Abstract
Haemonchus contortus is an infectious gastrointestinal nematode parasite of small ruminants. This study addresses the in vitro/in vivo anti-haemonchiasis potential, toxicological effects, and mechanism of action of nanoparticles. Online databases were used to search and retrieve the published literature (2000 to 2021). A total of 18 articles were selected and reviewed, out of which, 13 (72.2%) studies reported in vitro, 9 (50.0%) in vivo, and 4 (22.2%) both in vitro/in vivo efficacy of different nanoparticles. Mostly, organic nanoparticles (77.7%) were used including polymeric (85.7%) and lipid nanoparticles (14.3%). The highest efficacy, in vitro, of 100% resulted from using encapsulated bromelain against eggs, larvae, and adult worm mortality at 4, 2, and 1 mg/ml, respectively. While in vivo, encapsulated Eucalyptus staigeriana oil reduced worm burden by 83.75% and encapsulated Cymbopogon citratus nano-emulsion by 83.1%. Encapsulated bromelain, encapsulated Eucalyptus staigeriana oil, and encapsulated Cymbopogon citratus nano-emulsion were safe and non-toxic in vivo. Encapsulated bromelain damaged the cuticle, caused paralysis, and death. Nanoparticles could be a potential source for developing novel anthelmintic drugs to overcome the emerging issue of anthelmintic resistance in H. contortus. Studies on molecular effects, toxicological consequences, and different pharmacological targets of nanoparticles are required in future research.
Keywords: Haemonchus contortus, nanoparticles, anthelmintic, gastrointestinal nematode, toxicity, anthelmintic resistance
Introduction
Haemonchus contortus is a highly infectious gastrointestinal parasitic nematode of small ruminants. The parasite causes acute anemia, hemorrhagic gastroenteritis, diarrhea, edema, stunted growth, and death of severely affected animals. H. contortus affects millions of ruminants annually, resulting in substantial economic losses due to decreased milk, meat, and wool production, loss of body weight, and cost of anthelmintic drugs (1, 2). The available anthelmintic agents such as imidazothiazole, benzimidazole, and ivermectin among others are becoming ineffective due to the rising issue of chemoresistance in helminths (3–7).
Helminth resistance to multiple anthelmintic drugs is increasing at an alarming speed and has raised great public health concerns (8). In the near future, it would be very difficult to control some of the parasites with prevailing anthelmintic drugs. Some studies reported that sheep nematode populations are highly resistant to oxfendazole (88%), levamisole (41%), and ivermectin (59%) in farm animals (9). Therefore, it is indispensable and timely to develop novel anthelmintics, which are suitable, environmentally friendly, cost effective, and potentially active.
Nanoparticles, owing to their small size, remarkable surface reactivity, and their biomedical applications, are becoming the leading candidates for the development of new anthelmintic drugs (10). They are able to cross membranes and generate reactive oxygen species (ROS), leading to great reactivity and finally death of infectious agents (11, 12). Recently, anthelmintic potential of nanoparticles is being constantly evaluated for controlling parasitic infections (13).
Nanoparticles are widely used in modern medicines, such as vaccines, diagnostic procedures, medical devices, drug delivery, imaging, and antimicrobial therapies (14). Several applications of nanomaterials as anthelmintics have been reported, including inorganic and organic nanoparticles (13, 15–29). Since the anthelminthic use of nanoparticles, we aimed to systematically address the in vitro/in vivo anti-haemonchiasis potential of nanoparticles, toxicological effects, and mechanism of action. This review will also help to highlight the existing gaps in nanoparticle research against H. contortus.
Methodology
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (30). No protocol was followed for conducting this systematic review. The PRISMA checklist is provided in the supporting information section (Supplementary Table S1).
Searching Criteria
Different databases, e.g., ScienceDirect, Google Scholar, Scopus, and PubMed were searched to find relevant published literature (2000 to 2021). Research articles published in the English language were gathered for this systematic review. Keywords such as nanoparticles, nanoparticles nematicidal activity, anthelmintic activity of nanomaterials, in vitro/in vivo activity of nanoparticles, and nanoparticles mechanism of toxicity/inhibition. “Nanoparticles AND anthelmintics OR nematicidal,” “Nanoparticles AND Haemonchus contortus,” “Anthelmintic AND Haemonchus contortus,” “anthelmintic in vitro OR in vivo.” The list of references of published articles was carefully observed, and related titles were searched and downloaded. Moreover, other related literature was also searched and included to discuss and support the findings of the current review.
Inclusion/Exclusion Criteria
The inclusion criteria were (a) nanoparticles tested in vitro/in vivo, (b) articles containing information on assay types, concentration and time exposure used, inhibition/efficacy of nanoparticles, and size of nanoparticles, and (c) original research articles published in English. However, articles dealing with (a) molecular, prevalence, and epidemiological aspects of H. contortus, (b) nanoparticles studies dealing with parasites other than H. contortus, (c) studies that tested chemicals/drugs other than nanoparticles, (d) plant extracts used against H. contortus, and (e) nanoparticles used as a candidate for vaccine were out of the scope and were excluded from this review.
Data Extraction
Endnote (Thomson Reuters, San Francisco, CA, USA) was used to compile the articles. The selected articles were carefully reviewed by the researchers to extract the relevant information including nanoparticle(s) name and size, biological species used, time of exposure, concentration used, inhibition/efficacy, toxicological and pharmacological effects, author(s) name, country of study, and year of publication. Figures and tables were formulated to arrange the extracted data. Moreover, Inkscape (0.92) (https://inkscape.org/) was also used as a drawing tool.
Quantitative Analysis
Jaccard Similarity Index
Jaccard similarity index (JI) was calculated to determine the similarity between the two sets of studies reported in this review. One set of the study is the “in vitro pharmacological validation of nanoparticles” and the other one is the “in vivo pharmacological validation of nanoparticles.” The following formula was used for JI similarity (31):
where “a” is the total number of nanoparticles used in vitro, “b” is the total number of nanoparticles used in vivo as anthelmintic against H. contortus, and “c” is the number of nanoparticles common to both in vitro and in vivo studies.
Results
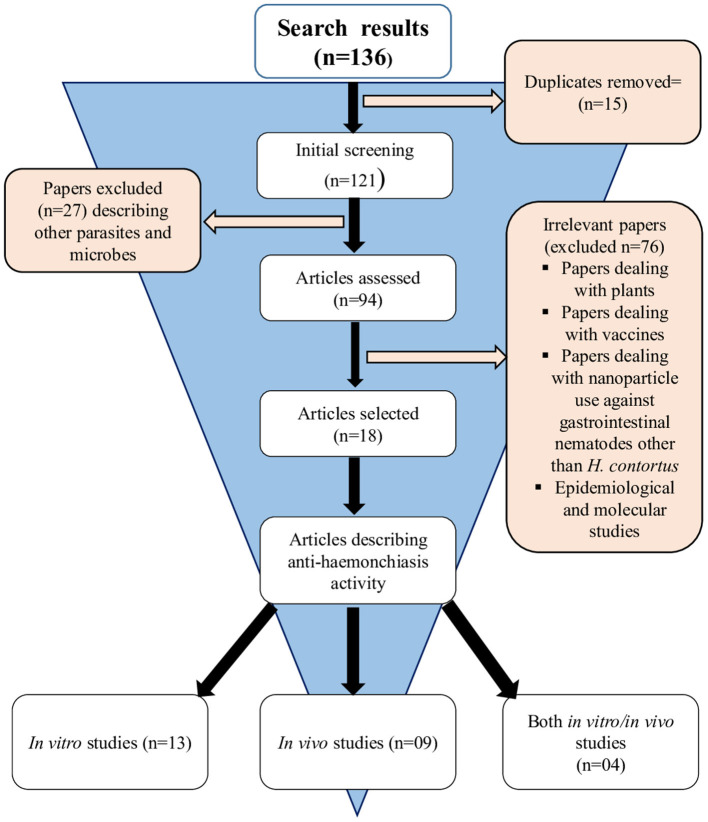
A total of 136 (n = 136) research articles were found and downloaded from online search databases. Eighteen (n = 18) articles were selected and thoroughly reviewed for this study. All the irrelevant and duplicate articles were removed (Figure 1). The quality of the selected articles was assessed, and the articles were summarized as author(s) name, nanoparticles used, biological species/compound used in combination with nanoparticles, source of nanoparticles, country name, release profile, reported quality control, as well as characterization (Table 1).
