Abstract
Bladder Urothelial Carcinoma (BLCA) is the major subtype of bladder cancer, and the prognosis prediction of BLCA is difficult. Ferroptosis is a newly discovered iron-dependent cell death pathway. However, the clinical value of ferroptosis-related genes (FRGs) on the prediction of BLCA prognosis is still uncertain. In this study, we aimed to construct a novel prognostic signature to improve the prognosis prediction of advanced BLCA based on FRGs. In the TCGA cohort, we identified 23 differentially expressed genes (DEGs) associated with overall survival (OS) via univariate Cox analysis (all P < 0.05). 8 optimal DEGs were finally screened to generate the prognostic risk signature through LASSO regression analysis. Patients were divided into two risk groups based on the median risk score. Survival analyses revealed that the OS rate in the high-risk group was significantly lower than that in the low-risk group. Moreover, the risk score was determined as an independent predictor of OS by the multivariate Cox regression analysis (Hazard ratio > 1, 95% CI = 1.724-2.943, P < 0.05). Many potential ferroptosis-related pathways were identified in the enrichment analysis in BLCA. With the aid of an external FAHWMU cohort (n = 180), the clinical predication value of the signature was further verified. In conclusion, the prognosis of advanced BLCA could be accurately predicted by this novel FRG-signature.
Keywords: ferroptosis, prognostic signature, bladder urothelial carcinoma, immune status, function analyses
Introduction
Bladder cancer (BC), ranking 9th in the incidence of malignant tumors, is a malignant tumor of the bladder mucosa with poor prognosis and high recurrence (1, 2). Usually, BC could be divided into muscle invasive bladder cancer (MIBC) and non-muscle invasive bladder cancer (NMIBC). In comparison with patients with NMIBC, patients with MIBC have a poor prognosis. Bladder Urothelial Carcinoma (BLCA), caused by cigarette smoking and occupational exposure, is the main BC subtype, accounting for 90–95% of cases (3, 4). However, clinical biomarkers that could accurately predict the prognosis of BLCA are still lacking. Most BLCA patients are diagnosed at the late stages, leading to the worse prognosis (4). Therefore, the need of a better predictive signature for the prognosis prediction of BCLA, especially for MIBC, is urgent.
Ferroptosis, results from the accumulation iron-dependent lipid peroxide (LPO), is a new discovered pathway of non-apoptotic cell death (5). Ferroptosis is mainly caused by iron-dependent oxidative damage (6). It has been reported to be involved in the vital biological functions, including a sequence of complex biochemical reactions, gene expression, and signal transduction events (7, 8). Ferroptosis-related genes (FRGs) has been demonstrated to be associated with the prognosis of various human cancers (9–12). However, the clinical value of FRGs in the prognosis of BLCA still remains unknown.
In this study, we constructed a novel prognostic signature to improve the prognosis prediction of BLCA. Due to the reason that most patients with BLCA in TCGA database are with advanced-stage disease, we focused on the clinical value of FRGs in patients with MIBC. With the aid of an extra cohort obtained from the First Affiliated Hospital of Wenzhou Medical University (FAHWMU), the accuracy of this prognostic signature was verified.
Materials and Methods
Data Collection
In this study, mRNA expression files [fragment per thousand base pairs per kilobase fragment normalization (FPKM) normalized] and clinical features from 406 BLCA patients were obtained from the TCGA database (https://portal.gdc.cancer. Government/Repository). Using the R package “limma”, gene expression profiles were standardized. Data from TCGA are publicly available and use standardized read count values. As this study follows the TCGA data access strategy and release guidelines, it does not require approval by the local ethics committee. The 259 FRGs were selected from the previous studies (13, 14). As an extra cohort data, the FAHWMU cohort (n = 180) was obtained from the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). BLCA samples in the FAHWMU cohort were collected from 2012 to 2020, and OS time was used as the main survival time indicator. The collection of this cohort was reviewed and approved by the human research ethics committee of the First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Generation and Validation of the Signature
In the TCGA cohort, differentially expressed genes (DEGs) between BLCA samples and adjacent nontumorous samples were identified using “limma” with a false discovery rate (FDR) < 0.05. Prognosis related DEGs were screened using univariate Cox analysis of overall survival (OS) (P < 0.05), P-values were adjusted using Benjamini & Hochberg (BH) correction. The STRING database (version 11.0) generated a protein-to-protein interaction (PPI) network for the interactions among prognostic DEGs (15). The prognostic signature was constructed using the combination of LASSO-penalized Cox regression and multivariate Cox regression (16, 17). The LASSO algorithm was used to select and compress variables. In the regression, the standardized expression matrix of the prognosis related DEGs constituted the independent variable, while the OS and patient status in the TCGA cohort constituted the response variables. Standard ten-fold cross-validation determined the model penalty parameter (λ). The standardized expression level and regression coefficients for each gene were used to calculate patient risk, where the score = sum (each gene’s expression × corresponding coefficient). In the TCGA cohort, as determined by the median risk score, all patients were divided into the high-risk and low-risk groups. By using the “prcomp” function from the R package “stats”, PCA was performed after the gene expression signature was obtained. t-SNE, produced by the R package “Rtsne”, explored group distribution. Time-dependent receiver operating characteristic (ROC) curve analysis was used to assess the performance of gene signatures. The Kaplan-Meier (K-M) survival analysis was performed using the log rank test.
