Abstract
Congestive heart failure (HF) is a progressive affliction defined as the inability of the heart to sufficiently maintain blood flow. Ventricular arrhythmias (VAs) are common in patients with HF, and conversely, advanced HF promotes the risk of VAs. Management of VA in HF requires a systematic, multimodality approach that comprises optimization of medical therapy and use of implantable cardioverter-defibrillator and/or device combined with cardiac resynchronization therapy. Catheter ablation is one of the most important strategies with the potential to abolish or decrease the number of recurrences of VA in this population. It can be a curative strategy in arrhythmia-induced cardiomyopathy and may even save lives in cases of an electrical storm. Additionally, modulation of the autonomic nervous system and stereotactic radiotherapy have been introduced as novel methods to control refractory VAs. In patients with end-stage HF and refractory VAs, an institution of the mechanical circulatory support device and cardiac transplant may be considered. This review aims to provide an overview of current evidence regarding management strategies of VAs in HF with an emphasis on interventional treatment.
Keywords: Catheter ablation, Heart failure, Implantable cardioverter-defibrillator, Sudden cardiac death, Ventricular arrhythmia
Key Findings.
-
▪
Management of ventricular arrhythmias (VAs) in heart failure requires a systematic, multimodality approach.
-
▪
Besides optimal medical therapy, implantable cardioverter-defibrillators remain the key component for secondary and primary prevention of sudden cardiac death.
-
▪
In patients with recurrent VAs, catheter ablation effectively decreases arrhythmia burden and represents life-saving therapy in patients with electrical storm or incessant VAs.
-
▪
Novel therapeutic modalities are currently under evaluation to control otherwise refractory VAs.
Introduction
Heart failure (HF) is a clinical syndrome characterized by typical symptoms and signs caused by structural and/or functional cardiac abnormalities, resulting in reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.1 Multiple studies have shown that a high proportion of deaths among patients with HF occur suddenly owing to ventricular arrhythmias (VAs).2,3 This is especially true for subjects with milder symptoms.4 Progress in the pharmacologic treatment of HF over the last 2 decades has improved or delayed the progression of cardiovascular disease and reduced the annual rate of sudden cardiac death (SCD). However, the risk of arrhythmic events remains. In this respect, the only effective protection against SCD is provided by implantable cardioverter-defibrillators (ICDs). These devices are effective in preventing bradycardia and abolishing potentially lethal VAs, in both primary and secondary prevention. However, ICDs do not prevent the occurrence of VAs. Recurrent VAs may lead to frequent appropriate shocks that require complex treatment. This includes pharmacological therapy, catheter ablation, and other treatment to minimize the risk of shocks. This review aims to provide an overview of current evidence regarding management strategies of VAs in HF with emphasis on interventional treatment. Although half of the patients with HF have a preserved ejection fraction (EF), there is no proven treatment by ICD for this population, and this review will focus mainly on patients with HF and reduced EF.
The interplay between VAs and HF
There is a complex interplay between HF and VAs. While HF can trigger arrhythmias and underlying heart disease forms an arrhythmogenic substrate, VAs may, in turn, accelerate HF.5 The structural changes that occur in patients with HF comprise replacement fibrosis, regional ventricular hypertrophy, and changes in myocyte mechanical and electrical function.6 Such a substrate is further modulated by neurohormonal activation, metabolic adaptation, and response to the neurohormonal blockade.
In some studies, the occurrence of appropriate shock for sustained VAs was associated with a significant increase in pump failure death.7 It was unclear whether the shocks were only a marker of disease progression or the cause of the adverse outcome. In the ATITUTIDE study,8 the question was answered, as the mortality risk was associated with underlying rhythm rather than the shock therapy itself. There was no significant difference in survival between no shocks and inappropriate shocks. The mechanisms owing to which VAs lead to the progression of HF are unclear, but it has been proposed that VAs may result in delayed recovery of myocardial function through the metabolic stunning.5 Another piece of evidence that shocks per se are not harmful was provided by the SIMPLE trial,9 which documented that ICD shocks during implant testing did not significantly increase the rate of adverse events.
Over time, large HF trials have documented that pharmacological treatment reduces both pump failure deaths and SCD. Thus, therapies that lead to the reversal of HF can reduce the incidence of VAs. On the other hand, it was shown that although resynchronization therapy improves HF symptoms and leads to reverse remodeling, it did not reduce the SCD and incidence of VAs.10,11 In the recent analysis of the RAFT trial, resynchronization therapy decreased the incidence of VAs only in patients with primary prophylactic indication, but not in those with secondary indication.12 Similarly, several studies have identified a very high prevalence of VAs after left ventricular assist device (LVAD) insertion.13,14 This occurs despite the improvement in HF that can be achieved with this therapy, mainly in patients with a preimplantation history of ventricular tachycardia (VT). Thus, it seems that improving hemodynamics in patients without previous history of VA delays the progression of arrhythmogenic substrate. But once the arrhythmogenic substrate is present, the hemodynamic benefit no longer prevents the occurrence of VAs.
Pharmacological therapy
For long-term management of HF, guidelines-directed medical treatment is crucial.1 The treatment should include angiotensin-converting enzyme inhibitors, beta-blockers (BBs), and mineralocorticoid receptor antagonists. Of these, BBs and mineralocorticoid receptor antagonists have been shown to prevent SCD.15,16 In the PARADIGM-HF trial,17 angiotensin neprilysin inhibitors were shown to reduce cardiovascular death and hospitalization. Concerning VAs, angiotensin neprilysin inhibitors were also shown to lower the incidence of SCD by 20% and are currently recommended in HF with reduced EF.
Antiarrhythmic drugs (AADs) are mainly used as adjunctive therapy to manage arrhythmias, especially in patients receiving ICD therapies. Until now, no AAD has demonstrated a reduction in all-cause mortality. Moreover, most AADs have a negative inotropic effect that can lead to the worsening of hemodynamic status. Class I drugs have been shown to increase mortality in structural heart disease owing to the proarrhythmic action.18 However, it should be noted that quinidine has been recently proposed for the treatment of polymorphic VAs in patients with ischemic heart disease19 and also as a bail-out therapy for patients with otherwise refractory VAs.20
Amiodarone as a class III drug is currently used as the primary drug for the treatment of VTs in advanced HF. When combined with BB, it has been shown to reduce the risk of VAs compared with BB alone and with sotalol.21 A systematic review and meta-analysis of 8 trials22 evaluating the effect of AADs showed that amiodarone significantly reduced appropriate ICD interventions (odds ratio [OR] 0.3, P < .001), while sotalol did not have a significant effect (OR 0.83, P = .59). However, it must be noted that amiodarone is associated with a 2- and 5-fold increase risk of pulmonary and thyroid toxicity,23 potentially increasing mortality.22 If VAs occur despite amiodarone therapy, mexiletine can be used as an adjunct, although catheter ablation is favored in this setting.24
In some specific diseases such as sarcoidosis25 or myocarditis,26 inflammation may play a crucial role in arrhythmogenesis, and immunosuppression therapy should be an integral part of the treatment. The current expert consensus recommendation is to consider assessment for active inflammation (eg, by positron emission tomography scanning) and administration of immunosuppression therapy with AADs, if active inflammation is present.27 In patients with myocarditis and active inflammatory phase, VAs are typically polymorphic and irregular, while monomorphic and regular VAs are typical for healed myocarditis.28
Device therapy
ICDs are indicated to provide antitachycardia pacing or deliver shocks in patients with a history of hemodynamically significant sustained VAs or aborted SCD (class I indication).29 A meta-analysis of the 3 ICD trials30, 31, 32 comparing ICD to medical therapy for secondary prevention of SCD demonstrated a 28% mortality reduction. Although most early trials have excluded patients with the presumed reversible cause of VAs, these may still have a high mortality rate.33 In a recent large observational study34 on survivors of SCA attributed to a reversible and correctable cause, subsequent ICD implantation was associated with lower all-cause mortality except for aborted cardiac arrest occurring in the presence of acute myocardial infarction. Thus, except for patients with VAs during acute coronary syndrome, the indication for an ICD for VAs occurring in the context of potentially reversible cause should be decided on an individual basis.
