Abstract
The interaction of nanoparticles with the biological system has increased with the increasing popularity of nanomedicines. Red blood cells (RBCs) are very sensitive, and abundant cells in the blood. They are highly prone to oxidative damage due to constant interaction with oxygen itself, foreign particles in the blood, and the lack of repair mechanism. The cell membrane of RBCs undergoes lipid peroxidation, protein oxidation, and heme degradation which results in altered membrane permeability, changes in the morphology, and functioning of RBCs. The nanoparticles induce oxidative stress, hemolysis, morphological changes, membrane deformability, and alterations in hemoglobin structure in RBCs. In this review, the effects of metallic nanoparticles and their modifications on the physiology, and life span of RBCs are discussed. The detailed analysis of the antioxidant enzymes-like activity of metal nanoparticles is expected to highlight the beneficial use of these metal nanoparticles in RBCs against oxidative stress and the development of new biosafe nanodrugs.
Keywords: Metal nanoparticles, Oxidative stress, Nanozymes, Red blood cells, Hemoglobin
Introduction
Red blood cells (RBCs) are the most abundant cells in the blood. RBCs are simple cells that lack a nucleus and other organelles. That’s why the shape and stability of cells depend mainly on the cell membrane. The primary function is to facilitate the transfer of gases throughout the body of an organism. Simultaneously, RBCs also maintain the redox balance, nitric oxide (NO) metabolism, the vascular tone which makes them highly sensitive cells to oxidative damage. The RBCs have a biconcave disc shape, peculiar for their function. They are very flexible cells, can change their shapes according to the environment. An RBC has a lifespan of 120 ± 20 days. RBCs are unable to synthesize any amino acids, fatty acids, or any metabolism due to the loss of organelles. The transport of gases is performed by hemoglobin (Hb), a red-colored protein with a molecular weight of 68 kD. Hb is the main intracellular content of RBCs. The lower concentration of Hb in blood and the lower number of RBCs in circulation leads to anemia. Anemia is a pathological condition that can be associated with several other complications such as aging, cardiovascular diseases, neurodegenerative diseases.
To maintain the primary function of RBCs i.e., transport of oxygen and carbon dioxide mainly, Hb has to be sustained in a reduced state. Hb in the oxidized form will drastically lose affinity to oxygen. Therefore, RBCs have to regulate redox balance. However, there are challenges like free iron that can facilitate the production of reactive oxygen species (ROS) via Fenton reaction, various oxidants including oxygen, poor repair mechanism due to loss of protein expression upon maturation. The RBCs are equipped with antioxidant systems to counter oxidative damage. Both enzymatic systems (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx)) and non-enzymatic systems (ascorbate/dehydroascorbate (vitamin C), reduced and oxidized glutathione (GSH/GSSG), and α-tocopherol (vitamin E)) are available to RBCs. The imbalance of oxidants and antioxidants has severe consequences on the function of Hb, other cytoplasmic proteins, ionic homeostasis, and membrane integrity. These damages are signals of abnormal conditions in the body. Many antioxidants like flavonoids have been applied during oxidative stress in RBCs. Two naturally occurring flavonoids, Quercetin (3,3’,4’,5’,7-pentahydroxyflavone) and myricetin (3,3’,4’,5’,5’,7-hexahydroxylflavone) were applied in RBCs to investigate their antioxidant role against oxidative stress (Maurya et al. 2016). Quercetin significantly reduced the malondialdehyde (MDA) content generated after the lipid peroxidation caused by t-BHP induced oxidative stress at the concentration of 10−8 M and 10−7 M. Myricetin reduced MDA content at 10−6 M and 10−5 M, hence proving to be a more efficient antioxidant. Both quercetin and myricetin showed an increase in GSH levels in RBCs.
With rapidly developing nanotechnology, nanomaterials have been applied in a wide range of biomedical areas such as drug delivery, bioimaging, biosensors, and nanozymes. Numerous metallic nanoparticles (NPs) have been synthesized and applied to RBCs. RBCs have proved to be an efficient model to evaluate the efficacy of NPs in-vitro (Wadhwa et al. 2019). Nanoparticles are particles in the size range of 1 to 100 nm. They are clusters of a few 100 atoms which behave differently from bulk material. Nanoparticles have unique physical and chemical properties. Some nanoparticles like zinc oxide NPs and silver NPs are well-known for their toxicity to induce oxidative stress in the biological system. The exposure of zinc oxide NPs led to DNA fragmentation, generation of ROS in human lung fibroblasts and led to the reduced viability of Drosophila melanogaster which was associated with zinc oxide NPs induced oxidative stress (Ng et al. 2017). Similarly, the polymer-coated silver NPs when incorporated in the diet of Drosophila melanogaster, induced heat shock proteins, oxidative stress that was assessed by the increased levels of ROS and decreased levels of glutathione (Ahamed et al. 2010). Moreover, the toxicity of silver NPs led to cellular apoptosis in flies, measured through the increased concentrations of caspase3 and caspase9 in cells. Whereas nanoparticles such as cerium oxide NPs (CeO NPs), gold NPs (AuNPs), iron oxide NPs (IONPs) have an intrinsic property to exhibit scavenging activity of ROS (Singh 2019). Hence, nanoparticles show antioxidant enzyme-like activity. They mimic superoxide dismutase enzyme to convert superoxide anions (O2•−) into hydrogen peroxide (H2O2), catalase enzyme to convert H2O2 into water (H2O) and oxygen (O2), and pro-oxidant enzymes like peroxidase and oxidase enzymes which generate free radicals from H2O2. The nanoparticles behaving as natural enzymes are popularly known as nanozymes. Nanozymes can be classified into two categories: oxidoreductase family and hydrolase family. The inorganic materials-based enzymes are more stable, durable and easy to synthesize than natural enzymes. Various novel metal, metal oxide, and metal sulfide nanozymes have been prepared and applied in biomedical fields (Alizadeh and Salimi 2021; Yadav and Maurya 2021; Murugan et al. 2019).
This review is aimed to review the structural changes in RBCs cell membrane, membrane proteins, changes in Hb due to oxidative stress. It also underlines the interaction of nanoparticles with RBCs, the effect of nanoparticles on the shape and function of RBCs. The antioxidant activity of nanoparticles against oxidative stress will also be discussed.
Oxidative stress in RBCs
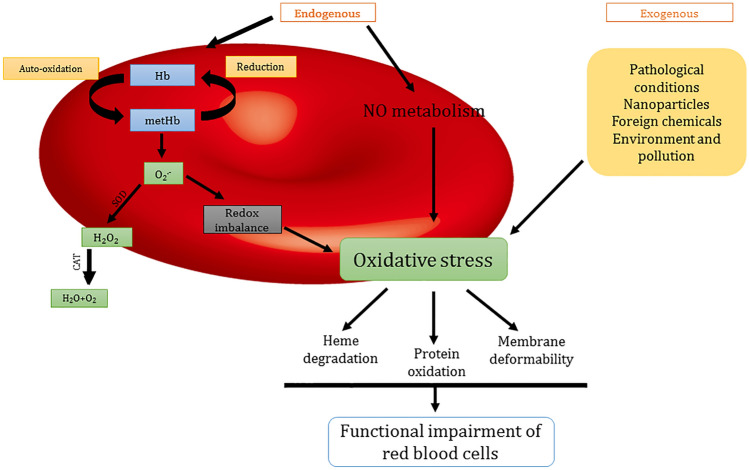
The primary physiological function of RBCs is to transport oxygen to tissues. However, to preserve the Hb structure from oxidation, RBCs also regulate redox balance (Kuhn et al. 2017). The auto-oxidation of Hb generates metHb (Fe3+Hb) and superoxide radical anion. The Fe3+ generally dissociates from the complex, this free iron then takes part in the Fenton reaction to produce hydroxyl radicals (OH•), H2O2, and catalyze the further production of ROS. The Nitric oxide is constantly produced by endothelial cells. Hb of RBCs is thought to be a sink for this nitric oxide generation. Nitric oxide further oxidizes the Hb and results in ROS generation. To counter oxidative stress, RBCs are enabled with enzymatic and non-enzymatic antioxidants. During pathological conditions, antioxidant systems fail to regulate oxidative damage levels. Oxidative stress in RBCs leads to heme degradation, lipid peroxidation in the membrane, membrane deformability which changes the functionality of RBCs. The schematic representation of RBCs alterations induced by oxidative stress is presented in Fig. 1.