Enrichment Analysis
DEGs (|log2FC|≥1, FDR < 0.05) were identified using the R package “clusterProfiler” based on the risk score. We used the BH method to adjust P values based on Gene Ontology (GO) function enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment analysis. The single-sample gene set enrichment analysis (ssGSEA) was used to calculate the activity of 13 immune-related pathways and the infiltration analysis data of 16 immune cells (18). The gene set of the 13 immune-related pathways and the 16 immune cells is shown in Table S2 .
Statistical Analysis
Student’s t-test was performed to compare gene expression between BLCA and controls. The Mann-Whitney test was used to compare ssGSEA scores between high- and low-risk groups. P values < 0.05 were considered statistically significant. R software (Version 3.5.3) was used for all statistical analyses.
Results
Identification of 23 OS-Related Differently Expressed Ferroptosis-Related Genes
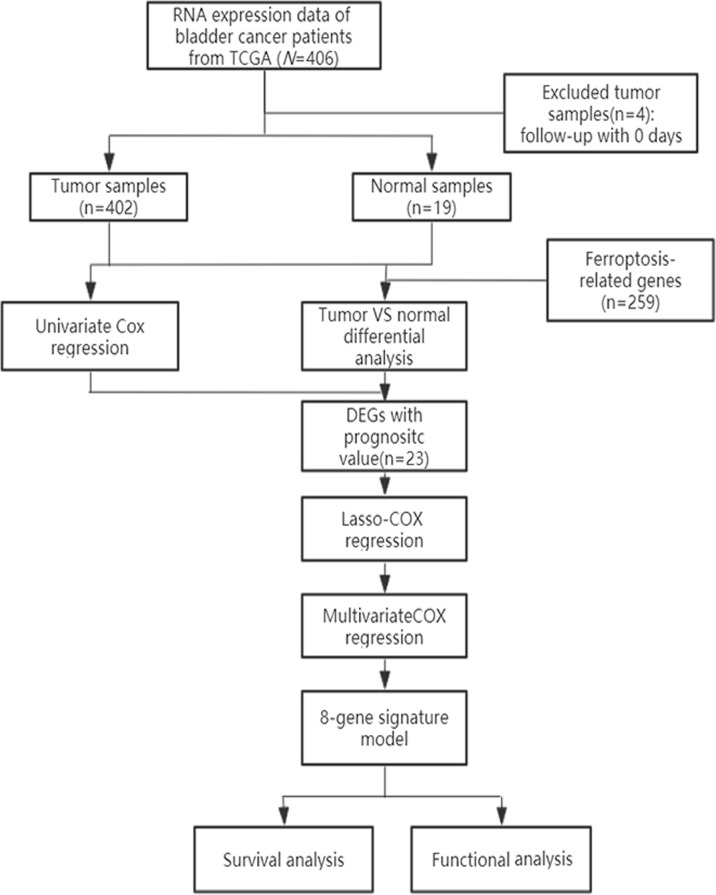
A schematic representation of data collection and analysis was shown in Figure 1 . We obtained data for BLCA patients from the TCGA databases. Clinical characteristics were summarized in Table 1 .
Figure 1.
Flowchart of data collection and analysis.
Table 1.
