Abstract
Background: Vitamin D deficiency is common in the general population worldwide, and the prevalence and severity of vitamin D deficiency increase in critically ill patients. The prevalence of vitamin D deficiency in a community-based cohort in Northern Taiwan was 22.4%. This multicenter cohort study investigated the prevalence of vitamin D deficiency and associated factors in critically ill patients in Northern Taiwan.
Methods: Critically ill patients were enrolled and divided into five groups according to their length of stay at intensive care units (ICUs) during enrolment as follows: group 1, <2 days with expected short ICU stay; group 2, <2 days with expected long ICU stay; group 3, 3-7 days; group 4, 8-14 days; and group 5, 15-28 days. Vitamin D deficiency was defined as a serum 25-hydroxyvitamin D (25(OH)D) level < 20 ng/ml, and severe vitamin D deficiency was defined as a 25(OH)D level < 12 ng/ml. The primary analysis was the prevalence of vitamin D deficiency. The exploratory analyses were serial follow-up vitamin D levels in group 2, associated factors for vitamin D deficiency, and the effect of vitamin D deficiency on clinical outcomes in critically ill patients.
Results: The prevalence of vitamin D deficiency was 59% [95% confidence interval (CI) 55-62%], and the prevalence of severe vitamin D deficiency was 18% (95% CI 15-21%). The median vitamin D level for all enrolled critically ill patients was 18.3 (13.7-23.9) ng/ml. In group 2, the median vitamin D levels were <20 ng/ml during the serial follow-up. According to the multivariable analysis, young age, female gender, low albumin level, high parathyroid hormone (PTH) level, and high sequential organ failure assessment (SOFA) score were significantly associated risk factors for vitamin D deficiency. Patients with vitamin D deficiency had longer ventilator use duration and length of ICU stay. However, the 28- and 90-day mortality rate were not associated with vitamin D deficiency.
Conclusions: This study demonstrated that the prevalence of vitamin D deficiency is high in critically ill patients. Age, gender, albumin level, PTH level, and SOFA score were significantly associated with vitamin D deficiency in these patients.
Keywords: vitamin D, critical care, deficiency, severity, mortality
Introduction
Vitamin D is a pleiotropic hormone that regulates several crucial physiological functions (1, 2). Vitamin D deficiency is related to multiple health issues, including cardiovascular disease, cancer, metabolic syndrome and infection [1]. In the general population, the prevalence of vitamin D deficiency exists ranges from 20 to 80% (3–5), and in critical ill patients, the prevalence and severity of vitamin D deficiency increase. In addition to baseline vitamin D deficiency, decreased intake and absorption, increased losses and decreased production of vitamin D (6), enhanced conversion of 25-hydroxyvitamin D (25(OH)D) to the active 1,25-dihydroxyvitamin D3 (1,25(OH)2D) (7), and acute fluid shifts (8) may result in severely low vitamin D levels. Several observational studies have shown that vitamin D deficiency is associated with sepsis severity, organ dysfunction, and even mortality (9–15). However, numerous studies have reported that the effect of vitamin D deficiency on mortality is uncertain (16, 17).
In a community-based cohort in Northern Taiwan, the mean serum concentration of 25(OH)D was 28.9 ng/ml, and the prevalence of vitamin D deficiency (25(OH)D level < 20 ng/L) was 22.4% (18). Moreover, vitamin D deficiency might be related to area specific health issues and patients' characteristics (16, 19–22). Because of the worldwide concern of a high prevalence vitamin D deficiency and its effect on clinical outcomes in critically ill patients (23, 24), we conducted this multicenter observational study in Northern Taiwan to investigate the prevalence of vitamin D deficiency in critical ill patients and the associated factors of vitamin D deficiency. In addition, we perform a series of examinations to determine vitamin D levels in specific critically ill patients to follow up the change in their vitamin D levels in intensive care units (ICUs).
