Abstract
The gut microbiome is impacted by environmental exposures and has been implicated in many physical and mental health conditions, including anxiety disorders, affective disorders, and trauma- and stressor-related disorders such as posttraumatic stress disorder (PTSD). United States (US) military Veterans are a unique population in that their military-related exposures can have consequences for both physical and mental health, but the gut microbiome of this population has been understudied. In this publication, we describe exposures, health conditions, and medication use of Veterans in the US Veteran Microbiome Project (US-VMP) and examine the associations between these characteristics and the gut microbiota. This cohort included 331 US Veterans seeking healthcare with the Veterans Health Administration who were 83% male with an average (±SD) age of 47.6 ± 13.4 years. The cohort displayed a high prevalence of PTSD (49.8%) and history of traumatic brain injuries (76.1%), and high current use of prescription medications (74.9%) to treat various acute and chronic conditions. We observed significant associations between the gut microbiota composition and gastroenteritis, peripheral vascular disease (PVD), bipolar disorders, symptoms of severe depression based on the Beck Depression Inventory, stimulant and opioid use disorders, beta-blockers, serotonin and norepinephrine reuptake inhibitor antidepressants, diabetes medications, and proton pump inhibitors. Many of the Veteran characteristics examined were associated with altered relative abundances of specific taxa. We found that PVD and cardiovascular disease were associated with lower microbiota diversity in the gut (i.e., α-diversity), while supplemental vitamin use was associated with higher α-diversity. Our study contributes novel insights as to whether the unique exposures of Veterans in this cohort correlate with gut microbiota characteristics and, in line with previous findings with other population-level studies of the microbiome, confirms associations between numerous health conditions and medications with the gut microbiome.
Keywords: Veteran, Microbiome, Gut microbiome, Bipolar disorder, Depression, Proton pump inhibitors, Medications, Cardiovascular disease
Highlights
-
•
Mental health disorders/symptoms were associated with gut microbiome composition.
-
•
Several medication classes were associated with gut microbiome characteristics.
-
•
Military deployments were associated with decreased abundance of Bifidobacterium.
-
•
Most military-related exposures were not related to microbiome characteristics.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ANCOM
analysis of compositions of microbiomes
- ARB
angiotensin II receptor blocker
- ATP
adenosine triphosphate
- BDI
Beck Depression Inventory
- BMI
body mass index
- CHF
congestive heart failure
- CPD
chronic pulmonary disease
- CVD
cardiovascular disease
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders Fifth Edition
- ECHCS
Eastern Colorado Health Care System
- EMR
Electronic Medical Record
- FDR
false discovery rate
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, Tenth Revision
- IL
interleukin
- IQR
interquartile range
- ISI
Insomnia Severity Index
- MDS
multidimensional scaling
- NSAIDs
nonsteroidal anti-inflammatory drugs
- OSU TBI-ID
The Ohio State University Traumatic Brain Injury-Identification Method
- PCS
Physical Health Composite Score
- PD
phylogenetic diversity
- PERMANOVA
permutational multivariate analysis of variance
- PiCrust
Phylogentic investigation of Communities by reconstruction of unobserved states
- PPI
proton pump inhibitor
- PTSD
posttraumatic stress disorder
- PVD
peripheral vascular disease
- QIIME2
Quantitative Insights Into Microbial Ecology v2
- SCFA
short-chain fatty acid
- SCID
Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders
- SD
standard deviation
- SEPP
SATé-enabled phylogenetic placement
- SF-36
36-Item Short Form Health Survey
- SNRI
serotonin and norepinephrine reuptake inhibitors
- SSRI
selective serotonin reuptake inhibitor
- TBI
traumatic brain injury
- TNFα
tumor necrosis factor alpha
- US
United States
- US-VMP
United States-Veteran Microbiome Project
- VA
Veteran Affairs
- VHA
Veterans Health Administration
1. Introduction
United States (US) military Veterans have exposures that can be different from those experienced by civilians. An unfortunate outcome of war and conflict can be disruptions to physical and mental health, which can result in changes to physiology and lifestyle that sometimes persist for the duration of Veterans’ lives. Many US Veterans have access to healthcare through the Veterans Health Administration (VHA). Electronic medical records (EMRs) within the VHA often contain comprehensive historical information including combat exposures, medical procedures, health conditions, and medication prescriptions. This information can provide valuable insight into health outcomes.
In recent years, evidence has come to light that environmental factors, health conditions, and medications may impact the gut microbiota (Cenit et al., 2017; Dinan and Cryan, 2017; Maier et al., 2018). There is also evidence that the gut microbiota may be associated with mental health disorders, such as depression, bipolar disorder, and schizophrenia (Cheung et al., 2019; Nguyen et al., 2018; Fond et al., 2020). Converging lines of evidence suggest that the connection between the gut microbiota and mental health disorders involves the immune system, specifically inflammatory pathways (Lowry et al., 2016). The gut microbiota has also been implicated in numerous inflammatory-related health conditions, including obesity and diabetes mellitus (Turnbaugh et al., 2009a; Sanna et al., 2019; Maruvada et al., 2017). The gut microbiota may also mediate the benefits of certain medications, such as metformin use for managing blood glucose in diabetes (Forslund et al., 2015; Jackson et al., 2018). Recent population-level studies have helped to quantify the relative importance of different correlates of the gut microbiota (Jackson et al., 2018; Falony et al., 2016), but it is unknown how well these results translate to a US Veteran population seeking care at the VHA, with unique environmental exposures, complex health conditions, and a high prevalence of medication use.
US Veteran Microbiome Project (US-VMP) investigators have been collecting microbiome samples from Veterans since 2015 (Brenner et al., 2018). Participants are recruited among those who are eligible to receive care within the Veterans Affairs (VA) Eastern Colorado Health Care System (ECHCS). The combination of microbiome and EMR data present a unique opportunity to examine the associations between the gut microbiota and various exposures among Veterans. In this study, we aimed to quantify the strength and nature of associations between Veteran characteristics, health conditions, and medications with the participants’ gut microbiota.
2. Materials and methods
2.1. Study design
This was a cross-sectional observational study of US military Veterans.
2.2. Study participants
The US-VMP was launched over six years ago in order to longitudinally assess the skin, oral, and fecal microbiomes of US Veterans, as well as collect validated measures of physical and mental health (Brenner et al., 2018). Participants were Veterans eligible to receive care within the ECHCS. This study includes data from all of the N = 331 Veterans who entered the study between June 2016 and November 2018, completed baseline measures, and provided a fecal sample that was successfully sequenced (N = 13 were excluded due to poor sequencing depth of the fecal samples).
2.3. Data collection
Data collection for the US-VMP has been previously described in detail, including the microbiome sample collection (Brenner et al., 2018). Information was collected during in-person visits and from the VA EMR. Height and weight were collected for calculation of body mass index (BMI). Mental health diagnoses (lifetime bipolar disorders, anxiety disorders and posttraumatic stress disorder [PTSD]) and current substance use disorders were identified via the Structured Clinical Interview for the Diagnostics and Statistical Manual of Mental Disorders (SCID) Version 5 (DSM-5) Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-5-I/P W/PSY SCREEN) (First et al., 2016). The SCID-5 is a comprehensive semi-structured clinical interview that is a reliable and valid means to acquire information regarding psychiatric disorders (Diagnostic and Statistical Manual of Mental Disorders, 2013). Presence of bipolar disorders was determined by a lifetime diagnosis of bipolar I, bipolar II, or other specified bipolar disorder. Presence of anxiety disorders was determined by a lifetime diagnosis of panic disorder, agoraphobia, social anxiety disorder, specific phobia, generalized anxiety disorder, separation anxiety disorder, other specified anxiety disorder, and anxiety disorder due to another medical condition. PTSD diagnosis required all necessary criteria including exposure to a traumatic event and sufficient symptoms. Presence of substance abuse was determined by a current diagnosis of abuse of cannabis, hallucinogens, inhalants, opioids, sedatives, hypnotics, anxiolytics, stimulants, or tobacco. Presence of alcohol use disorder was determined by a current diagnosis of alcohol use disorder. Depressive symptoms were identified by responses to the Beck Depression Inventory (BDI) (Beck et al., 1961). Additional measures included the Physical Health Composite Score (PCS) from the 36-Item Short Form Health Survey (SF-36) (Brazier et al., 1992) and the Insomnia Severity Index (ISI) (Morin, 1993). History of traumatic brain injury (TBI) was determined by the Ohio State University Traumatic Brain Injury Identification Method (OSU TBI-ID) (Bogner and Corrigan, 2009). For the individuals with data missing (<5%) from some of these surveys, single imputation was performed using fully conditional specification methods via PROC MI in SAS v9.4 (SAS Institute, Cary, NC, USA).
Health conditions were identified via National Health Interview Survey of Chronic Conditions (Kovar and Poe, 1985). In addition to querying the VA EMR (vital status, inpatient hospitalizations, outpatient clinic visits, and fee basis files) diagnoses of the following were identified using International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10) codes: cancer; congestive heart failure (CHF); peripheral vascular disease (PVD); cardiovascular disease (CVD); diabetes; liver disease; renal disease; chronic pulmonary disease (CPD); and gastroenteritis (ICD-9 and ICD-10 codes are lised in Appendix A; Table A1). Diagnoses were included in analyses if they were charted within one year prior to each participant's consent date in order to ensure that they were relatively current, and that the detection of health conditions was not overly biased towards older, long-term users of VA care. Additionally, a one year timeframe is often used for comorbidity assessment from EMRs (Casey et al., 2016; Lund et al., 2021). Medications were extracted from the VA EMR pharmacy prescriptions and refill records, including all medications with adequate supply to cover the two weeks preceding the fecal microbiota sample collection. Topical medications were excluded.
Table 1.
Characteristics of the US-VMP cohort.
| N | 331 | |
|---|---|---|
| Demographics | ||
| Age (years) | 47.6 (13.4) | |
| Male sex | 275 (83.1) | |
| Hispanic ethnicity | 53 (16.0) | |
| Race | ||
| Caucasian | 62 (18.7) | |
| African American | 218 (65.9) | |
| Other | 51 (15.4) | |
| Number of periods of homelessness | 1.6 (6.1) | |
| Periods of homelessness | ||
| Never | 192 (58.0) | |
| 1-2 times | 76 (23.0) | |
| 3+ times | 63 (19.0) | |
| Deployment history | ||
| Never | 100 (30.2) | |
| 1-2 times | 144 (43.5) | |
| 3+ times | 87 (26.3) | |
| Health Conditions and Metrics | ||
| Body mass index (kg/m2) | 29.0 (6.1) | |
| BMI category | ||
| Underweight | 1 (0.3) | |
| Normal weight | 90 (27.2) | |
| Overweight | 114 (34.4) | |
| Obese | 126 (38.1) | |
| Severe depressive symptoms (BDI) | 60 (18.1) | |
| Bipolar disorders (lifetime) | 17 (5.1) | |
| Anxiety disorders (lifetime) | 38 (11.5) | |
| Posttraumatic stress disorder (lifetime) | 165 (49.8) | |
| Number of traumatic brain injuries | 1.9 (1.8) | |
| Prevalence of TBI in cohort SF-36 Physical Health Composite score |
76.1 45.2 (10.2) |
|
| Insomnia Severity Index Prevalence of clinical insomnia |
11.9 (7.6) 35 |
|
| Cancer | 18 (5.4) | |
| Congestive heart failure | 8 (2.4) | |
| Peripheral vascular disease | 9 (2.7) | |
| Cardiovascular disease | 17 (5.1) | |
| Diabetes | 52 (15.7) | |
| Liver disease | 28 (8.5) | |
| Renal disease | 18 (5.4) | |
| Chronic pulmonary disease | 49 (14.8) | |
| Gastroenteritis | 41 (12.4) | |
| Alcohol use disorder (current) | 40 (12.1) | |
| Substance use disorder (current) | 25 (7.6) | |
| Cannabis | 20 (6.0) | |
| Opioids | 5 (1.5) | |
| Stimulants | 20 (6.0) | |
| Medications | ||
| Current VA medication | 248 (74.9) | |
Footnote. Numbers are presented as mean (SD) for continuous measures and prevalence (%) for categorical data. Severity of depressive symptoms was determined from the BDI. Bipolar disorders (lifetime diagnosis of bipolar I, bipolar II, or other specified bipolar disorder), anxiety disorders (lifetime diagnosis of panic disorder, agoraphobia, social anxiety disorder, specific phobia, generalized anxiety disorder, separation anxiety disorder, other specified anxiety disorder, or anxiety disorder due to another medical condition), posttraumatic stress disorder, current alcohol use disorder, and current substance use disorders (i.e., cannabis, opioids, and stimulants) were determined from the SCID-5-I/P W/PSY SCREEN. TBI history was determined by the OSU-TBI-ID. Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; OSU-TBI-ID, The Ohio State University Traumatic Brain Injury Identification Method; SCID-5-I/P W/PSY SCREEN, Structured Clinical Interview for the Diagnostic and Statistical Manual for Mental Health Disorders Version 5 (DSM-5) Axis I Disorders, Research Version, Patient Edition with Psychotic Screen; SD, standard deviation; SF-36, 36-Item Short Form Health Survey; TBI, traumatic brain injury.
