Abstract
We report a case of a pulmonary necrotizing sarcoid granulomatosis (NSG)-like lesion possibly associated with coinfection of Mycobacterium avium and Propionibacterium acnes. A solitary nodule in the right middle lobe of the lung was notable for coagulative necrosis with aggregates of sarcoid-like epithelioid granulomas. Small arteries were damaged by granulomas. Both M. avium and P. acnes were detected in the lesion. Furthermore, more P. acnes genomes were detected in the granulomas than in the non-lesion lung. These findings blur the pathophysiologic boundaries among NSG, sarcoidosis, and mycobacteriosis, and suggest that NSG needs to be recognized as continuous spectra of sarcoidosis/mycobcteriosis.
Keywords: mycobacteriosis, Mycobacterium avium, necrotizing sarcoid granulomatosis, propionibacteriosis, Propionibacterium acnes, sarcoidosis
Introduction
Necrotizing sarcoid granulomatosis (NSG) is a rare granulomatous disease detected primarily in the lungs by three diagnostic histopathological features: confluent granulomas, varying degrees of necrosis, and vasculitis with granulomas and giant cells obliterating the walls of the muscular arteries and veins. NSG shares many pathological, radiological, and clinical findings with sarcoidosis, especially with nodular sarcoidosis (NS), a type of sarcoidosis with at least one nodular lesion accounting for 1.6-4% of pulmonary sarcoidosis (1). The shared characteristics by NSG and NS indicate that NSG may be a variant of sarcoidosis (2). Although the precise pathogenic mechanisms that induce sarcoidosis are unknown, current hypotheses strongly suggest that this condition may occur in a genetically susceptible individual due to environmental exposure (3). The pathogenesis of sarcoidosis has been mainly associated with bacterial pathogens: mycobacterial and propionibacterial organisms (4). Recent studies, including polymerase chain reaction (PCR) and immune assay-based techniques, have successfully identified mycobacterial nucleic acids and insoluble mycobacterial proteins, respectively, in lesions associated with sarcoidosis (3,5,6). In addition, although Propionibacterium acnes (P. acnes), currently referred to as Cutibacterium acnes, are indigenous not only to the skin and mucosal surfaces but also to the lungs and lymph nodes (7), substantially more P. acnes genomic DNA was detected in patients with sarcoidosis than in the control samples (8,9), and genomic DNA and lipoteichoic acid of P. acnes have been detected inside granulomas in a lot of sarcoidosis patients (10,11). Recently, NSG cases with proven infectious pathogens, including M. tuberculosis or P. acnes have been reported (12,13). Furthermore, a case of NSG associated with coinfection of M. tuberculosis and P. acnes has also been reported (14). These findings not only make the boundaries among NSG, sarcoidosis, and mycobacteriosis indefinable, but also indicate a problem with the use of NSG as a diagnostic term. We herein report a unique case of a solitary NSG-like pulmonary lesion with evidence of coinfection by both M. avium and P. acnes.
Case Report
A Japanese man in his early 70s, with no history of dust exposure or inhalation toxicity, presented to our hospital with an abnormality in his right lung that was detected as part of a routine medical examination. The patient had no significant medical history or relevant family history. He had a smoking habit of 20 cigarettes per day when he was 20-35 years old, but he did not develop any pulmonary or systemic symptoms including fever, weight loss, night sweats, arthralgia, or fatigue. No superficial lymphadenopathy was detected on physical examination. Chest computed tomography revealed a solitary, a 15 mm nodule in the right middle lobe of the lung, without cavitation, and no hilar lymphadenopathy (Fig. 1A). A fluorodeoxyglucose-positron emission tomography study was notable for no uptake in the lung lesion. The serum levels of C-reactive protein and tumor markers, such as carcinoembryonic antigen, squamous cell carcinoma associated antigen, sialyl stage-specific embryonic antigen-1 (SSEA-1) antigen, neuron-specific enolase, and pro-gastrin-releasing peptide, were all within the normal range. QuantiFERONⓇ-TB Gold, hepatitis B antigen, hepatitis C antibody, and human immunodeficiency virus antibody tests were all negative, as well as an endobronchial brush biopsy for malignant cells and acid-fast bacilli. Serum levels of angiotensin-converting enzyme, anti-neutrophil cytoplasmic antibody, or lysozyme were not evaluated before surgical resection. Bronchoalveolar lavage was not performed. Wedge resection was performed by video-assisted thoracoscopic surgery.