Methods
Study Design and Patient Enrolment
This multicenter, prospective, observational cohort study was approved by the Research Ethics Committee of National Taiwan University Hospital (approval number: 201805087RINB) and registered on the ClinicalTrials.gov protocol registration system (ID: NCT03639584). It was conducted at four hospitals in Northern Taiwan (north latitude 25:01– 25:03) between August 2018 and July 2020. Patients admitted to the ICUs were eligible for enrollment. Patients were excluded if they met the following exclusion criteria: aged <20 years, lower body mass index (< 18 kg/m2), severe anemia (hemoglobin level < 7 g/dl), received additional vitamin D supplement (>3,000 IU/day) within 4 weeks, previous admission to ICU within 3 months, hyperparathyroidism, rickets, liver cirrhosis (Child-Pugh C). Written informed consent was obtained from critically ill patients or patients' legally authorized representatives before enrolment in the study. In the four participating hospitals, the usual supplementing vitamin D was only from daily regular nutrition, mostly ranged from 800 to 2,000 IU. There was no routine high-dose vitamin D therapy in the 4 hospitals.
Patient Grouping
For cross-sectional analysis of vitamin D deficiency, the enrolled critically ill patients were allocated to the following five groups according to their length of ICU stay at enrolment: group 1, <2 days and expected further ICU stay <5 days; group 2, <2 days and expected further ICU stay >5 days; group 3, ranging from 3 to 7 days; group 4, ranging from 8 to 14 days; and group 5, ranging from 15 to 28 days. Blood samples were obtained at enrolment, and serum 25(OH)D, parathyroid hormone (PTH), and cortisol levels were examined. In patients with sepsis, the procalcitonin level was examined. Subsequent time points (e.g., length of ICU-stay, 28-day mortality) were defined from the time on or after the day of enrolment.
For longitudinal follow-up of the vitamin D levels during the ICU stay since ICU admission, serial blood samples were obtained from patients in group 2 on day 7, 14, 21, and 28, and 25(OH)D levels were examined. If the patients died or were discharged from ICUs, further blood samples were not obtained. Patient characteristics, admission season, hemodynamic data, and laboratory data were recorded, and acute physiology and chronic health evaluation II (APACHE II) (25), Charlson et al. (26), and sequential organ failure assessment (SOFA) scores were calculated (27). The clinical outcomes were followed up for 90 days after patient enrolment, including duration of ventilator use, ICU-stay, and hospital stay, ICU-free days to day 28, and survival status.
Examination and Category of Vitamin D Level
Blood serum samples were stored at −80°C. The serum 25(OH)D level was measured with the commercially available TOTAL Liaison chemiluminescence assay (Liaison, Diasorin S.p.A., Saluggia, Italy) (28). Accordingly, patients were classified into to the following four categories: sufficiency (≥30 ng/ml), insufficiency (<30 ng/ml), deficiency (<20 ng/ml), moderate deficiency (12-19.9 ng/ml), and severe deficiency (<12 ng/ml) (29).
Primary Analysis and Exploratory Analyses
The primary analysis was the prevalence of vitamin D deficiency. The exploratory analyses included associated factors for vitamin D deficiency, serial vitamin D levels in group 2, and the effect of vitamin D deficiency on clinical outcomes of critically ill patients. Moreover, patients were divided into four groups according to their seasons at enrolment, and the median vitamin D levels were compared among the four seasons.
Statistical Analysis
All statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY, USA). Normally distributed numerical data were compared using one-way analysis of variance (ANOVA) and post hoc Tukey tests and are expressed as means (standard deviation). Non-normal distributed numerical data were compared using Kruskal-Wallis test with post hoc Mann-Whitney U-tests and are expressed as medians (interquartile range). Categorical variables were compared using chi-square tests or Fisher's exact tests as appropriate and are presented as percentages. Logistic regression models were used to estimate the relationship between the associated factors and vitamin D deficiency and the relationship between the vitamin D level and 28-day mortality rate. Backwards selection of variables was performed in the multivariable logistic regression. A P-value of <0.05 indicated a significant difference.
