Abstract
The group IV cytosolic phospholipase A2 (cPLA2) has been localized to the nucleus (M. R. Sierra-Honigmann, J. R. Bradley, and J. S. Pober, Lab. Investig. 74:684–695, 1996) and is known to translocate from the cytosolic compartment to the nuclear membrane (S. Glover, M. S. de Carvalho, T. Bayburt, M. Jonas, E. Chi, C. C. Leslie, and M. H. Gelb, J. Biol. Chem. 270:15359–15367, 1995; A. R. Schievella, M. K. Regier, W. L. Smith, and L. L. Lin, J. Biol. Chem. 270:30749–30754, 1995). We hypothesized that nuclear proteins interact with cPLA2 and participate in the functional effects of this translocation. We have identified a nuclear protein, cPLA2-interacting protein (PLIP), a splice variant of human Tip60, which interacts with the amino terminal region of cPLA2. Like Tip60, PLIP cDNA includes the MYST domain containing a C2HC zinc finger and well-conserved similarities to acetyltransferases. Both PLIP and Tip60 coimmunoprecipitate and colocalize with cPLA2 within the nuclei of transfected COS cells. A polyclonal antibody raised to PLIP recognizes both PLIP and Tip60. Endogenous Tip60 and/or PLIP in rat mesangial cells is localized to the nucleus in response to serum deprivation. Nuclear localization coincides temporally with apoptosis. PLIP expression, mediated by adenoviral gene transfer, potentiates serum deprivation-induced prostaglandin E2 (PGE2) production and apoptosis in mouse mesangial cells from cPLA2+/+ mice but not in mesangial cells derived from cPLA2−/− mice. Thus PLIP, a splice variant of Tip60, interacts with cPLA2 and potentiates cPLA2-mediated PGE2 production and apoptosis.
Phospholipase A2s (PLA2s) are a heterogeneous family of enzymes that are defined by their ability to cleave the fatty acid at the sn-2 position of phospholipids (9, 47). The group IVA 85-kDa cytosolic phospholipase A2, cPLA2, is distinguished from other PLA2s by its activation by submicromolar levels of Ca2+ and its preference for phospholipid substrates which contain arachidonic acid at the sn-2 position (18, 26). cPLA2 has been shown to play a role in many physiological processes, such as ion channel regulation, cell volume regulation, macrophage eicosanoid production, and parturition (9, 11, 73), as well as pathophysiologic processes, such as mitochondrial dysfunction (51), allergic responses involved in asthma, atopic dermatitis and anaphylaxis, and ischemia-reperfusion injury to the brain (11, 73). cPLA2 expression has been associated with cytotoxicity (30, 31, 65, 75).
Although primarily localized to the cytosol in resting cells, cPLA2 translocates to nuclear membranes when cellular [Ca2+] is increased to 300 nM (18, 26, 66). In one study, an intranuclear localization of cPLA2 has been proposed in subconfluent endothelial cells (67). This nuclear localization may be critical for physiological and pathophysiological actions of cPLA2. Arachidonic acid-metabolizing enzymes, such as prostaglandin H2 synthase-1 and -2 (COX-1 and -2) (70) and 5-lipoxygenase (77, 78), are localized on the nuclear membrane, and eicosanoids have been found to regulate transcription (3, 7, 8, 24). cPLA2 inhibitors result in reduced levels of group IIA secretory PLA2 mRNA (46), and cPLA2 expression has been correlated with COX-2 mRNA expression (2).
cPLA2 activity is regulated by cytosolic-free [Ca2+] and phosphorylation. Clark and others have shown that an amino-terminal [Ca2+]-dependent lipid-binding (CaLB) domain is both necessary and sufficient for the translocation of cPLA2 to cellular membranes (18, 52). The CaLB domain is homologous to domains found in Ras-GAP, phospholipase C, and PKCα (18). cPLA2 is phosphorylated at a number of sites, including Ser-505, and activated by ERK1/2 (48, 54) and p38 mitogen-activated protein kinase (75).
Despite the extensive data implicating cPLA2 in multiple physiological and pathophysiological processes, mechanisms by which cPLA2 acts at the nucleus are incompletely understood. We hypothesized that nuclear actions of cPLA2 may be facilitated by nuclear proteins which interact with cPLA2. A protein, PLIP, which colocalizes in the nucleus with cPLA2, was isolated using the yeast interaction trap two-hybrid system. PLIP enhances the cPLA2-dependent mesangial cell apoptosis and prostaglandin E2 (PGE2) production that occurs in response to serum deprivation.
MATERIALS AND METHODS
Plasmids.
pEG202-cPLA2 (1–215) was constructed by PCR amplification of the amino-terminal fragment of cPLA2 containing residues 1 to 215 by using the primers GGAATTCTAATGTCATTTATAGATCCT and CCCAAGCTTTGATTCGTATAATGCCTT and human cPLA2 as the template. The cDNA of cPLA2 was from pMT2-cPLA2 (obtained from James Clark, Genetics Institute, Cambridge, Mass.) (18). This was followed by cloning of an EcoRI/XhoI fragment of the PCR product into pEG202. The sequence was verified. A human fibroblast G0 library cloned into pJG4-5 was obtained from C. Sardet (then at the Whitehead Institute and Massachusetts Institute of Technology). pEG202, pSH18-34, pJK101, and pRFHM1 were obtained from Roger Brent of the Massachusetts General Hospital (28). PLIP cDNA was ligated from pJG4-5-PLIP into pBluescript and then into pMT3 EcoRI/Xba sites using the linkers AATTGAATTCCTCGAGT and CTAGACTCGAGGAATTC. PMT3-Tip60 was created by excising Tip60 cDNA (obtained from J. Kamine, Yale University, New Haven, Conn.) into pMT3 using EcoRI and XhoI sites.
Interaction trap two-hybrid screen.
The EGY48 strain of yeast (obtained from R. Brent), which contains an integrated copy of the LEU2 gene with upstream activating sequences replaced by 6 LexA operators (28), was transformed with both a bait plasmid, pEG202-cPLA2(1–215), and the reporter plasmid, pSH18-34, by using lithium acetate (25). Yeast colonies containing both bait and reporter plasmids were selected on Ura−, His−, glucose-containing medium and transformed with the library cDNA in a GAL1-inducible expression vector, pJG4-5. Transformants were selected on Ura−, His−, Trp− glucose-containing plates, and 106 CFU were plated onto Ura−, His−, Trp−, Leu−, galactose-raffinose medium. Positive colonies were grown up in Trp−, glucose-containing medium, and isolated prey plasmids were rescued using the method of Hoffman and Winston (34) and electroporated into KC8 strains of Escherichia coli. PLIP cDNA was cloned into pBluescript and pMT3 and amplified in XL-1 Blue strains of E. coli for sequencing and transfection experiments. DNA was sequenced completely on both strands by using customized oligonucleotides and standard techniques (5).
Screening of human placenta library.
A human placenta stretch library in λgt11 phage was screened in E. coli Y1090 cells as described previously (5, 64). Briefly, plaques were immobilized on Gene Screen Plus membranes (New England Nuclear, Boston, Mass.) with 0.5 N NaOH followed by neutralization in 1 M Tris (pH 7.5). Membranes were prehybridized at 55°C in 2× SDE (which contains 200 mM NaCl, 100 mM NaPO4 [pH 7.0], and 5 mM EDTA [pH 8.0]) with 5% sodium dodecyl sulfate (SDS), 100 μg of yeast tRNA/ml, and 100 μg of denatured salmon sperm DNA/ml and hybridized at 55°C with a 32P-labeled 900-bp fragment of the 5′ end of PLIP cDNA which had been amplified by PCR using the primers CCATTACATTGACTTCAACA and TTTCACTAATCTCATTGATG. Membranes were hybridized in 2% SDE overnight and washed in SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) as follows: 15 min (three times) in 2× SSC at room temperature, followed by 10 min (two times) in 1× SSC at 65°C, followed by 5 min (two times) in 0.1× SSC.
Coprecipitation experiments.
COS cells were transfected using DEAE-dextran. Cells were plated at 2.5 × 105 in 10-cm plates 24 h prior to transfection. For each 10-cm plate, 200 μl of 1× phosphate-buffered saline (PBS) containing DEAE-dextran (10 mg/ml) and chloroquine (2.5 mM) was added to 5 ml of Dulbecco modified Eagle medium (DMEM) containing 10% NuSerum (Collaborative Research, Bedford, Mass.). DNA (20 μg/plate) was added, and the chloroquine–DEAE-dextran–DNA mixture was layered onto cells. After a 4-h incubation at 37°C, the chloroquine–DEAE-dextran–DNA mixture was removed and cells were exposed to 10% dimethyl sulfoxide at room temperature for exactly 2 min. Cells were washed with 1× PBS, and fresh DMEM containing 10% fetal calf serum (FCS) was added. Forty-eight hours after transfection, confluent monolayers of transfected cells were harvested into lysis buffer containing 20 mM Tris (pH 8.0), 50 mM β-glycerophosphate, 2 mM EDTA, 1% triton, 200 μM vanadate, 100 μM phenylmethylsulfonyl fluoride, 2 μM leupeptin, 1 mM dithiothreitol, and 10% glycerol. Immunoprecipitation was done over 4 h at 4°C with a mouse monoclonal anti-HA antibody diluted 1:10 and protein G agarose beads. Precipitated proteins were run on a 10% SDS gel at 50 V and electrophoretically transferred onto Immobilon membranes (Millipore, Bedford, Mass.). Membranes were blotted with anti-cPLA2 antibody and developed by chemiluminescence.