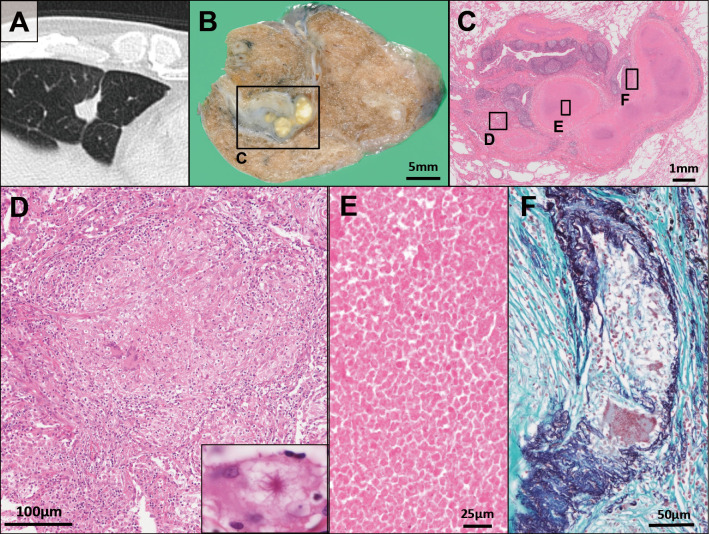
Figure 1.
(A) A solitary nodule in the right middle lobe of the lung detected by computed tomography. (B) Cut surface of the surgical specimen revealed a well-circumscribed, yellow-white-colored single nodule. (C) Whole histological image of the lesion; Hematoxylin and Eosin (H&E) staining. (D) Sarcoid-like non-caseating epithelioid granuloma with minute focal hemorrhages and multinucleated giant cells; inset: an asteroid body within a giant cell (H&E staining). (E) Approximately half the area of the lesion consisted of necrosis (H&E staining). (F) Destruction of a small pulmonary artery with Langerhans’ type multinucleated giant cells. The artery lumen is completely obstructed by epithelioid cell granuloma; Elastica-Masson stain.
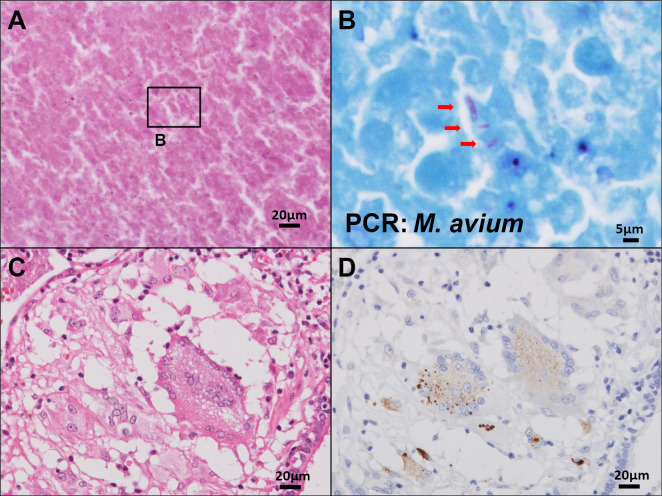
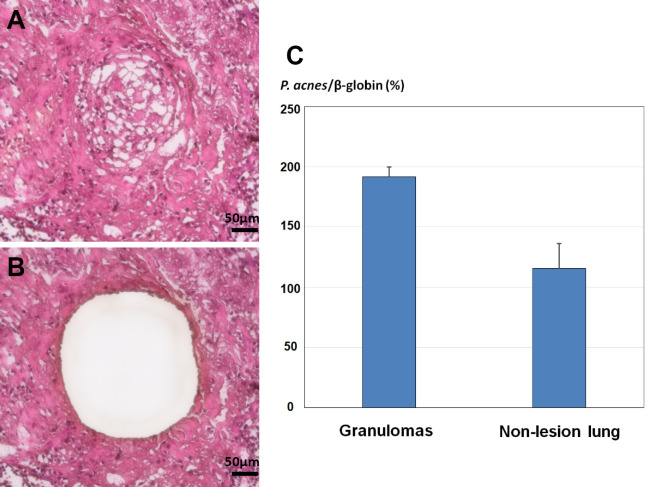
An intraoperative frozen section revealed multiple granulomas around a necrotic area with no atypical epithelium; the right middle lobe was partially resected. Macroscopic evaluations revealed a well-circumscribed, yellow-white single nodule (Fig. 1B). Microscopically, approximately half of the lesion underwent coagulative necrosis associated with chronic inflammation. The focus of necrosis lied adjacent to a bronchiole. Aggregates of sarcoid-like non-caseating epithelioid granulomas with minute focal hemorrhages were detected at the periphery of the lesion (Fig. 1C-E); most of the granulomas showed a centrilobular distribution and some were located close to subpleural or perilobular spaces. An asteroid body was identified within a giant cell (Fig. 1D inset). The tissue specimen revealed the obliteration of small pulmonary blood vessels by granulomas that included Langerhans' type multinucleated giant cells. The lumens of the affected blood vessels had either narrowed or were completely obstructed by multiple epithelioid cell granulomas (Fig. 1F). No granulocytic vasculitis, a hallmark of granulomatosis with polyangiitis, was detected. The histologic findings were consistent with giant cell vasculitis and were used to establish a diagnosis of NSG. Acid-fast bacilli detected within the lesion, especially in necrotic area, were identified as M. avium by a real-time