Abstract
Patients with atrial fibrillation (AF) were largely excluded from the major clinical trials of cardiac resynchronization therapy (CRT), despite the presence of AF in up to 40% of patients receiving CRT in clinical practice. AF appears to attenuate the response to CRT, by the combination of a reduction in biventricular pacing and the loss of atrioventricular synchrony. In addition, remodeling secondary to CRT may influence the progression of AF. Management options for patients with AF and CRT include rate control, with drugs or atrioventricular node ablation, or rhythm control, with electrical cardioversion and antiarrhythmic therapy, or AF catheter ablation. The evidence for these therapies in patients with CRT is largely limited to observational studies or inferred from randomized studies in the general heart failure population. In this review, we explore the complex interaction between AF, heart failure, and CRT and discuss the evidence for the treatment options in this difficult patient cohort.
Keywords: Atrial fibrillation, Cardiac resynchronization therapy, Rate control, Rhythm control, AV node ablation, AF ablation, Pulmonary vein isolation
Key Findings.
-
▪
Atrial fibrillation (AF) attenuates the response to cardiac resynchronization therapy (CRT) in patients with heart failure and electrical dyssynchrony owing to a combination of reduced biventricular pacing and loss of atrioventricular (AV) synchrony.
-
▪
AV node ablation increases biventricular pacing and has been shown to improve mortality after CRT in large observational studies.
-
▪
AF ablation in patients with CRT is feasible, and may provide a benefit over AV node ablation by restoring AV synchrony, but randomized trials are needed to determine if this theoretical benefit translates into improved clinical outcomes.
-
▪
CRT device programming in patients with AF is challenging, and multiple vendor-specific algorithms are available to try to optimize CRT delivery, achieve rate response, and potentially reduce AF burden.
Introduction
Heart failure is a major cause of morbidity and mortality worldwide, with an estimated prevalence in developed countries of 1%–2% of the general adult population.1 Atrial fibrillation (AF) is a common and complex problem in patients with heart failure, with an average prevalence of 25%.2 AF and heart failure form a complex synergistic interaction, with each influencing progression of the other. Chronically elevated left atrial pressures caused by left ventricular (LV) dysfunction induces structural and electrical atrial remodeling, creating the substrate for AF. The prevalence of AF rises with increasing heart failure severity, from 10% in New York Heart Association (NYHA) class II to 50% in NYHA class IV.3 In turn, AF can reduce cardiac output owing to loss of atrial systole and rapid irregular LV filling times, and is associated with poorer outcomes in patients with existing heart failure.2 The relationship is further complicated by associated pro-inflammatory comorbidities, such as diabetes, obesity, and hypertension, and complex neurohormonal interactions.2
Approximately a third of patients with heart failure are indicated for cardiac resynchronization therapy (CRT).4 The presence of AF in these patients brings additional challenges, with reduction in biventricular pacing owing to rapid intrinsic activation of the ventricles, and loss of atrioventricular (AV) resynchronization. Patients with AF were largely excluded from the major randomized controlled trials of CRT; however, up to 40% of patients receiving CRT in clinical practice have AF at the time of implantation,5 and new-onset AF has been reported in between 20% and 24% of patients after implantation.6,7 AF appears to attenuate the response to CRT, and patients with AF have a higher long-term mortality after CRT implant compared to those in sinus rhythm.8,9 The delivery of CRT may also alter the natural progression of AF in patients with heart failure. In this article we review the evidence for CRT in patient with AF, explore the relationship between AF and CRT delivery, and discuss the treatment options available in this patient cohort.
The evidence for cardiac resynchronization therapy in patients with atrial fibrillation
There is limited evidence from randomized trials on the use of CRT in patients with AF. In a sub-study of the Resynchronization/Defibrillation for Ambulatory Heart Failure Trial (RAFT) trial, 229 patients with permanent AF and dyssynchronous heart failure (NYHA class II–III, LV ejection fraction ≤30%, and QRS duration ≥120 ms) were randomized to either implantable cardioverter-defibrillator (ICD) or CRT-defibrillator.10 No difference in mortality was found between groups, though there was a borderline significant trend towards fewer heart failure hospitalizations in those who received a CRT-defibrillator (hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.38–1.01; P = .052). It should be noted, however, that only 1 patient received an AV node ablation and satisfactory biventricular pacing (>95%) in the 6-month follow-up period was achieved in only 34% of patients. The effect of paroxysmal AF, including new-onset in-trial AF, on CRT outcomes has been examined in several sub-analyses of key randomized trials. In a sub-study of the MADIT-CRT trial, previous or in-trial intermittent atrial tachyarrhythmias did not attenuate the reduction in heart failure events or death seen with CRT-defibrillators vs ICD.11 Similarly, in the sub-analysis of the RAFT trial, new-onset atrial arrhythmias (which remained paroxysmal in 69.5% of cases) did not affect the primary trial combined endpoint of death or heart failure hospitalization, though an increased risk of heart failure hospitalizations alone was reported.12 In contrast, a sub-analysis of the COMPANION trial showed that in patients with a prior history of intermittent AF or atrial flutter, CRT did not reduce death or hospitalizations over optimal medical therapy.13 The discrepancy between these sub-analyses may be related to the AF burden and effect on biventricular pacing. In the RAFT and MADIT-CRT sub-studies, in-trial atrial arrhythmias did not reduce biventricular pacing percentage compared to patients who remained in sinus rhythm. The effect of atrial arrhythmias on biventricular pacing percentage was not reported in the COMPANION sub-study. Notably, the atrial arrhythmia cohort in the COMPANION study had more advanced heart failure at baseline, with all included patients in NYHA functional class III or IV, whereas the majority of patients in the MADIT-CRT and RAFT studies were in NYHA class II. Furthermore, only 52% of patients with atrial arrhythmias in the COMPANION study were on a beta-blocker at baseline, vs 88% in MADIT-CRT and 84.7% in RAFT. It is therefore possible that the patients in the COMPANION sub-study had a higher AF burden, related to more advanced heart failure at baseline, and poorer rate control, with a resultant worsening of CRT delivery.
Wilton and colleagues8 performed a meta-analysis of observational trials incorporating 7495 patients and found that patients with AF had a higher risk of clinical nonresponse and all-cause mortality after CRT implant compared to those in sinus rhythm. A more recent meta-analysis of observational studies, which included 83,571 patients, also demonstrated a significantly higher mortality rate after CRT implant in patients with AF, compared to those in sinus rhythm.9 In addition, patients with heart failure and AF were not found to have a significant reduction in mortality or in a composite endpoint of mortality or heart failure hospitalization after CRT, when compared to either ICD or medical therapy. Importantly, in the sub-group of patients with AF who received an AV node ablation, mortality was significantly lower, and equivalent to patients with sinus rhythm, as discussed later in this article. These meta-analyses did not examine the different effects of permanent, persistent, or paroxysmal AF on CRT outcomes. Current guidelines have a class IIa recommendation for CRT for patients with AF and LV ejection fraction ≤35% who meet CRT criteria, provided a strategy to ensure biventricular capture is in place.14,15
More recently, conduction system pacing techniques (His bundle pacing and left bundle branch pacing) have emerged as novel methods of delivering CRT.16 Conduction system pacing in patients with AF is feasible, and has predominantly been reported as a means of delivering physiological pacing in nondyssynchronous patients with high-degree AV block or after AV node ablation.17,18 The use of conduction system pacing for ventricular resynchronization in patients with AF, heart failure, and electrical dyssynchrony has been demonstrated in small observational studies, with symptomatic and LV remodeling benefits, but randomized trials to compare against conventional biventricular pacing are lacking.19,20 Further study is required to assess the benefit of conduction system pacing in this patient cohort.
