Abstract
Background & Aims
Vascular liver diseases (VLDs) are represented mainly by portosinusoidal vascular disease (PSVD), noncirrhotic splanchnic vein thrombosis (SVT), and Budd Chiari syndrome (BCS). It is unknown whether patients with VLDs constitute a high-risk population for complications and greater coronavirus disease 2019 (COVID-19)-related mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Our objective was to assess the prevalence and severity of SARS-CoV-2 infection among patients with VLDs, as well as to assess its impact on hepatic decompensation and survival.
Methods
This is an observational international study analyzing the prevalence and severity of SARS-CoV-2 infection in VLDs between March 2020 and March 2021, compared with the general population (GP). Patients from Spain (5 centers; n = 493) and France (1 center; n = 475) were included.
Results
Nine hundred sixty-eight patients were included: 274 with PSVD, 539 with SVT, and 155 with BCS. Among them, 138 (14%) were infected with SARS-CoV-2: 53 with PSVD, 77 with SVT, and 8 with BCS. The prevalence of SARS-CoV-2 infection in patients with PSVD (19%) and SVT (14%) was significantly higher than in the GP (6.5%; P < .05), whereas it was very similar in patients with BCS (5%). In terms of infection severity, patients with VLDs also presented a higher need of hospital admission (14% vs 7.3%; P < .01), intensive care unit admission (2% vs 0.7%; P < .01), and mortality (4% vs 1.5%; P < .05) than the GP. Previous history of ascites (50% vs 8%; P < .05) and post-COVID-19 hepatic decompensation (50% vs 4%; P < .05) were associated with COVID-19 mortality.
Conclusions
Patients with PSVD and SVT could be at higher risk of infection by SARS-CoV-2 and at higher risk of severe COVID-19 disease.
Keywords: Budd Chiari Syndrome, COVID-19, Portosinusoidal Vascular Disease, SARS-CoV-2, Splanchnic Vein Thrombosis, Vascular Liver Diseases
Abbreviations used in this paper: BCS, Budd Chiari syndrome; COVID-19, coronavirus disease 2019; GP, general population; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; ICU, intensive care unit; PCR, polymerase chain reaction; PSVD, portosinusoidal vascular disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis; VLD, vascular liver disease
What You Need to Know.
Background
It is unknown whether patients with vascular liver diseases constitute a high-risk population for complications and greater mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Findings
Patients with portosinusoidal vascular disorder and patients with splanchnic vein thrombosis could be at higher risk of infection by SARS-CoV-2 and at higher risk of severe coronavirus disease 2019 (COVID-19).
Implications for patient care
Given the higher risk of severe COVID-19 disease in this population, it would seem reasonable to recommend checking the anti-SARS-CoV-2 antibody levels after full vaccination in patients with vascular liver diseases and administer a third dose to all patients not showing good antibody response.
The hasty spread of the global pandemic by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resultant coronavirus disease 2019 (COVID-19) has severely impacted global health with devastating effects. Certain populations have been reported as especially vulnerable to COVID-19 disease.1 Patients with advanced age, male sex, and comorbidities including arterial hypertension, obesity, heart disease, diabetes, or malignancy have been consistently reported to develop severe COVID-19.2 Although there is no clear evidence on SARS-CoV-2 hepatotropism, abnormalities in liver tests are present in 15% to 65% of patients with COVID-19, regardless of the presence of underlying liver disease.3 Indeed, SARS-CoV-2 is genetically related to Middle East Respiratory Syndrome Coronavirus, which is also known to affect liver tests. Abnormal liver tests in COVID-19 are probably multifactorial, due to a combination of immune-mediated inflammatory response, drug-induced liver injury, hepatic congestion, or direct infection of hepatocytes.4 Several studies have tried to elucidate if patients with chronic liver disease are at higher risk of SARS-CoV-2 infection. It has been shown that cirrhotic patients – specifically with decompensated cirrhosis – have a higher rate of hospitalization and mortality.5 , 6 Despite the concern raised by these studies, we currently do not know whether all chronic liver diseases are equally affected by COVID-19 or if there are specific subgroups with increased risk of mortality and morbidity. Immunosuppression does not seem to confer an increased risk for severe COVID-19 in specific subpopulations such as autoimmune hepatitis7 or liver transplant recipients.8
No studies have yet evaluated if patients with vascular liver diseases (VLDs) also represent a vulnerable population with a higher risk of complications and a higher mortality than general population. VLDs are a set of diseases that affect young and otherwise healthy patients that usually exhibit a preserved liver function and have a markedly different natural history from cirrhosis. Portosinusoidal vascular disease (PSVD, formerly known as idiopathic portal hypertension), chronic noncirrhotic splanchnic vein thrombosis (SVT), and Budd Chiari syndrome (BCS) are the most common VLDs. Although individually the prevalence of each of these rare diseases is less than 5 per 10,000 inhabitants,9 , 10 overall they affect a significant proportion of the population. A common feature of these VLDs is the presence of noncirrhotic portal hypertension and an underlying prothrombotic state,11 either per se (high incidence of SVT in patients with PSVD regardless of associated thrombophilia) or due to associated underlying diseases (ie, myeloproliferative disorders, inherited thrombophilia, antiphospholipid syndrome, or human immunodeficiency virus [HIV]). As one of the most feared complications of COVID-19 disease is the increased systemic inflammatory response leading to a higher risk of hypercoagulability and thromboembolic disease,12 , 13 it could be argued that SARS-CoV-2 may further deteriorate the clinical condition of patients with these VLDs.
The aim of the present work is to assess the prevalence and severity of SARS-CoV-2 infection among patients with VLDs. We provide the first international report detailing the impact of SARS-CoV-2 in VLDs, and we offer comparison with the corresponding data regarding the general population (GP) of Spain and France.
