Abstract
Background:
The Long-terM OUtcomes after the Multisystem Inflammatory Syndrome In Children (MUSIC) study aims to characterize the frequency and time course of acute and long-term cardiac and non-cardiac sequelae in MIS-C, which are currently poorly understood.
Methods:
This multicenter observational cohort study will enroll at least 600 patients <21 years old who meet the Centers for Disease Control and Prevention case definition of MIS-C across multiple North American centers over 2 years. The study will collect detailed hospital and follow-up data for up to 5 years, and optional genetic testing. Cardiac imaging at specific time points includes standardized echocardiographic assessment (all participants) and cardiac magnetic resonance imaging (CMR) in those with left ventricular ejection fraction (LVEF) <45% during the acute illness. The primary outcomes are the worst LVEF and the highest coronary artery z-score of the left anterior descending or right coronary artery. Other outcomes include occurrence and course of non-cardiac organ dysfunction, inflammation, and major medical events. Independent adjudication of cases will classify participants as definite, possible, or not MIS-C. Analysis of the outcomes will include descriptive statistics and regression analysis with stratification by definite or possible MIS-C. The MUSIC study will provide phenotypic data to support basic and translational research studies.
Conclusions:
The MUSIC study, with the largest cohort of MIS-C patients and the longest follow-up period to date, will make an important contribution to our understanding of the acute cardiac and non-cardiac manifestations of MIS-C and the long-term effects of this public health emergency.
Keywords: Multisystem inflammatory syndrome in children associated with COVID-19 (MIS-C), long-term outcomes
BACKGROUND
Coronavirus disease 2019 (COVID-19), triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected more than 174 million people and caused over 3.7 million deaths worldwide as of this writing.1 Acute respiratory failure, severe hypoxemia, and a profound inflammatory response with “cytokine storm” have been implicated in the high mortality rates seen in vulnerable adult populations. Early in the pandemic, children accounted for <2% of laboratory-confirmed cases of COVID-19 infection in the US, and among children admitted to pediatric intensive care units in the US, only 4% died despite >80% having pre-existing medical conditions.2 More recently, 11.5% of all US cases are <18 years old, but children continue to have the lowest rates of hospitalization.3 Thus, children exposed to SARS-CoV-2 are much less likely than adults to develop acute COVID-19 infection.
A small subset of children exposed to SARS-CoV-2, however, develop a multisystem inflammatory syndrome, termed by the US Centers for Disease Control and Prevention (CDC) as the multisystem inflammatory syndrome in children (MIS-C),4 often with features of toxic shock and Kawasaki disease.4–6 Whereas hospital admissions for acute COVID-19 have predominantly occurred in children with pre-existing morbidities, the majority of children with MIS-C have generally been healthy, with obesity and underlying lung conditions the most common co-morbidities, present in 29% and up to 18%, respectively.7, 8 The incidence of MIS-C is higher in those of Black and Hispanic race/ethnicity.7–10 MIS-C generally peaks approximately one month after COVID-19 infection, consistent with a post-infectious mechanism related to the host immune response and hyperinflammation.11–14
Cardiovascular Involvement
Cardiovascular involvement occurs in approximately 80% of children with MIS-C,7, 8 consisting of left ventricular (LV) dysfunction, shock, coronary artery dilation and/or aneurysms, valvulitis, pericardial effusions, arrhythmias, and conduction abnormalities.5, 7–9, 15–22 Few studies have characterized MIS-C-associated ventricular dysfunction or coronary artery anatomy using standardized assessments, and no data are available regarding long-term follow-up.
Myocardial Function
Early case series of MIS-C have reported LV dysfunction in up to 100% of patients, likely related to selection bias towards inclusion of the sickest patients;5, 15–17, 23 for example, some studies used ventricular dysfunction or shock as entry criteria.17, 23 In the three largest MIS-C case series to date, including 186 patients across the US,8 99 from New York State,7 and 286 across Europe,21 echocardiographic evidence of ventricular systolic dysfunction was present in 34–52%, vasoactive support was required in 30–62%, and ≤4% of children required extracorporeal membrane oxygenation (ECMO). The apparent recovery of LV systolic function in most cases suggests that systolic function may be affected largely by the cytokine milieu or stress. However, even with recovery of LV systolic function, a case series suggests that echocardiographic parameters of diastolic dysfunction persist in the early outpatient follow-up period.20 The time course of full myocardial recovery is currently unclear.
Studies using cardiac magnetic resonance imaging (CMR) in children with MIS-C are limited. In 3 case series, with 20 patients from the UK,22 4 from France, 24 and 5 from New York,25 50–80% showed myocardial edema, and evidence of late gadolinium enhancement was mixed, with none in one series24 to 40% in another.25 However, the majority of CMRs were performed within a month of illness onset, and longer-term data are unavailable. Of note, in adults who have recently recovered from COVID-19 (median 71 days after diagnosis), CMR has shown abnormalities (increased myocardial native T1, increased myocardial native T2, late gadolinium enhancement, or pericardial enhancement) in 78% of patients and ongoing myocardial inflammation in 60%. These findings were independent of pre-existing conditions, severity, overall course of the acute illness, or time from original diagnosis.26 Whereas this study of adults has been used to guide recommendations for resumption of sports and exercise after COVID-19 infection,27, 28 there are no evidence-based data to guide sports participation in patients with myocardial dysfunction associated with MIS-C, and longer-term implications for myocardial health are unknown.