Fig. 1.
Fig. 1 is showing the causes and effects of oxidative stress in red blood cells. The endogenous factors such as the auto-oxidation of hemoglobin, NO metabolism, and exogenous factors such as pathological conditions, interaction with nanoparticles, other foreign chemicals, environment, and pollutants are the main causes of oxidative stress in red blood cells. Oxidative stress causes hemoglobin degradation, oxidation of other proteins, the deformability of membrane, and other morphological changes which lead to functional impairment of red blood cells. (Hb—hemoglobin, SOD—superoxide dismutase, CAT—catalase, NO—nitric oxide)
The membrane of erythrocytes is the most prone to ROS and a secondary by-product is formed known as MDA. It has been observed that MDA levels were positively correlated with age i.e., the MDA levels in erythrocytes increased with increasing age (Rizvi and Maurya 2007). This study also reported the reduced levels of -SH membrane proteins due to reduced levels of reduced GSH, an intracellular enzyme, with increasing age. The alterations in -SH membrane proteins lead to the deformability of the RBC membrane and are related to various pathologies. The ROSs induce cell membrane deformability in RBCs which leads to the formation of induced microvesicles (MV). Sudnitsyna et al. observed that oxidative stress induced by tert-Butyl hydroperoxide (t-BOOH) in RBCs led to irreversible deformability of cell membrane, morphological transformation, MV formation, and band 3 clustering (Sudnitsyna et al. 2020). Hb is oxidized in RBCs during oxidative stress and formed HbFe3+ (ferric Hb), HbFe4+ (ferryl Hb), and •HbFe4+ (ferryl radical referred to as hemichromes, HbChr) and led to heme loss. The oxidation of Hb can be explained by various mechanisms (Wang and Zennadi 2020; Mohanty et al. 2014; Sudnitsyna et al. 2020). The autoxidation of ferrous hemoglobin (Fe2+-Hb) which normally carries oxygen results in HbFe3+ (cannot bind to oxygen) and superoxide anions.
The superoxide anions are dismutated into hydrogen peroxide and this hydrogen peroxide when reacts with Fe2+-Hb and HbFe3+ degrades Hb and frees the iron.
And, RBCs release the NO derivatives during the transport of oxygen to balance redox status. The NO results in vasodilation and reacts with Fe2+-Hb, forming HbFe3+ and nitrate.
Maurya et al. showed that the activity of enzyme GPx decreased in erythrocytes in an age-dependent manner (Maurya et al. 2010). There was a positive correlation between GPx activity and the total antioxidant status of plasma. The decrease in the antioxidant capacity of erythrocytes is due to the increased production of ROS with age. The increased ROS generation with age alters the transport proteins like calcium ATPases and sodium/potassium ATPases and their activities. Maurya and Prakash demonstrated a significant decrease in activities of both membrane-associated proteins, Ca2+ ATPases and Na+/K+ ATPases of erythrocytes in both males and females with increasing age (Maurya and Prakash 2013) and hypertensive patients (Kumar et al. 2012). The imaging by the two-photon fluorescence provided clear evidence of increased oxidative damage in human living RBCs in young, middle-aged, and elderly age groups (Tsakanova et al. 2020). Total RBC count decreased and the hemolysis rate increased with increasing age. This study showed that antioxidant enzymes like catalase, superoxide dismutase, and the ferroxidase activities of ceruloplasmin decreased significantly with age. Oxidative stress plays a major role in the early maturation of RBCs and their removal from circulation. The accumulation of oxidative damage and DNA damage in hematopoietic stem cells reduces the replicative properties of these stem cells (Ghaffari 2008). The aged stem cells lose their function and can become cancerous or die. Oxidative stress suppresses various pathways in stem cells such as nuclear factor erythroid 2-related factor 2 (Nrf2), p53 activity, Wnt/β-Catenin pathway, and NF-κB pathway (Chen et al. 2017). Bisphenol A (BPA) and its similar compounds like bisphenol S (BPS), bisphenol AF (BPAF), and bisphenol F (BPF) are broadly applied in the production of several everyday use products, leading to the day-to-day exposure of humans to such substances. The effect of bisphenols on oxidative stress in non-nucleated cells has been evaluated (Maćczak et al. 2017). The RBCs were incubated with compounds for 1, 4, or 24 h and in concentrations ranging from 0.1 to 500 μg/ml. It has been observed that compounds enhanced ROS formation, lipid peroxidation, depleted GSH levels, and altered the activities of SOD, CAT, and GSH-Px. It was established that BPAF induced the most notable alterations in the redox system of erythrocytes, which changed CAT and SOD activity even at 0.5 μg/ml. Misfolded specific proteins such as α-synuclein (a-syn), β-amyloid (Aβ), and tau were shown to be accumulated in RBCs when oxidative stress was induced in the presence of H2O2 (Iofrida et al. 2017). Accumulation of such proteins in the brain and peripheral tissues is a hallmark of neurodegenerative diseases. There is an urgent need to develop diagnostic and therapeutic tools to mitigate the oxidative damage in cells like RBCs which are integral for normal functioning of whole body.
Interaction of metallic NPs with RBCs
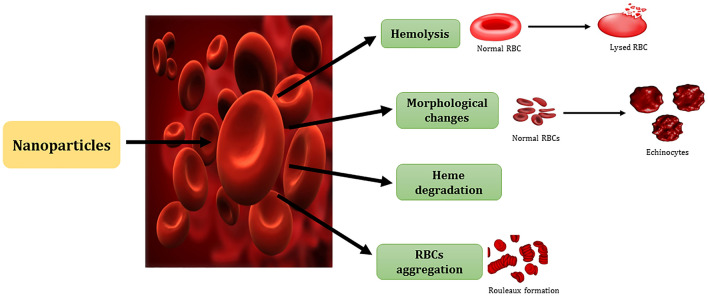
With the increasing popularity of NPs, they have been used extensively in biological systems. Intravenous is the most optimal route of administration of NPs for better bioavailability. Different administration routes such as intravenously (IV), perorally (PO), and intraperitoneally (IP) were observed in 8-week-old CD1 female mice for biodistribution of nanoceria (Hirst et al. 2013). It was found that IV deposited the greatest nanoceria, followed by IP and then PO. The spleen was the organ with the highest deposit, followed by the liver, lungs, and kidneys. Nanoceria were excreted through feces irrespective of the route, PO administered mice excreted 98% of nanoceria. Nanoceria did not induce any toxicological or pathological reactions in tissues of mice and it was established that the IV route is the optimal administration route for nanoceria. This study suggests that nanoceria due to their biocompatibility are potential antioxidant materials that are safe to apply in biological systems. Hence, the biocompatibility of NPs becomes a major concern. The RBCs are the first cell that directly interact with NPs. The direct toxicity of metal oxide nanoparticles like cerium oxide, zinc oxide, aluminum oxide, and tin oxide in erythrocytes was studied (Subramaniam et al. 2020). After the incubation of NPs for 3 h, it was observed that hemolysis percentage increased with an increase in concentration and decrease in size of NPs. The metal oxide nanoparticles showed hemocompatibility as the hemolytic percentage was less than 2% in the case of all NPs except zinc oxide which showed 3% hemolysis. The NPs distort the secondary structure of Hb and convert the α-helical structure into β-sheets. The effect of ZnO NPs of size 25 nm was studied on the osmotic fragility of the erythrocyte membrane, morphology, and as a result on hemolysis (Shirsekar et al. 2016). The effect of ZnO on the osmotic fragility of RBCs was negligible as 97% of the cells were stable even at the concentration of 100 µg/ml. The membrane did not break but NPs certainly affected the morphology of the membrane. In isotonic solutions, the hemolysis activity slightly increased with an increase in concentration but it varied in the case of hypotonic solution of NPs. RBCs showed aggregation to some degree in response to the interactions with higher concentrations of ZnO NPs. The NPs first and foremost come in contact with the lipid membrane of RBCs. NPs were shown to bind or get adsorbed on lipid bilayer by electrostatic and hydrophobic interactions due to the charge interaction on membrane and nanoparticles (Dias et al. 2019). Other than membrane lipid bilayer, NPs bind to membrane proteins like band 3 and spectrin and lead to changes in membrane integrity, permeability, and fluidity (Tian et al. 2021). The interaction itself and the oxidative stress induced by nanoparticles cause the structural deformation of membrane proteins, ultimately deforming the membrane of RBCs. The changes in the membrane lipid bilayer and proteins alter the morphology of erythrocytes from biconcave to echinocytes and discocytes. The oxidative stress caused by NPs also puts direct assault on membrane lipids inducing lipid peroxidation. This ongoing distortion of RBCs affects the microviscosity of cells which directly affects the lifespan of RBCs. The interactions of NPs with RBCs are schematically represented in Fig. 2.