For primary prevention of SCD, several randomized controlled trials35, 36, 37, 38 have defined the role of the ICD in HF patients with a left ventricular EF ≤35%, and the reported mortality reduction has recently been supported by large contemporary prospective registries.39,40 The evidence is most robust in patients with ischemic cardiomyopathy. The results of the recent DANISH trial have indicated that the mortality benefit may be less evident in contemporary patients with nonischemic cardiomyopathy.41 No reduction in the primary endpoint of all-cause death for patients randomized to ICD therapy (OR 0.87, P = .28) was observed, despite a significant decrease of SCD in the ICD group (OR 0.50, P = .005). Potential explanations may be optimized medical therapy, the high rate of cardiac resynchronization therapy, and low overall event rates. When grouped with other trials in a meta-analysis,42 primary prevention of SCD in nonischemic cardiomyopathy still demonstrated a reduction in overall mortality with ICD therapy (OR 0.76, P = .002). However, the benefit appears to be highest in younger patients.
Although low EF (≤35%) is now widely accepted for primary prevention ICD implantation, this practice has shortcomings. First, the risk of SCD has decreased in the last years,43 and only a minority of ICD recipients will need the therapy. Additionally, the absolute majority of SCD cases occur not in patients with reduced EF, but in the low-risk patients with moderately reduced or preserved EF.2 Thus, novel parameters for determining the risk of SCD are sought. These include the presence of fibrosis as identified by late gadolinium enhancement on cardiac magnetic resonance imaging, specific genetic mutations (ie, lamin A/C, phospholamban, truncating filamin, or SCN5A mutations), unexplained syncope, family history of SCD, and others. The goal is to establish a personalized assessment of the individual risk. This is the aim of the currently ongoing large PROFID project44 supported by an EU grant. Whether this approach will result in improved prevention of SCD is to be elucidated.
It is important to emphasize that appropriate programming for arrhythmia detection and therapy is also critical for the reduction of burden of ICD therapies and improvement of patient outcome.45,46 The strategies that effectively reduced shocks and improved mortality included prolonged and high rate detection rate, use of supraventricular/ventricular tachycardia discrimination algorithms, multizone programming, and systematic use of antitachycardia pacing. The evidence is more robust for primary rather than for secondary prevention indications. Although infrequently, ICD implantation may be adversely affected by severe complications. These include lead fractures, inappropriate therapies, and device infections. To address problems related to transvenous leads, a subcutaneous ICD (S-ICD) is now widely available. S-ICD has no intravascular lead and can therefore not deliver antitachycardia pacing. In a recent PRETORIAN trial, noninferiority of S-ICD to transvenous ICD for the primary endpoint of device-related complications and inappropriate shocks was demonstrated.47 S-ICD may therefore be used as an alternative to transvenous ICD when pacing, antitachycardia pacing, or resynchronization therapy are not required.
A different option represents the wearable external cardioverter-defibrillator.48 Although observational studies have indicated benefit of this therapy, in the randomized VEST trial49 in patients with low EF early after myocardial infarction, wearable cardioverter-defibrillator failed to reduce the primary endpoint of arrhythmic death. Thus, the use of the device seems to be appropriate only for select patients deemed to be at high risk for SCD (eg, after extraction of infected device during subsequent antibiotic treatment).
Finally, like any other product, ICDs and leads may be subject to device malfunction and recall. Once identified, the associated risks are classified depending on the chance of a device failure and possible damage.50 Severe device malfunctions requiring replacement are rather infrequent, and the benefits of ICD therapy still outweigh the potential risks.
Catheter ablation
ICDs do not prevent VAs, and many patients will experience symptomatic VA episodes leading to syncope or ICD shocks. In this respect, catheter ablation proved to be highly effective in the prevention of VAs.27 Historically, the method was limited to selected VAs as bundle branch reentry VT51 or mappable and tolerated VTs. However, the advent of electroanatomical mapping enabled mapping of the myocardial substrate even in sinus rhythm. In addition, cool tip technology improved the success of ablation by the creation of deeper lesions. This development resulted in a significant improvement in the efficacy of catheter ablation. It is now considered an effective treatment strategy for controlling incessant VTs and electric storm, and for reducing VA burden.27
Technique of catheter ablation
In patients with structural heart disease, most VAs are primarily due to a scar-related reentry.52, 53, 54 Ablation strategies vary depending on the underlying heart disease, the nature of the arrhythmogenic substrate and its location, and the hemodynamic tolerability of VA. The optimal target for catheter ablation is “the protected isthmus”—a corridor of surviving muscle bundles with slow conduction within a scar area. In hemodynamically tolerated VAs, activation and entrainment mapping are typically used to describe the reentrant circuit and locate the target ablation site. During monomorphic VT, the local ventricular electrogram at the protected isthmus is characterized by mid-diastolic timing and concealed entrainment without fusion with postpacing interval matching the VA cycle length. However, the majority of structural heart disease patients have unmappable VAs and thus, activation and entrainment mapping are not possible.55 Thus, the so-called “substrate mapping and modification” strategy (Figure 1) that comprises extended ablation of the arrhythmogenic substrate was proposed.56, 57, 58 Although there is currently no standardized approach for substrate-guided ablation, most existing strategies rely on elimination of abnormal tissue. Such is defined by a combination of lower bipolar/unipolar voltage and abnormal electrogram characteristics (ie, fragmented, split, and late electrograms). These abnormal electrogram features usually represent slow or delayed activation, constituting surrogate markers for potential VT circuits.27 The most commonly used substrate-guided ablation strategies include core scar isolation,59 late potential abolition,60 scar dechanneling,58 and scar homogenization.57 Some studies have shown a potential benefit of preprocedural imaging to identify the extent of the scar tissue and possibly even arrhythmogenic channels.61 Although noninducibility of VAs by programmed stimulation after ablation is the most commonly used endpoint,62 some studies demonstrated better long-term outcome when complete elimination of abnormal electrograms on remapping is achieved.56 In the VISTA trial, patients with tolerated VT were randomized to substrate-guided ablation vs ablation targeting only clinical and stable VT. The former, more extensive strategy was found to be superior for prevention of VAs recurrences, while the risk for complications remained comparable.