Coronary Artery Involvement
The severity of coronary artery involvement in MIS-C has varied among case series, with some only describing dilation,6, 17 and others reporting coronary artery aneurysms,5, 16, 18, 19 including giant aneurysms.16 In the three largest case series of MIS-C patients to date, 9% had coronary artery aneurysms as defined by a right coronary artery (RCA) or left anterior descending (LAD) z-score of ≥2.5,8 9% had documented coronary artery aneurysms though z-scores were not noted consistently,7 and 24.1% had z-score >2 in any coronary artery. Although rare, progression of coronary artery involvement to giant aneurysms at outpatient follow-up has been described,15 leading to recommendations for serial outpatient evaluations in these patients.29 It is not known whether the coronary artery enlargement in MIS-C is related to marked vasodilation in an inflammatory state30–33 versus the destruction of the arterial wall such as is seen in Kawasaki disease34, 35.
The extent and timing of coronary artery abnormalities in MIS-C is uncertain. Published case series have varied in their definitions of aneurysms and in the frequency of coronary artery imaging, and none have used centralized review of images to standardize assessment of the coronary arteries and account for the quality of imaging. The latter is particularly important in MIS-C given the inherent challenges of echocardiographic coronary artery imaging in these patients, as they are acutely ill and may be uncooperative, tachycardic, and obese7, 8 with poor acoustic windows. Furthermore, echocardiograms with targeted imaging are often obtained to prioritize safety and minimize the exposure of sonographers. Standardized protocols for acquisition of coronary artery images and core laboratory interpretation across centers, together with systematic longer-term surveillance, are needed to characterize the prevalence and progression of coronary artery abnormalities in MIS-C.
Arrhythmias
Arrhythmias and other electrocardiographic changes have been reported in 7–60% of the case series on MIS-C noted above. These have included premature atrial and ventricular beats; atrial fibrillation; first, second, and rarely third-degree atrioventricular block; prolongation of the QTc interval; and rarely sustained arrhythmias causing hemodynamic compromise and the need for ECMO support. Long-term data on persistence or recurrence of arrhythmias and other electrocardiographic changes are unknown.
Non-Cardiovascular Organ System Involvement
As implied by the syndrome name, multiple organ systems beyond the cardiovascular system have been involved in MIS-C. While any organ system is at risk, children with MIS-C most commonly present with gastrointestinal8, 9 and mucocutaneous symptoms and/or laboratory abnormalities,8, 9, 36 including vomiting, diarrhea, abdominal pain, transaminitis, and gall bladder hydrops, as well as rash and conjunctivitis, respectively.37 Hematologic abnormalities have included pulmonary embolism38 and deep vein thrombosi,37 or laboratory abnormalities including anemia and thrombocytopenia.8 Neurologic symptoms have included headache,7, 39 altered mental status,7 and encephalopathy.39, 40 Of note, a phenomenon termed “long COVID” has been used to describe those with previous COVID-19 infection who have persistent symptoms beyond the acute illness, with features similar to myalgic encephalomyelitis, chronic fatigue syndrome, and autonomic dysfunction.41–44 However, long-term neurocognitive or other organ system sequelae of MIS-C are unknown.
RATIONALE FOR THE STUDY
To better understand this emerging illness and its sequelae, the Long-TerM OUtcomes after the Multisystem Inflammatory Syndrome In Children (MUSIC) study was developed and will be the first to define long-term cardiovascular and other organ system health status in the largest population of children and adolescents with MIS-C to date. In addition, this study will contribute important new information on acute cardiac findings, including standardized measures of myocardial performance and coronary artery aneurysms in this mostly treated cohort. Through harmonization of data elements with other multicenter studies on MIS-C, this longitudinal study will provide a unique framework including phenotyping for basic and translational research studies. Specifically, we will provide clinical data from this study to investigators seeking to analyze the relationships of genomic or other fundamental biological factors with cardiac outcomes and facilitate collaboration across existing registries and center projects. Finally, risk stratification models and machine learning may identify personalized care pathways to both predict risk and improve outcomes.
STUDY DESIGN AND METHODS
This multicenter observational cohort study, funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute (NHLBI), will use routinely collected clinical and cardiac (electrocardiogram [ECG], echocardiogram, CMR, exercise testing) data to assess the association between MIS-C and cardiac and non-cardiac outcomes. The aims of this study are:
To characterize the frequency and course over time of LV dysfunction in MIS-C associated with COVID-19.
