Abstract
Arrhythmias and heart failure are among the most common complications encountered by adults with congenital heart disease (CHD). In this contemporary review, we explore the interactions between arrhythmias and heart failure and discuss management strategies. Major knowledge gaps are highlighted throughout. Interactions between arrhythmias and heart failure are complex and bidirectional, with one begetting the other. Arrhythmias can provoke heart failure through various mechanisms: conduction disturbances may contribute to inefficient ventricular filling and contraction patterns; bradyarrhythmias and tachyarrhythmias can result in a reduction in cardiac output; hypoxemia may be exacerbated by right-to-left shunting; and tachycardia-induced cardiomyopathy has potentially devastating consequences if the diagnosis is delayed. In turn, heart failure promotes arrhythmogenesis through various structural (eg, fibrosis, chamber dilation, hypertrophy) and electrical remodeling effects that include changes to ion currents and channels and connexin expression, along with shortening of atrial and ventricular refractory periods with increased heterogeneity. Several shared comorbidities can contribute to, and modulate the impact of, arrhythmias and heart failure. Preemptive arrhythmia management can potentially mitigate effects on heart failure exacerbations. Similarly, optimal heart failure control could curtail its impact on arrhythmogenesis. Treatment strategies to prevent or treat heart failure in adults with CHD encompass pharmacological agents, catheter ablation, and device therapies including defibrillators, cardiac resynchronization therapy, and His bundle pacing. High-priority research avenues with major knowledge gaps include tachycardia-induced cardiomyopathy, catheter ablation of atrial fibrillation, defibrillator indications in high-risk subsets, and the role of cardiac resynchronization therapy and His bundle pacing in diverse forms of CHD.
Keywords: Arrhythmias, Catheter ablation, Cardiac resynchronization therapy, Congenital heart disease, Heart failure, His bundle pacing, Implantable cardioverter-defibrillators
Key Findings.
-
▪
Interactions between arrhythmias and heart failure are complex and bidirectional, with one begetting the other, such that preemptive arrhythmia management can potentially mitigate heart failure exacerbations and optimal heart failure control could have a beneficial effect on arrhythmogenesis.
-
▪
Arrhythmias can provoke heart failure through various mechanisms including conduction disturbances, bradyarrhythmias and tachyarrhythmia-induced reductions in cardiac output, worsening hypoxemia, and tachycardia-induced cardiomyopathy. Heart failure promotes arrhythmogenesis through structural and electrical remodeling.
-
▪
The armamentarium of electrophysiological tools available to prevent or treat heart failure in adults with congenital heart disease include catheter ablation and device therapies for anti-bradycardia pacing, defibrillation, cardiac resynchronization therapy, and His bundle pacing.
-
▪
High-priority topics for research in which there are major knowledge gaps include tachycardia-induced cardiomyopathy, catheter ablation of atrial fibrillation, defibrillator indications in high-risk subsets, and the role of cardiac resynchronization therapy and His bundle pacing in diverse forms of congenital heart disease.
Introduction
By virtue of underlying congenital heart defects, surgical scars, postoperative sequelae, cyanosis, and hemodynamic perturbations, adults with congenital heart disease (CHD) are particularly prone to developing both bradyarrhythmias and tachyarrhythmias.1 In adults with CHD, arrhythmias are the leading cause of morbidity and hospitalizations and a major cause of mortality.1,2 Moreover, arrhythmias adversely impact quality of life and a host of varied patient-reported outcomes, including perceived health status, psychological well-being, and illness perception.3,4
Likewise, heart failure (HF) is highly prevalent in adults with CHD, particularly those over the age of 40 years, and is the most common cause of mortality. Importantly, HF in adults with CHD is not a uniform disease with a single pathway but, rather, a menacing clinical syndrome that is common to several processes that culminate in the inability of the cardiovascular circulation to meet the body’s metabolic needs. HF in adults with CHD encompasses unique phenotypes with different disease courses. In general, length of hospitalization and mortality are higher in adults with CHD hospitalized for HF than for other causes.5 Unique physiologic mechanisms include the inability of a systemic morphologic right ventricle or single-ventricle circulation to adequately meet metabolic needs and a distinctive dependence on pulmonary vascular integrity. Herein, we review the multifaceted bidirectional interactions between arrhythmias and HF, including scenarios in which arrhythmias contribute to HF and mechanisms for arrhythmogenesis secondary to HF. Management strategies for arrhythmias in adults with CHD and HF are discussed and knowledge gaps that provide opportunities for future research are highlighted throughout.
Bidirectional interactions between arrhythmias and HF in adults with CHD
Arrhythmias inducing HF in adults with CHD
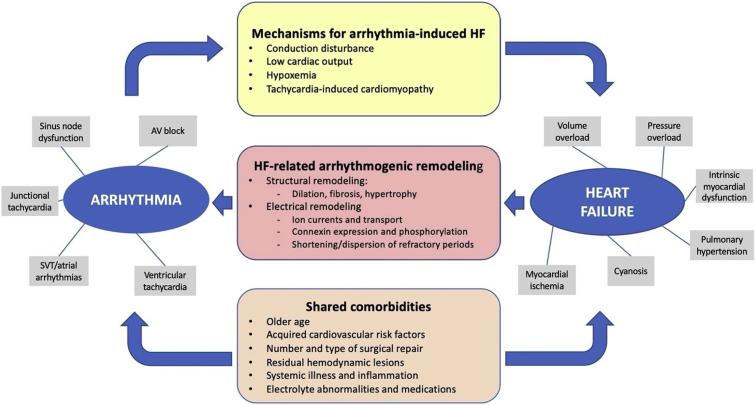
As schematically depicted in Figure 1, arrhythmias can provoke HF in adults with CHD through several mechanisms that may potentially overlap and interact, including conduction disturbances, low cardiac output, hypoxemia, and tachycardia-induced cardiomyopathy.
Figure 1.