2.4. Classification of exposures into four groups of predictors
We grouped the potential correlates of the gut microbiota into four broad categories of predictors: 1) demographics; 2) health conditions and metrics; 3) medication classes; and, 4) specific medications. Demographics included age, sex, ethnicity (Hispanic versus non-Hispanic), self-described race (Caucasian, African American, or Other), history of homelessness, history of combat exposure, and deployment history. Health conditions and metrics included BMI, severe depressive symptoms as measured by the BDI, lifetime bipolar disorders, lifetime anxiety disorders, lifetime PTSD, history of TBI, current alcohol use disorder, and current substance use disorders (i.e., cannabis, opioids, and stimulants), SF-36 PCS, ISI, cancer, CHF, PVD, CVD, diabetes, liver disease, renal disease, CPD, and gastroenteritis.
We focused our analyses on medications reported for at least 25 individuals in the cohort. Using the Epocrates drug classification system, (Epocrates) medications were grouped broadly by type of condition typically treated (though many medications are used for numerous conditions) and then into classes of medications, each of which include numerous specific types of medications: 1) psychiatric medications, including antidepressants (atypicals, selective serotonin reuptake inhibitors (SSRIs), and serotonin and norepinephrine reuptake inhibitors (SNRIs)), insomnia medications, and other psychiatric medications; 2) cardiovascular medications, including statins, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs), blood pressure medications, beta-blockers, antithrombotics, and other cardiovascular medications/supplements; 3) pain medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, muscle relaxers, and non-opioid analgesics; 4) gastrointestinal (GI) medications, including proton pump inhibitors (PPIs), laxatives, and other GI medications; 5) supplemental vitamins, including vitamin D; 6) neurologic seizure medications; 7) pulmonary medications, including bronchodilators; 8) endocrine medications, including diabetes and thyroid medications; and, 9) urogenital medications, including those for sexual function. Specific medications analyzed included the following, which were the most commonly prescribed: trazadone, bupropion, atorvastatin, lisinopril, prazosin, gabapentin, metformin, levothyroxine, sildenafil, albuterol, omeprazole, and cholecalciferol.
2.5. Microbiome sample processing
Sample DNA was extracted as previously described from fecal samples using the PowerSoil DNA extraction kit (Cat. No. 12955–4, Qiagen, Valencia, CA, USA). Marker genes in isolated DNA were PCR-amplified using GoTaq Master Mix (Cat. No. M5133, Promega, Madison, WI, USA) as described in Caporaso et al. (2012) PCR products were cleaned and normalized using the SequalPrep Normalization Kit (Cat. No. A1051001, ThermoFisher, Waltham, MA, USA) following manufacturer's instructions. The normalized amplicon pool was sequenced on an Illumina MiSeq. All library preparation and sequencing were conducted at the University of Colorado Boulder BioFrontiers Next-Gen Sequencing core facility.
Sequencing data were processed using the Quantitative Insights Into Microbial Ecology program (QIIME2 v. 2019.10 (Bolyen et al., 2019)). The Deblur (Amir et al., 2017a) algorithm was used to denoise demultiplexed sequences. SATé-enabled phylogenetic placement (SEPP (Janssen et al., 2018)) analysis was performed to remove sequences that were not 75% similar to any record in the tree. Quality-filtered sequences were assigned taxonomic classification based on the silva_128 database (Quast et al., 2013). Samples that were shipped to the research facility and had taxa that are known to “bloom” during shipping were removed as previously described by Amir et al. (2017b). Additionally, taxa that have been identified to “bloom” with increased time spent at room temperature were also considered for removal (Bokulich et al., 2019). Details of how we performed and assessed “deblooming” analysis can be found in the supplemental materials (Appendix B; Fig. B1; Table B1) (Amir et al., 2017b). For α- and β-diversity and taxonomic evaluations, samples were rarefied to a level of 2535 sequences per sample. The α-diversity metrics assessed were observed species, Shannon diversity, and Faith's phylogenetic diversity (PD). These measures capture different aspects of the microbiota diversity of each sample: observed species represents the richness of different types of microbiota detected, Shannon diversity gives equal weight to evenness and richness, and PD takes into account phylogenetic relatedness. The β-diversity metrics assessed were unweighted and weighted UniFrac. Jaccard and Bray-Curtis distance metrics were used to infer functional information (MetaCyc pathways (Caspi et al., 2014)) using Picrust2 v2.3.0-b (Douglas et al., 2019). These metrics capture the diversity across samples (differences in the microbiota between samples). Weighted UniFrac and Bray-Curtis are quantitative measures (i.e., take into account abundance of the microbes/functional pathways present). Unweighted UniFrac and Jaccard are qualitative (i.e., take into account only presence/absence of the microbes/functional pathways).
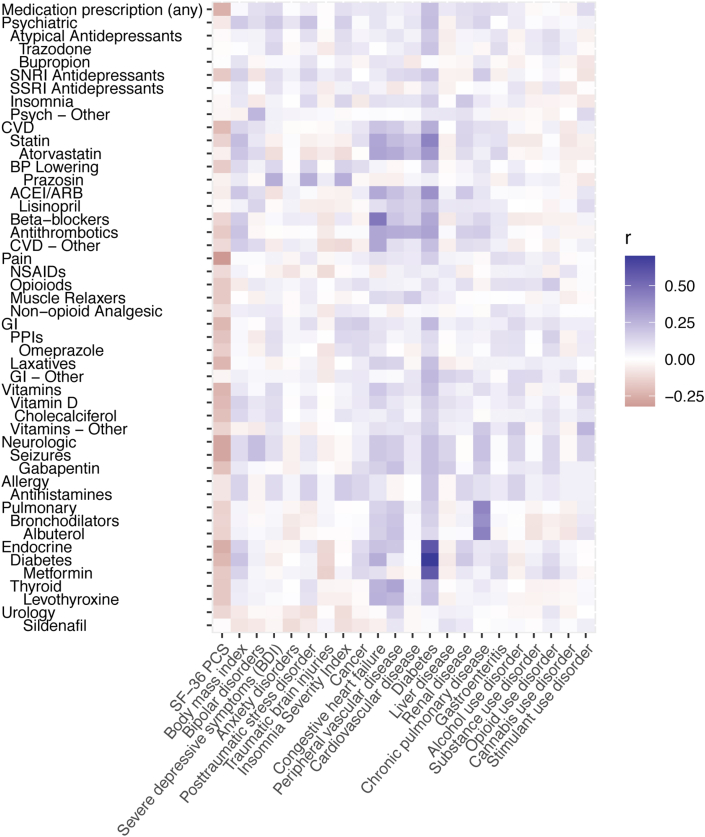
Fig. 1.
Correlation heat map of the associations between medications and health conditions and metrics. Prescription medications are shown on the y-axis, and health conditions and metrics are on the x-axis. The color indicates the strength of correlation with positive values shown in purple and negative values shown in red. Abbreviations: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; BDI, Beck Depression Inventory; BP, blood pressure; CVD, cardiovascular disease; GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs; PCS, Physical Health Composite Score; PPI, proton pump inhibitors; Psyc, psychiatric; SF-36, 36-Item Short Form Health Survey; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.6. Statistical analyses
We used Pearson's correlation coefficient to assess the strength of association between the health conditions and medications. We assessed the amount of variation in the gut microbiota composition explained by the four groups of predictors (described in detail above) using distance-based redundancy analyses of the unweighted and weighted UniFrac(Lozupone et al., 2011) distance metrics. In order to determine the strongest correlates of the gut microbiota composition, we used permutational multivariate analysis of variance (PERMANOVA) using the vegan package (Oksanen et al., 2018) and a stepwise model building tool for constrained ordination methods (ordistep) (Douglas et al., 2019) based on adjusted R2 using the UniFrac distance metrics. We applied the same methods to the inferred gut microbiota MetaCyc functional pathways (Caspi et al., 2014), quantifying the pathway abundance using Jaccard and Bray-Curtis distance metrics. In order to determine the strongest correlates of the three measures of the gut microbiota α-diversity, we used a similar model building tool for linear regression models, LEAPS (Miller, 1984). Analysis of Compositions of Microbiomes (ANCOM) (Mandal et al., 2015) was used to assess whether any gut microbiota taxa were differentially abundant with these predictors. For taxonomic analyses, we adjusted for multiple testing using a Benjamini-Hochberg (Benjamini and Hochberg, 1995) False Discovery Rate (FDR) with a two-tailed statistical significance threshold of 0.05, which was likewise used for two-tailed p-values in analyses of α- and β-diversity. Analyses were performed with QIIME2 v. 2019.10 (Bolyen et al., 2019), the open source statistical package R v.3.5.1 (R Core Team, 2018) and python v3.6.7 (Van Rossum and Drake, 2009).
3. Results
Among the 331 Veterans in the US-VMP cohort, over two-thirds experienced at least one deployment, and many of the Veterans (42%) reported at least one period of homelessness (Table 1). There was high prevalence of health conditions with: 1) over 35% having self-reported clinical insomnia; 2) almost 50% having a lifetime diagnosis of PTSD; and, 3) over three-quarters reporting at least one TBI of any severity (mild, moderate or severe). Approximately 12% of Veterans reported current alcohol use disorder and 7.6% reported some type of other current substance use disorder, such as cannabis, opioids, or stimulants.
3.1. The majority of veterans had multiple medication prescriptions
Three-quarters of the US-VMP cohort had at least one current medication prescription noted within the EMR. These individuals had an average of six prescriptions for unique medications (median = 4, IQR = 2–8). Psychiatric medications were the most prevalent class, with antidepressants being the most common sub-class (Table 2); 137 individuals (41.4% of the overall cohort) had an active prescription for antidepressants, most commonly atypical antidepressants (specifically, trazadone or bupropion), SSRIs, or SNRIs. CVD medications were also common (38.1% of the cohort), such as statins, ACEIs/ARBs, beta-blockers, and other blood pressure medications. Almost one-third (32.6%) of the cohort had some type of pain medication, with 13% taking prescription opioids.
Table 2.
