Abstract
A 73-year-old man previously treated with rituximab for his mucosa-associated lymphoid tissue lymphoma suffered a suboptimal humoral immune response against an acquired SARS-CoV-2 infection. A detailed serological description revealed discrepant antigen-specific humoral immune responses. The titer of spike-targeting, “viral-neutralizing” antibodies remained below the detection level, in contrast to the anti-nucleocapsid, “binding” antibody response, which was comparable in both magnitude and kinetics. Accordingly, viral neutralizability and clearance was delayed, leading to prolonged RNAemia and persistent pneumonia. The present case highlights the need to closely monitor this unique population of recipients of B-cell-targeted therapies for their neutralizing antibody responses against SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, rituximab, neutralizing activity, persistent RNAemia
Introduction
Increased death rates for the novel coronavirus infection (COVID-19) have been reported in patients with hematologic malignancies compared with the general population (1). Whether or not patients receiving B-cell-targeted therapies for their underlying malignancy can successfully develop SARS-CoV-2 specific antibodies (2-4), which are essential for achieving viral clearance and preventing future reinfections, is now of increasing concern. Furthermore, with the first generation of COVID-19 vaccines now being delivered, the vaccine response of subjects receiving anti-CD20-depleting therapy is the next issue to be addressed for the appropriate individualization of vaccination schedules (5).
We herein report a patient previously treated with B-cell-targeted, anti-CD20 monoclonal antibody therapy for mucosa-associated lymphoid tissue (MALT) lymphoma who suffered a suboptimal humoral immune response, resulting in SARS-CoV-2 dissemination and uniquely persistent COVID-19 pneumonia. Our detailed antigen-specific serological description highlights the need to closely monitor this particular vulnerable population for their neutralizing anti-spike antibody response, both after successful recovery and after vaccine administration.
Case Report
A 73-year-old man was diagnosed with esophageal MALT lymphoma by an upper gastrointestinal endoscopic needle biopsy. With metastases and bone marrow involvement ruled out, the patient was determined to have stage I disease. Following eight doses of anti-CD20-depleting rituximab therapy, localized irradiation was administered after observing residual positron emission tomography activity in the primary lesion.
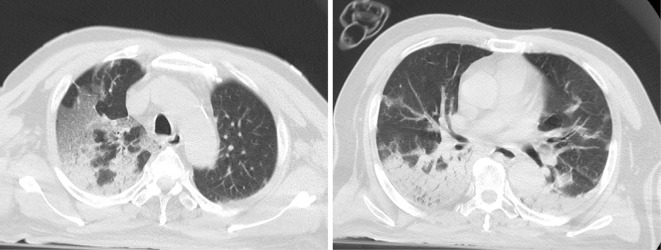
Twelve weeks after receiving his last dose of rituximab, the patient developed a fever and respiratory symptoms. Real-time reverse-transcriptase polymerase chain reaction (RT-PCR) testing performed on the fourth day of illness was positive for SARS-CoV-2. Not only respiratory specimens but also his serum was positive for SARS-CoV-2, indicating the presence of RNAemia resulting from SARS-CoV-2 dissemination. Chest computed tomography (CT) findings were significant for ground-glass opacities in the right upper lobe and atelectases/infiltrations in both dorsal lower lung fields (Fig. 1), indicative of a COVID-19 type H phenotype. Laboratory examinations showed lymphopenia (0.39×109 cells/L), and a flow cytometric analysis revealed complete depletion of CD19-positive circulating B cells.
Figure 1.
Chest CT findings. CT findings from day 4 of illness showed ground-glass opacities in the right upper lobe and atelectases/infiltrations in both dorsal lower lung fields.
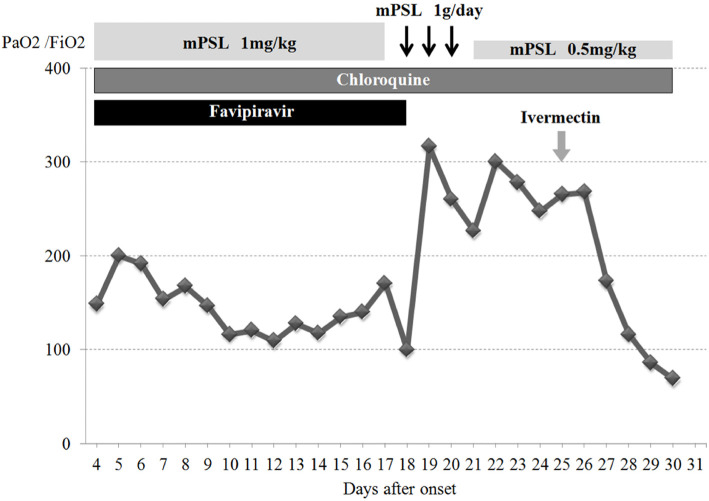
The patient's severe respiratory distress required management with mechanical ventilation. A combination of favipiravir (2 loading doses of 1,800 mg followed by 800 mg twice daily) and chloroquine (400 mg per day) was initiated but showed no apparent efficacy. High-dose methylprednisolone slightly improved his ventilation parameters, such as the PaO2/FiO2 ratio, but only in a transient manner. Additional treatment with a single dose of ivermectin (15 mg) was carried out. Sputum specimen RT-PCR performed on day 24 of illness remained positive for SARS-CoV-2.