Methods
Patients and Study Design
We conducted an observational multicentric international study including 5 centers in Spain belonging to the REHEVASC network (Hospital Clínic de Barcelona, Hospital Ramón y Cajal, Hospital Universitario Marqués de Valdecilla, Hospital Puerta de Hierro, Hospital de la Santa Creu i Sant Pau) and 1 center in France (Hôpital Beaujon). The 6 participating hospitals are the main tertiary referral centers in the field of VLDs in Spain and France, thus providing a good description of the VLD population of the 2 countries. Also, all the participating hospitals prospectively register all patients diagnosed with VLDs in their hospitals. The registries offer a comprehensive representation of all the stages of VLDs, given that both compensated patients (patients diagnosed during an outpatient study of hepatic liver disease) and patients that have required hospitalization are included in the registry. Given the nature of the study, to have a baseline registry was a necessary and limiting factor to participate in the study. The registries included baseline characteristics such as age, gender, and associated comorbidities. Using the registries of each hospital as a starting point, we reviewed their clinical records to investigate if they had been infected by SARS-CoV-2 in the period comprised between March 2020 and March 2021 as well as the clinical outcome. Positive cases were also contacted by phone or email and answered a questionnaire regarding detailed signs and symptoms of presentation. The follow-up period was stopped at March 2021 to avoid interference with the COVID-19 vaccination program. Indeed, no patient was vaccinated during the period of the study. Patients with VLDs different from PSVD, SVT, and BCS were not included in this study due to their extremely low prevalence. The study was performed in accordance with the International Guideline for Ethical Review of Epidemiological studies and principles of the Declaration of Helsinki, and was approved by the local ethics committees.
All cases of laboratory-confirmed SARS-CoV-2 infection with any symptom profile or disease severity were included in the analysis. Positive cases were defined as detection of SARS-CoV-2 by reverse-transcriptase polymerase chain reaction (PCR) on nasopharyngeal swabs, enzyme-linked immunosorbent assay serological testing for detection of antibodies to SARS-CoV-2, or antigen detection tests. Thirteen patients with conclusive clinical manifestations compatible with COVID-19 infection in which no tests had been performed due to lack of resources at the beginning of the pandemic were also included.
To provide a comparison with the GP, we used the data published by both the Spanish (ine.es) and the French government (santepubliquefrance.fr). From these websites, we established the GP prevalence, the stratification of prevalence by age group, and the severity of the disease in GP, defined by need of hospital admission, need of intensive care unit (ICU) admission and COVID-19-related death. Briefly, between March 2020 and March 2021, 3,042,127 people have been diagnosed with SARS-CoV-2 infection in Spain, accounting for 7% of the GP (current Spanish population 46,769,777 people). The proportion of patients needing hospital admission was 7.3%, whereas 0.7% needed ICU admission and 1.5% died. In France, the French population is estimated at 65,273,511 people, and between March 2020 and March 2021, there have been 5,498,044 confirmed cases of SARS-CoV2 infection, 8% of the French GP, which is similar and comparable to the Spanish data (P = .6). The French mortality rates were also comparable to that of the Spanish population (1.87% in France vs 1.5% in Spain; P = .86). We have focused on the Spanish GP data as reference to compare it with our VLD cohort.
Each VLD (PSVD, PVT, and BCS) has been analyzed separately. Additionally, we have performed a subanalysis stratifying patients by age groups (<40 years old, 40–59 years old, 60–69 years old, and >69 years old) to elucidate the possible role of this confounding factor. Supplementary Table 1 lists the points of comparison between the GP and our VLD cohort.
Statistical Analysis
Continuous variables are reported as mean + standard deviation, and categorical variables are reported as absolute and relative frequencies. Groups were compared using the t test or the Mann-Whitney test for continuous variables when appropriate, and the Fisher exact test was used for categorical variables. Logistic regression models were used to study the predictors of COVID-19 infection.
Results
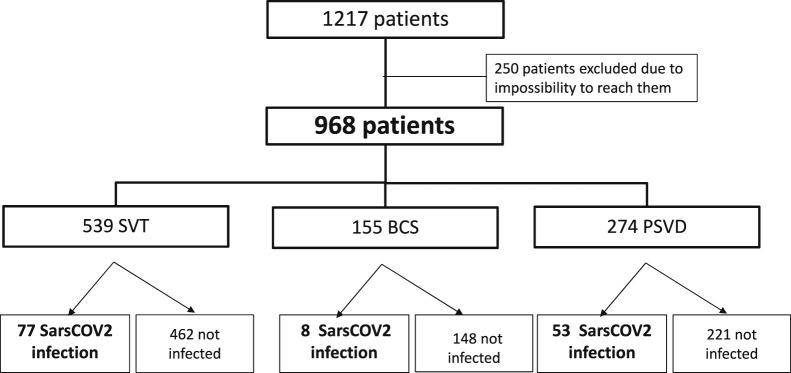
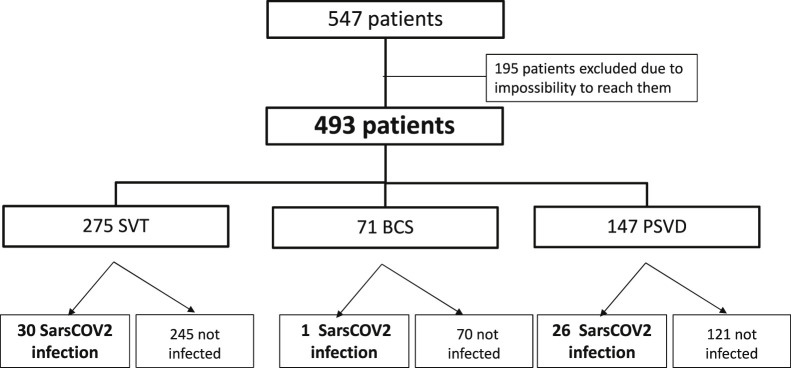
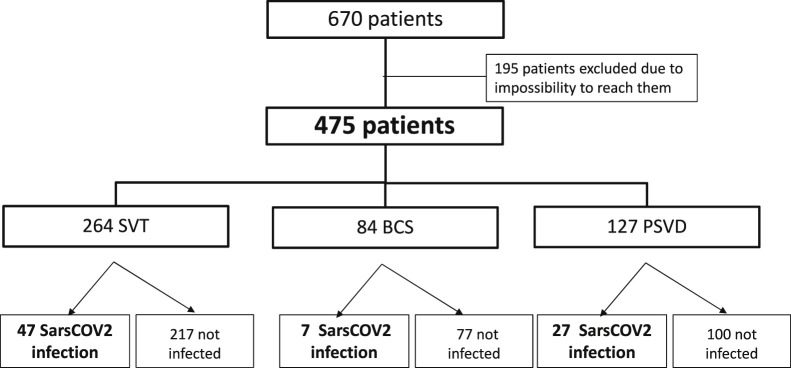
Five hundred forty-seven patients with VLDs were prospectively registered in the 5 Spanish centers (310 in Hospital Clínic de Barcelona, 123 in Hospital Ramon y Cajal, 50 in Hospital de la Santa Creu i Sant Pau, 43 in Hospital Universitario Marqués de Valdecilla, and 29 in Hospital Puerta de Hierro), and we obtained data from 493 (90%) (Supplementary Figure 1). In Hôpital Beaujoun, there were 670 patients with VLDs registered, and we obtained data from 475 patients (71%) (Supplementary Figure 2). Overall, of 1217 patients, 968 (80%) were included: 274 with PSVD, 539 with SVT, and 155 with BCS (Flowchart, Figure 1 ). Among these 968 patients, 138 (14%) were infected with SARS-CoV-2 between March 2020 and March 2021: 53 with PSVD (19%), 77 with SVT (14%), and 8 with BCS (5%).