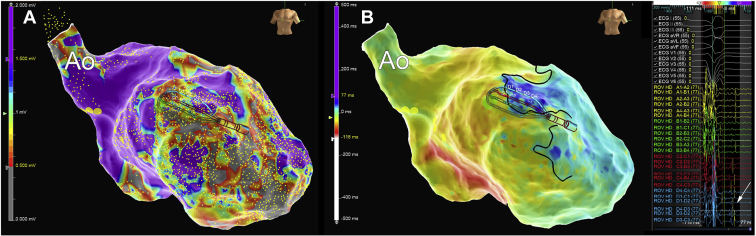
Figure 1.
Substrate mapping using a multipolar catheter in a patient after previous anterior myocardial infarction. A: Bipolar voltage map of the left ventricle in the right anterior oblique view. Voltage is color-coded, with violet representing healthy tissue and gray color dense scar. B: Activation map during sinus rhythm. Blue color corresponds to the late activated regions and areas of late potentials (arrow). A set of ablation lesions encircling the anterior scar led to the elimination of late potentials and suppressed ventricular tachycardia inducibility.
A portion of patients with HF may present with polymorphic VAs. These do not have a stable reentrant circuit, but may be initiated by similar premature ventricular complexes (PVCs). These commonly originate from the Purkinje network, the outflow tract region, and right and left ventricular papillary muscles,63 and may serve as a target for catheter ablation (Figure 2). Successful elimination of the triggering ectopy was shown to effectively prevent recurrent VAs.64
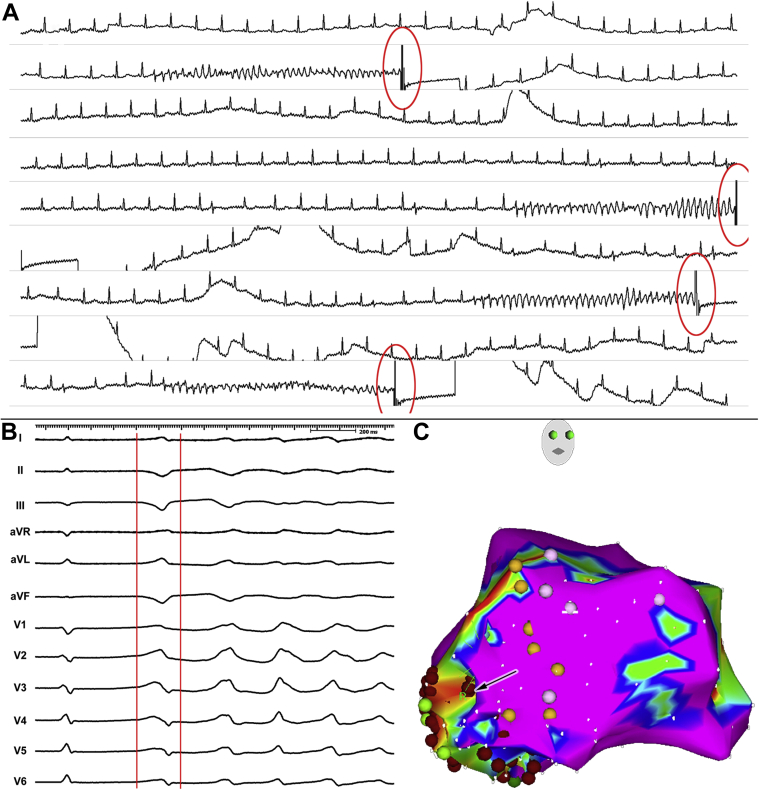
Figure 2.
Ablation of an electrical storm caused by recurrent episodes of polymorphic ventricular tachycardia/ventricular fibrillation (pVT/VF) in a patient after inferior myocardial infarction. A: Multiple episodes of pVT/VF requiring repeated shocks. All episodes were triggered by monomorphic premature ventricular contractions (triggering beat). B: The electrocardiogram morphology of the triggering beat. C: An electroanatomical voltage map of the left ventricle in the right anterior oblique view. The map shows localized scar in the posteroseptal region of the left ventricle; sites with conduction system were marked by yellow tags. The triggering ectopy was localized to the border zone of the scar area (arrow). Elimination of the triggering ectopy suppressed the episodes of pVT/VF.
Outcome of catheter ablation
The acute success rate in abolishing all VTs by ablation is reported around 70%, and the mean long-term success rate of VT ablation varies from 30% to 70%, depending on the severity of the HF and underlying structural heart disease.65, 66, 67 The clinical outcomes of VT ablation are better in ischemic heart disease as compared to nonischemic cardiomyopathy.68,69 An analysis of 780 patients with VT in nonischemic cardiomyopathy showed that the etiology of cardiomyopathy is a significant predictor of outcome. The best results were observed in arrhythmogenic right ventricular cardiomyopathy, myocarditis, and dilated cardiomyopathy as compared to hypertrophic cardiomyopathy, valvular heart disease, and sarcoidosis.70 This may be caused by the difference in the characteristics and localization of arrhythmogenic substrate in respect to the underlying heart disease. In patients with a postinfarction scar, the VT circuits are dominantly located endocardially and amenable to endocardial ablation. In patients with nonischemic cardiomyopathy, the location of the arrhythmogenic substrate is more variable,71,72 and epicardial involvement is more common. For such cases, a technique of dry pericardial puncture has been developed by Sosa and colleagues.73 This method uses a specialized needle with blunted tip (Tuohy) and subxiphoid puncture of the pericardial sack in the absence of pericardial fluid. In certain diseases (eg, arrhythmogenic right ventricular cardiomyopathy), the use of epicardial access (Figure 3) has dramatically improved the outcome.74 On the other hand, the arrhythmogenic substrate is in some diseases (eg, lamin A/C mutation) located intramyocardially, and despite the use of combined endo/epicardial approach, recurrences of VA are common.75 Another problem may be the inability to get into the pericardial space owing to previous cardiac surgery or postinflammatory adhesions. Some studies suggested that inaccessibility of the substrate can be observed in up to 10% of cases. For such cases, various bail-out strategies were proposed. These include transcoronary arterial or venous alcohol ablation,76,77 bipolar ablation,78 use of high-impedance irrigant,79 radiofrequency needle ablation,80 and surgical ablation.81 At this time, all of these techniques are used on an individual basis and can be considered investigational. Recently, radiotherapy (Figure 4), a standard oncology treatment for solid tumors, has been evaluated for the treatment of VAs.82,83 Although the concept of noninvasive ablation may look attractive and the initially reported outcome is favorable, it must be emphasized that the biological effects of radiation in nonmalignant myocardium are not fully understood, and many questions regarding optimal target volume and dose are to be determined.
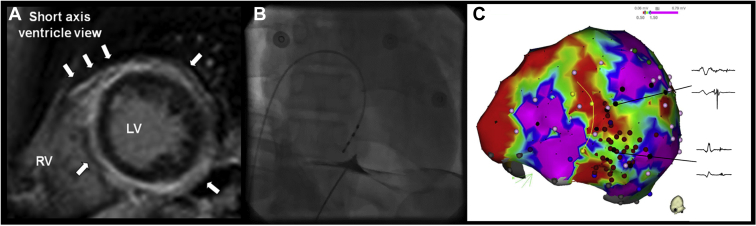
Figure 3.