Medication prescriptions among the US-VMP cohort.
| Medication class |
Specific medication |
N (%) |
|
|---|---|---|---|
| 331 | |||
| Psychiatric | 152 (45.9) | ||
| Antidepressants | 137 (41.4) | ||
| Atypical antidepressants | 72 (21.8) | ||
| Trazodone | 40 (12.1) | ||
| Bupropion | 29 (8.8) | ||
| SSRI antidepressants | 58 (17.5) | ||
| SNRI antidepressants | 37 (11.2) | ||
| Insomnia | 25 (7.6) | ||
| Other | 33 (10) | ||
| CVD | 126 (38.1) | ||
| Statins | 56 (16.9) | ||
| Atorvastatin | 40 (12.1) | ||
| ACEI/ARBs | 44 (13.3) | ||
| Lisinopril | 25 (7.6) | ||
| Blood pressure | 54 (16.3) | ||
| Prazosin | 26 (7.9) | ||
| Beta-blockers | 34 (10.3) | ||
| Antithrombotics | 29 (8.8) | ||
| Other | 41 (12.4) | ||
| Pain management | 108 (32.6) | ||
| NSAIDs | 43 (13) | ||
| Opioids | 43 (13) | ||
| Muscle relaxers | 39 (11.8) | ||
| Non-opioid analgesic | 30 (9.1) | ||
| Gastrointestinal (GI) | 83 (25.1) | ||
| PPI | 50 (15.1) | ||
| Omeprazole | 38 (11.5) | ||
| Laxatives | 30 (9.1) | ||
| GI - Other | 27 (8.2) | ||
| Supplemental vitamins | 83 (25.1) | ||
| Vitamin D | 54 (16.3) | ||
| Cholecalciferol | 44 (13.3) | ||
| Vitamins - Other | 50 (15.1) | ||
| Neurologic | Seizures | 59 (17.8) | |
| Gabapentin | 30 (9.1) | ||
| Pulmonary | 54 (16.3) | ||
| Bronchodilators | 48 (14.5) | ||
| Albuterol | 30 (9.1) | ||
| Endocrine | 52 (15.7) | ||
| Diabetes | Metformin | 26 (7.9) | |
| Thyroid | Levothyroxine | 24 (7.3) | |
| Urology | 50 (15.1) | ||
| Sexual function | Sildenafil | 28 (8.5) | |
| Other medications | 79 (23.9) | ||
Footnote: Medications ordered for Veterans covering the period within two weeks of their gut microbiota sample collection. Medications are grouped into broad disease categories that reflect their primary use, and then further grouped into general classes that include numerous specific medications. When medication classes were dominated by one specific medication (>80%), we report the numbers for the specific medication rather than the class and perform subsequent analyses at the specific medication level. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; GI, gastrointestinal; NSAID, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; US-VMP, United States Veteran Microbiome Project.
We examined the correlation between health conditions and medications (Fig. 1). The overall health metric of the SF-36 PCS correlated negatively with prescription medications (r = −0.25), as would be expected. The most strongly correlated disease-medication pair was diabetes and metformin (r = 0.58). Many individuals with diabetes were also prescribed CVD medications, particularly statins (r = 0.43). CHF correlated highly with beta-blockers (r = 0.47), and CPD with albuterol (r = 0.43).
3.1.1. The gut microbiota taxonomic compositions were correlated with numerous health conditions and medications
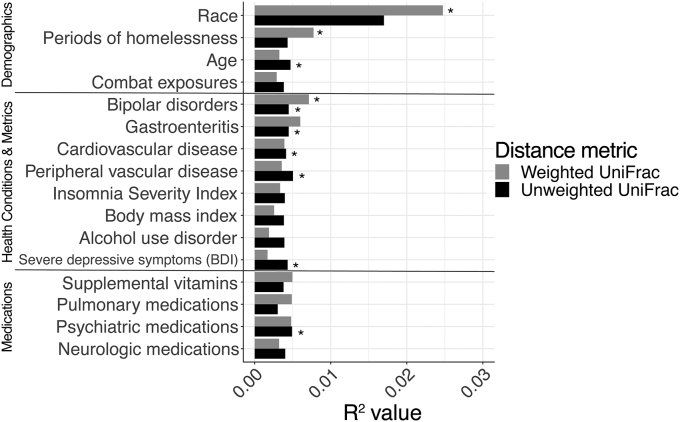
We evaluated associations between the overall gut microbiota composition, Veteran characteristics, and medications using two phylogenetic measures of composition that take into account the types of microbiota present or absent in a sample (unweighted UniFrac) and the relative abundance of the microbiota present (weighted UniFrac) (Lozupone et al., 2011). We identified the strongest correlates of the gut microbiome composition by examining each exposure independently using PERMANOVA (Fig. 2) and also through a stepwise constrained ordination approach (Table 3, Fig. 3) that identifies important sets of predictors. In the PERMANOVA analysis, race (P = 0.01), homeless frequency (P = 0.02), and bipolar disorders (P = 0.03) were correlated with weighted UniFrac; age (P = 0.02), bipolar disorders (P = 0.04), gastroenteritis (P = 0.03), CVD (P = 0.047), PVD (P = 0.02), BDI (P = 0.04), and psychiatric medications (P = 0.01) were most correlated with unweighted UniFrac (Fig. 2).
Fig. 2.
The variables most correlated with the gut microbial community composition. Bar graph showing univariate associations between demographics, health conditions/metrics and medications and the gut microbiota community composition, as quantified by weighted (light grey) and unweighted (black) UniFrac β-diversity indices. The variables shown had the highest levels of correlation with β-diversity determined by PERMANOVA across all of the examined variables, all with P < 0.1; asterisks represent variables showing nominally significant associations with P < 0.05. Abbreviatons: BDI, Beck Depression Inventory; PERMANOVA, permutational analysis of variance.
Table 3.
The subsets of variables demonstrating the strongest correlation with the gut microbial community composition.
| Distance metric | Exposure | Variable | P |
|---|---|---|---|
| Unweighted UniFrac | Demographics | Age | 0.014 |
| Health conditions/metrics | PVD | 0.018 | |
| Bipolar disorders | 0.038 | ||
| Stimulant use disorder | 0.046 | ||
| Gastroenteritis | 0.050 | ||
| Severe depressive symptoms (BDI) | 0.050 | ||
| Medication class | Beta blockers | 0.008 | |
| SNRI antidepressants | 0.014 | ||
| Diabetes medications | 0.028 | ||
| Weighted UniFrac | Demographics | Sex | 0.002 |
| Race | 0.012 | ||
| Health conditions/metrics | Stimulant use disorder | 0.022 | |
| Bipolar disorders | 0.028 | ||
| Opioid use disorder | 0.046 | ||
| Medication class | Other GI medications | 0.010 | |
| PPIs | 0.050 | ||
| Specific medications | Omeprazole | 0.032 |
Footnote. Microbial composition as measured by unweighted and weighted UniFrac distance metrics. The exposures were grouped into four categories: 1) demographics; 2) health conditions and metrics; 3) medication classes; and 4) specific medications. P-values were generated by a stepwise contrained ordination model. Abbreviations: BDI, Beck Depression Inventory; GI, gastrointestinal; PPI, proton pump inhibitor; PVD; peripheral vascular disease; SNRI, serotonin and norepinephrine reuptake inhibitor.
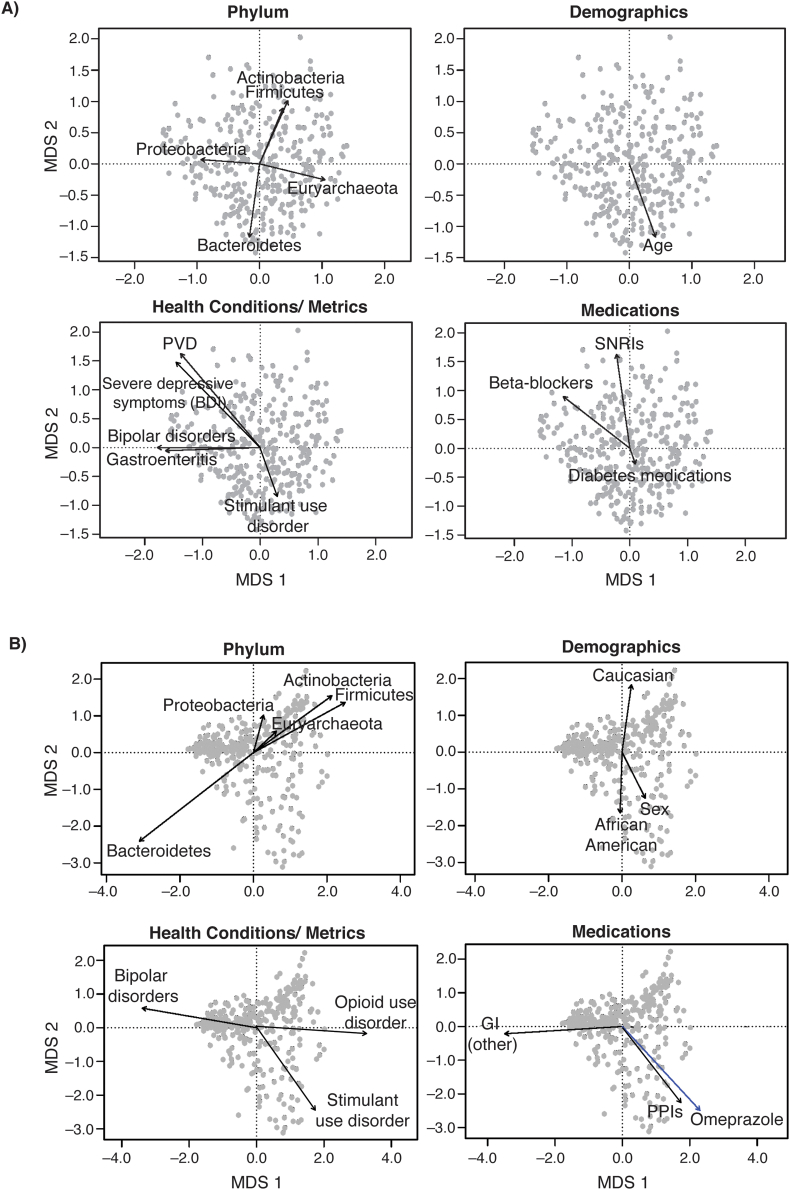
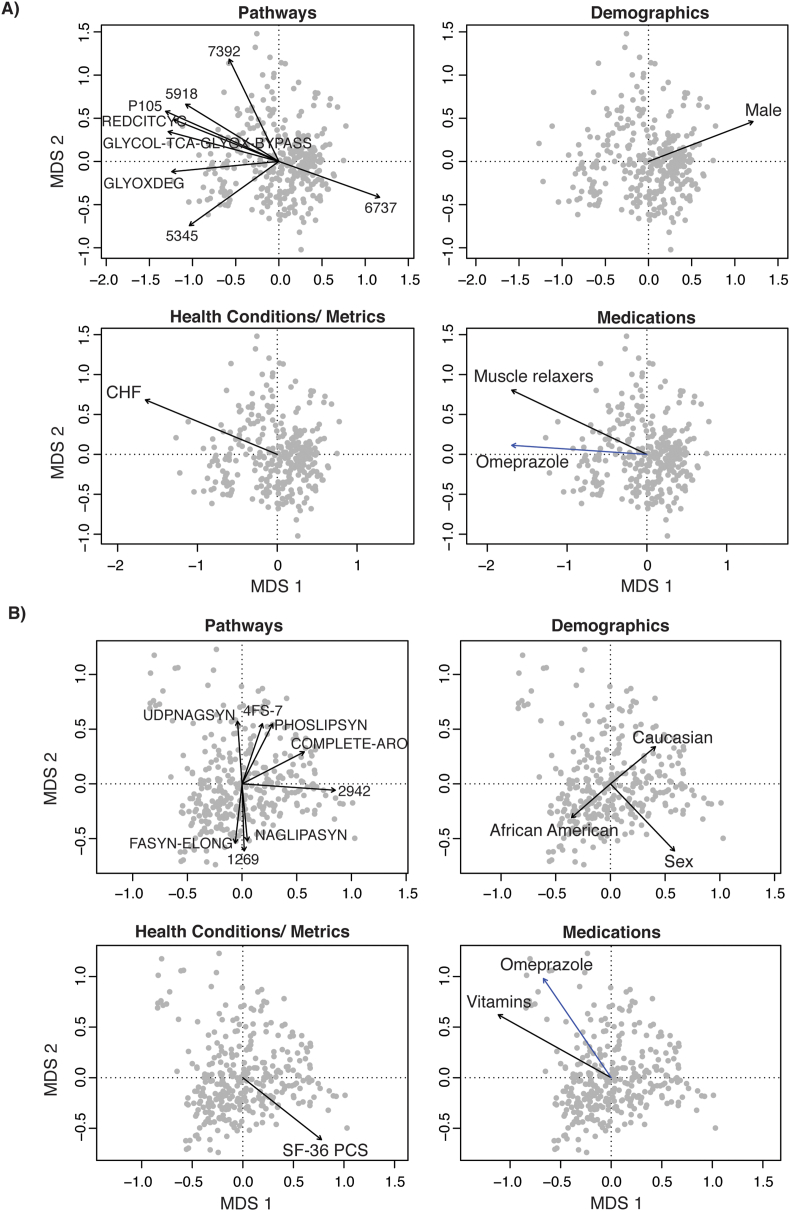
Fig. 3.