Fig. 2.
Fig. 2 is showing the interaction of nanoparticles with red blood cells. Nanoparticles cause hemolysis, morphological changes (conversion of discocytes into echinocytes), hemoglobin degradation, and red blood cells aggregation (rouleaux formation). (RBCs—red blood cells)
Some other nanoparticles such as aluminum dioxide (Al2O3), zirconium dioxide (ZrO2), and silica dioxide (SiO2) nanopowders were studied for their effect on microviscosity and morphology of erythrocyte membrane (Kozelskaya et al. 2016). ZrO2 increased the microviscosity of the membrane even at smaller concentrations whereas Al2O3 started to decrease the viscosity at very small concentrations. However, SiO2 did not affect the viscosity at lower concentrations but with increasing concentration, microviscosity of erythrocytes started to decrease slightly. 10% of the cells swelled up, 30% changed into echinocytes, 50% agglomerated and 10% remained intact after incubating with higher concentrations of SiO2 nanopowder shown by atomic force microscopy. The treatment of ZrO2 nanopowders too swelled up the cells and created cracks in the membrane of erythrocytes. In the case of Al2O3 nanopowder, all the erythrocytes changed into echinocytes from discocytes. Tiny vesicles with cube-like projections were observed on the surface along with cell adhesion alterations. It was shown that Al2O3 nanopowder was most toxic to RBCs. Nanoparticles interact with hemoglobin directly and affect their properties like changes in secondary, quaternary structures. Eskandari et al. showed the cerium oxide nanoparticles interact with hemoglobin through hydrophobic interactions (Eskandari et al. 2018). An NP-protein corona is formed that affects the structure of proteins, their aggregation, and this interaction also affects the availability of NPs (Mishra et al. 2021). A protein corona is formed around the inorganic nanomaterials when they are applied to a biological system. This protein corona helps the immune system to identify nanomaterials (Barbero et al. 2017). This is the major challenge for the biocompatibility of nanomaterials because it interferes with the targeting, specific activity, circulation time of nanoparticles (Pareek et al. 2018). However, this protein layer can be modified using biopolymers as capping agents on nanomaterials which can increase their biocompatibility (Berrecoso et al. 2020). The Nanozymes can also bind through electrostatic, hydrophobic, Vander Waal’s forces at specific sites on natural enzymes and inhibit their action. The NPs have been applied as inhibitors of various enzymes like R-chymotrypsin, β-galactosidase, mitochondrial ATPase (Huang et al. 2021). Cerium oxide NPs were shown to quench the fluorescence intensity of Hb. Other studies are summarized in Table 1. This wide range of studies aims to synthesize sensitive NPs towards biological systems. The harsh activities of NPs can be reduced by simple surface modifications. The coating of bovine serum albumin (BSA) on AuNPs has been shown to decrease the hemolysis percent of NPs in RBCs (Rahul et al. 2016). BSA-coated AuNPs were prepared in different sizes (15, 30, 50, and 70 nm) and showed negligible hemolysis in comparison to bare AuNPs. The hemolysis percent was maximum ~ 60% in the presence of 200 µg/ml of bare AuNPs whereas the same concentration and size of BSA-coated AuNPs exhibited only ~ 10% hemolysis in RBCs. This shows that the surface modifications on the surface of NPs can render the hemolytic activity of bare NPs and can protect the RBCs at the RBCs–NPs interface. Hence, it is important to study the effect of interactions of NPs with or without surface modifications on living cells.
Table 1.
Interactions of nanoparticles (NPs) and RBCs at cellular levels
| Nanoparticles | Interaction with membrane | Effect on hemoglobin | References |
|---|---|---|---|
| Zinc oxide nanoparticles with 0-307 nm | The hemolytic activity shown by only < 50 nm-sized NPs, showed structural and membrane damage which decreased with size increase | UV imaging showed significant changes in hemoglobin by < 50 nm-sized NPs, fluorescence intensity of hemoglobin decreased with increasing size and concentration of NPs, circular dichroism showed changes in the secondary structure of the protein with increasing size of NPs | (Preedia et al., 2017) |
| Amino-acid based gold nanoparticles ~ 20-25 nm | Less hemolytic than traditional AuNPs | No significant change in hemoglobin structure and function even at higher concentration | (Kumar et al, 2020) |
| Superparamagnetic iron oxide nanoparticles (SPIONs) with chitosan, hyaluronic acid, and polyacrylic acid in the size range of 5-6 nm | Polyacrylic acid-modified NPs showed the highest hemolysis in same-sized NPs and the rate increased with increasing concentration of NPs. Polyacrylic acid-modified NPs caused the greatest crenation of RBCs and led to morphological alterations with increasing size | During hemolysis, RBCs released hemoglobin which affected both structure and function of hemoglobin | (Liu et al, 2020a, b) |
| Gold and silver NPs ~ 50 nm | Both induced oxidative stress in RBCs but the thiol concentration was much lower in silver NPs treated cells. This resulted in a higher change in membrane permeability and membrane alterations in silver NPs treated cells than gold NPs treated cells | The interaction of NPs deoxygenated the hemoglobin studied by Raman spectra and resulted in the loss of functionality. It was established that silver NPs are more harmful than gold nanoparticles | (Barkur et al, 2020) |
| Bovine serum albumin (BSA)-conjugated gold NPs ~ 540 nm | BSA conjugated gold NPs demonstrated negligible hemolysis and morphological changes when compared with traditional gold NPs | Negligible hemolysis signifies no change in the structure and function of hemoglobin protein | (Hameed et al, 2018) |
Protective role of NPs during oxidative stress
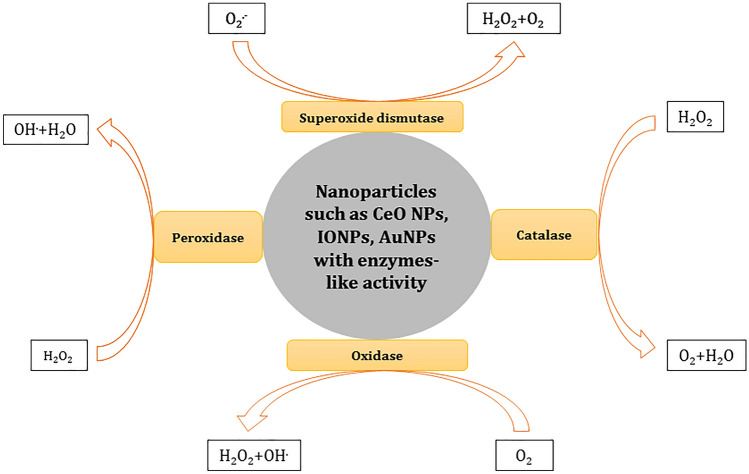
