Abstract
Background
Prevalence and clinical impact of increased liver function tests in patients affected by Coronavirus disease 2019 (COVID-19) is controversial.
Aims
This observational study evaluates the prevalence of transaminases elevation in hospitalized patients affected by COVID-19 and investigates the presence of factors associated with hepatocellular injury and with mortality.
Methods
Data of 292 adult patients with confirmed COVID-19 admitted to the Ente Ospedaliero Cantonale (Switzerland) were retrospectively analyzed.
Results
Transaminases were increased in about one-third of patients on hospital admission and two-thirds of patients during the hospital stay. On hospital admission, transaminases were more commonly elevated in younger patients, who also reported elevated C reactive protein and a higher degree of respiratory failure. Independent factors associated with abnormal transaminases during hospitalization were drugs, in particular paracetamol (OR=2.67; 95% CI=1.38–5.18; p = 0.004) and remdesivir (OR=5.16; 95% CI=1.10–24.26; p = 0.04). Mortality was independently associated to age (OR = 1.09; 95% CI=1.05–1.13; p<0.001), admission to intensive care unit (OR=5.22; 95% CI=2.28–11.90; p<0.001) and alkaline phosphatase peak (OR=1.01; 95% CI=1.00- 1.01; p = 0.01).
Conclusions
On hospital admission, factors associated with liver damage were linked to demographic and clinical characteristics (age, inflammation and hypoxia) while, during hospitalization, drug treatment was related to development and progression of hepatocellular damage. Mortality was associated with alkaline phosphate peak value.
Keywords: Liver injury, Liver function tests, Mortality, SARS-CoV-2
1. Introduction
In December 2019, a novel Coronavirus known as SARS-CoV-2 globally spread, causing Coronavirus Disease 2019 (COVID-19) [1]. The infection can have a totally asymptomatic course or manifest with a broad spectrum of clinical presentations up to acute respiratory distress syndrome (ARDS), multiple organ failure and death [1], [2], [3].
Among extrapulmonary manifestations, COVID-19 patients can experience some forms of liver injury. Previous studies showed that these patients presented increased liver function tests (LFTs) in up to 76% of the cases, more frequently with an elevation of aspartate aminotransferase (AST) rather than alanine aminotransferase (ALT) serum levels [4], [5], [6], [7], [8], [9]. Severe hepatitis with marked elevation of transaminases was unfrequently reported [10,11].
Serum markers of cholangiocyte potential injury such as gamma-glutamyltransferase (GGT) and alkaline phosphatase (ALP), were reported to be above the upper limit normal (ULN) value from 1.8% up to 50% of patients, respectively, according to the previous reports [11,12].
In this setting, a wide range of etiological factors can underlie liver injury. These include primarily drug-induced liver injury (DILI) secondary to administration of medications commonly used in the management of COVID-19, such as paracetamol, antiviral therapies, low molecular weight heparin, anti-interleukin-6 (IL-6) receptor agents, and antibiotic treatments. However, increased LFTs could also be caused by viral-induced cytokine storm, sinusoidal thrombotic events in the context of the COVID-19 associated coagulopathy, liver damage induced by hypoxia or could be secondary to alterations of blood outflow and inflow that may occur when positive end-expiratory pressure (PEEP) is applied [13], [14], [15]. Moreover, although expression of Angiotensin I Converting Enzyme 2 (ACE2), the gateway for SARS-CoV-2 entry into the host cell, is well represented on cholangiocytes and to a lesser extent on hepatocytes, only a few pathological studies on liver biopsy specimens from patients affected by COVID-19 were conducted, leading to inconclusive findings of the exact role of the virus on liver damage [16], [17], [18], [19], [20].
Identifying the etiological factor behind liver damage in SARS-CoV-2 infection remains a diagnostic challenge, and its impact on mortality in this setting is controversial. Indeed, while according to some studies elevated LFTs seemed to have a worse prognostic value, others have not confirmed this finding [4,[21], [22], [23]]. Therefore, we aimed at studying the prevalence and time-trend of hepatocellular liver injury in hospitalized patients affected by COVID-19, by detecting factors associated with hepatocellular damage and evaluating its impact on mortality.
2. Methods
2.1. Patients
We conducted a retrospective single-center study on adult patients admitted with COVID-19 at the Ente Ospedaliero Cantonale (EOC), a regional network of seven public hospitals in Southern Switzerland, serving a population of approximately 380,000 inhabitants. At the time of enrollment (25th February 2020 - 11th May 2020), all patients were confirmed positive for SARS-CoV-2 by at least one positive RT-PCR test performed on a nasopharyngeal swab using the commercial kit SARS-CoV-2 S Gene VIASURE Real-Time PCR detection kit by CerTest BIOTEC on a BD MAX Instrument (Becton Dickinson, New Jersey, USA).