Results
Patient Enrolment and Characteristics
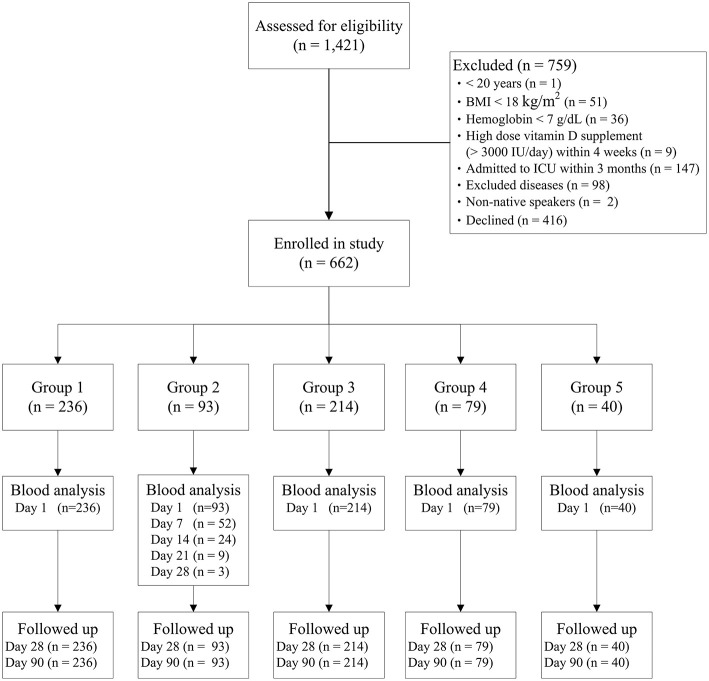
In total, 1,421 patients were assessed for enrolment eligibility, and 759 patients were excluded (Figure 1). Finally, 662 patients were enrolled and allocated to the five groups according to their length of ICU stay on the enrolment day, and all enrolled patients had completed their 90-day follow up. Characteristics of patients in the five groups are presented in Table 1. Of the patients enrolled, 50% were admitted to ICUs for postoperative care, and 18% had sepsis at enrolment. Furthermore, 55% of the enrolled patients were intubated and received mechanical ventilation at enrolment.
Figure 1.
Flow chart of patient enrolment and grouping. BMI, body mass index; ICU, intensive care unit.
Table 1.
Patient characteristics and clinical outcomes.
| Total | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| Number | 662 | 237 | 93 | 211 | 82 | 39 |
| Age (years) | 67 (56-78) | 68 (55-78) | 64 (54-74) | 68 (58-79) | 66 (57-80) | 70 (59-78) |
| Gender (female/male) | 257/405 | 106/131 | 29/64 | 76/135 | 32/50 | 14/25 |
| Height (cm) | 162 (155-169) | 162 (155-168) | 165 (158-171) | 163 (156-168) | 164 (155-170) | 161 (155-168) |
| Weight (kg) | 65 (56.1-74.8) | 64.7 (55-73.1) | 65 (56.8-77.8) | 65.6 (57.5-74.5) | 64.5 (55.4-77.1) | 64 (55.3-79) |
| BMI (kg/m2) | 24 (21.5-27.2) | 23.8 (21.6-27.2) | 24.1 (21-28.4) | 24.2 (21.7-26.6) | 23.8 (21.5-27.4) | 24.2 (22.5-28.9) |
| APACHE II | 15 (11-19) | 14 (10-19) | 21 (15-26) | 20 (16-25) | 19 (15-24) | 20 (15-24) |
| SOFA score | 5 (3-7) | 3 (2-5) | 7 (4-10) | 5 (3-8) | 5 (3-8) | 5 (4-9) |
| Charlson score | 2 (1-4) | 2 (1-4) | 2 (0-4) | 3 (1-4) | 2 (0-4) | 2 (1-5) |
| ICU admission | ||||||
| Sepsis | 117 (18%) | 16 (7%) | 26 (28%) | 39 (19%) | 24 (29%) | 12 (31%) |
| Postoperative | 330 (50%) | 150 (63%) | 34 (37%) | 98 (46%) | 35 (43%) | 13 (33%) |
| Respiratory | 54 (8%) | 14 (6%) | 10 (11%) | 23 (11%) | 3 (4%) | 4 (10%) |