Figure 1.
Article screening and selection process used for conducting this systematic review.
Table 1.
Quality assessment of articles selected for this systematic review.
| Country Name | Type of nanoparticles | Biological specie | Release profile | Quality reported? | Nanoparticle's characterization? | Reference |
|---|---|---|---|---|---|---|
| India | Silver nanoparticle (AgNPs) | Ziziphus jujuba | _ | _ | Transmission electron microscopy (TEM) and scanning electron microscope (SEM) | (20) |
| India | AgNPs | Azadirachta indica | _ | _ | TEM, and SEM | (22) |
| India | LAgNPs | Lansium parasiticum | _ | + | Surface plasmon resonance (SPR) | (25) |
| China | Chitosan nanoparticles | Carvacrol and carvacryl acetate | + | + | Fourier transform infrared spectroscopy (FTIR) | (24) |
| Iran | Zinc oxide nanoparticle (ZnO-NPs) | N/A | _ | + | XRD and TEM micrography | (13) |
| Brazil | Nanoemulsion | Eucalyptus staigeriana | _ | + | Beam of red light (ZetaSizer 3600, Malvern, United Kingdom) | (29) |
| Brazil | Solid lipid nanoparticle | Melaleuca alternifolia (Maiden & Betche) Cheel | _ | + | N/A | (19) |
| Brazil | Solid lipid nanoparticle | Melaleuca alternifolia | _ | + | N/A | (18) |
| Brazil | Chitosan-encapsulated | E. staigeriana essential oil (EsEO) | + | + | N/A | (17) |
| Brazil | Nanoemulsion | E. staigeriana | _ | + | Beam of red light (ZetaSizer 3600, Malvern, United Kingdom) | (21) |
| Brazil | Nanoencapsulated | Eucalyptus citriodora essential oils | + _ |
FTIR analysis | (16) | |
| Brazil | Encapsulated oil | E. staigeriana | _ | + | N/A | (15) |
| Brazil | Nanoemulsion | C. citratus essential oil Nanoemulsion | _ | + | Beam of red light (ZetaSizer 3600, Malvern, United Kingdom) | (23) |
| Brazil | Encapsulated oil | N/A | _ | + | N/A | (27) |
| Brazil | Polycaprolactone Thio1 nanoparticles (nano Thio1) | Tagetes patula L. | _ | + | Dynamic light scattering (DLS) (Zetasizer NanoZS™, Malvern Panalytical Instruments, UK) | (28) |
| Kenya | Chitosan encapsulated bromelain | N/A | _ | + | SEM and FTIR analysis | (26) |
| Kenya | Chitosan encapsulated bromelain | N/A | _ | + | FTIR analysis | (32) |
| Kenya | Encapsulated ethanolic extract | Prosopis juliflora | _ | – | N/A | (33) |
Key: N/A, data not available.
Out of 18 articles, 13 (72.2%) studies reported in vitro, 9 (50.0%) in vivo, and 4 (22.2%) reported both in vitro/in vivo efficacy of nanoparticles. In vitro studies were more than in vivo. Mostly, studies were carried out in Brazil (n = 10; 56.0%), Kenya, and India (n = 3; 17.0% each) (Figure 2). Most of the studies were reported in the year 2020 (n = 4) and 2017 (n = 3), followed by 2013 and 2016 (n = 2 each) (Figure 3). Mostly, organic nanoparticles (77.7%) were used including polymeric (85.7%) and lipid nanoparticles (14.3%). The remaining (22.2%) were non-organic nanoparticles comprised of metals (75.0%) and metal oxides (25.0%). Among metal and metal oxides nanoparticles, silver and zinc oxide were reported. The active substances were mainly encapsulated by using polycaprolactone and chitosan as a polymeric matrix. The release kinetics of chitosan encapsulated Eucalyptus staigeriana essential oil and chitosan nanoparticles loaded with carvacrol and carvacryl acetate were performed using dialysis membrane method. However, this important piece of information was found missing in most of the selected articles.
Figure 2.
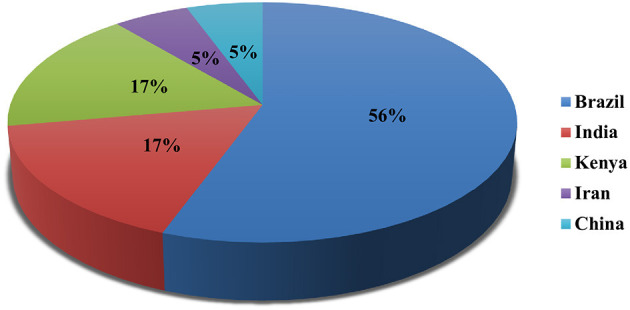
Country-wise studies of nanoparticles against H. contortus.
Figure 3.
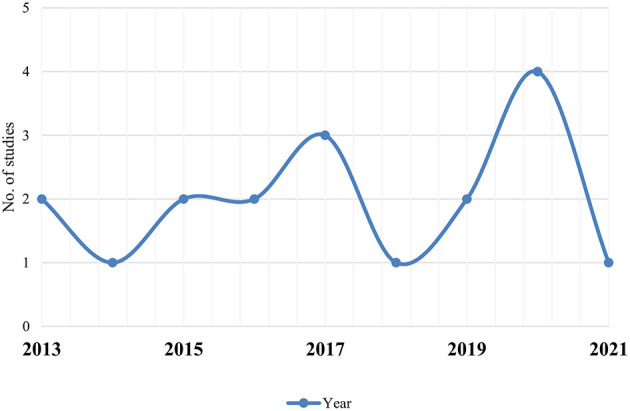
Year-wise studies of nanoparticles against H. contortus.
