Supplemental Digital Content is available in the text.
Abstract
Background.
Few reports have focused on newer coronavirus disease 2019 (COVID-19) therapies (remdesivir, dexamethasone, and convalescent plasma) in solid organ transplant recipients; concerns had been raised regarding possible adverse impact on allograft function or secondary infections.
Methods.
We studied 77 solid organ transplant inpatients with COVID-19 during 2 therapeutic eras (Era 1: March–May 2020, 21 patients; and Era 2: June–November 2020, 56 patients) and 52 solid organ transplant outpatients.
Results.
In Era 1, no patients received remdesivir or dexamethasone, and 4 of 21 (19.4%) received convalescent plasma, whereas in Era 2, remdesivir (24/56, 42.9%), dexamethasone (24/56, 42.9%), and convalescent plasma (40/56, 71.4%) were commonly used. Mortality was low across both eras, 4 of 77 (5.6%), and rejection occurred in only 2 of 77 (2.8%) inpatients; infections were similar in hypoxemic patients with or without dexamethasone. Preexisting graft dysfunction was associated with greater need for hospitalization, higher severity score, and lower survival. Acute kidney injury was present in 37.3% of inpatients; renal function improved more rapidly in patients who received remdesivir and convalescent plasma. Post–COVID-19 renal and liver function were comparable between eras, out to 90 d.
Conclusions.
Newer COVID-19 therapies did not appear to have a deleterious effect on allograft function, and infectious complications were comparable.
Coronavirus disease 2019 (COVID-19) therapies have evolved over time. Whereas hydroxychloroquine, azithromycin, lopinavir-ritonavir, and tocilizumab were commonly administered early in the pandemic, these have largely been replaced by therapies, such as remdesivir, dexamethasone, and convalescent plasma.1 However, published studies have included few solid organ transplant (SOT) recipients treated with these newer agents.1-11 In a multicenter cohort study of 482 SOT recipients through mid-May 2020, only 14 (2.9%) had received remdesivir and 15 (3.1%) had received convalescent plasma.7 Transplant clinicians have raised questions about remdesivir safety in relation to renal and liver function, dexamethasone risk for secondary infections, and whether convalescent plasma might trigger rejection by enhancing alloimmune responses.12 Although case series of 3 and 13 patients have suggested that convalescent plasma is well tolerated,10,11 targeted therapies are often given in combination, so a comparison of eras of therapy could be informative.
In order to assess effects of contemporary therapies, we sought to compare mortality, rejection, renal function, and infections, in a retrospective single-center cohort of SOT recipients with COVID-19 infection during 2 different therapeutic eras.
MATERIALS AND METHODS
Study Population and Definitions
This cohort of 151 SOT recipients with COVID-19 included 77 inpatients admitted to Johns Hopkins Medicine, 52 outpatients monitored at home, and 22 patients admitted to hospitals outside of Johns Hopkins Medicine. The 77 inpatients represented all adult SOT recipients admitted to Johns Hopkins Medicine with a diagnosis of COVID-19 between March 1, 2020, and November 30, 2020. These were divided into 2 eras: those admitted between March 1, 2020, and May 31, 2020 (Era 1, n = 21), and those admitted between June 1, 2020, and November 30, 2020 (Era 2, n = 56).
In addition, we collected data on demographics, comorbidities, graft outcomes, and survival, in outpatients (n = 52) diagnosed with COVID-19 between March 1, 2020, and November 30, 2020, who never required hospitalization. Finally, demographic data, comorbidities present immediately before the COVID-19 diagnosis, and survival data were collected on 22 patients who had received transplants at Johns Hopkins Hospital but were admitted to hospitals outside of Johns Hopkins Medicine. Because of limited data on their therapeutics and clinical courses, patients admitted to outside hospitals were not included in our inpatient analysis.
Acute cellular rejection (ACR) was defined as biopsy-proven ACR. Antibody-mediated rejection (AMR) was diagnosed by biopsy and by laboratory testing including increase in donor-specific antibody levels, although donor-specific antibody levels were not universally monitored post–COVID-19 in this cohort. Given that different clinical interpretations may occur, clinical notes, pathology reports, and discharge summaries were reviewed to insure that all episodes of ACR and AMR were captured.
Preexisting graft dysfunction was recorded as being present before the COVID-19 episode if clinical notes referred to graft dysfunction, and one of the following organ-specific criteria was present: for kidney transplant recipients, estimated glomerular filtration rate <45 mL/min per 1.73 m2; for liver transplant recipients, clinician interpretations of cholestatic abnormalities, international normalized ratio elevation, and liver biopsy results; for heart transplant recipients, cardiac allograft vasculopathy, or reduced ejection fraction on echocardiogram; and for lung transplant recipients, bronchiolitis obliterans syndrome/chronic lung allograft dysfunction of any stage.
Data Source
The primary data source was the Johns Hopkins electronic medical record (Epic Systems, Verona, WI). Data were collected manually on a patient-level basis by 3 transplant infectious disease clinicians. Data included demographics, transplant details, comorbidities, baseline renal and liver function, preexisting graft dysfunction, World Health Organization (WHO) severity scores on admission and changes in WHO score during the admission, sequential C-reactive protein (CRP), serum creatinine (SCr), liver function, and interleukin-6 values; use of antimicrobials and COVID-19 therapies; immunosuppression management; outcomes including ACR, AMR, incident infections, graft dysfunction, graft loss, and mortality. Outcomes were collected to 90 d, starting from the date of admission for COVID-19, or the date of COVID-19 diagnosis for outpatients. Any outcomes that might have occurred after February 14, 2021, were not included in the analysis. This study was approved with waiver of consent by the Institutional Review Board at Johns Hopkins University School of Medicine.
COVID-19 Diagnostic Tests
Diagnostic testing for COVID-19 was by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid amplification test (NAT) testing of nasopharyngeal swabs for all patients in this study, and no patient was diagnosed with COVID-19 infection on the basis of antibody testing. For inpatients, COVID-19 was diagnosed by nasopharyngeal NAT test performed in the Johns Hopkins Clinical Microbiology Laboratory, by one of the following assays: the Roche SARS-CoV-2 NAT RNA Assay, Cepheid Gene Xpert SARS-CoV-2 Assay, GenMark E-Plex SARS-CoV-2 Assay, or NeuMoDx SARS-CoV-2 Assay. For inpatients with a previous positive NAT test from an outside lab, this was repeated in the Johns Hopkins lab upon admission or transfer to our center. For outpatients, COVID-19 was diagnosed either by nasopharyngeal NAT testing performed in the Johns Hopkins Lab in 30 of 52 (58%) by one of the above assays or by nasopharyngeal NAT testing in outside labs in 22 of 52 (42%).
Infection Timing, Definitions, Prophylaxis, and Monitoring
Data on incident infections (including organism, site, and time of occurrence) were collected using standardized infection definitions.13-16 Infections were analyzed in the following time periods for inpatients: days 0–7, days 8–30, days 31–60, and days 61–90, with the day of hospital admission being day 0. Days 0–7 were chosen as the first category, as it appeared unlikely that COVID-19 therapies would affect very early infection risk. When clinical significance of a positive microbiologic test was uncertain according to accepted definitions,13-16 this was not considered an infection. Only infections occurring during a hospitalization, or requiring hospitalization, were recorded, with the exception of plasma cytomegalovirus (CMV) polymerase chain reaction (PCR), for which any positive results were recorded. Mild infections (such as outpatient urinary tract infections) were not counted.
SOT recipients treated with dexamethasone received prophylaxis with acyclovir or valacyclovir for 3 mo per our internal protocol to prevent reactivation of herpes simplex virus and varicella-zoster virus. CMV PCR were monitored weekly while in the hospital and per clinician discretion thereafter. Fungal biomarkers (serum 1,3 beta-d-glucan, galactomannan, cryptococcal antigen, and Histoplasma serum and urine antigen) were obtained after admission and per clinician discretion thereafter.