2.2. Data collection
Patient clinical data were obtained from electronic medical records collected daily during the hospital stay, including demographic, clinical, laboratory, treatment and outcome data. The 7 hospitals have the same computer system for medical records in each center; therefore, these data could be retrieved with high accuracy.
2.3. Ethical approval
The Ethics Committee of the Canton of Ticino approved this study (Project-ID 2020–01703) that was carried out in accordance with the principles of the 1975 Declaration of Helsinki (6th revision, 2008). Written informed consent was obtained prior to enrollment. Patient clinical data have been collected in coded form in a dataset by Clinical Trial Unit of EOC with approval by the Cantonal Ethics Committee.
2.4. Study design and definition of liver injury
Initial laboratory tests were performed within 24 h from admission and recorded as baseline values. The reference laboratory threshold limit for transaminases, GGT and ALP was considered the upper limit of normal (ULN), with a distinction between genders: for ALT and AST it was 50 U/l for male patients and 35 U/l for female patients, while for GGT and ALP it was respectively 71 U/l and 129 U/l for male and 42 U/l and 104 U/l for female patients. According to previous studies on COVID-19 and hepatic involvement, AST and/or ALT elevation up to 3x ULN was defined as abnormal, while values over 3x ULN were considered as liver injury [4].
Then, patients with a minimum of one transaminases (AST and/or ALT) control performed during hospital stay were compared with their respective baseline values. The degree of hepatocellular damage was defined according to the National Institute of Health within the Common Terminology Criteria for Adverse Events (CTCAE), namely the ratio between the peak value recorded during hospitalization and the ULN, when the value was normal on entry, or the baseline when transaminases were abnormal on admission [24].
2.4. Aim of the study
Our primary purpose was to evaluate the prevalence and time-trend of transaminases in patients with laboratory-confirmed SARS-CoV-2 infection hospitalized at our institution. Secondarily, we investigated potential risk co-factors for the occurrence of hepatocellular injury. Finally, our purpose was to identify independent risk factors associated with mortality in this population.
2.5. Statistical analysis
The mean with standard deviation (SD) or median with interquartile range (IQR) were used for quantitative data synthesis. Qualitative data were summarized as absolute numbers with percentages. Comparisons of the different variables among the three cohorts (patients with normal transaminases, abnormal transaminases and liver injury) were carried out with the parametric or the non-parametric analysis of variance (ANOVA), or the chi-square test, as appropriate. Indicated by significant p-value, post-hoc analysis was performed with the corresponding p adjustment for multiple comparisons. Prevalence of liver function test abnormality was estimated with the conventional formula and presented with 95%-Confidence Intervals (95%-CI). To identify potentially predictive variables for the occurrence of liver dysfunction, other than the virus infection itself, we first performed a univariate logistic regression, followed by a multivariable logistic regression model. Unadjusted and adjusted Odds Ratio (OR) with the corresponding 95%-CI were presented. A univariate and a multivariable logistic regression model were constructed to identify risk factors for mortality. All tests were conducted two-sided, and p-values < 0.05 were considered statistically significant. Stata version 15 (StatCorp. LP, College Station, TX, USA) was used for all statistical analysis.
3. Results
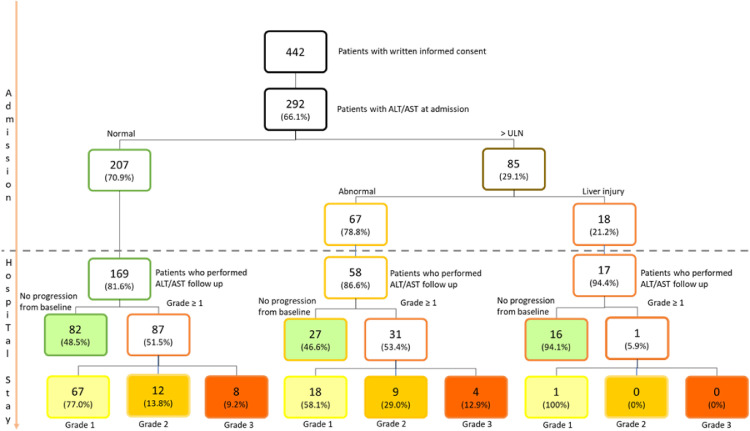
At least one determination of transaminases (ALT and/or AST) serum level within the first 24 h after admission to the hospital was available for 292 out of the 442 patients who signed the informed consent. Two-hundred-seven (70.9%) of these had normal transaminases, 67 (22.9%) had abnormal values (up to 3x ULN), and 18 (6.2%) had a liver injury (over 3x ULN) (Fig. 1 ). The median age of the total population was 74 years (IQR 58–79), two-thirds of the patients were male, and the median Body Mass Index (BMI) was 26.7 kg/m2 (IQR 24.7–30.5) (Table 1 ). The most frequent comorbidity was arterial hypertension (n = 137, 46.9%), followed by cardiovascular disease (n = 99, 33.9%) and chronic liver disease (CLD) (n = 69, 23.6%). Following a review of a liver imaging available during and before current hospitalization, the majority of patients with CLD (n = 61, 88%) had a previous diagnosis of fatty liver disease based on liver ultrasound, whereas patients with a known diagnosis of cirrhosis were only 2.4% of all patients with CLD (5 patients). Other comorbidities were diabetes (n = 63, 21.6%), lung disease (n = 44, 15.1%), cancer (n = 31, 10.6%) or other chronic diseases (n = 72, 24.6%).