Bidirectional interactions between arrhythmia and heart failure (HF) in adults with congenital heart disease (CHD). Bidirectional interactions between arrhythmia and HF are schematically shown. Arrhythmias induce HF through various mechanisms (yellow stadium) and, in turn, HF promotes arrhythmogenesis through structural and electrical remodeling (red stadium). They share various comorbidities (orange stadium) that may have potential contributory roles. Types and causes of arrhythmias and HF are depicted by the gray boxes. AV = atrioventricular; SVT = supraventricular tachycardia.
Conduction disturbances
Conduction disturbances are common in adults with CHD and include: (1) congenitally displaced and fragile atrioventricular (AV) conduction systems in the setting of congenitally corrected transposition of the great arteries (CCTGA), atrioventricular septal defects, and univentricular hearts, particularly those with L-looped ventricles, transposed great arteries (TGA), and/or AV septal malalignment; (2) postoperative bundle branch or AV block; and (3) pacing-induced ventricular dyssynchrony. Conduction disturbances can predispose to the development of HF through AV dyssynchrony, intra-atrial conduction delay, and intra- and interventricular dyssynchrony that contribute to inefficient ventricular filling and contraction associated with reduced cardiac output. Patients with CCTGA are particularly susceptible to developing sub–pulmonary ventricular pacing–induced systemic ventricular dysfunction, with a reported incidence that exceeds 50%.6
Low cardiac output
Sinus node dysfunction is likewise highly prevalent in adults with CHD. Although congenital absence of a sinus node is uncommon (eg, variants of heterotaxy syndrome and left atrial isomerism), sinus bradycardia is a frequent acquired late postoperative complication. It is particularly common following surgery in the vicinity of the superior vena cava to right atrial junction (eg, Glenn surgery, total cavopulmonary connection Fontan, Mustard or Senning baffle, and sinus venosus atrial septal defect [ASD]). Damage may occur as a result of direct injury to the sinus node or its surrounding tissue (thereby impairing propagation), or to its artery and/or neural inputs. Bradycardia can lead to a reduction in cardiac output in patients with fragile physiologies. This can be compounded by the loss of AV synchrony owing to a junctional escape rhythm, along with ineffective atrial hemodynamics from longstanding atrial remodeling.
At the other end of the spectrum, tachyarrhythmias of atrial, junctional, or ventricular etiology can lead to a reduction in stroke volume owing to decreased ventricular filling time and possibly altered coronary blood flow, AV dissociation, and/or a dyssynchronous pattern of contraction. The threshold heart rate at which cardiac output begins to decrease varies according to the underlying cardiac condition. Preexisting fragile physiologies with limited adaptive capacities such as a single ventricle, systemic right ventricle, or a major coexisting hemodynamic abnormality (eg, valvular stenosis/regurgitation, obstructed conduit or baffle) are particularly at risk. In adults with TGA and atrial switch surgery (ie, Mustard or Senning baffle), the tachycardia-induced reduction in ventricular filling is magnified by the rigid intra-atrial baffle, with poor atrial transport contributing importantly to the marked decline in stroke volume.7
Hypoxemia
Right atrial pressure generally increases in the context of a rapid tachyarrhythmia regardless of its underlying nature.8 In patients with a patent foramen ovale or an ASD with a predominant left-to-right shunt at rest, the tachycardia-induced increase in right atrial pressure can result in shunt reversal with hypoxemia and symptoms of HF. The classic example is the patient with Ebstein anomaly and exertional or tachycardia-induced oxygen desaturation. In patients with a preexisting right-to-left shunt and delicate baseline physiology (eg, Eisenmenger syndrome), tachycardia can lead to increased right ventricular filling pressure overload with worsening cyanosis and rapid hemodynamic collapse.
Tachycardia-induced cardiomyopathy
Finally, tachycardia-induced cardiomyopathy, a well-characterized clinical entity first described in the setting of persistent junctional reciprocating tachycardia, may be caused by any chronic sustained tachyarrhythmia or by frequent premature ventricular complexes. Although it is observed in adults with CHD, little is known about tachycardia-induced cardiomyopathy in this patient population beyond its potential devastating consequences.9 Considering that arrhythmias are the most common comorbidity in adults with CHD and that tachycardia-induced cardiomyopathy is not a rare complication, studies are required to elucidate high-risk substrates, associated factors, clinical course, propensity to recover myocardial function upon arrhythmia cessation, and overall prognosis.
Arrhythmogenesis secondary to HF
In patients without CHD, HF results in remodeling changes that enhance susceptibility to arrhythmias. Structural changes include increased cardiac mass, chamber distension, hypertrophy, and fibrosis.10 Heart failure–induced electrical modifications include downregulation of potassium currents that promote early afterdepolarizations leading to triggered activity, ion transport remodeling, abnormalities in connexin expression and phosphorylation, shortening of atrial and ventricular refractory periods, and dispersion of refractoriness.11 How these and other remodeling changes interact with CHD in arrhythmogenesis is unknown.
In a canine model mimicking Fontan surgery (ie, right atrium–to–pulmonary artery connection), changes in sodium, potassium, and calcium currents resulted in a net reduction in conduction velocity, shortening of the atrial action potential duration, and a reduction in the atrial effective refractory period, occasioning a higher proportion of inducible atrial arrhythmias.12 Similarly, in a porcine model mimicking tetralogy of Fallot, optical mapping revealed a reduction in ventricular conduction velocity with a prolonged ventricular conduction time and regions of slow conduction remote from surgical scar.13 Intramural heterogeneity in action potential duration and more frequent spontaneous arrhythmias were likewise noted. Moreover, similar changes were observed in the left ventricle with increased left ventricular fibrosis and a lower threshold for arrhythmia induction despite preserved systolic function.14 These remodeling changes likely underpin the propensity for ventricular arrhythmias and sudden death in patients with tetralogy of Fallot.