Biplots of the strongest correlates of the gut microbial community composition. These biplots show the results of the analysis described in Table 3 with each dot representing a gut microbiota sample from one individual. Dots that cluster together have more similar gut microbiota composition with arrows pointing in a similar direction characterized by similar types of microbiota. The gut microbiota composition was quantified based on a) unweighted and b) weighted UniFrac distance metrics. The strength of correlations between variables and the gut microbial community composition was determined by a stepwise contrained ordination model. The arrows represent the direction and strength of association with the strongest correlates of these measures of composition in terms of: 1) phylum-level taxonomy; 2) demographics; 3) health conditions and metrics; and 4) medication classes and specific medications (shown in blue). Unweighted UniFrac did not show any statistically significant correlation with specific medications. Abbreviations: BDI, Beck Depression Inventory; GI, gastrointestinal; MDS, multidimensional scaling; PPI, proton pump inhibitor; PVD, peripheral vascular disease; SNRI, serotonin and norepinephrine reuptake inhibitor. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
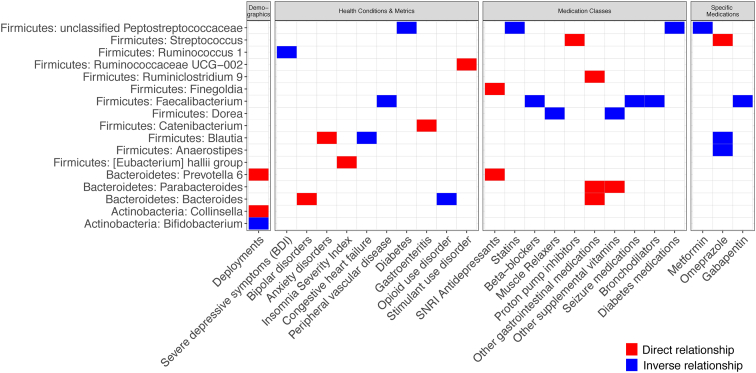
In the stepwise constrained ordination analysis, data were examined for both unweighted and weighted UniFrac in four groups: 1) demographics; 2) health conditions and metrics; 3) medication classes; and, 4) specific medications. In the demographics group, we found that age (P = 0.01) correlated most strongly with the unweighted UniFrac distance metric (Table 3, Fig. 3A). Race (P = 0.01) and sex (P = 0.002) correlated most strongly with the weighted UniFrac distance metric (Table 3, Fig. 3B). Among the health conditions and metrics, we found that PVD (P = 0.02), bipolar disorders (P = 0.04), stimulant use disorder (P = 0.05), gastroenteritis (P = 0.05), and severe depressive symptoms (based on BDI; P = 0.05) correlated with unweighted UniFrac (Table 3, Fig. 3A). Bipolar (P = 0.03) and stimulant use (P = 0.02) disorders also correlated significantly with weighted UniFrac, as did opioid use disorder (P = 0.05) (Table 3, Fig. 3B). Among medication classes and specific medications, beta-blockers (P = 0.01), SNRI antidepressants (P = 0.01), and diabetes medications (P = 0.03) correlated with unweighted UniFrac (Table 3, Fig. 3A). Other GI medications (P = 0.01), PPIs (P = 0.05) and omeprazole, which is a PPI (P = 0.03), correlated with weighted UniFrac (Table 3, Fig. 3B). We were able to identify specific taxa that differed between groups for many of the Veteran demographic characteristics and health metrics/conditions examined, including deployments, severe depressive symptoms, bipolar disorders, anxiety disorders, clinical insomnia, CHF, PVD, diabetes, gastroenteritis, opioid use disorder, and stimulant use disorder (Fig. 4). Likewise, both classes of and specific medications were associated with significant shifts in some taxa, including diabetes medications, SNRI antidepressants, statins, beta-blockers, muscle relaxers, PPIs, other GI medications (which included digestive enzymes and heart burn medications, most commonly ranitidine), other supplemental vitamins, seizure medications, bronchodilators, metformin, omeprazole, and gabapentin.
Fig. 4.
Taxonomic differences based on characteristics of US-VMP Veterans. These plots show the taxa that differed significantly (FDR < 0.05) with demographics, health conditions and metrics, medication classes, and specific medications. The color shows whether there is a direct (red) or inverse (blue) relationship between the exposure and taxonomic relative abundance determined by ANCOM. Abbreviations: ANCOM, analysis of composition of microbiomes; BDI, Beck Depression Inventory; FDR, false discovery rate; SNRI, serotonin and norepinephrine reuptake inhibitor; UCG, unclassified genus. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.1.2. The overall gut microbiota functional composition was correlated with numerous health conditions and medications
We used a similar approach in order to identify the strongest correlates of the gut microbiome functional pathways (Table 4; Fig. 5), using Jaccard and Bray-Curtis distance metrics based on functional information inferred using Phylogentic investigation of Communities by reconstruction of unobserved states v2 (PiCrust2) (Douglas et al., 2020), which are comparable to unweighted and weighted UniFrac, respectively. Sex (P = 0.002), CHF (P = 0.03), muscle relaxers (P = 0.01), and omeprazole (P = 0.02) (a PPI) were associated with differences in the types of functional pathways present or absent (Jaccard distance metric). Sex (P = 0.002) and omeprazole (P = 0.006) were likewise associated with the abundance of functional pathways (Bray-Curtis distance metric), as were race (P = 0.04), SF-36 PCS (P = 0.02), and other supplemental vitamins (P = 0.002). As shown in Fig. 5, samples from individuals with CHF or taking muscle relaxers are clustered together with functions related to the TCA cycle.
Table 4.
Variables most correlated with the gut microbiota functional pathways inferred using PiCrust2.
| Distance metric | Exposure | Variable | P |
|---|---|---|---|
| Jaccard | Demographics | Sex | 0.002 |
| Health conditions/metrics | Congestive heart failure | 0.026 | |
| Medication classes | Muscle relaxers | 0.008 | |
| Specific medications | Omeprazole | 0.016 | |
| Bray-Curtis | Demographics | Sex | 0.002 |
| Race | 0.038 | ||
| Health conditions/metrics | SF-36 PCS | 0.022 | |
| Medication classes | Other supplemental vitamins | 0.002 | |
| Specific medications | Omeprazole | 0.006 |
Footnote. β-diversity metrics Jaccard and Bray-Curtis were utilized with PiCrust2 to infer functional pathways most correlated to variables. Variables were grouped into four categories: 1) demographics; 2) health conditions; 3) medication classes; and, 4) specific medications. Abbreviations: PiCrust2, Phylogentic investigation of Communities by reconstruction of unobserved states v2; SF-36 PCS, 36-Item Short Form Health Survey Physical Health Composite Score.
Fig. 5.
Biplots of the strongest correlates of the gut microbial functional pathways. These biplots show the results of the same analysis as shown in Table 4 with each dot representing a gut microbiota sample from one individual. Dots that cluster together have more similar gut microbiota functional pathways with arrows pointing in a similar direction characterized by similar functions. The gut microbiota functional pathways were quantified based on: a) Jaccard and b) Bray-Curtis distance metrics. The strength of correlations between variables and the functional pathways was determined by a stepwise constrained ordination model. The arrows represent the direction and strength of association with the strongest correlates of these measures of composition in terms of: 1) functional pathways; 2) demographics; 3) health conditions and metrics; and 4) medication classes and specific medications (shown in blue). Functional pathway labels and pathway details can be found in Appendix C; Table C1. Abbreviations: CHF, congestive heart failure; SF-36 PCS, 36-Item Short Form Health Survey Physical Health Composite Score. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.1.3. The gut microbiota α-diversity metrics were correlated with numerous health conditions and medications
We also examined α-diversity of the gut microbiota taxa (Appendix C; Table C2). Most of the correlates of α-diversity showed an inverse relationship, including CVD (P = 0.04), PVD (P = 0.04), trazadone (P = 0.04), and albuterol (P = 0.02). However, this was not always the case; other supplemental vitamins (P = 0.03) (including all vitamins except for Vitamin D) and other CVD medications/supplements (P = 0.03) (e.g., aspirin, fish oil, etc.) were associated with higher α-diversity.
4. Discussion
In this study of Veterans with military-related exposures and associated health conditions, we found a number of associations with the microbial taxonomic composition of the gut, including bipolar disorders, severe depressive symptoms based on the BDI, PVD, gastroenteritis, opioid and stimulant use disorders, beta-blockers, SNRI antidepressants, diabetes medications, and GI medications including PPIs, and omeprazole, which is a specific type of PPI. Gut microbiota functional composition was associated with overall perceived physical health (SF-36 PCS), CHF, use of muscle relaxers, omeprazole, and supplemental vitamin use. Military service-related characteristics and conditions, such as deployment history, combat exposure, PTSD, and number of TBIs, were not among the strongest correlates of overall gut microbiota metrics, such as α- or β-diversity. However, there were several notable differences in taxonomic relative abundance associated with deployments, as well as with many of the health-related metrics and disorders that are highly prevalent among Veterans, including insomnia, bipolar and anxiety disorders, and depressive symptoms.
We observed that age, race, and sex correlated with the gut microbiota composition, as has been observed in many other population studies, including the Human Microbiome Project (Jackson et al., 2018; Falony et al., 2016; Human Microbiome Project, 2012). Mental health conditions were highly prevalent among the US-VMP cohort, and we found that bipolar disorders were associated with bacterial community structure. Previous findings on the community composition of the gut microbiota and bipolar disorders are mixed, with some showing community level differences in individuals with bipolar disorders (Coello et al., 2019; Evans et al., 2017) and others finding no such differences (Vinberg et al., 2019; Hu et al., 2019; Painold et al., 2019). There were many taxonomic differences cited by previous studies associated with bipolar disorders, specifically, increased Actinobacteria (Painold et al., 2019), increased Flavonifractor (Coello et al., 2019), and inconsistent associations with Faecalibacterium (Coello et al., 2019; Evans et al., 2017). Our study found that Bacteroides was increased in individuals with bipolar disorders, but this is somewhat difficult to interpret as this is a very common member of the human gut microbiota with wide-ranging correlations. Some prior studies separated bipolar disorders into the clinical subtypes of I and II (Coello et al., 2019; Evans et al., 2017; Aizawa et al., 2016), or differentiated bipolar individuals based on severity of current symptoms (Painold et al., 2019) or pharmacological intervention (Flowers et al., 2017, 2019), which we did not. Additionally, we found decreased Ruminococcus 1 in participants that suffered from severe depressive symptoms based on the BDI. One study that recruited 115 participants experiencing bipolar disorders reported a lower abundance of the family Ruminococcaceae compared to control participants (Evans et al., 2017), though the consistency of this result is difficult to assess as this is a broad and diverse family.
We showed an increase in Blautia genus of the Firmicutes phylum in individuals with anxiety disorders as determined by the SCID-5-I/P W/PSY SCREEN (First et al., 2016). Gacias et al. showed that Blautia was associated with increases in anxiety-like behavior (social avoidance) in mice (Gacias et al., 2016). Blautia has also been correlated with obesity and type 2 diabetes. (Ozato et al., 2019; Egshatyan et al., 2015). However, other work has suggested that Blautia may have protective effects in some contexts (Wong et al., 2016; Jenq et al., 2015), and a recent study found an inverse association in men between Blautia abundance and anxiety (Taylor et al., 2019) measured by the Depression, Anxiety, and Stress Scale (Lovibond and Lovibond, 1995) – although this was a pilot study, and the findings could be spurious due to the large number of comparisons made.