The deleterious effects of atrial fibrillation on cardiac resynchronization therapy
Suboptimal biventricular pacing
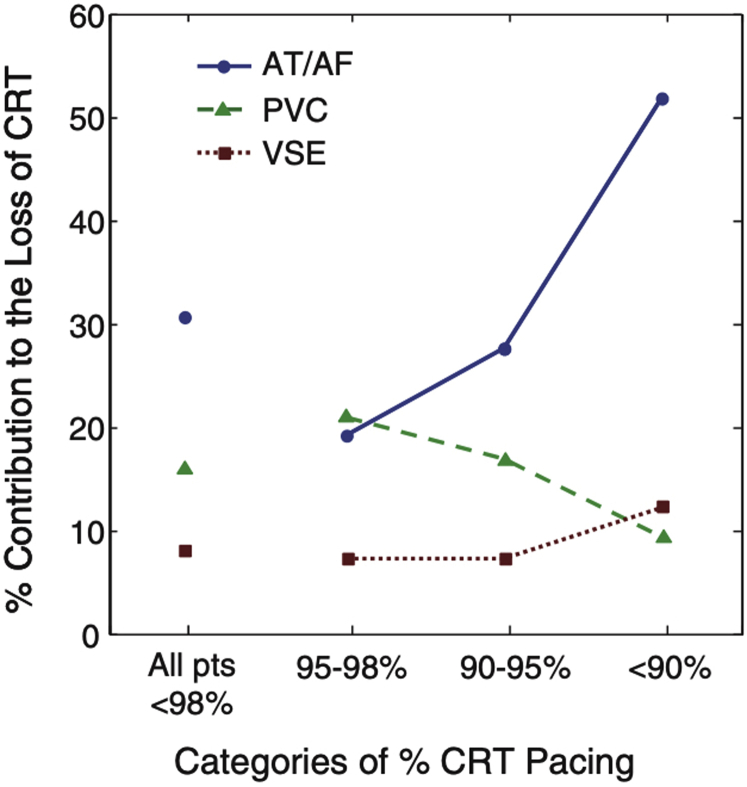
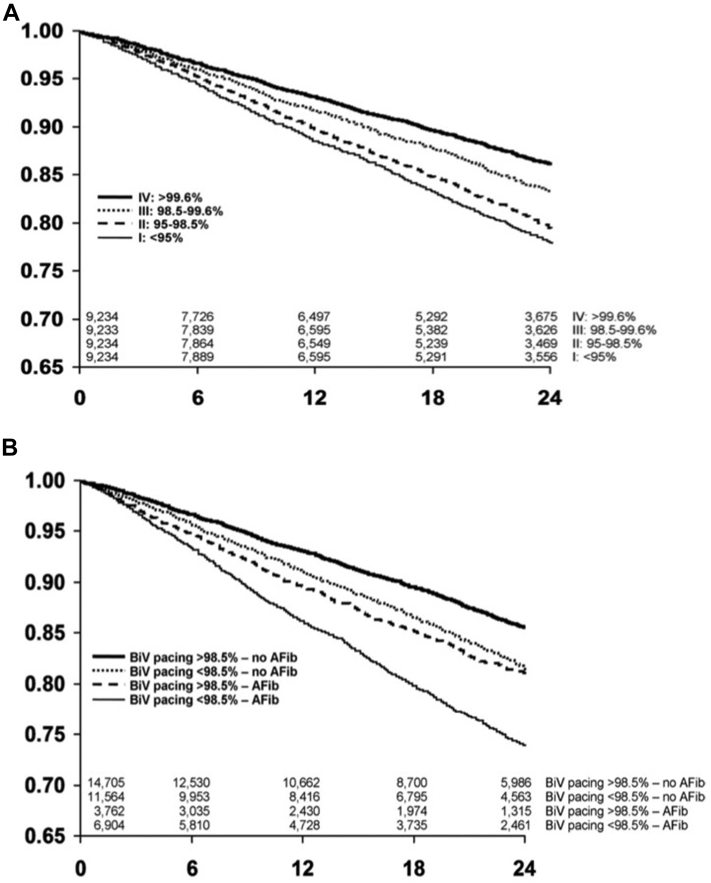
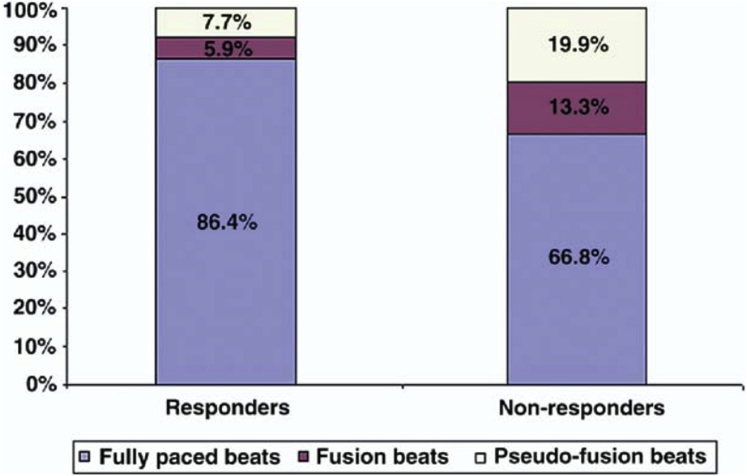
Rapid and irregular intrinsic activation of the ventricles in AF can reduce the delivery of biventricular pacing during CRT. In an observational study of over 32,000 patients with CRT devices, atrial arrhythmias were the most common reason for patients having a biventricular pacing percentage of less than 95% (Figure 1).21 Furthermore, ventricular rate during AF has a strong inverse correlation with biventricular pacing percentage22 and suboptimal biventricular pacing has been reported in up to 60% of patients with persistent or permanent AF.23 Suboptimal biventricular pacing is associated with a higher risk of mortality. This was most elegantly demonstrated by Hayes and colleagues24 in a large registry study of 36,935 patients in a remote monitoring network. There was a decremental reduction in survival seen with reducing biventricular percentage, with even patients receiving less than 99.6% pacing having a higher mortality, thus demonstrating the need to obtain as close to 100% pacing as possible (Figure 2A). Interestingly, patients with AF and biventricular pacing greater than 98.5% still had a higher mortality than patients in sinus rhythm (Figure 2B). This suggests that factors other than suboptimal biventricular pacing contribute to the attenuated response to CRT caused by AF. However, it should be noted that biventricular pacing percentages provided by device interrogation may be an overestimate of true CRT delivery. In a small study of 19 patients with permanent AF and CRT, despite all patients having greater than 90% biventricular pacing on device interrogation, only 53% of patients were found to have >90% true biventricular paced beats on Holter monitoring, with responders having a higher percentage of fully paced beats than nonresponders (86.4% ± 17.1% vs 66.8% ± 19.1%; P = .03) (Figure 3).25 This was due to the presence of fusion beats, which occur when a paced beat fuses with intrinsic activation of the ventricles, and pseudo-fusion beats, which occur when a pacing spike is delivered in the refractory period just after intrinsic activation, resulting in noncapture. This suggests that patients with AF who have seemingly adequate biventricular pacing on device interrogation may still be receiving suboptimal CRT. Automatic device algorithms, such as EffectivCRT™ (Medtronic), can use the morphology of the unipolar LV electrogram to determine if each paced beat was effective or ineffective and may provide a more accurate assessment of CRT delivery. In a study of 57 CRT patients, the use of this algorithm, which was validated with Holter monitoring, demonstrated that conventional device counters significantly underestimated the percentage of effective CRT pacing (94.8% ± 8% vs 87.5% ± 23%; P < .001), with AF being the primary cause of ineffective CRT.26
Figure 1.