The clinical features of TCGA cohort and the FAHWMU cohort.
| TCGA cohort | FAHWMU cohort | |
|---|---|---|
| No.of patients | 406 | 180 |
| gender | ||
| female | 106 (26.1%) | 47 (26.1%) |
| male | 300 (73.9%) | 133 (73.9%) |
| Age | ||
| >65 | 245 (60.3%) | 107 (59.4%) |
| ≤65 | 161 (39.7%) | 73 (40.5%) |
| status | ||
| alive | 227 (55.9%) | 93 (51.7%) |
| dead | 179 (44.1%) | 87 (48.3%) |
| stage | ||
| Stage I | 2 (0.5%) | 21 (11.7%) |
| Stage II | 129 (31.8%) | 56 (31.1%) |
| Stage III | 140 (34.5%) | 44 (24.4%) |
| Stage IV | 133 (32.8%) | 57 (31.7%) |
| unknown | 2 (0.5%) | 2 (1.1%) |
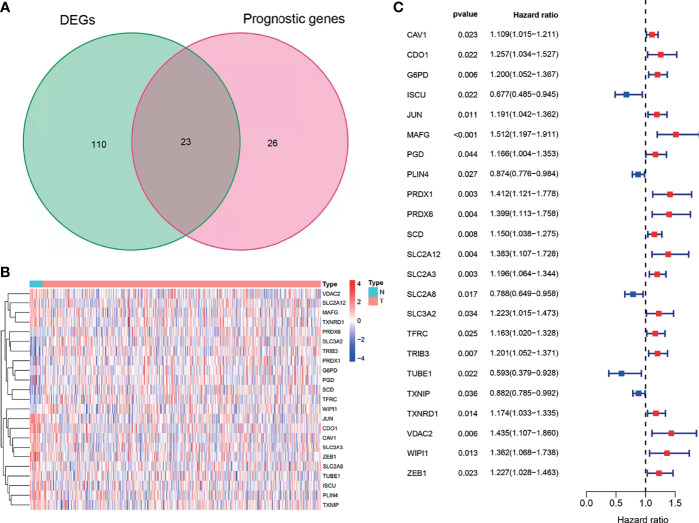
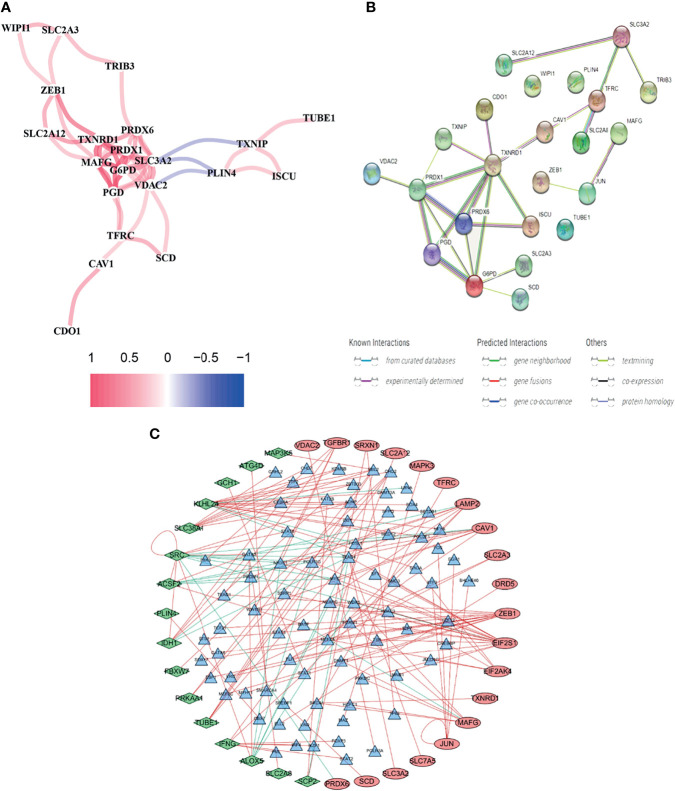
Using BLCA samples from the TCGA cohort, differential expression between BLCA samples and adjacent nontumorous samples was observed in most FRGs (133/259, 51.4%); 23 were related to OS via univariate Cox regression (all P < 0.05, Figures 2A – C ). The gene interaction network indicated that PRDX6, TXNRD1, PRDX6, and G6PD were the hub genes ( Figure 3A ). The correlations among genes were shown in Figure 3B . It was found that there were significant relationships between 193/318 TFs and DEGs (P < 0.05). A TF-based regulatory network was shown in Figure 3C .
Figure 2.
Identification of OS-related DEFRGs in the TCGA cohort. (A) Venn plot showing common genes from differential analysis and univariate Cox analysis. (B) Heat map of OS-related DEFRGs. (C) Forest plots showing OS-related DEFRGs (P < 0.05).
Figure 3.
