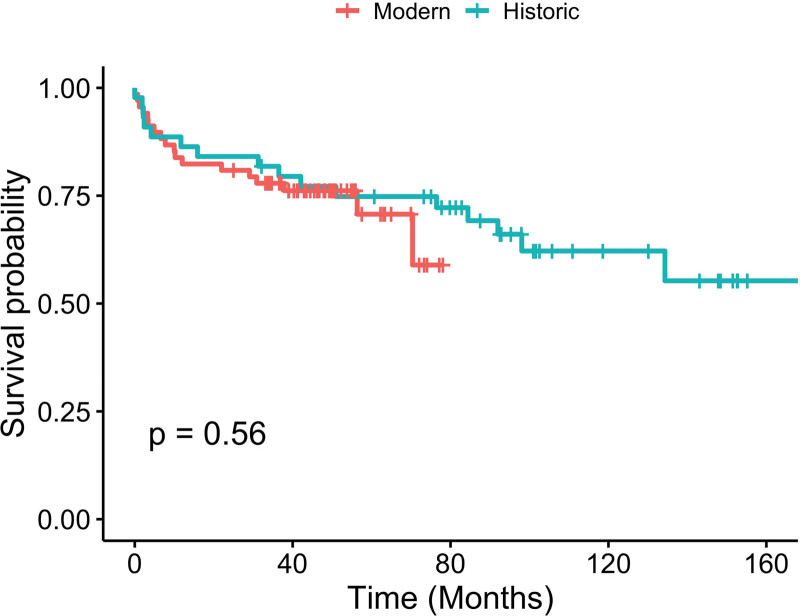
In the overall cohort, the 1- and 3-y patient survival was 84.8% and 78.1%. The 1- and 3-y liver allograft survival was 77.7% and 70.8%. In SLK recipients, renal allograft survival was 72.7% and 63.6% at 1 and 3 y (Figure 2). There was no statistical difference in patient and graft survival between the historic and modern groups (Figures 3 and 4).
FIGURE 2.
Kaplan-Meier curve for liver and overall survival.
FIGURE 3.
Kaplan-Meier curve for liver graft survival for historic and modern cohorts.
FIGURE 4.
Kaplan-Meier curve for overall survival for historic and modern cohorts.
Factors Associated With Development of IC
We used multivariate logistic regression with feature selection to evaluate the predictors for development of IC. WIT and aWIT were found to be strongly correlated (P = 0.86) and were, therefore, not tested together in the model. The most stable and predictive model occurred when DHT was dichotomized at <22 versus ≥22 min, with a C-statistic of 0.93. The longer extraction time confers an increased risk of IC (odds ratio, 14.3); however, recipient age and gender, physiological MELD, hepatocellular carcinoma status, donor gender, donor BMI, cause of death, solution, and WIT were not significant predictors for development of IC. The results of the logistic regression model showed that longer DHT, non-Caucasian donors, imported grafts, negative recipient HCV status, and transplantation before 2014 were associated with development of IC (Table 7).
TABLE 7.
Odds ratio associated with features included in the multivariate logistic regression to determine factors predictive for the development of ischemic cholangiopathy
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Recipient race (non-Caucasian) | 0.36 | 0.08-0.90 | 0.069 |
| Recipient hepatitis C status (positive) | 0.31 | 0.09-0.73 | 0.020 |
| Year of transplant (≥2014) | 0.2 | 0.05-0.56 | 0.005 |
| Donor age (y) | 1.92 | 0.87-4.73 | 0.123 |
| Donor race (non-Caucasian) | 2.28 | 1.08-5.33 | 0.038 |
| Location (import) | 3.53 | 1.3-12.88 | 0.026 |
| Cold ischemic time (h) | 0.52 | 0.20-1.18 | 0.135 |
| Donor hepatectomy time (≥22 min) | 14.29 | 2.22-137.58 | 0.009 |
CI, confidence interval.
DISCUSSION
More than 100 DCD liver transplants were performed during the 13-y study period, with more than half occurring during the “modern” era with a focus on rapid donor hepatectomy and cold time minimization. Although 14 patients (12.5%) developed IC, only 4 patients (6%) did so after changing our strategy.
As reported by the Oschner group and others,10-14 IC could be managed or eliminated with aggressive nonsurgical approaches. Less than half of patients with IC developed severe forms refractory to endoscopic or percutaneous interventions and required retransplantation. It should be noted that most patients required multiple interventions (on average, 3 ERCs), but with such management, the changes associated with IC resolved in some patients.
Most importantly, the decreased rate of IC was achieved despite improving graft utilization as reflected by higher donor age, donor BMI, and import rates. Patient and graft survival were not different between eras.