Fig. 1.
Time-trend analysis of transaminases in patients with COVID-19 pneumonia.
Table 1.
Characteristics of patients stratified by transaminases at admission. Statistically significancy differences are highlighted in bold.
| Characteristics | Total (n = 292) | Normal (n = 207) | Abnormal (n = 67) | Liver injury (n = 18) | p-value |
|---|---|---|---|---|---|
| Male, n (%) | 194 (66.4) | 139 (67.2) | 44 (65.7) | 11 (61.1) | 0.86 |
| Age, median (IQR) | 74 (58–79) | 74 (61–80)* | 68 (55–79) | 61 (57–70)* | 0.009 |
| BMI, median (IQR) | 26.7 (24.7–30.5) | 26.6 (24.4–30.0) | 27.5 (25.2–31.7) | 27.6 (26.5–30.1) | 0.11 |
| Symptoms and signs at admission | |||||
| Fever, n (%) | 237 (81.2) | 164 (79.2) | 59 (88.1) | 14 (77.8) | 0.26 |
| Cough, n (%) | 180 (61.6) | 126 (60.9) | 45 (67.2) | 9 (50.0) | 0.38 |
| Dyspnea, n (%) | 147 (50.3) | 96 (46.4) | 41 (61.2) | 10 (55.6) | 0.10 |
| Diarrhea, n (%) | 61 (20.9) | 43 (20.8) | 12 (17.9) | 6 (33.3) | 0.36 |
| Comorbidities | |||||
| Chronic liver disease, n (%) | 69 (23.6) | 46 (22.2) | 17 (25.4) | 6 (33.3) | 0.53 |
| Cirrhosis, n (%) | 5 (1.7) | 5 (2.4) | 0 (0) | 1 (5.6) | 0.27 |
| CV disease, n (%) | 99 (33.9) | 81 (39.3)* | 15 (22.4)* | 3 (16.7) | 0.01 |
| Diabetes, n (%) | 63 (21.6) | 50 (24.2) | 10 (14.9) | 3 (16.7) | 0.24 |
| Hypertension, n (%) | 137 (46.9) | 102 (49.3) | 30 (44.8) | 5 (27.8) | 0.20 |
| Pulmonary disease, n (%) | 44 (15.1) | 32 (15.5) | 10 (14.9) | 2 (11.1) | 0.88 |
| Malignancy, n (%) | 31 (10.6) | 24 (11.6) | 6 (9.0) | 1 (5.6) | 0.64 |
| Other comorbidities, n (%) | 72 (24.6) | 49 (23.7) | 18 (26.9) | 5 (27.8) | 0.83 |
| Home therapy | |||||
| Number of drugs, median (IQR) | 5 (3- 8) | 5 (3–9) | 4 (2–7) | 4 (2–7) | 0.14 |
| Antibiotics, n (%) | 14 (4.8) | 8 (9.4) | 5 (18.5) | 1 (14.3) | 0.43 |
| Paracetamol, n (%) | 19 (6.5) | 13 (15.3) | 5 (18.5) | 1 (14.3) | 0.92 |
| Metamizole, n (%) | 5 (1.7) | 3 (3.5) | 2 (7.4) | 0 (0) | 0.58 |
| Hospital therapy | |||||
| Heparin, n (%) | 245 (88.5) | 168 (87.1) | 62 (93.9) | 15 (83.3) | 0.25 |
| Any antibiotic, n (%) | 261 (89.4) | 181 (93.8) | 64 (97.0) | 16 (88.9) | 0.38 |
| Penicillin, n (%) | 128 (46.1) | 85 (44.0) | 33 (50.0) | 10 (55.6) | 0.50 |
| Cephalosporins, n (%) | 58 (20.9) | 40 (20.7) | 15 (22.7) | 3 (16.7) | 0.85 |
| Carbapenem, n (%) | 30 (10.8) | 14 (7.3)* | 11 (16.7)§ | 5 (27.8)*§ | 0.006 |
| Macrolid, n (%) | 29 (10.5) | 19 (9.8) | 8 (12.1) | 2 (11.1) | 0.87 |
| Fluoroquinolone, n (%) | 15 (5.4) | 12 (6.2) | 1 (1.5) | 2 (11.1) | 0.19 |
| Other antibiotics, n (%) | 35 (12.6) | 15 (7.8)*§ | 15 (22.7)* | 5 (27.8)§ | 0.001 |
| Acetaminophen, n (%) | 190 (68.6) | 123 (67.7)* | 53 (80.3)* | 14 (77.8) | 0.03 |
| Lopinavir/ritonavir, n (%) | 88 (31.8) | 59 (30.6) | 24 (36.4) | 5 (27.8) | 0.64 |
