Abstract
We herein report an 84-year-old woman with right middle cerebral artery (MCA) stenosis who presented with persistent left hemichorea preceding cerebral infarction. She visited our hospital on day 9 after the hemichorea onset. Magnetic resonance imaging (MRI) showed no acute cerebral infarction. Magnetic resonance angiography revealed right MCA stenosis. Her hemichorea persisted for 19 days and subsequently disappeared. On day 21, she developed left hemiplegia. Repeat MRI revealed a cerebral infarction in the right putamen. MCA stenosis can present with persistent hemichorea, even in the absence of cerebral infarction. Persistent hemichorea with MCA stenosis may presage cerebral infarction.
Keywords: chorea, hemichorea, cerebral infarction, middle cerebral artery, basal ganglia, putamen
Introduction
Chorea is a symptom characterized by abrupt involuntary movements resulting from a continuous flow of random muscle contractions (1). New-onset chorea can be caused by infections, autoimmune diseases, drug-induced disorders, metabolic diseases, neurodegenerative diseases, and stroke (1). Stroke is the most common cause of sporadic chorea (2), and hemichorea is the most common involuntary movement disorder with stroke (3). However, persistent hemichorea is a rare manifestation of the pre-stroke phase.
We herein report a patient with right middle cerebral artery (MCA) stenosis presenting with hemichorea that persisted for 19 days and preceded cerebral infarction. She developed cerebral infarction of the right putamen with facial palsy and hemiplegia, and her hemichorea disappeared.
Case Report
An 84-year-old woman, who was undergoing treatment for diabetes mellitus and hypertension complained of persistent involuntary movements in her left arm and leg. She visited our hospital on day 9 after the onset of involuntary movements. She had no history of smoking and drinking or family history of movement disorders. A neurological examination revealed hemichorea on the left side and no other signs of neurological impairment. Her hemichorea was persistent and was worsened by mental strain or calculation.
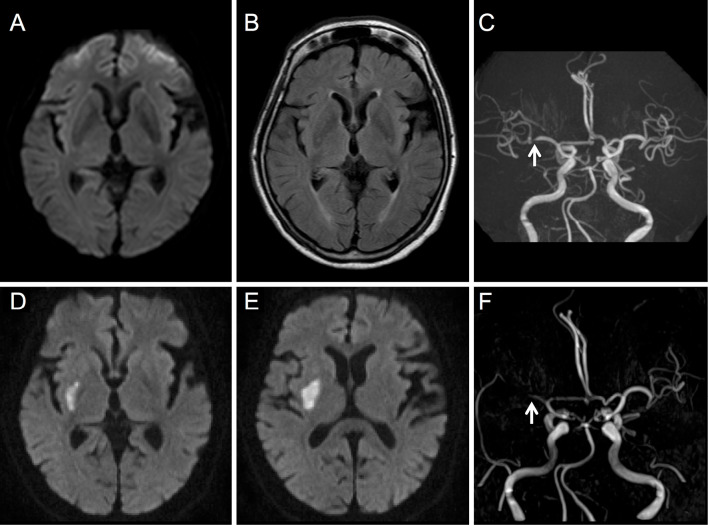
Brain diffusion-weighted imaging-magnetic resonance imaging (DWI-MRI) did not show any abnormalities (Fig. 1A). Fluid-attenuated inversion recovery (FLAIR) MRI showed white matter lesions around the anterior and posterior horn of the lateral ventricles (Fig. 1B). FLAIR and T2-weighted MRI revealed few small perivascular spaces in the bilateral putamen. Magnetic resonance angiography (MRA) revealed stenoses of the right MCA and posterior cerebral artery (Fig. 1C).
Figure 1.