Epicardial mapping and ablation in a patient with nonischemic cardiomyopathy. A: Cardiac magnetic resonance image with late gadolinium enhancement located dominantly in the epicardial circumference of the left ventricle (LV). The endocardium is spared. B: Puncture of the epicardial space using percutaneous subxiphoid access. Tenting of the parietal pericardium is marked by a small amount of contrast media. C: Electroanatomical voltage map in the inferior view. Large areas of late potentials (arrow) were located and abolished by radiofrequency ablation (red dots). RV = right ventricle.
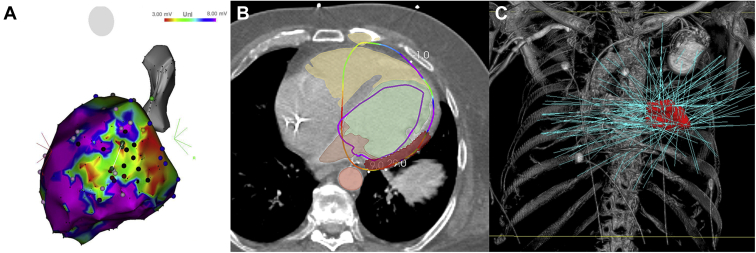
Figure 4.
Stereotactic body radioablation for refractory ventricular tachycardia (VT) in patient with nonischemic cardiomyopathy. A: Electroanatomical voltage map of the left ventricle in the left lateral view. Despite endo-epicardial radiofrequency ablation, VTs were still inducible. B: Integration of the electroanatomical map with planning computed tomography image with highlighted target for radioablation (red color). C: Prescribed radiotherapy doses.
In patients with ischemic heart disease, several randomized trials24,84,85 have demonstrated that catheter ablation prevents recurrent VA episodes and decreases the likelihood of subsequent ICD shocks, but not mortality. Thus far, there has not been a randomized controlled trial to compare catheter ablation to AAD therapy as a first-line treatment. The currently ongoing VANISH-2 trial (NCT02830360) is aimed to examine this question. The timing of the ablation was evaluated in the BERLIN VT86 trial, where patients were randomized for preventive ablation at the time of ICD implantation or deferred ablation after the third appropriate ICD shock. Although the study showed a reduction of likelihood of appropriate ICD interventions, it failed to reduce the composite endpoint of all-cause mortality and hospitalization for symptomatic VAs or worsening HF. On the contrary, the results of the PAUSE-SCD trial (NCT02848781) were recently presented. The trial has randomized patients to ICD implantation vs ICD implantation and prophylactic VT ablation and has demonstrated a reduction of combined endpoint of VT recurrences, cardiovascular hospitalization, and death (OR 0.58, P = .036). Other insights that VA ablation may have benefits beyond arrhythmia control come from observational studies. An international VT ablation center collaborative group has analyzed catheter ablation of VT in structural heart disease in 2061 patients and showed 70% freedom from VT recurrence, with an overall transplant and/or mortality rate of 15% at 1 year.87 Freedom from VT recurrence was associated with improved transplant-free survival, independent of HF severity. However, it should be noted that patients who did not have recurrent VT after ablation in this retrospective analysis were less sick than patients with VT recurrences, which illustrates the further need for randomized controlled trials.
Other adjunctive therapies
HF is associated with autonomic dysbalance in favor of sympathetic upregulation and parasympathetic withdrawal that correlates with the severity of HF and increases the risk of arrhythmias. Autonomic modulation is another emerging therapeutic strategy to modify the risk of VAs in HF. Multiple techniques of autonomic modulation do exist. Stellate ganglion blockade involves the injection of local anesthetic under ultrasound guidance into the left or bilateral stellate ganglia.88 Thoracic epidural anesthesia89 uses the injection of local anesthetics in thoracic epidural space. Both strategies can be used as a temporary measure to control VAs until more definitive treatment (eg, catheter ablation) is available. Left cardiac sympathetic denervation involves thoracoscopic or open surgical removal of the lower half of the left stellate and T2-T4 thoracic ganglia.90 Cardiac sympathetic denervation has been shown to reduce ICD shocks significantly in patients with otherwise resistant VT. Factors associated with worse outcomes were advanced HF, slower VTs, and left-sided-only denervation.
Specific clinical situations
Arrhythmia-induced/aggravated cardiomyopathy
One of the relatively frequent clinical scenarios is cardiomyopathy induced by frequent PVCs or runs of VT. It is essential to recognize this entity, as cardiomyopathy may be reversed by the elimination of arrhythmia.91 Although the pathogenesis related to this specific condition remains largely unknown, clinical experience highlights several points. First, the depressed left ventricular function has been observed with frequent PVCs from all common anatomic regions of origin. However, a higher risk of PVC-induced cardiomyopathy is observed for PVCs that lead to a higher degree of left ventricular dyssynchrony (eg, originating from epicardium).92 Second, the development of dysfunction appears to be triggered by a certain threshold of PVCs, with a minimum of 10% PVCs per day.93 Third, in some patients, the reversal of the left ventricular function is incomplete, despite complete elimination of PVCs.94,95
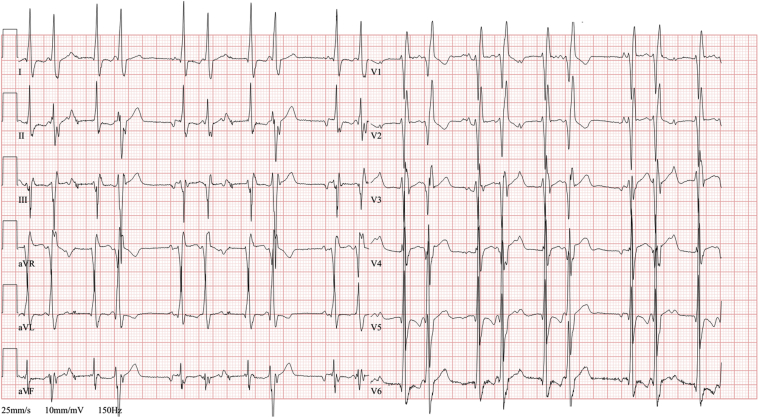
The latter scenario includes aggravation of preexisting left ventricular dysfunction by frequent ectopy in patients with heart disease. Progression of left ventricular dysfunction can either be a direct consequence of PVCs, as in PVC-induced cardiomyopathy, or be due to the interference of PVCs with biventricular pacing in cardiac resynchronization therapy patients (Figure 5). Several parameters were shown to predict the improvement of left ventricular function after the abolition of PVCs. These include smaller left ventricular end-diastolic diameter, shorter intrinsic QRS duration, and higher PVC burden.96 Additionally, cardiac magnetic resonance imaging may be used to exclude subtle forms of structural heart disease.97 The presence of late gadolinium enhancement suggests nonischemic cardiomyopathy with frequent PVCs rather than PVC-induced cardiomyopathy. The diagnosis of PVC-induced cardiomyopathy vs PVC-aggravated cardiomyopathy can sometimes be confirmed only after left ventricular EF improvement/normalization (reverse remodeling) following the suppression of PVCs.
Figure 5.
A: Ventricular bigeminy interfering with cardiac resynchronization therapy. The ectopic focus was successfully eliminated by catheter ablation on the basal left ventricular septum, which restored biventricular pacing to 100% (B) and led to improvement of left ventricular ejection fraction.