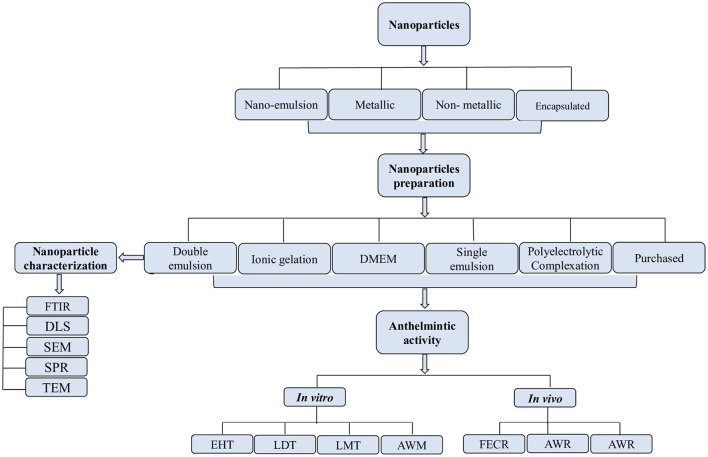
The effects of nanoparticles were evaluated using the egg hatching test (EHT), larval development test (LDT), adult worm mortality test (AWM), and adult worm motility test (AWM) in vitro, whereas in vivo efficacy was evaluated by using worm burden reduction and fecal egg count reduction tests (FECRT). Egg hatching test was the commonly used assay in vitro, while the worm burden reduction was common in vivo (Figure 4).
Figure 4.
Process of selection, preparation, and characterization of nanoparticles for in vitro/in vivo anthelmintic activity.
The results exhibited that the doses used in the in vitro studies ranging from 0.025 to 56 and 0.20 to 500 mg/kg for in vivo. The most common exposure time against eggs hatching was 48 h, whereas it was 24 h for larvae and adults. The highest efficacy of 100% was a result of using encapsulated bromelain against eggs, larvae, and adult worm mortality at a concentration of 4, 2, and 1 mg/ml, respectively (Table 2). Encapsulated E. staigeriana oil reduced worm burden by 83.75% and encapsulated Cymbopogon citratus nano-emulsion by 83.1% in vivo (Table 3).
Table 2.
In vitro efficacy of nanoparticles against H. contortus.
| Nanoparticle | Stage of parasite | Biological specie | Size (nm) | Concentration (mg/ml) | Time (h) | Efficacy (%) | Reference |
|---|---|---|---|---|---|---|---|
| Silver nanoparticle (AgNPs) | Eggs Adult | Ziziphus jujuba Mill. | 28–44 | 2 30 |
48 24 |
92 94 |
(20) |
| Eggs Adult | Azadirachta indica A. Juss | 15–25 | 0.025 0.025 |
48 24 |
85 87 |
(22) | |
| Eggs Larvae |
Lansium parasiticum | 300–700 | 15.8 nM 31.7 nM 63.5 nM 158.7 nM 15.8 nM 31.7 nM 63.5 nM 158.7 nM |
48 24 |
32.1 45 47.2 51.2 33.3 29.5 22.2 14.8 |
(25) | |
| Solid lipid nanoparticle | Egg Larval |
(nanoTTO) Essential oil of M. alternifolia |
N/A | 0.1 0.2 0.4 0.85 1.7 3.5 3.5 7 14 28 56 |
24 48 |
2.77 3.50 15.22 21.19 41.52 82.63 19.51 40.63 48.73 67.28 84.80 |
(18) |
| Zinc oxide nanoparticle (ZnO-NPs) | Adult Adult |
N/A | 20–30 | 8 12 16 8 12 16 8 12 16 12 16 12 16 |
16 20 24 20 24 |
Low motility Very low No motility Very low No motility No motility No motility No motility No motility 19.33/20 20/20 20/20 20/20 |
(13) |
| Nanoemulsion chitosan |
Eggs Larvae |
Eucalyptus staigeriana | 274 | 0.06 0.125 0.25 0.5 1 0.5 1 2 4 8 |
48 24 |
10.7 16.2 59.1 87.9 99 9.1 17.3 31.9 75.5 96.3 |
(29) |
| Eggs |
Citriodora citratus essential oil |
248 | 0.07 0.15 0.31 0.62 1.25 |
N/A | 34.9 49.4 58.1 73.3 97.1 |
(23) | |
| Encapsulated bromelain (chitosan) |
Eggs Larvae Adult | N/A | 200–700 | 4 2 2 |
48 24 24 |
100 | (26) |
| Adult | N/A | 1 | N/A | (32) | |||
| Chitosan encapsulated EcEO | Eggs L1 |
Eucalyptus citriodora essential oil | N/A | 0.125 0.25 0.5 1 2 4 0.5 1 2 4 8 |
N/A | 11.9 25.9 56.8 85.5 92.8 100 10 39.1 49.4 75.8 98.0 |
(16) |
| Chitosan-encapsulated EsEO | Larvae Eggs |
E. staigeriana essential oil (EsEO) | N/A | 0.72 1.45 2.9 5.8 0.18 0.37 0.75 1.5 |
3.6 23.53 54.6 96.59 19.88 39.23 78.42 97.19 |
(17) | |
| Chitosan nanoparticles | Adult | Carvacrol and carvacryl acetate | 271–276 | 0.15 | 6 12 | 66.6 8.3 |
(24) |
| Encapsulated leaves ethanolic extract (ELEE) | Eggs | Prosopis juliflora | N/A | 2 | N/A | 100 | (33) |
| Encapsulated root ethanolic extract (EREE) | 70 |
Keys: N/A, data not available; FECR, fecal egg count reduction.
Table 3.
In vivo efficacy of nanoparticles against H. contortus.
| Nanoparticle | Bioassay | Biological species | Size (nm) | Concentration (mg/kg) | Time (days) | Efficacy (%) | Model | Reference |
|---|---|---|---|---|---|---|---|---|
| Nanoemulsion-chitosan EO | FECR Worm burden | Cymbopogon citratus | 248 | 450 | 0 15 | 80 64 83.1 |
Sheep | (23) |
| Chitosan-encapsulated EO (EncEs) | Worm burden | Eucalyptus staigeriana | N/A | 500 | N/A | 40.51 | Mongolian gerbils | (17) |
| 365 | 30 | 83.75 | Sheep | (15) | ||||
| Solid lipid nanocarriers (nanoTTO) |
Worm burden | Melaleuca alternifolia | 287 | 0.20 0.50 |
N/A | 4.09 48.64 |
Mongolian gerbils | (19) |
| Encapsulated bromelain | FECR | N/A | N/A | 3 10 30 |
28 | 5 56.6 68.8 |
Goats | (32) |
| Chitosan encapsulated EcEO | FECR | Eucalyptus citriodora | N/A | 250 | 10 | 40.5 | Sheep | (16) |
| Encapsulated oils anethole + carvone | FECR | N/A | N/A | 50 | 45 | Significantly reduced FEC | Sheep | (27) |
| Polycaprolactone thio1 nanoparticles (nano thio1) | FECR | Tagetes patula L. | N/A | 2,5 | 30 | Kept the parasitic load stable | Sheep | (28) |
| EPG | 2.5 | 30 | 45% | Sheep | (28) | |||
| Nanoemulsion chitosan |
EPG | E. staigeriana | 277 | 0.25 | 180 | No significant difference was observed | Sheep | (21) |
Key: N/A, data not available; EO, essential oil; nanoTTO, nano tea tree oil; FECR, fecal egg count reduction; EPG, eggs per gram of feces.
The double emulsion method was the frequently used (n = 4) technique for nanoparticle preparation than the ionic gelation method (n = 2), and Dulbecco's modified eagle medium (DMEM), polyelectrolytic complexation system, and single emulsion method (n = 1 each). The methods used for nanoparticle characterization were Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), x-ray diffraction (XRD), and surface plasmon resonance (SPR).