| Hydroxychloquine, n (%) | 117 (42.2) | 80 (41.5) | 32 (48.5) | 5 (27.8) | 0.27 |
| Metamizole, n (%) | 150 (54.1) | 103 (53.4) | 32 (48.5)* | 15 (83.3)* | 0.03 |
| Tocilizumab, n (%) | 6 (2.2) | 4 (2.1) | 1 (1.5) | 1 (5.6) | 0.57 |
| Remdesivir, n (%) | 17 (6.1) | 10 (5.2) | 7 (10.6) | 0 (0) | 0.15 |
| Laboratory test at admission | |||||
| WBC (x10^9/l), median (IQR) | 6.4 (4.8–8.5) | 6.4 (4.9–8.6) | 6.2 (4.8–8.1) | 5.8 (4.1–9.8) | 0.76 |
| Lymphocytes (x10^9/l), median (IQR) | 0.87 (0.62–1.17) | 0.9 (0.6–1.3) | 0.9 (0.6–1.1) | 0.7 (0.5–1.0) | 0.10 |
| Albumin (g/l), median (IQR) | 38 (35–41) | 39 (35–42) | 37 (35–40) | 38 (35–40) | 0.34 |
| Total bilirubin (µmol/l), median (IQR) | 8.3 (5.7–12.7) | 8.3 (5.8–12.1) | 7.1 (5.0–11.8) | 12.6 (8.0–24.9) | 0.10 |
| LDH (IU/l), median (IQR) | 493 (393–644) | 486 (373–575)* | 624 (477–799)* | 860 (667–1114)* | 0.0001 |
| CRP (mg/l), median (IQR) | 65 (23–118) | 57 (18–109)* | 73 (44–146)* | 75 (39–127) | 0.01 |
| Ferritin (ng/ml), median (IQR) | 596 (513–1214) | 536 (226–971) | 806 (334–1243) | 1017 (*) | 0.34 |
| INR, median (IQR) | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.1–1.3) | 0.33 |
| D-dimer (mg/l), median (IQR) | 0.8 (0.5–1.3) | 0.8 (0.5–1.5) | 0.9 (0.6–1.2) | 0.8 (0.4–0.9) | 0.66 |
| PaO2/FiO2, median (IQR) | 296 (244–330) | 299 (207–342)* | 294 (227–321) | 226 (194–280)* | 0.02 |
| Outcome | |||||
| Death, n (%) | 68 (23.3) | 50 (24.2) | 14 (20.9) | 4 (22.2) | 0.85 |
| Admission to ICU, median (IQR) | 61 (20.9) | 36 (17.4)* | 18 (26.9) | 7 (38.9)* | 0.04 |
| Lenght of stay, median (IQR) | 11.1 (7.0–20–0) | 11.0 (6.8–18.0) | 11.9 (7.8–25.1) | 14.9 (8.3–23.5) | 0.15 |
*,§ Groups with statistical differences
IQR interquartile range, BMI body mass index, CV cardiovascular, WBC white blood cells, LDH lactate dehydrogenase, CRP C reactive protein, INR International Normalized ratio, paO2 partial pressure of oxygen, FiO2 fraction of inspired oxygen, ARDS acute respiratory distress syndrome, ICU intensive care unit.
119 patients took at least one home medication with a median number of 5 (IQR 3–8). 29 patients started at least one new medication after onset of symptoms of COVID-19 prior to hospitalization. In particular, the use of antibiotics and paracetamol was reported in 4.8% and 6.5% of patients. The most common in-hospital drugs were antibiotics (89.4%), heparins (88.5%) and antipyretics (paracetamol 68.6% and metamizole 54.1%). Only a small proportion of patients received remdesivir (17%) and tocilizumab (2.2%). By analyzing outcomes in the overall population, in-hospital mortality occurred in 23.3% of cases (n = 68), intensive care unit (ICU) admission in 20.9% (n = 61) and the median length of stay was 11 days (IQR 7–20).