WHO COVID-19 Severity Scale
We characterized the severity of COVID-19 using the WHO COVID-19 severity scale,17 an 8-point scale comprising the following scores: 1 (ambulatory, no limitations); 2 (limitation on activities); 3 (admitted to hospital, not on oxygen); 4 (oxygen mask or nasal prongs); 5 (high-flow oxygen or noninvasive positive pressure ventilation); 6 (intubation); 7 (intubation and advanced life support); and 8 (death). Advanced interventions included intravenous vasopressors, extracorporeal membrane oxygenation, and continuous renal-replacement therapy. We compared WHO severity scores between the 2 eras, both the WHO score on admission and the maximum WHO score attained during the admission, using Wilcoxon rank-sum testing. We compared the trajectory of WHO severity scores between patients with and without preexisting graft dysfunction (primary outcome) by multilevel ordinal logistic regression, with a model that included a patient-level random intercept, severity on admission, and an interaction between graft dysfunction and days since admission, assuming proportional odds.18 We also compared maximum WHO severity scores between patients who did or did not receive remdesivir, and those who did or did not receive convalescent plasma, using a Wilcoxon rank-sum test (Tables S1 and S2, SDC, http://links.lww.com/TXD/A395).
Laboratory Data
Laboratory data included inflammatory markers (peak and sequential levels of CRP and peak levels of interleukin-6). All values of SCr during the inpatient admission were recorded, as well as a baseline SCr value obtained at least 1 mo before the COVID-19 episode, and at 30, 60, and 90 d afterwards. Liver function tests (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and alkaline phosphatase) were recorded at baseline, at time of presentation, and at 30, 60, and 90 d after the COVID-19 admission. Acute kidney injury on admission was defined as a ≥0.3 mg/dL (or 50%) increase in SCr compared to baseline. Transaminitis was reported as the proportion with AST and ALT above 2× the upper limit of normal. Proteinuria was reported as the protein/creatinine ratio (PCrR), for any dates between 1 y before and >90 d after the COVID-19 admission, and as the designation 0 or trace, 1+, 2+, 3+ on urinalysis dipstick values at baseline, presentation, and 30, 60, and 90 d after the COVID-19 admission. Presence of proteinuria was defined as a positive dipstick, or PCrR over 0.2. Worsening of proteinuria was defined as >0.3 increase in PCrR.
The effects of remdesivir and convalescent plasma (primary exposures) on trajectory of SCr, ALT, and AST were analyzed using mixed effect linear regression, including a time-varying variable indicating days since earliest treatment or era indicator, adjusting for days since admission and adding a patient-level random intercept. Similarly, the effect of remdesivir and convalescent plasma on proteinuria through time was analyzed using multilevel logistic regression, including the same factors as mentioned above. The statistical significance of this time since treatment indicator reflects an increased (>1) or decreased (<1) odds of having proteinuria, comparing the posttreatment time to pretreatment or those not treated.
Medications
Use of a particular medication during the admission was reported as the proportion treated with that medication. We included immunosuppressive agents, such as tacrolimus, mycophenolate mofetil (MMF), and prednisone, as well as antibacterials, antivirals, and antifungal agents for inpatients.
Therapies for COVID-19 were administered per clinician choice, within Emergency Use Authorization (EUA) and center criteria for each therapeutic agent. Individual patients, therefore, could receive more than one targeted therapy, either simultaneously or sequentially. Access to remdesivir (other than in clinical trials) started after the EUA from the US Food and Drug Administration issued in May 2020.19 Renal dysfunction was not considered a contraindication to remdesivir at our center, based on previous reports.20,21 Dexamethasone use became widespread after publication of the Randomized Evaluation of COVID-19 Therapy trial in July 2020,22 and at our center, was used only in patients requiring supplemental oxygen. To assess effects of dexamethasone on infection risk, we compared the group of inpatients who had received dexamethasone (n = 24) to the group who had not received dexamethasone, but who had required supplemental oxygen (n = 24), since all patients who had received dexamethasone had required supplemental oxygen. Convalescent plasma was administered to inpatients under the August 2020 Food and Drug Administration EUA for convalescent plasma.23 Monoclonal antibody therapy for COVID-19 was not yet available during this time period. Medication use was compared between eras using the Fisher exact test.
Risk of Inpatient Admission
We examined baseline and preexisting comorbidities in the cohort and compared these characteristics between inpatients and outpatients by Wilcoxon rank-sum test for continuous variables, and Fisher exact test for binary or categorical variables.
Hospital Length of Stay, In-Hospital, and Late Mortality
Hospital length of stay was defined as the duration between the date of the index admission and the date of final discharge from the health system. Length of stay and in-hospital mortality were compared between eras using Fine and Gray competing-risks regression. Competing risks regression yields a subhazard ratio analogous to the hazard ratio from Cox regression, representing relative hazard of the outcome of interest after accounting for competing risks. Patients were followed from time of admission to discharge, in-hospital death, or administrative censorship on December 31, 2020, for all survival analyses.
Statistical Analysis
Since the Eras 1 and 2 populations were generally comparable in most characteristics, including age, race, and sex, all analyses were performed without adjustment unless otherwise specified above (Supplementary Methods, SDC, http://links.lww.com/TXD/A395). An α of 0.05 was used to determine statistical significance. All confidence intervals are 95% confidence intervals and are reported as per the method of Louis and Zeger.24 All analyses were performed using Stata 15.1/SE (College Station, TX).
RESULTS
Patient Demographics and Baseline Characteristics
Inpatients in both eras were generally comparable with respect to age, race, sex, type of transplant, and comorbidities (Table 1). As compared to outpatients, inpatients were more likely to have had preexisting graft dysfunction (47.5% versus 7.7%, P < 0.001), diabetes (56.6% versus 30.8%, P = 0.003), chronic kidney disease (38.4% versus 15.4%, P = 0.005), and lung disease (21.2% versus 5.8%, P = 0.018) (Table 2). Graft dysfunction before the COVID-19 episode occurred in 20 of 41 kidney (49%), 5 of 16 liver (31%), 9 of 12 lung (75%), 1 of 5 heart (20%), 0 of 1 hand, and 2 of 2 (100%) kidney/liver inpatients (P = 0.060 for lung recipients versus other organ recipients). Chronic kidney disease in nonkidney transplant inpatients before the COVID-19 episode occurred in 6 of 16 liver (38%), 6 of 12 lung (50%), and 3 of 5 (60%) of heart transplant recipients. Thus, chronic kidney disease occurred in 22 of 43 (51%) of kidney or kidney-liver recipients, and 15 of 34 (44%) of other organ recipients (P = 0.8).
TABLE 1.
Demographics and baseline characteristics of inpatients admitted to Johns Hopkins Medicine in eras 1 and 2
| Era 1 (March 1, 2020–May 31, 2020) | Era 2 (June 1, 2020–November 30, 2020) | P | |
|---|---|---|---|
| N | 21 | 56 | |
| Length of stay, median (IQR), d | 8 (5–9) (n = 21) | 6 (3–10) (n = 55) | 0.5 |
| Age, median (IQR), y | 55 (48–63) | 56 (43–67) | 0.9 |
| Biological female, n (%) | 9 (42.9) | 23 (41.1) | 1.0 |
| Gender, n (%) | 1.0 | ||
| Male | 12 (57.1) | 32 (57.1) | |
| Female | 9 (42.9) | 23 (41.1) | |
| Other | 0 (0.0) | 1 (1.8) | |
| Race, n (%) | 0.055 | ||
| Indigenous/Black | 0 (0.0) | 1 (1.8) | |
| Asian | 1 (4.8) | 0 (0.0) | |
| Black or African Descent | 11 (52.4) | 23 (41.1) | |
| White or Caucasian | 6 (28.6) | 30 (53.6) | |
| Other | 3 (14.3) | 2 (3.6) | |
| Hispanic, n (%) | 5 (23.8) | 7 (12.5) | 0.29 |
| Organ category, n (%) | 0.8 | ||
| Kidney | 12 (57.1) | 29 (51.8) | |
| Liver | 4 (19.0) | 12 (21.4) | |
| Heart | 2 (9.5) | 3 (5.4) | |
| Lung | 2 (9.5) | 10 (17.9) | |
| Hand | 0 (0.0) | 1 (1.8) | |
| Kidney/liver | 1 (4.8) | 1 (1.8) | |
| Mo since transplant, median (IQR) | 54 (18–119) | 63 (27–113) | 0.7 |
| Diabetes, n (%)a | 13 (61.9) | 28 (50.0) | 0.44 |
| HTN, n (%)a | 17 (81.0) | 43 (76.8) | 0.8 |
| CAD, n (%)a | 2 (9.5) | 7 (12.5) | 1.0 |
| CHF, n (%)a | 2 (9.5) | 8 (14.3) | 0.7 |
| Malignancy, n (%)a | 2 (9.5) | 5 (8.9) | 1.0 |
| CKD, n (%)a | 7 (33.3) | 21 (37.5) | 0.8 |
| Dialysis, n (%)a | 4 (19.0) | 5 (8.9) | 0.25 |
| Cirrhosis, n (%)a | 3 (14.3) | 1 (1.8) | 0.059 |
| Lung disease, n (%)a | 0 (0.0) | 16 (28.6) | 0.004 |
| HIV, n (%)a | 0 (0.0) | 4 (7.3) | 0.6 |
| Graft dysfunction, n (%)a | 9 (42.9) | 28 (50.0) | 0.6 |
| MMF use at baseline, n (%) | 13 (61.9) | 30 (53.6) | 0.6 |
| MMF discontinued, n (%) | 13 (100.0) | 30 (100.0) | n/a |
| Death, n (%) | 1 (4.8) | 4 (7.1) | 1.0 |
aComorbidities present immediately before the coronavirus disease 2019 episode, not pretransplant.