While HF-induced remodeling changes carry the potential to promote the gamut of arrhythmias most often encountered in adults with CHD (ie, intra-atrial reentrant tachycardia, nonautomatic focal atrial tachycardia, atrial fibrillation [AF], and macroreentrant ventricular tachycardia [VT]), the impact on new-onset arrhythmias, recurrences, and changing arrhythmia patterns according to underlying mechanisms remains to be elucidated. For example, as adults with CHD surpass the age of 50 years, AF becomes the most prevalent presenting atrial arrhythmia.15,16 A steeper slope of progression from paroxysmal to permanent AF is observed in adults with CHD compared to AF patients without CHD. It may very well be that HF-induced structural and electrical remodeling changes interact with CHD to contribute to this accelerated pattern of progression, although such a hypothesis remains speculative.
Management strategies for arrhythmias in adults with CHD and HF
It is a reasonable expectation that early and aggressive arrhythmia management may prevent substrate remodeling and HF exacerbations in adults with CHD, as demonstrated in other patient populations.17 This is particularly relevant to patients with more complex forms of CHD at greatest risk of developing HF. In the absence of high-quality evidence specific to the CHD population, the consensus is that rhythm control should generally be preferred to rate control as the initial treatment strategy for atrial arrhythmias owing to potential hemodynamic consequences.1 However, there are certain situations in which rate control becomes the preferred management approach, such as in the patient who has failed rhythm control and achieved targeted heart rates with rate control, experiences minimal symptoms, and has no appreciable deterioration in cardiac function upon serial testing.
Pharmacological therapy
Choice of pharmacological therapy should consider factors such as coexisting sinus node dysfunction, impaired AV node conduction, systemic or subpulmonary ventricular dysfunction, associated therapies, childbearing potential, and acquired comorbidities.1 A reasonable objective of antiarrhythmic drug therapy is to limit recurrences and render them better tolerated.
Beta-blockers and nondihydropyridine calcium channel antagonists (verapamil, diltiazem) are often prescribed as first-line agents but have limited efficacy in controlling tachyarrhythmias when administered alone. Beta-blockers should be introduced and titrated with care in the context of coexisting HF. Class I antiarrhythmic drugs (ie, flecainide and propafenone) are not recommended in patients with coronary artery disease or moderate/severe systolic dysfunction of the systemic or subpulmonary ventricle.1 Sotalol, a frequently used antiarrhythmic drug, is likewise not recommended in the setting of systolic dysfunction of the systemic or subpulmonary ventricle.1
Amiodarone is the most effective antiarrhythmic agent for maintaining sinus rhythm in patients with AF.18 Barring any contraindication, it is a drug of choice in the setting of HF. However, side effects increase with the cumulative dose such that long-term therapy in young adults is not ideal. Beyond the well-characterized pulmonary and liver toxicity, corneal microdeposits, photosensitivity, hypothyroidism, and adverse cardiac effects (eg, bradycardia, torsades de pointes), amiodarone-induced thyrotoxicosis is of particular concern in adults with CHD. It is more common in women with cyanotic heart disease or univentricular hearts with Fontan palliation, as well as in those with a body mass index <21 kg/m2.19 Amiodarone-induced thyrotoxicosis has also been linked to exacerbation of HF.20 Alternatively, dofetilide, another class III antiarrhythmic drug, is reasonably efficacious and well tolerated in adults with CHD if precautions are rigorously respected.21 It is contraindicated in the setting of congenital or acquired long QT syndrome or severe renal dysfunction (creatinine clearance <20 mL/min) and should only be initiated (or reloaded) in a healthcare facility that can provide continuous electrocardiographic monitoring, calculations of creatinine clearance, and cardiac resuscitation. The introduction of generic versions should, it is hoped, improve access, which has been limited by numerous obstacles, including supply shortages.
Catheter ablation
Nonpharmacological options should be thoughtfully considered prior to committing a young adult with CHD to long-term antiarrhythmic drug therapy, particularly amiodarone. Side effects and limited efficacy of pharmacological agents often tip the balance in favor of catheter ablation.1
Atrial arrhythmias
When performed in a tertiary center with dedicated expertise, acute success rates for catheter ablation of regular atrial arrhythmias exceed 75%.1,22 Long-term success rates depend, in part, on complexity of the underlying cardiac defect. For example, 5-year freedom from recurrent intra-atrial reentrant tachycardia is >70% in patients with simple forms of CHD, in contrast to <50% in those with Fontan palliation. Higher recurrence rates have been associated with non–cavotricuspid isthmus–dependent circuits, multiple atrial arrhythmias, and inducible AF.22 However, even in patients with complex CHD and/or multiple arrhythmias, catheter ablation can have beneficial palliative effects by decreasing the arrhythmia burden, improving quality of life, and reducing the need for, and number of, antiarrhythmic drugs.1,23,24 Arrhythmias postablation may consist of new substrates that could be targeted in repeat interventions to improve outcomes.25 The impact of catheter ablation on HF exacerbations and mortality merit further study.
Benefits associated with AF ablation in adults with CHD are less clear such that there is currently no broad consensus on indications and optimal management strategies. There is a paucity of data on the efficacy and safety of antiarrhythmic drug therapy in comparison to catheter ablation, with no randomized trial and limited cases series. It is the impression of the authors that non–pulmonary vein triggers are more common in the adult with CHD than in the general population with AF, in the form of focal or macroreentrant substrates in either atrium. While these extrapulmonary vein triggers should generally be targeted, there is currently no predefined ablation scheme that is applicable to the heterogeneous forms of CHD. An important avenue for research is to carefully assess and compare management strategies for AF in adults with CHD given that the scope of the problem will continue to increase in the foreseeable future.