Many of the physical and mental health conditions correlated with the gut microbiota in this study, such as gastroenteritis, CVD, anxiety disorders, and affective disorders, have been associated with underlying inflammation (Dickerson et al., 2016; Goldsmith et al., 2016; Vivinus-Nébot et al., 2014; Namazi et al., 2018; Hamjane et al., 2020). It is also worth noting that among military Veterans there is evidence linking exposure to traumatic stressors and subsequent diagnoses of mental health conditions (e.g., PTSD) to the development of future physical health conditions (e.g., CVD) (Dyball et al., 2019). Increased permeability of the GI lumen, commonly referred to as “leaky gut”, which also has been shown to lead to exaggerated inflammation (Camilleri, 2019), has been identified in those suffering from affective, GI (e.g., inflammatory bowel disorders [IBD]), and cardiovascular disorders (Ait-Belgnaoui et al., 2012; Bischoff et al., 2014; Novakovic et al., 2020). Similarly, it is common for those GI and cardiovascular disorders to have comorbid affective disorders (Wilmes et al., 2021; Cohen et al., 2015). Given the high rates of inflammatory disorders among Veterans (Tanielian et al., 2008; Breland et al., 2017), this is an important population within which to study the microbiota-gut-brain axis and physical and mental health. We predeominantly focused on lifetime diagnoses of mental health conditions because we were not adequately powered to examine current mental health conditions, or to compare short-term versus long-term conditions. However, this is an important area for future research, as there is some evidence that prolonged exposure to mental health conditions can lead to enhanced immunological and inflammatory changes (Dickerson et al., 2016; Goldsmith et al., 2016).
Number of deployments showed a positive relationship with Prevotella 6 and Collinsella and a negative relationship with Bifidobacterium. A deployment is a temporary relocation of a military service member, typically to an international location, and can vary in length from three to fifteen months and does not necessarily include combat. Relocation inherently involves the exposure to different environmental microbes (Barberán et al., 2015), changes in diet (Turnbaugh et al., 2009b), novel living quarters (Lax et al., 2014), and novel cohabitants (Song et al., 2013), all of which have been shown to influence the gut microbiome. Prevotella is a highly diverse genus with numerous clades, many of which are typically underrepresented in Westernized countries (Tett et al., 2019). Veterans with multiple deployments may have increased exposure to these types of microbes that are less common among Western populations, which could explain this finding. Collinsella has been associated with low dietary fiber intake and high circulating insulin (Gomez-Arango et al., 2018). There is high prevalence of insulin resistance and diabetes among Veterans (Liu et al., 2017), and the US-VMP cohort generally has a homogeneous, Western-style diet, low in dietary fiber, and high in animal foods, refined carbohydrates, and added sugars (Flowers et al., 2017). A reduction in dietary fiber intake could result in a general switch in the gut away from short-chain fatty acid (SCFA) production and toward more lactate fermentation processes (Pylkas et al., 2005). Bifidobacterium has many known probiotic strains and has been largely considered a genus comprised of beneficial microbes (Azad et al., 2018). Bifidobacterium species are generally known for their ability to catabolize complex carbohydrates and produce the necessary substrates for the Bif Shunt metabolic pathway (O'Callaghan and van Sinderen, 2016). The Bif Shunt pathway breaks down phosphates to generate adenosine triphosphate (ATP) and produces SCFAs as end products (O'Callaghan and van Sinderen, 2016). SCFAs are important signaling molecules, and they can influence systemic inflammation by reducing inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor alpha (TNF-⍺) and increasing anti-inflammatory cytokines such as IL-10 (Vinolo et al., 2011). However, they have also been associated with obesity and may reflect an increased capacity to harvest energy from food (Gentile and Weir, 2018).
Our results show that the most common medications among Veterans in the US-VMP were comparable to adults in the US as a whole, antidepressants and CVD-related medications, including lipid-lowering medications, beta-blockers, and ACEIs (Hales et al., 2019). The prevalence of antidepressants and other mental health medication prescriptions was higher in the US-VMP than in the US generally, which is likely due to recruitment of Veterans that were actively seeking healthcare from a VHA medical center. It is important to note that some of the commonly prescribed medications in this cohort were classified as treatment for a specific condition based on the classification system from Epocrates, (Epocrates) but that in this population, it is likely that certain medications were being used off-label to address other conditions (Leslie et al., 2009). For example, 18% of the cohort was prescribed “seizure medications,” but drugs within this class of neurological medications (e.g., gabapentin) are often used in the VHA at a lower dosage as mood stabilizers or for pain control (Ketter et al., 2003). Another example is prazosin, which in Fig. 1 was classified as a blood pressure lowering medication, but is frequently used among Veterans to treat PTSD-related nightmares (Raskind et al., 2018).
We found that PPIs, particularly omeprazole, as well as other GI medications (which included digestive enzymes and heart burn medications, most commonly ranitidine) showed a strong correlation with overall gut microbiota composition and functional pathways. This is not surprising, as these medications target the gut function. PPIs increase the gut pH, which alters the types of microbes that can survive and grow (Duncan et al., 2009). This finding is consistent with prior literature showing that these commonly prescribed medications are associated with alterations in the gut microbiota, such as lower microbial diversity and increases in potentially commensal microbes, specifically the genera of Enterococcus, Streptococcus, Staphylococcus, and the potentially pathogenic species Escherichia coli. We likewise saw an association between increased Streptococcus and PPIs (Imhann et al., 2016; Jackson et al., 2016). These alterations in the gut microbiota composition are likely not entirely benign, as use of PPIs is also associated with an increased risk of enteric infections, including C. difficile (Janarthanan et al., 2012), which carries a high mortality risk (Oake et al., 2010) and is known to respond well to restoration of a healthy microbiota composition through fecal microbiota transplant therapy (Youngster et al., 2014). Moreover, these findings are particulary relevant in light of recent wide-spread PPI de-implementation strategies, based on findings suggesting that many PPI prescriptions are inappropriate (Orelio et al., 2021).
In some cases, it was difficult to isolate whether a condition or its treatment was more strongly associated with the gut microbiota. For example, we saw that PVD, SNRIs, and beta-blockers were associated with differences in the gut microbiota composition. There were only nine individuals with PVD in the cohort; almost half of them were on beta-blockers, and one-third were on SNRIs, making it difficult to tease apart these effects. This is an important area for further research as PVD showed some of the most pervasive associations with gut microbiota characteristics, including with ⍺-diversity, overall gut microbiota community composition and relative abundance of Faecalibacterium. In a recent study of atherosclerotic CVD, disease status was more strongly associated with the gut microbiota than CVD medication (Jie et al., 2017). However, the relationships among disease, medications, and the gut microbiota are complex, and the efficacy of some medications may be dependent on interactions with the gut microbiota. This is thought to be the case with metformin, the most frequently prescribed medication for type 2 diabetes (Vallianou et al., 2019). Our results confirm those of other studies that diabetes medications are associated with alterations in the overall gut microbiota (Jackson et al., 2018; Vallianou et al., 2019).
This study has some notable limitations and strengths. The participants were mainly recruited through the VA medical facilities in Eastern Colorado, and do not fully represent the Veteran population as a whole, particularly younger, healthier Veterans who are less likely to regularly use VA health services. Fecal samples were collected in-person for some of the participants, while other participants collected samples at home and shipped the samples to the study center. Sample transit time (time between when the sample was collected and when the sample was placed into an ultra-low temperature freezer at the research facility) was variable and conditions before and during shipment may have varied among samples. We tried to address this issue using previously applied deblooming methods, but it is likely impossible to fully correct for this issue. The samples were sequenced based on the 16S rRNA gene, which limits our ability to accurately classify species-level taxonomy or to understand gut microbiota functions. We examined gut microbiota functional pathways that were inferred from taxonomy, but these analyses are preliminary in nature since microbiota functions would be more accurately assessed through shotgun metagenomic sequencing of samples, which we hope to do in the future. A record of a medication prescription in the VA EMR is indicative of a provider order, but it does not necessarily mean the Veteran has taken the medication. However, medication adherence levels tend to be better among those getting care at the VA than outside the VA (Gaffney et al., 2020). The medications recorded in the VA EMR may underestimate the true prevalence of medication use among these individuals since Veterans may get some of their medications outside the VA, particularly for medications that are available over-the-counter, such as those for heartburn. It is difficult to isolate the effects of medication from the effects of health conditions, and medications may have interaction effects, which are difficult to estimate without larger sample sizes. It is also inherently challenging to classify medications into their predominant therapeutic indications, as numerous medications can be used to treat different conditions (Raskind et al., 2018). We chose to use Epocrates classification system, which is one of the most commonly used sources of drug information by physicians that does not require a subscription (Apidi et al., 2017). This study has unique strengths as well, including the validated measures of health conditions and substance use disorders and the comprehensive information about medical conditions and medications available through the VA EMRs. As the recruited population in the US-VMP increases over time, we will be better powered to more thoroughly characterize the associations between individual characteristics (i.e., current vs lifetime psychological conditions) and the gut microbiota of US Veterans.
5. Conclusions
In this study, we leveraged the extensive information collected in the VA EMR in order to examine the relationships of the gut microbiota to demographic factors, common health-related conditions, military-specific exposures, and medications. This work will help to inform decisions about which types of information are important to collect and consider in microbiome studies, and it adds to the growing body of literature supporting associations between gut microbiota features and numerous health conditions, particularly those that tend to be characterized by underlying inflammation, such as gastroenteritis and affective disorders.
Data availability
The VHA has rules about data security that aim to protect the privacy of Veteran health information. Thus, the personal health data used for this study cannot be made publicly available. Please contact the authors for further information or collaboration.
Disclaimer
The views, opinions, and/or findings contained in this article are those of the author(s) and should not be construed as an official Department of Defense or Veterans Affairs position, policy, or decision unless so designated by other documentation.
Funding
Funding for this project was provided by the Department of Veteran Affairs, Rocky Mountain Mental Illness Research Education and Clinical Center.
Declaration of competing interest
C.A.L. serves on the Scientific Advisory Board of Immodulon Therapeutics, Ltd. and is a member of the faculty of the Integrative Psychiatry Institute, Boulder, Colorado. The remaining authors have no conflicts of interest to report.
Acknowledgements
We would like to thank Jared D. Heinze for performing the molecular processing and assistance in the preparation of the samples used in this manuscript.
Appendix A.
Table A.1.
International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10) codes for health conditions
| Health condition | ICD-9 code | ICD-10 code |
|---|---|---|
| Cancer | 140-165, 170–195, 200–208, 230.3, 230.4, 231.2, 233.0, 233.2, 233.4, 233.6, 273.0, 273.3, V10.05, V10.06, V10.11, V10.3, V10.42, V10.46 | C00–C26, C30–C34, C37–C41, C43, C45–C58, C60–C76, C81–C86, C88, C90–C96, D01.0, D01.1, D01.2, D02.2, D03, D05, D07.0, D07.5, D45, D47.Z9, D89.0, Z85.038, Z85.048, Z85.118, Z85.3, Z85.42, Z85.46 |
| Congestive heart failure (CHF) | 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.13, 404.91, 404.93, 425, 428, 429.3 | A18.84, I09.81, I11.0, I13.0, I13.2, I42, I43, I50, I51.7 |
| Peripheral vascular disease (PVD) | 093.0, 437.3, 440–442, 443.1, 443.2, 443.8, 443.9, 447.1, 557.1, 557.9, 785.4, V43.4 | A52.01, E08.51, E08.52, E09.51, E09.52, E10.51, E10.52, E11.51, E11.52, E13.51, E13.52, I67.0, I67.1, I70–I72, I73.01, I73.1, I73.8, I73.9, I77.1, I77.71, I77.72, I77.73, I77.74, I77.79, 179, I96, K55.1, K55.8, K55.9, Z95.820, Z95.828 |
| Cardiovascular disease (CVD) | 362.34, 430–438, 781.4, 784.3, 997.0, 410, 412 | G03.8, G45, G46, G97.0, G97.2, G97.3, G97.8, H34.0, I60–I63, I65–I69, I97.81, I97.82, R295, R47.01, I21, I22, I25.2 |
| Diabetes | 249, 250, 357.2, 362.01, 362.02, 362.03, 362.04, 362.05, 362.06, 366.41 | E11.65, E13.2, E13.31, E13.32, E13.33, E13.34, E13.351, E13.359, E13.36, E13.39, E13.4, E13.5, E13.610, E13.65, E13.69, E13.8, E13.9 |
| Liver disease | 571.2, 571.5, 572.2, 572.4, 567.23, 589.5, 589.51, 589.59, 456.0, 456.2, 456.20, 456.21 | K7030, K74.0, K74.60, K74.69, K72.90, K76.7, K65.2, R18.0, R18.8, I85.01, I85.11, I85.10 |
| Renal disease | 016.0, 095.4, 223.0, 236.91, 271.4, 274.10, 283.11, 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 580–582, 583.1, 583.2, 583.4, 583.6, 583.7, 584–588, 591, 753.12–753.17, 753.19, 753.2, 794.4, V42.0, V45.1, V56 | A18.11, A52.75, B52.0, D30.0, D41.0, D41.1, D41.2, D59.3, E08.2, E09.2, E74.8, I12.0, I13.11, M10.3, N00–N04, N05.2. N05.3, N05.4, NO5.5, N05.9, N06.2, N06.3, N06.4, N06.5, N07.2, N07.3, N07.4, N07.5, N08, N13.1, N13.2, N13.3, N17–N19, N25, N26.1, N26.9, Q61.02, Q61.1-Q61.5, Q61.8, Q62.0-Q62.3, R94.4, Z48.22, Z49, Z91.15, Z94.0, Z99.2 |
| Chronic pulmonary disease (CPD) | 415.0, 416.8, 416.9, 490–496, 500–505, 506.4, 508.1, 508.8 | J47, J60-J67, J68.4, J70.1-J70.4, J70.8 |
| Gastroenteritis | 558, 558.1, 558.3, 558.41 | K52, K52.0, K52.2, K52.21, K52.22, K52.29, K52.81, K52.9, A09 |
Footnote: CHF, congestive heart failure; ICD, International Classification of Diseases; PVD, peripheral vascular disease; CVD, cardiovascular disease; CPD, chronic pulmonary disease.