Comparative Analysis of Common Nanoparticles
Nanoparticles evaluated for both in vitro and in vivo efficacies were compared to know their effectiveness against the parasite. Four nanoparticles were commonly evaluated for in vitro as well as in vivo efficacy (Table 4). Since minimum concentration used and maximum efficacy obtained, the encapsulated bromelain was highly effective (100%) in vitro; however, the in vivo efficacy was not satisfactory (68.8%) at the tested concentration. Similarly, the chitosan-encapsulated EO (EncEs) were potentially active against eggs and larvae in vitro (98.0 and 97.0%, respectively), while high activity (84.0%) was also reported in vivo with a relatively higher concentration. Moreover, the nanoparticles were more effective in vitro compared with in vivo.
Table 4.
Comparative analysis of common nanoparticles used against H. contortus.
| Nanoparticle | In vitro | In vivo | Toxicology | Mechanism of action | References | |||
|---|---|---|---|---|---|---|---|---|
| Conc. (mg/ml) | Eff. (%) | Conc. (mg/kg) | Eff. (%) | Dose. (mg/ml) | T. level | |||
| Chitosan-encapsulated EO (EncEs) |
5.8b 1.5a |
96.59 7.19 |
365c | 83.75 | 500 | Non | N/A | (17, 23) |
| Solid lipid nanocarriers (nanoTTO) |
3.5a 56b |
82.63 84.80 |
0.50c | 48.64 | 0.20 | Non | N/A | (18, 19) |
| Encapsulated bromelain | 1c 2b 4a |
100 | 30d | 68.8 | 3–30 | Non | N/A | (26, 32) |
| Chitosan encapsulated EcEO | 4a 8b |
100 98.0 |
250d | 40.5 | N/A | N/A | N/A | (16) |
Keys: a, eggs; b, larvae; c, adult worm; d, fecal egg count reduction; Conc., concentration; Eff., efficacy; T. level, toxicity level; EO, essential oil; TTO, tea tree oil; N/A, data not available.
Toxicity Evaluation
Toxicity and toxic doses of different nanoparticles were reviewed and reported (Table 5). Among the tested nanoparticles, nano-tea tree oil (TTO), EncEs, EcEOn, nanoencapsulated carvacryl acetate (nCVA), and encapsulated bromelain were reported as non-toxic at the tested concentrations, while AgNPs and EsNano were moderately and mildly toxic in HEK293 cell lines and female Swiss albino mice, respectively. Zinc oxide nanoparticles were not evaluated for their toxicity. Nanoparticles were either orally administered or through esophageal gavage. The LC50 for AgNPs, CcEOn, and nano-TTO was not calculated.
Table 5.
Toxicity of nanoparticles used against H. contortus.
| Nanoparticles (symbol) | Concentration (mg/kg) | Exposure time (days) | Mode of administration | Model/cell line | Toxicity level | LC-50 value (mg/ml) | Physiological changes | References |
|---|---|---|---|---|---|---|---|---|
| Silver nanoparticles (AgNPs) | 31.7 nM 63.5 nM 158.7 nM |
1 | N/A | HEK293 | Moderate | N/A | Viability of cell was decreased. | (25) |
| Solid lipid nanoparticles (nanoTTO) | 0.20 | 5 7 9 |
Oral | Gerbils (Meriones unguiculatus) | Non | N/A | Non-toxic to liver and kidneys since hepatic and renal functions were not affected. | (18) |
| Nano emulsion Eucalyptus staigeriana (EsNano) |
1,000 1,500 2,000 2,500 3,000 |
1–14 | Esophageal gavage | Female Swiss albino mice (Mus musculus) Female Wistar albino rats |
Mild | 1,603.9 | No significant differences were found in the body weights or the histological morphologies of organs between the treatment and control groups. | (29) |
| Nano-encapsulated EcEOn | 2,000 2,500 3,000 3,500 |
15 | Esophageal gavage | Female Swiss albino mice (Mus musculus) | Non | 1,680.7 | No behavioral changes and mortality were observed. | (16) |
| Nanoencapsulated carvacryl acetate (nCVA) | 0.00156 0.3 |
1 | N/A | Murine fibroblast L929 | Non | 0.3 | No cytotoxic and genotoxic effects were observed. | (24) |
| Encapsulated bromelain | 3–30 | 14 | Oral | Goats | Non | 0.155 | No treatment related pathological changes of internal organs were observed after necropsy. No changes in the histology of heart, kidney, or hematology parameters were recorded. | (32) |
| Chitosan encapsulated Eucalyptus staigeriana EO (EnEsEO) | 500 | 3 | Oral | M. unguiculatus | Non | N/A | No hematological and biochemical alterations were reported. | (17) |
| Zinc oxide nanoparticles | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (13) |
| Cymbopogon citratus EO nanoemulsion | 450 | 3 | Oral | Sheep | Toxic | N/A | One sheep out of ten died, treated with CcEOn. The sheep presented sialorrhea before death. | (23) |
| Encapsulated oils anethole + carvone | 50 | 45 | Oral | Sheep | Non | N/A | No effect on kidney and liver function. | (27) |
Key: N/A, data not available.
Jaccard Similarity Index
The two datasets, i.e., in vitro and in vivo use of nanoparticles was checked for their similarity by using JI similarity formula and 22.2% similarity was found.
Discussion
The main constraints of profitable products in the livestock sector are parasites and parasitic resistance to anthelmintic drugs around the world. To resolve the huge economic losses, it is important to improve the control of main parasitic diseases through alternative, less harmful, biodegradable, and ecologically safe anthelmintic strategies. Nanoparticles may reduce the risk of resistance of H. contortus to the anthelmintic drugs and overcome the resistance mechanisms adapted by the parasite, potentiating the drug target, and increasing bioavailability of the drug. The current systematic review assessed the in vitro/in vivo nematicidal potential and toxicological implications along with the mechanism of action of various nanoparticles against H. contortus. The results of this study will help to identify potential approaches to design new nanoparticulate drugs and ways to meet the current research limitations in prospective studies.
Nanoparticles were commonly evaluated in vitro, and only few studies had reported in vivo effectiveness of different nanoparticles. Previously, in vitro studies were mostly reported than in vivo and justify the current findings (34–36). In vitro studies are inexpensive and less time consuming, and anthelmintic effects at different life stages of the parasite can easily be studied (37). After initial screening, effective substance/product can further be evaluated for in vivo efficacy (38). In vivo studies are useful to know the host immune response to a particular anthelmintic agent, toxicological and pharmacological effects, and in vivo efficacy. However, in vivo studies are expensive and difficult to reproduce the results, required long experimental duration, and has lower precision (39). The research field is highly inundated with in vitro studies, and in vivo studies are insufficient; therefore, in vivo evaluation of nanoparticles would be of great importance in future studies (36).