| Cardiovascular | 33 (5%) | 16 (7%) | 1 (1%) | 11 (5%) | 3 (4%) | 2 (5%) |
| Neurologic | 63 (10%) | 15 (6%) | 10 (11%) | 25 (12%) | 10 (12%) | 3 (8%) |
| Others | 65 (10%) | 26 (11%) | 12 (13%) | 15 (7%) | 7 (9%) | 5 (13%) |
| Comorbidities | ||||||
| Hypertension | 90 (14%) | 24 (10%) | 18 (19%) | 28 (13%) | 16 (20%) | 4 (10%) |
| Diabetes | 202 (31%) | 64 (27%) | 25 (27%) | 78 (37%) | 21 (26%) | 14 (36%) |
| Liver diseases | 108 (16%) | 40 (17%) | 14 (15%) | 40 (19%) | 11 (13%) | 3 (8%) |
| Renal diseases | 100 (15%) | 26 (11%) | 17 (18%) | 31 (15%) | 17 (21%) | 9 (23%) |
| Hemodialysis | 35 (5%) | 11 (5%) | 6 (7%) | 10 (5%) | 5 (6%) | 3 (8%) |
| Metastasis | 48 (7%) | 24 (10%) | 4 (4%) | 15 (7%) | 2 (2%) | 3 (8%) |
| PTH (pg/ml) | 30 (19-49) | 29 (19-42) | 37 (24-65) | 30 (18-48) | 29 (18-59) | 28 (17-44) |
| Vitamin D (ng/ml) | 18.3 (13.7-23.9) | 19.9 (14.8-26.1) | 18.1 (13-23.1) | 17.5 (12.4-22.7) | 17.5 (14.2-24.9) | 18.1(13.5-22.9) |
| >30 | 66 (10%) | 35 (15%) | 6 (7%) | 18 (8%) | 5 (6%) | 2 (5%) |
| 20.1-30 | 208 (31%) | 80 (34%) | 32 (34%) | 58 (28%) | 26 (32%) | 12 (31%) |
| 12.1-20 | 268 (41%) | 92 (39%) | 35 (38%) | 88 (42%) | 35 (43%) | 18 (46%) |
| ≤ 12 | 120 (18%) | 30 (12%) | 20 (21%) | 47 (22%) | 16 (19%) | 7 (18%) |
| After enrolment | ||||||
| ICU stay (days) | 8 (4-8) | 3 (2-4) | 9 (6-18) | 10 (6-19) | 20 (14-26) | 28 (21-39) |
| Hospital stay (days) | 28 (17-47) | 18 (11-29) | 30 (17-48) | 34 (21-52) | 43 (27-60) | 53 (36-75) |
| 28-day ICU free days | 25 (18-27) | 26 (25-27) | 21 (15-25) | 24 (16-27) | 23 (14-26) | 18 (5-26) |
| 28-day mortality | 59 (9%) | 9 (4%) | 14 (15%) | 21 (10%) | 12 (15%) | 3 (8%) |
| 90-day mortality | 105 (16%) | 17 (7%) | 21 (23%) | 38 (18%) | 21 (26%) | 10 (26%) |
Data are presented as number, number (%), or median (interquartile range).
APACHE, acute physiology and chronic health evaluation; BMI, body mass index; ICU, intensive care unit; PTH, parathyroid hormone; SOFA, sequential organ failure assessment.