Ventricular arrhythmias
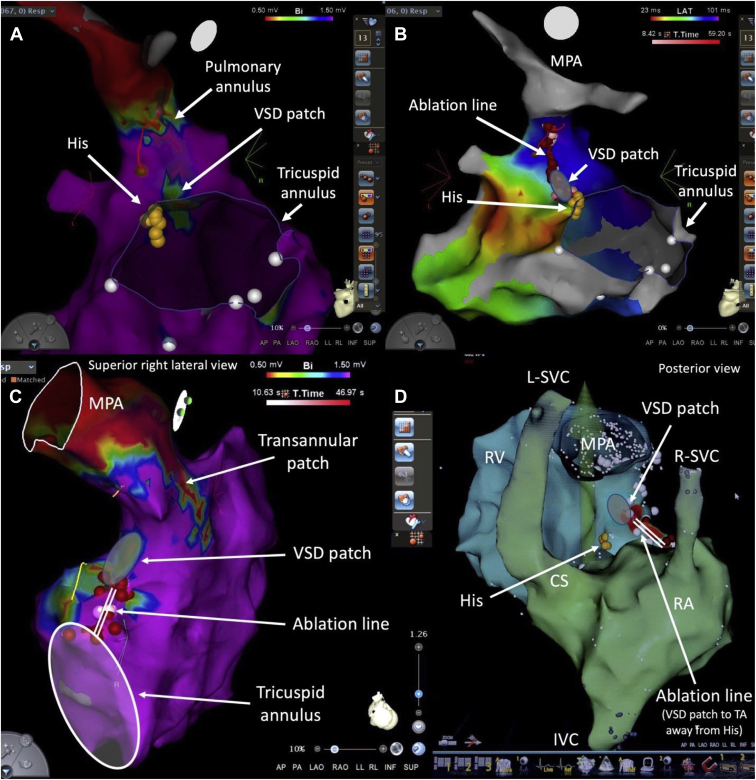
In the context of HF, some ventricular arrhythmias may be amenable to catheter ablation, especially as a means of reducing the likelihood of receiving shocks in implantable cardioverter-defibrillator (ICD) recipients. Tetralogy of Fallot (TOF) is the archetypal example in adults with CHD. Electromechanical interactions are well described, and potential discrete critical isthmuses for macroreentrant VT have been characterized, ie, between surgical scars (eg, ventriculotomy or ventricular septal defect [VSD] patch) and tricuspid or pulmonary annulus (Figure 2).26 Similar critical isthmuses can be targeted in “TOF-like” defects, including double-outlet right ventricle with a subaortic VSD. Any ventricular incision has the potential to be arrhythmogenic and give rise to peri-incisional VT. Moreover, the risk for VT increases with extent of ventricular fibrosis and is compounded by left ventricular dysfunction. Despite multiple potential contributors to HF in some adults with CHD, the potential role of frequent premature ventricular complexes in tachycardia-induced cardiomyopathy should not be overlooked. Eliminating this addressable trigger can have a major impact on ventricular function and quality of life in selected candidates.
Figure 2.
Ventricular tachycardia (VT) ablation in tetralogy of Fallot (TOF). Shown are catheter ablation procedures for VT in 2 adults with TOF. In the first patient, the critical isthmus targeted for ablation was between the ventricular septal defect (VSD) patch and pulmonary annulus, identified by voltage mapping in panel A. The ablation line connecting these 2 electrically inert structures is depicted in panel B. In the second patient, the critical isthmus was between the VSD patch and tricuspid annulus (TA), as identified by voltage mapping in panel C. The ablation line, shown in panel D, was anchored on the VSD patch and directed away from the His bundle. Note that this patient had bilateral superior vena cavae (SVC), with a left SVC (L-SVC) draining into the coronary sinus (CS). IVC = inferior vena cava; MPA = main pulmonary artery; RA = right atrium; R-SVC = right SVC; RV =right ventricle.
Technical considerations
Careful preprocedural planning is essential in maximizing safety and efficacy of catheter ablation in adults with CHD and HF. Prior operative notes should be meticulously reviewed, along with an analysis of documented arrhythmias and imaging tests. It is essential to plan the access strategy, rule out thrombosis, and anticipate location of the AV conduction system so as to minimize risks of AV block.27,28 Access can be limited by venous anomalies, vascular obstructions, stents, conduits, baffles, patches, and prosthetic material. In certain situations, obstacles to access can be overcome by remote magnetic navigation. Three-dimensional electroanatomic mapping systems are now considered essential for catheter ablation in adults with CHD. High-density mapping tools allow a large number of points to be collected in a brief span of time, thereby improving procedural efficiency and accuracy. Irrigated-tip radiofrequency ablation catheters have contributed importantly to the creation of effective ablation lesions.
The patient’s HF status and potential eligibility for mechanical support or transplant must be discussed and clarified prior to undertaking the procedure. Deep sedation vs general anesthesia should be considered on a case-by-case basis while balancing factors such as hemodynamic considerations, patient stability/comfort, and arrhythmia inducibility. In exceptional situations in which severe hemodynamic instability is predictable, proactive periprocedural extracorporeal membrane oxygenation support could be considered.29
Primary prevention ICDs
Selecting appropriate candidates for primary prevention ICDs is an important management issue in adults with CHD and HF. In general, systemic left ventricular dysfunction (ie, ejection fraction ≤35%) with biventricular physiology and New York Heart Association functional class II or III symptoms is considered an ICD indication in much the same way as it is for a patient without CHD.1 For example, an adult with a repaired ASD, VSD, patent ductus arteriosus, or aortic coarctation who also has ischemic cardiomyopathy would not have been excluded from participating in ICD trials such as the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II).30 Such patients should, therefore, not be denied proven effective therapies on the basis of coexisting CHD.