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; HIV, human immunodeficiency virus; HTN, hypertension; IQR, interquartile range; MMF, mycophenolate mofetil.
TABLE 2.
Characteristics of study participants, stratified by whether or not they were admitted to the hospital (including all hospital admissions both in and outside of Johns Hopkins Health System)
| Not admitted | Admitted | P | |
|---|---|---|---|
| N | 52 | 99 | |
| Age, median (IQR), y | 55 (43–65) (n = 52) | 58 (46–66) (n = 99) | 0.31 |
| Biological female, n (%) | 27 (51.9) | 41 (41.4) | 0.23 |
| Gender, n (%) | 0.45 | ||
| Male | 25 (48.1) | 57 (57.6) | |
| Female | 27 (51.9) | 41 (41.4) | |
| Other | 0 (0.0) | 1 (1.0) | |
| Race, n (%) | 0.20 | ||
| Indigenous/Black | 0 (0.0) | 1 (1.0) | |
| Asian | 2 (3.8) | 1 (1.0) | |
| Black or African Descent | 26 (50.0) | 44 (44.4) | |
| White or Caucasian | 23 (44.2) | 48 (48.5) | |
| Other | 0 (0.0) | 5 (5.1) | |
| Unknown | 1 (1.9) | 0 (0.0) | |
| Hispanic, n (%) | 4 (7.7) | 13 (13.1) | 0.42 |
| Transplanted organ, n (%) | 0.20 | ||
| Kidney | 37 (71.2) | 56 (56.6) | |
| Liver | 12 (23.1) | 21 (21.2) | |
| Heart | 1 (1.9) | 6 (6.1) | |
| Lung | 1 (1.9) | 12 (12.1) | |
| Hand | 0 (0.0) | 1 (1.0) | |
| Kidney/liver | 1 (1.9) | 2 (2.0) | |
| Kidney/pancreas | 0 (0.0) | 1 (1.0) | |
| Mo since transplant, median (IQR) | 58 (30–104) | 60 (27–135) | 0.9 |
| Diabetes, n (%)a | 16 (30.8) | 56 (56.6) | 0.003 |
| HTN, n (%)a | 46 (88.5) | 80 (80.8) | 0.26 |
| CAD, n (%)a | 5 (9.6) | 20 (20.2) | 0.11 |
| CHF, n (%)a | 1 (1.9) | 15 (15.2) | 0.011 |
| Malignancy, n (%)a | 5 (9.6) | 8 (8.1) | 0.8 |
| CKD, n (%)a | 8 (15.4) | 38 (38.4) | 0.005 |
| Dialysis, n (%)a | 4 (7.7) | 13 (13.1) | 0.42 |
| Cirrhosis, n (%)a | 0 (0.0) | 6 (6.1) | 0.094 |
| Lung disease, n (%)a | 3 (5.8) | 21 (21.2) | 0.018 |
| HIV, n (%)a | 1 (1.9) | 5 (5.1) | 0.7 |
| Graft dysfunction at baseline, n (%)a | 4 (7.7) | 48 (48.5) | <0.001 |
| MMF use at baseline, n (%) | 29 (55.8) | 59 (59.6) | 0.7 |
| Death, n (%) | 1 (1.9) | 13 (13.1) | 0.035 |
aComorbidities present immediately before the coronavirus disease 2019 episode, not pretransplant.
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; HIV, human immunodeficiency virus; HTN, hypertension; IQR, interquartile range; MMF, mycophenolate mofetil.
Laboratory Data
Renal Function at Baseline, During the COVID-19 Episode, and After (to 90 Days)
Nine patients were on dialysis before the COVID-19 admission. Acute kidney injury was present on admission in 6 (37.5%) of Era 1 and 18 (36.0%) of Era 2 patients not already on dialysis (P > 0.9, Table 3). These included 13 of 36 (36%) of kidney, 4 of 15 liver (27%), 4 of 10 lung (40%), 0 of 5 heart, 1 of 1 hand, and 2 of 2 kidney/liver recipients. Of these patients with acute kidney injury (AKI) on admission, 13 of 24 (54%) had diabetes as a preexisting comorbidity, and 11 of 24 (46%) did not. The prevalence of diabetes in the inpatient cohort as a whole was 41 of 77 or 53%, so diabetes was not significantly more common in the AKI group than in the group as a whole (P > 0.9).
TABLE 3.
Inpatient characteristics of patients hospitalized for coronavirus disease 2019, in eras 1 and 2
| Era 1 (March 1, 2020–May 31, 2020) | Era 2 (June 1, 2020–November 30, 2020) | P | |
|---|---|---|---|
| n | 21 | 56 | |
| Upon admission | |||
| WHO score on admission, n (%) | 0.28 | ||
| Mild, no O2 | 19 (90.5) | 36 (64.3) | |
| Mild, mask, or nasal | 2 (9.5) | 15 (26.8) | |
| Severe, noninvasive | 0 (0.0) | 3 (5.4) | |
| Severe, intubated, or ventilated | 0 (0.0) | 1 (1.8) | |
| Ventilated, plus IVP, ECMO, or CRRT | 0 (0.0) | 1 (1.8) | |
| WHO score on admission, median (IQR) | 3 (3–3) (n = 21) | 3 (3–4) (n = 56) | 0.021 |
| WHO score on admission, mean (SD) | 3.1 (0.3) (n = 21) | 3.5 (0.8) (n = 56) | 0.033 |
| Transaminitis on admission, n (%) | 4 (19.0) | 8 (14.3) | 0.7 |
| ALT >2× upper limit of normal, n (%) | 3 (14.3) | 6 (10.9) | 0.7 |
| ALT >5× upper limit of normal, n (%) | 0 (0.0) | 4 (7.3) | 0.6 |
| AST >2× upper limit of normal, n (%) | 2 (9.5) | 7 (12.7) | 1.0 |
| AST >5× upper limit of normal, n (%) | 0 (0.0) | 4 (7.3) | 0.6 |
| Elevated alkaline phosphatase on admission, n (%) | 4 (19.0) | 10 (17.9) | 1.0 |
| Acute kidney injury on admission, n (%) | 6 (37.5) (n = 16) | 18 (36.0) (n = 50) | 1.0 |
| Any proteinuria on admission, n (%) | 10 (58.8) (n = 17) | 21 (50.0) (n = 42) | 0.6 |
| During the entire admission | |||
| Highest WHO score achieved, n (%) | 0.9 | ||
| Mild, no O2 | 7 (33.3) | 22 (39.3) | |
| Mild, mask, or nasal | 10 (47.6) | 20 (35.7) | |
| Severe, noninvasive | 2 (9.5) | 5 (8.9) | |
| Severe, intubated, or ventilated | 0 (0.0) | 4 (7.1) | |
| Ventilated, plus IVP, ECMO, or CRRT | 1 (4.8) | 2 (3.6) | |
| Death | 1 (4.8) | 3 (5.4) | |
| Highest WHO score achieved, median (IQR) | 4 (3–4) (n = 21) | 4 (3–5) (n = 56) | 1.0 |
| Highest WHO score achieved, mean (SD) | 4.1 (1.3) (n = 21) | 4.2 (1.4) (n = 56) | 0.9 |
| Ventilated or intubated during admission, n (%) | 2 (9.5) | 8 (14.3) | 0.7 |
| CMV result, n (%) | 0.43 | ||
| Negative | 13 (61.9) | 39 (69.6) | |
| Positive | 3 (14.3) | 5 (8.9) | |
| N/A | 5 (23.8) | 12 (21.4) | |
| Peak CRP, median (IQR) | 7 (2–14) (n = 21) | 5 (2–8) (n = 53) | 0.20 |
| Peak IL-6 (before tocilizumab if given), median (IQR) | 62 (27–174) (n=16) | 27 (11–73) (n = 45) | 0.048 |
| Length of stay, median (IQR) | 8 (5–9) (n = 21) | 6 (3–10) (n = 56) | 0.51 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CMV, cytomegalovirus; CRP, C-reactive protein; CRRT, continuous renal-replacement therapy; ECMO, extracorporeal membrane oxygenation; IL-6, interleukin-6; IQR, interquartile range; IVP, intravenous vasopressors; N/A, not applicable; WHO, World Health Organization.