To test for coprecipitation of endogenous PLIP and cPLA2, renal mesangial cells were grown to confluence and harvested into buffer containing 10 mM potassium phosphate (pH 7.4), 5 mM EGTA, 50 mM β-glycerophosphate, 1 mM vanadate, 1 mM dithiothreitol, 2 μM leupeptin, 2 μM pepstatin, 0.5% NP-40, and 0.1% Brij 35. Supernatants were immunoprecipitated with anti-PLIP antibody, and precipitants were analyzed by Western blotting as described above.
Immunofluorescent microscopy.
COS cells were grown to 50% confluence on glass coverslips and transiently transfected using DEAE-dextran as described above. Forty-eight hours after transfection, cells were washed with ice-cold PBS and fixed with 4% paraformaldehyde–0.1% triton over 30 min on ice. Fixed cells were blocked at room temperature in 40% calf serum and exposed to primary and secondary antibodies over 1 h at room temperature with copious washing with PBS in between exposure to antibodies. Primary antibodies were rabbit polyclonal anti-cPLA2 used at 1:100 and mouse monoclonal anti-HA used at 1:5. Secondary antibodies were goat fluorescein isothiocyanate (FITC)-conjugated anti-mouse, rhodamine-conjugated anti-rabbit, and Cy3-conjugated anti-rabbit, all used at 1:100. Cell nuclei were stained with 0.5 μg of Hoechst dye/ml. FITC, rhodamine, and Cy3-conjugated antibodies were obtained from Jackson Immuno Research (West Grove, Pa.).
Production of PLIP antibody.
Supercompetent X-L-1 Blue E. coli cells were transformed with pGexKg-PLIP. A 5-ml culture was grown to an optical density at 600 nm of 0.50, induced with 0.2 mM isopropyl-β-Δ-thiogalactopyranoside (IPTG; Sigma, St. Louis, Mo.), and incubated overnight at 37°C with agitation. Cells were harvested and resuspended in 1× binding buffer containing 50 mM Tris (pH 8.0), 150 mM KCl, and 1% Triton X-100. The suspension was sonicated at 375W and centrifuged for 20 min at 10,000 × g at 4°C. The supernatant was run over a glutathione agarose column and washed with 10 volumes of binding buffer. The fusion protein was eluted with 10 mM glutathione in 1× binding buffer and dialyzed against 1× binding buffer overnight at 4°C. A rabbit antibody was made to this protein by SeraSource (Royalston, Mass.).
Cell culture.
Rat mesangial cell cultures were derived from 6-week-old Sprague Dawley rats. Cortices of decapsulated, bisected kidneys were minced and forced through a 106-μm sieve (Bellco Glass Co., Vineland, N.J.) followed by passage through a 53-μm sieve. The washed, sieved glomeruli were resuspended in minimal essential medium with d-valine, l-glutamine and Earle's salts (Mediatech, Inc., Herndon, Va.). After excluding fibroblasts by growth in d-valine-containing medium, cells were grown in RPMI with 20% FCS (Mediatech, Inc.). Homogenous cell cultures have been demonstrated using this method (10, 12–14). Mesangial cell cultures were similarly derived from 5-week-old cPLA2-knockout mice and from wild-type control animals (11). Cardiac myocytes were isolated from day-old rats using the neonatal cardiomyocyte isolation system (Worthington Biochemical Corp., Lakewood, N.J.) as described previously (29, 42).
Adenoviral infection.
PLIP cDNA was subcloned into the NotI and XhoI sites of pADRSV4, which contains adenoviral sequences from the 0 to 1.2 and 9.2 to 16.1 map units, the Rous sarcoma virus long-terminal-repeat promoter, and the simian virus 40 early polyadenylation signal to generate pAdRSV4-PLIP. The position and orientation of the insert were confirmed by sequencing of the 5′ ends of the constructs using a pADRSV4 primer. pADRSV4-PLIP was cotransfected into 293 cells with pJM17, which contains adenoviral cDNA. Homologous recombinants between pADRSV4-PLIP and pJM17 contain exogenous DNA substituted for E1. Individual plaques were purified, and protein expression was confirmed by immunoblotting. The recombinant adenovirus was prepared in high titer by propagation in 293 cells and purification by CsCl gradient. Optimal expression of protein was determined to occur at 48 to 72 h after infection with 200 to 350 PFU/cell. Infectivity was approximately 50%. A recombinant adenovirus carrying the E. coli LacZ gene (Ad-Lac) encoding β-galactosidase was used as a control. In other experiments Ad-GFP, expressing green fluorescent protein, was used as a control.
[3H]arachidonic acid release.
Mesangial cells were plated in six-well dishes and grown to confluence in DMEM and either 0.1 or 10% FCS. Cells were labeled overnight with [3H]arachidonic acid (New England Nuclear). Cells were washed twice in serum-free DMEM containing 0.2% albumin and incubated in DMEM. At the end of 30 min of incubation at 37°C, medium was removed and floating cells were removed from the medium by centrifugation at 20,800 × g. Supernatant was counted. Adherent cells were dissolved in 0.1% triton and counted. Data are expressed as supernatant counts over cellular plus supernatant counts.
Measurement of supernatant PGE2.
Anti-PGE2 antibody and PGE2 standard were obtained from Sigma Immunochemicals (St. Louis, Mo.). Supernatant PGE2 was measured by radioimmunoassay per the protocol described by the manufacturer. Briefly, PGE2 standard was diluted to 15 to 1,000 pg/100 μl in buffer containing 0.01 M sodium PBS (pH 7.4), 0.1% bovine serum albumin, and 0.1% sodium azide. One hundred microliters of sample or standard was vortex mixed with 500 μl of anti-PGE2 antibody and incubated at 4°C for 30 min. 3H-PGE2 (New England Nuclear) was added for 1 h at 4°C followed by 200 μl of dextran-coated charcoal suspension. Samples were centrifuged at 2,000 × g for 5 min, and radioactivity in supernatants was determined.
RNA isolation and RT-PCR.
RNA was harvested from BALB/c mice using the RNA easy mini kit (Qiagen, Inc., Valencia, Calif.) following the manufacturer's protocol. One-step reverse transcriptase PCR (RT-PCR) (Clontech, Palo Alto, Calif.) was performed using primers TGAGCGGCTGGACCTAAAGAAG and GAATACCGTCAGCACCACGCAT.
Nucleotide sequence accession number.
Nucleotide sequence data for PLIP have been submitted to DDBJ/EMBL/GenBank under the accession number U67734.
RESULTS
Identification of a cPLA2-interacting protein, PLIP.
A fragment of cPLA2 cDNA encoding amino acids 1 to 215, which includes the CaLB domain, was cloned into the bait vector, pEG202, to create a fusion protein with the DNA-binding domain of LexA. Using LexA-cPLA2(1–215), we screened 106 clones of a G0 human fibroblast library, which had been cloned into an expression vector, pJG4-5 (28). Restriction analysis and partial sequencing revealed three distinct clones, one of which (no. 55) is the focus of this report.
The interaction between clone 55 and cPLA2(1–215) is specific since cotransformation of yeast with pJG4-5-55 (expressing the interactor) and the bait vector, pEG202-Bicoid, encoding an unrelated LexA fusion protein, does not allow growth on Leu− medium and does not activate β-galactosidase transcription (data not shown). In yeast, cPLA2(1–215) coimmunoprecipitates with the interactor when the latter is immunoprecipitated with an antibody to the hemagglutinin (HA) epitope tag (data not shown).
PLIP cDNA and amino acid sequence.