Figure 1.
Study flowchart.
Table 1 shows that there is a high variability in baseline characteristics. However, most of these differences can be explained by the intrinsic characteristics of each disease (ie, PSVD is usually associated with HIV, inflammatory bowel disease [IBD], or history of neoplasm and chemotherapy; BCS is more frequent in women and usually decompensates as ascites and not as variceal bleeding). Supplementary Table 2 summarizes the diagnostic method (PCR, serologies, antigen test, or clinical signs) used for COVID-19 diagnosis, as well as the reason motivating the diagnostic test. Most patients were diagnosed by PCR (69%) or serologies (11%). The reason prompting the diagnostic test was only collected in the Spanish cohort, and in most patients it was the presence of symptoms (n = 37; 65%), followed by contact tracing screening program (n = 15; 26%).
Table 1.
Baseline Characteristics of the VLD Cohort
| PSVD (n = 274) | SVT (n = 539) | BCS (n = 155) | P value | |
|---|---|---|---|---|
| Age, y | 53.2 ± 15.4 | 52.5 ± 15.1 | 50.5 ± 14.5 | .22 |
| Sex (male) | 155 (57) | 301 (56) | 51 (33) | < .01 |
| Excessive alcohol consumptiona | 25 (9) | 65 (12) | 13 (9) | .25 |
| Smoking | 25 (9) | 68 (13) | 12 (8) | .48 |
| Obesity | 19 (7) | 81 (15) | 19 (12) | < .01 |
| Arterial hypertension | 48 (17) | 98 (18) | 14 (9) | .02 |
| Diabetes mellitus | 32 (12) | 51 (10) | 10 (6.5) | .21 |
| HIV | 29 (11) | 0 | 0 | < .01 |
| Neoplasm | 16 (6) | 7 (1) | 2 (1) | < .01 |
| MPN | 12 (4) | 116 (22) | 73 (47) | < .01 |
| IBD | 15 (6) | 13 (2) | 0 | < .01 |
| Any comorbidity | 149 (54) | 218 (40) | 62 (40) | < .01 |
| Any previous PH complication | 71 (26) | 129 (24) | 62 (40) | < .01 |
| Previous variceal bleeding | 52 (19) | 77 (14) | 12 (8) | .06 |
| Previous ascites | 32 (12) | 73 (14) | 51 (33) | < .01 |
| Previous HE | 7 (3) | 9 (2) | 7 (4.5) | .23 |
| TIPS | 22 (8) | 13 (2) | 59 (38) | < .01 |
Note: Data are presented as mean ± standard deviation or number (%).
Note: Boldface P values indicate statistical significance.
BCS, Budd Chiari syndrome; HE, hepatic encephalopathy; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; MPN, myeloproliferative neoplasm; PH, portal hypertension; PSVD, portosinusoidal vascular liver disorder; SVT, splanchnic vein thrombosis; TIPS transjugular intrahepatic portosystemic shunt; VLD, vascular liver disease.
Excessive alcohol consumption is defined as more than 4 units of alcohol per day.
Prevalence
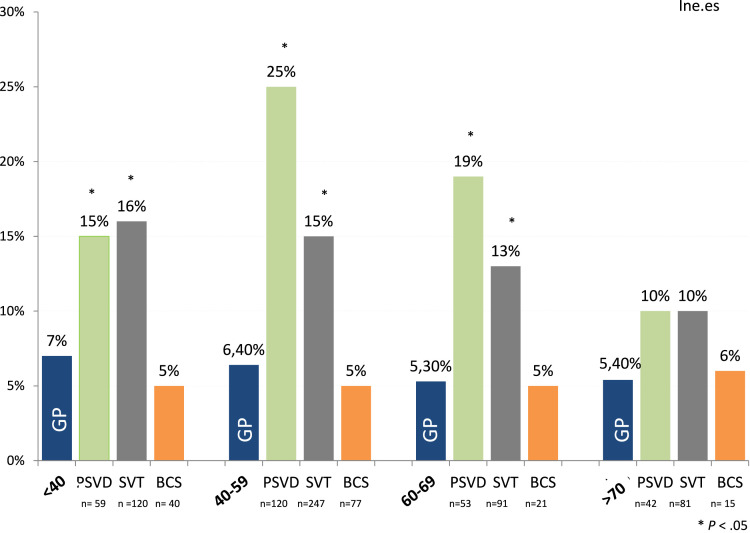
PSVD. Of 274 patients with PSVD, 53 were infected with SARS-CoV-2 (19%), a prevalence significantly higher than in the GP (6.5%; P < .05). This was observed in all age groups (Figure 2 ), except in the >70 year group. Indeed, the prevalence reached 15%, 25%, and 19% in age groups <40, 40 to 59, and 60 to 69 years, respectively.