To characterize the frequency and time course of coronary artery dilation (z ≥2.0 to <2.5) and coronary artery aneurysm (z ≥2.5) in MIS-C associated with COVID-19.
To characterize the frequency and time course of non-cardiac organ dysfunction, inflammation, and major medical events, including death.
To develop clinical risk stratification models for worse LV systolic function or higher coronary artery maximum z-scores and for their evolution over time.
To determine genetic associations with MIS-C manifestations by collecting DNA specimens from participants and their parents for whole genome sequencing (WGS) for future research.
The MUSIC study (https://covidmusicstudy.com/) is a component of the Department of Health and Human Services strategy to understand MIS-C and pediatric COVID-19 as quickly as possible. The study uses the research infrastructure of the Pediatric Heart Network (PHN), a pediatric cardiology research consortium funded by NHLBI, and its data coordinating center, HealthCore Inc. This study is supported by grants (HL135680, HL135685, HL135683, HL135689, HL135646, HL135665, HL135678, HL135682, HL135666, HL135691, HL068270) from the NHLBI, NIH. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Inclusion Criteria
Eligibility criteria for the MUSIC study align with the current CDC definition for MIS-C4
Age <21 years.
Fever ≥38°C for ≥24 hours, or report of subjective fever lasting ≥24 hours.
Laboratory evidence of inflammation, including, but not limited to, one or more of the following: an elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, d-dimer, ferritin, lactate dehydrogenase (LDH), interleukin-6 (IL-6) or neutrophils, reduced lymphocytes and low albumin.
Evidence of clinically severe illness requiring hospitalization, with multisystem (≥2) organ involvement, based on clinical judgment from record review, discharge diagnosis, laboratory or diagnostic tests. Organ system involvement includes but is not limited to cardiac, renal, respiratory, hematologic including coagulopathy, gastrointestinal including liver, dermatologic or neurological.
Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms.
Some individuals may fulfill American Heart Association (AHA) complete or incomplete criteria for KD,35 but they will be eligible for inclusion in this study if they meet the case definition for MIS-C. We will consider MIS-C in any deaths in children or adolescents with evidence of SARS-Cov-2 infection if they otherwise meet criteria for enrollment.
Exclusion Criterion
A plausible alternative diagnosis, such as culture-positive bacterial sepsis, murine typhus, staphylococcal or streptococcal shock syndromes.
Recruitment and Enrollment
As of March 31, 2021, 33 centers in North America have committed to participating in the MUSIC study: 10 PHN core centers and 23 auxiliary centers, which were chosen based on geographic variation, racial diversity and number of MIS-C cases (Supplement 1). We plan to enroll at least 600 participants in 2 years and will include all eligible patients who meet the CDC case definition of MIS-C starting from March 2020. Follow-up will be for up to 5 years. The MUSIC study launched in October 2020, and as of May 26, 2021, we have enrolled over 600 participants.
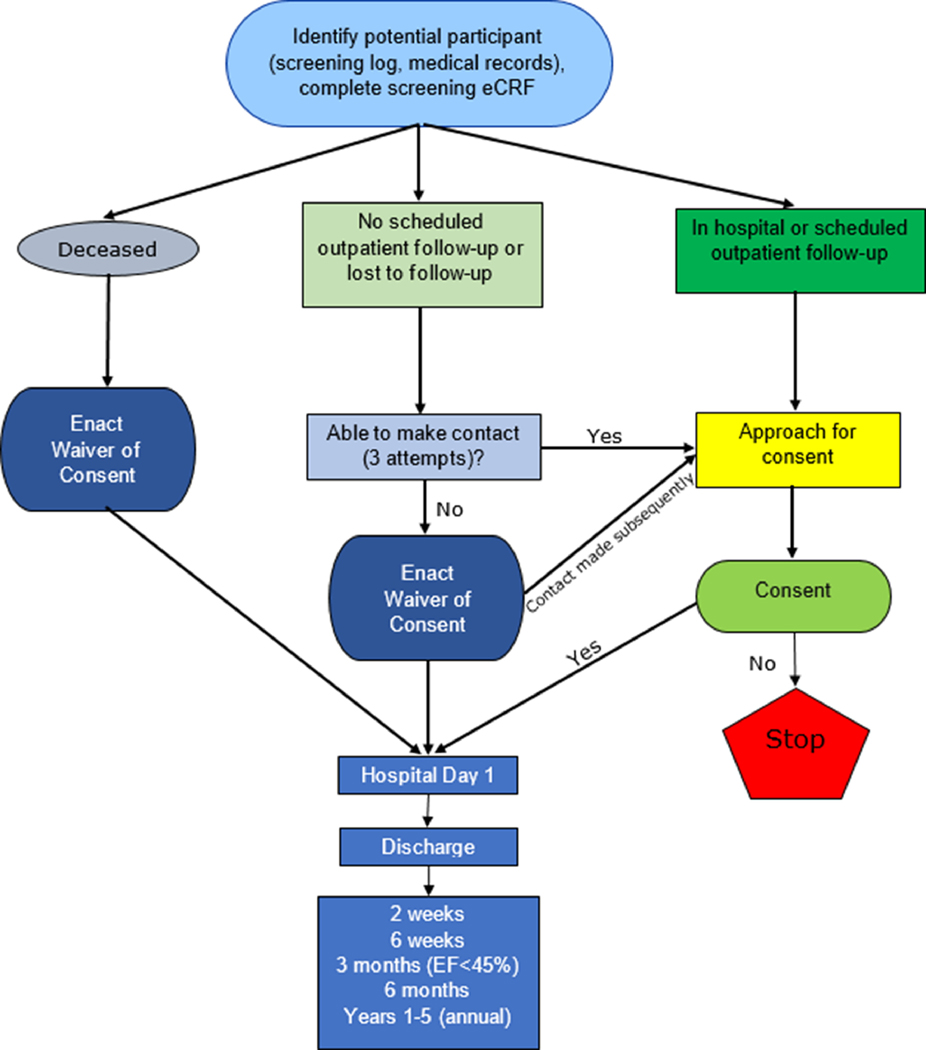