Prevalence and Characteristics of Patients With Vitamin D Deficiency
The prevalence of vitamin D deficiency and clinical outcomes of the five groups are shown in Table 1. The overall prevalence of vitamin D deficiency was 59% [95% confidence interval (CI) 55-62%], and the prevalence of severe vitamin D deficiency was 18% (95% CI 15-21%). The median vitamin D level for all enrolled critically ill patients was 18.3 (13.7-23.9) ng/ml. The proportion of sepsis is higher in the patients with severe deficiency vitamin D than in those with sufficiency of vitamin D (30 vs. 15%, P = 0.032). The median vitamin D levels differed significantly among patients enrolled during different seasons (Spring 17.6 [13-23.6]; Summer 17.5 [12.4-22.4]; Fall 19.2 [14.8-23.6]; and Winter 19.1 [14.8-27] ng/ml; P = 0.028). More patients with sepsis were admitted in Spring than in Fall (25 vs. 11%, P = 0.001), and more patients with neurologic diseases were admitted in Winter than in Summer (17 vs. 6%, P < 0.001).
Moreover, patients were allocated to four categories according to their vitamin D levels at enrolment, and patient characteristics and clinical outcomes are shown in Table 2. The procalcitonin levels in patients with sepsis did not differ significantly between the four groups (sufficiency, 3.9 [0.5–60.6]; insufficiency, 4.5 [0.4-26.1]; moderate deficiency, 4.7 [0.6-14.6]; and severe deficiency, 2.1 [0.5-11.2] ng/ml; P = 0.722). The 28- and 90-day mortality did not differ significantly among the four categories. Patients with vitamin D deficiency were associated with longer duration of ventilator use and longer length of ICU stay. Furthermore, ICU-free days to day 28 were more in the vitamin D sufficiency group than in the vitamin D insufficiency, moderate deficiency, and severe deficiency groups. Length of hospital stay did not differ significantly among the four categories.
Table 2.
Characteristics and clinical outcomes of patients in different categories of vitamin D level.
| Vitamin D level | Sufficiency | Insufficiency | Moderate deficiency | Severe Deficiency | |
|---|---|---|---|---|---|
| (ng/ml) | >30 | 20.1-30 | 12.1-20 | ≤12 | P |
| Number | 66 | 208 | 268 | 120 | |
| Age (years) | 73 (65-83) | 70 (60-79)* | 67 (55-78)* | 61 (50-72)* | <0.001 |
| ≤ 56 | 9 (14%) | 39 (19%) | 73 (27%) | 50 (42%) | <0.001 |
| >56, ≤ 67 | 10 (15%) | 44 (21%) | 60 (22%) | 32 (27%) | 0.330 |
| >67, ≤ 78 | 19 (29%) | 64 (31%) | 64 (24%) | 19 (16%) | 0.021 |
| >78 | 28 (42%) | 61 (29%) | 71 (27%) | 19 (16%) | 0.001 |
| BMI (kg/m2) | 24.3 (3.6) | 24.5 (4.6) | 25.2 (5.4) | 24.8 (5.3) | 0.458 |
| ICU admission | |||||
| Sepsis | 10 (15%) | 30 (14%) | 41 (15%) | 36 (30%) | 0.002 |
| Postoperative | 37 (56%) | 113 (54%) | 137 (51%) | 43 (36%) | 0.006 |
| Respiratory | 8 (12%) | 13 (6%) | 23 (9%) | 10 (8%) | 0.482 |
| Cardiovascular | 4 (6%) | 9 (4%) | 13 (5%) | 7 (6%) | 0.909 |
| Neurologic | 7 (11%) | 25 (12%) | 22 (8%) | 9 (8%) | 0.440 |