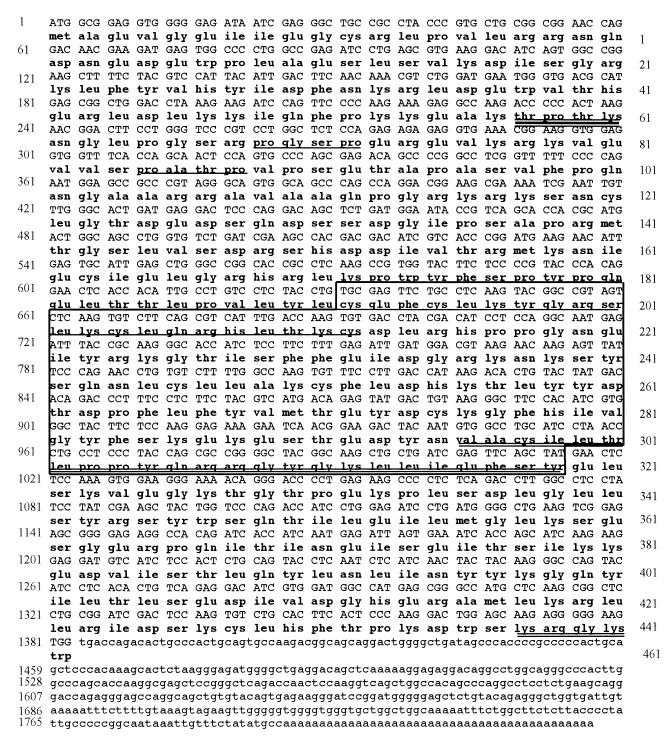
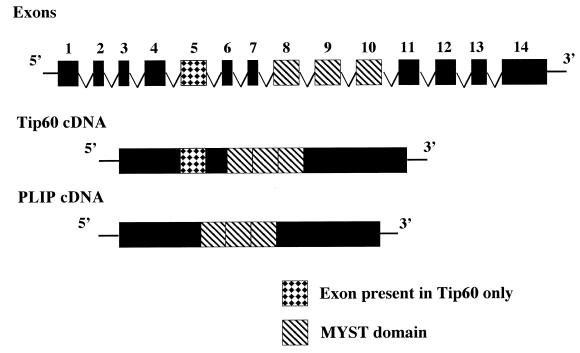
The cDNA of clone 55 is 1,840 bp in length with a poly(A) tail (Fig. 1). The cDNA contains a putative initiator ATG within an optimal Kozak consensus sequence (45). There is an open reading frame of 1,383 bp encoding a protein of 461 amino acids with a predicted Mr of 53,000. The protein was named PLIP for PLA2 interacting protein. A human placenta library (Clontech) was screened using a 900-bp PCR fragment from the 5′ end of the cDNA. Eight clones were isolated, each of which had a 5′ origin at, or 3′ to, the 5′ end of the pJG4-5-55 cDNA insert and was identical in size. The nonredundant database of the National Center for Biotechnology Information (NCBI) was searched with the PLIP cDNA sequence using the GAPPED BLAST program (1). PLIP is identical to the human TAT-interacting protein, Tip60 (41), except for a 52-amino-acid fragment that is present in Tip60 but not PLIP. PLIP and Tip60 cDNA were compared to the human genomic clone, RP11-856B14 (accession number, AP001362), which has been partially sequenced and is located on chromosome 11 and mapped to 11q13. Alignment of PLIP and Tip60 cDNA to a fragment of clone RP11-856B145 predicted the genomic structure which is schematically shown in Fig. 2. Clone RP11-856B14 comprises 14 exons. The fifth exon is present in Tip60 but not PLIP.
FIG. 1.
Nucleotide and predicted amino acid sequence of PLIP. The MYST domain is boxed, and within the box the acetyltransferase domain is double underlined. A C2HC domain, also within the boxed MYST domain, is single underlined. A potential nuclear localization sequence at the carboxy terminus is double underlined. Potential ERK1/2 kinase phosphorylation sites (PGSP and PATP) are single underlined. The potential CDK phosphorylation site is triple underlined.
FIG. 2.
Structure of PLIP locus. Partial genomic structure is represented by exons consecutively numbered 1 to 14. Tip60, but not PLIP cDNA, contains exon 5. Both Tip60 and PLIP contain the MYST domain.
The amino acid sequences of both Tip60 and PLIP contain the MYST domain that is homologous to the yeast silencing proteins, SAS2 and SAS3 (63); the Drosophila transcription regulator protein, MOF (males absent on the first) (33); the human monocytic leukemia zinc finger protein, MOZ (15); and ESA1 (essential SAS2-related acetyltransferase) (68). The MYST domain comprises a putative histone acetyltransferase domain and a C2HC zinc finger domain (63). PLIP and Tip60 contain two potential ERK1/2 kinase phosphorylation sites (19) and a potential cyclin-dependent kinase phosphorylation site (55, 56, 69).
Both PLIP and Tip60 interact and colocalize with cPLA2 in mammalian cells.
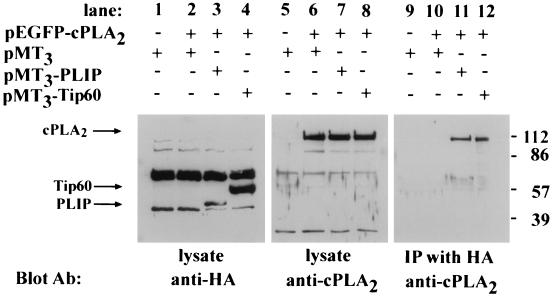
To evaluate whether PLIP interacts with cPLA2 in mammalian cells, PLIP or Tip60 cDNA was cloned into the mammalian expression vector pMT3, which encodes the protein with a HA tag at its NH2 terminus. COS cells were cotransfected with either pEGFP-cPLA2 (for coimmunoprecipitation experiments) or pMT2-cPLA2 (for immunofluorescent microscopy) and with either pMT3-PLIP or pMT3-Tip60. cPLA2 coimmunoprecipitates with HA-tagged PLIP and Tip60 but not with HA alone (Fig. 3).
FIG. 3.
PLIP interacts with cPLA2. COS cells were transfected as follows: empty pMT3 vector alone; pEGFP-cPLA2 and pMT3; pEGFP-cPLA2 and pMT3-PLIP; and pEGFP-cPLA2 and pMT3-Tip60. Cell lysate proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and analyzed by Western blot analysis. Western blot with anti-cPLA2 antibody demonstrates a band at approximately 112 kDa in lanes 6, 7, and 8, which contain lysates of pEGFP-cPLA2-transfected COS cells (arrow). Western blot with anti-HA antibody demonstrates a band at approximately 50 kDa in lane 3, which contains the lysate of pMT3-PLIP-transfected cells (arrow), and at 60 kDa in lane 4, which contains the lysate of pMT3-Tip60-transfected COS cells (arrow). After immunoprecipitation of lysates with anti-HA antibody and resolution of the precipitants by SDS-PAGE, Western blot analysis with the anti-cPLA2 antibody revealed a band corresponding to GFP-cPLA2 in lysates of cells in which GFP-cPLA2 was coexpressed with HA-PLIP (lane 11) and HA-Tip60 (lane 12) but not in lysates of cells transfected with pEGFP-cPLA2 and pMT3 (lane 10).
The fragment of cPLA2 that was used as the “bait” in the original two-hybrid screen contains the CaLB domain, which shares homology with domains of other proteins, including the GTPase-activating protein, Ras-GAP, which translocate to cell membranes in response to increases in cytosolic [Ca2+] (18). While cPLA2 coimmunoprecipitates with PLIP, Ras-GAP does not, suggesting specificity of the interaction between PLIP or Tip60 and cPLA2 (data not shown).
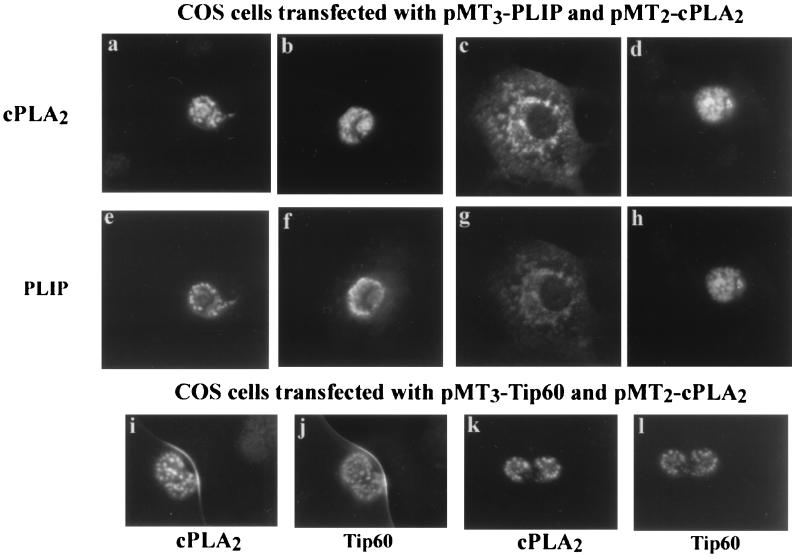
cPLA2 and either PLIP or Tip60 were localized by immunofluorescence on transfected COS cells using mouse HA antibody followed by FITC-conjugated anti-mouse antibody and anti-cPLA2 antibody followed by Cy3-conjugated anti-rabbit antibody. Representative photographs demonstrating localization of cPLA2 and either PLIP or Tip60 are shown in Fig. 4. Fields were viewed with a Cy3 filter (panels a, b, c, d, i, and k) and with an FITC filter (panels e, f, g, h, j, and l). PLIP and cPLA2 localize to a nuclear or perinuclear region of cells that express both proteins (panels a to h). Tip60 also colocalizes with cPLA2 in the nucleus of cells which coexpress both proteins (panels i to l).