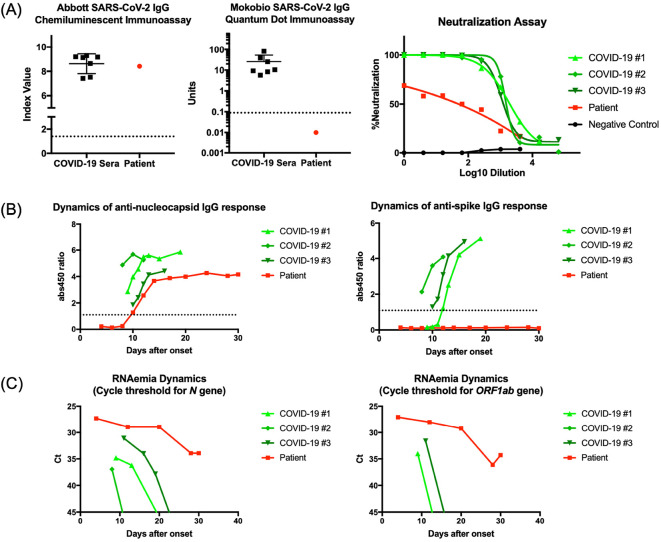
The extraordinarily delayed viral clearance prompted detailed analyses of his serological status, in order to gain insight into the pathophysiology of the persistent COVID-19 infection in this patient. SARS-CoV-2 specific antibody testing of the serum sample obtained after 25 days from the onset showed discrepant results; Architect SARS-CoV-2 IgG (Abbott Laboratories, Chicago, United States) targeting the nucleocapsid antigen showed an elevated titer, while a SARS-CoV-2 IgG Quantum Dot Immunoassay (Mokobio Biotechnology R&D center, Rockville, United States) targeting the spike-antigen detected no significant signal (Fig. 2A). Accordingly, as antibodies targeting the spike antigen receptor-binding domain are used to determine viral neutralizability (6,7), the in vitro viral neutralization assay confirmed that the patient's serum showed negligible viral neutralizability (Fig. 2A). A time-course serial sample analysis revealed that the patient had developed an anti-nucleocapsid antibody response comparable to that of other immunocompetent severe COVID-19 subjects, both in magnitude and kinetics (Fig. 2B). In contrast, the serum anti-spike antibody titer remained below the detection level throughout his clinical course (Fig. 2B). Thus, the viral clearance was significantly delayed, and the level of RNAemia in the patient remained persistently high, while that of the other immunocompetent COVID-19 subjects gradually declined as their illness subsided (Fig. 2C).
Figure 2.
Detailed serological description of the patient. (A) The anti-nucleocapsid IgG response of the patient measured by the Abbott Architect immunoassay resembled that of other immunocompetent COVID-19 subjects (left). The anti-spike IgG titer of the patient was below the detection limit of the Mokobio immunoassay (middle). The neutralizing activity of the patient’s serum against in vitro SARS-CoV-2 infection was negligible, correlating with the attenuated anti-spike IgG response. Neutralizability results were plotted against three seroconverted immunocompetent COVID-19 subjects (COVID-19 #1–3) and a SARS-CoV-2 negative control (negative control) (right). The patient’s serum was obtained 25 days after the symptom onset. Dashed lines indicate the cut-off values for assays. (B) A time-course analysis of the patient’s serum showed anti-nucleocapsid antibody responses comparable to those of other immunocompetent severe COVID-19 subjects (COVID-19 #1–3), both in magnitude and kinetics, while the anti-spike IgG titer remained below the detection limit of the EUROIMMUN Anti-SARS-CoV-2 ELISA assay. (C) The level of RNAemia, determined by the cycle threshold for N and ORF1ab gene amplification, remained persistently high in the patient, while that of the other immunocompetent COVID-19 subjects (COVID-19 #1–3) gradually declined as their illness subsided. The ORF1ab gene amplicon was never amplified in subject COVID-19 #2.
On day 29 of illness, the patient unfortunately died due to poor oxygenation despite maximized ventilator mechanics. The patient's clinical course is summarized in Figure 3.
Figure 3.
Clinical course of the patient. mPSL: methylprednisolone
Discussion
The majority of patients receiving anti-CD20 B-cell-targeted therapies still successfully clear the virus, resulting in a mere self-limited infection, although some have reported severe respiratory distress or even fatal COVID-19 disease in patients treated with rituximab or ocrelizumab (5), resembling the case presented here. Irrespective of the clinical course or severity, however, this specific population seems to suffer a suboptimal SARS-CoV-2 antibody response (2-4).