| Others | 4 (6%) | 22 (11%) | 38 (14%) | 18 (15%) | 0.203 |
| Seasons | |||||
| Spring | 21 (32%) | 45 (22%) | 68 (25%) | 39 (33%) | 0.117 |
| Summer | 11 (17%) | 46 (22%) | 70 (26%) | 35 (29%) | 0.201 |
| Fall | 10 (15%) | 71 (34%) | 72 (27%) | 22 (18%) | 0.002 |
| Winter | 22 (36%) | 46 (22%) | 58 (22%) | 24 (20%) | 0.054 |
| Hospitals | |||||
| Site 1 | 38 (58%) | 113 (54%) | 132 (49%) | 44 (37%) | 0.009 |
| Site 2 | 15 (23%) | 50 (24%) | 64 (24%) | 43 (36%) | 0.069 |
| Site 3 | 9 (14%) | 25 (12%) | 48 (18%) | 18 (15%) | 0.359 |
| Site 4 | 4 (6%) | 20 (10%) | 24 (9%) | 15 (13%) | 0.546 |
| APACHE II | 15 (10-19) | 15 (10-19) | 15 (11-20) | 16 (11-20) | 0.419 |
| SOFA score | 5 (2-6) | 4 (2-7) | 5 (3-8) | 5 (3-8) | 0.127 |
| MAP (mm Hg) | 84 (76-92) | 87 (78-96)# | 88 (78-98)# | 92 (82-99)* | 0.016 |
| Heart rate (breaths/min) | 87 (75-101) | 86 (74-100)# | 86 (75-100)# | 92 (82-102) | 0.045 |
| Creatinine (mg/dl) | 1.1 (0.8-1.8) | 0.9 (0.7-1.4) | 1 (0.6-1.7) | 1 (0.6-2.4) | 0.131 |
| White blood cells (k/μl) | 11.3 (7.9-14.2) | 10.6 (8.3-14.3) | 10.7 (8-15) | 10.4 (7.9-13.5) | 0.922 |
| Hematocrit (%) | 30.8 (28-34) | 30.8 (27.4-35.5) | 30.1 (27.4-34.4) | 29.6 (26.4-33.6) | 0.125 |
| Platelet (k/μl) | 202 (141-273) | 181 (130-253) | 178 (123-253) | 162 (110-227) | 0.075 |
| Lactate (mmol/L) | 1.6 (1-2.5) | 1.8 (1.2-6.6) | 2 (1.3-7.3)* | 2.2 (1.3-11.6) | 0.045 |
| Total bilirubin (mg/dl) | 0.8 (0.5-1.2) | 0.9 (0.6-1.3) | 0.8 (0.5-1.4) | 0.7 (0.5-1.8) | 0.940 |
| Albumin (g/dl) | 3.1 (2.8-3.5) | 3.1 (2.7-3.6)# | 3.1 (2.7-3.5)# | 2.9 (2.4-3.3)* | 0.007 |
| C-reactive protein (mg/L) | 3.8 (2.4-20.1) | 6.2 (1.6-15.8) | 7.3 (2.6-16.9) | 8.9 (3.4-17.1) | 0.308 |
| PTH (pg/ml) | 26 (17-42) | 25 (17-40) | 31 (21-55)* | 35 (25-65)* | <0.001 |
| Cortisol (μg/dl) | 21 (13-28) | 19 (12-27) | 18 (12-27) | 18 (13-29) | 0.877 |
| Total Calcium (mmol/L) | 2.1 (2-2.2) | 2.1 (2-2.2) | 2.1 (2-2.2) | 2.1 (2-2.2) | 0.021 |
| Duration of ventilator use (day) | 2 (1-9) | 5 (1-15) | 8 (2-17)* | 9 (3-26)* | 0.001 |
| ICU stay (days) | 5 (3-8) | 9 (3-19)* | 9 (4-18)* | 9 (4-19)* | 0.001 |
| 28-day ICU free days | 26 (24-27) | 24 (18-27)* | 25 (18-27)* | 24 (18-27)* | 0.011 |
| Hospital stay (days) | 27 (15-37) | 26 (17-47) | 28 (16-50) | 32 (17-49) | 0.444 |
| 28-day mortality | 5 (8%) | 17 (8%) | 28 (10%) | 9 (8%) | 0.714 |
| 90-day mortality | 10 (15%) | 32 (15%) | 42 (16%) | 23 (19%) | 0.727 |
Data are presented as number, number (%), mean (standard deviation), or median (interquartile range). P values are for one-way analysis of variance among the four categories. Duration of ventilator use in intubated patients are compared among the four categories.
compared with group sufficiency (>30) and
compared with group severe insufficiency (≤12) represent a significant difference between the two groups using post hoc analysis with Tukey test or Mann–Whitney U-test.