Figure 2.
Age distribution of SARS-CoV-2 infection among patients with VLDs compared with the GP.
SVT. The prevalence of SARS-CoV-2 was also significantly higher in patients with SVT than in the GP. Of 539 patients with SVT, 77 were infected with SARS-CoV-2 (14% vs 6.5% in GP; P < .05). Regarding age distribution (Figure 2), it was also significantly different from GP (P < .05): SARS-CoV-2 infection was present in 16% of patients aged <40 years, in 15% of patients between 40 and 59 years, and in 13% of patients in the 60 to 69 year age group. In patients over 70 years, the prevalence was also higher than in GP (10% vs 5.4%), but it did not reach statistical significance.
Importantly, if we exclude the 13 patients that were clinically diagnosed without a confirmation test, the prevalence and severity of SARS-CoV-2 infection in patients with VLDs was still higher than in the GP (125 of 968; 13%; P < .05) (Supplementary Table 3). Importantly, this was observed both in the Spanish and in the French cohort (data not shown).
BCS. In our cohort, only 8 patients of 155 with BCS presented with SARS-CoV-2 (5%). Thus, the global prevalence of SARS-CoV-2 in patients with BCS was very similar to that of the GP, even stratified by age groups.
Signs and Symptoms
Among the 138 patients, only 21 (15%) remained completely asymptomatic throughout the infection period. Table 2 shows the most frequent symptoms developed in this cohort and dissects them according to the VLD type. Fever and asthenia were present in 60% of patients, followed by cough in 46%. Cephalea, anosmia, dysgeusia, or other symptoms were less frequent.
Table 2.
Symptoms Associated With SARS-CoV-2 Infection
| Symptoms | All VLD (N = 138) | SVT (n = 77) | BCS (n = 8) | PSVD (n = 53) | P value |
|---|---|---|---|---|---|
| Fever | 84 (61) | 48 (62) | 4 (50) | 32 (60) | .87 |
| Asthenia | 83 (60) | 48 (62) | 4 (50) | 31 (58) | .81 |
| Cough | 64 (46) | 32 (41) | 3 (37) | 29 (54) | .34 |
| Cephalea | 47 (34) | 28 (36) | 2 (25) | 17 (32) | .80 |
| Anosmia | 44 (32) | 25 (32) | 2 (25) | 17 (32) | 1 |
| Dysgeusia | 37 (27) | 23 (30) | 2 (25) | 12 (22) | .67 |
| Dyspnea | 26 (18) | 15 (19) | 1 (12) | 10 (18) | 1 |
| Diarrhea | 21 (15) | 11(14) | 0 | 10 (18) | .42 |
| Asymptomatic | 21 (15) | 8 (10) | 3 (37) | 10 (18) | .07 |
Note: Data are presented as number (%).
BCS, Budd Chiari syndrome; PSVD, portosinusoidal vascular liver disorder; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis; VLD, vascular liver disease.
Risk Factors of SARS-CoV-2 Infection in Patients With VLDs
PSVD. The median age of infected patients was 49.7 ± 17 years, and 50% of them were men. There were no statistically significant differences in age and sex distribution among patients infected or not by SARS-CoV-2. We assessed if previous decompensation, variceal bleeding, ascites, hepatic encephalopathy, sex, age, comorbidities (arterial hypertension, diabetes mellitus, obesity, history of neoplasms, IBD, HIV infection), use of anticoagulation, use of transjugular intrahepatic portosystemic shunt, previous SVT in PSVD, or baseline liver function were potential risk factors for COVID-19 in the VLD population. Only a higher baseline bilirubin was observed in patients with PSVD acquiring SARS-CoV-2 (2.5 ± 2.6 vs 1.5 ± 1.5 mg/dL; P < .05) without significant differences in the remaining assessed parameters (Supplementary Table 4).
SVT. The median age of infected patients was 48.3 ± 18 years, and 38% of them were male. History of arterial hypertension (28% vs 16%; P < .05), IBD (8% vs 2%; P < .01) higher bilirubin (2.6 ± 2.8 vs 1.5 ± 1.9 mg/dL; P < .01), higher protrombin time (38% ± 44% vs 55% ± 41%; P < .05), and use of anticoagulation (79% vs 67%; P < .05) were associated with SARS-CoV-2 infection, but only IBD (hazard ratio, 4.3; 95% confidence interval, 1.1–17.2; P < .05) and bilirubin levels (hazard ratio, 1.2; 95% confidence interval, 1.1–1.4; P < .01) were independently associated with it (Supplementary Tables 5 and 6).
BCS. No further analysis was performed in patients with BCS given the low number of cases recorded and the similar prevalence to the GP.
Severity of COVID-19 Disease in Patients With VLDs
Table 3 summarizes the severity of COVID-19 disease in patients with VLDs using the need of hospital admission, need for ICU care, and mortality as indicators. The analysis was performed either globally or categorized by age groups (Supplementary Table 7). As shown, patients with VLDs presented a higher need of hospital admission (14% vs 7.3%; P < .01), ICU admission (2% vs 0.7%; P < .01), and COVID-19-related mortality (4% vs 1.5%; P < .05) than the GP. However, COVID-19 infection increase in severity was not homogeneously distributed. Indeed, although the low sample size when subanalyzing by age groups reduces the strength of the finding, our results suggest that the increase of COVID-19 severity in patients with VLDs focuses in those patients below the age of 60.
Table 3.
Severity of SARS-CoV-2 Infection and Need for Hospital Admission, ICU Admission, and Mortality
| GP (N = 3,042,127) | VLD (n = 138) | P | PSVD (n = 53) | P | BCS (n = 8) | P | SVT (n = 77) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Admission | 221,118 (7.3) | 20 (14) | .01 | 5 (9) | .57 | 1 (12) | .60 | 14 (18) | .01 |
| ICU | 20,362 (0.7) | 4 (2) | .01 | 2 (4) | .01 | 0 | .81 | 2 (3) | .04 |
| Mortality | 45,797 (1.5) | 4 (4) | .04 | 1 (2) | .8 | 0 | .72 | 3 (6) | .01 |
Note: Data are presented as number (%).