Through detailed molecular and serological analyses, we investigated whether or not a B-cell-depleted patient could develop significant levels of antibodies targeting variable viral antigens. Our results revealed not a mere attenuation but a unique discrepancy in antigen-specific antibody responses. His persistent RNAemia and fatal phenotype were associated with a below-detection anti-spike antibody titer and negligible in vitro viral neutralizability despite an anti-nucleocapsid antibody response comparable to that of other immunocompetent COVID-19 subjects. One explanation for the specifically attenuated anti-spike IgG response may be that it reflects the differences in immunogenicity between the two antigens, with the spike antigen harboring less immunogenicity than the nucleocapsid antigen. A similar heterogenous attenuation in antibody response has been observed in the strain- or serotype-specific antibody responses to influenza and pneumococcal vaccines (8). These facts raise concerns that the immunogenicity of the first generation of COVID-19 vaccines targeting the whole spike antigen may fall short of effective protection.
Regarding the treatment of an immunosuppressed population possessing low-level neutralization antibodies, since the report of the initial experience with convalescent plasma therapy for B-cell-ablated severe COVID-19 patients (9), an increasing number of case series and one recently published retrospective matched-control study have now provided evidence suggesting its clinical benefit (10,11). The boost in neutralizability following convalescent plasma transfusion is thought to dampen the viral replication at an earlier stage, thus leading to mortality benefit. For this reason, convalescent plasma therapy and, more recently, monoclonal antibody therapies, namely casirivimab-imdevimab and sotrovimab, have now been approved for emergency usage by the U.S. Food and Drug Administration, with special emphasis placed on the targeting of patients with impaired humoral immunity (12-14). While the benefits of early casirivimab-imdevimab therapy for the prevention of severe COVID-19 have been demonstrated in a phase 3 trial (15), an anecdotal case report now indicates its potential use as a later treatment option, especially when B-cell-depleted patients are targeted (16). Although the clinical consequences of a suboptimal SARS-CoV-2 antibody response warrant further investigation, the evidence altogether support the notion that humoral immunity plays the central role in SARS-CoV-2 clearance and prevention.
Our observations indicate that B-cell-depleted patients on anti-CD20 therapy are not only excellent candidates for convalescent plasma, as well as the other forms of antibody therapy, but also an at-risk population meriting close monitoring for reinfection and post-vaccination responses.
Analyses were conducted in accordance with the principles of the Declaration of Helsinki. The work was approved by the institutional ethics committee. Consent for publication was obtained from the patient's family.
Author's disclosure of potential Conflicts of Interest (COI).
Yu Nakagama: Stock ownership or options; Quantum Molecular Diagnostics, an Osaka City University spinout. Yasutoshi Kido: Stock ownership or options; Quantum Molecular Diagnostics, an Osaka City University spinout. Masayuki Hino: Honoraria; Pfizer Japan, Kyowa Kirin, Novartis and Takeda Pharmaceutical.
Financial Support
This research was supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP20wm0125003 (YK), JP20he1122001(YK), JP20nk0101627(YK) and JP20jk0110021 (YN). We are grateful for the COVID-19 Private Fund granted from the Shinya Yamanaka laboratory, CiRA, Kyoto University.
Teruhito Takakuwa and Yu Nakagama equally contributed to this work.
Supplementary Material
This supplement describes the detailed method of the assay used in this paper.
Acknowledgement
ELISA data was acquired at the Research Support Platform, Graduate School of Medicine, Osaka City University. Serological tests were provided by Mokobio Biotechnology R&D, USA, and Abbott Japan, Japan.
References
- 1.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21: 335-337, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasuda H, Tsukune Y, Watanabe N, et al. Persistent COVID-19 pneumonia and failure to develop anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk 20: 774-776, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton JR, Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult Scler Relat Disord 44: 102341, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maillart E, Papeix C, Lubetzki C, et al. Beyond COVID-19: DO MS/NMO-SD patients treated with anti-CD20 therapies develop SARS-CoV2 antibodies? Mult Scler Relat Disord 46: 102482, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker D, Roberts CAK, Pryce G, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol 202: 149-161, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 26: 1033-1036, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science 369: 650-655, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology 95: e1999-e2008, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betrains A, Godinas L, Woei-A-Jin FJSH, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol 192: 1100-1105, 2021. [DOI] [PubMed] [Google Scholar]
- 10. Libster R, Pérez Marc G, Wappner D, et al. ; Fundación INFANT-COVID-19 Group. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson MA, Henderson JP, Shah PK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fact sheet for health care providers - emergency use authorization (EUA) of COVID-19 convalescent plasma for treatment of hospitalized patients with COVID-19 [Internet]. Available from: https://www.fda.gov/media/141478/download
- 13.Fact sheet for health care providers - emergency use authorization (EUA) of REGEN-COV (casirivimab with imdevimab) [Internet]. Available from: https://www.fda.gov/media/145611/download
- 14.Fact sheet for health care providers - emergency use authorization (EUA) of sotrovimab [Internet]. Available from: https://www.fda.gov/media/149534/download
- 15.O'Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination for COVID-19 prevention. medRxiv. Forthcoming. [Google Scholar]
- 16.D'Abramo A, Vita S, Maffongelli G, et al. Prolonged and severe SARS-CoV-2 infection in patients under B-cell-depleting drug successfully treated: a tailored approach. Int J Infect Dis 107: 247-250, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This supplement describes the detailed method of the assay used in this paper.