FIG. 4.
PLIP and Tip60 colocalize with cPLA2. Panels a to h are representative photographs which demonstrate colocalization of HA-tagged PLIP and cPLA2 by immunofluorescence in transfected COS cells. Expressed proteins were identified with anti-cPLA2 antibody followed by Cy3-conjugated anti-rabbit antibody. This was followed by anti-HA antibody followed by FITC-conjugated anti-mouse antibody. COS cells transfected with both pMT3-PLIP and pMT2-cPLA2 are visualized with an FITC filter in panels e to h and a Cy3 filter in panels a to d. Panels i to l demonstrate cells expressing both Tip60 and cPLA2. cPLA2 is visualized with a Cy3 filter in panels i and k, and Tip60 is visualized with an FITC filter in panels j and l.
PLIP protein is expressed in multiple cell types.
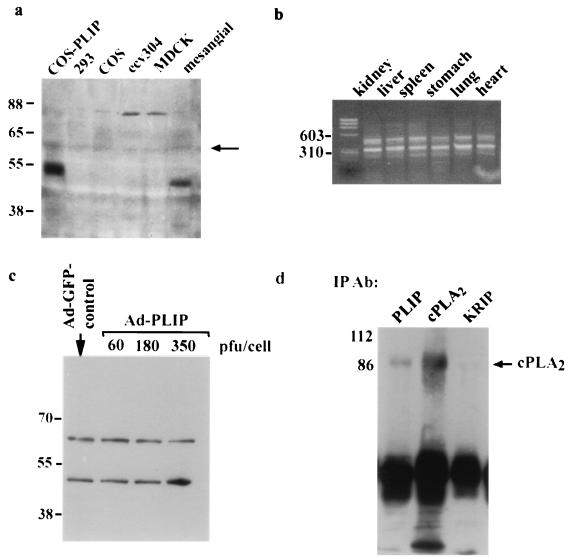
An antibody to full-length PLIP was generated in rabbits using a glutathione S-transferase fusion protein, and cell lines were screened by Western blot analysis (Fig. 5a). A faint band at 60 kDa can be seen in all lanes and likely represents Tip60 (arrow). The lane containing lysate of renal mesangial cells demonstrates a striking band at approximately 50 kDa, which indicates that the smaller molecular mass PLIP is also expressed in these cells. This is shown in comparison to lysates of HA-PLIP transfected COS cells. A larger band at approximately 80 kDa also appears in lanes containing lysates of MDCK and ecv304 cells, suggesting the possibility of the existence of larger proteins homologous to Tip60 and PLIP. Both Tip60 and PLIP RNA are present in mouse tissue (Fig. 5b). RNA was harvested and purified from BALB/c mouse organs, and RT-PCR was performed using flanking primers to exon 5. Gel electrophoresis demonstrates two bands which conform to predicted sizes of 456 and 300, representing cDNAs of Tip60 and PLIP, respectively. To determine whether PLIP protein was present in nonrenal primary cultured cells, myocytes were harvested and infected with Ad-PLIP (Fig. 5c). Western blot analysis of lysates of both Ad-PLIP-infected and Ad-GFP-infected myocytes shows two bands at 60 to 65 kDa and at 50 kDa, likely representing Tip60 and PLIP. Ad-PLIP-infected myocytes show an increase in PLIP-related signal.
FIG. 5.
Detection and coimmunoprecipitation of Tip60-PLIP and cPLA2 in cultured mesangial cells. (a) Western blot of lysates from COS, 293, ecv304, MDCK, and mesangial cells using a polyclonal antibody raised in rabbits against GST-PLIP. A band at approximately 50 kDa is present in the lane containing lysates of HA-PLIP-transfected COS cells. A band of approximately similar size is present in the lane corresponding to rat renal mesangial cells but not in those lanes containing protein from 293, ECV304, MDCK, or nontransfected COS cells. There is a light band in each lane at 60 kDa which likely reflects Tip60. (b) Gel electrophoresis of DNA obtained by RT-PCR of RNA harvested from BALB/c mouse tissue. Two bands of 456 and 300 bp, representing Tip60 and PLIP DNA, respectively, were obtained using primers which flanked exon 5. Molecular mass markers are indicated. (c) Western blot of rat neonatal myocytes infected with Ad-GFP or with increasing amounts of Ad-PLIP. Two bands are present in all lysates at approximately 60 and 50 kDa, representing Tip60 and PLIP, respectively. Signal corresponding to PLIP is enhanced in lysates of Ad-PLIP-infected myocytes at 350 PFU/cell. (d) Mesangial cell lysate was immunoprecipitated over 4 h with anti-PLIP antibody versus a control antibody which recognizes the nuclear protein, KRIP (43), or with anti-cPLA2 antibody. Immunoprecipitants separated by SDS-polyacrylamide gel electrophoresis and transferred to an Immobilon membrane were blotted with cPLA2 antibody. A band corresponding to cPLA2 appears in lanes containing precipitants with either anti-PLIP or anti-cPLA2 antibody but not with anti-KRIP antibody.
We used renal mesangial cells to determine whether endogenous PLIP and cPLA2 coprecipitate in vivo. Lysates from confluent mesangial cells were precipitated over 4 h using anti-PLIP antibody. Western blot analysis of precipitates, using cPLA2 antibody but not an unrelated antibody (KRIP) (43), demonstrates coimmunoprecipitation of endogenous cPLA2 (Fig. 5d), although the PLIP band is less intense than that seen in transfected cells, as expected.
Serum deprivation results in nuclear localization of PLIP and causes apoptosis.
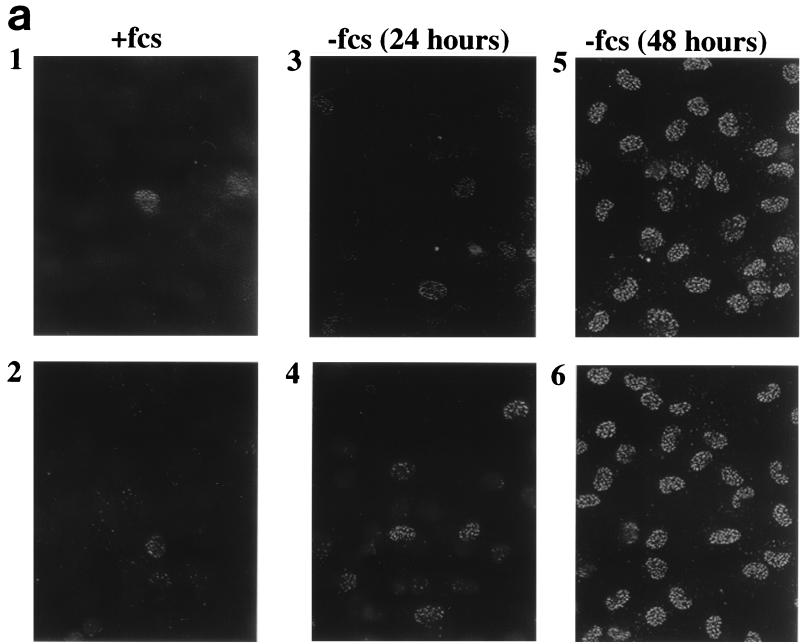
Mutations of SAS2 are associated with loss of viability under conditions of nutrient limitation (63). Similarly, ESA1 is required for cell growth in yeast (68). Given PLIP's and Tip60's homology with these proteins, we evaluated whether PLIP expression is modulated by the presence or absence of growth factors in serum. Incubation of mesangial cells in 0.25% FCS induces growth arrest and eventually apoptosis. Using anti-PLIP antibody, a striking pattern of immunofluorescence was detected in the nuclei of greater than 95% of renal mesangial cells grown in 0.25% FCS (−fcs) for 48 h (Fig. 6a, panels 5 and 6). By contrast, this strong signal was demonstrated in the nuclei of only 1 or 2 in 50 mesangial cells grown in 10% FCS (+fcs) (Fig. 6a, panels 1 and 2). There is a small but significant increase in PLIP-associated signal after only 24 h of incubation in 0.25% FCS (Fig. 6a, panels 3 and 4). Multiple fields were examined, and two representative fields are shown. As our antibody recognizes both PLIP and Tip60, this pattern of immunofluorescence may reflect either PLIP or Tip60. Nonspecific staining of the nucleus was ruled out by the observation that preincubation of anti-PLIP antibody with purified PLIP protein prevented antibody staining of the nucleus whereas preincubation with albumin had no effect on the staining pattern (data not shown).