Comprehensive networks of 23 OS-related DEFRGs. (A) PPI network (B) Correlation network of 23 OS-related DEFRGs. (C) Regulatory network of TFs and DEFRGs (green nodes: DEFRGs with low risk; red nodes: DEFRGs with high risk, only nodes with correlation coefficient > 0.4 and P < 0.05 were selected).
Generation of the 8 Ferroptosis-Related Prognostic Gene Signature
To prevent over fitting of the model, Lasso-Cox regression was used to screen the identified 21 genes ( Figures 4A, B ). Then, multivariate Cox regression analysis was used to determine the optimal regulatory genes and 8 FRGs were identified to generate the prognostic signature ( Figure 4C ). The risk scores of BLCA patients were calculated using mRNA levels and the estimated regression coefficients. The resulting formula was used:
Figure 4.
Construction of the prognostic model. (A, B). Lasso-Cox analysis of 23 OS-related DEFRGs. (C) Forest plot showing the eight genes in the prognostic risk model.
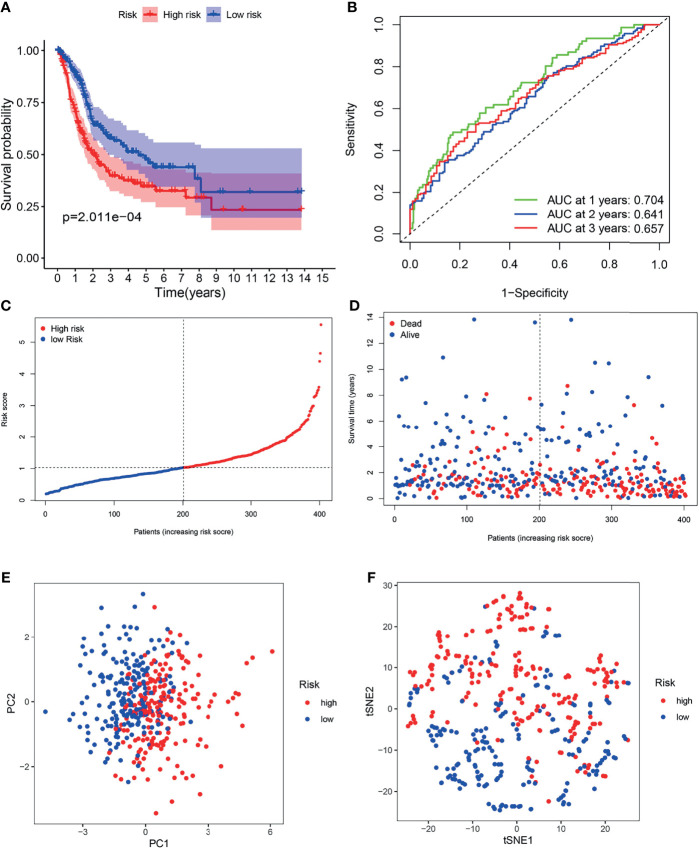
Patients in the TCGA cohort were partitioned into high-risk (n = 203) and low-risk groups (n = 203) according to the median risk score ( Figure 5C ). K-M survival curves indicated that the OS of the low-risk group was higher than that of the high-risk group ( Figure 5A , P < 0.05). Time-dependent ROC curves indicated that area under the curve (AUC) values were 0.704, 0.641, and 0.657 at 1st years, 2nd years and 3rd years ( Figure 5B ). As shown in Figure 5D , worse OS was found in high-risk patients, consistent with the results of the K-M curve. PCA and t-SNE analysis indicated two directions in the patient distribution of the two risk groups ( Figures 5E, F ).
Figure 5.
Survival analysis of the TCGA cohort prognostic signature. (A) Kaplan-Meier survival curve. (B) time-dependent ROC curve. (C) The distribution and median value of the risk scores. (D) OS-related scatter plot. (E) PCA plot. (F) t-SNE plot.