Participants can be enrolled at any time in the first year after MIS-C onset, either retrospectively or prospectively. Three potential pathways for patient recruitment include: 1) parental/guardian or participant consent during the hospitalization, at outpatient follow-up, or via a virtual platform after contacting the family by mail, phone, or email; 2) waiver of consent after three unsuccessful attempts to contact a family; or 3) waiver of consent due to death prior to study enrollment to avoid bias in the data (Figure 1). Because MIS-C surged in many centers before the MUSIC study began, each center will identify past cases who were hospitalized with MIS-C. For those enrolled under a waiver of consent, the waiver will cover clinical data for research elements which meet the specific regulatory criteria that the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context. The waiver of consent and collection of retrospective data on cardiac and non-cardiac organ dysfunction are considered vital because MIS-C particularly affects underrepresented minorities and underserved populations who may be more difficult to reach. Omission of these data would carry substantial risk of selection bias, affecting the generalizability and validity of the results.
Figure 1.
Pathways for enrollment of participants in the MUSIC study. For participants enrolled under a waiver of consent due to death or lost to follow-up, follow-up data beyond discharge may not be available.
Outcome Measures and Schedule of Measurements
Our principal goal is to determine the spectrum and early time course of LV systolic dysfunction, coronary artery involvement, and arrhythmias or conduction system abnormalities, and to define associated clinical and laboratory factors using these data. The primary and secondary cardiac outcomes are listed in Table 1.
Table 1.
Primary and Secondary Cardiac Outcomes
| Primary Outcomes |
| 1. Worst-ever LVEF |
| 2. Worst-ever maximum z-score of the proximal LAD or RCA |
| Secondary Cardiac Outcomes |
| 1. Occurrence of a proximal LAD or RCA z-score of ≥2.5 on any echocardiogram |
| 2. Occurrence of aneurysms by Japanese Ministry of Health criteria |
| 3. Individual z-scores for the LMCA, RCA, and LAD |
| 4. Left ventricular size and function: LVEDV z-score, LVEF, LVSF |
| 5. Percentage of patients who had LVEF <55% on any echocardiogram |
| a. LVEF 45–54% (mildly depressed systolic function) |
| b. LVEF 35–44% (moderately depressed systolic function) |
| c. LVEF <35% (severely depressed systolic function |
| 6. LV strain |
| a. Global longitudinal strain from apical view |
| b. Global circumferential strain from parasternal short-axis view |
| 7. Right ventricular systolic function (qualitative assessment) |
| a. If possible, right ventricular global longitudinal strain |
| 8. Presence and degree of mitral and aortic valve insufficiency |
| 9. LV diastolic function, i.e., tissue Doppler imaging and mitral valve inflow |
| 10. Presence and size of pericardial effusion |
| 11. The occurrence of arrhythmias and conduction system disturbances |
| 12. Exercise testing (age and maturing permitting) at ~3 months in those who had at least moderate systolic dysfunction |
| 13. CMR outcomes |
| a. LVEF and RVEF |
| b. Valvar regurgitation |
| c. Percent of participants with |
| i. Myocardial late gadolinium enhancement, and its distribution |
| ii. Abnormal LV T2-weighted imaging |
| iii. Elevated LV T2 |
| iv. Elevated LV native T1 |
| v. Elevated LV extracellular volume fraction |
| vi. Coronary artery dilation |
| vii. Final interpretation of CMR as abnormal, equivocal, or normal |
LVEF=Left ventricular ejection fraction; LAD=Left anterior descending; RCA=Right coronary artery; LMCA=Left main coronary artery; LVEDV=Left ventricular end-diastolic volume; LVSF=Left ventricular shortening fraction; CMR=Cardiac magnetic resonance imaging; RVEF=Right ventricular ejection fraction
The secondary non-cardiac outcomes are listed in Table 2.
Table 2.