Biological systems like RBCs are enabled with enzymatic and non-enzymatic antioxidants. Metal nanozymes can behave like pro-oxidant and antioxidant enzymes. Pro-oxidant enzymes are the ones that induce oxidative stress while the antioxidant enzymes such as catalase, superoxide dismutase, peroxiredoxin, glutathione peroxidase, scavenge free radicals formed during oxidative stress (Lewandowska et al. 2021). Two nanoemulsions Ethyl acetate fraction-loaded pomegranate seed oil nanoemulsion (EAF-PSO-NE) and EAF-loaded medium-chain triglyceride nanoemulsion (EAF-MCT-NE) were prepared in the size range of 201 to 205 nm (Baccarin et al. 2015). The synthesized nanoemulsions protected the RBCs from H2O2 and 2,2′-azobis (amidinopropane dihydrochloride) (AAPH)-induced oxidative stress. The free EAF, EAF-loaded PSO, and MCT NE maintained the erythrocyte membrane fluidity in both inner and outer sides by maintaining the baseline levels of membrane proteins. The AAPH and H2O2 induced oxidative stress led to the loss of spectrin proteins and band 3 proteins. Further SEM analysis showed the changed morphology of RBCs due to oxidative stress. AAPH treatment led to the formation of echinocytes. Therefore, this in-vitro study suggests that EAF-loaded PSO and EAF-loaded MCT nanoemulsions can be applied as an antioxidant. The intrinsic ability of nanoparticles to act as antioxidant enzymes was discovered in 2007. Gao et al. for the first time demonstrated the peroxidase activity of iron oxide NPs (Gao et al. 2007). After this discovery, various nanoparticles have been studied and applied to scavenge free radicals. Here, we examined the antioxidant properties of mainly cerium oxide nanoparticles, iron oxide nanoparticles, and gold nanoparticles. The protective effect of nanoparticles has been widely studied as shown in Table 2. These metal nanoparticles are the most extensively applied in biomedical fields. The antioxidant properties of different NPs are presented in Fig. 3.
Table 2.
Studies on the protective effect of nanoparticles in biological systems
| Nanoparticles | Size | Results | References | |
|---|---|---|---|---|
| Bovine serum albumin-coated magnesium nanoparticles | ~ 4–8 nm | BSA-coated magnesium nanoparticles showed catalase-like activity. The synthesized nanoparticles protected the hepatocytes from the 3-AT induced oxidative stress and degraded the hydrogen peroxide into water and oxygen. The 3-AT exposure could not affect the catalase-like activity of the nanoparticle | (Shah et al, 2020) | |
| Nanoceria–gold nanoparticles conjugates with a coating of polyoxometalate, 12-phosphotungstic acid | Nanoceria ~ 27 nm and gold NPs ~ 10 nm | The nanoceria and gold NPs form a multienzyme complex in synthesized conjugates. Polyoxometalate,12-phosphotunstic acid-coated nanoceria improved the catalytic activity of gold NPs and showed 82% better efficiency in converting the 4-nitrophenol into 4-aminophenol in vitro. The gold NPs showed increased peroxidase-like activity in nanocomposites | (Shah et al, 2021) | |
| Nanocomposites of cerium oxide NPs, sulforaphane loaded on silk fibroin NPs (SFSNPs), and carbon dots (CDs) | ~ 365–385 nm | CeNPs–CDs–SFSNPs nanocomposites, multifunctional formulations which showed diagnostic and therapeutic properties, were prepared. Sulforaphane is an antioxidant drug. The CeNPs catalyzed the neutralization of the ROS, whereas CDs acted as a probe for fluorescence imaging of cells, simultaneously. Hence, the nanocomposites behaved as carriers, antioxidants, and probes for imaging | (Passi et al, 2020) | |
| Poly (ethylene glycol) and poly (acrylic acid) coated cerium oxide NPs | ~ 7.8 nm | The synthesized cerium oxide NPs have been studied for their catalytic activities to mimic antioxidative enzymes. The coatings of poly (ethylene glycol) and poly (acrylic acid) on cerium oxide NPs inhibited the catalase-like activity, impaired oxidase-like activity, had not affected the superoxide dismutase-like activity, and enhanced the peroxidase-like activity of NPs | (Baldim et al. 2020) | |
| Cerium vanadate nanorods | ~ 50–150 nm | Superoxide dismutase-like activity of synthesized nanorods was investigated in the SH-SY5Y, neuronal cells. They neutralized the superoxides which were generated due to the exposure of diethyldithiocarbamate (DDC), a SOD inhibitor. The nanorods also retained the structural integrity of mitochondria | (Singh et al, 2021) | |
| A PTEN plasmid and a siRNA carrying cerium oxide nanoparticles | ~ 98–136 nm | The PTEN is a tumor suppressor gene and its loss results in overexpression of phosphatidylinositol 3-kinase (PI3K)/AKT pathway which leads to increased cellular proliferation. The cerium oxide nanoliposomes incorporating the PTEN plasmid and AKT3 siRNA were studied internalized in the prostate cancer cell culture model (PC-3) and shown to internalize by endocytosis. The nanoliposomes induced DNA fragmentation then apoptosis which ultimately led to cell death. The cerium oxide NPs in the formulation behaved as the SOD enzyme and reduced the excess levels of free radicals from cancer cells | (Bhagat and Singh, 2020) | |
| Cerium oxide nanorods | ~ 1.88 nm | The WRL-68 cells (hepatocyte model) were treated with Buthionine sulfoximine (BSO). BSO depletes the cells of GSH and induces redox imbalance. This study showed the SOD-like activity of synthesized cerium oxide nanorods which catalyzed the neutralization of free radicals generated during the BSO exposure in cells | (Yadav and Singh, 2021a, b) | |
| Cobalt-doped iron oxide nanozymes | ~ 94.6 ± 8.6 nm | The Co-Fe3O4 nanoparticles when applied in human renal cancer cells, killed the cells by inducing oxidative stress. The synthesized nanoconjugates switched on the oxidative stress in the presence of H2O2, which led to the bursting and killing of the cancer cells. The nanoparticles typically behaved as peroxidase enzyme activity and killed the cancer cells | (Wang et al, 2019) | |
Fig. 3.