Appendix B. Supplemental Methods
Bloom analysis for US Veteran Microbiome Project (VMP)
Our sampling procedures allowed participants to provide samples in one of two ways: 1) participants could provide samples during their in-person visit; or 2) participants could take a sampling kit to their home to collect their sample and ship the sample back to the research facility. Although the participants were provided with an ice pack and instructed to ship the sample back to the research facility with the ice pack, adherence to this protocol was not always followed. Therefore, samples shipped back to the research facility had the potential to be above freezing temperature for extended periods of time. In contrast, samples collected by participants that elected to provide their sample during the in-person visit were immediately frozen and stored at −80 °C.
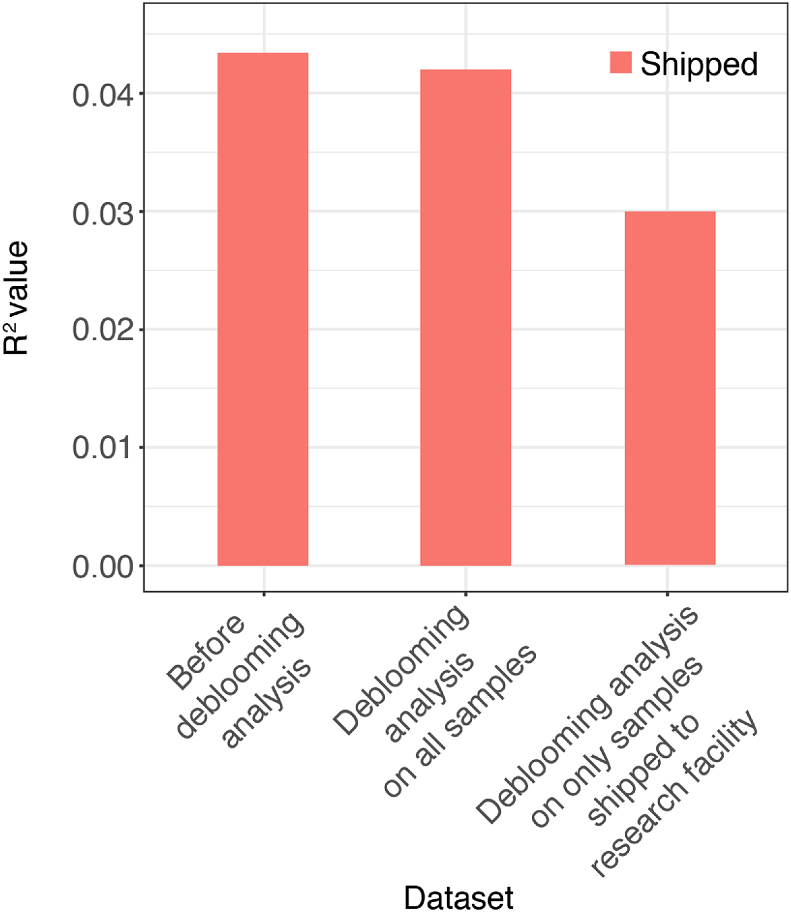
Amir et al. were the first to report on the phenomena of “blooming”, where certain taxa that are not obligate anerobic bacteria can bloom in fecal samples if not quickly refrigerated or frozen (Amir et al., 2017b). Their publication provided detailed explanations, files, and code to remove taxa that had been determined to have artificially “bloomed” in the American Gut Project data. We followed these methods to perform debloooming of our data. A total of N = 95 samples was provided during in-person visits, whereas a total of N = 236 samples were shipped. The R2 values from constrained analysis of principal coordinates of the weighted UniFrac distance matrix were used as a means to objectively determine how deblooming analysis impacted measures of bacterial community structure. We examined R2 values of both the dichotomous variable of shipped (yes, N = 236; no, N = 95) and the continuous variable of transit days (number of days between when the sample was collected by the participant and when the sample was placed into the −80 °C freezer at the research facility; mean ± SD (2.94 ± 3.82 days)). We performed this analysis on the data before deblooming analysis, after deblooming analysis of all samples (N = 331), and after deblooming analysis of only samples shipped back to the research facility (N = 236). The results of this analysis are shown in Figure B1.
The R2 values from the constrained analysis of principal coordinates is a measure of the variance explained by the factor “shipping” or “transit time” on the bacterial community composition. Ideally, after deblooming, these variables would no longer explain any variation in the gut microbiota composition (i.e., R2 = 0). While this was not the case, the R2 values were substantially reduced by performing the deblooming analysis on only samples shipped back to the research facility relative to no deblooming or deblooming all samples, supporting that this method helped to correct for the effects of sample transit conditions.
Additionally, Bokulich et al. showed that the taxa that artificially bloom in fecal samples exposed to room temperature conditions for extended periods of time are overrepresented by members of the Enterobacteriaceae family (Bokulich et al., 2019). For this reason, we also examined all the sub-operational taxonomic unit (sOTUs) in our dataset belonging to the Enterobacteriaceae family for significant correlation with transit days to ensure that our initial deblooming analysis did not overlook other taxa that may have bloomed. We found 21 sOTUs in our dataset belonging to the Enterobacteriaceae family. The results for the correlations run for each sOTU against transit days can be found in Supplemental Table B1.
There was only 1 sOTU from the Enterobacteriaceae family that was significantly correlated with transit days (sOTU: 4c8288bfbd76958c0c094d87b97650f8; Genus: Escherichia-Shigella; Species: Unknown). This sOTU was also identified by Amir et al. (Amir et al., 2017b) and therefore was already removed from the dataset during the initial deblooming analysis. Consequently, in this report we have used data derived from deblooming of only the samples shipped back to the research facility.
Figure B.1.
Bar plots of the R2 values from constrained analysis of principal coordinates of the weighted UniFrac distance matrix for the shipped samples. The analysis was performed on the data before deblooming analysis, after deblooming analysis on all samples, and after deblooming analysis on only samples shipped to the research facility.
Table B.1.
Table of correlation results of sOTUs belonging to the family of Enterobacteriaceae against transit days.
| Taxonomy | Correlation results | ||||
|---|---|---|---|---|---|
| sOTU | Genus | Species | R-values | p -value | Significance |
| 03156252fe6d4e8f09aeafc3bef8622f | Enterobacter | 0.058 | 0.29 | ||
| 054e27bdfbc9ec73099088c1ad200dfc | Providencia | Providencia vermicola | −0.03 | 0.59 | |
| 1aed9737c554992f5e99137008f672b0 | Citrobacter | uncultured | −0.056 | 0.31 | |
| 26d8f09d7fc0ca9b34c16f29516675b8 | Rahnella | uncultured | −0.0095 | 0.86 | |
| 2c9ba6e5f77c3128bab581f724f48d26 | Morganella | −0.021 | 0.7 | ||
| 3bef2d36c2b2da1c7ca58212c72a9864 | Proteus | ASC10 | −0.016 | 0.78 | |
| 411bb521b1feec2b25d0128518f057bb | Escherichia-Shigella | Escherichia coli Nissle 1917 | 0.068 | 0.22 | |
| 4c8288bfbd76958c0c094d87b97650f8 | Escherichia-Shigella | 0.17 | 0.0014 | ∗∗ | |
| 57f3e001eec8fb0909c4ea3524aa27eb | Citrobacter | 0.093 | 0.092 | ||
| 583cc45144c321d815f2d4d90a55f4f8 | Klebsiella | uncultured | 0.035 | 0.52 | |
| 64ee8ff8e0185065c7d02eb2488d90aa | Morganella | BAB-5304 | −0.021 | 0.7 | |
| 83182c1c91133da26653aeb58052e7cc | Raoultella | −0.03 | 0.59 | ||
| 9a5171a5b50ffc0b6b48abc366e0076b | Klebsiella | Oxytoca KA-2 | 0.017 | 0.75 | |
| aa1e3f1e251b633baa135e028732abc3 | Enterobacter | mixed culture X17-21 | −0.0091 | 0.87 | |
| bad443c798b38eed5f2008d150376715 | Unclassified | −0.043 | 0.44 | ||
| be011200bb48abc86a552828c40c6c8c | Cronobacter | Sakazakii | 0.039 | 0.48 | |
| bf8b84f3b4506cf8bed2c2b22fddb848 | Hafnia-Obesumbacterium | Bta3-1 | 0.0092 | 0.87 | |
| c0a0588789c30c5f413216dfef6cc8f0 | Morganella | 0.049 | 0.37 | ||
| e42382ea3fa1149094d507e3167e462b | Rosenbergiella | 0.0084 | 0.88 | ||
| ec9ed9b5acb1ccd3cdfd196b3632904c | Unclassified | 0.021 | 0.71 | ||
| fc5860decf6565201a343d865b30951f | Serratia | Pantoea agglomerans | −0.069 | 0.21 | |
Appendix C. Supplemental tables
Table C.1.
Functional pathway labels and pathway details from Fig. 5.
| Pathway label | Pathway details/webpage link |
|---|---|
| GLYOXDEG | Superpathway of glycol metabolism and degradation |
| GLYCOL-TCA-GLYOX-BYPASS | Superpathway of glycolysis, pyruvate dehydrogenase, TCA, and glyoxylate bypass |
| P105 | TCA cycle IV (2-oxoglutarate decarboxylase) |
| 1269 | CMP-3-deoxy-D-manno-octulosonate biosynthesis |
| 2942 | L-lysine biosynthesis III |
| 5345 | Superpathway of L-methionine biosynthesis (by sulfhydrylation) |
| 5918 | Superpathway of heme b biosynthesis from glutamate |
| 6737 | Starch degradation V |
| 7392 | Taxadiene biosynthesis (engineered) |
| 4FS-7 | Phosphatidylglycerol biosynthesis I (plastidic) |
| REDCITCYC | TCA cycle VI (Helicobacter) |
| COMPLETE-ARO | Superpathway of aromatic amino acid biosynthesis |
| FASYN-ELONG | Fatty acid elongation -- saturated |
| NAGLIPASYN | Lipid IVA biosynthesis (E. coli) |
| PHOSLIPSYN | Superpathway of phospholipid biosynthesis I (bacteria) |
| UDPNAGSYN | UDP-N-acetyl-D-glucosamine biosynthesis I |
Table C.2.