Secondary Non-Cardiac Outcomes
| 1. Other organ abnormalities: Immunologic, rheumatologic, renal, pulmonary, hematologic, gastrointestinal, dermatologic or neurologic |
| 2. Trends in laboratory markers of inflammation |
| 3. Admission to the intensive care unit |
| 4. Maximal vasoactive inotrope score |
| 5. Hospital length of stay |
| 6. Symptom duration |
| 7. Major medical events (e.g. stroke, need for extracorporeal therapies such as renal replacement therapy, plasma exchange, ECMO, ventricular assist device) |
| 8. Mortality |
| 9. Global Health |
| a. Functional Status Score45 |
| b. Patient-Reported Outcomes Measurement Information Systems (PROMIS) Instrument46 |
ECMO=Extracorporeal membrane oxygenation
The schedule of measurements is based on standard clinical follow-up in MIS-C patients (Table 3), with study timepoints at Day 1 of hospitalization, hospital discharge, follow-up at 2 weeks, 6 weeks, and 6 months after discharge, and annually at years 1–5. In those with LVEF <45%, we expect that CMR and exercise testing would be obtained as part of routine clinical care at ~3 months. We included wide windows for each timepoint to optimize data capture.
Table 3.
Schedule of Measurements
| Variable | Hospital Day 1 | Discharge | 2 wks (1<3 wks) | 6 wks (3–9 wks) | 3 mo (1–6 mo) | 6 mo (9 wks–1 yr) | 1–5 Years |
|---|---|---|---|---|---|---|---|
| Demographics | X | ||||||
| Medical History1 | X | X | X | X | X | X | |
| Echocardiogram | X | X | X | X | X2 | X2 | |
| ECG | X | X | X | X | X | ||
| CMR and exercise test (LVEF <45%) | X | ||||||
| Clinical Labs3 | X | X | X | X | X | ||
| Research Labs4 | X | X | X | X | X |
ECG=Electrocardiogram; CMR=Cardiac magnetic resonance imaging; LVEF=Left ventricular ejection fraction
Medical history will include complete cardiac and non-cardiac systems review; Functional Status Score (FSS) will be measured Day 1, Discharge, and 2 weeks, 6 weeks, and 6 months; PROMIS instrument at 2 weeks, 6 weeks, 6 months, and annually.
Echocardiograms at 6 months and annually between years 1 and 5 are optional in patients who have previously had two consecutive normal echocardiograms, though the study will collect information on clinical cardiology visits beyond the 6-month window, and also will complete annual medical history forms for years 1–5, to bring follow-up to up to 5 years after presentation.
We will collect and analyze all data obtained as part of routine care. Although we will not mandate specific laboratory tests in the research protocol, we recommend based upon best clinical evidence to date that patients have lab testing that includes at a minimum the following tests until they have normalized: CBC and differential (for absolute lymphocyte count and neutrophil/lymphocyte ratio), platelets, ESR, CRP, ferritin, D-Dimers, procalcitonin (if the center performs this), ALT, BNP and troponin.
Biospecimens for the PHN biobank for future whole genome sequencing in patients who give consent for this can be obtained at any point within the first year of enrollment. Other research laboratory tests may be drawn at the time of blood draws based upon the patient’s participation in protocols in addition to the MUSIC study within their clinical center if they have signed the study-specific informed consent form.
Adjudication of MIS-C Diagnosis
The CDC case definition of MIS-C is broad and nonspecific, and confirmatory laboratory testing by serologic testing can be unreliable. Furthermore, as increasing seroprevalence from exposures or vaccination becomes more common, accurate diagnosis of MIS-C will become more complicated and inaccurate diagnoses may increase. An Adjudication Committee of three experts from pediatric rheumatology, pediatric infectious disease, and pediatric cardiology, will classify cases as definite, possible, or not MIS-C. They will use data from within the first 6 months after initial presentation, based upon the state of current knowledge.
Medical History and Annual Health Assessment
Besides extensive data collection during the hospital period, including labs, treatment and medication, participants will undergo a medical history at each timepoint, including at the annual health assessment that will either occur in-person, or via virtual platform or telephone starting 1 year from MIS-C diagnosis for up to 5 years from diagnosis. The medical history includes a series of questions to better understand the participant’s current health status. The broad range of questions covers general health and well-being with specific questions on various organ systems including cardiovascular, respiratory, neurologic, rheumatologic, immunologic, hematologic, genitourinary, renal, gastrointestinal, and dermatologic. (Supplement 2)
Tools to assess general health and well-being include the Functional Status Score45 (FSS) and the Patient-Reported Outcomes Measurement Information Systems (PROMIS) Global Health46 instrument. The FSS assesses 5 domains of functioning: mental status, sensory functioning, communication, feeding, and respiratory status,45 and will be assessed at hospital admission and discharge. The PROMIS Global Health assesses an individual’s physical, mental, and social health in a general manner, rather than in disease-specific terms.47 The age appropriate Pediatric, Parent Proxy, or Adult PROMIS Global Health instrument46 will be assessed at the 2 week, 6 week, 6 month, and annual follow-up time points.