FIG. 6.
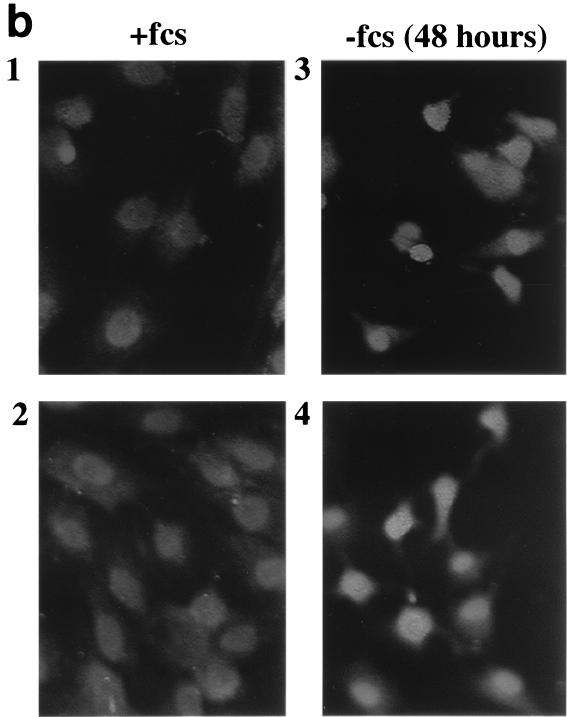
Serum deprivation alters the nuclear localization of Tip60 or PLIP and cPLA2. (a) Rat renal mesangial cells grown on coverslips were fixed and exposed to anti-PLIP antibody followed by rhodamine-conjugated anti-rabbit antibody. Greater than 95% of mesangial cells grown in 0.25% FCS (−fcs) (panels 5 and 6) demonstrate nuclear localization of Tip60 or PLIP by immunofluorescence microscopy as compared to 2 to 4% of cells grown in 10% FCS (+fcs) for 48 h (panels 1 and 2). PLIP appears in the nuclei of approximately 12 to 24% of cells grown in 0.25% FCS for 24 h. (b) Mesangial cells were fixed and exposed to anti-cPLA2 antibody followed by rhodamine-conjugated anti-rabbit antibody; cPLA2-associated signal is seen in the cytosol and nucleus in cells grown in 10% FCS (panels 1 and 2) but is lost from the cytosol and accentuated at the nucleus in cells grown in 0.25% FCS for 48 h (panels 3 and 4).
Western blot analysis of lysates of serum-deprived cells showed no difference in either PLIP or Tip60 expression (data not shown), ruling out changes in total cellular protein expression of Tip60 or PLIP as an explanation for the changes seen by immunofluorescence. We examined cPLA2 expression using anti-cPLA2 antibody in cells grown in 10% FCS or 0.25% FCS for 48 h (Fig. 6b). Whereas cPLA2 is detected in both the cytosol and nuclei of cells grown in 10% FCS (Figure 6b, panels 1 and 2), cytosolic staining is diminished and nuclear staining is accentuated in cells grown in 0.25% FCS (Fig. 6b, panels 3 and 4). Although the particulate nature of the cPLA2-associated signal is not nearly as striking as that seen with PLIP antibody, a similar, particulate pattern can be seen in the nuclei of a number of cells. Western blot analysis showed no change in total expression of cPLA2 in lysates of serum-deprived compared to serum-replete cells. [3H]arachidonic acid release into supernatant of serum-deprived cells was 1.71 ± 0.45% of total cellular [3H]arachidonic acid compared to 0.22 ± 0.05% from serum-replete cells (P < 0.05) (data not shown). [3H]arachidonic acid release was similar after 24 and 48 h of serum deprivation.
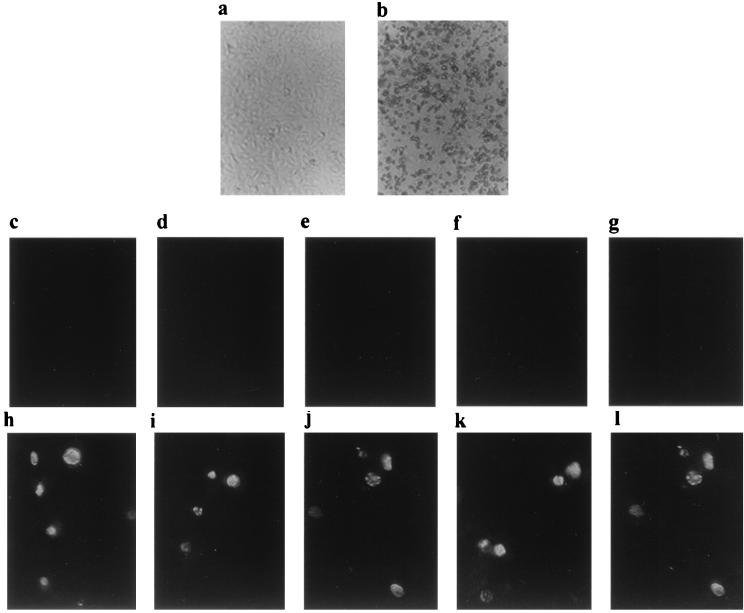
Mesangial cells incubated in 0.25% FCS for 50 h undergo cell death and detachment, which is demonstrated by light microscopy (Fig. 7b), whereas cells grown in the presence of 10% FCS remain viable and attached (Fig. 7a). As a marker for apoptosis, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) at 36 h is modestly positive in fixed cells grown in 0.25% FCS (Fig. 7h to l) but negative in cells grown in 10% FCS (Fig. 7c to g). TUNEL of adherent cells likely underestimates the degree of apoptosis as apoptotic cells rapidly detach from plastic, as described in other models of apoptosis (38).
FIG. 7.
Serum deprivation causes apoptosis. (a and b) Mesangial cells grown in 0.25% FCS die at approximately 50 h. These photographs demonstrate light microscopy of cells grown in 10% FCS (a) versus 0.25% FCS (b) for 50 h. (c to l) TUNEL stain of adherent cells grown in 10% FCS (c to g) or 0.25% FCS (h to l) at 36 h.
PLIP expression enhances PGE2 production susceptibility to apoptosis in +/+ but not −/− cPLA2 mesangial cells.
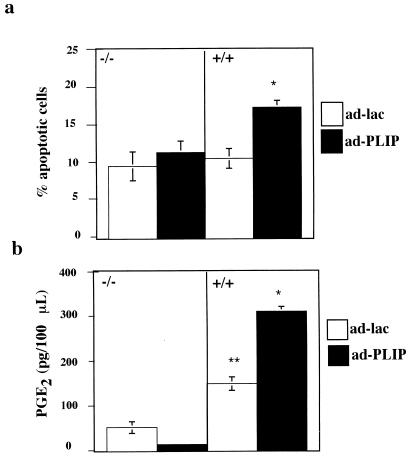
Although the nuclear localization of PLIP is temporally related to the onset of apoptosis, these data do not demonstrate that PLIP is causally related to apoptosis. cPLA2 has been associated with apoptosis, however (30, 49, 74, 76, 79), and our data suggest that loss of cPLA2 from the cytosol and its accentuation in the nucleus may also be induced by serum deprivation. To determine whether the PLIP-cPLA2 interaction is functionally relevant to apoptosis, we examined the effect of adenovirus-mediated PLIP expression in serum-deprived mesangial cells derived from cPLA2+/+ and cPLA2−/− mice (11). Although mouse mesangial cells were resistant to the apoptotic effects of 0.25% FCS, they were susceptible to apoptosis induced by incubation in serum-free medium. Ad-PLIP-infected cPLA2+/+ and cPLA2−/− cells were incubated in serum-free medium for 2 days. PLIP expression, which was equivalent in cPLA2+/+ and cPLA2−/− cells (data not shown), results in a consistent increase in the number of apoptotic cells in cPLA2+/+ but not cPLA2−/− cells after serum deprivation (Fig. 8a).
FIG. 8.