Atrial fibrillation (AF) / atrial tachycardia (AT) is the most common etiology for loss of effective cardiac resynchronization therapy (CRT) pacing. As the percentage of CRT pacing decreases, the contribution of AT/AF to the loss increases. PVC = premature ventricular contraction; VSE = ventricular sensing episodes. Reproduced with permission from Cheng et al (2012).
Figure 2.
Survival decreases with reducing biventricular (BiV) pacing in an observational analysis of 36,935 patients with cardiac resynchronization therapy defibrillators. A: Survival analysis by biventricular pacing percentage. B: Survival analysis by biventricular pacing percentage and by the presence of significant atrial fibrillation (AFib), defined as average daily burden >0.5%. Reproduced with permission from Hayes et al 2011.
Figure 3.
Nonresponders have a low percentage of effective cardiac resynchronization therapy paced beats on Holter monitoring despite high (>90%) biventricular pacing percentage on device interrogation. Reproduced with permission from Kamath et al 2009.
Loss of atrioventricular synchrony
AF removes the contribution of atrial systole to cardiac output, which is estimated to be as high as 20%–30%.27 It also eliminates the benefit of AV resynchronization during CRT. Small observational studies have demonstrated acute hemodynamic benefits of optimizing AV delays during CRT.28, 29, 30 Although the use of echocardiography-based AV optimization was not shown to provide clinical benefit over empirical AV delays in a large randomized trial,31 studies using dynamic device-based algorithms to optimize AV delays have shown more promise.32,33 Interestingly, in a recently reported mechanistic study of 19 patients undergoing temporary His bundle pacing at the time of CRT implant, the majority of the hemodynamic benefit derived from CRT was found to be secondary to shortening of the AV delay, rather than ventricular resynchronization.34 It therefore follows that the loss of AV synchrony during AF is likely to attenuate the hemodynamic benefit provided by CRT.
Inappropriate defibrillator therapies
For patients who receive a CRT-defibrillator, the presence of AF also has a significant impact on the risk of ICD shocks. Multiple observational studies have demonstrated that the presence of AF significantly increases the risk of inappropriate ICD therapies.35, 36, 37, 38, 39 AF also appears to increase the risk of appropriate therapies for ventricular arrhythmias.36,37 In addition to a significant effect on quality of life, inappropriate shocks have been independently associated with increased long-term mortality.35,37,38
The effects of cardiac resynchronization therapy on atrial fibrillation
Conversely, CRT may alter disease progression in patients with AF and heart failure. A meta-analysis examining the effect of CRT on AF demonstrated that restoration of sinus rhythm occurred in 10.7% of patients (95% CI 6.9%–16.3%) with persistent or permanent AF after CRT implant.40 Overall, 10 out of 12 studies demonstrated a beneficial effect of CRT on AF; however, data were predominantly limited to observational studies. This may be related to the effect of reverse LV remodeling on left atrial hemodynamics, and improved left atrial function has been demonstrated in CRT responders who are in sinus rhythm.41 Furthermore, up to 40% of patients indicated for CRT have significant functional mitral regurgitation, owing to combination of LV dyssynchrony and dilatation, and improvement in mitral regurgitation severity has been reported in 23%–49% of patients after CRT in clinical trials.42 Mitral regurgitation has a complex interaction with AF, and has been associated with poorer outcomes after ablation. In a retrospective study of 216 patients with longstanding persistent AF who underwent ablation, mitral regurgitation was an independent predictor of atrial tachyarrhythmia recurrence.43 Furthermore, the rate of recurrence increased with the grade of mitral regurgitation severity.
There is also evidence that CRT reduces the elevated sympathetic activity associated with heart failure. In a study of 36 patients who received CRT for heart failure, average skin sympathetic nerve activity was significantly reduced in CRT responders (defined by improvement in LV ejection fraction ≥5%), but not in nonresponders.44 AF ablation may also affect the autonomic nervous system, owing to coincidental modification of ganglionated plexi during pulmonary vein isolation, and elevated sympathetic tone has been shown to predict AF recurrence after ablation.45 It could therefore be postulated that CRT may help restore and maintain sinus rhythm in patients with AF, owing to a combination of reverse LV remodeling, reduction in mitral regurgitation, and a potential reduction in sympathetic nerve activity.
Management of atrial fibrillation in patients with cardiac resynchronization therapy
Rate control
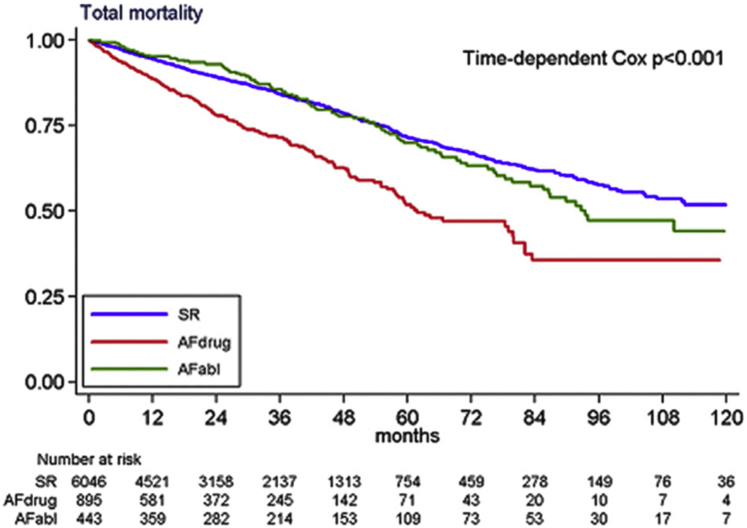
Rate control strategies minimize the rapid, irregular ventricular activation that can occur in AF. This can be achieved with pharmacological AV nodal blocking agents such as beta-blockers and digoxin, or by performing an AV node ablation. In contrast to medical therapy, AV node ablation completely eliminates AV conduction, thus rendering the patient dependent on pacing from the CRT device. This can often achieve biventricular pacing of close to 100%, though it should be noted that the presence of ventricular arrhythmias or ectopy can still result in suboptimal CRT delivery. Although no randomized studies have been performed to compare AV node ablation with medical rate control, observational studies have consistently shown superior CRT response and improved long-term mortality after AV node ablation in patients with AF and CRT (Table 1).8,46, 47, 48, 49, 50 This was most notably demonstrated in a large prospective multicenter trial of 7384 patients undergoing CRT (CERTIFY Study), where patients with AF who were treated with medical rate control alone had a higher all-cause mortality compared to those who received AV node ablation (HR 1.52; 95% CI 1.28–1.82; P < .001), as shown in Figure 4.47 Indeed, patients with AF who had an AV node ablation had comparable mortality to patients in sinus rhythm (HR 0.93; 95% CI 0.74–1.67), a finding that has also been demonstrated in a recent meta-analysis of over 80,000 patients.9 This is reflected in current European guidelines, where AV node ablation after CRT in the case of incomplete biventricular pacing has a class IIa indication.14 AV node ablation has also been shown to significantly reduce the incidence of both inappropriate and appropriate ICD shocks in patients with AF and CRT-defibrillators.49
Table 1.