Organic nanoparticles were among the frequently used nanoparticles. Nanoparticles can easily be produced in large quantities using different approaches and are highly biodegradable and biocompatible. These nanoparticles possess the capacity to solve, absorb, and encapsulate a drug in a polymer matrix and are excellent nanocarriers for the controlled and sustained release of drugs (40, 41). Among metal and metal oxides, AgNPs and ZnO were reported. AgNPs have profound antiparasitic and antibacterial activities. Antiparasitic activity of AgNPs was inhibition of metabolic activities and cell proliferation of Leishmania spp. promastigotes. Antiviral activities of AgNPs have also been demonstrated to stop viral replication process and prevent binding of virus particles to host cell receptors. These nanoparticles have promising efficacy as anticancer agents and could be a reason that they have attracted more attention as an anthelmintic agent (42–46). ZnO nanoparticles are widely used and important candidates for developing novel drugs due to their non-toxic, antiparasitic, antifungal, and antimicrobial effects. These can also be used for gene delivery and can cause death of cancerous cells without effecting normal healthy cells (47, 48).
Nanoparticles were encapsulated using a polymeric matrix, mainly chitosan and polycaprolactone, to improve the controlled drug release. Encapsulation of bioactive substances also improves the absorption and bioavailability by facilitating the diffusion through epithelium. The most common are aliphatic polyesters and their copolymers. Polycaprolactone (PCL) is a synthetic aliphatic polyester approved by the FDA and has some advantages: it is hydrophobic, biodegradable, biocompatible, and relatively inexpensive (49). In addition, due to its low toxicity, it is suitable for intravenous or oral administration (50, 51). Chitosan, a natural polymer obtained by the deacetylation of chitin, was the frequently used encapsulating matrix. The chitosan microsphere formulation for the controlled release of drugs improves their dissolution and bioavailability (52, 53). Hence, increases in efficient drug delivery may increase the overall effectiveness of the targeted drug/compound. Furthermore, its excellent biodegradability and non-toxic nature were the core reasons for which chitosan was selected as an encapsulating agent for evaluating anti-haemonchiasis nanoparticles (21).
The release kinetics of nanoparticles was the neglected aspect and barely studied in the reviewed articles. It is an important and critical aspect that helps to understand the dosage form behavior, and assess the safety and efficacy of a desired drug during the various stages of development. To maximize the effectiveness of nanoparticle targeting, drug release from nanoparticles needs to be slow enough to avoid substantial drug loss before the carrier reaches the site of action thereby reducing toxicity (54, 55). After nanoparticle accumulation at the target site, optimizing efficacy will require tunability of the drug release rate (56). Therefore, determination of product quality and performance becomes a crucial aspect during nanoparticulate dosage form development. When designed appropriately, an in vitro release profile can reveal fundamental information on the dosage form and its behavior, as well as provide details on the release mechanism and kinetics, enabling a rational and scientific approach to drug product development (57). Thus, the kinetics of drug release from nanoparticles should be an essential feature of their design and a property monitored for the quality control of nanoparticle formulations (58).
The frequently reported assay was egg hatching test (EHT) followed by larval development test (LDT). The possible reason for such an extensive use of these assays may be attributed to the fact that these tests take into account variations in the habits, behavior, and sensibility of these life forms of the parasite and permit the exposure of different potential pharmacological sites for future pharmacodynamics investigation (59). Moreover, APMT and AWMT were less likely to be used in in vitro studies for anthelmintic evaluation. It is because of the lack of a culture system yielding adults of this nematode parasite, which prevents a preliminary investigation of the efficacy of anthelmintics at this stage (60); hence, the in vitro tests using free-living stages of nematode parasites are considered as the best means of screening the anthelmintic activity of new substances/products (61).
The most widely utilized in vivo assessment of nanoparticles against H. contortus was the FECR test. The major benefit of this examination is that, regardless of their mode of operation, it can be carried out with all anthelmintics (59). Gerbils and sheep have been primarily used in in vivo experiments as animal models. Using sheep as a model can be explained by the fact that domestic animals are a valuable component of clinical studies for several purposes, including simple availability and management, accessibility to early examine diseased tissues as well as the models, and also require disease characteristics to be explored at an early level (62). However, a study also evaluated the efficacy of plants against intestinal nematode parasites by using mice as models and reported high anthelmintic efficacy (63). Rodent use as an animal model may have some drawbacks; rodents provide a completely different internal environment (habitat) to the nematodes than small ruminants; thus, the drug efficacy may be lower or higher based on the habitat and drug absorption site of the host (64). Rodents are monogastric, and sheep are polygastric animals, which can also alter the drug mechanism of distribution and biotransformation. However, efficacy test on rodents can help researchers deduce the prescriptions to be used on sheep and goats (63).
Toxicity
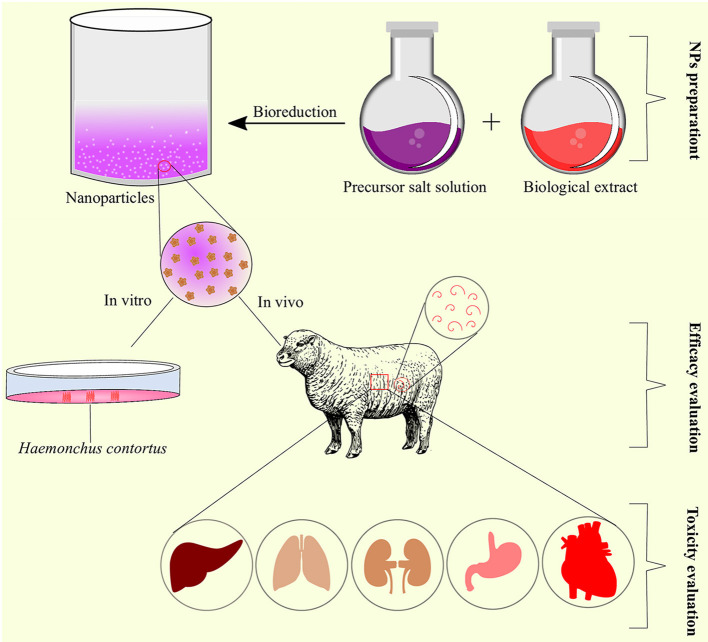
LAgNPs were moderately toxic when tested on HEK293 cell lines. The HEK293 cell viability decreased in a dose- and time-dependent manner. The lowest concentration produced no significant toxic effects; however, with an increase in concentration, i.e., 31.7, 63.5, and 158.7 nM, the observed decrease in the viability was 77.5 ± 5.06, 71.3 ± 9.8, and 62.1 ± 9.3%, respectively (25). Oral administration of CcEOn at 450 mg/kg concentration resulted in the death of 1 sheep out of 10. The sheep suffered from sialorrhea before death (23). However, the death of the sheep was not confirmed through necropsy and was assumed that the sheep may have aspirated the essential oil, and the wrong route of administration was attributed as the cause of death. Other studies also supported the non-toxic nature of CcEO at 1 and 800 mg/kg in rats and gerbils, respectively (65, 66) (Figure 5).
Figure 5.
Schematic representation of nanoparticles preparation, efficacy, and toxicity evaluation as anthelmintic agents.