Primary prevention ICD indications in adults with CHD and less conventional forms of HF are less clear. It is reasonable to implant an ICD in selected adults with TOF who have multiple risk factors for sudden death, such as left ventricular dysfunction, nonsustained VT, QRS duration ≥180 ms, extensive ventricular scarring, or inducible sustained VT.1 Indications in patients with failing systemic right ventricles and single ventricles remain to be defined. Sudden death may be due to be factors other than primary ventricular arrhythmias, including electromechanical dissociation and atrial arrhythmias that provoke subendocardial ischemia.31 In patients eligible for cardiac transplantation, ICDs can be used as bridge therapy. For many patients in whom transvenous devices are contraindicated or not desirable (eg, intracardiac shunt, lack of access, prior endocarditis), the subcutaneous ICD is the preferred option.
Arrhythmia-related management strategies for HF in adults with CHD
Just as arrhythmias must be controlled to limit the burden on HF, so too must HF be controlled to minimize the impact on arrhythmias. This includes optimizing hemodynamics and addressing potentially reversible concomitant lesions, such as valvular stenosis or regurgitation, according to guideline-directed therapies. There is a dire need for future research to develop pharmacological options, demonstrate efficacy, and standardize management approaches to HF in adults with CHD. Whereas clinical trials inform management paradigms for HF in the absence of CHD, no pharmacological therapies have been proven to improve survival in patients with failing systemic right ventricles or single-ventricle circulations. Promising initial observational studies with angiotensin receptor–neprilysin inhibitors32,33 provide the impetus to pursue more definitive studies. Similar arguments could be made for mineralocorticoid receptor antagonists such as spironolactone, eplerenone, or finerenone. Beta-blockers are the cornerstone of therapy for patients with HF and a reduced ejection fraction. Intriguing results suggest a protective role against sudden death in patients with TGA and Mustard or Senning baffles.34 More conclusive studies are required, particularly in light of the frequent coexistence of sinus node dysfunction that may limit dose titration.
Cardiac resynchronization therapy
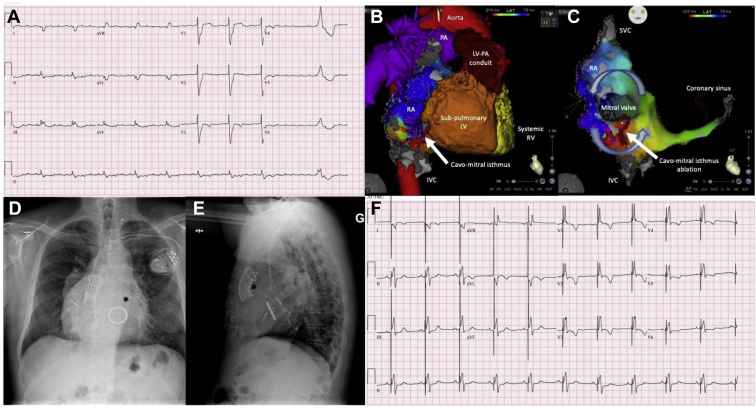
Although cardiac resynchronization therapy (CRT) is an established therapy in symptomatic patients with HF and a prolonged QRS duration (especially left bundle branch block),35 there is far less supportive evidence in the CHD population. Underlying phenotypes are diverse and include dysfunction of a systemic right ventricle, subpulmonary right ventricle, and single ventricle. Moreover, right is far more common than left bundle branch block in adults with CHD. In some patients, such as those with TGA and Mustard or Senning baffles, the coronary sinus is often inaccessible from the systemic venous atrium and leads to the subpulmonary ventricle, such that an epicardial lead is required to pace the systemic ventricle. These particularities and unique considerations limit the extrapolation of data from trials in patients with acquired heart disease. Nevertheless, encouraging results have been reported from observational studies in patients with heterogeneous forms of CHD,36, 37, 38, 39, 40, 41, 42, 43, 44 including systemic right ventricular dysfunction.45, 46, 47 In the CHD population that deviates from conventional criteria, the most established indication for CRT is the patient with CCTGA who requires ventricular pacing (Figure 3). In the setting of AV block, primary biventricular pacing appears effective in preserving systemic ventricular function.6 Moreover, systemic ventricular function improves in 50% of patients following upgrade from initial univentricular pacing.6
Figure 3.
Combined arrhythmia procedures in an adult with congenital heart disease (CHD) and heart failure (HF). A 58-year-old man with congenitally corrected transposition of the great arteries, mesocardia, sub–pulmonary stenosis, atrial septal defect, and ventricular septal defect underwent several cardiac surgeries, culminating in a left ventricle (LV)–to–pulmonary artery (PA) conduit with multiple stents and a Melody valve, along with a mechanical tricuspid valve for severe regurgitation. A: He presented with increased dyspnea, peripheral edema, and mild systemic right ventricular (RV) systolic dysfunction in the context of a persistent atrial tachyarrhythmia with a slow ventricular response. His native QRS duration was 178 ms, with a nonspecific intraventricular conduction delay. He underwent a catheter ablation procedure. B: Anatomical features are depicted. C: Intra-atrial reentrant tachycardia with a cycle length of 280 ms propagated counterclockwise around the mitral valve of the subpulmonary LV and was dependent on the cavomitral isthmus. Ablation across this isthmus successfully interrupted tachycardia and produced bidirectional conduction block. D, E: Pathology of the atrioventricular (AV) conduction system was confirmed post ablation, with 2:1 to 3:2 AV block. A cardiac resynchronization therapy device was, therefore, implanted. Asterisks mark the tip of the coronary sinus lead in posteroanterior (D) and lateral (E) radiographic views, placed in an anterolateral branch to pace the systemic RV. F: A postprocedural 12-lead electrocardiogram is presented showing an atrial-sensed rhythm with biventricular pacing that resulted in a narrower QRS complex. IVC = inferior vena cava; SVC = superior vena cava.