Validation of the Prognostic Value of Gene Signature in the FAHWMU Cohort
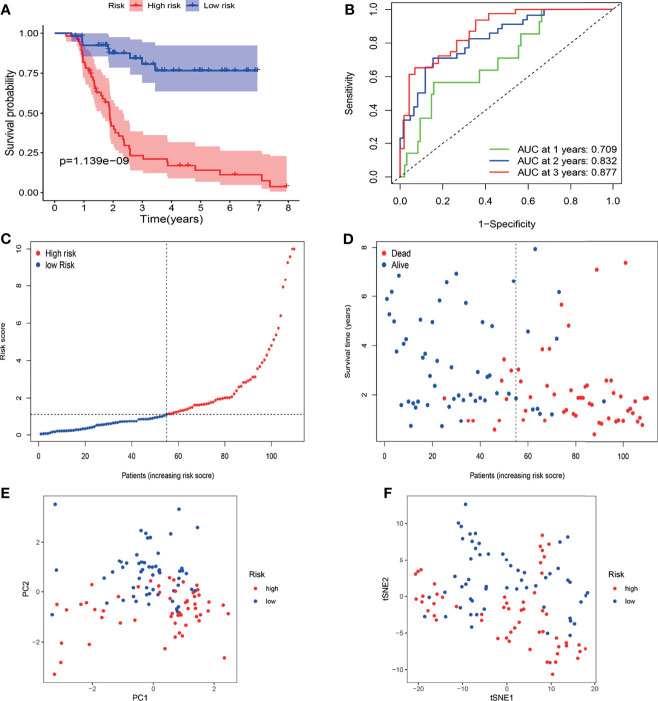
The FAHWMU cohort was used to verify prognostic signature. Considering that most patients with BLCA in TCGA database are at the advanced-stage, only patients with MIBC in the FAHWMU cohort (n = 110) were used for the next analysis. The risk score was calculated based on the expressions of eight risk genes: CDO1, JUN, MAFG, PRDX6, SCD, SLC2A12, TUBE1 and TXNRD1. According to the calculated median risk score of the FAHWMU cohort, all patients were divided into high-risk (n=55) or low-risk (n=55) groups ( Figures 6C, D ). Similarly, the AUC in 1st years, 2nd years and 3rd years in the FAHWMU cohort was 0.709, 0.832, and 0.877 ( Figure 6B ). K-M survival curve indicated that compared to low-risk patients, the OS of high-risk patients was worse ( Figure 6A , P < 0.05). The results of PCA and t-SNE analysis in the FAHWMU cohort were similar to the results of the TCGA cohort ( Figures 6E, F ). Taken together, our results preliminarily suggest the prognosis prediction of our signature in BLCA.
Figure 6.
Validation of gene signature in patients with MIBC in the FAHWMU cohort (n = 110). (A) Kaplan-Meier survival curve. (B) time-dependent ROC curve. (C) The distribution and median value of the risk scores. (D) OS-related scatter plot. (E) PCA plot. (F) t-SNE plot.
The Risk Score Was Identified as an Independent Prognostic Predictor
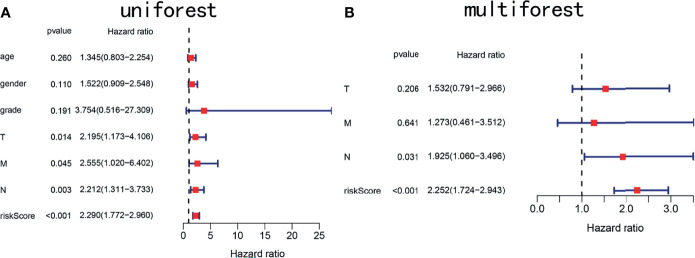
To determine whether the risk score is an independent prognostic marker, univariate and multivariate Cox regression analyses were performed. Univariate Cox regression indicated that the risk score of TCGA cohort was closely related to OS (P < 0.001, Figure 7A ). Multivariate Cox regression analysis further confirmed the risk score as an independent prognostic factor in TCGA cohort (P < 0.01, Figure 7B ).
Figure 7.
Forest plots showing key clinical factors under the signature. (A) Univariate Cox regression analysis of the TCGA cohort. (B) Multivariate Cox regression analysis of the TCGA cohort.
Function Enrichment Analyses Revealed Potential Molecular Mechanisms
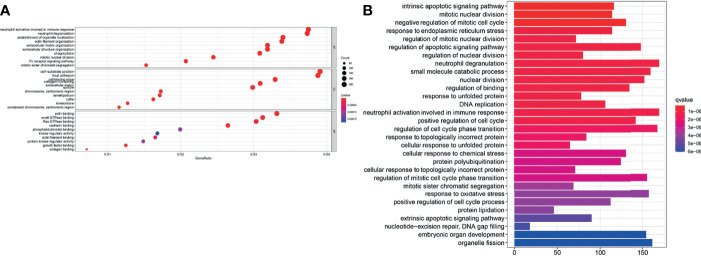
GO enrichment and KEGG pathway analysis were performed to reveal ferroptosis-related potential molecular mechanisms. In the TCGA cohort, various neutrophil-related molecular functions and mitotic nuclear division molecular functions were enriched in the GO analysis (P < 0.05, Figure 8A ). KEGG pathway analysis indicated that DNA replication pathways were enriched in the TCGA cohort (P < 0.05, Figure 8B ).