Fig. 3 is showing the enzyme-like activity of metal nanoparticles. Metal nanoparticles behave as superoxide dismutase, catalase, oxidase, and peroxidase enzymes
Cerium oxide NPs
The antioxidant ability of cerium oxide nanoparticles has been attributed to the auto-regenerative cycling of Ce3+, and Ce4+ ions on their surface. This interchangeability causes oxygen vacancies at the surface of CeO NPs and catalyzes the neutralization of free radicals (Sarnatskaya et al. 2020). CeO NPs which are also known as nanoceria, show both SOD and catalase-like activity due to high Ce3+ ions and Ce4+ ions, respectively (Singh et al. 2018). The enzyme mimicking the activity of CeO NPs has been well researched in-vitro and in almost all kinds of models including cell culture, animal models, and RBCs. The cerium oxide NPs were shown to exhibit peroxidase activity but inhibited the H2O2 induced killing of both Gram-negative and Gram-positive bacteria (Zhu et al. 2021). Hence, it is clear that CeO NPs show multiple enzyme-like properties due to their shape, size, and environment interaction. The oxidative damage induced by 3-Amino-1,2,4-triazole (3-AT) toxicity inhibited the antioxidant enzyme catalase irreversibly that led to the deposition of free radicals and ROS (Singh and Singh 2019). A hepatocyte WRL-68 cell-culture model was treated with 3-AT that caused cytotoxicity. However, when the WRL-68 cells which were pre-incubated with CeO NPs exposed to 3-AT did not die and endured the toxicity very well. The cellular morphology was also altered from elongated to the sphere by the exposure of 3-AT which was retained in the elongated form in pre-incubated cells with CeO NPs. The study demonstrated that CeO NPs protected the cells from oxidative DNA fragmentation by ROS and apoptosis induced by 3-AT. The CeO NPs are highly recognized nanoparticles and have been applied in biological systems to scavenge oxidative damage. The surface of these CeO nanoparticles needs to be modified using chemical ligands to change the activity as well as the interaction of nanoparticles with biological systems. Phosphomolybdic acid (PMA) and phosphotungstic acid (PTA), two surface ligands altered the CAT and SOD-like activity of CeO NPs (Yadav and Singh 2021a). The PMA did not affect the CAT-like activity but repressed the SOD-like activity of NPs, whereas PTA improved the ability of cerium oxide NPs to mimic antioxidant enzymes. Cerium oxide nanoparticles protected RBCs from damages caused by hyperthermia (Liu et al. 2020b). This short-term hypothermia increased the density of older cells, increased the generation of ROS, hence, induced apoptosis. Phosphatidylserine was found to be on the surface of RBCs that showed induced apoptosis due to hypothermia. The pre-treated erythrocytes with CeO NPs showed less generation of ROS and less apoptosis. CeO NPs have been applied to the whole blood and shown to improve the life span and ATP content of stored RBCs (Rzigalinski et al. 2020). The NPs preserved the ATP content of RBCs for 42 days. The maximum decline was up to 27% on the 42nd day. The 10 nM and 100 nM of CeO NPs also retained the morphology and number of RBCs. CeO NPs helped the RBCs endure oxidative stress. Cerium oxide nanoparticles embedded scaffolds for bone grafts were prepared to investigate the role of CeO NPs in angiogenesis by increasing calcium ion channels of mesenchymal stem cells (MSCs) (Xiang et al. 2016). CeO NPs as an antioxidant promoted the growth of MSCs and improved the co-culture of EPCs which enhanced the blood vessel formation. The underlying mechanism was the increase in VEGF secretion which indirectly depends on the calcium channels of MSCs. This study established that CeO NPs increased the free calcium levels in pre-incubated cells through activation of calcium channels at the plasma membrane of MSCs. Cerium oxide NPs inhibited the effect of oxidative stress caused by NADPH oxidase (NOX) activation in UV-B irradiated skin cells (Peloi et al. 2021). CeO NPs behaved as superoxide dismutase and catalase enzymes and mitigated the neutrophils' oxidative damage, by decreasing ROS production, therefore, decreasing the cellular damages. This study suggests the potential utility of CeO NPs in controlling NOX-associated oxidative stress in erythrocytes. Acid hemolysis generates oxidative stress in RBCs of old rats. The nanoceria was applied to mitigate the oxidative damage generated by acid hemolysis in erythrocytes (Kotsuruba et al. 2016). A dose of 0.1 mg/kg of nanoceria was given for 14 days to old rats, nanoceria fully stabilized the RBCs, reduced the ROS generation in RBCs and plasma. Cerium oxide nanoparticles are hemocompatible and show antioxidative enzymes-like activity which makes them safe to treat the oxidative stress complications of RBCs.
Iron oxide NPs
The IONPs like Fe2O3, Fe3O4 have been thoroughly researched. They have been used in probes during magnetic resonance imaging (MRI), near-infrared fluorescence (NIRF) imaging, Positron emission tomography (PET). IONPs have also been applied as biosensors in the living system to detect glucose during cancer diagnosis (Vallabani and Singh 2018). IONPs show peroxidase and oxidase-like activities. The peroxidase activity of IONPs is in the presence of H2O2 and depends on the pH, temperature of the solutions. ATP enhanced the catalytic property of IONPs to measure increased glucose levels in the blood serum of patients (Vallabani et al. 2017). This one-step detection has been used to detect glucose from diabetic to cancer patients. The IONPs incorporated into the diet of Drosophila melanogaster, enhanced the life span of the fruit flies (Zhang et al. 2016). The daily consumption of 200 µg IONPs improved the climbing capacity of 6-week-old flies by reducing oxidative damage at cellular levels. The IONPs treatment additionally improved the life span of flies from normally 49 days to 57 days. Iron oxide nanozymes can be applied as multifunctional enzymes. Guo and Guo showed that IONPs intrinsically exhibit antioxidative enzymes such as CAT, SOD, peroxidase-like activity (Guo and Guo 2019). IONPs behaving as CAT catalyze the degradation of hydrogen peroxide into oxygen and water.
Three possible reaction mechanisms, base-like dissociative mechanism, acid-like dissociative mechanism, and bihydrogen peroxide associative mechanism of CAT-like activity of IONPs were described. Similarly, IONPs behaving SOD enzyme, catalyze the conversion of OOH radicals into oxygen and hydrogen peroxide.
And two possible reaction mechanisms Langmuir–Hinshelwood mechanism and Eley–Rideal mechanism of SOD-like activity of IONPs were explored. IONPs behaving like peroxidase oxidize the TMB in the presence of H2O2. The catalytic activities of IONPs depended on different morphologies and sizes of NPs. IONPs due to effective peroxidase-like activity received great attention for antibacterial applications (Vallabani et al. 2020). The peroxidase activity of IONPs is pH-dependent and restricted to acidic pH. At near neutral or neutral pH, the IONPs lose peroxidase activity, which is the major hurdle to their antibacterial activity. The IONPs were coated with citrate and ATP was used as a synergistic agent to endure the wide range of pH and maintain the peroxidase activity i.e., to generate free radical OH•. The novel IONPs with their synergistic combination of 30 µg/mL IONPs and 2.5 mM ATP showed high bactericidal activity against both Gram-negative and Gram-positive bacteria even at neutral pH in the presence of H2O2. Similarly, the effects of buffers, pH, and ATP on the peroxidase activity of IONPs were studied. This study established mechanisms to enhance the activity of IONPs even at neutral pH (Vallabani et al. 2019). It was elucidated that generation of OH• radical due to the synergistic combination of nucleotides and IONPs gave rise to peroxidase activity at neutral pH. These findings overcome the limitations of IONPs to be used only in acidic pH. Hence, the IONPs act as all antioxidant enzymes and are applied as a versatile detection tool, antibacterial activity, to enhance cellular longevity. IONPs usually induce oxidative stress in RBCs. However, the IONPs can overcome these limitations with surface modifications, different sizes, or different morphologies during further research.