For most patients, SCr returned to baseline during follow-up (Table 4), with 1 (6.3%) from Era 1 and 4 (9.1%) from Era 2 having persistent AKI at 30 d (P > 0.9). Of these 5 patients with persistent AKI at 30 d, there were 2 kidney, 1 liver, 1 lung, and 1 kidney/liver recipient. Two of those 5 patients (40%) had diabetes.
TABLE 4.
Postdischarge complications, laboratory values, and outcomes in inpatients in eras 1 and 2
| Era 1 (March 1, 2020–May 31, 2020) | Era 2 (June 1, 2020–November 30, 2020) | P | |
|---|---|---|---|
| Latest (after 30 d) or postdischarge follow-up | |||
| n | 21 | 56 | |
| ALT elevation 2× ULN, new/persistent, n (%) | 0 (0.0) | 4 (7.1) | 0.57 |
| ALT elevation 5× ULN, new/persistent, n (%) | 0 (0.0) | 0 (0.0) | N/A |
| AST elevation 2× ULN, new/persistent, n (%) | 2 (9.5) | 5 (8.9) | 1.0 |
| AST elevation 5× ULN, new/persistent, n (%) | 0 (0.0) | 2 (3.6) | 1.0 |
| Persistent AKI at follow-up (>4 wk), n (%) | 1 (6.3) (n = 17) | 4 (9.1) (n = 44) | 1.0 |
| Change of SCr: last vs admission, mean (SD) | −0.3 (0.4) (n = 17) | −0.1 (0.9) (n = 44) | 0.7 |
| Change of SCr: last vs admission, median (IQR) | −0.1 (−0.3 to −0.0) (n = 17) | −0.0 (−0.2 to 0.1) (n = 44) | 0.18 |
| Change of SCr: last vs baseline, mean (SD) | 0.0 (0.2) (n = 16) | −0.1 (0.9) (n = 44) | 0.7 |
| Change of SCr: last vs baseline, median (IQR) | 0.1 (−0.0 to 0.1) (n = 16) | −0.0 (−0.2 to 0.1) (n = 44) | 0.18 |
| PCrR ever higher than 0.2, n (%) | 9 (42.9) | 17 (30.4) | 0.42 |
| Significant increase (≥0.3) in PCrR between last and first, n (%) | 3 (21.4) (n = 14) | 3 (8.8) (n = 34) | 0.34 |
| Acute cellular rejection at 90 d, n (%) | 1 (5.0) | 0 (0.0) (n = 55) | 0.28 |
| Antibody-mediated rejection at 30 d, n (%) | 1 (5.0) | 0 (0.0) (n = 55) | 0.27 |
| Antibody-mediated rejection at 60 d, n (%) | 1 (5.0) | 0 (0.0) (n = 55) | 0.28 |
| Antibody-mediated rejection at 90 d, n (%) | 2 (10.0) | 0 (0.0) (n = 55) | 0.07 |
| Graft dysfunction, n (%) | 6 (28.6) | 19 (33.9) | 0.8 |
| ICU, n (%) | 4 (19.0) | 13 (23.2) | 0.8 |
| ARDS, n (%) | 0 (0.0) | 3 (5.4) | 0.56 |
| Septic shock, n (%) | 1 (4.8) | 2 (3.6) | 1.0 |
| Acute liver injury, n (%) | 1 (4.8) | 4 (7.1) | 1.0 |
| Myocarditis, n (%) | 0 (0.0) | 1 (1.8) | 1.0 |
| Encephalopathy, n (%) | 0 (0.0) | 1 (1.8) | 1.0 |
AKI, acute kidney injury; ALT, alanine aminotransferase; ARDS, adult respiratory distress syndrome; AST, aspartate aminotransferase; ICU, intensive care unit; IQR, interquartile range; N/A, not applicable; PCrR, protein-creatinine ratio; SCr, serum creatinine; ULN, upper limit of normal.
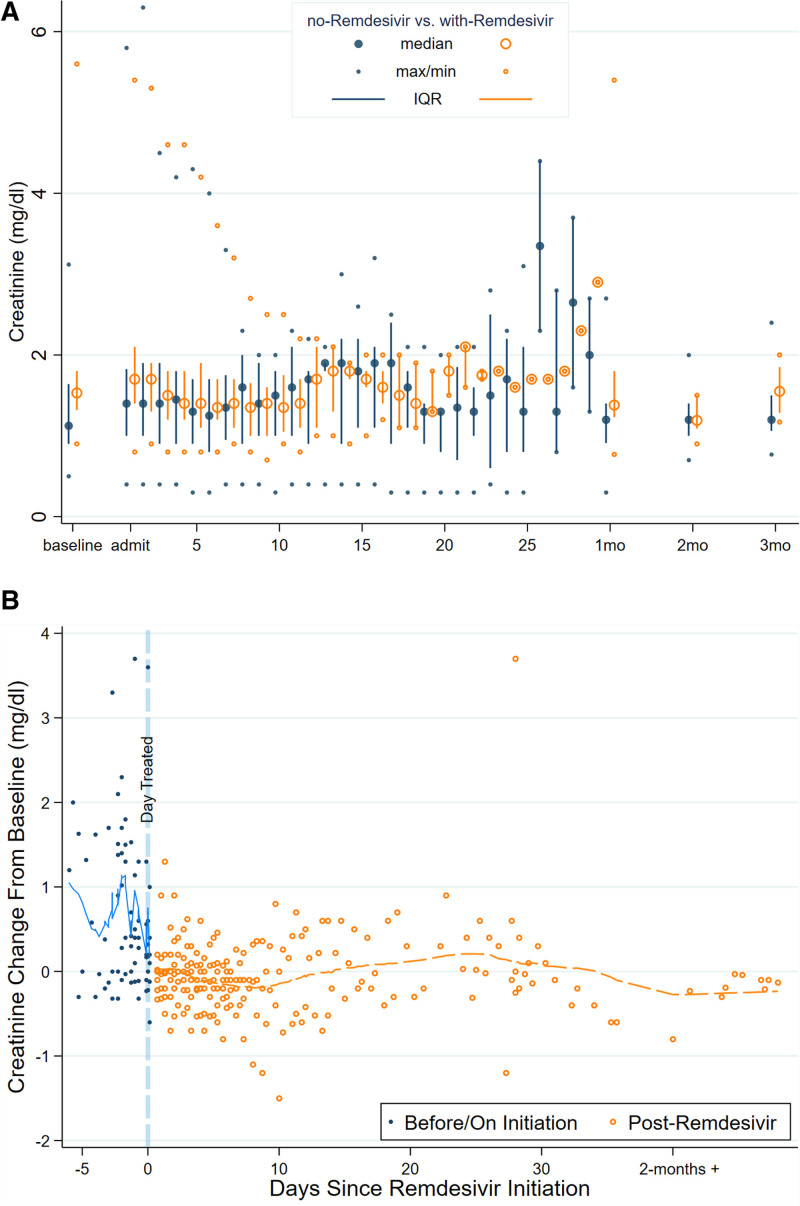
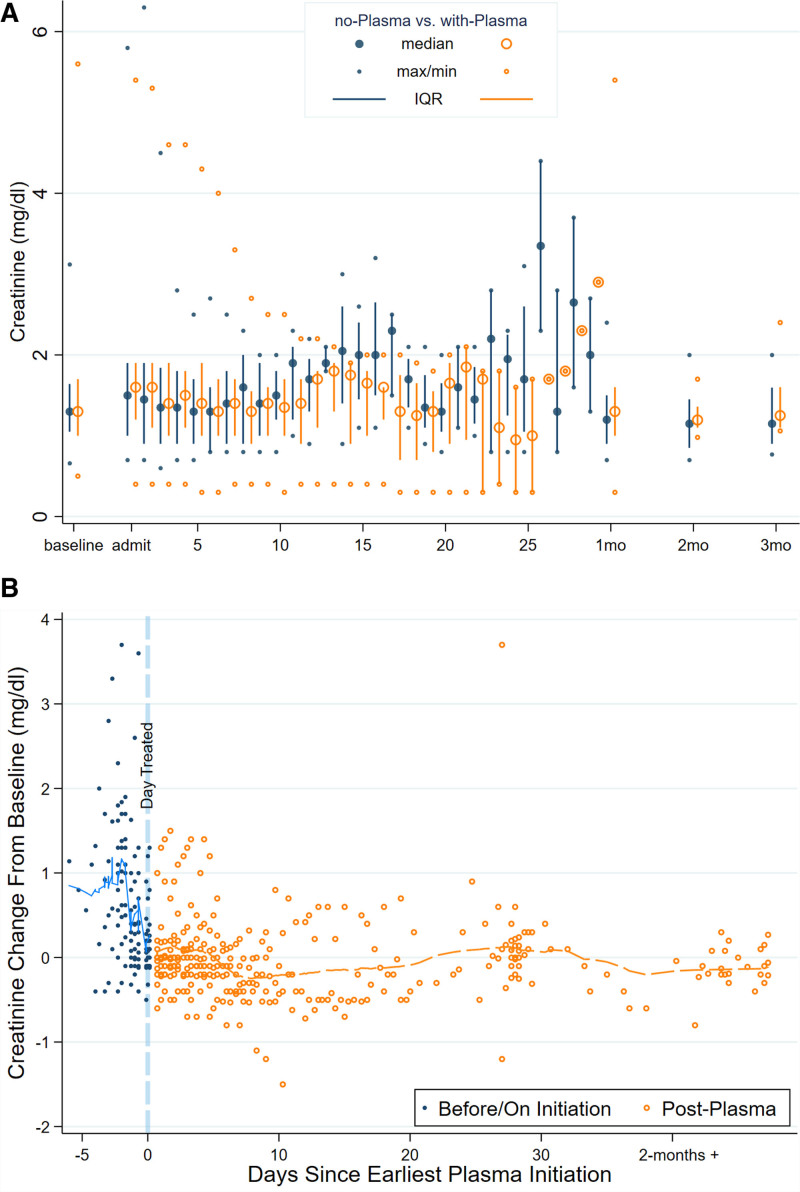
For patients not receiving remdesivir, there was no evidence of change in SCr over time (average change in SCr = −0.02 −0.01 0.01 units per day, P = 0.4). Patients receiving remdesivir tended to experience a decline in SCr over time following initiation of therapy (average change = −0.12 −0.10 −0.09 units per day, P < 0.001, interaction P < 0.001). Similarly, for patients not receiving convalescent plasma, there was no evidence of change in SCr over time (average change in SCr = −0.02 −0.001 0.02 units per day). Patients receiving convalescent plasma tended to experience a decline in SCr over time (average change in SCr = −0.11 −0.10 −0.08 units per day, P < 0.001, interaction P < 0.001). Figures 1A and 2A summarize the trajectory of SCr stratified by treatment with remdesivir (Figure 1A) and treatment with convalescent plasma (Figure 2A) in daily median, interquartile range (IQR), and range. There was no evidence of increased SCr following initiation of remdesivir (Figure 1B) or convalescent plasma (Figure 2B).
FIGURE 1.
Trajectories of serum creatinine (SCr) for solid organ transplant recipients hospitalized for coronavirus disease 2019, stratified by remdesivir use. A, Distribution of SCr by time since admission stratified by use/nonuse of remdesivir. B, Lowess plot of change over time in SCr for patients who received remdesivir stratified by days before/after initiation of treatment (normalized so that baseline SCr obtained before onset of illness = 0; eg, a value of 1 represents SCr 1 mg/dL higher than baseline). IQR, interquartile range.