EsNano was found non-toxic after acute and subchronic toxicity evaluation in rats. The tested concentrations did not produce any change in the hematological parameters except a slight increase in the white blood cells (WBCs) after subchronic toxicity (29). There was no change in the body weight and histological morphologies of organs in treated rats (29). Nano encapsulated carvacryl acetate (nCVA) had no cytotoxic and genotoxic/mutagenic effects on murine fibroblast cell lines at the tested concentration. The non-toxic effects of nanoparticles were attributed to CVA and the biopolymers as they had no toxicity (24). Esophageal gavage administration of nanoencapsulated EcEO was safe, and no behavioral changes and mortality were recorded after acute toxicity evaluation (16). Similarly, nanoTTO produced non-significant differences in the hematological and serum biochemical profiles of the treated and untreated groups (18). Encapsulated bromelain was non-toxic as no pathological and histological changes were observed at concentrations ranging from 3 to 30 mg/kg after necropsy. The hematological parameters also remained unchanged at the same concentrations, confirming the non-toxic effect of bromelain in vivo (32). Encapsulated E. staigeriana essential oil (EnEsEO) was found non-toxic when orally administered to gerbils at 500 mg/kg concentration (17). However, the toxicity of ZnO-NPs was not evaluated and should be evaluated in future studies.
Mechanism of Action
The pharmacological activity of a drug depends on how it interacts with the targeted biomolecules, i.e., receptors (67). Pharmacological activity is an important phenomenon to know the precise target of the nanoparticle with anthelmintic efficacy against the parasite or other organism/pathogen under observation (36).
Exposure of H. contortus to LAgNPs produced morphological and physiological effects. Morphologically, LAgNPs caused complete distortion of the cuticle and shrank the body. Physiologically, levels of reactive oxygen and nitrogen species were significantly increased, which resulted in oxidative stress and caused physical damage to tissues of the worm (25). In response to oxidative stress, a sharp increase in stress-responsive activities of enzymes, like catalase, superoxide dismutase, and glutathione peroxidase activities, along with the concentration of glutathione, was observed in worm tissue, which indicated a LAgNPs-responsive alteration of metabolism (25). Moreover, AgNPs also depleted the levels of glycogen, lipids, and protein contents of H. contortus. Parasites produce energy from stored carbohydrates (glycogen) to perform major metabolic processes (20). Glycogen is the chief energy reserve in most of the nematodes that exist in environments of low oxygen tension (68). Lipids are the chief functional and structural components of nematode parasites. Plasma membranes and eggs contain lipids as an important energy source in the free-living stages, any depletion or damage to lipid constituents may lead to mortality of the parasite. Therefore, lipid biosynthesis inhibition could be a potential target to develop an effective anti-haemonchiasis drug (20).
Proteins, like enzymes, are very important for normal physiological functioning and to carry out key metabolic activities. Hence, reduced protein content would hamper the normal physiological activities of the worms and may be accounted for mortality at higher concentrations. Egg morphological alterations justify the disintegration and shrinkage of H. contortus larvae development (20). Some studies reported, a drastic decrease in 5′ nucleosidase, ATPase, alkaline, and acid phosphatases of intestinal cestodes treated with AuNPs (69). Encapsulated bromelain is highly effective against nematode parasites (70), and it was found that bromelain damages the cuticle of H. contortus leading to paralysis and death (71).
The ZnO-NPs completely paralyzed the parasites. These nanoparticles can adversely affect the antioxidant systems of H. contortus by inducing severe oxidative stress resulting in denaturation of the antioxidant enzymes. Various concentrations of ZnO-NPs imposed controversial alteration on the activities of the antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) (13). This increase in the oxidative stress and reactive oxygen species (ROS) can damage proteins, carbohydrates, lipids, and DNA of the parasite (72). Therefore, disruption of the antioxidant system of the parasite unables H. contortus to survive against the host generated free radicals.
Conclusion and Future Recommendations
Nanoparticles could be a potential source for developing novel anthelmintic drugs to overcome the emerging issue of anthelmintic resistance in H. contortus. Mostly, in vitro studies have reported the anthelmintic efficacy of nanoparticles. More studies are required to evaluate and describe the effects of nanoparticles on a molecular level, toxicological consequences, and different pharmacological targets along with exact mechanism of action using suitable animal models. Furthermore, the size of the nanoparticles was not determined in some of the studies, which is one of the crucial aspects of nanoparticles, and should be considered in future studies to provide more in-depth information of the nanoparticles under consideration.
Chitosan-encapsulated EO and encapsulated bromelain were highly effective both in vitro and in vivo with no observed toxic effects at the tested concentration. However, the release profile of mostly nano-encapsulated compound(s) was missing, and hence, the controlled and sustained drug release properties are unknown. These nanoparticles should further be evaluated and could be alternative sources of anti-haemonchiasis agents.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
RA and NA conceptualized the study, conducted the formal analysis, curated the data, and wrote the original draft. RA and SM formulated the methodology. RA sourced the software and provided supervision for the study. RA, SNK, AM, and SFA reviewed and edited the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.789977/full#supplementary-material
References
- 1.Wang C, Li F, Zhang Z, Yang X, Ahmad AA Li X, et al. Recent research progress in China on Haemonchus contortus. Front Microbiol. (2017) 8:1509. 10.3389/fmicb.2017.01509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tariq KA. A review of the epidemiology and control of gastrointestinal nematode infections of small ruminants. Proc Natl Acad Sci India B Biol Sci. (2015) 85:693–703. 10.1007/s40011-014-0385-9 [DOI] [Google Scholar]
- 3.Easwaran C, Harikrishnan TJ, Raman M. Multiple anthelmintic resistance in gastrointestinal nematodes of sheep in Southern India. Vet Arh. (2009) 79:611–20. [Google Scholar]
- 4.Kaplan RM, Vidyashankar AN. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol. (2012) 186:70–8. 10.1016/j.vetpar.2011.11.048 [DOI] [PubMed] [Google Scholar]
- 5.Das G. Levamisole and fenbendazole resistance among gastrointestinal nematodes in goats at Jabalpur, Madhya Pradesh. J Vet Parasitol. (2015) 29:98–102. [Google Scholar]
- 6.Dixit AK, Das G, Dixit P, Singh AP, Kumbhakar NK, Sankar M, et al. An assessment of benzimidazole resistance against caprine nematodes in Central India. Trop Anim Health Prod. (2017) 49:1471–8. 10.1007/s11250-017-1349-x [DOI] [PubMed] [Google Scholar]
- 7.Furgasa W, Abunna F, Yimer L, Haile G. Review on anthelmintic resistance against gastrointestinal nematodes of small ruminants: its status and future perscpective in Ethiopia. J Vet Sci Ani Husb. (2018) 6:407. [Google Scholar]