Patients with elevated transaminases at baseline were younger (median=61 years old, p = 0.009), with a lower prevalence of cardiovascular comorbidities (p = 0.01), presenting with higher levels of LDH (p = 0.0001) and C reactive protein (CRP) (p = 0.01) values and had a more advanced degree of respiratory failure expressed by lower values of arterial partial pressure of oxygen/fraction of inspired oxygen ratio (PaO2/FiO2) (p = 0.02). Furthermore, during hospitalization, these patients were more likely to receive antibiotic treatments with carbapenems (p = 0.006) or other classes of antibiotics (p = 0.001), as well as antipyretic drugs (p = 0.03). In addition, they showed a higher rate of intensive care unit (ICU) admission (p = 0.04).
Of the 207 patients who had normal transaminases on admission, 169 repeated laboratory tests, including transaminases during hospitalization: 82 (48.5%) maintained values within the ULN, while 87 (52.5%) showed an increase in ALT and/or AST values. Considering the 67 patients with liver injury at baseline, 58 (87%) performed successive LFTs during hospitalization. 27 (46.6%) did not show any further increase in LFTs, while 31 (53.4%) showed liver damage progression. Finally, of the 18 patients (6.2%) who had elevated transaminases up to 3x ULN on admission, almost all (94.1%) did not show a significant increase in hepatocellular damage indices, while there was only one case of a further worsening.
Finally, considering the development of hepatocellular damage during the hospital stay, we focused on patients with baseline transaminase values up to 3xULN, and stratified them between patients who had a development or progression of liver damage (grade ≥ 1) and those who did not show a significant rise from baseline (grade 0). We performed univariate and multivariable logistic regression to identify factors associated with the development of liver damage during hospitalization (Table 2 ) and with mortality (Table 3 ). The only variables independently associated with the progression of in-hospital liver injury were the administration of paracetamol (OR = 2.67; 95% CI = 1.38–5.18; p = 0.004) and remdesivir (OR = 5.16; 95% CI = 1.10- 24.26; p = 0.04). Those factors that were independently associated with mortality, after being adjusted for comorbidities, included: age (OR = 1.09; 95% CI = 1.05–1.13; p < 0.001), admission to ICU (OR = 5.22; 95% CI = 2.28–11.90; p < 0.001) and peak value of alkaline phosphatase (ALP) (OR = 1.01; 95% CI = 1.00- 1.01; p = 0.01).
Table 2.
Univariate Analysis and Multivariable logistic regression model considering hepatocellular damage during hospital stay. Statistically significancy comparisons are highlighted in bold.
| Univariate |
Multivariable |
|||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age, median (IQR) | 0.96 (0.94–0.98) | < 0.001 | 0.98 (0.95–1.00) | 0.06 |
| BMI, median (IQR) | 0.98 (0.94–1.03) | 0.44 | ||
| Comorbidities | ||||
| CLD, n (%) | 0.64 (0.36–1.14) | 0.13 | ||
| CV disease, n (%) | 0.37 (0.21–0.64) | < 0.001 | 0.70 (0.36–1.38) | 0.30 |
| Diabetes, n (%) | 0.50 (0.27–0.93) | 0.03 | 0.78 (0.39–1.59) | 0.50 |
| Hypertension, n (%) | 0.57 (0.34–0.94) | 0.03 | 0.76 (0.41–1.42) | 0.39 |
| Hospital therapy | ||||
| Heparin, n (%) | 3.42 (1.21–9.66) | 0.02 | 2.28 (0.75–6.95) | 0.15 |
| Any antibiotic, n (%) | 1.47 (0.40–5.36) | 0.56 | ||
| Penicillin, n (%) | 0.84 (0.50–1.40) | 0.50 | ||
| Cephalosporin, n (%) | 1.61 (0.87–2.96) | 0.13 | ||
| Carbapenem, n (%) | 1.04 (0.47–2.29) | 0.92 | ||
| Macrolid, n (%) | 2.59 (1.02–6.54) | 0.05 | 1.95 (0.69–5.53) | 0.21 |
| Fluoroquinolone, n (%) | 0.63 (0.20–1.99) | 0.43 | ||
| Other antibiotics, n (%) | 1.04 (0.50–2.15) | 0.91 | ||
| Acetaminophen, n (%) | 2.39 (1.32–4.34) | 0.004 | 2.67 (1.38–5.18) | 0.004 |
| Lopinavir/ritonavir, n (%) | 1.40 (0.80–2.44) | 0.24 | ||
| Hydroxychloquine, n (%) | 1.02 (0.60–1.72) | 0.94 | ||
| Tocilizumab, n (%) | 2.11 (0.38–11.74) | 0.40 | ||
| Remdesivir, n (%) | 8.77 (1.96–39.30) | 0.005 | 5.16 (1.10–24.26) | 0.04 |
| Peak laboratory test | ||||
| Total bilirubin (µmol/l), median (IQR) | 1.02 (1.00–1.04) | 0.08 | ||
| CRP (mg/l), median (IQR) | 1.00 (0.99–1.00) | 0.23 | ||
| Ferritin (ng/ml), median (IQR) | 1.00 (0.99–1.00) | 0.25 | ||
| INR, median (IQR) | 1.68 (0.59–4.73) | 0.33 | ||
| D-dimer (mg/l), median (IQR) | 1.03 (0.97–1.09) | 0.41 | ||
| paO2/FiO2, median (IQR) | 1.00 (0.99–1.00) | 0.41 | ||
| Illness severity | ||||
| Moderate ARDS | 0.84 (0.44–1.61) | 0.61 | ||
| Severe ARDS | 1.56 (0.74–3.43) | 0.24 | ||
IQR interquartile range, BMI body mass index, CLD chronic liver disease, CV cardiovascular, CRP C reactive protein, INR International Normalized Ratio, paO2 partial pressure of oxygen, FiO2 fraction of inspired oxygen, ARDS acute respiratory distress syndrome.