FIGURE 2.
Trajectories of serum creatinine (SCr) for solid organ transplant recipients hospitalized for coronavirus disease 2019, stratified by convalescent plasma use. A, Distribution of SCr by time since admission stratified by use/nonuse of convalescent plasma. B, Lowess plot of change over time in SCr for patients who received convalescent plasma stratified by days before/after initiation of treatment (normalized so that baseline SCr obtained before onset of illness = 0; eg, a value of 1 represents SCr 1 mg/dL higher than baseline). IQR, interquartile range.
Proteinuria was present at baseline in 8 of 20 (40%) of those who received remdesivir and 13 of 44 (29.5%) who did not receive remdesivir (P = 0.57, Table S1, SDC, http://links.lww.com/TXD/A395). There was no statistically significant difference between patients who did and did not receive remdesivir in terms of an increase in PCrR of > 0.3 at last follow-up on or after 30 d (P = 0.34, Table S1, SDC, http://links.lww.com/TXD/A395), however, only 48 patients had data available on proteinuria after the COVID-19 episode. Initiation of remdesivir was associated with slightly increased risk of having proteinuria through time (odds ratio [1.002 1.03 1.05], P = 0.033).
Liver Function Tests at Baseline, During the COVID-19 Episode, and After (to 90 Days)
Transaminitis was present at the time of admission in 12 of 77 inpatients (16%), including 6 of 16 liver (38%), 5 of 41 kidney (12%), and 1 of 2 kidney/liver recipients, and was statistically significantly more common in liver or kidney/liver recipients as compared with all other organs (P = 0.005). Patients with transaminitis on admission included 4 (19%) patients in Era 1 and 8 (14.3%) in Era 2 (Table 3). Persistent elevation of AST to >2× upper limit of normal was seen in 2 (9.5%) patients in Era 1 and 5 (8.9%) in Era 2; for ALT this occurred in 0 patients in Era 1, and 4 in Era 2 (Table 4). There were no differences in changes of ALT or AST after initiation of either remdesivir or convalescent plasma, comparing posttreatment to pretreatment values, or to those not treated with these therapies (Figures S1A through S4B, SDC, http://links.lww.com/TXD/A395).
Inflammatory Markers
There were no statistically significant differences in peak CRP between Eras 1 and 2 (Table 4), nor between patients who did or did not receive remdesivir, nor those who did or did not receive convalescent plasma (Tables S1 and S2, SDC, http://links.lww.com/TXD/A395). Interleukin-6 was slightly higher in Era 1 compared with Era 2 (P = 0.048, Table 4). Peak CRP was slightly higher among dexamethasone-treated patients (median, IQR, 7 [5–11] versus 4 [1–8] mg/dL, P = 0.04).
Medications
MMF was discontinued in 43 of 43 (100%) of patients who were receiving MMF on admission (Table 1), whereas tacrolimus was typically continued, and prednisone was continued unless replaced by dexamethasone. Only 2 patients in this cohort were on mTOR-inhibitor containing regimens (1 sirolimus and 1 everolimus).
For targeted therapies directed at COVID-19, hydroxychloroquine was used only in Era 1 (11/21 or 52.4%, Table 5). Remdesivir and dexamethasone were used only in Era 2. In Era 1, no patients received remdesivir or dexamethasone, whereas in Era 2, 24 of 56 (42.9%) received remdesivir and 24 of 56 (42.9%) received dexamethasone (Table 5). Only patients receiving supplemental oxygen were candidates for dexamethasone; in Era 2, 24 of 34 (70.6%) of those requiring supplemental oxygen received dexamethasone. Era 1 patients did not receive augmented steroids, except for the sole patient who died in Era 1, who expired shortly after admission with Pseudomonas septic shock, and briefly received stress-dose hydrocortisone. Convalescent plasma was used mainly in Era 2 (in 4/21 [19%] in Era 1, and in 40/56 [71.4%] in Era 2), for a total of 44 of 77 (57.1%) overall. Tocilizumab was used primarily in Era 1 (5/21 patients or 23.8%) and in only one patient in Era 2 (1.8%). Use of antibacterial, antiviral, and antifungal therapies are recorded in Table 5.
TABLE 5.