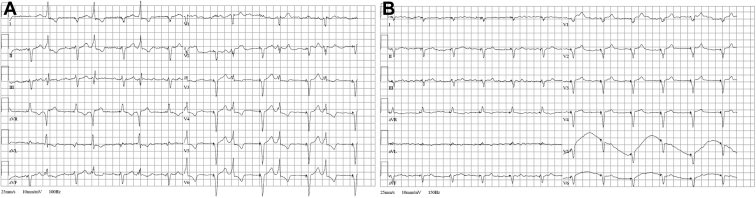
In some rare cases, a genetic cause of PVC-induced cardiomyopathy and HF can be recognized. One of these clinical entities is multifocal ectopic Purkinje-related premature contractions, characterized by the presence of polytopic PVCs (Figure 6), decreased LV systolic function, and increased risk of SCD.98 The other monogenic disease that may manifest as frequent PVCs associated with HF is Andersen-Tawil syndrome.99 Owing to the multifocal nature of PVCs in these diseases, radiofrequency catheter ablation is generally not recommended. Conversely, treatment with flecainide, hydroquinidine, or amiodarone may suppress the ectopy and improve systolic function.
Figure 6.
Resting electrocardiogram in a patient with multifocal ectopic Purkinje-related premature ventricular contractions (PVCs). The typical feature of multifocal ectopic Purkinje-related PVCs is that the PVCs are very “narrow” and may be mistaken for aberrant conduction of premature atrial beats. The underlying mechanism is pathogenic mutations in the SCN5A gene, which leads to a gain of function of the sodium channel and hyperexcitability of the fascicular-Purkinje system. The high burden of ventricular ectopy led to PVC-induced cardiomyopathy. The treatment with hydroquinidine suppressed the ectopy and improved left ventricular ejection fraction.
Electrical storm
An electrical storm has been defined as 3 or more episodes of sustained VA occurring within 24 hours, requiring either antitachycardia pacing or cardioversion/defibrillation, with each event separated by at least 5 minutes.100 Patients who experience an electrical storm are prone to psychological disorders, HF decompensation, and increased mortality.101,102 Electrical storm often manifests as a life-threatening situation with electrical instability and VAs that frequently recurs after multiple shocks or that is incessant despite repeated termination attempts. Such a situation of an ongoing electrical storm when only short periods of stable rhythm can be achieved requires rapid intervention. A multifaceted approach is required for management, consisting of ICD reprogramming when appropriate, AAD therapy, sedation, catheter ablation, autonomic modulation, and mechanical circulatory support (MCS). It must be emphasized that in the context of an electrical storm, catheter ablation is often life-saving therapy. It was shown that acute procedural success of ablation in patients with an electrical storm is associated with a significant reduction in VT recurrence and improved 1-year survival.103 Therefore, based on these data and our own experiences,104 we believe that the threshold for catheter ablation of recurrent VAs should be, in an experienced center, low. Earlier ablation could not only prevent VAs in these patients, but also potentially improve long-term survival.
VAs in advanced HF
Patients with advanced HF and otherwise refractory VAs might be considered for cardiac replacement therapies, such as heart transplant or long-term mechanical assist device. When catheter ablation of VAs is considered in this population, there is a risk of periprocedural hemodynamic decompensation. The potential benefit of MCS in patients undergoing VT ablation has been evaluated in multiple observation series. Overall, MCS allows the possibility to map during ongoing VT to better define the arrhythmia circuit with a higher chance of acute VT termination. Some studies proposed scoring systems to predict the risk of acute decompensation. One of them is called PAINESD and was used to evaluate the prophylactic use of percutaneous support to improve the outcome of catheter ablation in high-risk patients.105 In an observational study comparing patients with a high and low risk score, prophylactic MCS device placement was associated with a reduced risk of acute hemodynamic decompensation and death/transplant during follow-up without affecting VT-free survival. Rescue use of MCS during ablation was, on the other hand, found to be associated with a very high mortality rate.106 In a recent meta-analysis107 that included 2465 patients, prophylactic MCS was associated with improved survival compared to rescue or no MCS treatment among patients suffering from an electrical storm or those with high-risk PAINESD score. However, the benefit of MCS for ablation of VAs was not universally confirmed by other studies that showed higher risk of complications and longer fluoroscopy and procedure time associated with MCS.108,109 The potential explanation may be that available studies were observational and compared mostly percutaneous support for mapping during VT vs mapping during VT without the support. There is no real comparison of the use of percutaneous support for activation mapping vs substrate-based ablation in sinus rhythm. Thus, a prospective randomized controlled trial is required to identify if and in which patients periprocedural MCS will impact clinical outcomes.
Another proposed scoring system is called I-VT.110 It aims to predict 1-year recurrence and mortality after VT ablation and uses parameters such as left ventricular EF, the presence of an ICD or cardiac resynchronization therapy device, previous VT ablation, or electrical storm. The first 3 parameters were the best predictors of VT recurrence, while left ventricular EF, previous VT ablation, and electrical storm were identified as the best predictors of mortality.
Patients with LVAD
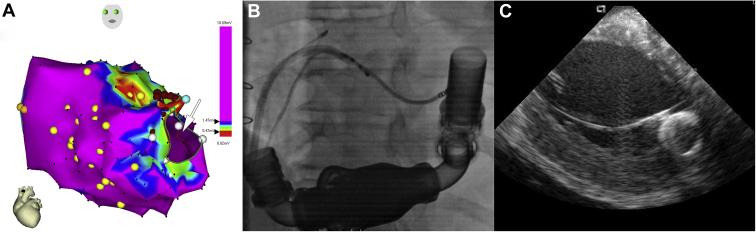
Implantation of LVAD currently serves as a bridge to transplant or as destination therapy in patients with the most advanced HF, including those with intractable VAs. On one side, VAs are usually well tolerated, since LVADs maintain adequate cardiac output and prevent circulatory collapse. However, sustained untreated VAs may worsen right ventricular dysfunction and adversely affect patient outcome. VAs pre- and post-LVAD implantation have been associated with an increased risk of cardiovascular and all-cause mortality.111, 112, 113 The patients with a history of VAs before LVAD implantation are at the highest risk of recurrent VAs following LVAD implantation. Thus, perioperative cryoablation at the time of LVAD implantation has been suggested. Probably a more viable option is catheter ablation of VA after LVAD implantation.13 Based on our experience and several other observational studies, this ablation has a high success rate with a good safety profile (Figure 7). However, several specific considerations have to be taken into account. Since the patients show no peripheral pulsation, ultrasound-guided vascular access is typically used. Owing to the closed aortic valve, transseptal access is the preferred strategy. Electromagnetic interference disturbs the function of electroanatomic mapping systems with magnetic sensor, and intracardiac echocardiography may be very useful to map the substrate close to the inflow cannula.
Figure 7.
Catheter ablation of recurrent ventricular tachycardia in a patient after implantation of the left ventricular assist device. A: Electroanatomical map of the left ventricle in the right oblique view. Arrow marks the location of the inflow cannula. B: Fluoroscopic image with an ablation catheter inserted transseptally in the left ventricle close to the inflow cannula. C: Position of the ablation catheter close to the inflow cannula shown by intracardiac echocardiography.
Conclusion
Management of VA in HF requires a systematic, multimodality approach that comprises optimization of medical therapy and the use of ICD and/or device combined with cardiac resynchronization therapy. Catheter ablation is one of the most important strategies with the potential to abolish or decrease the number of recurrences of VAs in this population. It can be a curative therapy in arrhythmia-induced cardiomyopathy and may even save lives in cases of an electrical storm. Catheter ablation may be used even in patients with VAs after implantation of LVAD.