Table 3.
Univariate Analysis and Multivariable logistic regression model considering death as outcome. Statistically significancy comparisons are highlighted in bold.
| Univariate |
Multivariable |
|||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Gender | 1.31 (0.83–2.06) | 0.24 | ||
| Age | 1.08 (1.06–1.10) | <0.001 | 1.09 (1.05–1.13) | <0.001 |
| BMI | 1.00 (0.96–1.04) | 0.84 | ||
| Comorbidities | ||||
| Number of comorbidities | 1.61 (1.37–1.88) | <0.001 | 1.50 (0.98–2.29) | 0.06 |
| Chronic liver disease, n (%) | 1.44 (0.89–2.34) | 0.14 | ||
| CV disease, n (%) | 3.76 (2.40–5.89) | <0.001 | 1.26 (0.55–2.88) | 0.58 |
| Diabetes, n (%) | 1.96 (1.21–3.18) | 0.006 | 0.75 (0.31–1.82) | 0.53 |
| Hypertension, n (%) | 1.58 (1.03–2.44) | 0.04 | 0.53 (0.24–1.18) | 0.12 |
| Pulmonary disease, n (%) | 1.46 (0.83–2.55) | 0.19 | ||
| Malignancy, n (%) | 3.59 (1.98–6.50) | <0.001 | 2.44 (0.90–6.61) | 0.08 |
| Other comorbidities, n (%) | 2.00 (1.26–3.18) | 0.003 | ||
| Laboratory test at admission | ||||
| GGT at admission | 1.00 (0.99–1.00) | 0.89 | ||
| ALT at admission | 0.99 (0.98–1.00) | 0.14 | ||
| Peak GGT | 1.00 (0.99–1.00) | 0.17 | ||
| Peak ALP | 1.005 (1.001–1.008) | 0.001 | 1.01 (1.00–1.01) | 0.01 |
| Peak ALT | 0.99 (0.98–0.99) | 0.004 | 0.99 (0.99–1.00) | 0.07 |
| Peak AST | 0.99 (0.99–1.00) | 0.65 | ||
| Peak GGT ≥ 5X | 1.38 (0.58–3.25) | 0.47 | ||
| Peak ALT ≥ 5X | 0.52 (0.15–1.83) | 0.31 | ||
| Peak AST ≥ 5X | 0.77 (0.25–2.40) | 0.65 | ||
| Peak transaminases | 0.74 (0.27–2.05) | 0.56 | ||
| Grade ≥ 1 | 0.60 (0.32–1.13) | 0.11 | ||
| Illness gravity | ||||
| Severe ARDS | 2.73 (1.36–5.49) | 0.005 | ||
| Admission to ICU | 2.99 (1.82–4.92) | <0.001 | 5.22 (2.28–11.90) | < 0.001 |
OR odds ratio, BMI body mass index, CV cardiovascular, GGT gamma-glutamyl transferase, ALT alanine aminotransferase, ALP alkaline phosphatase, AST aspartate aminotransferase, ARDS acute respiratory distress syndrome, ICU intensive care unit.
Discussion
This study shows that hepatocellular damage is a clinically relevant manifestation in hospitalized patients affected by COVID-19. It occurred in about one-third of patients already on admission. However, the elevation of transaminases was usually mild, and only in one-fifth of cases, laboratory patterns of liver injury could be observed. Nonetheless, during hospitalization, almost half of the patients developed liver injury de novo or had a significant progression from baseline, while only slightly more than a quarter of them (27.1%) developed a grade 2 or a higher rise in transaminases.