Echocardiographic Core Laboratory
The MUSIC study uses an echocardiographic imaging protocol for prospectively-performed imaging studies and an Echocardiographic Core Laboratory (Boston Children’s Hospital) to ensure standardized echocardiographic assessment for the outcome measures. The Echocardiographic Core Laboratory will interpret all echocardiograms 1) performed at the study time points; 2) with the worst LVEF and worst-ever LAD or RCA z-score obtained outside of the study time points; 3) performed beyond the 6-month visit because of persistent abnormalities. Research funds are available for unsedated echocardiograms to be performed in participants at the designated time points if they are not deemed to be clinically indicated by the local study site.
CMR Core Laboratory
The CMR Core Laboratory (Cincinnati Children’s Hospital) will ensure uniform assessment of CMR outcomes, measured according to standard established conventions.48–50. We anticipate that CMRs will be performed approximately 3 months (range 1–6 months) after illness onset in children who have at least moderately depressed LV function (i.e., LVEF <45%). Research funds will be available for CMR (unsedated and without a gadolinium-based contrast agent) if not ordered for clinical reasons in participants who had decreased LVEF <45%. The CMR Core Laboratory will also interpret all CMRs ordered for any clinical reason, including mild decrease in function or arrhythmias, as such studies could potentially identify subclinical issues.
Assessment of Arrhythmias and Conduction Disturbances
To assess for arrhythmias and conduction disturbances, we will collect data from resting electrocardiograms (ECG) obtained during the hospitalization and follow-up of participants, with research funds available for ECGs if not ordered for clinical reasons at the study timepoints. Data will also be collected from any ambulatory monitoring, as well as any exercise stress testing performed for clinical purposes, both of which may be ordered as part of assessment prior to return to sports in those with significant ventricular dysfunction or concerns for myocarditis.51
Optional Genetic Testing and Biorepository
Participants who consent for optional genetic testing will provide blood or saliva samples for future whole genome sequencing. Biological parents will also be approached for blood or saliva samples for optional genetic testing to better understand genetic variation within the MIS-C population. Samples will be stored at the PHN biorepository (University of Michigan). We will leverage the careful phenotyping of participants in the MUSIC study to provide an opportunity for future genotype-phenotype studies using whole genome sequencing from those samples, and to make these data available for hypothesis-driven studies aimed at identifying genetic determinants of outcome in MIS-C. By aligning the study with treatment trials and bench research, and in using common data elements and access to clinical data, the structure of this study will provide a resource for investigators across disciplines and funding agencies. The Data Coordinating Center (HealthCore) will provide necessary infrastructure, harmonization, data synthesis, and quality control.
Statistical Considerations
In this observational study, we will exclude cases later adjudicated as “not MIS-C” from analyses of MIS-C outcomes, and will describe the alternative diagnoses. Coronary artery dimensions will be normalized for BSA as z-scores using the Boston Z-score system52 (primary, for consistency with the definition of coronary artery aneurysms in the AHA guideline), as well as the PHN53 (secondary). We will characterize the study population, treatment course, medical history, and study outcomes over time using descriptive statistics. Before beginning statistical modelling, outcomes will be examined for normality and transformations considered. Trends over time will be examined through non-linear curve fitting, e.g., LOESS curves; this will inform further modelling, e.g., treating time as linear, quadratic, or categorical.
For the analysis of the primary outcome measures, we will use mixed effects linear regression analysis for repeated measures to examine trends in LVEF and coronary artery z- scores over time, as well as to identify independent factors associated with lower LVEF or higher coronary artery z-scores. As factors such as a known prior history of decreased LVEF or coronary artery dilation have a potential to confound the results, we will first calculate the proportion of those with a known prior history of decreased LVEF or coronary artery dilation (with or without Kawasaki disease), but will exclude them from the main analysis of LV dysfunction and/or coronary artery outcomes. Predictors significant at level 0.20 in bivariate analyses (with time included in all models) will be entered into a multivariable model with backwards selection. Care will be taken to investigate possible collinearity of predictors and to choose from among the highly correlated predictors for inclusion in the multivariable model.
For secondary outcome measures, we will similarly use linear, logistic, or Cox regression analyses to assess trends over time and multivariable associations of other outcomes, such as LVEF at the time of presentation, presence of conduction system abnormalities, or symptom duration, respectively. A random effect of site will be used for outcomes that may be affected by differences in practice between sites, e.g., length of stay. Risk stratification models will also be developed by applying machine learning (random forest) to dichotomized outcomes. We will train both random forest and gradient boosting algorithms, selecting the best performing models. The dataset will be split into training and test sets using stratified random sampling to ensure balanced samples.