Figure 8.
Enrichment analyses of the signature. (A) GO enrichment plot of the TCGA cohort. (B) KEGG pathway enrichment plot of the TCGA cohort.
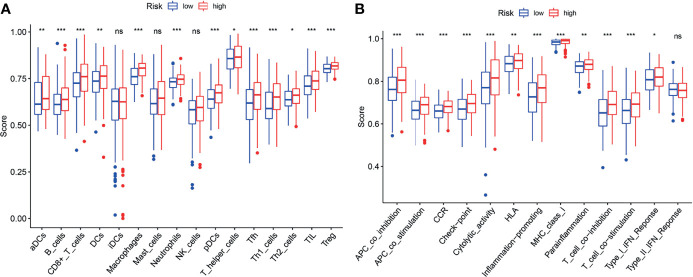
ssGSEA was performed to analyze the relationships between the risk scores and immune-related cells and pathways in TCGA. In the TCGA cohort, there were significant differences between the low-risk and high-risk groups (all adjusted P < 0.05, Figures 9A, B ), with the differences in aDC, Tfh, Inflammation-promoting and MHC class I.
Figure 9.
ssGSEA analyses of the signature. (A, B) The block diagram showing the scores of 16 immune cells (A) and 13 immune-related functions (B) of the TCGA cohort. *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significance.
Discussion
It has been reported that FRGs are involved in the regulation of drug-induced ferroptosis in BLCA (11). Additionally, FRG-related inducers have been found to suppress BC with the help of mTOR inhibitors (19). Recently, ferroptosis participates in the inhibition of cell cycle in BC by obstructing the G0/G1 phase (11). Combined with these, ferroptosis plays a key role in BLCA development. However, the clinical value of FRGs in predicting the OS of BLCA patients was still unknown. In this study, a novel FRG-signature for BLCA was generated. The relationships between FRGs and prognosis of BLCA were explored. Next, the hub FRGs were identified and used to construct this FRG-signature. Our data suggest that this signature could improve the prognosis of advanced BLCA. Notably, the clinical prediction value of our FRG-signature was confirmed in an external FAHWMU cohort.
Herein, 8 FRGs (CDO1, JUN, MAFG, PRDX6, SCD, SLC2A12, TUBE1, TXNRD1) were used to establish the prognostic signature. These FRGs could be summarized as four types: iron metabolism (CDO1), lipid metabolism (JUN, TUBE1), (anti)oxidant metabolism (TXNRD1, SCD, SLC2A12) (20) and energy metabolism (MAFG, PRDX6). CDO1, silenced by promoter methylation in BLCA, induces a reduction in the onset of ferroptosis, leading to the inhibition of BLCA invasion (21). JUN has been reported to reduce lipid peroxidation and inhibit ferroptosis, which contributes to the invasion and metastasis of BC (22). Pitsava et al. found that TUBE1 suppresses BLCA metastasis by promoting ferroptosis through lipid metabolism (23). High expression of SLC2A12 has been found to be associated with glucose metabolism in ferroptosis, which exacerbates the invasion of BLCA (24). PRDX6 could inhibit the high oxidative damage caused by ferroptosis and thus promote the proliferation of BLCA (25). In addition, SCD promotes the proliferation of BLCA via fatty acid metabolic pathway in ferroptosis (26). In sum, these 8 FRGs may participate in the key biological processes such as proliferation, invasion and metastasis of BLCA through ferroptosis pathway.
Increasing studies have shown that there is an association between ferroptosis and tumor immunity (27). In this study, it was found that biological functions associated with immunity such as neutrophil activation in immune response, are enriched in the GO analysis. Moreover, it was found that the proportion of macrophages (28, 29) and Treg cells (29, 30) is increased in the high-risk group. Furthermore, several anti-tumor immunity factors, like NK cells, type I IFN response and type II IFN response were decreased in the high-risk group. Recent studies have demonstrated that high proportion of tumor-related macrophages or Treg cells is associated with poor prognosis of BLCA patients. Accordingly, our results also showed that the high-risk patients with the high proportion of macrophages and Treg cells, were associated with poor prognosis, suggesting the existence of the association of ferroptosis and tumor immunity.