Magnetic resonance imaging (MRI) results on day 9 and day 21 after the onset of hemichorea. The upper panels show the initial MRI findings on day 9 after the onset of hemichorea, and the lower panels show the MRI findings on day 21. A: Diffusion-weighted imaging (DWI) shows no acute lesions. B: Fluid-attenuated inversion recovery MRI reveals white matter lesions around the anterior and posterior horn of the lateral ventricles. C: Magnetic resonance angiography (MRA) reveals stenosis in the distal portion of the M1 segment of the right middle cerebral artery (MCA) (arrow). D, E: DWI reveals a fresh infarct in the right putamen. F: MRA reveals occlusion in the proximal portion of M1 segment of the right MCA.
Blood tests revealed normal renal, hepatic, and thyroid functions. Her serum levels of glucose, iron, copper, and ceruloplasmin were also normal. Her serum levels of low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides were 94 mg/dL, 51 mg/dL, and 119 mg/dL, respectively. Immunologic tests were negative for anti-nuclear and anti-cardiolipin-beta2-glycoprotein I complex antibodies. Since there was no evidence of acute cerebral infarction and since she refused to take anti-dopaminergic agents, we did not prescribe any anti-platelet or anti-dopaminergic agents.
On day 19 after the onset of hemichorea, she developed left facial palsy. Hemichorea persisted for 19 days and subsequently disappeared. On day 21, she visited our hospital with left facial palsy and hemiplegia. Her blood pressure was 113/69 mmHg, pulse rate 55 beats/minute, and body temperature 36.7℃. Her muscle strength was grade 2 in the left arm and grade 3 in the left leg on the Medical Research Council (MRC) Muscle scale. There were no marked differences in the bilateral tendon reflexes, and her plantar reflexes were absent in both of her feet.
Repeat brain DWI-MRI revealed an acute cerebral infarction in the right putamen (Fig. 1D, E). According to MRA, the stenosis of the right MCA developed into an occlusion (Fig. 1F). No stenoses or occlusions were observed on a carotid ultrasound.
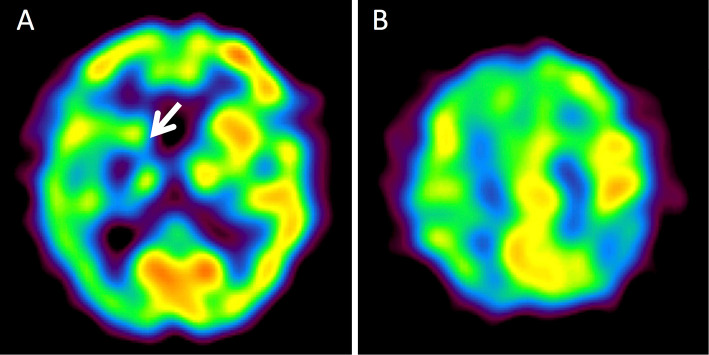
She was admitted to our hospital and treated with anti-platelet therapy. On day 29, single photon-emission computed tomography (SPECT) using technetium-99m ethylcysteinate dimer revealed hypoperfusion in the right MCA territory, including the basal ganglia (Fig. 2A, B). At discharge on day 75, her muscle strength in the left limb had improved to grade 4 on the MRC scale, but mild dysarthria and left facial palsy remained.
Figure 2.
Single-photon emission computed tomography images. A, B: Single-photon emission computed tomography using technetium-99m ethylcysteinate dimer on day 29 after the onset of hemichorea demonstrates hypoperfusion in the territory of the right middle cerebral artery, predominantly in the right basal ganglia (arrow).
Discussion
We encountered a patient with right MCA stenosis presenting with hemichorea that persisted for 19 days and preceded cerebral infarction. She developed a cerebral infarction of the right putamen with facial palsy and hemiplegia, and her hemichorea disappeared.
Hemichorea is a rare manifestation of cerebral vascular diseases (3,4), and post-stroke hemichorea can develop within five days of the stroke onset but usually develops within the first day of the onset (4). Some case reports have described hemichorea-associated MCA stenosis without cerebral infarction (5,6). However, persistent hemichorea in patients with MCA stenosis preceding cerebral infarction has not been reported.