- 8.Torres-Acosta JF. Mendoza-de-Gives P, Aguilar-Caballero AJ, Cuéllar-Ordaz JA. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Vet Parasitol. (2012) 189:89–96. 10.1016/j.vetpar.2012.03.037 [DOI] [PubMed] [Google Scholar]
- 9.Melo ACFL, Bevilaqua CML, Reis IF. Resistance to benzimidazoles anthelmintics in gastrointestinal nematodes of small ruminants from the Brazilian northeastern semiarid region. Braz Anim Sci. (2009) 10:294–300. [Google Scholar]
- 10.Adeyemi OS, Whiteley CG. Interaction of nanoparticles with arginine kinase from Trypanosoma brucei: kinetic and mechanistic evaluation. Int J Biol Macromol. (2013) 62:450–6. 10.1016/j.ijbiomac.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Butkus MA, Labare MP, Starke JA, Moon K, Talbot M. Use of aqueous silver to enhance inactivation of coliphage MS-2 by UV disinfection. Appl Environ Microbiol. (2004) 70:2848–53. 10.1128/AEM.70.5.2848-2853.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhardwaj R, Saudagar P, Dubey V. Nanobiosciences: a contemporary approach in antiparasitic drugs. Mol Cell Pharmacol. (2012) 4:97–103. [Google Scholar]
- 13.Esmaeilnejad B, Samiei A, Mirzaei Y, Farhang-Pajuh F. Assessment of oxidative/nitrosative stress biomarkers and DNA damage in Haemonchus contortus, following exposure to zinc oxide nanoparticles. Acta Parasitol. (2018) 63:563–71. 10.1515/ap-2018-0065 [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Radovic-Moreno AF, Wu J, Langer R, Shi J. Nanomedicine in the management of microbial infection - overview and perspectives. Nano Today. (2014) 9:478–98. 10.1016/j.nantod.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Aquino Mesquita M, JB ESJ, Panassol AM, de Oliveira EF, Vasconcelos AL, de Paula HC, et al. Anthelmintic activity of Eucalyptus staigeriana encapsulated oil on sheep gastrointestinal nematodes. Parasitol Res. (2013) 112:3161–5. 10.1007/s00436-013-3492-2 [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro J, Ribeiro W, Camurça-Vasconcelos A, Macedo I, Santos J, Paula H, et al. Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet Parasitol. (2014) 204:243–8. 10.1016/j.vetpar.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro WL, Macedo IT, dos Santos JM, de Oliveira EF, Camurça-Vasconcelos AL, de Paula HC, et al. Activity of chitosan-encapsulated Eucalyptus staigeriana essential oil on Haemonchus contortus. Exp Parasitol. (2013) 135:24–9. 10.1016/j.exppara.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 18.Grando T, De Sá M, Baldissera M, Oliveira C, De Souza M, Raffin R, et al. In vitro activity of essential oils of free and nanostructured Melaleuca alternifolia and of terpinen-4-ol on eggs and larvae of Haemonchus contortus. J Helminthol. (2016) 90:377–82. 10.1017/S0022149X15000401 [DOI] [PubMed] [Google Scholar]
- 19.Grando TH, Baldissera MD, Gressler LT, de Sá MF, Bortoluzzi BN, Schafer AS, et al. Melaleuca alternifolia anthelmintic activity in gerbils experimentally infected by Haemonchus contortus. Exp Parasitol. (2016) 170:177–83. 10.1016/j.exppara.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 20.Preet S, Tomar RS. Anthelmintic effect of biofabricated silver nanoparticles using Ziziphus jujuba leaf extract on nutritional status of Haemonchus contortus. Small Rumin Res. (2017) 154:45–51. 10.1016/j.smallrumres.2017.07.002 [DOI] [Google Scholar]
- 21.Ribeiro WL, Camurça-Vasconcelos AL, dos Santos JM, Macedo IT., Ribeiro JdC, Oliveira EFd, et al. The use of Eucalyptus staigeriana nanoemulsion for control of sheep haemonchosis. Braz J Vet Res. (2017) 37:221–6. 10.1590/s0100-736x2017000300004 [DOI] [Google Scholar]
- 22.Tomar R, Preet S. Evaluation of anthelmintic activity of biologically synthesized silver nanoparticles against the gastrointestinal nematode, Haemonchus contortus. J Helminthol. (2017) 91:454. 10.1017/S0022149X16000444 [DOI] [PubMed] [Google Scholar]
- 23.Macedo ITF, Oliveira LMB, André WPP, Araújo Filho JV, Santos J, Rondon FCM, et al. Anthelmintic effect of Cymbopogon citratus essential oil and its nanoemulsion on sheep gastrointestinal nematodes. Rev Bras Parasitol Vet. (2019) 28:522–7. 10.1590/s1984-29612019065 [DOI] [PubMed] [Google Scholar]
- 24.André WP, Paiva JR, Cavalcante GS, Ribeiro WL., Araújo JVd, Cavalcanti BC, et al. Chitosan nanoparticles loaded with carvacrol and carvacryl acetate for improved anthelmintic activity. J Braz Chem Soc. (2020) 31:1614–22. 10.21577/0103-5053.20200047 [DOI] [Google Scholar]
- 25.Goel V, Kaur P, Singla LD, Choudhury D. Biomedical evaluation of Lansium parasiticum extract-protected silver nanoparticles against Haemonchus contortus, a parasitic worm. Front Mol Biosci. (2020) 7:595646. 10.3389/fmolb.2020.595646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunduza A, Kagira J, Maina N, Andala D, Cheruiyot K, Kahiro S. In vitro anthelmintic activity of chitosan encapsulated bromelain against eggs, larval and adult stages of Haemonchus contortus. J Appl Life Sci Int. (2020) 23:28–38. 10.9734/jalsi/2020/v23i330151 [DOI] [Google Scholar]
- 27.Katiki LM, Araujo RC, Ziegelmeyer L, Gomes ACP, Gutmanis G, Rodrigues L, et al. Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus. Exp Parasitol. (2019) 197:36–42. 10.1016/j.exppara.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 28.Politi FAS, Bueno RV, Zeoly LA, Fantatto RR, Eloy JO, Chorilli M, et al. Anthelmintic activity of a nanoformulation based on thiophenes identified in Tagetes patula L. (Asteraceae) against the small ruminant nematode Haemonchus contortus. Acta Trop. (2021) 219:105920. 10.1016/j.actatropica.2021.105920 [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro WL, Camurça-Vasconcelos AL, Macedo IT, dos Santos JM, de Araújo-Filho JV, Ribeiro Jde C, et al. In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Vet Parasitol. (2015) 212:444–7. 10.1016/j.vetpar.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. (2009) 89:873–80. 10.1093/ptj/89.9.873 [DOI] [PubMed] [Google Scholar]
- 31.Kayani S, Ahmad M, Sultana S, Khan Shinwari Z, Zafar M, Yaseen G, et al. Ethnobotany of medicinal plants among the communities of Alpine and Sub-alpine regions of Pakistan. J Ethnopharmacol. (2015) 164:186–202. 10.1016/j.jep.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Wasso S, Maina N, Kagira J. Toxicity and anthelmintic efficacy of chitosan encapsulated bromelain against gastrointestinal strongyles in Small East African goats in Kenya. Vet World. (2020) 13:177–83. 10.14202/vetworld.2020.177-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kipyegon C, Helen KL, Patrick KG, Francis NK, Odhiambo RS, Jackson MK, et al. In vitro ovicidal activity of encapsulated ethanolic extract of Prosopis juliflora against Haemonchus contortus eggs. J Pharm Biol Sci. (2015) 10:18e22. 10.9790/3008-10541822 [DOI] [Google Scholar]