APACHE, acute physiology and chronic health evaluation; BMI, body mass index; MAP, mean arterial pressure; ICU, intensive care unit; PTH, parathyroid hormone; SOFA, sequential organ failure assessment.
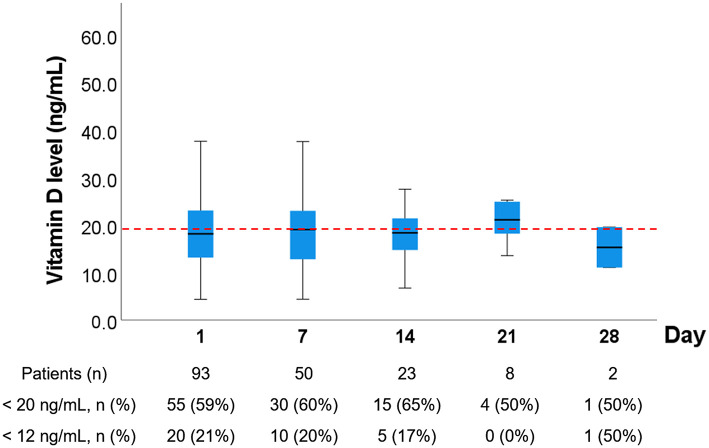
Serial Follow-Up of Vitamin D Levels
The vitamin D levels at day 1, 7, 14, 21, and 28 of group 2 are shown in Figure 2. The prevalence of vitamin D deficiency was 59% on day 1, 60% on day 7, 55% on day 14, 50% on day 21, and 100% on day 28. Five patients in group 2 receive high-dose vitamin D supplement. Three patients were discharged before day 7. One patient received high-dose vitamin D (560,000 IU) therapy within Day 7, and the vitamin D levels of this patient on day 1 and 7 was 12.4 and 78.6 ng/ml, respectively. This patient was discharger before day 14. One patient received high-dose vitamin D between day 7 and 14 and had a complete serial follow-up of vitamin D levels as follows: day 1, 13.1 ng/ml; day 7, 18.8 ng/ml; day 14, 92 ng/ml; day 21, 68.2 ng/ml, and day 28, 64.6 ng/ml.
Figure 2.
Serial follow-up of vitamin D levels in Group 2. Serial blood samples were obtained from patients in group 2 on day 7, 14, 21, and 28. If the patients died or were discharged from ICUs, further blood samples were not obtained. If the patients received high-dose vitamin D therapy, the subsequent vitamin D level of these patients were excluded One patient received high-dose vitamin D (560,000 IU) therapy within Day 7, and the vitamin D levels of this patient on day 1 and 7 was 12.4 and 78.6 ng/ml, respectively. This patient was discharger before day 14. Another patient received high-dose vitamin D (560,000 IU) therapy between Day 7 and 14, and the vitamin D levels of this patient on day 1, 7, 14, 21, and 28 were 13.1, 18.8, 92, 68.2, and 64.6 ng/ml, respectively.
Associated Factors for Vitamin D Deficiency and Mortality
The associated factors for vitamin D deficiency based on univariable and multivariable analyses are presented in Table 3. In the multivariable analysis, young age, female gender, low albumin level, high PTH level, and high SOFA scores were significantly associated risk factors for vitamin D deficiency. The associated factors for 28-day mortality based on univariable and multivariable analyses are presented in Table 4. Both univariable and multivariable analyses revealed no association between the vitamin D level and 28-day mortality. In the multivariable analysis, age, high heart rate, and high SOFA score were significantly associated risk factors for 28-day mortality.
Table 3.
Associated factors for vitamin D deficiency (<20 ng/ml).