Use of coronavirus disease 2019 targeted therapies, immunosuppression, and antimicrobials during admission in eras 1 and 2
| Era 1 (March 1, 2020–May 31, 2020), n (%) | Era 2 (June 1, 2020–November 30, 2020), n (%) | P | |
|---|---|---|---|
| n | 21 | 56 | |
| MMF use at baseline | 13 (61.9) | 30 (53.6) | 0.6 |
| MMF resumed | 8 (66.7) | 9 (30.0) | 0.041 |
| Remdesivir | 0 (0.0) | 24 (42.9) | <0.001 |
| Hydroxychloroquine | 11 (52.4) | 0 (0.0) | <0.001 |
| Convalescent plasma | 4 (19.0) | 40 (71.4) | <0.001 |
| Tocilizumab | 5 (23.8) | 1 (1.8) | 0.005 |
| Dexamethasone | 0 (0.0) | 24 (42.9) | <0.001 |
| Empiric antibiotics | 19 (90.5) | 42 (75.0) | 0.21 |
| Antiviral | 8 (38.1) | 32 (57.1) | 0.20 |
| Antiviral: acyclovir | 6 (28.6) | 24 (42.9) | 0.30 |
| Antiviral: valacyclovir | 2 (9.5) | 13 (23.2) | 0.21 |
| Antiviral: ganciclovir | 2 (9.5) | 4 (7.1) | 0.7 |
| Antiviral: HIV antiretroviral therapy | 0 (0.0) | 3 (5.4) | 0.56 |
| Antiviral: entecavir | 0 (0.0) | 2 (3.6) | 1.0 |
| Antiviral: letermovir | 0 (0.0) | 3 (5.4) | 0.56 |
| Antifungal | 6 (28.6) | 17 (30.4) | 1.0 |
| Antifungal: isavuconazole | 3 (14.3) | 7 (12.5) | 1.0 |
| Antifungal: micafungin | 4 (19.0) | 5 (8.9) | 0.25 |
| Antifungal: posaconazole | 0 (0.0) | 6 (10.7) | 0.18 |
| Antifungal: fluconazole | 2 (9.5) | 3 (5.4) | 0.6 |
| Antifungal: voriconazole | 0 (0.0) | 2 (3.6) | 1.0 |
MMF, mycophenolate mofetil.
Additional comparisons between patients who did and did not receive remdesivir are listed in Table S1 (SDC, http://links.lww.com/TXD/A395), and between patients who did and did not receive convalescent plasma, in Table S2 (SDC, http://links.lww.com/TXD/A395). Although convalescent plasma was administered in both eras, it was only administered to 4 patients in Era 1 and to 40 of 77 patients in Era 2, so the use was predominantly in Era 2. There were no statistically significant differences between patients who did or did not receive convalescent plasma in terms of maximum WHO score, inflammatory markers, liver function tests, rejection, secondary infections, graft dysfunction, or death (Table S2, SDC, http://links.lww.com/TXD/A395).
Hospital Length of Stay, Severity of Illness, Inpatient Mortality, and Late Mortality (to 90 Days)
Three SOT recipients died during the initial COVID-19 hospitalization, and one died on a subsequent hospitalization, for a total of 4 inpatient deaths (4/77, 5.6%). These included one (4.7%) death in Era 1, and 3 (5.4%) deaths in Era 2, which was not statistically significantly different (Table 4). Similarly, accounting for the competing risk of in-hospital mortality, there was no difference in time to alive-at-discharge, that is, length of stay or discharge rate (subhazard ratio Era 2 compared to Era 1 [0.68 1.07 1.66], P = 0.8). The median (IQR) length of stay for Era 1 was 8 d (5–9), and for Era 2 was 6 d (3–10) (P = 0.43, Table 4).
The WHO severity score on admission was slightly higher in Era 2 (rank-sum P = 0.033), but the maximum WHO severity score was similar between eras (Table 3). The proportion who required mechanical ventilation was similar between eras: 2 (9.5%) in Era 1 and 8 (14.3%) in Era 2 (P = 0.7). As our center’s criteria for remdesivir included hypoxemia, the maximum WHO severity score was higher for patients who received remdesivir compared with those who did not, with the median (IQR) highest WHO score being 5 (4–6) for patients treated with remdesivir versus 3 (3–4) for those not treated with remdesivir (P < 0.001, Table S1, SDC, http://links.lww.com/TXD/A395). Similarly, as dexamethasone was restricted to patients requiring supplemental oxygen, the maximum WHO severity score was higher for patients who received dexamethasone compared with those who did not (5 [4–6] and 4 [4–5], P = 0.047). The maximum WHO severity score was not different comparing those who received convalescent plasma to those who did not (4 [3–5] and 4 [3–4], P = 0.9, Table S2, SDC, http://links.lww.com/TXD/A395).
Outcomes in Patients With and Without Preexisting Graft Dysfunction
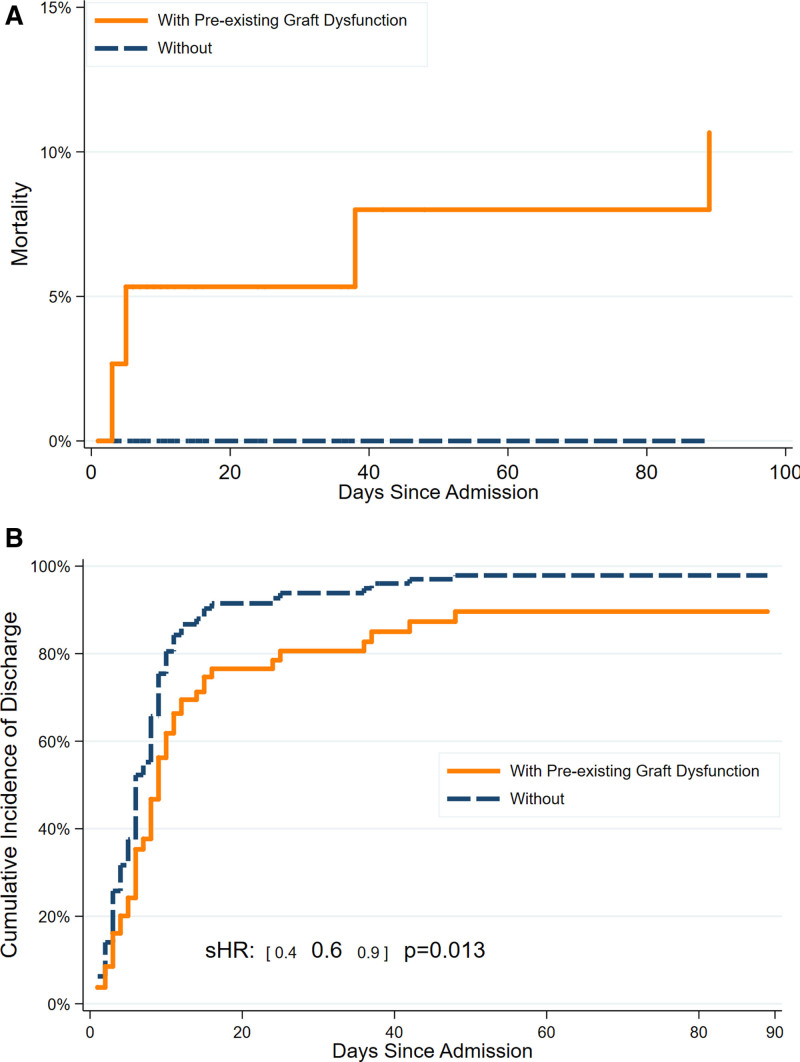
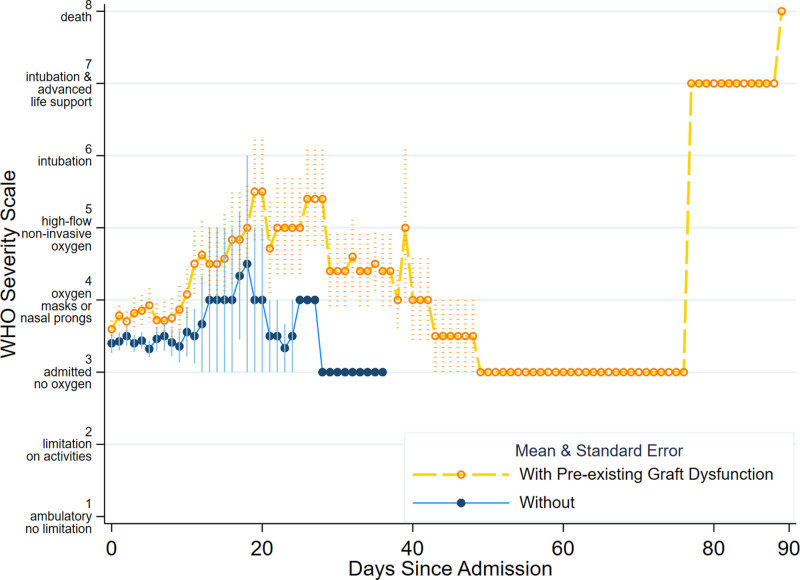
As mentioned above, graft dysfunction before the COVID-19 episode occurred in 20 of 41 kidney (49%), 5 of 16 liver (31%), 9 of 12 lung (75%), 1 of 5 heart (20%), and 2 of 2 (100%) kidney/liver inpatients (P = 0.060 for lung recipients versus other organ recipients). Preexisting graft dysfunction was associated with increased risk for mortality in inpatients (Figure 3A), longer length of stay (Figure 3B, subhazard ratio = 0.4 0.6 0.9, P = 0.013), and slower recovery by WHO scale during the admission, across both eras (odds ratio from ordinal logistic regression 1.13 1.19 1.27, P < 0.001) (Figure 4). In terms of infection testing, patients with prior graft dysfunction were more likely to have a positive galactomannan antigen (4/37 or 10.8% versus 0/40 or 0%, P = 0.048), a diagnostic test that may indicate aspergillosis, although other fungal biomarkers and CMV PCR results were not statistically significantly different between those with and without prior graft dysfunction. Additional comparisons between inpatients with and without graft dysfunction are listed in Table S3 (SDC, http://links.lww.com/TXD/A395).