PLIP expression potentiates cPLA2-induced cell susceptibility to injury by serum withdrawal. (a) Wild-type (+/+) and cPLA2 knockout (−/−) mouse mesangial cells were infected with Ad-PLIP or Ad-Lac. Forty-eight hours after infection, cells were incubated in serum-free medium for 48 h. Ad-PLIP-infected (+/+) cells showed a greater number of apoptotic cells than Ad-Lac-infected (+/+) cells or either Ad-PLIP- or Ad-Lac-infected (−/−) cells. Ad-PLIP resulted in no change in the number of apoptotic cells in the (−/−) cells compared to Ad-Lac-infected (−/−) cells. (b) PGE2 levels were greater in the supernatants of Ad-PLIP-infected (+/+) cells than in those of Ad-PLIP-infected (−/−) cells and Ad-Lac-infected (+/+) or (−/−) cells. PGE2 levels were greater in supernatants of Ad-Lac-infected (+/+) cells than in those of Ad-Lac-infected (−/−) cells. ∗, P < 0.05 versus (+/+) cells infected with Ad-Lac and (−/−) cells infected with either Ad-PLIP or Ad-Lac; ∗∗, P < 0.05 versus (−/−) cells infected with Ad-Lac.
cPLA2 expression has also been closely linked to PGE2 generation (2, 11, 32, 49, 53), which has been shown to have both pro- (17, 49, 60) and anti- (58) apoptotic effects. We compared PGE2 generation in Ad-PLIP-infected cPLA2+/+ and cPLA2−/− mesangial cells incubated in serum-free medium for 48 h. PLIP expression markedly increases PGE2 production in cPLA2+/+ but not cPLA2−/− cells (Fig. 8b).
DISCUSSION
We report that Tip60 and PLIP, a novel splice variant of Tip60, interact and colocalize with group IVA cPLA2. This is a potentially important observation because cPLA2 plays a large role in the production of arachidonic acid, a precursor of eicosanoid-derived metabolites, which are mediators of many physiologic and pathologic cellular processes including inflammation (9, 11, 73). A cPLA2-interacting protein may play a role to modulate the activity and/or intracellular localization of cPLA2 and may serve as a target for antiinflammatory therapies.
Tip60 has been associated with DNA repair (35, 71) and with both positive and negative transcriptional regulation (16, 20). PLIP and Tip60 belong to a family of proteins characterized by a conserved MYST domain, which consists of a C2HC zinc finger domain and an acetyltransferase domain (15, 33, 63, 68). Tip60 has histone acetyltransferase activity in vitro (80). Tip60 interacts with Bcl-3, a nuclear member of the IκB family (20), and colocalizes with the interleukin-9 receptor α-chain, suggesting both intranuclear and extranuclear roles (81).
The role of PLIP may or may not be similar to that of Tip60. The biological significance of the 52-amino-acid fragment present in Tip60 but not in PLIP is not yet apparent. Our data indicate that PLIP is a splice variant of Tip60 that is expressed under physiologic conditions. PLIP mRNA was isolated from two libraries, including a human fibroblast G0 and a human placenta library. Both PLIP and Tip60 mRNAs are present in mouse tissue, and proteins consistent in size with Tip60 and PLIP are expressed in primary mouse neonatal myocytes as well as primary rat renal mesangial cells.
The striking increase in nuclear immunocytochemical staining with anti-PLIP antibody in serum-deprived mesangial cells may be due to either PLIP or Tip60 as the PLIP antibody recognizes both proteins. Western blot analysis of whole-cell lysates does not demonstrate upregulation of total cell PLIP protein, nor of Tip60, after serum deprivation. Thus, neither Tip60 nor PLIP expression is induced by serum deprivation. While the observed enhanced nuclear signal by immunofluorescence after serum deprivation in the absence of a change in total cellular Tip60 and PLIP protein may be related to epitope unmasking, its appearance suggests translocation of the protein from another intracellular site to the nucleus. The model of serum deprivation-induced apoptosis is well described in mesangial cells (72) and nonmesangial cells (27, 36, 37, 39). Apoptosis in mesangial cells in vivo may play an important role in determining the outcome of glomerulonephritis (6). Prominent Tip60 or PLIP nuclear staining correlates temporally with the onset of apoptosis in serum-deprived cells, although the rat mesangial cell model does not permit us to draw a causal relationship between the appearance of Tip60 or PLIP and apoptosis.
cPLA2 has been implicated in tumor necrosis factor alpha (TNF-α) induced apoptosis, though not in other models. Nuclear localization of cPLA2 is more evident in serum-deprived than in serum-replete mesangial cells. We do not believe this is related to serum deprivation-associated catalytic cleavage of cytosolic cPLA2 (4) since total expression is not changed. Additionally, there is a seven- to eightfold increase in [3H]arachidonic acid release from serum-deprived compared to serum-replete rat mesangial cells, suggesting activation of phospholipases. However, multiple phospholipases are active in the mesangial cell (44, 59). In particular, apoptotic agents have been shown to increase group II phospholipase A2 (44). In order to determine the biologic relevance of the PLIP- or Tip60-cPLA2 interaction, it was thus first necessary to identify the cPLA2-specific contribution to serum deprivation-induced mesangial cell apoptosis. By selectively expressing PLIP in cells derived from cPLA2+/+ and cPLA2−/− mice, we were able to identify an effect specific to PLIP and cPLA2. Although the murine mesangial cells are more resistant to the apoptotic effects of serum deprivation than rat mesangial cells, apoptosis is potentiated in serum-deprived cells expressing both PLIP and cPLA2 compared to cells expressing either cPLA2 or PLIP alone. Cells expressing either PLIP or cPLA2 alone show no increase in apoptosis compared to cells expressing neither protein, indicating that the proteins have a synergistic effect on susceptibility to apoptosis. The synergistic effect of the expression of both proteins supports the biologic relevance of the PLIP-cPLA2 interaction.
The effect of PLIP should be placed in the context of other data which implicate cPLA2 in apoptosis secondary to TNF-α (30, 74, 76, 79) but not Fas (4, 23). Hayakawa isolated TNF-α-resistant derivatives of L929 cells and demonstrated that these cells had a marked decrease in TNF-α-induced arachidonic acid release and a decrease in cPLA2 expression. Expression of murine cPLA2 restored both TNF-α-induced arachidonic acid release and cytotoxicity (30). In a wide variety of human melanoma-derived cell lines, normal epidermal melanocytes, and murine cell lines, cell susceptibility to apoptosis induced by TNF-α in the presence of inhibitors of transcription and translation directly correlates with cPLA2 expression (74) and activity (79). Inhibitors of caspases inhibit TNF-α-induced arachidonic acid release and cytotoxity (76), suggesting that TNF-α-induced cPLA2 activity may require caspase activity.
cPLA2, although not previously implicated in serum deprivation-associated apoptosis, may influence apoptosis via its role in sphingomyelin signaling (38). cPLA2 is necessary for the generation of ceramide, a sphingomyelinase product (57). High levels of ceramide have been demonstrated in Molt-4 leukemia cells in which cycle arrest and apoptosis have been induced by serum withdrawal. The administration of exogenous cell-permeable ceramide to these cells results in cell cycle arrest and apoptosis comparable to that seen after serum withdrawal (40).
TNF-α-induced ceramide accumulation has been demonstrated in cells susceptible to TNF-α-induced apoptosis. An increase in arachidonic acid release precedes the TNF-α-induced increase in ceramide in HL-60 cells, and the administration of arachidonic acid activates sphingomyelinase in these cells (35a). TNF-α-induced arachidonic acid release and ceramide generation is decreased in a cPLA2-deficient derivative of a murine fibroblast cell line, suggesting a cPLA2-specific role in sphingomyelinase activation (38).
PLIP expression markedly increases serum deprivation-induced PGE2 generation in cPLA2+/+ cells but has no effect in cPLA2−/− cells. Changes in renal eicosanoid synthesis may contribute to the matrix production and cell proliferation seen in glomerular diseases such as diabetes (21, 50, 61). In contrast to the observed effect on apoptosis, cPLA2 alone but not PLIP alone also increases PGE2 production. PGE2 has been shown to have both anti- (22, 58) and pro- (17, 49, 60) apoptotic effects. The experimental model does not permit us to determine whether PGE2 contributes to or protects against serum deprivation-induced apoptosis.
In summary, we have found that Tip60 and a novel splice variant, PLIP, interact with cPLA2. PLIP potentiates apoptosis and prostaglandin production in renal mesangial cells, two processes critical to the role of mesangial cells in physiologic and pathophysiologic states. Recognition of this interaction may lead to therapeutic approaches which target the PLIP/Tip60-cPLA2 protein complex.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DK02356, DK 39773, DK 38452, NS 10828, and DK 54741 and American Heart Association grant-in-aid 9950460N.
We thank R. Brent, E. Golemis, J. Gyuris, and S. Hanes for the vectors used in the two-hybrid interaction trap; C. Sardet for the fibroblast Go library and for invaluable advice on the two-hybrid system; R. Finley for the anti-LexA antibody; J. Settleman for pRc/CMV-GAP plasmid; A. Cybulsky for the anti-cPLA2 antibody; and J. Clark for the pMT2-cPLA2 plasmid. We also thank D. A. Dichek (Gladstone Institute for Cardiovascular Disease) for the pADRSV4-LacZ construct, James Kamine for Tip60 cDNA, and S. Breton for assistance with immunofluorescence microscopy.