Subgroup analysis will be performed based on adjudication as “definite” vs. “possible” MIS-C, changing definitions of MIS-C over time, and by cohorts over time. At this time, there are no plans for subgroup analyses for specific pre-existing conditions, including congenital heart disease; however, if ≥10% of participants have a particular pre-existing condition, subgroup analysis will be considered. Moreover, patients with forms of congenital heart disease that might affect left ventricular size or function, or coronary artery dimension, will be excluded from analyses of those variables; in particular, congenital heart disease except for bicommissural aortic valve with <mild stenosis and/or insufficiency, mitral valve prolapse with <mild insufficiency, and hemodynamically insignificant atrial septal or ventricular septal defects will be excluded from analyses of left ventricular or coronary artery outcomes. Differential effects of treatment strategies on outcomes will be explored via propensity score adjustment.
DISCUSSION
Importance of the Knowledge to be Gained
While MIS-C is a rare complication of COVID-19, the multiorgan involvement leads to critical illness in the majority of patients, many of whom had been previously healthy. The results of the MUSIC study will make an important contribution to our understanding of the cardiac and non-cardiac manifestations of MIS-C and its long-term effects through a systematic and standardized history and imaging assessment for up to 5 years across North American centers. Currently available practices and algorithms are based on limited data and extrapolated from other illnesses, such as myocarditis. The results of the MUSIC study are expected to provide vital data to inform clinical pathways and algorithms for best therapies and clinical practice during the acute illness, as well as for long-term follow-up and return to sports. This study will also share phenotypic data with translational and basic researchers studying MIS-C biology, thereby expanding our understanding of MIS-C disease mechanisms.
STUDY LIMITATIONS
This study has several potential limitations. The number of eligible patients is unpredictable, because we do not know whether the current surge will be maintained or the location of future hot spots. For this reason, we have chosen sites in diverse geographic locations. It is possible that the CDC definition for MIS-C will change during the course of the study. MUSIC study entry criteria will change in accordance with the most current CDC definition, and we will account for the change in definition in the study analysis plan. We anticipate that some families of children who had MIS-C prior to the study launch at their local center may be difficult to reach. Moreover, it is possible that return for clinical follow-up will be lower in this previously healthy population of low socioeconomic status than in our usual studies in children with a history of heart disease and a pre-existing relationship with pediatric cardiologists. Participants with mild disease may also be less inclined to return for follow-up at the recommended time points. Each center will work closely with social work and pediatric offices to maximize return for scheduled visits. We will request a waiver of consent for collection of information related to clinical care when we are unable to reach patients after three attempts. As the study is observational, centers and providers could vary considerably with respect to depth of testing or timing of follow-up, we have allotted funding for echocardiograms and CMR in some patients. We have constructed our study protocol in accordance with current norms of routine practice; therefore, most prospectively-enrolled patients should have relatively complete data. Data will be reviewed periodically to search for patterns of missingness, and we will determine ways to address gaps in data collection. Our study can serve as a scaffold for basic and translational research of other investigators. If patients have not given consent to contribute biospecimens, this could limit study utility for correlation of clinical phenotype with fundamental bench findings.
CONCLUSIONS
The emergence of MIS-C during the COVID-19 pandemic has created a public health emergency that necessitates multicenter collaboration to better understand this new illness and its long-term outcomes. The MUSIC study will gather systematic follow-up data using standardized protocols for cardiac imaging, independent assessment in Echocardiographic and CMR Core Laboratories and surveillance for non-cardiac morbidities and global health in a large population of patients with MIS-C. Through harmonization of data elements with other studies on MIS-C, this longitudinal study will provide a unique framework including phenotype for basic and translational research studies. In the future, risk stratification models and machine learning may identify personalized care pathways to both predict risk and improve outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The study was supported by grants (HL135680, HL135685, HL135683, HL135689, HL135646, HL135665, HL135678, HL135682, HL135666, HL135691, HL068270) from the National Heart, Lung, and Blood Institute, NIH.
Footnotes
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclaimer: The views expressed in this manuscript are those of the authors, and do not necessarily represent the views of the National Heart, Lung, and Blood Institute or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.WHO Health Emergency Dasboard. In: https://covid19whoint/; Access date: June 10, 2021.
- 2.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr 2020;174(9):868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID Data Tracker. In: https://covidcdcgov/covid-data-tracker/#demographics; Access Date: March 23, 2021.
- 4.CDC Health Advisory: Multi-system Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). In; 2020.