Summary of studies comparing atrioventricular node ablation with medical therapy in patients with atrial fibrillation and cardiac resynchronization therapy
| Study (year) | Study design | Inclusion criteria | Comparator groups | N | F/U (mo) | Baseline characteristics | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Gasparini et al (2006) | Multicenter prospective observational | CRT for
|
Perm AF + AVNA∗ Perm AF + drugs |
48 114 |
24.6 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class (% III–IV) |
66.0 (8.3) 85.8 26.3 (6.7) 165.0 (35.5) 96.9 |
AVNA group superior in:
|
| ∗Performed if BiVp% ≤85% at 2 months | Higher mortality in AF + drugs group (OR 11.1; 95% CI 4.03–25.35; P < .001) | |||||||
| Gasparini et al (2008) | Multicenter prospective observational | All patients who received CRT | Perm AF + AVNA∗ Perm AF + drugs |
118 125 |
34 | Age Sex (% M) LVEF QRSd NYHA class |
66.2 (8.9) 81.9 26.0 (8.0) 161 (32) 3.12 |
Lower mortality in AF + AVNA group (HR 0.31; 95% CI 0.1–0.99; P = .048) |
| ∗Performed if BiVp% ≤85% at 2 months | ||||||||
| Dong et al (2010) | Single-center prospective observational | CRT-D for
|
AF + AVNA AF + drugs |
45 109 |
25.2 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class |
70.5 (9.8) 86.3 23.4 (7.0) 170.7 (35.1) 3.0 (0.4) |
AVNA group superior in:
|
| AVNA independently associated with survival (HR 0.13; 95% CI 0.03–0.58; P = .008) and freedom from death, transplant, and LVAD (HR 0.19; 95% CI 0.06–0.62; P = .006) | ||||||||
| No difference in LVEF, LVEDD, HFH | ||||||||
| CERTIFY (2013) | Multicenter prospective observational | CRT for
|
AF + AVNA AF + drugs |
443 895 |
37 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class (% III–IV) |
69.3 (9.3) 85.1 26.3 (6.8) 156.7 (35.0) 79.2 |
Lower all-cause mortality in AF + AVNA group (HR 0.67; 95% CI 0.52–0.85; P = .001) |
| Lower cardiac mortality in AF + AVNA group (HR 0.63; 95% CI 0.46–0.86; P = .003) | ||||||||
| Gasparini et al (2018) | Multicenter prospective observational | CRT-D for
|
Perm AF + AVNA∗ Perm AF + drugs |
262 402 |
18 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class (% III–IV) |
69 (9.4) 86.0 27.4 (5.6) 142.4 (30.2) 71.6 |
Lower annual rates of all-cause ICD shocks (IRR 0.18; 95% CI 0.10–0.32; P < .001) and all-cause hospitalizations (IRR 0.57; 95% CI 0.41–0.79; P < .001) in AF + AVNA group |
| (pooled analysis from 2 RCTs and 1 observational trial) | ∗Performed if BiVp% <95% at 2 months | Both inappropriate and appropriate ICD shock rates lower in AVNA group | ||||||
Continuous baseline characteristics expressed as mean (standard deviation).
AF = atrial fibrillation; AVNA = atrioventricular node ablation; BiVp% = biventricular pacing percentage; CI = confidence interval; CRT = cardiac resynchronization therapy; F/U = mean follow-up; HFH = heart failure hospitalization; HR = hazard ratio; ICD = implantable cardioverter-defibrillator; IRR = incidence rate ratio; LVAD = left ventricular assist device; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; NYHA = New York Heart Association; OR = odds ratio; perm AF = permanent atrial fibrillation; QRSd = QRS duration; RCT = randomized controlled trial.
Figure 4.
Atrioventricular node ablation improves mortality in patients with atrial fibrillation and cardiac resynchronization therapy in a prospective observational study (CERTIFY trial). AFabl = patients with atrial fibrillation treated with atrioventricular node ablation; AFdrug = patients with atrial fibrillation treated with drugs alone; SR = patients in sinus rhythm. Reproduced with permission from Gasparini et al 2013.
Rhythm control
Restoration of sinus rhythm can be achieved with electrical cardioversion and/or antiarrhythmic drug therapy. However, long-term success likely requires invasive catheter-based left atrial ablation, comprising pulmonary vein isolation with or without additional lesions. Small studies of non-ablation-based strategies have shown variable benefit for rhythm control in patients with CRT (Table 2). In a nonrandomized study of patients with permanent AF, 28 patients were scheduled for internal electrical cardioversion 3 months after CRT implant (group A), though cardioversion was not performed in 6 patients owing to the presence of left atrial appendage thrombus.51 Outcomes were compared with a control group of 27 patients (group B). After 12 months, 58% of the patients in group A (and 78% of those who actually underwent cardioversion) were in sinus rhythm, compared to 4% of patients in group B. AV node ablation was performed at 3 months if biventricular pacing was <90%, which occurred in 1 patient in group A and 2 patients in group B. Although improvements in LV ejection fraction were observed in both groups, a significant reduction in LV end-systolic volume was only found in group A. In a subsequent randomized study of 52 patients with heart failure, persistent AF, and left bundle branch block, all patients underwent CRT implantation, AV node ablation, and cardioversion.52 Patients randomized to rhythm control were discharged in sinus rhythm (with or without the initiation of antiarrhythmic drugs), while those in the rate control group had AF reinduced by rapid atrial pacing. At 1 year there was no difference between groups in a variety of echocardiographic and symptom-based endpoints, despite excluding patients in whom rhythm control was not achieved from the analysis. Notably, patients in the rhythm control group had a significantly higher number of hospital encounters, owing to the requirement for repeat cardioversions and the initiation and monitoring of antiarrhythmic drugs. More recently, the PilotCRAfT study randomized 43 patients with CRT and persistent or permanent AF to a rate control or rhythm control (via external electrical cardioversion).53 Both groups received amiodarone therapy. At 12 months, both groups had similar improvements in biventricular pacing percentage. In a per-protocol analysis (only 19 of 22 patients in the rhythm control group underwent electrical cardioversion), LV ejection fraction was higher in the rhythm control group than the rate control group at follow-up (36.8% vs 29.9%; P = .039). It should be noted, however, that only 1 patient in the rate control group received an AV node ablation, and no patients in the rhythm control group underwent AF ablation.
Table 2.
Summary of studies of rhythm control in patients with atrial fibrillation and cardiac resynchronization therapy
| Study (year) | Study design | Inclusion criteria | Comparator groups | N | F/U (mo) | Baseline characteristics | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Non-ablation strategies | ||||||||
| Turco et al (2012) | Multicenter prospective observational | Permanent AF CRT for
|
Rhythm control (group A)∗ Standard care (group B) |
28 27 |
12 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class (% IV) |
70.5 (10.0) 80 24 (5.5) 132 (16.3) 5.5 |
No difference in mortality between groups (P = .469) |
| ∗DCCV via ICD + amiodarone | Lower mortality for patients in SR at follow-up vs those in AF (P = .048) | |||||||
| NB groups from different centers | Improvement in LVEF seen in both groups, but LVESV reduction only seen in group A (P = .018 vs baseline) | |||||||
| Schwartzman et al (2015) | Single-center RCT | Persistent AF NYHA class III Mean HR >85 LVEF ≤35% LVEDD >55 mm LBBB QRSd >130 ms |
Rhythm control∗ Standard care$ |
26 26 |
12 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class |
70.0 (8.0) 71.2 28 (7.6) 143.5 (12.6) 3.3 (0.5) |
No significant difference between groups for incidence of CRT response or change in: NYHA class, MLWHF score, 6MWT, LVEF, LVEDD |
|
∗DCCV ± AAD $DCCV + reinduction of AF |
Higher hospital encounters in rhythm control group (11.7% vs 3.2%; P = .002) | |||||||
| NB 4 patients with failed rhythm control excluded from analysis | ||||||||
| PilotCRAfT (2021)∗ | Single-center RCT |
CRT Perm AF or pers AF lasting >6 months BiVp% <95% |
Rhythm control∗ Rate control$ |
22 21 |
12 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class |
68.4 (8.3) 97.7 30 (8) NA NA |
No difference between groups in improvement in BiVp%, VO2max, QOL / clinical endpoints |
| ∗Abstract |
∗DCCV $Drugs ± AVNA |
In per-protocol analysis, higher LVEF in rhythm control group at follow-up (36.8% vs 29.9%; P = .039) | ||||||
| NB both groups received amiodarone | ||||||||
| AF Ablation | ||||||||
| Fink et al (2019) | Single-center retrospective observational | AF and CRT nonresponse∗ who underwent AF ablation | No control group | 38 | 12 | Age Sex (% M) LVEF (%) QRSd (ms) NYHA class |
67.8 (9.8) 78.9 30.4 (7.2) NA 3.0 |
68% in sinus rhythm at follow-up Significant improvements from baseline in:∗ BiVp% (Δ7.5%; P < .001) LVEF (Δ2.2; P = .0225) NYHA class (P < .0001) |
∗at least 1 of:
|
∗6 patients underwent AVNA during follow-up and were excluded in analysis | |||||||
| CASTLE-AF (2018) | Multicenter RCT (subgroup analysis) | Symptomatic pers AF or pAF LVEF ≤35% Failed AAD Biotronik ICD / CRT-D |
AF ablation Medical therapy |
48 52 |
37.6 | Not reported for subgroup of patients with CRT-D | No significant difference in primary endpoint of death or HFH (HR 0.65; 95% CI 0.43–0.98) | |
| Subgroup of total cohort who had CRT-D | NB No significant interaction between presence of CRT-D vs ICD on primary endpoint on Cox logistic regression analysis (P = .6) | |||||||
Continuous baseline characteristics expressed as mean (standard deviation).