The strengths of this study are that an external FAHWMU cohort was used to further verify the clinical value of our FRG-signature in the prognosis prediction of advanced BLCA. Meanwhile, there are several limitations in this study. First, cell biological experiments should be performed to further validate the functions of 8 FRGs in the future. Second, more sample validation is needed for clinical value of our prognostic signature.
Conclusion
In conclusion, we generate a novel prognostic FRG-signature, which contributes to the improvement in the prognosis prediction of advanced BLCA.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/https://portal.gdc.cancer.gov/.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research Ethics Committee in The First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XL and WG designed the study and analyzed the data. JH, JC, YTZ, and RZ revised the images. EL, CL, YXZ, and YW performed the literature search and collected data for the manuscript. YL and JZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The project was supported by the Zhejiang Provincial Research Center for Cancer Intelligent Diagnosis and Molecular Technology (JBZX-202003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.726486/full#supplementary-material
Abbreviations
BC, Bladder cancer; LASSO, Least absolute shrinkage and selection operator; TCGA, The Cancer Genome Atlas; DEGs, Differentially expressed genes; OS, Overall survival; ROC, Receiver operating characteristic; FDR, False discovery rate; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; ssGSEA, Single-sample gene set enrichment analysis; PCA, Principal component analysis; t-SNE, t-distributed stochastic neighbor embedding; AUC, Area under the curve; HR, Hazard ratio; CI, Confidence interval; APC, Antigen presenting cell; AFP, alpha fetoprotein; aDC, Activated dendritic cell; iDC, Immature dendritic cell; pDC, Plasmacytoid dendritic cell; Tfh, T follicular helper cell; TIL, Tumor Infiltrating Lymphocyte; HLA, Human leukocyte antigen; CCR, Cytokine-cytokine receptor.
References
- 1. Patel V, Oh W, Galsky M. Treatment of Muscle-Invasive and Advanced Bladder Cancer in 2020. CA: Cancer J Clin (2020) 70(5):404–23. doi: 10.3322/caac.21631 [DOI] [PubMed] [Google Scholar]
- 2. Gruba N, Stachurski L, Lesner AJC. Biodiversity. Non-Proteasomal Urine Activity in Bladder Cancer. (2021) 18: (3):e2000981. doi: 10.1002/cbdv.202000981 [DOI] [PubMed] [Google Scholar]
- 3. Gan J, Skipper P, Gago-Dominguez M, Arakawa K, Ross RK, Yu MC, et al. Alkylaniline-Hemoglobin Adducts and Risk of Non-Smoking-Related Bladder Cancer. J Natl Cancer Institute (2004) 96(19):1425–31. doi: 10.1093/jnci/djh274 [DOI] [PubMed] [Google Scholar]
- 4. Amara C, Vantaku V, Lotan Y, Putluri N. Recent Advances in the Metabolomic Study of Bladder Cancer. Expert Rev Proteomics (2019) 16(4):315–24. doi: 10.1080/14789450.2019.1583105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu H, Guo P, Xie X, Wang Y, Chen G. Medicine M. Ferroptosis, a New Form of Cell Death, and Its Relationships With Tumourous Diseases. (2017) 21: (4):648–57. doi: 10.1111/jcmm.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ubellacker J, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, et al. Lymph Protects Metastasizing Melanoma Cells From Ferroptosis. Nature (2020) 585(7823):113–8. doi: 10.1038/s41586-020-2623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu S, He Y, Lin L, Chen P, Chen M, Zhang S. The Emerging Role of Ferroptosis in Intestinal Disease. Cell Death Dis (2021) 12(4):289. doi: 10.1038/s41419-021-03559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Z, Zhang L, Zheng J, Sun H, Shao C. Ferroptosis: Biochemistry and Biology in Cancers. Front Oncol (2021) 11:579286. doi: 10.3389/fonc.2021.579286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Yin X, He W, Xue W, Zhang J, Huang Y. SUV39H1 Deficiency Suppresses Clear Cell Renal Cell Carcinoma Growth by Inducing Ferroptosis. Acta Pharm Sinica B (2021) 11(2):406–19. doi: 10.1016/j.apsb.