PCR-based genome DNA amplification (CobasⓇ TaqMan MTB Test, Roche Diagnostics, Basel, Switzerland) using the fresh sample collected intraoperatively, with no amplification of genomic material from M. tuberculosis or M. intracellulare (Fig. 2A, B). No microorganism was identified by either Gram or Grocott staining, and the culture results were negative for bacteria or fungi. Immunohistochemistry was performed manually according to the original protocol using a P. acnes-specific monoclonal antibody (PAB antibody, clone: TMDU2, MBL: Nagoya, Japan) that reacts with the cell-membrane-bound lipoteichoic acid of the bacterium (10). P. acnes were mainly detected within granulomas in the pulmonary lesion; positive signals were found in multinucleated giant cells and epithelioid cells (Fig. 2C, D). Following selective laser microdissection of the granulomas from three different formalin-fixed paraffin-embedded (FFPE) blocks of the case using a Leica LMD6 (Leica Microsystems, Germany) (Fig. 3A, B), genomic DNA was extracted from each FFPE tissue by QIAamp DNA FFPE Tissue Kit (Qiagen, Chatsworth, USA) and subjected to real-time PCR as previously described (9). In short, primers were used for real-time PCR quantification to amplify 16S rRNA of P. acnes and the human β-globin gene. Primers PA-F (5'-GCGTGAGTGACGGTAATGGGTA-3') and PA-R (5'-TTCCGACGCGATCAACCA-3') were designed to amplify a 131-bp portion of P. acnes 16S rRNA. Primers BG-F (5'-TGCCTATCAGAAAGTGGTGGCT-3') and BG-R (5'-GCTCAAGGCCCTTCATAATATCC-3') were designed to amplify a 150-bp portion of the human β-globin gene. PCR was performed with THUNDERBIRDⓇ Next SYBRⓇ qPCR Mix (Toyobo, Tokyo, Japan) according to standard protocols. Genomic DNA of P. acnes (JGD07462) as a positive control was provided by the RIKEN BRC through the National BioResource Project of the MEXT/AMED, Japan. The relative DNA levels for P. acnes to β-globin in granulomas were (1.92±0.08) ×102% and those in the non-lesion lung parenchyma (1.16±0.21) ×102% (Fig. 3C). The P. acnes genomes showed a larger average accumulation level in the granulomas than in the non-lesion lung. The patient had an uneventful postoperative course. No additional therapy was required, and no sign of relapse was noted for 24 months.