Previous studies showed elevated LFTs in up to 76% of cases in COVID-19 patients, with higher involvement of AST rather than ALT [4,7,8]. In our study, the prevalence and damage pattern of transaminases were in line with these literature data, with 66.4% of patients developing abnormal transaminases during their hospital stay, showing an AST/ALT ratio > 1 (median peak AST 54 IU/L, median peak ALT 47 IU/L). This particular pattern of hepatocellular damage is more typical in toxic (including alcohol or drugs) or ischemic liver damage, although extra-hepatic causes of AST elevation may originate from muscle or heart involvement due to the virus. However, a previous prospective observational study on patients with COVID-19 showed that AST elevation correlated with the trend of ALT but not with creatine phosphokinase, which supports a hepatic origin of AST [6]. Recent studies have suggested that the direct virus action at the mitochondrial level may be a possible mechanism behind the prevalent rise in AST [25].
When examining factors associated with liver damage during hospitalization, we focused our analysis on patients who had transaminases values at baseline up to 3x ULN because, for baseline values above this cut-off, we noticed that the determination of hepatocellular damage was a less sensitive parameter in detecting transaminases elevation (Fig. 1).
In this analysis, we observed that transaminases at baseline were most commonly elevated in younger patients, with less cardiovascular comorbidities, higher inflammatory parameters and a higher degree of respiratory failure. These results suggest a more robust systemic inflammatory response and elevated transaminase levels could represent its hepatic involvement [7]. Interestingly, the association between hypertransaminasemia and inflammation was lost during hospitalization. Indeed, the only factors independently associated with the development of de novo hepatocellular damage or its progression during hospitalization were drugs.
Only a few studies longitudinally analyzed the trend of transaminases markers in patients with COVID-19, and none of them did it on a European cohort [6,26]. Moreover, while several studies are available in cirrhosis patients, few studies described the liver steatosis impact on liver damage and mortality in patients with COVID-19 [27,28]. In this study, patients with CLD represented 23.6% of our cohort, a reliable representative percentage of the general population estimated to be from 20% to 25%, according to a previous study conducted in a neighboring region [29]. Interestingly, in that study, liver steatosis occurred in 54% of patients with elevated transaminases. These findings were not confirmed in our study, but the prevalence of liver damage on hospital admission may be due to underlying CLD also in our population. Despite this, we did not appreciate a different prevalence of CLD among the groups of patients stratified by liver test elevation on admission. Moreover, CLD does not appear to be a risk factor for the development of hepatocellular damage. This is in accord with literature where fatty liver disease, the most representative etiology of chronic liver disease in our population, represents a risk factor for drug-induced hepatotoxicity only for some medication [30]. However, it is possible that the change of the therapeutic regimens in the management of COVID-19 could play a relevant role in the genesis of liver damage.
Alkaline phosphatase peak was an independent factor associated with death in hospitalized patients with COVID-19, whereas gamma-GT and transaminases, both on admission and at peak, were not (Table 3). This finding could identify ALP as a value of global clinical severity [22]. Considering the cholestasis pathophysiology, this could indicate a mechanism of adaptation to hyperinflammation and/or hypoxic damage of biliary cells, as described in critical-illness induced cholestasis [31]. However, in this context, the contribution of DILI or direct viral damage could not be excluded.
Our findings are new, and their implications may be relevant in clinical practice and management of patients hospitalized for SARS-CoV-2 infection. Firstly, we characterized liver damage as a function of time during the whole hospitalization. Once a careful longitudinal analysis of transaminases was carried out, we considered the different factors associated with liver injury. Furthermore, the possibility of using a fully detailed, centralized multicenter clinical data management system allowed us to obtain accurate and reliable data. Indeed, an exhaustive study that takes into consideration the role of drugs prior hospitalization as well as the most common drugs medication administered during hospitalization is still missing [32]. Even if remdesivir and paracetamol can cause alterations in transaminases, we had demonstrated the pivotal role that they play in the progression of liver injury in hospitalized patients affected by COVID-19.
This study has several limitations, mainly because we had to exclude a significant number of patients who provided written informed consent but who did not perform liver function tests on admission or during hospitalization. Further intrinsic limitations lie in the study retrospective nature, specifically due to the unique emergency setting in which the study was conducted, where the patient clinical management was clearly a priority over comprehensive data collection. Moreover, as the liver damage causes are also linked to therapeutic interventions and systemic factors, the prevalence may be different for non-hospitalized patients, and our considerations are valid for patients affected by COVID-19 with in-hospital management. Therefore, our results should be reassessed with future new therapeutic protocols because of the potentially different pharmacological profile of the disease hepatotoxic risk and clinical course. For this reason we haven't extended our study to the following pandemic wave where the different therapeutic protocols applied would have entailed the inclusion of an heterogeneous group of patients. Finally, our study evaluated only hepatocellular injury as a liver damage pattern since both GGT and ALP dosage at baseline and during hospital stay were available only in a small proportion of patients. Further studies investigating the role of cholestasis parameters would provide further information on liver involvement.