FIGURE 3.
Cumulative incidence of mortality and discharge for solid organ transplant recipients hospitalized for coronavirus disease 2019, stratified by presence of preexisting graft dysfunction. A, Mortality after hospital admission. B, Hospital discharge. sHR, subhazard ratio.
FIGURE 4.
Summarized trajectory of inpatient World Health Organization (WHO) severity scale, stratified by presence of preexisting graft dysfunction (mean and SE).
ACR and AMR
ACR occurred in 1 patient in Era 1 and none in Era 2. AMR occurred in 2 patients in Era 1 and none in Era 2. One of these patients, a lung transplant recipient who had received convalescent plasma, had both ACR and AMR before the COVID-19 episode and again during follow-up. The other patient with AMR is a kidney transplant recipient from Era 1 who had not received remdesivir, dexamethasone, or convalescent plasma and was considered to likely have had chronic AMR before the COVID-19 episode as well. Thus, only 2 of 77 inpatients (2.6%) experienced rejection during 90-d follow-up, despite universal discontinuation of MMF.
Graft Loss
There were no instances of de novo graft loss in any patients in this cohort. Two patients had returned to dialysis shortly before the COVID-19 admission.
Infections During and After Inpatient Admission
Infections occurring during days 0–7 of hospital admission were considered less likely to be attributable to effects of targeted COVID-19 therapies, such as dexamethasone. There was no statistically significant difference in incidence of infections occurring during days 0–7 between eras. Secondary infections after the COVID-19 episode were uncommon in both eras and were not significantly different in incidence between eras during days 8–30, 31–60, and 61–90 after hospital admission (Table S4, SDC, http://links.lww.com/TXD/A395).
Specific types of infections, by era, are recorded in Table S4 (SDC, http://links.lww.com/TXD/A395), the majority of which were bacterial. Invasive fungal infections occurred in 7 patients. CMV PCR monitoring during and after the COVID-19 episode revealed 8 episodes of low-level CMV reactivation. Epstein-Barr virus PCR were not routinely monitored, but 2 episodes of low-positive viral load Epstein-Barr virus DNAemia were noted, without posttransplant lymphoproliferative disease. BK virus PCR were monitored in the aftermath of the COVID-19 episode in 30 of 41 (73%) of kidney recipients, but only one patient developed quantifiable BK virus DNAemia, with a peak viral load of 1250 copies/mL, and the other BK virus PCR were all undetectable or below limit of quantitation.
There were no statistically significant differences between dexamethasone-treated patients and a comparator group of patients requiring supplemental oxygen who did not receive dexamethasone, in any of the time periods, in terms of total, bacterial, fungal, or viral infections. There was a numerical difference in infections in the days 0–7 time period, in that 12 of 24 (50%) of dexamethasone-treated patients had infections during that time, compared with 5 of 24 (21%) in the comparator group, but this did not reach statistical significance (P = 0.069). Between days 8 and 30, 6 (25%) patients treated with dexamethasone and 1 (4.2%) patient in the comparator group experienced infections (P = 0.097, Table 6). Three patients (3/24, 12.5%) in the dexamethasone group had infections between days 31 and 60 (P = 0.23) and 2 of 24 (8.3%) in the dexamethasone group had infections between days 61 and 90 (P = 0.5), so in no time period was there a statistically significant difference in infections between the dexamethasone and comparator groups.
TABLE 6.
Infection stratified by dexamethasone use, among inpatients who received supplementary oxygen
| Did not receive, n (%) | Received dexamethasone, n (%) | P | |
|---|---|---|---|
| n | 24 | 24 | |
| Days 0–7 total infections | 5 (20.8) | 12 (50.0) | 0.069 |
| Days 8–30 total infections | 1 (4.2) | 6 (25.0) | 0.097 |
| Days 8–30 bacterial | 1 (4.2) | 4 (16.7) | 0.35 |
| Days 8–30 viral | 0 (0.0) | 2 (8.3) | 0.49 |
| Days 8–30 fungal | 0 (0.0) | 1 (4.2) | 1.0 |
| Days 31–60 total infections | 0.23 | ||
| None | 24 (100) | 21 (87.5) | |
| Yes | 0 (0.0) | 2 (8.3) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 31–60 bacterial | 0.5 | ||
| None | 24 (100) | 22 (91.7) | |
| Yes | 0 (0.0) | 1 (4.2) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 31–60 viral | 0.5 | ||
| None | 24 (100) | 22 (91.7) | |
| Yes | 0 (0.0) | 1 (4.2) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 31–60 fungal | N/A | ||
| None | 24 (100) | 23 (95.8) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 61–90 total infections | 0.5 | ||
| None | 24 (100) | 22 (91.7) | |
| Yes | 0 (0.0) | 1 (4.2) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 61–90 bacterial | 0.5 | ||
| None | 24 (100) | 22 (91.7) | |
| Yes | 0 (0.0) | 1 (4.2) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 61–90 viral | N/A | ||
| None | 24 (100) | 23 (95.8) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Days 61–90 fungal | 0.5 | ||
| None | 24 (100) | 22 (91.7) | |
| Yes | 0 (0.0) | 1 (4.2) | |
| N/A | 0 (0.0) | 1 (4.2) | |
| Any infection after 7th day of admission | 1 (4.2) | 6 (25.0) | 0.097 |
N/A, not applicable.
Outcomes of COVID-19 in Outpatients Monitored at Home
There were 52 SOT outpatients monitored at home for COVID-19, who were followed closely with telemedicine visits, telephone calls, and home pulse oximetry (Table 2).25,26 Outpatients were similar to inpatients in terms of age, race, ethnicity, gender, and type of organ transplant. However, outpatients were less likely to have diabetes, chronic kidney disease, and lung disease. Graft dysfunction before the COVID-19 episode was present in only 4 (7.7%) of outpatients, as compared to 47 (47.5%) of inpatients (P < 0.001) (Table 2).
In terms of therapies, outpatients did not receive augmented doses of steroids. MMF was used in 29 (55.8%) of outpatients at baseline, and 15 (53.6%) of those patients had MMF discontinued, as compared to 100% MMF discontinuation in inpatients who were on MMF at the time of admission (P < 0.001). Other than stopping MMF, the rest of the immunosuppressive regimen was generally continued. Vitamin D supplementation was administered (to both inpatients and outpatients). There were no instances of new-onset graft dysfunction or graft loss in outpatients. As this cohort of patients was diagnosed before the availability of EUA monoclonal antibody infusion for outpatients, we did not include any information on monoclonal antibodies in the current study, although this later became our standard of care for SOT outpatients with COVID-19.
Outcomes of Patients Admitted to Other Hospitals
There were 22 patients who had received transplants at Johns Hopkins Hospital, who were admitted to hospitals outside Johns Hopkins Medicine for COVID-19. These patients were not included in the analyses of inpatients because of limited data availability. Of these, 8 of 22 (36.4%) died during the COVID-19 admission to the outside hospital.