- 5.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet 2020;395(10239):1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med 2020;383(4):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020;383(4):334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shana Godfred-Cato D, Bobbi Bryant M, Jessica Leung M, Matthew E. Oster M, Laura Conklin M, Joseph Abrams P, et al. COVID-19–Associated Multisystem Inflammatory Syndrome in Children —United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020;69 (32):1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javalkar K, Robson VK, Gaffney L, Bohling AM, Arya P, Servattalab S, et al. Socioeconomic and Racial/Ethnic Disparities in Multisystem Inflammatory Syndrome. Pediatrics 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapid risk assessment: Paediatric inflammatory multisystem syndrome and SARS –CoV-2 infection in children. European Centre for Disease Prevention and Control 2020. [Google Scholar]
- 12.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020;183(4):968–981 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vella L, Giles JR, Baxter AE, Oldridge DA, Diorio C, Alanio C, et al. Deep Immune Profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020;183(4):982–995 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet 2020;395(10237):1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020. [DOI] [PubMed] [Google Scholar]
- 18.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionne A, Mah DY, Son MBF, Lee PY, Henderson L, Baker AL, et al. Atrioventricular Block in Children With Multisystem Inflammatory Syndrome. Pediatrics 2020;146(5). [DOI] [PubMed] [Google Scholar]
- 20.Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. Echocardiographic Findings in Pediatric Multisystem Inflammatory Syndrome Associated With COVID-19 in the United States. J Am Coll Cardiol 2020;76(17):1947–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute Cardiovascular Manifestations in 286 Children with Multisystem Inflammatory Syndrome Associated with COVID-19 Infection in Europe. Circulation 2020. [DOI] [PubMed] [Google Scholar]
- 22.Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 2020;10(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, et al. Cardiac MRI in Children with Multisystem Inflammatory Syndrome Associated with COVID-19. Radiology 2020;297(3):E283–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S, Nolan SM, Singh AR, Lovig L, Biller R, Kamat A, et al. Myocarditis in Multisystem Inflammatory Syndrome in Children Associated With Coronavirus Disease 2019. Cardiol Rev 2020;28(6):308–311. [DOI] [PubMed] [Google Scholar]
- 26.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5(11):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelan D, Kim JH, Chung EH. A Game Plan for the Resumption of Sport and Exercise After Coronavirus Disease 2019 (COVID-19) Infection. JAMA Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, et al. Coronavirus Disease 2019 and the Athletic Heart: Emerging Perspectives on Pathology, Risks, and Return to Play. JAMA Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 29.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. European Journal of Pediatrics 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratincsak A, Reddy VD, Purohit PJ, Tremoulet AH, Molkara DP, Frazer JR, et al. Coronary artery dilation in acute Kawasaki disease and acute illnesses associated with Fever. Pediatr Infect Dis J 2012;31(9):924–6. [DOI] [PubMed] [Google Scholar]
- 31.Binstadt BA, Levine JC, Nigrovic PA, Gauvreau K, Dedeoglu F, Fuhlbrigge RC, et al. Coronary artery dilation among patients presenting with systemic-onset juvenile idiopathic arthritis. Pediatrics 2005;116(1):e89–93. [DOI] [PubMed] [Google Scholar]
- 32.Cevik C, Otahbachi M, Nugent K, Jenkins LA. Coronary artery aneurysms in Behcet’s disease. Cardiovasc Revasc Med 2009;10(2):128–9. [DOI] [PubMed] [Google Scholar]
- 33.Kikuta H, Taguchi Y, Tomizawa K, Kojima K, Kawamura N, Ishizaka A, et al. Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 1988;333(6172):455–7. [DOI] [PubMed] [Google Scholar]
- 34.Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One 2012;7(6):e38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease; 2017. [DOI] [PubMed] [Google Scholar]
- 36.Young TK, Shaw KS, Shah JK, Noor A, Alperin RA, Ratner AJ, et al. Mucocutaneous Manifestations of Multisystem Inflammatory Syndrome in Children During the COVID-19 Pandemic. JAMA Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. 2020. [Google Scholar]
- 38.Winant AJ, Blumfield E, Liszewski MC, Kurian J, Foust A, Lee EY. Thoracic Imaging Findings of Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19: What Radiologists Need to Know Now Radiology: Cardiothoracic Imaging 2020;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-Mannan O, Eyre M, Lobel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome. JAMA Neurol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nath A. Long-Haul COVID. Neurology 2020;95(13):559–560. [DOI] [PubMed] [Google Scholar]
- 42.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020;69(30):993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary Evidence on Long COVID in children. medRxiv 2021:2021.01.23.21250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics 2009;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.PROMIS Health In: https://wwwhealthmeasuresnet/explore-measurementsystems/promis; Access date: February 28, 2021.
- 47.Forrest CB, Bevans KB, Pratiwadi R, Moon J, Teneralli RE, Minton JM, et al. Development of the PROMIS (R) pediatric global health (PGH-7) measure. Qual Life Res 2014;23(4):1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging 2013;6(3):373–83. [DOI] [PubMed] [Google Scholar]
- 50.Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease. Pediatrics International 2005;47:711–732. [DOI] [PubMed] [Google Scholar]
- 51.Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA 3rd, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2015;132(22):e273–80. [DOI] [PubMed] [Google Scholar]
- 52.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985) 2005;99(2):445–57. [DOI] [PubMed] [Google Scholar]
- 53.Lopez L, Colan S, Stylianou M, Granger S, Trachtenberg F, Frommelt P, et al. Relationship of Echocardiographic Z Scores Adjusted for Body Surface Area to Age, Sex, Race, and Ethnicity: The Pediatric Heart Network Normal Echocardiogram Database. Circulation Cardiovascular imaging 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.