His bundle pacing
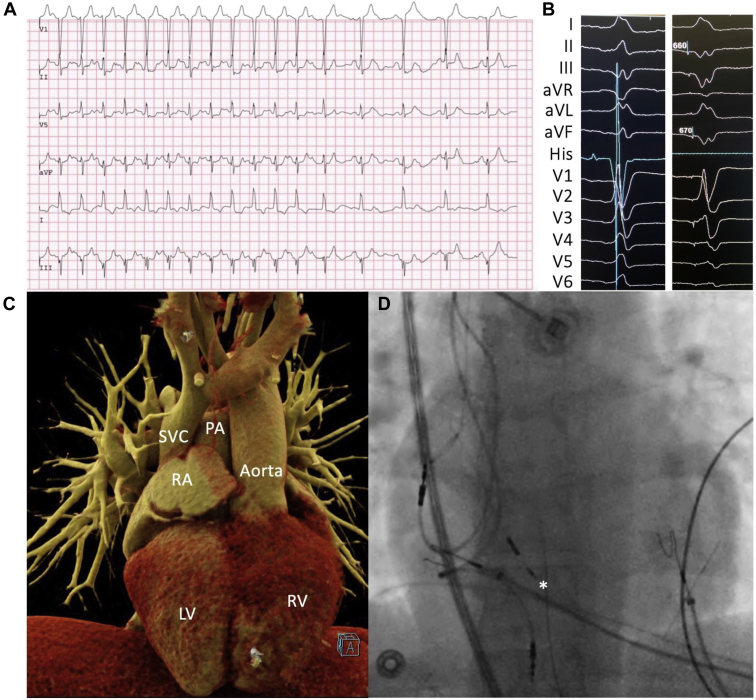
His bundle pacing is an alternative to standard subpulmonary ventricular and biventricular pacing that is performed to maintain physiological ventricular activation via the native His-Purkinje system (Figure 4). An additional theoretical advantage is a reduction in functional AV valve regurgitation owing to the fact that the lead does not cross the AV valve.48 In a recent multicenter study, His bundle pacing was shown to be safe and acutely successful in >85% of patients with CCTGA after detailed 3-dimensional electroanatomic mapping of the His bundle area.49 Considering that intra-Hisian block is common in patients with CCTGA, long-term efficacy remains to be established. Safety and feasibility have yet to be demonstrated in patients with other forms of CHD in whom access to, or identification of, the His bundle for pacing could be problematic (eg, Mustard or Senning baffle, atrioventricular septal defects, Ebstein anomaly). If His bundle pacing is combined with an ICD, lead connections and programming will depend on whether it is used in lieu of right ventricular or biventricular pacing. His bundle pacing is also compatible with the subcutaneous ICD.50
Figure 4.
His bundle pacing in adult with congenitally corrected transposition of the great arteries (CCTGA). A 28-year-old woman with CCTGA, mesocardia, moderate tricuspid regurgitation, and systemic right ventricle (RV) dilation experienced recurrent dyspnea and dizziness on exertion. A: The electrocardiographic tracing shows a 2:1 atrioventricular block occurred during exercise testing, with a sudden drop in heart rate from 150 to 75 beats per minute that reproduced her symptoms. Leads were placed in the right atrium (RA), near the His bundle, and in the septal subpulmonary left ventricle (LV). B: Twelve-lead surface electrocardiogram tracings along with an intracardiac His bundle signal (in the nonpaced beat) with and without pacing at the site of the best His recording. Nonselective capture is noted with a QRS complex that remains narrow and a similar, but not identical, QRS morphology. C, D: Computed tomography (C) and fluoroscopic (D) images are shown side-by-side in anteroposterior views to better visualize lead positioning. C: Mesocardia with an aortic valve that is anterior and to the left of the pulmonary valve. D: Asterisk marks the position of the His pacing lead. PA = pulmonary artery; SVC = superior vena cava.
AV node ablation
After exhausting all other options, AV node ablation could be considered as a last resort in adults with CHD, HF, and intractable atrial arrhythmias. Importantly, loss of intrinsic conduction via the native His-Purkinje system with single subpulmonary ventricular pacing could result in rapid deterioration of ventricular function in susceptible individuals. As such, careful consideration must be given to combining AV node ablation with His bundle pacing or CRT in adults with CHD. Ablating the AV node, a seemingly straightforward procedure, can be quite challenging in adults with CHD. The AV junction may be displaced, inaccessible from a standard femoral venous approach (eg, left-sided), or blocked by patches or prosthetic material. To our knowledge, there are no published studies on AV junction ablation in adults with CHD.
Conclusion
In adults with CHD, arrhythmias and HF are both highly prevalent, frequently coexist, and have multifaceted and complex bidirectional interactions, with one begetting the other. Arrhythmias can induce HF through various mechanisms that include conduction disturbances, low cardiac output, hypoxemia, and tachycardia-induced cardiomyopathy. In turn, HF promotes arrhythmogenesis through structural and electrical remodeling. Early and aggressive arrhythmia management can potentially mitigate the impact of arrhythmias on HF exacerbations. Likewise, optimal control of HF could minimize its effect on arrhythmias. Treatment strategies to prevent or treat HF in adults with CHD encompass pharmacological agents, catheter ablation, ICDs, and pacemakers, including CRT and His bundle pacing. High-priority research avenues relevant to the adult with CHD and HF where major knowledge gaps exist include tachycardia-induced cardiomyopathy, catheter ablation of AF, ICD indications in patients with single or systemic right ventricles, and the role of CRT and His bundle pacing in the diverse forms of CHD.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Acknowledgments
Dr Khairy is supported by the André Chagnon Research Chair in Electrophysiology and Congenital Heart Disease.