Funding Sources
This work was supported by grant project AZV NU20-02-00244 from the Ministry of Health of the Czech Republic.
Disclosures
Petr Peichl has received speaker honoraria from Abbott and Biosense Webster and has served as a consultant for Biotronik and Medtronic. Adam Rafaj has no financial disclosures. Josef Kautzner reports personal fees from Bayer, Biosense Webster, Boehringer Ingelheim, Medtronic, and Abbott for participation in scientific advisory boards, and has received speaker honoraria from Bayer, Biosense Webster, Biotronik, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Mylan, Pfizer, ProMed, and Abbott.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
References
- 1.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Myerburg R.J., Junttila M.J. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 3.Stecker E.C., Reinier K., Marijon E., et al. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol. 2014;7:212–217. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MERIT-HF Study Group Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 5.Santangeli P., Rame J.E., Birati E.Y., Marchlinski F.E. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. 2017;69:1842–1860. doi: 10.1016/j.jacc.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Tomaselli G.F., Rose J. Molecular aspects of arrhythmias associated with cardiomyopathies. Curr Opin Cardiol. 2000;15:202–208. doi: 10.1097/00001573-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Saxon L.A., Bristow M.R., Boehmer J., et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2006;114:2766–2772. doi: 10.1161/CIRCULATIONAHA.106.642892. [DOI] [PubMed] [Google Scholar]
- 8.Powell B.D., Saxon L.A., Boehmer J.P., et al. Survival after shock therapy in implantable cardioverter-defibrillator and cardiac resynchronization therapy-defibrillator recipients according to rhythm shocked. The ALTITUDE survival by rhythm study. J Am Coll Cardiol. 2013;62:1674–1679. doi: 10.1016/j.jacc.2013.04.083. [DOI] [PubMed] [Google Scholar]
- 9.Healey J.S., Hohnloser S.H., Glikson M., et al. Cardioverter defibrillator implantation without induction of ventricular fibrillation: a single-blind, non-inferiority, randomised controlled trial (SIMPLE) Lancet. 2015;385:785–791. doi: 10.1016/S0140-6736(14)61903-6. [DOI] [PubMed] [Google Scholar]
- 10.Carson P., Anand I., O’Connor C., et al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2329–2334. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 11.van der Heijden A.C., Höke U., Thijssen J., et al. Super-responders to cardiac resynchronization therapy remain at risk for ventricular arrhythmias and benefit from defibrillator treatment. Eur J Heart Fail. 2014;16:1104–1111. doi: 10.1002/ejhf.152. [DOI] [PubMed] [Google Scholar]
- 12.Sapp J.L., Parkash R., Wells G.A., et al. Cardiac resynchronization therapy reduces ventricular arrhythmias in primary but not secondary prophylactic implantable cardioverter defibrillator patients: insight from the Resynchronization in Ambulatory Heart Failure Trial. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004875. [DOI] [PubMed] [Google Scholar]
- 13.Sacher F., Reichlin T., Zado E.S., et al. Characteristics of ventricular tachycardia ablation in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol. 2015;8:592–597. doi: 10.1161/CIRCEP.114.002394. [DOI] [PubMed] [Google Scholar]
- 14.Efimova E., Fischer J., Bertagnolli L., et al. Predictors of ventricular arrhythmia after left ventricular assist device implantation: a large single-center observational study. Heart Rhythm. 2017;14:1812–1819. doi: 10.1016/j.hrthm.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Al-Gobari M., El Khatib C., Pillon F., Gueyffier F. β-Blockers for the prevention of sudden cardiac death in heart failure patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2013;13:52. doi: 10.1186/1471-2261-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitt B., Remme W., Zannad F., et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 17.McMurray J.J.V., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 18.Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 19.Viskin S., Chorin E., Viskin D., et al. Quinidine-responsive polymorphic ventricular tachycardia in patients with coronary heart disease. Circulation. 2019;139:2304–2314. doi: 10.1161/CIRCULATIONAHA.118.038036. [DOI] [PubMed] [Google Scholar]
- 20.Li D.L., Cox Z.L., Richardson T.D., et al. Quinidine in the management of recurrent ventricular arrhythmias: a reappraisal. JACC Clin Electrophysiol. 2021;S2405-500X(21) doi: 10.1016/j.jacep.2021.03.024. 00327-3. [DOI] [PubMed] [Google Scholar]
- 21.Connolly S.J., Dorian P., Roberts R.S., et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006;295:165–171. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 22.Santangeli P., Muser D., Maeda S., et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552–1559. doi: 10.1016/j.hrthm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Piccini J.P., Berger J.S., O’Connor C.M. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J. 2009;30:1245–1253. doi: 10.1093/eurheartj/ehp100. [DOI] [PubMed] [Google Scholar]
- 24.Sapp J.L., Wells G.A., Parkash R., et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 25.Papageorgiou N., Providência R., Bronis K., et al. Catheter ablation for ventricular tachycardia in patients with cardiac sarcoidosis: a systematic review. Europace. 2018;20:682–691. doi: 10.1093/europace/eux077. [DOI] [PubMed] [Google Scholar]
- 26.Peretto G., Sala S., Rizzo S., et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16:793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Cronin E.M., Bogun F.M., Maury P., et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: executive summary. Heart Rhythm. 2020;17:e155–e205. doi: 10.1016/j.hrthm.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peretto G., Sala S., Basso C., Della Bella P. Programmed ventricular stimulation in patients with active vs previous arrhythmic myocarditis. J Cardiovasc Electrophysiol. 2020;31:692–701. doi: 10.1111/jce.14374. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. J Am Coll Cardiol. 2018;72:1677–1749. doi: 10.1016/j.jacc.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 30.Connolly S.J., Gent M., Roberts R.S., et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 31.Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 32.Kuck K.H., Cappato R., Siebels J., Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 33.Wyse D.G., Friedman P.L., Brodsky M.A., et al. Life-threatening ventricular arrhythmias due to transient or correctable causes: high risk for death in follow-up. J Am Coll Cardiol. 2001;38:1718–1724. doi: 10.1016/s0735-1097(01)01597-2. [DOI] [PubMed] [Google Scholar]
- 34.Ladejobi A., Pasupula D.K., Adhikari S., et al. Implantable defibrillator therapy in cardiac arrest survivors with a reversible cause. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005940. [DOI] [PubMed] [Google Scholar]
- 35.Moss A.J., Zareba W., Hall W.J., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 36.Buxton A.E., Lee K.L., Fisher J.D., Josephson M.E., Prystowsky E.N., Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 37.Bardy G.H., Lee K.L., Mark D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 38.Moss A.J., Hall W.J., Cannom D.S., et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 39.Zabel M., Willems R., Lubinski A., et al. Clinical effectiveness of primary prevention implantable cardioverter-defibrillators: results of the EU-CERT-ICD controlled multicentre cohort study. Eur Heart J. 2020;41:3437–3447. doi: 10.1093/eurheartj/ehaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrage B., Uijl A., Benson L., et al. Association between use of primary-prevention implantable cardioverter-defibrillators and mortality in patients with heart failure: a prospective propensity score-matched analysis from the Swedish Heart Failure Registry. Circulation. 2019;140:1530–1539. doi: 10.1161/CIRCULATIONAHA.119.043012. [DOI] [PubMed] [Google Scholar]