Model selection results for linear regressions of α-diversity. Three measures of α-diversity (Observed species, Shannon diversity, and Faith's Phylogenetic Diversity (PD)) were modeled as a function of predictors from four groups of exposures: 1) demographics; 2) health conditions and metrics; 3) medication classes; and 4) specific medications. The regression effect estimates (beta) and 95% confidence interval (CI) are shown for the selected predictors that are statistically significant. Most of the diseases and medications showing a significant association with α-diversity show an inverse relationship, but there are some exceptions. For example, vitamins and “other CVD medications,” which includes aspirin and fish oil, are associated with higher α-diversity. The beta estimates correspond to 1 standard deviation change in each metric of α-diversity.
| Predictor group | α-diversity metric | Selected covariates | Estimate | 95% CI | P |
|---|---|---|---|---|---|
| Demographics | Observed species | Non-significant: Race | |||
| Shannon | Non-significant: Race | ||||
| Faith's PD | Non-significant: Race | ||||
| Health conditions & metrics | Observed species | CVD | −0.5 | (-0.98, -0.01) | 0.046 |
| Non-significant: BMI, Bipolar disorders, Severe depressive symptoms (BDI), Gastroenteritis, Cannabis use disorder | |||||
| Shannon | PVD | −0.7 | (-1.36, -0.04) | 0.039 | |
| Non-significant: BMI, Bipolar disorders, Severe depressive symptoms (BDI), Gastroenteritis, Cannabis use disorder | |||||
| Faith's PD | CVD | −0.531 | (-1.03, -0.03) | 0.039 | |
| Non-significant: BMI, Bipolar disorders, Severe depressive symptoms (BDI), SF-36 PCS, Cancer, PVD, Gastroenteritis, Cannabis use disorder | |||||
| Medication classes | Observed species | Other Vitamins | 0.37 | (0.05, 0.71) | 0.028 |
| Non-significant: Antithrombotics, Beta blockers, Other CVD medications, Diabetes medications, Bronchodilators, NSAIDs, SSRIs, Other Psychiatric medications, Sexual function medications, Vitamin D | |||||
| Shannon | Bronchodilators | −0.34 | (-0.65, -0.02) | 0.036 | |
| Other supplemental vitamins | 0.38 | (0.05, 0.71) | 0.025 | ||
| Non-significant: Antithrombotics, Other CVD medications, Seizure medications, Other Psychiatric medications, Sexual function medications, Vitamin D | |||||
| Faith's PD | Other CVD medications | 0.38 | (0.03, 0.73) | 0.031 | |
| Non-significant: Antithrombotics, Beta blockers, Bronchodilators, Non-opioid analgesics, NSAIDs, SSRIs, Other psychiatric medications, Sexual function medications, Other supplemental vitamins, Vitamin D | |||||
| Specific medications | Observed species | Trazadone | −0.35 | (-0.68, -0.02) | 0.038 |
| Non-significant: Albuterol, Bupropion, Sildenafil, Cholecalciferol | |||||
| Shannon | Albuterol | −0.44 | (-0.81, -0.07) | 0.021 | |
| Non-significant: Bupropion, Trazadone, Sildenafil, Cholecalciferol | |||||
| Faith's PD | Non-significant: Bupropion, Trazadone, Sildenafil, Cholecalciferol | ||||
Footnote. Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; CVD, cardiovascular disease, NSAIDs, nonsteroidal anti-inflammatory drugs; SF-36 PCS, 36-Item Short Form Health Survey Physical Health Composite Score; PD, phylogenetic diversity; PVD, peripheral vascular disease; SSRI, selective serotonin reuptake inhibitor.
References
- Ait-Belgnaoui A., Durand H., Cartier C., Chaumaz G., Eutamene H., Ferrier L., Houdeau E., Fioramonti J., Bueno L., Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Aizawa E., Tsuji H., Asahara T., Takahashi T., Teraishi T., Yoshida S., Ota M., Koga N., Hattori K., Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016;202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Amir A., McDonald D., Navas-Molina J.A., Kopylova E., Morton J.T., Xu Z.Z., Kightley E.P., Thompson L.R., Hyde E.R., Gonzalez A., Knight R. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems. 2017;2 doi: 10.1128/mSystems.00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A., McDonald D., Navas-Molina J.A., Debelius J., Morton J.T., Hyde E., Robbins-Pianka A., Knight R. Correcting for microbial blooms in fecal samples during room-temperature shipping. mSystems. 2017;2 doi: 10.1128/mSystems.00199-16. 16,/msys/2/2/e00199-16.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidi N.A., Murugiah M.K., Muthuveloo R., Soh Y.C., Caruso V., Patel R., Ming L.C. Mobile medical applications for dosage recommendation, drug adverse reaction, and drug interaction: review and comparison. Drug Inf. J. 2017;51:480–485. doi: 10.1177/2168479017696266. [DOI] [PubMed] [Google Scholar]
- Azad MdAK., Sarker M., Li T., Yin J. Probiotic species in the modulation of gut microbiota: an overview. BioMed Res. Int. 2018:1–8. doi: 10.1155/2018/9478630. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberán A., Ladau J., Leff J.W., Pollard K.S., Menninger H.L., Dunn R.R., Fierer N. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Natl. Acad. Sci. U.S.A. 2015;112:5756–5761. doi: 10.1073/pnas.1420815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961 doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. J. Roy. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.-D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14 doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner J, Corrigan J. Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. J. Head Trauma Rehab. 2009;24:279–291. doi: 10.1097/HTR.0b013e3181a66356. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Maldonado J., Kang D.-W., Krajmalnik-Brown R., Caporaso J.G. Rapidly processed stool swabs approximate stool microbiota profiles. mSphere. 2019;4 doi: 10.1128/mSphere.00208-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.-X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft JJJ, Vargas F., Vázquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier J, Harper R, Jones N, O’Cathain A, Thomas K, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland J.Y., Phibbs C.S., Hoggatt K.J., Washington D.L., Lee J., Haskell S., Uchendu U.S., Saechao F.S., Zephyrin L.C., Frayne S.M. The obesity epidemic in the veterans health administration: prevalence among key populations of women and men veterans. J. Gen. Intern. Med. 2017;32:11–17. doi: 10.1007/s11606-016-3962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner L.A., Hoisington A.J., Stearns-Yoder K.A., Stamper C.E., Heinze J.D., Postolache T.T., Hadidi D.A., Hoffmire C.A., Stanislawski M.A., Lowry C.A. Military-related exposures, social determinants of health, and dysbiosis: the United States-veteran microbiome project (US-VMP) Front Cell Infect Microbiol. 2018;8 doi: 10.3389/fcimb.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N., Owens S.M., Betley J., Fraser L., Bauer M., Gormley N., Gilbert J.A., Smith G., Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J.A., Schwartz B.S., Stewart W.F., Adler N.E. Using electronic health records for population health research: a review of methods and applications. Annu. Rev. Publ. Health. 2016;37:61–81. doi: 10.1146/annurev-publhealth-032315-021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R., Altman T., Billington R., Dreher K., Foerster H., Fulcher C.A., Holland T.A., Keseler I.M., Kothari A., Kubo A., Krummenacker M., Latendresse M., Mueller L.A., Ong Q., Paley S., Subhraveti P., Weaver D.S., Weerasinghe D., Zhang P., Karp P.D. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2014;42:D459–D471. doi: 10.1093/nar/gkt1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenit M.C., Sanz Y., Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017;23:5486–5498. doi: 10.3748/wjg.v23.i30.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S.G., Goldenthal A.R., Uhlemann A.-C., Mann J.J., Miller J.M., Sublette M.E. Systematic review of gut microbiota and major depression. Front. Psychiatr. 2019;10 doi: 10.3389/fpsyt.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coello K., Hansen T.H., Sørensen N., Munkholm K., Kessing L.V., Pedersen O., Vinberg M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav. Immun. 2019;75:112–118. doi: 10.1016/j.bbi.2018.09.026. [DOI] [PubMed] [Google Scholar]
- Cohen B.E., Edmondson D., Kronish I.M. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am. J. Hypertens. 2015;28:1295–1302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders . Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Association; Arlington: 2013. [Google Scholar]
- Dickerson F., Stallings C., Origoni A., Schroeder J., Katsafanas E., Schweinfurth L., Savage C., Khushalani S., Yolken R. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophr. Bull. 2016;42:134–141. doi: 10.1093/schbul/sbv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Brain–gut–microbiota axis — mood, metabolism and behaviour. 2. Nat. Rev. Gastroenterol. Hepatol. 2017;14:69–70. doi: 10.1038/nrgastro.2016.200. [DOI] [PubMed] [Google Scholar]
- Douglas G.M., Maffei V.J., Zaneveld J., Yurgel S.N., Brown J.R., Taylor C.M., Huttenhower C., Langille M.G.I. 2019. PICRUSt2: an Improved and Extensible Approach for Metagenome Inference. bioRxiv 672295. [Google Scholar]
- Douglas G.M., Maffei V.J., Zaneveld J.R., Yurgel S.N., Brown J.R., Taylor C.M., Huttenhower C., Langille M.G.I. PICRUSt2 for prediction of metagenome functions. 6. Nat. Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Louis P., Thomson J.M., Flint H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009;11:2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- Dyball D., Evans S., Boos C.J., Stevelink S.A.M., Fear N.T. The association between PTSD and cardiovascular disease and its risk factors in male veterans of the Iraq/Afghanistan conflicts: a systematic review. Int. Rev. Psychiatr. 2019;31:34–48. doi: 10.1080/09540261.2019.1580686. [DOI] [PubMed] [Google Scholar]
- Egshatyan L., Kashtanova D., Popenko A., Tkacheva O., Tyakht A., Alexeev D., Karamnova N., Kostryukova E., Babenko V., Vakhitova M., Boytsov S. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2015;8:19–29. doi: 10.1530/EC-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epocrates 2020. http://online.epocrates.com (Accessed March 2020)
- Evans S.J., Bassis C.M., Hein R., Assari S., Flowers S.A., Kelly M.B., Young V.B., Ellingrod V.E., McInnis M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017;87:23–29. doi: 10.1016/j.jpsychires.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D., Tito R.Y., Chaffron S., Rymenans L., Verspecht C., De Sutter L., Lima-Mendez G., D’hoe K., Jonckheere K., Homola D., Garcia R., Tigchelaar E.F., Eeckhaudt L., Fu J., Henckaerts L., Zhernakova A., Wijmenga C., Raes J. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. American Psychiatric Association Publishing; Arlington,VA: 2016. Structured Clinical Interview for DSM-5 Disorders: Scid-5-Cv: Clinician Version. [Google Scholar]
- Flowers S.A., Evans S.J., Ward K.M., McInnis M.G., Ellingrod V.L. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37:261–267. doi: 10.1002/phar.1890. [DOI] [PubMed] [Google Scholar]
- Flowers S.A., Baxter N.T., Ward K.M., Kraal A.Z., McInnis M.G., Schmidt T.M., Ellingrod V.L. Effects of atypical antipsychotic treatment and resistant starch supplementation on gut microbiome composition in a cohort of patients with bipolar disorder or schizophrenia. Pharmacotherapy. 2019;39:161–170. doi: 10.1002/phar.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G.B., Lagier J.-C., Honore S., Lancon C., Korchia T., Verville P.-L.S.D., Llorca P.-M., Auquier P., Guedj E., Boyer L. Microbiota-orientated treatments for major depression and schizophrenia. 4. Nutrients. 2020;12:1024. doi: 10.3390/nu12041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Krogh Pedersen H., Arumugam M., Kristiansen K., Yvonne Voigt A., Vestergaard H., Hercog R., Igor Costea P., Roat Kultima J., Li J., Jørgensen T., Levenez F., Dore J., Bjørn Nielsen H., Brunak S., Raes J., Hansen T., Wang J., Dusko Ehrlich S., Bork P., Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. 7581. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacias M., Gaspari S., Santos P.-M.G., Tamburini S., Andrade M., Zhang F., Shen N., Tolstikov V., Kiebish M.A., Dupree J.L., Zachariou V., Clemente J.C., Casaccia P. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife. 2016;5 doi: 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney A., Bor D.H., Himmelstein D.U., Woolhandler S., McCormick D. The Effect of Veterans Health Administration Coverage on Cost-Related Medication Nonadherence: the effect of the Veterans Health Administration's low-cost drug coverage on cost-related medication nonadherence among people participating in the system. Health Aff. 2020;39:33–40. doi: 10.1377/hlthaff.2019.00481. [DOI] [PubMed] [Google Scholar]