ADDENDUM IN PROOF
While the manuscript was in preparation Tip60(β), which is identical to PLIP, was isolated by the laboratory of Pereira-Smith (62).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angel J, Berenbaum F, Le Denmat C, Nevalainen T, Masliah J, Fournier C. Interleukin-1-induced prostaglandin E2 biosynthesis in human synovial cells involves the activation of cytosolic phospholipase A2 and cyclooxygenase-2. Eur J Biochem. 1994;226:125–131. doi: 10.1111/j.1432-1033.1994.tb20033.x. [DOI] [PubMed] [Google Scholar]
- 3.Atluru D, Gudapaty S, O'Donnell M P, Woloschak G E. Inhibition of human mononuclear cell proliferation, interleukin synthesis, mRNA for IL-2, IL-6, and leukotriene B4 synthesis by a lipoxygenase inhibitor. J Leukoc Biol. 1993;54:269–274. doi: 10.1002/jlb.54.4.269. [DOI] [PubMed] [Google Scholar]
- 4.Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley and Sons, Inc.; 1995. [Google Scholar]
- 6.Baker A J, Mooney A, Hughes J, Lombardi D, Johnson R J, Savill J. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Investig. 1994;94:2105–2116. doi: 10.1172/JCI117565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Menahem D, Shraga-Levine Z, Limor R, Naor Z. Arachidonic acid and lipoxygenase products stimulate gonadotropin alpha-subunit mRNA levels in pituitary alpha T3–1 cell line: role in gonadotropin releasing hormone action. Biochemistry. 1994;43:12795–12799. doi: 10.1021/bi00209a010. [DOI] [PubMed] [Google Scholar]
- 8.Benoit H, Jordan M, Wagner H, Wagner P D. Effect of NO, vasodilator prostaglandins, and adenosine on skeletal muscle angiogenic growth factor gene expression. J Appl Physiol. 1999;86:1513–1518. doi: 10.1152/jappl.1999.86.5.1513. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre J V. PLA2 and signal transduction. J Am Soc Nephrol. 1992;3:128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre J V, Gronich J H, Nemenoff R A. Epidermal growth factor enhances glomerular mesangial cell soluble phospholipase A2 activity. J Biol Chem. 1990;265:4934–4938. [PubMed] [Google Scholar]
- 11.Bonventre J V, Huang Z, Taheri M R, O'Leary E, Li E, Moskowitz M A, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 12.Bonventre J V, Skorecki K L, Kreisberg J I, Cheung J Y. Vasopressin increases cytosolic free calcium concentration in glomerular mesangial cells. Am J Physiol. 1986;251:F94–F102. doi: 10.1152/ajprenal.1986.251.1.F94. [DOI] [PubMed] [Google Scholar]
- 13.Bonventre J V, Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Investig. 1988;82:168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonventre J V, Weber P C, Gronich J H. PAF and PDGF increase cytosolic [Ca2+] and phospholipase activity in mesangial cells. Am J Physiol. 1988;254:F87–F94. doi: 10.1152/ajprenal.1988.254.1.F87. [DOI] [PubMed] [Google Scholar]
- 15.Borrow J, Stanton V P, Andresen J M, Becher R, Behm F G, Chaganti R S, Civin C I, Disteche C, Dube I, Frischauf A M, Horsman D, Mitelman F, Volinia S, Watmore A E, Housman D E. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 16.Brady M E, Ozanne D M, Gaughan L, Waite I, Cook S, Neal D E, Robson C N. Tip60 is a nuclear hormone receptor coactivator. J Biol Chem. 1999;274:17599–17604. doi: 10.1074/jbc.274.25.17599. [DOI] [PubMed] [Google Scholar]
- 17.Brown D M, Phipps R P. Bcl-2 expression inhibits prostaglandin E2-mediated apoptosis in B cell lymphomas. J Immunol. 1996;157:1359–1370. [PubMed] [Google Scholar]
- 18.Clark J D, Lin L L, Kriz R W, Ramesha C S, Sultzman L A, Lin A Y, Milona N, Knopf J L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 19.Clark-Lewis I, Sanghera J S, Pelech S L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991;266:15180–15184. [PubMed] [Google Scholar]
- 20.Dechend R, Hirano F, Lehmann K, Heissmeyer V, Ansieau S, Wulczyn F G, Scheidereit C, Leutz A. The Bcl-3 oncoprotein acts as a bridging factor between NF-kappaB/Rel and nuclear co-regulators. Oncogene. 1999;18:3316–3323. doi: 10.1038/sj.onc.1202717. [DOI] [PubMed] [Google Scholar]
- 21.DeRubertis F R, Craven P A. Eicosanoids in the pathogenesis of the functional and structural alterations of the kidney in diabetes. Am J Kidney Dis. 1993;22:727–735. doi: 10.1016/s0272-6386(12)80439-2. [DOI] [PubMed] [Google Scholar]
- 22.Di Popolo A, Memoli A, Apicella A, Tuccillo C, di Palma A, Ricchi P, Acquaviva A M, Zarrilli R. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene. 2000;19:5517–5524. doi: 10.1038/sj.onc.1203952. [DOI] [PubMed] [Google Scholar]
- 23.Enari M, Hug H, Hayakawa M, Ito F, Nishimura Y, Nagata S. Different apoptotic pathways mediated by Fas and the tumor-necrosis-factor receptor. Cytosolic phospholipase A2 is not involved in Fas-mediated apoptosis. Eur J Biochem. 1996;236:533–538. doi: 10.1111/j.1432-1033.1996.t01-1-00533.x. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima M, Kato T, Narumiya S, Mizushima Y, Sasaki H, Terashima Y, Nishiyama Y, Santoro M G. Prostaglandin A and J: antitumor and antiviral prostaglandins. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:415–418. [PubMed] [Google Scholar]
- 25.Gietz D, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover S, de Carvalho M S, Bayburt T, Jonas M, Chi E, Leslie C C, Gelb M H. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 27.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law P Y, Hebbel R P. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 28.Gyuris J, Golemis E, Chertkov H, Brent R. Cdil, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 29.Haq S, Choukroun G, Kang Z B, Ranu H, Matsui T, Rosenzweig A, Molkentin J D, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3 beta is a negative regulator of cardiac hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayakawa M, Ishida N, Takeuchi K, Shibamoto S, Hori T, Oku N, Ito F, Tsujimoto M. Arachidonic acid-selective cytosolic phospholipase A2 is crucial in the cytotoxic action of tumor necrosis factor. J Biol Chem. 1993;268:11290–11295. [PubMed] [Google Scholar]
- 31.Hayakawa M, Jayadev S, Tsujimoto M, Hannun Y A, Ito F. Role of ceramide in the stimulation of the transcription of cytosolic phospholipase A2 and cycloxygenase 2. Biochem Biophys Res Commun. 1996;220:681–686. doi: 10.1006/bbrc.1996.0464. [DOI] [PubMed] [Google Scholar]
- 32.Higashi S, Ohishi H, Kudo I. Augmented prostaglandin E2 generation resulting from increased activities of cytosolic and secretory phospholipase A2 and induction of cyclooxygenase-2 in interleukin-1 beta-stimulated rat calvarial cells during the mineralizing phase. Inflamm Res. 2000;49:102–111. doi: 10.1007/s000110050566. [DOI] [PubMed] [Google Scholar]
- 33.Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi J C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16:2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 35.Ikura T, Ogryzko V V, Grigoriev M, Groishman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. Involvement of the Tip60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 35a.Jayadev S, Linardic C M, Hannun Y A. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 36.Jayadev S, Hannun Y A. Ceramide: role in growth inhibitory cascades. J Lipid Mediat Cell Signal. 1996;14:295–301. doi: 10.1016/0929-7855(96)00538-x. [DOI] [PubMed] [Google Scholar]
- 37.Jayadev S, Barret J C, Murphy E. Elevated ceramide is downstream of altered calcium homeostasis in low serum-induced apoptosis. Am J Physiol Cell Physiol. 2000;279:C1640–C1647. doi: 10.1152/ajpcell.2000.279.5.C1640. [DOI] [PubMed] [Google Scholar]
- 38.Jayadev S, Hayter H L, Andrieu N, Gamard C J, Liu B, Balu R, Hayakawa M, Ito F, Hannun Y A. Phospholipase A2 is necessary for tumor necrosis factor α-induced ceramide generation in L929 cells. J Biol Chem. 1997;272:17196–17203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- 39.Jayadev S, Petranka J G, Cheran S K, Biermann J A, Barrett J C, Murphy E. Reduced capacitative calcium entry correlates with vesicle accumulation and apoptosis. J Biol Chem. 1999;274:8261–8268. doi: 10.1074/jbc.274.12.8261. [DOI] [PubMed] [Google Scholar]
- 40.Jayadev S, Liu B, Bielawska A E, Lee J Y, Nazaire F, Pushkareva M Y, Obeid L M, Hannun Y A. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 41.Kamine J, Elangovan B, Subramanian T, Coleman D, Chinnadurai G. Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-Tat transactivator. Virology. 1996;216:357–366. doi: 10.1006/viro.1996.0071. [DOI] [PubMed] [Google Scholar]