6MWT = 6-minute walk test; AAD = antiarrhythmic drug; AF = atrial fibrillation; AVNA = atrioventricular node ablation; BiVp% = biventricular pacing percentage; CI = confidence interval; CRT = cardiac resynchronization therapy; DCCV = DC cardioversion; F/U = mean follow-up; HFH = heart failure hospitalization; HR = hazard ratio; ICD = implantable cardioverter-defibrillator; LBBB = left bundle branch block; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; MLWHF = Minnesota Living with Heart Failure Questionnaire; NA = not available; NYHA = New York Heart Association; pAF = paroxysmal AF; pers AF = persistent atrial fibrillation; QOL= quality of life; QRSd = QRS duration; RCT = randomized controlled trial SR = sinus rhythm.
Performing AF ablation in patients with CRT is feasible. In a small observational study by Fink and colleagues,54 38 patients with AF and nonresponse to CRT (defined as at least 1 of the following: <95% biventricular pacing, <1 point improvement in NYHA class, or <5% improvement in LV ejection fraction) underwent AF ablation. Significant improvements in biventricular pacing percentage, LV ejection fraction, and NYHA class were demonstrated and 67% of patients were free from AF at 24 months, though 45.9% of patients required at least 1 redo ablation and 16.2% of patients underwent AV node ablation during follow-up. Randomized studies of AF ablation in patients with CRT and AF are lacking, though evidence may be inferred from studies in the general heart failure population. In the PABA-CHF study, 81 patients with AF and heart failure (LV ejection fraction ≤40%) were randomized to either AF ablation or AV node ablation + CRT implantation, with superior improvements in LV ejection fraction, heart failure symptoms, and 6-minute walk test performance demonstrated in the AF ablation group.55 However, it should be noted that this patient cohort did not have electrical dyssynchrony at baseline (QRS duration of 91 ± 9.5 ms), and thus differs from the population of patients who receive CRT for dyssynchronous heart failure. Therefore, while this suggests a potential benefit for a rhythm control strategy, the AV node ablation + CRT group here may have experienced the negative impact of electrical dyssynchrony induced by CRT in patients with underlying narrow QRS.56,57 Further small randomized studies have also demonstrated that AF catheter ablation improves LV ejection fraction58, 59, 60 and exercise capacity61 compared to medical rate control in patients with heart failure. The multicenter AATAC study randomized 203 patients with persistent AF and LV impairment to either AF ablation or amiodarone.62 In addition to a significant reduction in the primary endpoint of AF recurrence in the ablation arm, significant reductions in the predefined secondary endpoints of hospitalization and all-cause mortality were also demonstrated. While all patients enrolled in the AATAC study had an existing ICD or CRT-defibrillator, the proportion of patients with CRT were not reported, nor were specific outcomes in this subgroup. In the CASTLE-AF study, 398 patients with severe LV impairment and symptomatic paroxysmal or persistent AF were randomized to either AF ablation or medical therapy.63 There was a significant reduction in the primary composite endpoint of death or heart failure hospitalization in the ablation group (HR 0.62; 95% CI 0.43–0.87; P = .007) and 63% of patients were free from AF on device interrogation after 60 months of follow-up. 27.5% of patients had a CRT-defibrillator and there was a nonsignificant trend towards a lower primary endpoint event rate in the AF ablation arm compared to medical therapy in this subgroup (HR 0.54; 95% CI 0.28–1.04). There was also no significant interaction found between the primary endpoint and the presence of CRT-defibrillator (vs ICD) on Cox logistic regression analysis (P = .60). However, all patients required a device manufactured by Biotronik to be included, and overall only 13% of patients screened for the study were deemed eligible for inclusion, which has raised questions about the real-world applicability of the findings.
Rate vs rhythm control
Previous studies attempting to compare rate vs rhythm control in patients with CRT have produced variable results and had significant limitations, as previously discussed.51, 52, 53 Importantly, these studies focused on nonablation techniques for rhythm control. While there is evidence that both AV node ablation and AF ablation are beneficial in patients with AF and CRT, the question of which treatment is superior remains unanswered, and randomized studies are greatly needed. Patients with heart failure and AF are a heterogeneous cohort, and there are multiple factors that may determine the optimal treatment strategy. As with any patient with AF, CRT patients with symptomatic, paroxysmal AF are likely to benefit from a rhythm control strategy, while those with longstanding “permanent” AF are more likely to benefit from rate control. There does, however, remain a significant proportion of patients with CRT who have persistent AF, or paroxysmal AF with sufficiently high AF burden to significantly reduce biventricular pacing, in whom the optimal treatment strategy remains unclear. Although AV node ablation will undoubtedly achieve close to 100% biventricular pacing, and has been shown to improve mortality, the additional benefit gained by restoring atrial systole and AV resynchronization with rhythm control remains unclear.