In conclusion, liver involvement in SARS-CoV-2 infection was a frequent complication in patients admitted to the hospital. The pathogenic mechanisms are multifactorial and their contribution varies during the course of hospitalization.
At admission, liver damage factors were linked to demographic and clinical characteristics (inflammation and hypoxia), while during hospitalization, drugs played a predominant role in the development or progression of liver damage. In this context, whenever transaminases elevation is appreciated, a DILI should always be taken into consideration and ruled out.
Monitoring liver function tests, particularly when worsening of disease occurs and/or new drugs are administered, may help understand the etiology of a possible increase in transaminases. Alkaline phosphatase, an independent factor associated with mortality, should be monitored regularly as its rise may reflect a negative disease evolution.
Conflict of Interest
Massimo Leo, Antonio Galante, Alberto Pagnamenta, Lorenzo Ruinelli, Francesca Romana Ponziani, Antonio Gasbarrini and Andrea De Gottardi declare that there are no conflicts of interest.
Funding
This work was funded by a grant from the Scientific Committee Covid-19 AFRI of Ente Ospedaliero Cantonale.
Acknowledgements
We thank the Scientific Committee Covid-19 AFRI of Ente Ospedaliero Cantonale for its support in this study. A special thank you to all the patients enrolled in the study and to all the healthcare staff involved in the care of COVID-19 patients. We thank Ettore Marzocchi for his valuable support in processing the data. These results were partly presented as a poster at the 53rd Annual Meeting of the Italian Association for the Study of the Liver, 3–5 March 2021, Italy.
References
- 1.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Mao R., Qiu Y., He J.S., et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Q., Huang D., Yu H., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom P.P., Meyerowitz E.A., Reinus Z., et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. 2020;73:890–900. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 7.Phipps M.M., Barraza L.H., LaSota E.D., et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Zompo F., De Siena M., Ianiro G., et al. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072–13088. doi: 10.26355/eurrev_202012_24215. [DOI] [PubMed] [Google Scholar]
- 10.Weber S., Mayerle J., Irlbeck M., et al. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69:1365–1367. doi: 10.1136/gutjnl-2020-321350. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer E.A.K., Arvind A., Bloom P.P., et al. Interrelationship between coronavirus infection and liver disease. Clin Liver Dis (Hoboken) 2020;15:175–180. doi: 10.1002/cld.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald B., Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology. 2016;151:1087–1095. doi: 10.1053/j.gastro.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., Liu J., Lu M., et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brienza N., Revelly J.P., Ayuse T., et al. Effects of PEEP on liver arterial and venous blood flows. Am J Respir Crit Care Med. 1995;152:504–510. doi: 10.1164/ajrccm.152.2.7633699. [DOI] [PubMed] [Google Scholar]
- 16.Chai X., Hu L., Zhang Y., et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 [Google Scholar]
- 17.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q., Wang R., Qu G., et al. General anatomy report of novel coronavirus pneumonia patients. Journal of Forensic Medicine. 2020;36:21–23. [Google Scholar]
- 19.Wang Y., Liu S., Liu H., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philips C.A., Ahamed R., Augustine P. SARS-CoV-2 related liver impairment - perception may not be the reality. J Hepatol. 2020;73:991–992. doi: 10.1016/j.jhep.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan Z., Chen L., Li J., et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponziani F.R., Del Zompo F., Nesci A., et al. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060–1068. doi: 10.1111/apt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Z., Li G., Chen L., et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295–13024. doi: 10.1016/j.jhep.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institutes of Health, National Cancer Institute, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published November 27, 2017.
- 25.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei F., Liu Y.M., Zhou F., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palomar-Lever A., Barraza G., Galicia-Alba J., et al. Hepatic steatosis as an independent risk factor for severe disease in patients with COVID-19: a computed tomography study. JGH Open. 2020;4:1102–1107. doi: 10.1002/jgh3.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedogni G., Miglioli L., Masutti F., et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 30.Massart J., Begriche K., Moreau C., et al. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017;3:212–232. doi: 10.18053/jctres.03.2017S1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenniskens M., Langouche L., Van den Berghe G. Cholestatic Alterations in the Critically Ill: some New Light on an Old Problem. Chest. 2018;153:733–743. doi: 10.1016/j.chest.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Bertolini A., Van de Peppel I.P., Bodewes F.A.J.A., et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]