Abstract
The spontaneous regression (SR) of cancer is defined as either partial or complete, and temporary or permanent, disappearance without appropriate treatment for the disease, and this phenomenon is rare in the case of small cell lung carcinoma (SCLC). We herein report an 83-year-old woman who presented with left-sided hemichorea associated with anti-SOX1 (SOX1-Ab) and -CV2/CRMP5 (CV2/CRMP5-Ab) antibodies with SR following a 7-year interval free of disease progression of SCLC. Hemichorea can present with the coexistence of anti-SOX1 and CV2/CRMP5-Ab with SR after a long interval free of SCLC. The immune response associated with these onco-neural antibodies may become independent of the original tumor trigger and remain active for many years.
Keywords: anti-CV2/CRMP5 antibody, anti-SOX antibody, chorea, paraneoplastic neurological syndrome, small cell lung carcinoma, spontaneous regression
Introduction
The spontaneous regression (SR) of cancer is defined as either partial or complete, and temporary or permanent, disappearance without appropriate treatment for the disease, and this is rare in the case of small cell lung carcinoma (SCLC) (1). Although tumor regression may be an immune-mediated event especially through inhibiting tumor growth, the mechanism underlying SR of cancer remains controversial (2). Previous reports revealed a relatively high incidence of paraneoplastic neurological syndrome (PNS) associated with SR of cancer. These suggest an anti-tumor immune-mediated response as a potential mechanism for regression. Owing to its very rare incidence, only a few cases with a long period of SR in SCLC have so far been reported (3-5). We herein describe a case of left-sided hemichorea associated with anti-SOX1 (SOX1-Ab) and -CV2/CRMP5 (CV2/CRMP5-Ab) antibodies with a 7-year interval free of disease progression of SCLC.
Case Report
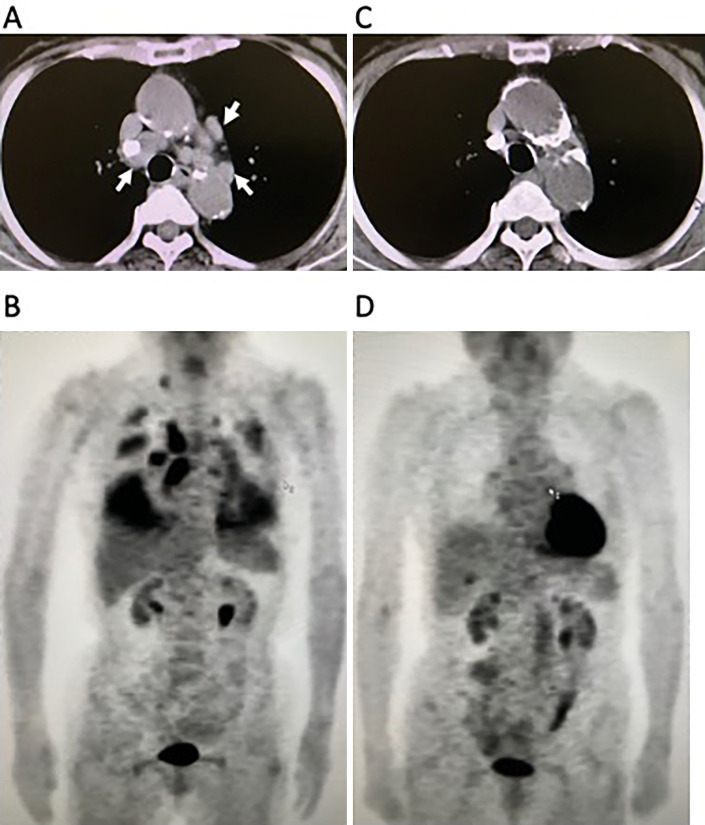
An 83-year-old woman was referred to our hospital with a 4-day period of progressive involuntary movements of the limbs. Seven years before the current admission, the patient had been investigated for a cough that persisted several months with abnormal hilar masses on chest radiography. Chest computed tomography (CT) revealed abnormal hilar masses of the mediastinum (Fig. 1A) and whole-body 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) showed an abnormal uptake in the mediastinum, lung, and right subclavicular lesions (Fig. 1B). An elevated level of gastrin-releasing peptide precursor (pro-GRP) at 138.0 pg/mL (normal range: <46.0), and tumor sampling of paratracheal lymph node obtained by endobronchial ultrasound-guided transbronchial needle aspiration confirmed the histological diagnosis of SCLC (Fig. 2).
Figure 1.