Figure 2.
(A, B) Acid-fast bacilli detected in a necrotic area (indicated by the arrows in Fig. 2B) were identified as Mycobacterium avium (M. avium) by polymerase chain reaction (PCR); A: Hematoxylin and Eosin (H&E) staining, B: Ziehl-Neelsen stain. (C, D) Positive signals within multinucleated giant cells and epithelioid cells of the granuloma detected by immunohistochemistry; C: H&E staining, D: Propionibacterium ances-specific monoclonal antibody (PAB antibody).
Figure 3.
(A, B) A representative histological image of a laser-microdissected granuloma (Hematoxylin and Eosin staining). (C) Comparison of the relative DNA levels for Propionibacterium acnes (P. acnes) to β-globin between granulomas and the non-lesion lung parenchyma.
Discussion
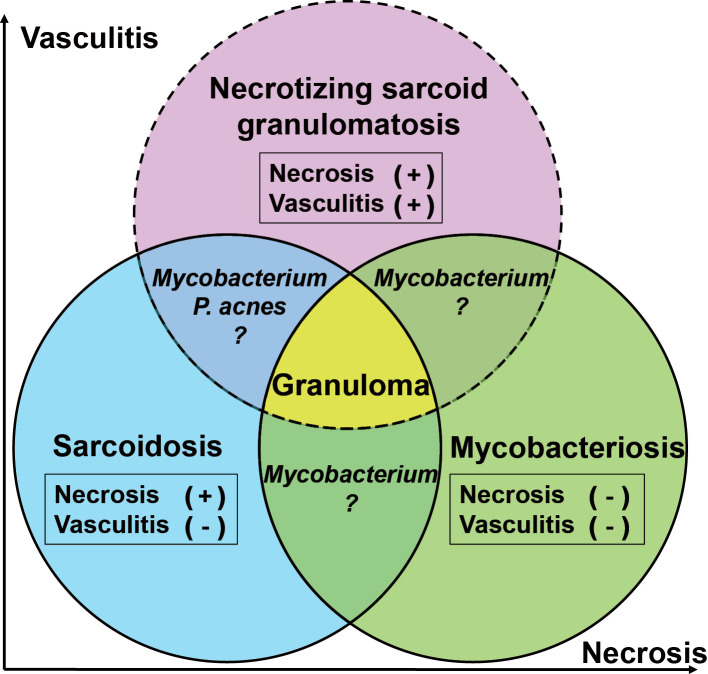
By its original definition, a diagnosis of sarcoidosis requires the exclusion of all known causes of granulomas, including beryllium and mycobacterial or fungal infection (15). Traditionally, granulomas associated with sarcoidosis have been considered non-necrotizing because the presence of necrosis within the lesions typically indicates infection (16). However, 4.7% of the open-lung biopsy specimens from patients with well-documented sarcoidosis have been associated with confluent nodule-forming granulomas, granulomatous angiitis, and necrosis (16). Moreover, minimal to moderate central necrosis has been reported in up to one-third of cases of sarcoidosis with surgically resected specimens (16). Therefore, at this time, a diagnosis of sarcoidosis, whether necrotizing or non-necrotizing, can only be made after the exclusion of infectious etiologies. However, this diagnostic paradigm is complicated by recent molecular evidence that the presence of microorganisms, including Mycobacterium spp. and P. acnes, is associated with characteristic sarcoidosis lesions (1,17). Although necrosis and obstruction of vascular lumens were considered the only histological distinctions between NS and NSG, one or both of these characteristics have been associated with sarcoidosis (2,16). In addition, NSG caused by P. acnes and/or M. tuberculosis have also been reported (12-14). All these findings blur the distinctions among NSG, sarcoidosis, and mycobacteriosis (Fig. 4). Technological advances and expansion of histological findings warrant a re-evaluation of the definition and the histological diagnostic criteria for NSG as well as sarcoidosis. In fact, the distinctions may be arbitrary and could depend on sampling, the time course of each disease, the examination methods used, and the inclinations of the individual surgical pathologist.
Figure 4.