- 42.Kang J X, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S S, Chen Y M, O'Leary E, Witzgall R, Bonventre J V. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of the zinc finger proteins. Proc Natl Acad Sci USA. 1996;93:15299–15304. doi: 10.1073/pnas.93.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konieczkowski M, Sedor J R. Cell-specific regulation of type II phospholipase A2 expression in rat mesangial cells. J Clin Investig. 1993;92:2524–2532. doi: 10.1172/JCI116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 46.Kuwata H, Nakatani Y, Murakami M, Kudo I. Cytosolic phospholipase A2 is required for cytokine-induced expression of type IIA secretory phospholipase A2 that mediates optimal cyclooxygenase-2-dependent delayed prostaglandin E2 generation in rat 3YI fibroblasts. J Biol Chem. 1998;273:1733–1740. doi: 10.1074/jbc.273.3.1733. [DOI] [PubMed] [Google Scholar]
- 47.Leslie C C. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- 48.Lin L L, Lin A Y, Knopf J L. Cytosolic PLA2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci USA. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo H H, Teichmann P, Furstenberger G, Gimenez-Conti I, Fischer S M. Suppression or elevation of cytosolic phospholipase A2 alters keratinocyte prostaglandin synthesis, growth and apoptosis. Cancer Res. 1998;58:4624–4637. [PubMed] [Google Scholar]
- 50.Mahadevan P, Larkins R G, Fraser J R, Dunlop M E. Effect of prostaglandin E2 and hyaluronan on mesangial cell proliferation. A potential contribution to glomerular hypercellularity in diabetes. Diabetes. 1996;45:44–50. doi: 10.2337/diab.45.1.44. [DOI] [PubMed] [Google Scholar]
- 51.Malis C D, Weber P C, Leaf A, Bonventre J V. Incorporation of marine lipids into mitochondrial membranes increases susceptibility to damage by calcium and reactive oxygen species: evidence for enhanced activation of phospholipase A2 in mitochondria enriched with n-3 fatty acids. Proc Natl Acad Sci USA. 1990;87:8845–8849. doi: 10.1073/pnas.87.22.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nalefski E A, Sultzman L A, Martin D M, Kriz R W, Towler P S, Knopf J L, Clark J D. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid-binding domain and a Ca2+-independent catalytic domain. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 53.Naraba H, Murakami M, Matsumoto H, Shimbara S, Ueno A, Kudo I, Ohishi S. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J Immunol. 1998;160:2974–2982. [PubMed] [Google Scholar]
- 54.Nemenoff R A, Winitz S, Qian N X, Van Putten V V, Johnson G L, Heasley L E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 55.Nigg E A. The substrates of the cdc2 kinase. Semin Cell Biol. 1991;2:261–270. [PubMed] [Google Scholar]
- 56.Nigg E A. Targets of cyclin-dependent protein kinases. Curr Opin Cell Biol. 1993;5:187–193. doi: 10.1016/0955-0674(93)90101-u. [DOI] [PubMed] [Google Scholar]
- 57.Obeid L M, Hannun Y A. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995;58:191–198. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- 58.Oshima M, Dinchuk J E, Kargman S L, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 59.Pfeilschifter J, Huwiler A. Phospholipase A2 in mesangial cells: control mechanisms and functional importance. Exp Nephrol. 1997;5:189–193. [PubMed] [Google Scholar]
- 60.Pica F, Franzese O, D'Onofrio D, Bonmassar E, Favalli C, Garaci E. Prostaglandin E2 induces apoptosis in resting immature and mature human lymphocytes: a c-Myc-dependent and Bcl-2-independent associated pathway. J Pharmacol Exp Ther. 1996;277:1793–1800. [PubMed] [Google Scholar]
- 61.Pricci F, Pugliese G, Mene P, Romeo G, Romano G, Galli G, Casini A, Rotella C M, DiMario U, Pugliesi F. Regulatory role of eicosanoids in extracellular matrix overproduction induced by long-term exposure to high glucose in cultured rat mesangial cells. Diabetologia. 1996;39:1055–1062. doi: 10.1007/BF00400654. [DOI] [PubMed] [Google Scholar]
- 62.Ran Q, Pereira-Smith O M. Identification of an alternatively spliced form of the Tat interactive protein (Tip60), Tip60 (β) Gene. 2000;258:141–146. doi: 10.1016/s0378-1119(00)00410-8. [DOI] [PubMed] [Google Scholar]
- 63.Reifsnyder C, Lowell J, Clarke A, Pillus L. Yeast SAS silencing genes and human genes associated with AML and HIV-1 Tat interactions are homologous with acetyltransferases. Nat Genet. 1996;14:42–49. doi: 10.1038/ng0996-42. [DOI] [PubMed] [Google Scholar]
- 64.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 65.Sapirstein A, Spech R A, Witzgall R, Bonventre J V. Cytosolic phospholipase A2 (PLA2), but not secretory PLA2, potentiates hydrogen peroxide cytotoxicity in kidney epithelial cells. J Biol Chem. 1996;271:21505–21513. doi: 10.1074/jbc.271.35.21505. [DOI] [PubMed] [Google Scholar]
- 66.Schievella A R, Regier M K, Smith W L, Lin L L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 67.Sierra-Honigmann M R, Bradley J R, Pober J S. “Cytosolic” phospholipase A2 is in the nucleus of subconfluent endothelial cells but confined to the cytoplasm of confluent endothelial cells and redistributes to the nuclear envelope and cell junctions upon histamine stimulation. Lab Investig. 1996;74:684–695. [PubMed] [Google Scholar]
- 68.Smith E R, Eisen A, Gu W, Sattah M, Pannuti A, Zhou J, Cook R G, Lucchesi J C, Allis C D. ESA1 is a histone acetyltransferase that is essential for growth in yeast. Proc Natl Acad Sci USA. 1998;95:3561–3565. doi: 10.1073/pnas.95.7.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Songyang Z, Blechner S, Hoagland N, Hoekstra M F, Piwnica-Worms H, Cantley L C. Use of an oriented peptide library to determine the optimal substrate of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 70.Spencer A G, Woods J W, Arakawa T, Singer I I, Smith W L. Subcellular localization of prostaglandin endoperoxide H synthase-1 and -2 by immunoelectron microscopy. J Biol Chem. 1998;16:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- 71.Sterner D E, Berger S L. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugiyama H, Kashihara N, Maeshima Y, Okamoto K, Kanao K, Sekikawa T, Makino H. Regulation of survival and death of mesangial cells by extracellular matrix. Kidney Int. 1998;54:1188–1196. doi: 10.1046/j.1523-1755.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 73.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata N, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 74.Voelkel-Johnson C, Thorne T E, Laster S M. Susceptibility to TNF in the presence of inhibitors of transcription or translation is dependent on the activity of cytosolic phospholipase A2 in human melanoma tumor cells. J Immunol. 1996;156:201–207. [PubMed] [Google Scholar]
- 75.Waterman W H, Sha'afi R I. A mitogen-activated protein kinase independent pathway involved in the phosphorylation and activation of cytosolic phospholipase A2 in human neutrophils stimulated with tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1995;209:271–278. doi: 10.1006/bbrc.1995.1499. [DOI] [PubMed] [Google Scholar]
- 76.Wissing D, Mouritzen H, Egeblad M, Poirier G G, Jaatela M. Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:5073–5077. doi: 10.1073/pnas.94.10.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woods J W, Coffey M J, Brock T G, Singer I I, Peters-Golden M. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J Clin Investig. 1995;95:2035–2046. doi: 10.1172/JCI117889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woods J W, Evans J F, Ethier D, Scott S, Vickers P J, Hearn L, Heibein J A, Charleson S, Singer I I. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y L, Jiang X R, Newland A C, Kelsey S M. Failure to activate cytosolic phospholipase A2 causes TNF resistance in human leukemic cells. J Immunol. 1998;160:5929–5935. [PubMed] [Google Scholar]
- 80.Yamamoto T, Horikoshi M. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J Biol Chem. 1997;272:30595–30598. doi: 10.1074/jbc.272.49.30595. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Y X, Sun H B, Tsang M L, McMahel J, Grigsby S, Yin T, Yang Y C. Critical cytoplasmic domains of human interleukin-9 receptor alpha chain in interleukin-9-mediated cell proliferation and signal transduction. J Biol Chem. 1997;272:21334–21340. doi: 10.1074/jbc.272.34.21334. [DOI] [PubMed] [Google Scholar]