Chest CT and whole-body FDG-PET findings. (A, B) Initial CT and FDG-PET findings showed abnormal hilar masses in the mediastinum (arrowheads) (A), and an abnormal uptake in the mediastinum and lung, which partially suggest carcinomatous pleuritis, and right subclavicular lesions (B). (C, D) Seven years after the first examination, chest CT and FDG-PET revealed the absence of the original abnormal hilar lesion (C), and no evidence of abnormal uptake (D). CT: computed tomography, FDG-PET: 18F-fluorodeoxyglucose-positron emission tomography
Figure 2.
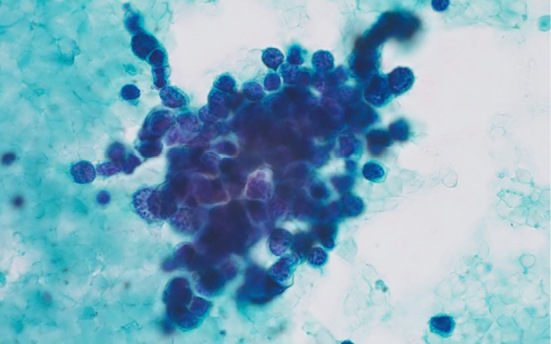
A histological diagnosis of small cell lung carcinoma was established on tissue specimens obtained by endobronchial ultrasound-guided transbronchial needle aspiration (Papanicolaou staining, ×100).
Immediately prior to the initiation of treatment for limited SCLC disease, the patient refused to undergo treatment; thereafter, the patient received no cancer therapy and was lost to the follow-up. On arrival at our hospital after a 7-year interval, the patient was taking antihypertensive drugs, but had not received any vaccines within the previous several months. She had no weight loss. On examination, the patient was afebrile and alert; a neurological examination revealed choreic movements in the left upper and lower limbs as well as her face. Cranial nerves including visual acuity, taste, smell sensation, and extraocular movement; muscle strength; tendon reflexes; and sensory examination were well preserved with flexor plantar reflexes. The patient scored 24 in the mini-mental state examination with mistakes in calculation.
The results of laboratory analyses like blood glucose, antiphospholipid antibody, lupus anticoagulant antibody, thyroid function, rheumatic factor, and antinuclear antibody were all normal. There were no morphological abnormalities of erythrocytes in the peripheral blood. Pro-GRP was normal at 25.2 pg/mL. A serum analysis for paraneoplastic autoantibodies (anti-AMPH, -CRMP5/CV2, -PNMA2, -Ri, -Yo, -Hu, -recoverin, -SOX1, -titin, -zic4, -GAD65, -Tr) was performed by immunoblot assay (Euroimmun, Lübeck, Germany), and it was strongly positive for anti-SOX1 and positive for anti-CRMP5/CV2. Anti-NMDA antibody was not examined.
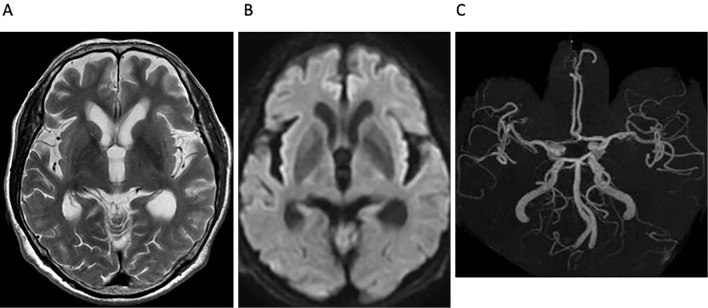
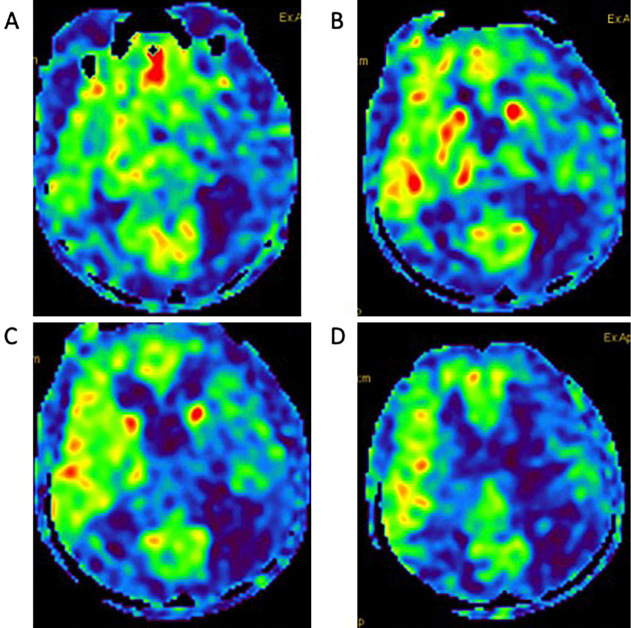
Chest CT and PET scans revealed no evidence of any original abnormal hilar lesions and no signs of primary or metastatic tumors (Fig. 1C, D). Thymoma and ovarian teratoma were not detected by whole-body CT scans. A cerebrospinal fluid evaluation revealed 13 cells/μl with negative cytology and an IgG index of 0.4. Electroencephalography, nerve conduction, and repetitive nerve stimulation studies were normal. Magnetic resonance imaging (MRI) of T2- and diffusion-weighted imaging (DWI), and magnetic resonance angiography (MRA), revealed no abnormalities (Fig. 3); however, arterial spin labeling (ASL) perfusion showed hyperperfusion in the right thalamus, putamen, and frontotemporal lesions (Fig. 4).