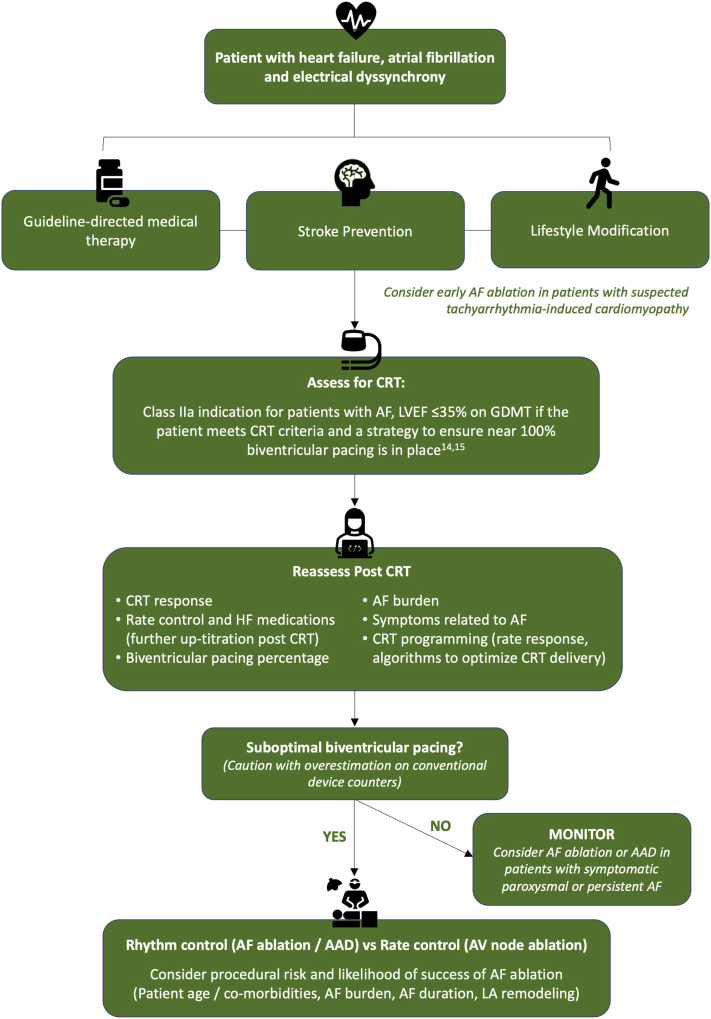
Though AF ablation may offer the additional benefits of AV synchrony over AV node ablation, a significant proportion of patients are likely to require multiple procedures, with associated expense and risk, and AF recurrence at some stage in the disease process is almost inevitable. AF ablation success rates from randomized studies in heart failure vary between 50% and 88%,64 and an international multicenter registry has demonstrated that the presence of heart failure significantly reduces success rates for catheter ablation in patients with persistent AF (57.3% vs 75.8%), though there was no significant difference for patients with paroxysmal AF (78.7% vs 85.7%).65 Prediction of which patients are likely to respond to AF ablation with a low risk of recurrence is therefore important, with a multitude of factors requiring consideration, including age, duration of AF, the presence of clinical comorbidities, and structural and electrical left atrial remodeling.64 Careful patient selection is likely to be key in determining the optimal treatment strategy in this challenging cohort. A flow diagram with a proposed clinical approach to patients with AF and CRT is shown in Figure 5.
Figure 5.
Clinical flowchart of the management of patients with atrial fibrillation (AF) and cardiac resynchronization therapy (CRT). AAD = antiarrhythmic drugs; AV = atrioventricular; GDMT = guideline-directed medical therapy; HF = heart failure; LA = left atrial; LVEF = left ventricular ejection fraction.
Device programming
AF brings additional challenges in device programming, and various strategies and algorithms have been developed to overcome these and optimize CRT delivery. The use of atrial pacing has also been proposed as a potential method of preventing AF. As discussed previously in this article, conventional device counters can overestimate CRT delivery in patients with AF, owing to fusion or pseudo-fusion beats. Algorithms such as EffectivCRT (Medtronic) can use the morphology of the LV electrogram to determine effective CRT delivery, and may provide a more accurate assessment than conventional counters. Irregular R-R intervals during AF lead to intermittent intrinsic ventricular activation. Most CRT devices feature an algorithm to trigger LV pacing in response to an intrinsically conducted right ventricular-sensed beat. This aims to increase biventricular pacing in AF, though many of these beats are likely to be fusion beats, as slow LV activation across the interventricular septum may have already commenced. No randomized trials have assessed the benefits of LV triggered pacing on CRT outcomes in patients with AF; however, there is evidence for hemodynamic benefit in a small echocardiographic-based observational study of patients in sinus rhythm.66 Furthermore, there have been concerns about the pro-arrhythmic risk of LV trigger pacing owing to R-on-T phenomenon.67
Several vendors also feature algorithms to automatically adjust the pacing rate to increase CRT delivery in response to ventricular-sensed events. Examples include Ventricular Rate Regulation (Boston Scientific) and Conducted AF Response (Medtronic). However, the benefit of these algorithms has not been assessed in clinical studies. The EffectivCRT during AF algorithm (Medtronic) uses the LV lead electrogram morphology to adjust pacing rate, thus allowing the system to detect ineffective fusion or pseudo-fusion beats and increase pacing rate accordingly. The use of this algorithm in a crossover randomized trial of 54 patients with CRT, AF and intact AV conduction, resulted in a significant increase in the percentage of effective CRT (87.7% ± 7.8% vs 80.8% ± 14.3%; P < .001).68
Another important consideration in device programming for patients with AF is the use of activity sensors to achieve rate response. Whereas patients in sinus rhythm can track the atrial activity to vary their biventricular pacing rate in response to exercise, this is not achievable in patients with AF, particularly in those who have undergone AV node ablation. The use and optimization of rate response algorithms are therefore important this group. Various rate response algorithms are available, including minute ventilation systems and accelerometers. A full discussion of rate response programming is beyond the scope of this article, and is discussed in detail elsewhere.69
The delivery of atrial overdrive pacing or atrial antitachycardia pacing (ATP) has been proposed as a potential method of preventing AF in patients with cardiac devices. Atrial overdrive pacing algorithms deliver pacing in response to atrial sensed events, but their use has not been shown to reduce frequency or duration of AF.70 Device algorithms can also be used to deliver atrial pacing after long sinus pauses (Atrial Rhythm Stabilization; Medtronic) or after termination of an AF episode (Post Mode-Switch Overdrive Pacing; Medtronic) in an attempt to prevent induction of atrial arrhythmias. Atrial ATP has been theorized to terminate atrial tachycardia re-entry circuits and prevent deterioration into AF. Early trials demonstrated that, while atrial ATP can terminate atrial tachycardia episodes in some cases, there was no overall effect on AF burden.70 More recently, a newer generation of “reactive ATP,” which delivers therapy timed to changes in the atrial arrhythmia cycle length or regularity, has been shown to reduce the incidence of persistent or permanent AF.71 However, this therapy was assessed in combination with an algorithm to reduce right ventricular pacing in patients with bradycardia, and has not been assessed in CRT.
Conclusion
The presence of AF attenuates the response to CRT in patients with heart failure, likely owing to a combination of suboptimal biventricular pacing and a loss of AV synchrony. AV node ablation improves biventricular pacing percentage and has been demonstrated to improve mortality after CRT implantation in large observational studies. AF ablation is feasible in patients with CRT, and may provide a benefit over AV node ablation by allowing AV resynchronization; however, randomized trials are required to demonstrate if this theoretical benefit improves clinical outcomes. The requirement for repeat AF ablation procedures, with associated cost and risk to the patient, must also be considered. A randomized study of AV node ablation vs AF ablation in patients with heart failure, CRT, and suboptimal biventricular pacing secondary to AF is currently enrolling (NCT04664686) and will hopefully help address this important question. Device programming in patients with AF is challenging, and various algorithms are available to help optimize CRT delivery and potentially reduce AF burden.
Funding Sources
The department is supported by the Wellcome/EPSRC Centre for Medical Engineering (WT203148/Z/16/Z).