Note: Boldface P values indicate statistical significance.
BCS, Budd Chiari syndrome; GP, general population; ICU, intensive care unit; PSVD, portosinusoidal vascular liver disorder; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis; VLD, vascular liver disease.
Regarding BCS, only one patient required hospital admission, which makes it difficult to draw any conclusions from this subpopulation. This single patient presented a full recovery afterwards without further complications.
Liver Decompensation After SARS-CoV2 Infection
Six patients (2 with PSVD and 4 with SVT) developed hepatic decompensation during COVID-19 disease: splanchnic thrombosis and variceal bleeding in the 2 patients with PSVD; ascites, variceal bleeding, and 2 episodes of re-thrombosis in the 4 patients with SVT. Noteworthy, according to their clinical characteristics, presence or not of underlying prothrombotic factors, or previous development of splanchnic thrombosis in patients with PSVD, at inclusion 89% of patients with BCS, 68% of patients with SVT, and 31% of patients with PSVD were on long-term anticoagulation treatment. Based on the local clinical guidelines of each center, 3 of 53 patients with PSVD (5.7%) and 4 of 77 patients with SVT (5.2%) received anticoagulation as part of the treatment of SARS-CoV-2 infection. The 3 episodes of thrombosis were the only post-COVID-19 thrombotic recorded events in our cohort, and they were all in the splanchnic territory. The 2 patients with SVT had associated underlying thrombophilia that conferred them a higher risk of thrombosis and were accordingly under long-term anticoagulation.
Given the low number of events, to assess the risk of hepatic decompensation and risk of mortality due to COVID-19, we evaluated the VLD cohort globally. We found that history of diabetes mellitus (12% vs 42%; P < .05), previous ascites (8% vs 42%; P <0 .01), or hepatic encephalopathy (3% vs 28%; P < .05) were significantly more frequent in patients presenting further hepatic decompensation after SARS-COV-2 infection.
Four patients died due to COVID-19 pneumonia and respiratory insufficiency (n = 1 with PSVD; n = 3 with SVT). Previous history of ascites (50% vs 8%; P < .05) and post-COVID-19 hepatic decompensation (50% vs 4%; P < .05) were more frequent among these patients.
Discussion
The huge daily death toll, fast infection rates, and the burden on ICUs of the COVID-19 pandemic rapidly lead to a worldwide crisis with a dramatic impact on global health care systems. This pandemic made it clear that health care systems need to be improved and have to be more resilient to emergencies. One of the most important items to improve the approach to the present – and, eventually, future – pandemics is the identification of vulnerable patients at higher risk of adverse outcomes. Several studies have shown that chronic health conditions can increase the risk of presenting severe COVID-19. Liver disease has also been identified as an important risk factor for severe COVID-19, being especially virulent in those patients with cirrhosis and hepatic decompensation. However, the term liver disease encompasses a broad range of pathologies that have few things in common and that require an individualized approach. Among them, VLDs deserve special mention. Despite their relatively low prevalence, they represent a significant health problem in the field of liver disease. They usually affect young patients and cause portal hypertension, which, in turn, leads to increased morbidity and mortality.
Although fortunately, in some countries, the current pandemic situation is beginning to improve thanks to the development of vaccines, the emergence of multiple new SARS-CoV-2 strains make it difficult to predict the long-term protective effects of the available shots. It is therefore clear that one of the best existing strategies is the identification of vulnerable populations. Thus, the aim of the present study is to focus on VLDs and characterize the effects of SARS-CoV-2 infection in this population.
The strength of our study rests in the approach and methodology used. For this study, a thorough investigation was performed, and we were able to contact 80% of our patients, thus making a selection bias highly unlikely. Estimations of the real prevalence of the disease, as well as detecting asymptomatic patients, would have required testing of all patients with VLDs. However, this is not a real option, and we consider that our approach is a good surrogate given that the GP underwent the same testing policy. Our results can be summarized in 3 important messages:
-
(1)
The prevalence of SARS-CoV-2 infection among patients with PSVD and SVT seems to be higher than in the GP. On the contrary, in patients with BCS, the prevalence was very similar to the GP. Of note, as detailed in Table 1, the prevalence of arterial hypertension was significantly lower in patients with BCS than in patients with PSVD and SVT, which could confer a lower risk of severe COVID-19 disease. There were no statistically significant differences in other factors such as diabetes, smoking, or obesity. The higher prevalence in patients with PSVD and SVT seems to not be influenced by patients’ age. Only in the age group >70 years was the prevalence similar to the GP, probably due to the low representation of older patients in our cohort and to a higher concern of these patients in preventing infection. A similar finding regarding absence of differences in older groups was observed in the study by Marjot et al.5 This study showed that, although patients without cirrhosis display an age-related gradient for mortality, mortality in patients with cirrhosis was higher in patients under 40 years.
Higher bilirubin and IBD were the only features identified as risk factors for SARS-CoV-2 infection. However, the clinical relevance of these findings is debatable. Liver function is usually well-preserved in both PSVD and SVT and generally is not a matter of concern in these patients. Indeed, the only slightly higher levels of bilirubin found in patients with SARS-CoV-2 infection have no clinical relevance. Regarding IBD, recent publications specifically focused on assessing the incidence and severity of COVID-19 in patients with Crohn’s disease and ulcerative colitis suggest that patients with IBD are not at higher risk of COVID-19 than the GP.14 Thus, currently we are compelled to consider these results cautiously and wait for validation in future cohorts.
-
(2)
Our data suggests that patients with SVT and PSVD could have a more severe course of COVID-19 infection than the GP. This stronger severity is more evident at younger ages, which could be explained by the fact that VLDs predominantly affect younger patients.