- 41.Køber L., Thune J.J., Nielsen J.C., et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 42.Beggs S.A.S., Jhund P.S., Jackson C.E., McMurray J.J.V., Gardner R.S. Non-ischaemic cardiomyopathy, sudden death and implantable defibrillators: a review and meta-analysis. Heart. 2018;104:144–150. doi: 10.1136/heartjnl-2016-310850. [DOI] [PubMed] [Google Scholar]
- 43.Shen L., Jhund P.S., Petrie M.C., et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 44.Dagres N., Peek N., Leclercq C., Hindricks G. The PROFID project. Eur Heart J. 2020;41:3781–3782. doi: 10.1093/eurheartj/ehaa645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan V.H., Wilton S.B., Kuriachan V., Sumner G.L., Exner D.V. Impact of programming strategies aimed at reducing nonessential implantable cardioverter defibrillator therapies on mortality: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:164–170. doi: 10.1161/CIRCEP.113.001217. [DOI] [PubMed] [Google Scholar]
- 46.Scott P.A., Silberbauer J., McDonagh T.A., Murgatroyd F.D. Impact of prolonged implantable cardioverter-defibrillator arrhythmia detection times on outcomes: a meta-analysis. Heart Rhythm. 2014;11:828–835. doi: 10.1016/j.hrthm.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Knops R.E., Olde Nordkamp L.R.A., Delnoy P.-P.H.M., et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383:526–536. doi: 10.1056/NEJMoa1915932. [DOI] [PubMed] [Google Scholar]
- 48.Masri A., Altibi A.M., Erqou S., et al. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: a systematic review and meta-analysis. JACC Clin Electrophysiol. 2019;5:152–161. doi: 10.1016/j.jacep.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olgin J.E., Lee B.K., Vittinghoff E., et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: as-treated and per-protocol analyses. J Cardiovasc Electrophysiol. 2020;31:1009–1018. doi: 10.1111/jce.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirian K.B., Sears S.F., Shea J.B. How to respond to an implantable cardioverter-defibrillator recall. Circulation. 2009;119:e189–e191. doi: 10.1161/CIRCULATIONAHA.108.810606. [DOI] [PubMed] [Google Scholar]
- 51.Caceres J., Jazayeri M., McKinnie J., et al. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation. 1989;79:256–270. doi: 10.1161/01.cir.79.2.256. [DOI] [PubMed] [Google Scholar]
- 52.Stevenson W.G., Khan H., Sager P., et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–1670. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 53.de Bakker J.M., van Capelle F.J., Janse M.J., et al. Slow conduction in the infarcted human heart. “Zigzag” course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 54.Hsia H.H., Callans D.J., Marchlinski F.E. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson W.G., Wilber D.J., Natale A., et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 56.Jaïs P., Maury P., Khairy P., et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 57.Di Biase L., Burkhardt J.D., Lakkireddy D., et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy. J Am Coll Cardiol. 2015;66:2872–2882. doi: 10.1016/j.jacc.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 58.Berruezo A., Fernández-Armenta J., Andreu D., et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol. 2015;8:326–336. doi: 10.1161/CIRCEP.114.002386. [DOI] [PubMed] [Google Scholar]
- 59.Tzou W.S., Frankel D.S., Hegeman T., et al. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythm Electrophysiol. 2015;8:353–361. doi: 10.1161/CIRCEP.114.002310. [DOI] [PubMed] [Google Scholar]
- 60.Vergara P., Trevisi N., Ricco A., et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23:621–627. doi: 10.1111/j.1540-8167.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 61.Andreu D., Penela D., Acosta J., et al. Cardiac magnetic resonance–aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm. 2017;14:1121–1128. doi: 10.1016/j.hrthm.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Yokokawa M., Kim H.M., Baser K., et al. Predictive value of programmed ventricular stimulation after catheter ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2015;65:1954–1959. doi: 10.1016/j.jacc.2015.02.058. [DOI] [PubMed] [Google Scholar]
- 63.Haïssaguerre M., Shoda M., Jaïs P., et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 64.Knecht S., Sacher F., Wright M., et al. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54:522–528. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 65.Dukkipati S.R., Koruth J.S., Choudry S., Miller M.A., Whang W., Reddy V.Y. Catheter ablation of ventricular tachycardia in structural heart disease. J Am Coll Cardiol. 2017;70:2924–2941. doi: 10.1016/j.jacc.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 66.Zeppenfeld K. Ventricular tachycardia ablation in nonischemic cardiomyopathy. JACC Clin Electrophysiol. 2018;4:1123–1140. doi: 10.1016/j.jacep.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Guandalini G.S., Liang J.J., Marchlinski F.E. Ventricular tachycardia ablation. JACC Clin Electrophysiol. 2019;5:1363–1383. doi: 10.1016/j.jacep.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Dinov B., Fiedler L., Schönbauer R., et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation. 2014;129:728–736. doi: 10.1161/CIRCULATIONAHA.113.003063. [DOI] [PubMed] [Google Scholar]
- 69.Proietti R., Essebag V., Beardsall J., et al. Substrate-guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome. Europace. 2015;17:461–467. doi: 10.1093/europace/euu326. [DOI] [PubMed] [Google Scholar]
- 70.Vaseghi M., Hu T.Y., Tung R., et al. Outcomes of catheter ablation of ventricular tachycardia based on etiology in nonischemic heart disease: an International Ventricular Tachycardia Ablation Center collaborative study. JACC Clin Electrophysiol. 2018;4:1141–1150. doi: 10.1016/j.jacep.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tung R., Raiman M., Liao H., et al. Simultaneous endocardial and epicardial delineation of 3D reentrant ventricular tachycardia. J Am Coll Cardiol. 2020;75:884–897. doi: 10.1016/j.jacc.2019.12.044. [DOI] [PubMed] [Google Scholar]
- 72.Shirai Y., Liang J.J., Santangeli P., et al. Comparison of the ventricular tachycardia circuit between patients with ischemic and nonischemic cardiomyopathies: detailed characterization by entrainment. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007249. [DOI] [PubMed] [Google Scholar]
- 73.Sosa E., Scanavacca M., D’Avila A., et al. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998;9:229–239. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 74.Santangeli P., Zado E.S., Supple G.E., et al. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1413–1421. doi: 10.1161/CIRCEP.115.003562. [DOI] [PubMed] [Google Scholar]
- 75.Kumar S., Baldinger S.H., Gandjbakhch E., et al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68:2299–2307. doi: 10.1016/j.jacc.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 76.Tavares L., Lador A., Fuentes S., et al. Intramural venous ethanol infusion for refractory ventricular arrhythmias: outcomes of a multicenter experience. JACC Clin Electrophysiol. 2020;6:1420–1431. doi: 10.1016/j.jacep.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 77.Tokuda M., Sobieszczyk P., Eisenhauer A.C., et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol. 2011;4:889–896. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]