The importance of minimizing DHT and its impact on patient and graft outcomes has been emphasized in recent studies. Farid et al15 found that a DHT >60 min was associated with an increased risk of PNF in patients undergoing DCD liver transplantation.15 Jochmans et al16 found that DCD liver grafts are more susceptible to poor outcomes compared with DBD grafts when subjected to longer DHT. Additionally, DHT has been found to be an independent risk factor associated with development of biliary complications following DCD liver transplantation.17 With an average of <20 min, we acknowledge that the DHT in our cohort is much shorter than previous reports; however, we do not know at which point the DHT starts to have a determinantal effect on the graft. Gilbo et al21 suggested that there is a linear relationship between DHT and IC and that there is a 19% increment in the rate of IC for every 10 min increase in DHT. This effect is similar to 1 h increase in CIT. In an effort to standardize DCD liver procurement, the American Society of Transplant Surgeons/Association of Organ Procurement Organizations DCD recovery practices advocated to limit DHT to <30 min.22 In our cohort, patients who developed IC had a significantly longer DHT (24.5 versus 15.5 min, P < 0.001) compared with patients without IC, and longer DHT (≥22 min) was an independent factor associated with the development of IC.
Animal and human studies have shown that the liver continues to receive heat from the abdominal cavity via conduction as long as it is still in the remains in the abdomen, and this occurs despite the initiation of cold perfusion and the application of topical ice slush during procurement. Failure to promptly extract the liver from the donor delays sufficient cooling of the graft to a temperature low enough to halt most metabolic activity (<4C).23,24
The incidence of IC improved greatly over the study period. As noted above, only 6.1% of recipients developed IC after 2014 as opposed to 23.3% in the earlier era. These improved outcomes coincided with strategies adopted during the second part of the study. CIT was generally limited to 4 to 6 h by avoiding patients who anticipated having a difficult hepatectomy (retransplantation, history of complex portal vein thrombosis, and patients with extensive upper abdominal surgeries) and by adopting strategies to enhance transport and implant efficiency. Our average CIT in our cohort was 4.9 h, with only 19 patients with a CIT longer than 6 h (most of the latter were transplanted before 2011). DCD recipient selection was made with special attention to cardiopulmonary reserve. Despite having 18 patients with a MELD >30 at the time of transplantation and 5 status A1 patients, we avoided patients who were perceived to lack adequate reserve to tolerate physiologically challenging reperfusion as well as potential EAD. WIT was generally limited to 30 min with special attention to aWIT. The average aWIT for our cohort was 18 min with only 9 patients having a WIT >30 min. Rapid donor hepatectomy was facilitated without compromising graft quality or injuring accessory vessels through the use of adequate staffing during a DCD recovery. All of our DCD procurements were performed by an experienced transplant surgeon along with a transplant fellow and surgical resident. We believe that appropriate staffing during DCD procurements is of paramount importance not only in reducing DHT but also in standardizing and optimizing the procurement process by assuring the adequacy of organ and flush quality, appropriateness of the ischemic times, and preservation of accessory vessels before final organ acceptance.25 Finally, as with reports from other centers, advances in endoscopic and percutaneous biliary interventions allowed us to salvage >50% of the grafts that developed milder forms of IC.26
Eleven patients in our cohort underwent SLK with a 1-y renal allograft survival of 72.7% that is lower than more contemporary reports.27,28 All 3 graft losses occurred because of patient death; 2 of them happened early on in our experience (2005 and 2009) and were the result of graft versus host disease in 1 patient and cardiopulmonary arrest in the other.
Our study has several limitations. First, we did not examine biopsy results at the time of procurement. Our center’s practice is to obtain biopsies at the discretion of the donor surgeon, and, typically, this is not performed in DCD donors to minimize CIT. Biopsy results could have proven informative to the understanding of several poor outcomes despite appropriate CIT, WIT, and DHT. A more challenging limitation is the inherent time bias in the study. It is conceivable that the reduced incidence of IC in the latter group could be the reflection of the center’s staffing or learning curve in the perioperative management of DCD liver transplant recipients rather than just the modifications implemented after 2014. As a single-center study, these results are not generalizable.
In conclusion, DCD liver transplantation can be performed with an acceptable rate of IC when performed in a high-volume center focused on minimization of DHT and CIT. Appropriate recipient selection likely contributed to these results.
Footnotes
The authors declare no funding or conflicts of interest.
N.G., J.A.-C., and J.C.L. participated in research design and article writing. N.G., J.A.-C., and W.X. participated in data collection. N.D. participated in data analysis and figure preparation. N.G., J.A.-C., S.M., S.H.G., R.N.B., and J.C.L. participated in revision.
This study was approved by the institutional review board of University of Maryland, and consent was waived because of the retrospective nature of the study.