Gold NPs
The inorganic AuNPs have intrinsic enzyme-like biological activities such as peroxidase-like activity, oxidase-like activity. AuNPs have been widely explored in biomedical fields due to the well-known surface chemistry of AuNPs. Novel gold nanoparticles reduced and capped with gallnut extract (GNE) were synthesized (Deshmukh et al. 2021). The novel AuNPs were shown to have peroxidase and glucose oxidase-like activity to catalyze multienzyme cascade reactions. First, the glucose oxidase-like activity of AuNPs was assessed. The AuNPs catalyzed the glucose oxidation into gluconic acid and H2O2 with an apparent Michaelis constant value of 0.089 mM. Second, in the presence of the H2O2 generated during in situ oxidation of glucose, the peroxidase-like activity of AuNPs was measured. AuNPs oxidized the TMB in the presence of H2O2 with an apparent Michaelis constant value of 0.118 mM. GNE-based AuNPs showed the optimum catalytic efficiency within a pH range of 6–8 at 40 °C. The Km values were low which contends for the great affinity of the AuNPs and both substrates. These multienzyme AuNPs can substitute peroxidase and glucose oxidase enzymes which are generally used to develop detection methods, like glucose level detection. AuNPs have found applications in the development of several biosensors. Shah et al. demonstrated the influence of ATP, ions, and other molecules on the peroxidase-mimicking activity of AuNPs (Shah et al. 2015). It is widely accepted that NPs usually lose the catalytic property when exposed to biomolecules. On the Contrary, the ATP and ADP addition improved the peroxidase-like activity of AuNPs whereas, they did not affect the horseradish peroxidase activity, the natural peroxidase enzyme. Free phosphates, carbonate anions, and sulfates did not alter the intrinsic peroxidase-like activity, however, ascorbic acid reduced the catalytic efficiency of AuNPs, irrespective of ATP and ADP. In addition to this study, Shah and Singh also revealed the effect of surface charge on the peroxidase-like activity of AuNPs (Shah and Singh 2018). AuNPs were coated with PEG, citrate, and Cetyl-N, N, N-trimethylammonium bromide (CTAB). These newly synthesized AuNPs exhibited a varying degree of peroxidase-like activity even the boosting effect of ATP was different. It was found that the catalytic activity of citrate and PEG-coated AuNPs depends on hydroxyl radical formation and ATP improved the peroxidase activity of both citrate and PEG-coated AuNPs. However, the activity of CTAB coated AuNPs did not depend on OH• radical formation neither ATP has any boosting effect on the activity of CTAB AuNPs. The porphyrinic metal–organic framework (MOF) was conjugated with AuNPs nanohybrid and used to produce chemoradiotherapy in tumors (He et al. 2019). The MOFs are stabilized by AuNPs decorated on their surface and act as radiosensitizers, here the MOF scaffolds served as the container for the chemotherapeutic drug doxorubicin. The catalase activity of AuNPs in the nanohybrids enhanced the radiotherapy effect, alleviated tumor hypoxia, and achieved synergistic anticancer efficacy in in-vitro and in-vivo studies. This study opens new horizons for the next generation of theranostic nanomedicines. Gold nanoparticles due to their extensive surface chemistry studies are the most familiar nanoparticles with a vast variety of applications in different biomedical fields.
Conclusion
RBCs are the first cells to be encountered with any foreign particles entering the blood. They are very simple cells, without any proper repair mechanism. Hence, they are the most sensitive and most prone to damages. Oxidative stress is a central pathway of nearly all pathological conditions and aging. The first signs of oxidative damage in erythrocytes are lipid peroxidation of the lipid bilayer. Then, oxidative stress affects the structure and the function of Hb protein. The metal NPs have been widely applied in organisms. Therefore, it becomes important to resolve the problems arising at the bio interface of these nanozymes. The metal nanozymes are haematologically safe and applied in RBCs to protect them from the complications of oxidative damage. The increasing studies and use of nanoparticles in living systems call for the understanding of NPs interactions with RBCs. There is still a need to explore the multiple enzyme-like activities of nanozymes, intrinsic catalytic mechanisms of the inorganic nanomaterials, a solution to the long-term standing issue of in-vivo toxicity of NPs. In conclusion, the unique ability of NPs to change their properties with the surface, or ligand modifications can be exploited to make them biocompatible and apply in sensitive systems like RBCs.
Acknowledgements
This study was supported by Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India to Somu Yadav (09/1152(0013)/2019-EMR-I). This agency had no role in the interpretation, or writing the manuscript.
Funding
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
Not applicable.
References
- Ahamed M, Posgai R, Gorey TJ, Nielsen M, Hussain SM, Rowe JJ. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol Appl Pharmacol. 2010;242(3):263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Alizadeh N, Salimi A. Multienzymes activity of metals and metal oxide nanomaterials: applications from biotechnology to medicine and environmental engineering. J Nanobiotechnology. 2021;19(1):26–26. doi: 10.1186/s12951-021-00771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarin T, Mitjans M, Lemos-Senna E, Vinardell MP (2015) Protection against oxidative damage in human erythrocytes and preliminary photosafety assessment of Punica granatum seed oil nanoemulsions entrapping polyphenol-rich ethyl acetate fraction. Toxicology in Vitro 30 (1, Part B):421–428. doi:10.1016/j.tiv.2015.09.020 [DOI] [PubMed]
- Baldim V, Yadav N, Bia N, Graillot A, Loubat C, Singh S, Karakoti AS, Berret J-F. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl Mater Interfaces. 2020;12(37):42056–42066. doi: 10.1021/acsami.0c08778. [DOI] [PubMed] [Google Scholar]
- Barbero F, Russo L, Vitali M, Piella J, Salvo I, Borrajo ML, Busquets-Fité M, Grandori R, Bastús NG, Casals E, Puntes V. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin Immunol. 2017;34:52–60. doi: 10.1016/j.smim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Barkur S, Lukose J, Chidangil S. Probing Nanoparticle-cell interaction using micro-Raman spectroscopy: silver and gold nanoparticle-induced stress effects on optically trapped live red blood cells. ACS Omega. 2020;5(3):1439–1447. doi: 10.1021/acsomega.9b02988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrecoso G, Crecente-Campo J, Alonso MJ. Unveiling the pitfalls of the protein corona of polymeric drug nanocarriers. Drug Deliv Transl Res. 2020;10(3):730–750. doi: 10.1007/s13346-020-00745-0. [DOI] [PubMed] [Google Scholar]
- Bhagat S, Singh S. Co-delivery of AKT3 siRNA and PTEN plasmid by antioxidant nanoliposomes for enhanced antiproliferation of prostate cancer cells. ACS Appl Bio Mater. 2020;3(7):3999–4011. doi: 10.1021/acsabm.9b01016. [DOI] [PubMed] [Google Scholar]