Disclosures
M.K.E. and V.S.M. have received fellowship funding from Abbott. B.S.S. is supported by a project grant from NIHR and has received speaker fees from EBR systems. S.N. acknowledges support from the UK Engineering and Physical Sciences Research Council (EP/M012492/1, NS/A000049/1, and EP/P01268X/1), the British Heart Foundation (PG/15/91/31812, PG/13/37/30280, SP/18/6/33805), US National Institutes of Health (NIH R01-HL152256), European Research Council (ERC PREDICT-HF 864055), and Kings Health Partners London National Institute for Health Research (NIHR) Biomedical Research Centre. C.A.R. receives research funding and/or consultation fees from Abbott, Medtronic, Boston Scientific, Spectranetics, and MicroPort outside of the submitted work.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
References
- 1.Groenewegen A., Rutten F.H., Mosterd A., Hoes A.W. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlisle M.A., Fudim M., DeVore A.D., Piccini J.P. Heart failure and atrial fibrillation, like fire and fury. JACC: Heart Fail. 2019;7:447–456. doi: 10.1016/j.jchf.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Maisel W.H., Stevenson L.W. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2–8. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 4.Lyons K.J., Ezekowitz J.A., Liang L., et al. Impact of current versus previous cardiac resynchronization therapy guidelines on the proportion of patients with heart failure eligible for therapy. JACC Heart Fail. 2017;5:388–392. doi: 10.1016/j.jchf.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein K., Normand C., Auricchio A., et al. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients—who is doing what to whom and how? Eur J Heart Fail. 2018;20:1039–1051. doi: 10.1002/ejhf.1142. [DOI] [PubMed] [Google Scholar]
- 6.Buck S., Rienstra M., Maass A.H., Nieuwland W., van Veldhuisen D.J., van Gelder I.C. Cardiac resynchronization therapy in patients with heart failure and atrial fibrillation: importance of new-onset atrial fibrillation and total atrial conduction time. Europace. 2008;10:558–565. doi: 10.1093/europace/eun064. [DOI] [PubMed] [Google Scholar]
- 7.Santini M., Gasparini M., Landolina M., et al. Device-detected atrial tachyarrhythmias predict adverse outcome in real-world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;57:167–172. doi: 10.1016/j.jacc.2010.08.624. [DOI] [PubMed] [Google Scholar]
- 8.Wilton S.B., Leung A.A., Ghali W.A., Faris P., Exner D.V. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8:1088–1094. doi: 10.1016/j.hrthm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa U., Atkins J., Mina G., et al. Outcomes of cardiac resynchronisation therapy in patients with heart failure with atrial fibrillation: a systematic review and meta-analysis of observational studies. Open Heart. 2019;6:1–12. doi: 10.1136/openhrt-2018-000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healey J.S., Hohnloser S.H., Exner D.V., et al. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the Resynchronization for Ambulatory Heart Failure Trial (RAFT) Circ Heart Fail. 2012;5:566–570. doi: 10.1161/CIRCHEARTFAILURE.112.968867. [DOI] [PubMed] [Google Scholar]
- 11.Ruwald A.C., Pietrasik G., Goldenberg I., et al. The effect of intermittent atrial tachyarrhythmia on heart failure or death in cardiac resynchronization therapy with defibrillator versus implantable cardioverter-defibrillator patients: a MADIT-CRT substudy (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2014;63:1190–1197. doi: 10.1016/j.jacc.2013.10.074. [DOI] [PubMed] [Google Scholar]
- 12.Wilton S.B., Exner D.V., Wyse D.G., et al. Frequency and outcomes of postrandomization atrial tachyarrhythmias in the resynchronization/defibrillation in ambulatory heart failure trial. Circ Arrhythm Electrophysiol. 2016;9:1–9. doi: 10.1161/CIRCEP.115.003807. [DOI] [PubMed] [Google Scholar]
- 13.Kalscheur M.M., Saxon L.A., Lee B.K., et al. Outcomes of cardiac resynchronization therapy in patients with intermittent atrial fibrillation or atrial flutter in the COMPANION trial. Heart Rhythm. 2017;14:858–865. doi: 10.1016/j.hrthm.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Glikson M., Nielsen J., Kronborg M., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA) Eur Heart J. 2021;Aug 29 doi: 10.1093/eurheartj/ehab364. eha. [DOI] [PubMed] [Google Scholar]
- 15.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 16.Upadhyay G.A., Vijayaraman P., Nayak H.M., et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of the His-SYNC Pilot Trial. Heart Rhythm. 2019;16:1797–1807. doi: 10.1016/j.hrthm.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Ma P.P., Yang Y.H., Dai B.L., et al. Brady-arrhythmias in patients with atrial fibrillation and heart failure of reduced ejection fraction: is his-bundle pacing superior to biventricular pacing? Pacing Clin Electrophysiol. 2021;44:1193–1199. doi: 10.1111/pace.14289. [DOI] [PubMed] [Google Scholar]
- 18.Muthumala A., Vijayaraman P. His-Purkinje conduction system pacing and atrioventricular node ablation. Herzschrittmacherther Elektrophysiol. 2020;31:117–123. doi: 10.1007/s00399-020-00679-7. [DOI] [PubMed] [Google Scholar]
- 19.Qi J., Jia X., Wang Z. His bundle pacing for cardiac resynchronization therapy: a systematic literature review and meta-analysis. J IntervCard Electrophysiol. 2020;59:463–470. doi: 10.1007/s10840-020-00827-6. [DOI] [PubMed] [Google Scholar]
- 20.Boczar K., Sławuta A., Ząbek A., et al. Cardiac resynchronization therapy with His bundle pacing. Pacing Clin Electrophysiol. 2019;42:374–380. doi: 10.1111/pace.13611. [DOI] [PubMed] [Google Scholar]
- 21.Cheng A., Landman S.R., Stadler R.W. Reasons for loss of cardiac resynchronization therapy pacing: insights from 32 844 patients. Circ Arrhythm Electrophysiol. 2012;5:884–888. doi: 10.1161/CIRCEP.112.973776. [DOI] [PubMed] [Google Scholar]
- 22.Boriani G., Gasparini M., Landolina M., et al. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:868–876. doi: 10.1093/eurjhf/hfr046. [DOI] [PubMed] [Google Scholar]
- 23.Ousdigian K.T., Borek P.P., Koehler J.L., Heywood J.T., Ziegler P.D., Wilkoff B.L. The epidemic of inadequate biventricular pacing in patients with persistent or permanent atrial fibrillation and its association with mortality. Circ Arrhythm Electrophysiol. 2014;7:370–376. doi: 10.1161/CIRCEP.113.001212. [DOI] [PubMed] [Google Scholar]
- 24.Hayes D.L., Boehmer J.P., Day J.D., et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Kamath G.S., Cotiga D., Koneru J.N., et al. The utility of 12-lead Holter monitoring in patients with permanent atrial fibrillation for the identification of nonresponders after cardiac resynchronization therapy. J Am Coll Cardiol. 2009;53:1050–1055. doi: 10.1016/j.jacc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Hernández-Madrid A., Facchin D., Klepfer R.N., et al. Device pacing diagnostics overestimate effective cardiac resynchronization therapy pacing results of the hOLter for Efficacy analysis of CRT (OLÉ CRT) study. Heart Rhythm. 2017;14:541–547. doi: 10.1016/j.hrthm.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Fang F., Lee A.P.W., Yu C.M. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr Opin Cardiol. 2014;29:430–436. doi: 10.1097/HCO.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 28.Auricchio A., Stellbrink C., Block M., et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 29.Meluzín J., Novák M., Müllerová J., et al. A fast and simple echocardiographic method of determination of the optimal atrioventricular delay in patients after biventricular stimulation. Pacing Clin Electrophysiol. 2004;27:58–64. doi: 10.1111/j.1540-8159.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferchaud V, Garcia R, Bidegain N, et al. Non-invasive hemodynamic determination of patient-specific optimal pacing mode in cardiac resynchronization therapy [published online ahead of print October 30, 2020]. J Interv Card Electrophysiol. https://doi.org/10.1007/s10840-020-00908-6 [DOI] [PubMed]
- 31.Ellenbogen K.A., Gold M.R., Meyer T.E., et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 32.Starling R.C., Krum H., Bril S., et al. Impact of a novel adaptive optimization algorithm on 30-day readmissions. evidence from the Adaptive CRT Trial. JACC Heart Fail. 2015;3:565–572. doi: 10.1016/j.jchf.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Varma N., Hu Y., Connolly A.T., et al. Gain in real-world cardiac resynchronization therapy efficacy with SyncAV dynamic optimization: heart failure hospitalizations and costs. Heart Rhythm. 2021;18:1577–1585. doi: 10.1016/j.hrthm.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Arnold A., Mj S., Ali N., et al. Atrioventricular shortening is the dominant mechanism of benefit of biventricular pacing in left bundle branch block. Europace. 2021;23(Supplement 3) [Google Scholar]