BCS seems to relate differently to SARS-CoV-2 than the rest of the VLDs. Noticeably, the severity of SARS-CoV-2 infection in patients with BCS is strictly comparable to that of the GP. We hypothesize that the absence of portal hypertension in compensated BCS could explain this important difference with PSVD and SVT. Although BCS entails of course the development of portal hypertension, in chronic compensated stages, patients will have either developed collateral circulation that effectively decompresses the splanchnic system or will have required the placement of a transjugular portosystemic shunt. This is also suggested by the fact that 37% of our patients with BCS were asymptomatic for COVID-19, which is a higher frequency than in patients with SVT and PSVD.
-
(3)
We carefully assessed if well-known high-risk factors such as age, diabetes, arterial hypertension, obesity, or malignancy, among others, played a role in the evolution and final outcome of the SARS-CoV-2 infection. We also assessed if other concomitant diseases, especially the association with myeloproliferative diseases (very frequent in patients with SVT and BCS) could interfere in its severity. Of all the parameters evaluated, diabetes mellitus and previous hepatic decompensation were the only factors associated with post-infection liver decompensation and with mortality. Admittedly, due to the low number of deaths in our cohort, the strength of these results is reduced. However, these results support and point in the direction that the higher prevalence and higher severity of COVID-19 in patients with PSVD and SVT is due to the VLDs per se and is not influenced by associated comorbidities (with the exception of diabetes mellitus facilitating hepatic decompensation). Of note, although the interpretation of these results is limited by not having a comparable control group, comorbidities such as obesity, diabetes mellitus, or arterial hypertension had a lower prevalence in our cohort than in both the Spanish and French GP (in our cohort, the prevalence of diabetes mellitus, arterial hypertension, and obesity was 10%, 17%, and 12%, respectively, vs 13.8%, 42%, and 23% in the Spanish population and 8%, 30%, and 26% in the French population), which can explain why metabolic factors known to increase the risk of severe COVID-19 had a low impact in the outcome of our cohort.
Finally, it is remarkable that, although most VLDs are associated with a hypercoagulable state and COVID-19 is also considered a prothrombotic condition,15 the rate of thromboembolic events was unexpectedly low. Although this is an important finding, in this observational study, no systemic imaging tests looking for thrombotic events were performed, and thus asymptomatic thrombotic events could have been underdiagnosed. Accordingly, no strong recommendations regarding venous thromboembolism prophylaxis and anticoagulation can be made based on our results.
Conclusions
In conclusion, this international study shows that patients with PSVD and SVT could be at higher risk of infection by SARS-CoV-2 and, importantly, point in the direction that they could also be at higher risk of severe COVID-19 disease. Our results have significant implications for the management and risk stratification of patients with VLDs, for both the present pandemic and for future hypothetical outbreaks of new SARS-CoV-2 strains. Future research should be directed at vaccination programs, as data regarding duration of protection, tolerability, and long-term safety of COVID-19 vaccines in patients with VLDs has yet to be generated. In this regard, as it is known that some patients do not mount a strong immune response to the vaccines and taking into consideration the higher risk of severe COVID-19 disease in VLDs, it would seem reasonable to recommend checking the anti-SARS-CoV-2 antibody levels after full vaccination in this population and administer a third dose to all patients not showing good antibody response.
Acknowledgments
CRediT Authorship Contributions
Anna Baiges (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead)
Eira Cerda (Data curation: Equal; Writing – review & editing: Equal)
Caroline Amicone (Data curation: Equal; Writing – review & editing: Equal)
Luis Téllez (Data curation: Equal; Writing – review & editing: Equal)
Edilmar Alvarado-Tapias (Data curation: Equal; Writing – review & editing: Equal)
Angela Puente (Data curation: Equal; Writing – review & editing: Equal)
Jose Ignacio Fortea (Data curation: Equal; Writing – review & editing: Equal)
Elba Llop (Data curation: Equal; Writing – review & editing: Equal)
Filipa Rocha (Data curation: Equal; Writing – review & editing: Equal)
Lara Orts (Data curation: Equal; Writing – review & editing: Equal)
Oliva Ros-Fargas (Data curation: Equal; Writing – review & editing: Equal)
Pamela Vizcarra (Data curation: Equal; Writing – review & editing: Equal)
Kamal Zekrini (Data curation: Equal; Writing – review & editing: Equal)
Ould Amara Lounes (Data curation: Equal; Writing – review & editing: Equal)
Ghiles Touati (Data curation: Equal; Writing – review & editing: Equal)
Natalia Jimenez (Data curation: Equal; Writing – review & editing: Equal)
Maria Jose Serrano (Data curation: Equal; Writing – review & editing: Equal)
Angels Falgà (Data curation: Equal; Writing – review & editing: Equal)
Marta Magaz (Data curation: Equal; Writing – review & editing: Equal)
Pol Olivas (Data curation: Equal; Writing – review & editing: Equal)
Fabian Betancourt (Data curation: Equal; Writing – review & editing: Equal)
Valeria Perez-Campuzano (Data curation: Equal; Writing – review & editing: Equal)
Fanny Turon (Data curation: Equal; Writing – review & editing: Equal)
Audrey Payancé (Data curation: Equal; Writing – review & editing: Equal)
Odile Goria (Data curation: Equal; Writing – review & editing: Equal)
Pierre-Emmanuel Rautou (Data curation: Equal; Writing – review & editing: Equal)
Virginia Hernández-Gea (Data curation: Equal; Writing – review & editing: Equal)
Candid Villanueva (Data curation: Equal; Writing – review & editing: Equal)
Agustin Albillos (Data curation: Equal; Writing – review & editing: Equal)
Aurélie Plessier (Conceptualization: Lead; Data curation: Equal; Methodology: Equal; Supervision: Equal; Writing – review & editing: Equal)
Juan Carlos Garcia-Pagan, MD, PhD (Conceptualization: Lead; Data curation: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Validation: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was sponsored by the Instituto de Salud Carlos III FIS PI20/00569 , SAF PID2019-105/48RB-100, Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR-SGR2017-517) a grant from Generalitat de Catalunya, Fondo Europeo de Desarrollo Regional (FEDER) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), funded by Instituto de Salud Carlos III. Anna Baiges and Edilmar Alvarado-Tapias are funded by a “Juan Rodes” contract from the Instituto de Salud Carlos III. Marta Magaz is funded by a “Rio Hortega” contract from the Instituto de Salud Carlos III. Pol Olivas is funded by Contractes Clínic de Recerca “Emili Letang-Josep Font’’ 2020, granted by Hospital Clínic de Barcelona.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.12.032.