Chorea results from dysfunctional neuronal networks interconnecting the basal ganglia and frontal cortical motor areas (1). In these networks, the striatal direct and indirect pathways modulate the activity of the globus pallidus internus, which controls motor facilitation and inhibition (1). In previous reports, it has been suggested that hemichorea accompanied by internal carotid artery or MCA stenosis is caused by hypoperfusion derangement in the contralateral basal ganglia (5,7,8), frontal cortical and subcortical motor pathways (9,10), or both (6). Our patient developed cerebral infarction only in the right basal ganglia within the territory of the right MCA. The SPECT analysis after her stroke revealed hypoperfusion of the right MCA territory, and her cerebral hypoperfusion was more severe in the right basal ganglia than in the right cerebral cortex or subcortical white matter. In this case, we did not evaluate the cerebral blood flow during hemichorea before the onset of the cerebral infarction. However, we speculate that the patient's hemichorea was caused by mild hypoperfusion in the basal ganglia, which did not develop into cerebral infarction. The hypoperfusion probably reduced the activation of the striatal indirect pathway to a greater extent than that of the striatal direct pathway, resulting in excessive thalamocortical motor facilitation.
In the present case, persistent hemichorea with MCA stenosis, which is similar to the limb shaking observed in temporary ischemic attacks with carotid artery stenosis (11), preceded cerebral infarction. Previously reported patients were treated with anti-platelet therapy or surgical reconstruction, and their hemichorea improved (5,6). In patients with hemichorea and MCA stenosis, the prompt administration of anti-platelet agents should be considered to prevent cerebral infarction. However, further studies are required to determine whether or not anti-platelet agents prevent cerebral infarction in patients with persistent hemichorea and MCA stenosis.
In conclusion, MCA stenosis without cerebral infarction can present with persistent hemichorea. Therefore, MCA stenosis should be a differential diagnosis of hemichorea, even in the absence of cerebral infarction. Hemichorea may presage cerebral infarction with MCA stenosis.
Written informed consent was obtained from the patient for publication of the case history and the accompanying images.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Cardoso F, Seppi K, Mair KJ, Menning GK, Poewe W. Seminar on choreas. Lancet Neurol 5: 589-602, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Piccolo I, Defanti CA, Soliveri P, Volontè MA, Cislaghi G, Girotti F. Cause and course in a series of patients with sporadic chorea. J Neurol 250: 429-435, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Ghika-Schmid F, Ghika J, Regli F, Bogousslavsky J. Hyperkinetic movement disorders during and after acute stroke: the Lausanne Stroke Registry. J Neurol Sci 146: 109-116, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical-radiological correlation. J Neurol 6: 725-729, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Irioka T, Ayabe J, Mizusawa H. Hemichorea improved by extracranial-intracranial bypass surgery for middle cerebral artery occlusion. J Neurol 257: 1756-1758, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Chung SJ, Lee HS, Yoo HS, et al. A case of isolated middle cerebral artery stenosis with hemichorea and moyamoya pattern collateralization. J Mov Disord 6: 13-16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JM, Kim JS, Cho AH, et al. Angioplasty of middle cerebral artery stenosis improves recurrent hemichorea caused by basal ganglia hypoperfusion. J Stroke Cerebrovasc Dis 15: 69-71, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Galea I, Norwood F, Phillips MJ, Shearman C, McMonagle P, Gibb WR. Pearls & Oy-sters: resolution of hemichorea following endarterectomy for severe carotid stenosis. Neurology 71: e80-e82, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Im SH, Oh CW, Kwon OK, Cho BK, Chung YS, Han DH. Involuntary movement induced by cerebral ischemia: pathogenesis and surgical outcome. J Neurosurg 100: 877-882, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Morigaki R, Uno M, Suzue A, Nagahiro S. Hemichorea due to hemodynamic ischemia associated with extracranial carotid artery stenosis. Report of two cases. J Neurosurg 105: 142-147, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Baquis GD, Pessin MS, Scott RM. Limb shaking--a carotid TIA. Stroke 16: 444-448, 1985. [DOI] [PubMed] [Google Scholar]