References
- 1.Khairy P., Van Hare G.F., Balaji S., et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD) Heart Rhythm. 2014;11:e102–e165. doi: 10.1016/j.hrthm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Verheugt C.L., Uiterwaal C.S., van der Velde E.T., et al. The emerging burden of hospital admissions of adults with congenital heart disease. Heart. 2010;96:872–878. doi: 10.1136/hrt.2009.185595. [DOI] [PubMed] [Google Scholar]
- 3.Casteigt B., Samuel M., Laplante L., et al. Atrial arrhythmias and patient-reported outcomes in adults with congenital heart disease: an international study. Heart Rhythm. 2021;18:793–800. doi: 10.1016/j.hrthm.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Yang H., Kuijpers J.M., de Groot J.R., et al. Impact of atrial arrhythmias on outcome in adults with congenital heart disease. Int J Cardiol. 2017;248:152–154. doi: 10.1016/j.ijcard.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 5.Moussa N.B., Karsenty C., Pontnau F., et al. Characteristics and outcomes of heart failure-related hospitalization in adults with congenital heart disease. Arch Cardiovasc Dis. 2017;110:283–291. doi: 10.1016/j.acvd.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Hofferberth S.C., Alexander M.E., Mah D.Y., Bautista-Hernandez V., del Nido P.J., Fynn-Thompson F. Impact of pacing on systemic ventricular function in L-transposition of the great arteries. J Thorac Cardiovasc Surg. 2016;151:131–138. doi: 10.1016/j.jtcvs.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 7.Derrick G.P., Narang I., White P.A., et al. Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load-independent indexes of right ventricular performance after the Mustard operation. Circulation. 2000;102:III154–III159. doi: 10.1161/01.cir.102.suppl_3.iii-154. [DOI] [PubMed] [Google Scholar]
- 8.Kaye G.C., Astridge P., Perrins J. Tachycardia recognition and diagnosis from changes in right atrial pressure waveform--a feasibility study. Pacing Clin Electrophysiol. 1991;14:1384–1392. doi: 10.1111/j.1540-8159.1991.tb02884.x. [DOI] [PubMed] [Google Scholar]
- 9.Stampfli S.F., Plass A., Muller A., Greutmann M. Complete recovery from severe tachycardia-induced cardiomyopathy in a patient with Ebstein's anomaly. World J Pediatr Congenit Heart Surg. 2014;5:484–487. doi: 10.1177/2150135114528222. [DOI] [PubMed] [Google Scholar]
- 10.Ravelli F., Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 11.Escudero C., Khairy P., Sanatani S. Electrophysiologic considerations in congenital heart disease and their relationship to heart failure. Can J Cardiol. 2013;29:821–829. doi: 10.1016/j.cjca.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Wu J., Zhou W., Wu L., Qian Y., Lu Y., Li F. Ionic mechanisms underlying atrial electrical remodeling after a Fontan-style operation in a canine model. Heart Vessels. 2020;35:731–741. doi: 10.1007/s00380-019-01544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoist D., Dubes V., Roubertie F., et al. Proarrhythmic remodelling of the right ventricle in a porcine model of repaired tetralogy of Fallot. Heart. 2017;103:347–354. doi: 10.1136/heartjnl-2016-309730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubes V., Benoist D., Roubertie F., et al. Arrhythmogenic remodeling of the left ventricle in a porcine model of repaired tetralogy of Fallot. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldmann V., Laredo M., Abadir S., Mondesert B., Khairy P. Atrial fibrillation in adults with congenital heart disease. Int J Cardiol. 2019;287:148–154. doi: 10.1016/j.ijcard.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 16.Labombarda F., Hamilton R., Shohoudi A., et al. Increasing prevalence of atrial fibrillation and permanent atrial arrhythmias in congenital heart disease. J Am Coll Cardiol. 2017;70:857–865. doi: 10.1016/j.jacc.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Marrouche N.F., Brachmann J., Andresen D., et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 18.Roy D., Talajic M., Dorian P., et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 19.Stan M.N., Hess E.P., Bahn R.S., et al. A risk prediction index for amiodarone-induced thyrotoxicosis in adults with congenital heart disease. J Thyroid Res. 2012:210529. doi: 10.1155/2012/210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawada S., Chakraborty P., Roche L., et al. Role of amiodarone in the management of atrial arrhythmias in adult Fontan patients. Heart. 2021;107:1062–1068. doi: 10.1136/heartjnl-2020-317378. [DOI] [PubMed] [Google Scholar]
- 21.El-Assaad I., Al-Kindi S.G., Abraham J., et al. Use of dofetilide in adult patients with atrial arrhythmias and congenital heart disease: a PACES collaborative study. Heart Rhythm. 2016;13:2034–2039. doi: 10.1016/j.hrthm.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Roca-Luque I., Rivas-Gandara N., Dos Subira L., et al. Long-term follow-up after ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease: types and predictors of recurrence. JACC Clin Electrophysiol. 2018;4:771–780. doi: 10.1016/j.jacep.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 23.de Groot N.M., Atary J.Z., Blom N.A., Schalij M.J. Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with congenital heart disease and characteristics of atrial tachyarrhythmia recurrences. Circ Arrhythm Electrophysiol. 2010;3:148–154. doi: 10.1161/CIRCEP.109.909838. [DOI] [PubMed] [Google Scholar]
- 24.Triedman J.K., Alexander M.E., Love B.A., et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39:1827–1835. doi: 10.1016/s0735-1097(02)01858-2. [DOI] [PubMed] [Google Scholar]
- 25.Combes N., Derval N., Hascoet S., et al. Ablation of supraventricular arrhythmias in adult congenital heart disease: a contemporary review. Arch Cardiovasc Dis. 2017;110:334–345. doi: 10.1016/j.acvd.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Zeppenfeld K., Schalij M.J., Bartelings M.M., et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.723551. [DOI] [PubMed] [Google Scholar]