Supplementary Material
Supplementary Figure 1.
Spanish VLD cohort.
Supplementary Figure 2.
French VLD cohort.
Supplementary Table 1.
Variables Compared Between the GP and the VLD Cohort
| Variable | GP, % | VLD cohort, % | P |
|---|---|---|---|
| Prevalence of SARS-CoV-2 infection | 6.5 | 14 | < .05 |
| Need of hospital admission | 7.3 | 14 | < .05 |
| Need for ICU care | 0.7 | 2 | < .05 |
| Mortality | 1.5 | 4 | < .05 |
| Prevalence of diabetes mellitus | 13.8 | 10 | .4 |
| Prevalence of obesity | 23 | 12 | < .05 |
| Prevalence of arterial hypertension | 42 | 17 | < .05 |
GP, General population; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VLD, vascular liver disease.
Supplementary Table 2.
SARS-CoV-2 Infection Diagnostic Method and Reason Leading to Test
| Diagnostic method (total cohort, n = 138) | n (%) |
| PCR | 96 (69) |
| Serology | 15 (11) |
| Antigens test | 14 (10) |
| Clinical | 13 (10) |
| Reason for diagnosis (Spanish cohort, n = 57) | |
| Symptoms | 37 (65) |
| High-risk contact | 15 (26) |
| Screening | 5 (8) |
PCR, Polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 3.
Severity of SARS-CoV-2 Infection and Need for Hospital Admission, ICU Admission, and Mortality Excluding the 13 Patients That Were Diagnosed Clinically Without SARS-CoV-2 Diagnostic Test
| GP (N = 3,042,127) | VLD (n = 125) | P | PSVD (n = 46) | P | BCS (n = 8) | P | SVT (n = 71) | p | |
|---|---|---|---|---|---|---|---|---|---|
| Admission | 221,118 (7.3) | 18 (14) | .01 | 4 (9) | .57 | 1 (12) | .60 | 13 (18) | .01 |
| ICU | 20,362 (0.7) | 4 (3) | .01 | 2 (4) | .01 | 0 | .81 | 2 (3) | .04 |
| Mortality | 45,797 (1.5) | 4 (3) | .04 | 1 (2) | .8 | 0 | .72 | 3 (8) | .01 |
Note: Data are presented as number (%).
Note: Boldface P values indicate statistical significance.
BCS, Budd Chiari syndrome; GP, general population; ICU, intensive care unit; PSVD, portosinusoidal vascular liver disorder; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis; VLD, vascular liver disease.
Supplementary Table 4.
Risk Factors for SARS-COV-2 Infection in PSVD
| PSVD | No infection (n = 221), n ± SD | Infection (n = 53), n ± SD | P | No infection (n = 221), n ± SD | Infection (n = 53), n ± SD | P | |
|---|---|---|---|---|---|---|---|
| Age, y | 53 ± 18 | 50 ± 17 | .27 | Anticoagulation | 72 | 13 | .25 |
| Sex (male) | 128 | 27 | .36 | TIPS | 18 | 4 | 1 |
| Alcohol | 18 | 7 | .58 | IBD | 11 | 4 | .5 |
| Tobacco | 21 | 2 | .07 | Bilirubin, mg/dL | 1.5 ± 1.5 | 2.5 ± 2.6 | .02 |
| Decompensation | 57 | 14 | 1 | Albumin, mg/dL | 38 ± 4.7 | 39 ± 5.5 | .30 |
| Variceal bleeding | 39 | 13 | .33 | PT, % | 39 ± 36 | 62 ± 34 | .85 |
| Ascites | 28 | 4 | .35 | AST, U/L | 38 ± 30 | 39 ± 16 | .44 |
| HE | 5 | 2 | 1 | ALT, U/L | 39 ± 32 | 33 ± 19 | .15 |
| Thrombosis | 59 | 12 | .60 | AP, U/L | 146 ± 119 | 97 ± 60 | .99 |
| Comorbidities | 119 | 30 | .76 | GGT, U/L | 73 ± 72 | 57 ±48 | .35 |
| Arterial hypertension | 38 | 10 | .81 | Platelets | 116 ± 67 | 113 ± 89 | .32 |
| DM | 24 | 8 | .47 | Cr, mg/dL | 0.95 ± 0.3 | 1.1 ± 1 | .35 |
| Obesity | 15 | 4 | 1 | Leucocytes | 5.2 ± 2.1 | 4.2 ± 1.9 | .01 |
| Neoplasm | 13 | 3 | 1 | Hb, mg/dL | 131 ± 20 | 128 ± 18 | .37 |
| HIV | 23 | 6 | 1 |
Note: Boldface P values indicate statistical significance.
ALT, Alanine aminotransferase, AP, alkaline phosphatase; AST, aspartate aminotransferase; Cr, creatinine; DM, diabetes mellitus; GGT, gamma glutamil transpeptidase; Hb, hemoglobin; HE, hepatic encephalopathy; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; PSVD, portosinusoidal vascular disorder; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; TIPS, transjugular intrahepatic portosystemic shunt.
Supplementary Table 5.