Figure 3.
Brain MRI and MRA findings. MRI on (A) T2WI, (B) DWI, and (C) MRA revealed no abnormalities. DWI: diffusion-weighted imaging, MRA: magnetic resonance angiography, MRI: magnetic resonance imaging, T2WI: T2-weighted imaging
Figure 4.
Brain ASL perfusion findings. (A-D) ASL perfusion showed hyperperfusion in the right thalamus, putamen, frontotemporal lesions. ASL: arterial spin labeling
Despite being treated with intravenous immunoglobulin, her choreic movements remained unchanged; she improved with haloperidol. After 31 days of hospitalization, the patient was discharged. The patient was free of SCLC and neurological signs after a two-year follow-up.
Discussion
There have been several cases of PNS associated with SR in SCLC (4,6,7), including two cases without any cancer therapy (6,7). These were primarily diagnosed as sensory polyneuropathy with anti-Hu antibodies (Hu-Ab), which detected cancer around the same time. The interval from the diagnosis of SCLC until SR was up to 7 months, and the disease-free survival period ranged from 18 months to 8 years. To date, the mechanism of tumor regression in PNS related to anti-Hu-Ab remains unknown. To the best of our knowledge, this is the first case of autoimmune hemichorea associated with the coexistence of anti-SOX1-Ab and anti-CV2/CRMP5-Ab with SR with a long interval free of SCLC.
PNS are autoimmune disorders associated with specific cancers and are marked by specific autoantibodies. Anti-CV2/CRMP5-Ab is a marker of paraneoplastic autoimmunity related to SCLC, renal cell carcinoma, or thymoma (8). Chorea in PNS is typical in patients with anti-CV2/CRMP5-Ab, presenting unilaterally in some patients, and it is frequently associated with SCLC (8-10). In the PNS EuroNetwork study, 11 out of 13 patients with chorea showed the onset of abnormal movement antedated the tumor diagnosis or tumor recurrence (mean 4.9 months, 1-16 months), whereas the tumor preceded the chorea in two cases by 1 and 17 months, respectively (9).
While anti-SOX1-Ab was found in up to 40% of patients with SCLC, its pathogenic role in PNS is still unclear, except for paraneoplastic Lambert-Eaton myasthenic syndrome (11,12). Anti-SOX1-Ab was found in eight of 55 (14.5%) patients with PNS, and the coexistence of anti-SOX1-Ab and anti-CV2/CRMP5-Ab was detected in one patient with paraneoplastic cerebellar degeneration in non-SCLC (12). There has only been one case of autoimmune chorea with anti-SOX1-Ab, but without anti-CV2/CRMP5-Ab, who had a 6-year prior history of lung adenocarcinoma with no recurrence or other cancer (13). In this case, hemichorea with anti-SOX1-Ab and anti-CV2/CRMP5-Ab was suggested to result from a tumor-induced immune response of SR associated with SCLC.
In a study of paraneoplastic autoimmune chorea in adults, two out of four cases with anti-CV2/CRMP5-Ab showed SR. One patient with SR without cancer therapy developed chorea 14 years after squamous-cell tonsillar carcinoma was diagnosed; detection of anti-CV2/CRMP5-Ab at chorea presentation predicted SCLC, but none was found (10). As in the present case, definitive confirmation was not obtained for the association of SR in SCLC and developing hemichorea. Nonetheless, hemichorea can present with the coexistence of anti-SOX1 and anti-CV2/CRMP5-Ab with SR after a long interval free of SCLC. This case suggests that the immune response associated with the onco-neural antibodies may become independent of the original tumor trigger and remain active for many years. We cannot exclude the possibility of another cancer related to paraneoplastic chorea; therefore, further follow-up is needed. In PNS, the relationship between the coexistence of anti-SOX1-Ab and anti-CV2/CRMP5-Ab and the SR of SCLC needs to be further clarified.