- Chen F, Liu Y, Wong N-K, Xiao J, So K-F. Oxidative stress in stem cell aging. Cell Transplant. 2017;26(9):1483–1495. doi: 10.1177/0963689717735407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh AR, Aloui H, Kim BS. Novel biogenic gold nanoparticles catalyzing multienzyme cascade reaction: glucose oxidase and peroxidase mimicking activity. Chem Eng J. 2021;421:127859. doi: 10.1016/j.cej.2020.127859. [DOI] [Google Scholar]
- Dias A, Werner M, Ward KR, Fleury J-B, Baulin VA. High-throughput 3D visualization of nanoparticles attached to the surface of red blood cells. Nanoscale. 2019;11(5):2282–2288. doi: 10.1039/C8NR09960J. [DOI] [PubMed] [Google Scholar]
- Eskandari N, Nejadi Babadaei MM, Nikpur S, Ghasrahmad G, Attar F, Heshmati M, Akhtari K, Rezayat Sorkhabadi SM, Mousavi SE, Falahati M. Biophysical, docking, and cellular studies on the effects of cerium oxide nanoparticles on blood components: in vitro. Int J Nanomedicine. 2018;13:4575–4589. doi: 10.2147/IJN.S172162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10(11):1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Guo L. Unraveling the multi-enzyme-like activities of iron oxide nanozyme via a first-principles microkinetic study. J Phys Chem C. 2019;123(50):30318–30334. doi: 10.1021/acs.jpcc.9b07802. [DOI] [Google Scholar]
- Hameed MK, Ahmady IM, Alawadhi H, Workie B, Sahle-Demessie E, Han C, Chehimi MM, Mohamed AA. Gold-carbon nanoparticles mediated delivery of BSA: Remarkable robustness and hemocompatibility. Colloids Surf, A. 2018;558:351–358. doi: 10.1016/j.colsurfa.2018.09.004. [DOI] [Google Scholar]
- He Z, Huang X, Wang C, Li X, Liu Y, Zhou Z, Wang S, Zhang F, Wang Z, Jacobson O, Zhu J-J, Yu G, Dai Y, Chen X. A catalase-like metal-organic framework nanohybrid for O2-evolving synergistic chemoradiotherapy. Angew Chem Int Ed. 2019;58(26):8752–8756. doi: 10.1002/anie.201902612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst SM, Karakoti A, Singh S, Self W, Tyler R, Seal S, Reilly CM. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol. 2013;28(2):107–118. doi: 10.1002/tox.20704. [DOI] [PubMed] [Google Scholar]
- Huang Y, Jiang J, Wang Y, Chen J, Xi J. Nanozymes as Enzyme Inhibitors. Int J Nanomedicine. 2021;16:1143–1155. doi: 10.2147/IJN.S294871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iofrida C, Daniele S, Pietrobono D, Fusi J, Galetta F, Trincavelli ML, Bonuccelli U, Franzoni F, Martini C. Influence of physical exercise on β-amyloid, α-synuclein and tau accumulation: an in vitro model of oxidative stress in human red blood cells. Arch Ital Biol. 2017;155(1–2):33–42. doi: 10.12871/000398292017124. [DOI] [PubMed] [Google Scholar]
- Kotsuruba AV, Kopyak BS, Sagach VF, Spivak MY. Nanocerium restores erythrocyte stability to acid hemolysis by inhibition of oxygen and nitrogen reactive species in old rats. Int J Physiol Pathophysiol. 2016;7(1):41–49. doi: 10.1615/IntJPhysPathophys.v7.i1.50. [DOI] [PubMed] [Google Scholar]
- Kozelskaya AI, Panin AV, Khlusov IA, Mokrushnikov PV, Zaitsev BN, Kuzmenko DI, Vasyukov GY. Morphological changes of the red blood cells treated with metal oxide nanoparticles. Toxicol in Vitro. 2016;37:34–40. doi: 10.1016/j.tiv.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Kuhn V, Diederich L, Keller TCSt, Kramer CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M, Cortese-Krott MM, Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism. Anemia Antioxid Redox Signal. 2017;26(13):718–742. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Kant R, Maurya PK, Rizvi SI. Concentration dependent effect of (−)-Epicatechin on Na+/K+-ATPase and Ca2+-ATPase inhibition induced by free radicals in hypertensive patients: comparison with L-ascorbic acid. Phytother Res. 2012;26(11):1644–1647. doi: 10.1002/ptr.4624. [DOI] [PubMed] [Google Scholar]
- Kumar S, Jha I, Mogha NK, Venkatesu P. Biocompatibility of surface-modified gold nanoparticles towards red blood cells and haemoglobin. Appl Surf Sci. 2020;512:145573. doi: 10.1016/j.apsusc.2020.145573. [DOI] [Google Scholar]
- Lewandowska H, Wójciuk K, Karczmarczyk U (2021) Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Applied Sciences 11 (19). doi:10.3390/app11199019
- Liu T, Bai R, Zhou H, Wang R, Liu J, Zhao Y, Chen C. The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv. 2020;10(13):7559–7569. doi: 10.1039/C9RA10969B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Han S, Pang M, Li J, Wang J, Luo X, Wang Y, Liu Z, Yang X, Ye Z. Cerium oxide nanoparticles protect red blood cells from hyperthermia-induced damages. J Biomater Appl. 2020 doi: 10.1177/0885328220979091. [DOI] [PubMed] [Google Scholar]
- Maćczak A, Cyrkler M, Bukowska B, Michałowicz J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study) Toxicol in Vitro. 2017;41:143–149. doi: 10.1016/j.tiv.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Maurya PK, Prakash S. Decreased activity of Ca++-ATPase and Na+/K+-ATPase during aging in humans. Appl Biochem Biotechnol. 2013;170(1):131–137. doi: 10.1007/s12010-013-0172-8. [DOI] [PubMed] [Google Scholar]
- Maurya PK, Kumar P, Siddiqui N, Tripathi P, Rizvi SI. Age-associated changes in erythrocyte glutathione peroxidase activity: correlation with total antioxidant potential. Indian J Biochem Biophys. 2010;47(5):319–321. [PubMed] [Google Scholar]
- Maurya PK, Kumar P, Nagotu S, Chand S, Chandra P. Multi-target detection of oxidative stress biomarkers in quercetin and myricetin treated human red blood cells. RSC Adv. 2016;6(58):53195–53202. doi: 10.1039/C6RA05121A. [DOI] [Google Scholar]
- Mishra RK, Ahmad A, Vyawahare A, Alam P, Khan TH, Khan R. Biological effects of formation of protein corona onto nanoparticles. Int J Biol Macromol. 2021;175:1–18. doi: 10.1016/j.ijbiomac.2021.01.152. [DOI] [PubMed] [Google Scholar]
- Mohanty J, Nagababu E, Rifkind J (2014) Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Frontiers in Physiology 5 (84). 10.3389/fphys.2014.00084 [DOI] [PMC free article] [PubMed]
- Murugan C, Murugan N, Sundramoorthy AK, Sundaramurthy A. Nanoceria decorated flower-like molybdenum sulphide nanoflakes: an efficient nanozyme for tumour selective ROS generation and photo thermal therapy. Chem Commun. 2019;55(55):8017–8020. doi: 10.1039/C9CC03763B. [DOI] [PubMed] [Google Scholar]
- Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, Baeg GH. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomedicine. 2017;12:1621–1637. doi: 10.2147/IJN.S124403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek V, Bhargava A, Bhanot V, Gupta R, Jain N, Panwar J. Formation and characterization of protein corona around nanoparticles: a review. J Nanosci Nanotechnol. 2018;18(10):6653–6670. doi: 10.1166/jnn.2018.15766. [DOI] [PubMed] [Google Scholar]
- Passi M, Kumar V, Packirisamy G. Theranostic nanozyme: Silk fibroin based multifunctional nanocomposites to combat oxidative stress. Mater Sci Eng, C. 2020;107:110255. doi: 10.1016/j.msec.2019.110255. [DOI] [PubMed] [Google Scholar]
- Peloi KE, Ratti BA, Nakamura CV, Neal CJ, Sakthivel TS, Singh S, Seal S, de Oliveira Silva Lautenschlager S (2021) Engineered nanoceria modulate neutrophil oxidative response to low doses of UV-B radiation through the inhibition of reactive oxygen species production. Journal of Biomedical Materials Research Part A n/a (n/a). doi:10.1002/jbm.a.37251 [DOI] [PubMed]