- 27.Moore J.P., Khairy P. Adults with congenital heart disease and arrhythmia management. Cardiol Clin. 2020;38:417–434. doi: 10.1016/j.ccl.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Avila P., Bessiere F., Mondesert B., et al. Cryoablation for perinodal arrhythmia substrates in patients with congenital heart disease and displaced atrioventricular conduction systems. JACC Clin Electrophysiol. 2018;4:1328–1337. doi: 10.1016/j.jacep.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Carmichael T.B., Walsh E.P., Roth S.J. Anticipatory use of venoarterial extracorporeal membrane oxygenation for a high-risk interventional cardiac procedure. Respir Care. 2002;47:1002–1006. [PubMed] [Google Scholar]
- 30.Moss A.J., Zareba W., Hall W.J., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 31.Chaix M.A., Chergui M., Leduc C., Khairy P. Sudden death in transposition of the great arteries with atrial switch surgery: autopsy evidence of acute myocardial ischemia despite normal coronary arteries. Int J Cardiol. 2019;288:65–67. doi: 10.1016/j.ijcard.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 32.McCollum J.C., Ephrem G.A., Guglin M.E., Rao R.A. Tolerability and effect of angiotensis receptor neprilysin inhibitors in adult congenital patients with systemic right ventricle. J Card Fail. 2020;26:S100. [Google Scholar]
- 33.Lluri G., Lin J., Reardon L., Miner P., Whalen K., Aboulhosn J. Early experience with sacubitril/valsartan in adult patients with congenital heart disease. World J Pediatr Congenit Heart Surg. 2019;10:292–295. doi: 10.1177/2150135119825599. [DOI] [PubMed] [Google Scholar]
- 34.Khairy P., Harris L., Landzberg M.J., et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baflles: a multicenter study. Circ Arrhythm Electrophysiol. 2008;1:250–257. doi: 10.1161/CIRCEP.108.776120. [DOI] [PubMed] [Google Scholar]
- 35.Abraham W.T., Fisher W.G., Smith A.L., et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 36.Janousek J., Tomek V., Chaloupecky V.A., et al. Cardiac resynchronization therapy: a novel adjunct to the treatment and prevention of systemic right ventricular failure. J Am Coll Cardiol. 2004;44:1927–1931. doi: 10.1016/j.jacc.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Jauvert G., Rousseau-Paziaud J., Villain E., et al. Effects of cardiac resynchronization therapy on echocardiographic indices, functional capacity, and clinical outcomes of patients with a systemic right ventricle. Europace. 2009;11:184–190. doi: 10.1093/europace/eun319. [DOI] [PubMed] [Google Scholar]
- 38.Thambo J.B., De Guillebon M., Dos Santos P., et al. Electrical dyssynchrony and resynchronization in tetralogy of Fallot. Heart Rhythm. 2011;8:909–914. doi: 10.1016/j.hrthm.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 39.Thambo J.B., De Guillebon M., Xhaet O., et al. Biventricular pacing in patients with Tetralogy of Fallot: non-invasive epicardial mapping and clinical impact. Int J Cardiol. 2013;163:170–174. doi: 10.1016/j.ijcard.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Kubus P., Materna O., Tax P., Tomek V., Janousek J. Successful permanent resynchronization for failing right ventricle after repair of tetralogy of Fallot. Circulation. 2014;130:e186–e190. doi: 10.1161/CIRCULATIONAHA.114.012205. [DOI] [PubMed] [Google Scholar]
- 41.Dubin A.M., Janousek J., Rhee E., et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol. 2005;46:2277–2283. doi: 10.1016/j.jacc.2005.05.096. [DOI] [PubMed] [Google Scholar]
- 42.Koyak Z., de Groot J.R., Krimly A., et al. Cardiac resynchronization therapy in adults with congenital heart disease. Europace. 2018;20:315–322. doi: 10.1093/europace/euw386. [DOI] [PubMed] [Google Scholar]
- 43.Kharbanda R.K., Moore J.P., Taverne Y., Bramer W.M., Bogers A., de Groot N.M.S. Cardiac resynchronization therapy for the failing systemic right ventricle: a systematic review. Int J Cardiol. 2020;318:74–81. doi: 10.1016/j.ijcard.2020.06.052. [DOI] [PubMed] [Google Scholar]
- 44.Khairy P., Fournier A., Thibault B., Dubuc M., Therien J., Vobecky S.J. Cardiac resynchronization therapy in congenital heart disease. Int J Cardiol. 2006;109:160–168. doi: 10.1016/j.ijcard.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 45.Yeo W.T., Jarman J.W., Li W., Gatzoulis M.A., Wong T. Adverse impact of chronic subpulmonary left ventricular pacing on systemic right ventricular function in patients with congenitally corrected transposition of the great arteries. Int J Cardiol. 2014;171:184–191. doi: 10.1016/j.ijcard.2013.11.128. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki A., Sakaguchi H., Kagisaki K., et al. Optimal pacing sites for cardiac resynchronization therapy for patients with a systemic right ventricle with or without a rudimentary left ventricle. Europace. 2016;18:100–112. doi: 10.1093/europace/euu401. [DOI] [PubMed] [Google Scholar]
- 47.Moore J.P., Cho D., Lin J.P., et al. Implantation techniques and outcomes after cardiac resynchronization therapy for congenitally corrected transposition of the great arteries. Heart Rhythm. 2018;15:1808–1815. doi: 10.1016/j.hrthm.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Lewis A.J.M., Foley P., Whinnett Z., Keene D., Chandrasekaran B. His bundle pacing: a new strategy for physiological ventricular activation. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore J.P., Gallotti R., Shannon K.M., et al. Permanent conduction system pacing for congenitally corrected transposition of the great arteries: A Pediatric and Congenital Electrophysiology Society (PACES)/International Society for Adult Congenital Heart Disease (ISACHD) Collaborative Study (published online ahead of print) Heart Rhythm. 2020.03.13 doi: 10.1016/j.hrthm.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 50.Gauthey A., Calle S., Accinelli S., et al. His bundle pacing for newly acquired pacing needs in patients implanted with a subcutaneous implantable cardioverter defibrillator: a feasibility study based on the automated screening score and clinical cases. J Cardiovasc Electrophysiol. 2020;31:1793–1800. doi: 10.1111/jce.14566. [DOI] [PubMed] [Google Scholar]