- Gentile C.L., Weir T.L. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. 12. Mol. Psychiatr. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arango L.F., Barrett H.L., Wilkinson S.A., Callaway L.K., McIntyre H.D., Morrison M., Nitert M.D. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microb. 2018;9:189–201. doi: 10.1080/19490976.2017.1406584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C., Servais J., Martin C., Kohen D. CDC; 2019. Prescription Drug Use Among Adults Aged 40–79 in the United States and Canada. 347. [PubMed] [Google Scholar]
- Hamjane N., Benyahya F., Nourouti N.G., Mechita M.B., Barakat A. Cardiovascular diseases and metabolic abnormalities associated with obesity: what is the role of inflammatory responses? A systematic review. Microvasc. Res. 2020;131:104023. doi: 10.1016/j.mvr.2020.104023. [DOI] [PubMed] [Google Scholar]
- Hu S., Li A., Huang T., Lai J., Li J., Sublette M.E., Lu H., Lu Q., Du Y., Hu Z., Ng C.H., Zhang H., Lu J., Mou T., Lu S., Wang D., Duan J., Hu J., Huang M., Wei N., Zhou W., Ruan L., Li M.D., Xu Y. Gut microbiota changes in patients with bipolar depression. Adv. Sci. 2019:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhann F., Bonder M.J., Vich Vila A., Fu J., Mujagic Z., Vork L., Tigchelaar E.F., Jankipersadsing S.A., Cenit M.C., Harmsen H.J.M., Dijkstra G., Franke L., Xavier R.J., Jonkers D., Wijmenga C., Weersma R.K., Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.A., Goodrich J.K., Maxan M.-E., Freedberg D.E., Abrams J.A., Poole A.C., Sutter J.L., Welter D., Ley R.E., Bell J.T., Spector T.D., Steves C.J. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.A., Verdi S., Maxan M.-E., Shin C.M., Zierer J., Bowyer R.C.E., Martin T., Williams F.M.K., Menni C., Bell J.T., Spector T.D., Steves C.J. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018;9:2655. doi: 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janarthanan S., Ditah I., Adler D.G., Ehrinpreis M.N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- Janssen S., McDonald D., Gonzalez A., Navas-Molina J.A., Jiang L., Xu Z.Z., Winker K., Kado D.M., Orwoll E., Manary M., Mirarab S., Knight R. Phylogenetic placement of exact amplicon sequences improves associations with clinical information. mSystems. 2018;3:e00021. doi: 10.1128/mSystems.00021-18. 18,/msystems/3/3/msys.00021-18.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq R.R., Taur Y., Devlin S.M., Ponce D.M., Goldberg J.D., Ahr K.F., Littmann E.R., Ling L., Gobourne A.C., Miller L.C., Docampo M.D., JU Peled, Arpaia N., Cross J.R., Peets T.K., Lumish M.A., Shono Y., Dudakov J.A., Poeck H., Hanash A.M., Barker J.N., Perales M.-A., Giralt S.A., Pamer E.G., van den Brink M.R.M. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie Z., Xia H., Zhong S.-L., Feng Q., Li S., Liang S., Zhong H., Liu Z., Gao Y., Zhao H., Zhang D., Su Z., Fang Z., Lan Z., Li J., Xiao L., Li J., Li R., Li X., Li F., Ren H., Huang Y., Peng Y., Li G., Wen B., Dong B., Chen J.-Y., Geng Q.-S., Zhang Z.-W., Yang H., Wang J., Wang J., Zhang X., Madsen L., Brix S., Ning G., Xu X., Liu X., Hou Y., Jia H., He K., Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter T., Wang Po, Becker O., Nowakowska C., Yang Y.-S. The diverse roles of anticonvulsants in bipolar disorders. Ann. Clin. Psychiatr. 2003;15:95–108. doi: 10.1023/a:1024636309185. [DOI] [PubMed] [Google Scholar]
- Kovar M, Poe G, National Center for Health Statistics . The National Health Interview Survey Design, 1974-84, and Procedures, 1975-83. Public Health Service; Washington DC: 1985. [Google Scholar]
- Lax S., Smith D.P., Hampton-Marcell J., Owens S.M., Handley K.M., Scott N.M., Gibbons S.M., Larsen P., Shogan B.D., Weiss S., Metcalf J.L., Ursell L.K., Vazquez-Baeza Y., Van Treuren W., Hasan N.A., Gibson M.K., Colwell R., Dantas G., Knight R., Gilbert J.A. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345:1048–1052. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie D.L., Mohamed S., Rosenheck R.A. Off-label use of antipsychotic medications in the department of veterans Affairs health care system. PS. 2009;60:1175–1181. doi: 10.1176/ps.2009.60.9.1175. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sayam S., Shao X., Wang K., Zheng S., Li Y., Wang L. Prevalence of and trends in diabetes among veterans, United States, 2005–2014. Prev. Chronic Dis. 2017;14 doi: 10.5888/pcd14.170230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond P.F., Lovibond S.H. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav. Res. Ther. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- Lowry C.A., Smith D.G., Siebler P.H., Schmidt D., Stamper C.E., Hassell J.E., Yamashita P.S., Fox J.H., Reber S.O., Brenner L.A., Hoisington A.J., Postolache T.T., Kinney K.A., Marciani D., Hernandez M., Hemmings S.M.J., Malan-Muller S., Wright K.P., Knight R., Raison C.L., Rook G.A.W. The microbiota, immunoregulation, and mental health: implications for public health. Curr Envir Health Rpt. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Lladser M.E., Knights D., Stombaugh J., Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E.M., Hostetter T.A., Forster J.E., Hoffmire C.A., Stearns-Yoder K.A., Brenner L.A., Tahmasbi Sohi M. Suicide among veterans with amyotrophic lateral sclerosis. Muscle Nerve. 2021;63(6):807–811. doi: 10.1002/mus.27181. [DOI] [PubMed] [Google Scholar]
- Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., Patil K.R., Bork P., Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S., Van Treuren W., White R.A., Eggesbø M., Knight R., Peddada S.D. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015;26 doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada P., Leone V., Kaplan L.M., Chang E.B. The human microbiome and obesity: moving beyond associations. Cell Host Microbe. 2017;22:589–599. doi: 10.1016/j.chom.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Miller Alan. Selection of Subsets of Regression Variables. J. Roy. Stat. Soc. 1984;147(3):389–425. [Google Scholar]
- Morin C. Insomnia: Psychological Assessment and Management. Gilford Press; New York: 1993. [Google Scholar]
- Namazi N., Larijani B., Azadbakht L. Dietary inflammatory index and its association with the risk of cardiovascular diseases, metabolic syndrome, and mortality: a systematic review and meta-analysis. Horm. Metab. Res. 2018;50:345–358. doi: 10.1055/a-0596-8204. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T., Kosciolek T., Eyler L.T., Knight R., Jeste D.V. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J. Psychiatr. Res. 2018;99:50–61. doi: 10.1016/j.jpsychires.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic M., Rout A., Kingsley T., Kirchoff R., Singh A., Verma V., Kant R., Chaudhary R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020;12:110–122. doi: 10.4330/wjc.v12.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oake N., Taljaard M., Walraven C van, Wilson K., Roth V., Forster A.J. The effect of hospital-acquired Clostridium difficile infection on in-hospital mortality. Arch. Intern. Med. 2010;170:1804–1810. doi: 10.1001/archinternmed.2010.405. [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R., O'Hara R.B., Simpson G.L., Solymos P., Stevens M.H.H., Szoecs E., Wagner H. 2018. Vegan: Community Ecology Package. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: the R Project for Statistical Computing. [Google Scholar]
- Orelio C.C., Heus P., Kroese-van Dieren J.J., Spijker R., van Munster B.C., Hooft L. Reducing inappropriate proton pump inhibitors use for stress ulcer prophylaxis in hospitalized patients: systematic review of de-implementation studies. J. Gen. Intern. Med. 2021:14–16. doi: 10.1007/s11606-020-06425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato N., Saito S., Yamaguchi T., Katashima M., Tokuda I., Sawada K., Katsuragi Y., Kakuta M., Imoto S., Ihara K., Nakaji S. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. 1. npj Biofilms and Microbiomes. 2019;5:1–9. doi: 10.1038/s41522-019-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan A., van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painold A., Mörkl S., Kashofer K., Halwachs B., Dalkner N., Bengesser S., Birner A., Fellendorf F., Platzer M., Queissner R., Schütze G., Schwarz M.J., Moll N., Holzer P., Holl A.K., Kapfhammer H.-P., Gorkiewicz G., Reininghaus E.Z. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21:40–49. doi: 10.1111/bdi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylkas A.M., Juneja L.R., Slavin J.L. Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J. Med. Food. 2005;8:113–116. doi: 10.1089/jmf.2005.8.113. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind M.A., Peskind E.R., Chow B., Harris C., Davis-Karim A., Holmes H.A., Hart K.L., McFall M., Mellman T.A., Reist C., Romesser J., Rosenheck R., Shih M.-C., Stein M.B., Swift R., Gleason T., Lu Y., Huang G.D. Trial of prazosin for post-traumatic stress disorder in military veterans. N. Engl. J. Med. 2018;378:507–517. doi: 10.1056/NEJMoa1507598. [DOI] [PubMed] [Google Scholar]
- Sanna S., Zuydam NR van, Mahajan A., Kurilshikov A., Vila A.V., Võsa U., Mujagic Z., Masclee A.A.M., Jonkers D.M.A.E., Oosting M., Joosten L.A.B., Netea M.G., Franke L., Zhernakova A., Fu J., Wijmenga C., McCarthy M.I. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019;51:600. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.J., Lauber C., Costello E.K., Lozupone C.A., Humphrey G., Berg-Lyons D., Caporaso J.G., Knights D., Clemente J.C., Nakielny S., Gordon J.I., Fierer N., Knight R. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2 doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian T.L., Tanielian T., Jaycox L. Rand Corporation; 2008. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. [Google Scholar]
- Taylor A.M., Thompson S.V., Edwards C.G., Musaad S.M., Khan N.A., Holscher H.D. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr. Neurosci. 2019;23(12):983–992. doi: 10.1080/1028415X.2019.1582578. [DOI] [PubMed] [Google Scholar]
- Tett A., Huang K.D., Asnicar F., Fehlner-Peach H., Pasolli E., Karcher N., Armanini F., Manghi P., Bonham K., Zolfo M., De Filippis F., Magnabosco C., Bonneau R., Lusingu J., Amuasi J., Reinhard K., Rattei T., Boulund F., Engstrand L., Zink A., Collado M.C., Littman D.R., Eibach D., Ercolini D., Rota-Stabelli O., Huttenhower C., Maixner F., Segata N. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe. 2019;26:666–679. doi: 10.1016/j.chom.2019.08.018. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., Egholm M., Henrissat B., Heath A.C., Knight R., Gordon J.I. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallianou N.G., Stratigou T., Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Basel) 2019;18:141–144. doi: 10.1007/s42000-019-00093-w. [DOI] [PubMed] [Google Scholar]
- Van Rossum G., Drake F.L. CreateSpace; Scotts Valley, CA: 2009. Python 3 Reference Manual. [Google Scholar]
- Vinberg M., Ottesen N.M., Meluken I., Sørensen N., Pedersen O., Kessing L.V., Miskowiak K.W. Remitted affective disorders and high familial risk of affective disorders associate with aberrant intestinal microbiota. Acta Psychiatr. Scand. 2019;139:174–184. doi: 10.1111/acps.12976. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A.R., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivinus-Nébot M., Frin-Mathy G., Bzioueche H., Dainese R., Bernard G., Anty R., Filippi J., Saint-Paul M.C., Tulic M.K., Verhasselt V., Hébuterne X., Piche T. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- Wilmes L., Collins J.M., O'Riordan K.J., O'Mahony S.M., Cryan J.F., Clarke G. Of bowels, brain and behavior: a role for the gut microbiota in psychiatric comorbidities in irritable bowel syndrome. Neuro Gastroenterol. Motil. 2021;33 doi: 10.1111/nmo.14095. [DOI] [PubMed] [Google Scholar]
- Wong M.L., Inserra A., Lewis M.D., Mastronardi C.A., Leong L., Choo J., Kentish S., Xie P., Morrison M., Wesselingh S.L., Rogers G.B. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngster I., Russell G.H., Pindar C., Ziv-Baran T., Sauk J., Hohmann E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. J. Am. Med. Assoc. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The VHA has rules about data security that aim to protect the privacy of Veteran health information. Thus, the personal health data used for this study cannot be made publicly available. Please contact the authors for further information or collaboration.