REFERENCES
- 1.Goldberg DS, Abt PL. Improving outcomes in DCDD liver transplantation: there can only be strength in numbers. Am J Transplant. 2014;14:1016–1020. [DOI] [PubMed] [Google Scholar]
- 2.Merion RM, Pelletier SJ, Goodrich N, et al. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abt PL, Desai NM, Crawford MD, et al. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaghan CJ, Charman SC, Muiesan P, et al. ; UK Liver Transplant Audit. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: a cohort study. BMJ Open. 2013;3:e003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jay C, Ladner D, Wang E, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant —an analysis of the national registry. J Hepatol. 2011;55:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathur AK, Heimbach J, Steffick DE, et al. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512–2519. [DOI] [PubMed] [Google Scholar]
- 7.Croome KP, Lee DD, Keaveny AP, et al. Improving national results in liver transplantation using grafts from donation after cardiac death donors. Transplantation. 2016;100:2640–2647. [DOI] [PubMed] [Google Scholar]
- 8.Bellingham JM, Santhanakrishnan C, Neidlinger N, et al. Donation after cardiac death: a 29-year experience. Surgery. 2011;150:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley DP, Fernandez LA, Leverson G, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle MB, Collins K, Vachharajani N, et al. Outcomes using grafts from donors after cardiac death. J Am Coll Surg. 2015;221:142–152. [DOI] [PubMed] [Google Scholar]
- 11.Bohorquez H, Seal JB, Cohen AJ, et al. Safety and outcomes in 100 consecutive donation after circulatory death liver transplants using a protocol that includes thrombolytic therapy. Am J Transplant. 2017;17:2155–2164. [DOI] [PubMed] [Google Scholar]
- 12.Mihaylov P, Mangus R, Ekser B, et al. Expanding the donor pool with the use of extended criteria donation after circulatory death livers. Liver Transpl. 2019;25:1198–1208. [DOI] [PubMed] [Google Scholar]
- 13.Kollmann D, Sapisochin G, Goldaracena N, et al. Expanding the donor pool: donation after circulatory death and living liver donation do not compromise the results of liver transplantation. Liver Transpl. 2018;24:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croome KP, Lee DD, Perry DK, et al. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transpl. 2017;23:342–351. [DOI] [PubMed] [Google Scholar]
- 15.Farid SG, Attia MS, Vijayanand D, et al. Impact of donor hepatectomy time during organ procurement in donation after circulatory death liver transplantation: the United Kingdom experience. Transplantation. 2019;103:e79–e88. [DOI] [PubMed] [Google Scholar]
- 16.Jochmans I, Fieuws S, Tieken I, et al. The impact of hepatectomy time of the liver graft on post-transplant outcome: a Eurotransplant cohort study. Ann Surg. 2019;269:712–717. [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen OB, van Reeven M, van der Helm D, et al. Donor hepatectomy time influences ischemia-reperfusion injury of the biliary tree in donation after circulatory death liver transplantation. Surgery. 2020;168:160–166. [DOI] [PubMed] [Google Scholar]
- 18.Khorsandi SE, Giorgakis E, Vilca-Melendez H, et al. Developing a donation after cardiac death risk index for adult and pediatric liver transplantation. World J Transplant. 2017;7:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available at http://www.R-project.org. Accessed October 2020. [Google Scholar]
- 21.Gilbo N, Fieuws S, Meurisse N, et al. Donor hepatectomy and implantation time are associated with early complications after liver transplantation: a single-center retrospective study. Transplantation. 2021;105:1030–1038. [DOI] [PubMed] [Google Scholar]
- 22.Hobeika MJ, Glazner R, Foley DP, et al. A step toward standardization: results of two national surveys of best practices in donation after circulatory death liver recovery and recommendations from The American Society of Transplant Surgeons and Association of Organ Procurement Organizations. Clin Transplant. 2020;34:e14035. [DOI] [PubMed] [Google Scholar]
- 23.Hertl M, Howard TK, Lowell JA, et al. Changes in liver core temperature during preservation and rewarming in human and porcine liver allografts. Liver Transpl Surg. 1996;2:111–117. [DOI] [PubMed] [Google Scholar]
- 24.Villa R, Fondevila C, Erill I, et al. Real-time direct measurement of human liver allograft temperature from recovery to transplantation. Transplantation. 2006;81:483–486. [DOI] [PubMed] [Google Scholar]
- 25.Croome KP, Taner CB. The changing landscapes in DCD liver transplantation. Curr Transplant Rep. 2020;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croome KP, McAlister V, Adams P, et al. Endoscopic management of biliary complications following liver transplantation after donation from cardiac death donors. Can J Gastroenterol. 2012;26:607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunez-Nateras R, Reddy KS, Aqel BA, et al. Simultaneous liver-kidney transplantation from donation after cardiac death donors: an updated perspective. Am J Transplant. 2020;20:3582–3589. [DOI] [PubMed] [Google Scholar]
- 28.LaMattina JC, Mezrich JD, Fernandez LA, et al. Simultaneous liver and kidney transplantation using donation after cardiac death donors: a brief report. Liver Transpl. 2011;17:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]