- 78.Igarashi M., Nogami A., Fukamizu S., et al. Acute and long-term results of bipolar radiofrequency catheter ablation of refractory ventricular arrhythmias of deep intramural origin. Heart Rhythm. 2020;17:1500–1507. doi: 10.1016/j.hrthm.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen D.T., Tzou W.S., Sandhu A., et al. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Clin Electrophysiol. 2018;4:1176–1185. doi: 10.1016/j.jacep.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Stevenson W.G., Tedrow U.B., Reddy V., et al. Infusion needle radiofrequency ablation for treatment of refractory ventricular arrhythmias. J Am Coll Cardiol. 2019;73:1413–1425. doi: 10.1016/j.jacc.2018.12.070. [DOI] [PubMed] [Google Scholar]
- 81.Anter E., Hutchinson M.D., Deo R., et al. Surgical ablation of refractory ventricular tachycardia in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:494–500. doi: 10.1161/CIRCEP.111.962555. [DOI] [PubMed] [Google Scholar]
- 82.Cuculich P.S., Schill M.R., Kashani R., et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377:2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neuwirth R., Cvek J., Knybel L., et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace. 2019;21:1088–1095. doi: 10.1093/europace/euz133. [DOI] [PubMed] [Google Scholar]
- 84.Reddy V.Y., Reynolds M.R., Neuzil P., et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–2665. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuck K.-H., Schaumann A., Eckardt L., et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 86.Willems S., Tilz R.R., Steven D., et al. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (BERLIN VT): a multicenter randomized trial. Circulation. 2020;141:1057–1067. doi: 10.1161/CIRCULATIONAHA.119.043400. [DOI] [PubMed] [Google Scholar]
- 87.Tung R., Vaseghi M., Frankel D.S., et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. doi: 10.1016/j.hrthm.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fudim M., Boortz-Marx R., Ganesh A., et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017;28:1460–1467. doi: 10.1111/jce.13324. [DOI] [PubMed] [Google Scholar]
- 89.Do D.H., Bradfield J., Ajijola O.A., et al. Thoracic epidural anesthesia can be effective for the short-term management of ventricular tachycardia storm. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaseghi M., Barwad P., Malavassi Corrales F.J., et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017;69:3070–3080. doi: 10.1016/j.jacc.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duffee D.F., Shen W.K., Smith H.C. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clin Proc. 1998;73:430–433. doi: 10.1016/S0025-6196(11)63724-5. [DOI] [PubMed] [Google Scholar]
- 92.Walters T.E., Rahmutula D., Szilagyi J., et al. Left ventricular dyssynchrony predicts the cardiomyopathy associated with premature ventricular contractions. J Am Coll Cardiol. 2018;72:2870–2882. doi: 10.1016/j.jacc.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 93.Baman T.S., Lange D.C., Ilg K.J., et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 94.Mountantonakis S.E., Frankel D.S., Gerstenfeld E.P., et al. Reversal of outflow tract ventricular premature depolarization–induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–1614. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 95.Deyell M.W., Park K.-M., Han Y., et al. Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm. 2012;9:1465–1472. doi: 10.1016/j.hrthm.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 96.Penela D., Fernández-Armenta J., Aguinaga L., et al. Clinical recognition of pure premature ventricular complex-induced cardiomyopathy at presentation. Heart Rhythm. 2017;14:1864–1870. doi: 10.1016/j.hrthm.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 97.Yokokawa M., Siontis K.C., Kim H.M., et al. Value of cardiac magnetic resonance imaging and programmed ventricular stimulation in patients with frequent premature ventricular complexes undergoing radiofrequency ablation. Heart Rhythm. 2017;14:1695–1701. doi: 10.1016/j.hrthm.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 98.Laurent G., Saal S., Amarouch M.Y., et al. Multifocal ectopic Purkinje-related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol. 2012;60:144–156. doi: 10.1016/j.jacc.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto K., Aiba T., Kimura H., et al. Efficacy and safety of flecainide for ventricular arrhythmias in patients with Andersen-Tawil syndrome with KCNJ2 mutations. Heart Rhythm. 2015;12:596–603. doi: 10.1016/j.hrthm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 100.Kowlgi G.N., Cha Y.-M. Management of ventricular electrical storm: a contemporary appraisal. Europace. 2020;22:1768–1780. doi: 10.1093/europace/euaa232. [DOI] [PubMed] [Google Scholar]
- 101.Guerra F., Shkoza M., Scappini L., Flori M., Capucci A. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. 2014;16:347–353. doi: 10.1093/europace/eut304. [DOI] [PubMed] [Google Scholar]
- 102.Noda T., Kurita T., Nitta T., et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol. 2018;255:85–91. doi: 10.1016/j.ijcard.2017.11.077. [DOI] [PubMed] [Google Scholar]
- 103.Vergara P., Tung R., Vaseghi M., et al. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm. 2018;15:48–55. doi: 10.1016/j.hrthm.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 104.Aldhoon B., Wichterle D., Peichl P., Čihák R., Kautzner J. Outcomes of ventricular tachycardia ablation in patients with structural heart disease: the impact of electrical storm. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muser D., Liang J.J., Castro S.A., et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: a propensity-score matched analysis. Heart Rhythm. 2018;15:1500–1506. doi: 10.1016/j.hrthm.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 106.Mathuria N., Wu G., Rojas-Delgado F., et al. Outcomes of pre-emptive and rescue use of percutaneous left ventricular assist device in patients with structural heart disease undergoing catheter ablation of ventricular tachycardia. J Interv Card Electrophysiol. 2017;48:27–34. doi: 10.1007/s10840-016-0168-8. [DOI] [PubMed] [Google Scholar]
- 107.Mariani S., Napp L.C., Lo Coco V., et al. Mechanical circulatory support for life-threatening arrhythmia: a systematic review. Int J Cardiol. 2020;308:42–49. doi: 10.1016/j.ijcard.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 108.Luni F.K., Zungsontiporn N., Farid T., et al. Percutaneous left ventricular assist device support during ablation of ventricular tachycardia: a meta-analysis of current evidence. J Cardiovasc Electrophysiol. 2019;30:886–895. doi: 10.1111/jce.13907. [DOI] [PubMed] [Google Scholar]
- 109.Turagam M.K., Vuddanda V., Koerber S., et al. Percutaneous ventricular assist device in ventricular tachycardia ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2019;55:197–205. doi: 10.1007/s10840-018-0477-1. [DOI] [PubMed] [Google Scholar]
- 110.Vergara P., Tzou W.S., Tung R., et al. Predictive score for identifying survival and recurrence risk profiles in patients undergoing ventricular tachycardia ablation: the I-VT score. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cikes M., Jakus N., Claggett B., et al. Cardiac implantable electronic devices with a defibrillator component and all-cause mortality in left ventricular assist device carriers: results from the PCHF-VAD registry. Eur J Heart Fail. 2019;21:1129–1141. doi: 10.1002/ejhf.1568. [DOI] [PubMed] [Google Scholar]
- 112.Makki N., Mesubi O., Steyers C., Olshansky B., Abraham W.T. Meta-analysis of the relation of ventricular arrhythmias to all-cause mortality after implantation of a left ventricular assist device. Am J Cardiol. 2015;116:1385–1390. doi: 10.1016/j.amjcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 113.Galand V., Flécher E., Auffret V., et al. Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin Electrophysiol. 2018;4:1166–1175. doi: 10.1016/j.jacep.2018.05.006. [DOI] [PubMed] [Google Scholar]