In chorea with anti-CV2/CRMP5-Ab related to PNS, MRI is normal in 60% of cases. Nonetheless, in some cases, hyperintensity in T2-fluid-attenuated inversion recovery sequences of the basal ganglia can be transitory and then disappear after a few months, or can be absent at the time of the onset of chorea (8-10). To the best of our knowledge, there has been no report of single-photon emission computed tomography (SPECT) or ASL perfusion in paraneoplastic chorea with anti-CV2/CRMP5-Ab. In the main connection of the motor circuit of basal ganglia, chorea is suggested to be mainly characterized by hyperactivity of the subthalamic nucleus leading to disinhibition of the internal segment of the globus pallidus output to the thalamocortical projection (14). The study of immune-mediated chorea, namely Sydenham chorea, by SPECT revealed hyperperfusion in the striatum and thalamus, which suggests the presence of a subcortical inflammatory process (15). The ASL perfusion changes, in this case, may be epiphenomena, suggesting that both the contralateral thalamus and basal ganglia reflect the loss of pallidal inhibitory input (16). The abnormal perfusion of ASL contralateral to the side of hemichorea may reflect the immune process in the basal ganglia by anti-CV2/CRMP5-Ab thus leading to hyperactivity of thalamocortical projection.
Conclusion
Hemichorea can present with the coexistence of anti-SOX1 and anti-CV2/CRMP5-Ab with SR of a long interval free of SCLC. The immune response associated with these onco-neural antibodies may become independent of the original tumor trigger and remain active. Neurological symptoms and onco-neural antibodies in SR are important for recognizing tumor immunity in PNS.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Everson TC. Spontaneous regression of cancer. Ann NY Acad Sci 114: 712-735, 1964. [PubMed] [Google Scholar]
- 2.Zhang J, Wang H, Li Chunxiao, Qian H. Chance to rein in a cancer - spontaneous regression of lung carcinoma (1988-2018): a 30-year perspective. Int J Clin Exp Pathol 13: 1190-1196, 2020. [PMC free article] [PubMed] [Google Scholar]
- 3.Lowy AD Jr, Erickson ER. Spontaneous 19-year regression of oat cell carcinoma with scalene node metastasis. Cancer 58: 978-980, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet 341: 21-22, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Kang HM, Jang PS, et al. Spontaneous regression of small cell lung cancer. Respirology 13: 615-618, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Gill S, Murray N, Dalmau J, Thiessen B. Paraneoplastic sensory neuronopathy and spontaneous regression of small cell lung cancer. Can J Neurosci 30: 269-271, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Mawhinney E, Gray OM, McVerry F, McDonnell GV. Paraneoplastic sensorimotor neuropathy associated with regression of small cell lung carcinoma. BMJ Case Rep 2010: bcr0120091486, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernino S, Tuite P, Adler CH, et al. Paraneoplastic chorea associated with CRMP-5 neuronal antibody and lung carcinoma. Ann Neurol 51: 625-630, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Vigliani MC, Honnorat J, Antoine JC, et al. Chorea and related movement disorders of paraneoplastic origin: the PNS EuroNetwork experience. J Neurol 258: 2058-2068, 2011. [DOI] [PubMed] [Google Scholar]
- 10.O'Toole O, Lennon VA, Ahlskog JE, et al. Autoimmune chorea in adults. Neurology 80: 1133-1144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabater L, Titulaer M, Saiz A, Verschuuren J, Güre AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 70: 924-928, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Stich O, Klages E, Bischler P, et al. SOX1 antibodies in sera from patients with paraneoplastic neurological syndromes. Acta Neurol Scand 125: 326-333, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi T, Inoue K, Ouchi H, Shibano K, Hara K. A case of anti-SRY-Related HMG-Box Gene 1 (SOX1) antibody-positive chorea. Rinsho Shinkeigaku (Clin Neurol) 60: 852-856, 2020(in Japanese). [DOI] [PubMed] [Google Scholar]
- 14.Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Arbizu J, Giménez-Amaya JM. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci 17: 51-55, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Lee PH, Nam HS, Lee KY, Lee BI, Lee JD. Serial brain SPECT images in a case of Sydenham chorea. Arch Neurol 56: 237-240, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Lee KS, Lee KH, et al. Evidence of thalamic disinhibition in patients with hemichorea: semiquantitative analysis using SPECT. J Neurol Neurosurg Psychiatry 72: 329-333, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]