- 34.Albalawi AE, Alanazi AD, Baharvand P, Sepahvand M, Mahmoudvand H. High potency of organic and inorganic nanoparticles to treat cystic echinococcosis: an evidence-based review. Nanomaterials (Basel). (2020) 10:2538. 10.3390/nano10122538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali R, Khan S, Khan M, Adnan M, Ali I, Khan TA, et al. A systematic review of medicinal plants used against Echinococcus granulosus. PLoS One. (2020) 15:e0240456. 10.1371/journal.pone.0240456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali R, Rooman M, Mussarat S, Norin S, Ali S, Adnan M, et al. A systematic review on comparative analysis, toxicology, and pharmacology of medicinal plants against Haemonchus contortus. Front Pharmacol. (2021) 12:644027. 10.3389/fphar.2021.644027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demeler J, Gill JH, von Samson-Himmelstjerna G, Sangster NC. The in vitro assay profile of macrocyclic lactone resistance in three species of sheep trichostrongyloids. Int J Parasitol Drugs Drug Resist. (2013) 3:109–18. 10.1016/j.ijpddr.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zips D, Thames HD, Baumann M. New anticancer agents: in vitro and in vivo evaluation. In Vivo. (2005) 19:1–7. [PubMed] [Google Scholar]
- 39.Lacey E, Redwin J, Gill J, Demargheriti V, Waller P. A larval development assay for the simultaneous detection of broad spectrum anthelmintic resistance. In: Resistance of Parasites to Antiparasitic Drugs Round Table Conference Held at the 7th International Congress of Parasitology. Paris: Merck; (1990). p. 177–84. [Google Scholar]
- 40.Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomedicine. (2015) 10:1001–18. 10.2147/IJN.S56932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatia D, Mittal A, Malik DK. Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians. 3 Biotech. (2016) 6:196. 10.1007/s13205-016-0514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, et al. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int J Nanomedicine. (2011) 6:2705–14. 10.2147/IJN.S23883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. (2011) 16:8894–918. 10.3390/molecules16108894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M, et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. (2014) 103:1931–44. 10.1002/jps.24001 [DOI] [PubMed] [Google Scholar]
- 45.Gaafar M, Mady R, Diab R. Shalaby ThI. Chitosan and silver nanoparticles: promising anti-toxoplasma agents. Exp Parasitol. (2014) 143:30–8. 10.1016/j.exppara.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 46.Wei L, Lu J, Xu H, Patel A, Chen ZS, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. (2015) 20:595–601. 10.1016/j.drudis.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomed. (2011) 6:1463–73. 10.2147/IJN.S22021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzaei H, Darroudi M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceramics Int. (2017) 43(1 Part B):907–14. 10.1016/j.ceramint.2016.10.051 [DOI] [Google Scholar]
- 49.Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. Int J Pharm. (2004) 278:1–23. 10.1016/j.ijpharm.2004.01.044 [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Huang C, Chen Z. Study on the biocompatibility and toxicology of biomaterials-poly(epsilon-caprolactone). J Biomed Eng. (2000) 17:380–2. [PubMed] [Google Scholar]
- 51.Hernán Pérez de, la Ossa D, Ligresti A, Gil-Alegre ME, Aberturas MR, Molpeceres J, Di Marzo V, et al. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: development, characterization and in vitro evaluation of their antitumoral efficacy. J Control Release. (2012) 161:927–32. 10.1016/j.jconrel.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 52.Jayakumar R, Nwe N, Tokura S, Tamura H. Sulfated chitin and chitosan as novel biomaterials. Int J Biol Macromol. (2007) 40:175–81. 10.1016/j.ijbiomac.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 53.Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. (2011) 36:981–1014. 10.1016/j.progpolymsci.2011.02.001 [DOI] [Google Scholar]
- 54.Loew S, Fahr A, May S. Modeling the release kinetics of poorly water-soluble drug molecules from liposomal nanocarriers. J Drug Deliv. (2011) 2011:376548. 10.1155/2011/376548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng L, An L, Wu X. Modeling drug-carrier interaction in the drug release from nanocarriers. J Drug Deliv. (2011) 2011:370308. 10.1155/2011/370308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston MJ, Semple SC, Klimuk SK, Edwards K, Eisenhardt ML, Leng EC, et al. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta. (2006) 1758:55–64. 10.1016/j.bbamem.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 57.D'Souza S A. Review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. (2014) 2014:304757. 10.1155/2014/30475721028937 [DOI] [Google Scholar]
- 58.Modi S, Anderson BD. Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol Pharm. (2013) 10:3076–89. 10.1021/mp400154a [DOI] [PubMed] [Google Scholar]
- 59.Demeler J, Kleinschmidt N, Küttler U, Koopmann R, von Samson-Himmelstjerna G. Evaluation of the egg hatch assay and the larval migration inhibition assay to detect anthelmintic resistance in cattle parasitic nematodes on farms. Parasitol Int. (2012) 61:614–8. 10.1016/j.parint.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 60.Geary TG, Sangster NC, Thompson DP. Frontiers in anthelmintic pharmacology. Vet Parasitol. (1999) 84:275–95. 10.1016/S0304-4017(99)00042-4 [DOI] [PubMed] [Google Scholar]
- 61.Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds MS. Ethnobotanical study of some Ghanaian anti-malarial plants. J Ethnopharmacol. (2005) 99:273–9. 10.1016/j.jep.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 62.Sadia S, Tariq A, Shaheen S, Malik K, Ahmad M, Qureshi H, et al. Ethnopharmacological profile of anti-arthritic plants of Asia-a systematic review. J Herb Med. (2018) 13:8–25. 10.1016/j.hermed.2018.08.003 [DOI] [Google Scholar]
- 63.Camurça-Vasconcelos A, Bevilaqua C, Morais S, Maciel M, Costa C, Macedo I, et al. Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet Parasitol. (2007) 148:288–94. 10.1016/j.vetpar.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 64.Hennessy D. Modifying the formulation or delivery mechanism to increase the activity of anthelmintic compounds. Vet Parasitol. (1997) 72:367–90. 10.1016/S0304-4017(97)00106-4 [DOI] [PubMed] [Google Scholar]
- 65.Fandohan P, Gnonlonfin B, Laleye A, Gbenou JD, Darboux R, Moudachirou M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem Toxicol. (2008) 46:2493–7. 10.1016/j.fct.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 66.Macedo IT, Oliveira LM, Ribeiro WL, Santos JM, Silva K, Araújo Filho JV, et al. Anthelmintic activity of Cymbopogon citratus against Haemonchus contortus. Rev Bras Parasitol Vet. (2015) 24:268–75. 10.1590/S1984-29612015059 [DOI] [PubMed] [Google Scholar]
- 67.Roy J. 9 - Pharmacological concepts and drugs. In: Roy J, editor. An Introduction to Pharmaceutical Sciences. Sawston: Woodhead Publishing; (2011). p. 205–30. 10.1533/9781908818041.205 [DOI] [Google Scholar]
- 68.Sood M. Histochemical, biochemical and immunological studies in Haemonchus contortus (Nematoda: Trichostrongyloidea)– an Indian perspective. J Parasit Dis. (2006) 30:4–15. [Google Scholar]
- 69.Kar PK, Murmu S, Saha S, Tandon V, Acharya K. Anthelmintic efficacy of gold nanoparticles derived from a phytopathogenic fungus, Nigrospora oryzae. PLoS One. (2014) 9:e84693. 10.1371/journal.pone.0084693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Domingues LF, Giglioti R, Feitosa KA, Fantatto RR, Rabelo MD, de Sena Oliveira MC, et al. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet Parasitol. (2013) 197:263–70. 10.1016/j.vetpar.2013.04.031 [DOI] [PubMed] [Google Scholar]
- 71.Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology. (2006) 132(Pt 5):681–9. 10.1017/S003118200500973X [DOI] [PubMed] [Google Scholar]
- 72.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. (2002) 30:620–50. 10.1080/01926230290166724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.