DISCUSSION
With additional clinical and published experience regarding COVID-19 therapeutics, remdesivir, dexamethasone, and convalescent plasma have largely replaced hydroxychloroquine and other therapies used earlier in the pandemic,1,14,27-30 yet little is known about safety and efficacy of these newer therapies in SOT recipients. In this study of 77 SOT inpatients with COVID-19, we observed no differences in length of stay, in-hospital or late mortality, rejection, renal or liver function, or secondary infections out to 90 d after the COVID-19 episode, comparing 2 different eras of therapeutics. Renal and liver function were not adversely affected after receiving remdesivir and convalescent plasma, and dexamethasone was not associated with excess infection risk, although the sample size of this study does not allow for definitive statements about safety. It is possible that statistically significant differences may emerge in larger studies. Renal function actually improved more rapidly in patients who had received remdesivir than those who had not received it, despite a higher severity of illness (reflected in maximal WHO severity score) in patients treated with remdesivir in this cohort. In our anecdotal experience with patients transferred from other centers, some clinicians remain hesitant to use newer targeted therapies in SOT recipients, particularly remdesivir in patients with renal dysfunction, so these results may help to provide support for administering these therapies to SOT inpatients with COVID-19.
Of note, across both eras, preexisting allograft dysfunction was associated with greater need for hospital admission and for mortality, although overall mortality in this cohort was relatively low. This observation may be helpful in triaging SOT recipients with newly diagnosed COVID-19 infection, and for targeting this group for prevention efforts in the future.
In the group of 52 outpatients successfully monitored at home, preexisting graft dysfunction was uncommon, and there were no cases of rejection during follow-up. Our center had developed methods for outpatient management early in the pandemic, incorporating telemedicine visits, frequent telephone follow-up, discontinuation of mycophenolate, provision of a pulse oximeter free of charge, and Vitamin D supplementation.25,26 Although 36.4% of 22 patients admitted to outside hospitals died (as compared to 5.6% admitted to our health system), this may have represented a more severely ill group of patients.
In summary, previous studies of SOT recipients with COVID-19 have included relatively few who received remdesivir, dexamethasone, and convalescent plasma. We report the use of all 3 of these therapies in SOT inpatients with COVID-19, with follow-up to 90 d after the COVID-19 episode. In both earlier and later therapeutic eras, we observed an overall low mortality and low incidence of late rejection.
Limitations
Limitations of this study include those inherent to a retrospective study. Case ascertainment of outpatients and those admitted to outside hospitals may have been incomplete, particularly patients who had transitioned to follow-up outside our health system. Antibody titers of convalescent plasma units were not known during that time period. The number of patients who had data on proteinuria after the COVID-19 episode was limited. Donor-specific antibody was not universally monitored in the aftermath of the COVID-19 episode, so it is possible that AMR was under-reported. Over half of patients in this study were kidney transplant recipients, so results may be less generalizable to other organ transplant recipients. Patients received targeted therapies per clinician choice and thus were not limited to a single therapeutic agent, so outcomes are not attributed to remdesivir alone or to convalescent plasma alone as would be the case in a randomized trial. A large randomized trial of remdesivir (or convalescent plasma) in SOT recipients would be a more reliable way to assess the benefits and risks of these therapies in the SOT population. However, since such trials are unlikely to be done, we feel that the kinds of information on these therapies that we can derive from our retrospective cohort is still of value to clinicians.
CONCLUSIONS
Outcomes were favorable across both the earlier and later eras of therapeutics at this center, with low mortality, low incidence of rejection, and return of renal and liver function to baseline in most patients after the COVID-19 episode. Patients with preexisting graft dysfunction had a higher risk for inpatient and intensive care unit admission and mortality and represent a group deserving of special attention. Since Era 1 patients already had low mortality at our center, comparative effectiveness of newer therapies in Era 2 cannot be assessed. We did not detect any associations of remdesivir, dexamethasone, or convalescent plasma with adverse patient or allograft outcomes. Although larger studies will be needed to confirm safety, we encourage use of these therapies in SOT inpatients with COVID-19 who meet appropriate criteria, including those with renal dysfunction or acute kidney injury at presentation.
Supplementary Material
Footnotes
A.S.S. and T.P.-Y.C. contributed equally.
This work was supported by grant numbers K24DK101828 (D.L.S.), K01DK101677 (A.B.M.), and K23DK115908 (J.G.-W.) from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and a grant (gSAN-201C0WW) from the Transplantation and Immunology Research Network of the American Society of Transplantation (W.A.W.). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This research was made possible with generous support of the Ben-Dov family (D.L.S.).
K.A.M. reports consulting/advisory board income from Amplyx, Cidara, Merck, and Sfunga. Equity and licensing revenue came from MycoMed Technologies. Research grant came from Merck. D.C.B. reports consulting/speaking honoraria from Allovir, Amplyx, Argenyx, CareDx, Natera, Sanofi, and Veloxis. Research support to Johns Hopkins from CareDx. N.P. reports the study support from Health Systems Research Institute, Ministry of Public Health, Thailand. T.J. reports consultancy with Targeted Oncology and the advisory board for CareDx and Bristol Myer Squibb. D.L.S. reports consulting/speaking honoraria from Sanofi, Novartis, CSL Behring, and Veloxis. R.K.A. reports study support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire.
A.S.S., T.P.-Y.C., K.A.M., A.B.M., W.C., P.S., T.J., J.G.-W., and D.L.S. participated in research design. All authors participated in substantial contributions to the conception of the work. A.S.S., W.C., and R.K.A. participated in data collection. T.P.-Y.C., A.B.M., A.G.T., and C.-Y.H. participated in data analysis. T.P.-Y.C., A.B.M., A.G.T., and C.-Y.H. contributed new analytic tools. A.S.S., T.P.-Y.C., A.B.M., and R.K.A. participated in writing of the paper. All authors participated in critical review and revisions of the paper and final approval.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Avery RK. COVID-19 therapeutics for solid organ transplant recipients; 6 months into the pandemic: where are we now? Transplantation. 2021;105:56–60. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Chen V, Fung CM, et al. COVID-19 outcomes among solid organ transplant recipients: a case-control study. Transplantation. 2021;105:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespo M, Mazuecos A, Rodrigo E, et al. ; Spanish Society of Nephrology COVID-19 Group. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104:2225–2233. [DOI] [PubMed] [Google Scholar]
- 4.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laracy JC, Verna EC, Pereira MR. Antivirals for COVID-19 in solid organ transplant recipients. Curr Transpl Rep. 2020;7:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Ruiz M, Aguado JM. Immunomodulatory therapies for COVID-19 in solid organ transplant recipients. Curr Transpl Rep. [Epub ahead of print. October 23, 2020]. doi:10.1007/s40472-020-00306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kates OS, Haydel BM, Florman SS, et al. ; UW COVID-19 SOT Study Team. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. [Epub ahead of print. August 7, 2020]. doi:10.1093/cid/ciaa1097. [Google Scholar]
- 8.Caillard S, Anglicheau D, Matignon M, et al. ; French SOT COVID Registry. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahalingasivam V, Craik A, Tomlinson LA, et al. A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 2021;6:24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naeem S, Gohh R, Bayliss G, et al. Successful recovery from COVID-19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2021;23:e13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman F, Liu STH, Taimur S, et al. Treatment with convalescent plasma in solid organ transplant recipients with COVID-19: Experience at large transplant center in New York City. Clin Transplant. 2020;34:e14089. [DOI] [PubMed] [Google Scholar]
- 12.Cravedi P, Schold JD, Safa K, et al. The COVID-19 pandemic: a community approach. Clin Transplant. 2020;34:e14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman JD, Julian K. Urinary tract infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13507. [DOI] [PubMed] [Google Scholar]
- 16.Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64:87–91. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. COVID-19 therapeutic trial synopsis. February 18, 2020. Available at https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed November 4, 2021.
- 18.Avery RK, Chiang TP, Marr KA, et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: a retrospective cohort. Am J Transplant. 2021;21:2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. October 22, 2020. Available at https://www.fda.gov/media/137564/download. Accessed January 20, 2021.
- 20.Thakare S, Gandhi C, Modi T, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep. 2021;6:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamsick ML, Gandhi RG, Bidell MR, et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31:1384–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. February 11, 2021. (referencing the EUA of August 2020). Available at https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed March 1, 2021.
- 24.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abuzeineh M, Muzaale AD, Crews DC, et al. Telemedicine in the care of kidney transplant recipients with coronavirus disease 2019: case reports. Transplant Proc. 2020;52:2620–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MY, Brennan DC, Shah P. General approach to the clinical care of solid organ transplant recipients with COVID-19 infection. Curr Transpl Rep. 2020;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19—Final Report. N Engl J Med. 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med. 2021;384:1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horby P, Mafham M, Linsell L, et al. ; RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;383:2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miarons M, Larrosa-García M, García-García S, et al. ; Vall d’Hebron COVID-19 Working Group. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation. 2021;105:138–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.