- 35.van Rees J.B., Borleffs C.J.W., de Bie M.K., et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–562. doi: 10.1016/j.jacc.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 36.Borleffs C.J.W., Ypenburg C., van Bommel R.J., et al. Clinical importance of new-onset atrial fibrillation after cardiac resynchronization therapy. Heart Rhythm. 2009;6:305–310. doi: 10.1016/j.hrthm.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Poole J.E., Johnson G.W., Hellkamp A.S., et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daubert J.P., Zareba W., Cannom D.S., et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II. Frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 39.Fischer A., Ousdigian K.T., Johnson J.W., Gillberg J.M., Wilkoff B.L. The impact of atrial fibrillation with rapid ventricular rates and device programming on shocks in 106,513 ICD and CRT-D patients. Heart Rhythm. 2012;9:24–31. doi: 10.1016/j.hrthm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Hess P.L., Jackson K.P., Hasselblad V., Al-Khatib S.M. Is cardiac resynchronization therapy an antiarrhythmic therapy for atrial fibrillation? A systematic review and meta-analysis. Curr Cardiol Rep. 2013;15:330. doi: 10.1007/s11886-012-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bytyçi I., Bajraktari G., Lindqvist P., Henein M.Y. Improved left atrial function in CRT responders: a systematic review and meta-analysis. J Clin Med. 2020;9:298. doi: 10.3390/jcm9020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavazzoni M., Taramasso M., Zuber M., et al. Functional mitral regurgitation and cardiac resynchronization therapy in the “era” of trans-catheter interventions: is it time to move from a staged strategy to a tailored therapy? Int J Cardiol. 2020;315:15–21. doi: 10.1016/j.ijcard.2020.03.071. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L., Jiang W., Zhou L., et al. The role of valvular regurgitation in catheter ablation outcomes of patients with long-standing persistent atrial fibrillation. Europace. 2014;16:848–854. doi: 10.1093/europace/eut252. [DOI] [PubMed] [Google Scholar]
- 44.Xiao P.L., Cai C., Zhang P., et al. Cardiac resynchronization therapy modulates peripheral sympathetic activity. Heart Rhythm. 2020;17:1139–1146. doi: 10.1016/j.hrthm.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Masuda M., Yamada T., Mizuno H., et al. Impact of atrial fibrillation ablation on cardiac sympathetic nervous system in patients with and without heart failure. Int J Cardiol. 2015;199:65–70. doi: 10.1016/j.ijcard.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Gasparini M., Auricchio A., Metra M., et al. Long-term survival in patients undergoing cardiac resynchronization therapy: the importance of performing atrio-ventricular junction ablation in patients with permanent atrial fibrillation. Eur Heart J. 2008;29:1644–1652. doi: 10.1093/eurheartj/ehn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasparini M., Leclercq C., Lunati M., et al. Cardiac resynchronization therapy in patients with atrial fibrillation. The CERTIFY Study (Cardiac Resynchronization Therapy in Atrial Fibrillation Patients Multinational Registry) JACC Heart Fail. 2013;1:500–507. doi: 10.1016/j.jchf.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Gasparini M., Auricchio A., Regoli F., et al. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression. The importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006;48:734–743. doi: 10.1016/j.jacc.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 49.Gasparini M., Kloppe A., Lunati M., et al. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter-defibrillator therapies and hospitalizations. Eur J Heart Fail. 2018;20:1472–1481. doi: 10.1002/ejhf.1117. [DOI] [PubMed] [Google Scholar]
- 50.Dong K., Shen W.K., Powell B.D., et al. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm. 2010;7:1240–1245. doi: 10.1016/j.hrthm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Turco P., D’Onofrio A., Stabile G., et al. Feasibility and efficacy of electrical cardioversion after cardiac resynchronization implantation in patients with permanent atrial fibrillation. J Interv Card Electrophysiol. 2012;35:331–336. doi: 10.1007/s10840-012-9713-2. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzman D., Housel D., Bazaz R., et al. A pilot study to assess benefit of atrial rhythm control after cardiac resynchronization therapy and atrioventricular node ablation. Pacing Clin Electrophysiol. 2015;38:275–281. doi: 10.1111/pace.12535. [DOI] [PubMed] [Google Scholar]
- 53.Jb C., Tajstra M., Kowalik I., et al. Rhythm or rate control strategy in CRT recipients with long-standing persistent atrial fibrillation - preliminary results of the PilotCRAfT study. Europace. 2021;23(Supplement 3):i515. [Google Scholar]
- 54.Fink T., Rexha E., Schlüter M., et al. Positive impact of pulmonary vein isolation on biventricular pacing in nonresponders to cardiac resynchronization therapy. Heart Rhythm. 2019;16:416–423. doi: 10.1016/j.hrthm.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 55.Khan M.N., Jaïs P., Cummings J., et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 56.Ploux S., Eschalier R., Whinnett Z.I., et al. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm. 2015;12:782–791. doi: 10.1016/j.hrthm.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 57.Ruschitzka F., Abraham W.T., Singh J.P., et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 58.Prabhu S., Taylor A.J., Costello B.T., et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI Study. J Am Coll Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 59.Hunter R.J., Berriman T.J., Diab I., et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial) Circ Arrhythm Electrophysiol. 2014;7:31–38. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald M.R., Connelly D.T., Hawkins N.M., et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 61.Jones D.G., Haldar S.K., Hussain W., et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 62.di Biase L., Mohanty P., Mohanty S., et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 63.Marrouche N.F., Brachmann J., Andresen D., et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee R.K., Williams S.E., Niederer S.A., O’Neill M.D. Atrial fibrillation ablation in patients with heart failure: one size does not fit all. Arrhythm Electrophysiol Rev. 2018;7:84–90. doi: 10.15420/aer.2018.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ullah W., Ling L.H., Prabhu S., et al. Catheter ablation of atrial fibrillation in patients with heart failure: impact of maintaining sinus rhythm on heart failure status and long-term rates of stroke and death. Europace. 2016;18:679–686. doi: 10.1093/europace/euv440. [DOI] [PubMed] [Google Scholar]
- 66.Aktas M.K., Jeevanantham V., Sherazi S., et al. Effect of biventricular pacing during a ventricular sensed event. Am J Cardiol. 2009;103:1741–1745. doi: 10.1016/j.amjcard.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 67.Fujimoto M., Ikeda T., Tuchida M., et al. A case of biventricular pacing with a spike on T-wave caused by the algorithm maintaining biventricular pacing rate. J Arrhythmia. 2012;28:56–60. [Google Scholar]
- 68.Plummer C.J., Frank C.M., Bári Z., et al. A novel algorithm increases the delivery of effective cardiac resynchronization therapy during atrial fibrillation: the CRTee randomized crossover trial. Heart Rhythm. 2018;15:369–375. doi: 10.1016/j.hrthm.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 69.Trohman R.G., Huang H.D., Larsen T., Krishnan K., Sharma P.S. Sensors for rate-adaptive pacing: how they work, strengths, and limitations. J Cardiovasc Electrophysiol. 2020;31:3009–3027. doi: 10.1111/jce.14733. [DOI] [PubMed] [Google Scholar]
- 70.Chutani S.K., Shah A.N., Kantharia B.K. Pacing to prevent atrial fibrillation. Curr Opin Cardiol. 2017;32:22–26. doi: 10.1097/HCO.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 71.Padeletti L., Pürerfellner H., Mont L., et al. New-generation atrial antitachycardia pacing (Reactive ATP) is associated with reduced risk of persistent or permanent atrial fibrillation in patients with bradycardia: results from the MINERVA randomized multicenter international trial. Heart Rhythm. 2015;12:1717–1725. doi: 10.1016/j.hrthm.2015.04.015. [DOI] [PubMed] [Google Scholar]