Risk Factors for SARS-COV-2 Infection in SVT
| SVT | No infection (n = 462), n ± SD | Infection (n = 77) n ± SD | P | No infection (n = 462), n ± SD | Infection (n = 77), n ± SD | P | |
|---|---|---|---|---|---|---|---|
| Age | 52 ± 16 | 48 ± 19 | .6 | Anticoagulation | 310 (67%) | 61 (79%) | .03 |
| Sex (male) | 208 | 30 | .38 | TIPS | 10 | 3 | .41 |
| Alcohol | 52 | 13 | .29 | IBD | 7 | 6 | .01 |
| Tobacco | 59 | 9 | .07 | Bilirubin, mg/dL | 1.5 ± 1.9 | 2.6 ±2.8 | .01 |
| Decompensation | 115 | 14 | .19 | Albumin, mg/dL | 40 ± 4.7 | 39 ± 5 | .06 |
| Variceal bleeding | 66 | 11 | 1 | PT, % | 38 ± 44 | 55 ± 41 | .04 |
| Ascites | 67 | 6 | .11 | AST, U/L | 26 ± 12 | 27 ± 13 | .78 |
| HE | 7 | 2 | 1 | ALT, U/L | 29 ± 17 | 29 ± 16 | .43 |
| Comorbidities | 180 | 38 | .16 | AP, U/L | 146 ± 119 | 97 ± 60 | .47 |
| Arterial hypertension | 76 | 22 | .02 | GGT, U/L | 57 ± 99 | 62 ± 77 | .75 |
| DM | 40 | 11 | .21 | Platelets | 184 ± 97 | 210 ± 110 | .06 |
| Obesity | 64 | 17 | .08 | Cr, mg/dL | 0.95 ± 0.30 | 1.1 ± 1.0 | .72 |
| Neoplasm | 7 | 0 | .60 | Leucocytes | 5.5 ± 2.3 | 5.1 ± 2.7 | .21 |
| Myeloproliferative neoplasm | 101 | 15 | .68 | Hb, mg/dL | 138 ± 20.8 | 130 ± 26 | .37 |
| HIV | 0 | 0 | 1 | APS | 6 | 0 | .59 |
| Thrombophilia | 64 | 11 | .59 |
Note: Boldface P values indicate statistical significance.
ALT, Alanine aminotransferase, AP, alkaline phosphatase; APS, antiphospholipid syndrome; AST, aspartate aminotransferase; Cr, creatinine; DM, diabetes mellitus; GGT, gamma glutamil transpeptidase; Hb, hemoglobin; HE, hepatic encephalopathy; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SVT, splanchnic vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt.
Supplementary Table 6.
Multivariate Analysis of Risk Factors for SARS-CoV-2 Infection in SVT
| Variables | Univariate analysis |
Multivariate analyisis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Arterial hypertension | 2.0 (1.2–3.5) | .02 | ||
| Anticoagulation | 1.87 (1.0–3.3) | .03 | ||
| Inflammatory bowel disease | 5.5 (1.8–16.8) | .01 | 4.3 (1.1–17.2) | .04 |
| Bilirubin, mg/dL | 1.2 (1.1–1.4) | .01 | 1.2 (1.1–1.4) | < .01 |
| Protrombin time, % | 1.0 (1.0–1.0) | .01 | ||
CI, Confidence interval; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis.
Supplementary Table 7.
Severity of SARS-CoV-2 Infection and Need for Hospital Admission, ICU Admission, and COVID-19-related Mortality, Stratified by Age Groups
| Admission, n (%) | P | ICU, n (%) | P | Death, n (%) | P | |
|---|---|---|---|---|---|---|
| Age group <40 y | ||||||
| GP (n = 1,410,888) | 17,069 (1.2) | 1217 (0.1) | 191 (0) | |||
| PSVD (n = 9) | 1 (11) | .00 | 1 (11) | .00 | 1 (11) | .00 |
| SVT (n = 19) | 3 (16) | .00 | 1 (5) | .00 | 0 | 1 |
| Age group 40–59 y | ||||||
| GP (n = 966,713) | 54,529 (5.60) | 6026 (0.6) | 2059 (0.20) | |||
| PSVD (n = 30) | 3 (10) | .23 | 0 | .66 | 0 | .8 |
| SVT (n = 38) | 6 (16) | < .01 | 1 (2.6) | .12 | 2 (5) | < .01 |
| Age group 60–69 y | ||||||
| GP (n = 285,591) | 38,881 (13.6) | 6346 (2.2) | 4247 (1.5) | |||
| PSVD (n = 10) | 0 | .24 | 0 | .78 | 0 | .69 |
| SVT (n = 12) | 3 (25) | .32 | 0 | .75 | 0 | .65 |
| Age group >70 y | ||||||
| GP (n = 369,143) | 10,5840 (28) | 6724 (1.8) | 39,132 (10.6) | |||
| PSVD (n = 4) | 1 (25) | .87 | 1 (25) | .00 | 0 | .49 |
| SVT (n = 8) | 2 (25) | .81 | 0 | .7 | 2 (25) | .33 |
Note: Boldface P values indicate statistical significance.
COVID-19, Coronavirus disease 2019; GP, general population; ICU, intensive care unit; PSVD, portosinusoidal vascular disorder; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis.
References
- 1.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sultan S., Altayar O., Siddique S.M., et al. AGA Institute AGA Institute Rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jothimani D., Venugopal R., Abedin M.F., et al. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marjot T., Webb G.J., Barritt A.S., et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjot T., Buescher G., Sebode M., et al. Contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linares L., Cofan F., Diekmann F., et al. Hospital Clínic COVID-19 research group. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One. 2021;16:e0247251. doi: 10.1371/journal.pone.0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valla D.C., Cazals-Hatem D. Vascular liver diseases on the clinical side: definitions and diagnosis, new concepts. Virchows Arch. 2018;473:3–13. doi: 10.1007/s00428-018-2331-3. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Gea V., De Gottardi A., Leebeek F.W.G., et al. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol. 2019;71:175–199. doi: 10.1016/j.jhep.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Gea V., Baiges A., Turon F., et al. Idiopathic portal hypertension. Hepatology. 2018;68:2413–2423. doi: 10.1002/hep.30132. [DOI] [PubMed] [Google Scholar]
- 12.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiezia L., Boscolo A., Poletto F., et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A.K., Jena A., Kumar-M P., et al. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J. 2021;9:159–176. doi: 10.1177/2050640620972602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buso G., Becchetti C., Berzigotti A. Acute splanchnic vein thrombosis in patients with COVID-19: a systematic review. Dig Liver Dis. 2021;53:937–949. doi: 10.1016/j.dld.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]