- Preedia Babu E, Subastri A, Suyavaran A, Premkumar K, Sujatha V, Aristatile B, Alshammari GM, Dharuman V, Thirunavukkarasu C. Size dependent uptake and hemolytic effect of zinc oxide nanoparticles on erythrocytes and biomedical potential of ZnO-Ferulic acid conjugates. Sci Rep. 2017;7(1):4203–4203. doi: 10.1038/s41598-017-04440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahul P, Srikanth VN, K. SR, Ashutosh K, Sanjay S, Effect of gold nanoparticle size and surface coating on human red blood cells. Bioinspired, Biomimetic and Nanobiomaterials. 2016;5(3):121–131. doi: 10.1680/jbibn.15.00018. [DOI] [Google Scholar]
- Rizvi SI, Maurya PK. Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci. 2007;1100(1):373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Giovinco HM, Cheatham BJ (2020) Cerium Oxide Nanoparticles Improve Lifespan of Stored Blood. Military Medicine 185 (Supplement_1):103–109. doi:10.1093/milmed/usz210 [DOI] [PubMed]
- Sarnatskaya V, Shlapa Y, Yushko L, Shton I, Solopan S, Ostrovska G, Kalachniuk L, Negelia A, Garmanchuk L, Prokopenko I, Khudenko N, Maslenny V, Bubnovskaya L, Belous A, Nikolaev V. Biological activity of cerium dioxide nanoparticles. J Biomed Mater Res, Part A. 2020;108(8):1703–1712. doi: 10.1002/jbm.a.36936. [DOI] [PubMed] [Google Scholar]
- Shah J, Purohit R, Singh R, Karakoti AS, Singh S. ATP-enhanced peroxidase-like activity of gold nanoparticles. J Colloid Interface Sci. 2015;456:100–107. doi: 10.1016/j.jcis.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Shah J, Pandya A, Goyal P, Misra SK, Singh S. BSA-decorated magnesium nanoparticles for scavenging hydrogen peroxide from human hepatic cells. ACS Applied Nano Materials. 2020;3(4):3355–3370. doi: 10.1021/acsanm.0c00088. [DOI] [Google Scholar]
- Shah F, Yadav N, Singh S. Phosphotungstate-sandwiched between cerium oxide and gold nanoparticles exhibit enhanced catalytic reduction of 4-nitrophenol and peroxidase enzyme-like activity. Colloids Surf, B. 2021;198:111478. doi: 10.1016/j.colsurfb.2020.111478. [DOI] [PubMed] [Google Scholar]
- Shah J, Singh S (2018) Unveiling the role of ATP in amplification of intrinsic peroxidase-like activity of gold nanoparticles. 3 Biotech 8 (1):67. 10.1007/s13205-017-1082-1 [DOI] [PMC free article] [PubMed]
- Shirsekar P, Kanhe N, Mathe V, Lahir YK, Dongre P. Interaction of zinc oxide nanoparticles with human red blood cells. Bionano Frontier. 2016;9:99–104. [Google Scholar]
- Singh S. Nanomaterials exhibiting enzyme-like properties (nanozymes): current advances and future perspectives. Front Chem. 2019;7:46–46. doi: 10.3389/fchem.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Singh S. Redox-dependent catalase mimetic cerium oxide-based nanozyme protect human hepatic cells from 3-AT induced acatalasemia. Colloids Surf, B. 2019;175:625–635. doi: 10.1016/j.colsurfb.2018.12.042. [DOI] [PubMed] [Google Scholar]
- Singh S, Asal R, Bhagat S. Multifunctional antioxidant nanoliposome-mediated delivery of PTEN plasmids restore the expression of tumor suppressor protein and induce apoptosis in prostate cancer cells. J Biomed Mater Res, Part A. 2018;106(12):3152–3164. doi: 10.1002/jbm.a.36510. [DOI] [PubMed] [Google Scholar]
- Singh N, NaveenKumar SK, Geethika M, Mugesh G. A cerium vanadate nanozyme with specific superoxide dismutase activity regulates mitochondrial function and ATP synthesis in neuronal cells. Angew Chem Int Ed. 2021;60(6):3121–3130. doi: 10.1002/anie.202011711. [DOI] [PubMed] [Google Scholar]
- Subramaniam VD, Murugesan R, Pathak S. Assessment of the cytotoxicity of cerium, tin, aluminum, and zinc oxide nanoparticles on human cells. J Nanopart Res. 2020;22(12):373. doi: 10.1007/s11051-020-05102-3. [DOI] [Google Scholar]
- Sudnitsyna J, Skverchinskaya E, Dobrylko I, Nikitina E, Gambaryan S, Mindukshev I (2020) Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 9 (10). doi:10.3390/antiox9100929 [DOI] [PMC free article] [PubMed]
- Tian Y, Tian Z, Dong Y, Wang X, Zhan L. Current advances in nanomaterials affecting morphology, structure, and function of erythrocytes. RSC Adv. 2021;11(12):6958–6971. doi: 10.1039/D0RA10124A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakanova G, Arakelova E, Ayvazyan V, Ayvazyan A, Tatikyan S, Grigoryan R, Sargsyan N, Arakelyan A. Two-photon imaging of oxidative stress in living erythrocytes as a measure for human aging. Biomed Opt Express. 2020;11(7):3444–3454. doi: 10.1364/BOE.393898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabani NVS, Karakoti AS, Singh S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH. Colloids Surf, B. 2017;153:52–60. doi: 10.1016/j.colsurfb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Vallabani NVS, Singh S, Karakoti AS. Investigating the role of ATP towards amplified peroxidase activity of Iron oxide nanoparticles in different biologically relevant buffers. Appl Surf Sci. 2019;492:337–348. doi: 10.1016/j.apsusc.2019.06.177. [DOI] [Google Scholar]
- Vallabani NVS, Vinu A, Singh S, Karakoti A. Tuning the ATP-triggered pro-oxidant activity of iron oxide-based nanozyme towards an efficient antibacterial strategy. J Colloid Interface Sci. 2020;567:154–164. doi: 10.1016/j.jcis.2020.01.099. [DOI] [PubMed] [Google Scholar]
- Vallabani NVS, Singh S (2018) Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 8 (6):279. doi:10.1007/s13205-018-1286-z [DOI] [PMC free article] [PubMed]
- Wadhwa R, Aggarwal T, Thapliyal N, Kumar A, Priya, Yadav P, Kumari V, Reddy BSC, Chandra P, Maurya PK (2019) Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech 9 (7):279. doi:10.1007/s13205-019-1807-4 [DOI] [PMC free article] [PubMed]
- Wang Y, Li H, Guo L, Jiang Q, Liu F. A cobalt-doped iron oxide nanozyme as a highly active peroxidase for renal tumor catalytic therapy. RSC Adv. 2019;9(33):18815–18822. doi: 10.1039/C8RA05487H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zennadi R (2020) Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. International Journal of Molecular Sciences 21 (12). doi:10.3390/ijms21124259 [DOI] [PMC free article] [PubMed]
- Xiang J, Li J, He J, Tang X, Dou C, Cao Z, Yu B, Zhao C, Kang F, Yang L, Dong S, Yang X. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl Mater Interfaces. 2016;8(7):4489–4499. doi: 10.1021/acsami.6b00158. [DOI] [PubMed] [Google Scholar]
- Yadav S, Maurya PK (2021) Biomedical applications of metal oxide nanoparticles in aging and age-associated diseases. 3 Biotech 11 (7):338. doi:10.1007/s13205-021-02892-8 [DOI] [PMC free article] [PubMed]
- Yadav N, Singh S. Polyoxometalate-mediated vacancy-engineered cerium oxide nanoparticles exhibiting controlled biological enzyme-mimicking activities. Inorg Chem. 2021;60(10):7475–7489. doi: 10.1021/acs.inorgchem.1c00766. [DOI] [PubMed] [Google Scholar]
- Yadav N, Singh S. SOD mimetic cerium oxide nanorods protect human hepatocytes from oxidative stress. Emergent Materials. 2021 doi: 10.1007/s42247-021-00220-7. [DOI] [Google Scholar]
- Zhang Y, Wang Z, Li X, Wang L, Yin M, Wang L, Chen N, Fan C, Song H. Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in drosophila. Adv Mater. 2016;28(7):1387–1393. doi: 10.1002/adma.201503893. [DOI] [PubMed] [Google Scholar]
- Zhu W, Wang L, Li Q, Jiao L, Yu X, Gao X, Qiu H, Zhang Z, Bing W (2021) Will the Bacteria Survive in the CeO2 Nanozyme-H2O2 System? Molecules 26 (12). 10.3390/molecules26123747 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.