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang Y, Lin J, Guo S, Xue X, Wang Y, Qiu S, et al. RRM2 Protects Against Ferroptosis and Is a Tumor Biomarker for Liver Cancer. Cancer Cell Int (2020) 20(1):587. doi: 10.1186/s12935-020-01689-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao F, Vakhrusheva O, Markowitsch SD, Slade KS, Tsaur I, Cinatl J, Jr, et al. Artesunate Impairs Growth in Cisplatin-Resistant Bladder Cancer Cells by Cell Cycle Arrest, Apoptosis and Autophagy Induction. Cells (2020) 9(12). doi: 10.3390/cells9122643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang X, Stockwell B, Conrad M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat Rev Mol Cell Biol (2021) 22(4):266–82. doi: 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stockwell B, Friedmann Angeli J, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell (2017) 171(2):273–85. doi: 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell (2019) 35(6):830–49. doi: 10.1016/j.ccell.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 15. Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, et al. The STRING Database in 2011: Functional Interaction Networks of Proteins, Globally Integrated and Scored. (2011) 39:D561–8. doi: 10.1093/nar/gkq973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon N, Friedman J, Hastie T, Tibshirani R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J Stat Softw (2011) 39(5):1–13. doi: 10.18637/jss.v039.i05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tibshirani R. The Lasso Method for Variable Selection in the Cox Model. Stat Med (1997) 16(4):385–95. doi: [DOI] [PubMed] [Google Scholar]
- 18. Rooney M, Shukla S, Wu C, Getz G, Hacohen NJC. Molecular and Genetic Properties of Tumors Associated With Local Immune Cytolytic Activity. (2015) 160:48–61. doi: 10.1016/j.cell.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Y, Berleth N, Wu W, Schlutermann D, Deitersen J, Stuhldreier F, et al. Fin56-Induced Ferroptosis Is Supported by Autophagy-Mediated GPX4 Degradation and Functions Synergistically With mTOR Inhibition to Kill Bladder Cancer Cells. Cell Death Dis (2021) 12(11):1028. doi: 10.1038/s41419-021-04306-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wohlhieter C, Richards A, Uddin F, Hulton CH, Quintanal-Villalonga A, Martin A, et al. Concurrent Mutations in STK11 and KEAP1 Promote Ferroptosis Protection and SCD1 Dependence in Lung Cancer. Cell Rep (2020) 33(9):108444. doi: 10.1016/j.celrep.2020.108444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brait M, Ling S, Nagpal JK, Chang X, Park HL, Lee J, et al. Cysteine Dioxygenase 1 Is a Tumor Suppressor Gene Silenced by Promoter Methylation in Multiple Human Cancers. PloS One (2012) 7(9):e44951. doi: 10.1371/journal.pone.0044951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren S, Zhang N, Shen L, Lu Y, Chang Y, Lin Z, et al. Lnc00892 Competes With C-Jun to Block NCL Transcription, Reducing the Stability of RhoA/RhoC mRNA and Impairing Bladder Cancer Invasion. Oncogene (2021). doi: 10.1038/s41388-021-02033-8 [DOI] [PubMed] [Google Scholar]
- 23. Pitsava G, Feldkamp M, Pankratz N, Lane J, Kay DM, Conway KM, et al. Exome Sequencing of Child-Parent Trios With Bladder Exstrophy: Findings in 26 Children. Am J Med Genet Part A (2021) 185(10):3028–41. doi: 10.1002/ajmg.a.62439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiong Y, Lei F. SLC2A12 of SLC2 Gene Family in Bird Provides Functional Compensation for the Loss of SLC2A4 Gene in Other Vertebrates. Mol Biol Evol (2021) 38(4):1276–91. doi: 10.1093/molbev/msaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao L, Meng J, Yue C, Wu X, Su Q, Wu H, et al. Integrative Analysis the Characterization of Peroxiredoxins in Pan-Cancer. Cancer Cell Int (2021) 21(1):366. doi: 10.1186/s12935-021-02064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun J, Yue W, You J, Wei X, Huang Y, Ling Z, et al. Identification of a Novel Ferroptosis-Related Gene Prognostic Signature in Bladder Cancer. Front Oncol (2021) 11:730716. doi: 10.3389/fonc.2021.730716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li D, Li Y. The Interaction Between Ferroptosis and Lipid Metabolism in Cancer. Signal Transduction Targeted Ther (2020) 5(1):108. doi: 10.1038/s41392-020-00216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell (2019) 179(4):829–45.e20. doi: 10.1016/j.cell.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 29. Zhou S, Zhou Z, Hu Z, Huang HW, Wang Z, Chen EB, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology (2016) 150(7):1646–58.e17. doi: 10.1053/j.gastro.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 30. Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased Regulatory T Cells Correlate With CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology (2007) 132(7):2328–39. doi: 10.1053/j.gastro.2007.03.102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/https://portal.gdc.cancer.gov/.