A schematic illustration depicting overlap in the disease concepts and histological findings among necrotizing sarcoid granulomatosis, sarcoidosis, and mycobacteriosis.
In this case, M. avium was mainly detected in the necrotic area of the lesion by acid-fast stain and PCR. In addition, immunohistochemistry by PAB antibody identified P. ances mainly within the granulomas. Moreover, quantitative PCR detected a larger amount of P. acnes genomes within the non-caseating granulomas in the lesion. Although M. avium seems to have been a predominant contributor in this case, the accumulation of P. acnes in non-caseating granulomas demonstrates an etiologic link between the indigenous bacterium and the NSG-like lesion (17). Non-caseating granulomas caused by P. acnes might have coincided with mycobacterial infection (14). In this sense, NSG might be a rare disease caused by the coinfection of Mycobacterium spp. and P. acnes (14). Alternatively, as Rosen argues, the use of NSG as a diagnostic term might have to be discontinued because vasculitis and necrosis are the common histological findings that can be detected in both sarcoidosis and mycobacteriosis (16,18). Further clinical studies are thus required for clarification.
The diagnosis of granulomatous lesion, particularly in sarcoidosis, is based on clinical, radiological, and histopathological findings; however, current evidence suggests that it may become difficult to determine a histologic differentiation of sarcoidosis and mycobacteriosis because infectious agents are likely to be involved in the etiologies of sarcoidosis as well. Therefore, because the sensitivity of tissue acid-fast staining is relatively low (16), comprehensive methods, including culture, immunohistochemistry with specific antibodies, and molecular techniques, should be incorporated into the diagnostic criteria of granulomatous lesions whenever possible to confirm the presence of pathogens. In addition, overall consensus on factors contributing to sarcoidosis needs to be established prior to any discussions regarding the significance of NSG as a distinct disease.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Karpathiou G, Batistatou A, Boglou P, Stefanou D, Froudarakis ME. Necrotizing sarcoid granulomatosis: a distinctive form of pulmonary granulomatous disease. Clin Respir J 12: 1313-1319, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Popper HH, Klemen H, Colby TV, Churg A. Necrotizing sarcoid granulomatosis - is it different from nodular sarcoidosis? Pneumologie 57: 268-271, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol 49: 6-18, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Esteves T, Aparicio G, Garcia-Patos V. Is there any association between sarcoidosis and infectious agents?: a systematic review and meta-analysis. BMC Pulm Med 16: 165, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake WP, Dhason MS, Nadaf M, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun 75: 527-530, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 30: 508-516, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ishige I, Eishi Y, Takemura T, et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 22: 33-42, 2005. [PubMed] [Google Scholar]
- 8.Ishige I, Usui Y, Takemura T, Eishi Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 354: 120-123, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 40: 198-204, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negi M, Takemura T, Guzman J, et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol 25: 1284-1297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada T, Eishi Y, Ikeda S, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol 198: 541-547, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Arai T, Inoue Y, Eishi Y, Yamamoto S, Sakatani M. Propionibacterium acnes in granulomas of a patient with necrotising sarcoid granulomatosis. Thorax 63: 90-91, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Hsiung TC, Kuo CH, Kuo HP. A rare case of the coexistence of latent tuberculosis and necrotizing sarcoid granulomatosis with atypical presentation. J Formos Med Assoc 113: 662-663, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Hibi M, Nkao A, Sumida A, et al. An operated case of necrotizing sarcoid granulomatosis showing positive immunostaining for Propionibacterium acnes. Jpn J Sarcoidosis Granulomatous Disord 31: 33-40, 2011. [Google Scholar]
- 15. Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee, February 1999. Am J Respir Crit Care Med 160: 736-755, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Rosen Y. Four decades of necrotizing sarcoid granulomatosis: what do we know now? Arch Pathol Lab Med 139: 252-262, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Eishi Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. Biomed Res Int 2013: 935289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchevsky A, Damsker B, Gribetz A, Tepper S, Geller SA. The spectrum of pathology of nontuberculous mycobacterial infections in open-lung biopsy specimens. Am J Clin Pathol 78: 695-700, 1982. [DOI] [PubMed] [Google Scholar]