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 0.979 | 0.969-0.989 | <0.001 | 0.969 | 0.958-0.980 | <0.001 |
| Female | 1.676 | 1.212-2.317 | 0.002 | 2.098 | 1.479-2.976 | <0.001 |
| Albumin (g/dl) | 0.692 | 0.530-0.903 | 0.007 | 0.646 | 0.487-0.857 | 0.002 |
| PTH (pg/ml) | 1.005 | 1.002-1.009 | 0.003 | 1.006 | 1.002-1.010 | 0.002 |
| SOFA score | 1.059 | 1.012-1.107 | 0.012 | 1.066 | 1.014-1.120 | 0.012 |
| APACHE II score | 1.022 | 0.996-1.048 | 0.104 | |||
| BMI (kg/m2) | 1.024 | 0.992-1.057 | 0.182 | |||
| Heart rate (bpm) | 1.009 | 0.999-1.018 | 0.071 | |||
| MAP (mmHg) | 1.009 | 0.998-1.020 | 0.118 | |||
| Platelet (k/μl) | 0.999 | 0.997-1.000 | 0.152 | |||
| Creatinine (mg/dl) | 1.080 | 0.993-1.174 | 0.072 | |||
| Sepsis | 1.448 | 0.954-2.200 | 0.082 | |||
| Post-operation | 0.715 | 0.524-0.976 | 0.034 | |||
Backwards selection of variables was performed in the multivariable logistic regression. APACHE, acute physiology and chronic health evaluation; BMI, body mass index; CI, confidence interval; MAP, mean arterial pressure; OR, odds ratio; PTH, Parathyroid Hormone; SOFA, sequential organ failure assessment.
Table 4.
Associated factors for 28-day mortality.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 1.039 | 1.019-1.060 | <0.001 | 1.043 | 1.020-1.066 | <0.001 |
| Heart rate (bpm) | 1.039 | 1.022-1.056 | <0.001 | 1.052 | 1.033-1.071 | <0.001 |
| SOFA score | 1.274 | 1.189-1.364 | <0.001 | 1.302 | 1.205-1.405 | <0.001 |
| Albumin (g/dl) | 0.493 | 0.316-0.769 | 0.002 | |||
| Vitamin D (ng/ml) | 0.990 | 0.959-1.023 | 0.551 | |||
| PTH (pg/mL) | 1.006 | 1.002-1.009 | 0.001 | |||
| APACHE II score | 1.051 | 1.008-1.097 | 0.020 | |||
| Charlson score | 1.140 | 1.037-1.253 | 0.006 | |||
| Total bilirubin (mg/dl) | 1.133 | 1.057-1.214 | <0.001 | |||
| Lactate (mmol/L) | 1.059 | 1.090-1.089 | <0.001 | |||
| C-reactive protein (mg/L) | 1.038 | 1.010-1.068 | 0.009 | |||
| Cortisol (μg/dl) | 1.018 | 1.004-1.032 | 0.013 | |||
| Creatinine (mg/dl) | 1.065 | 0.977-1.160 | 0.151 | |||
| MAP (mmHg) | 0.975 | 0.956-0.995 | 0.015 | |||
| Hematocrit (%) | 0.949 | 0.900-1.000 | 0.049 | |||
| Platelet (k/μl) | 0.995 | 0.992-0.999 | 0.004 | |||
Backwards selection of variables was performed in the multivariable logistic regression. APACHE, acute physiology and chronic health evaluation; CI, confidence interval; MAP, mean arterial pressure; OR, odds ratio; PTH, parathyroid Hormone; SOFA, sequential organ failure assessment.
Discussion
This study shows that the prevalence of vitamin D deficiency is high (59%) in critically ill patients, and only 10% of critically ill patients had a vitamin D level > 30 ng/ml. Furthermore, we found that vitamin D deficiency is associated with long durations of ventilator use and ICU stay, but is not associated with 28- or 90-day mortality. Moreover, this study revealed that young age, female gender, low albumin level, high PTH level, and high SOFA score are associated with vitamin D deficiency. In the serial examinations of vitamin D level, we further found that the median vitamin D level remained <20 ng/dl in most critically ill patients during their stay in ICUs.
The median vitamin D level (18.3 ng/ml) in the critically ill patients of this study was <28.9 ng/ml in the participants of a community-based cohort study in Northern Taiwan (18). The prevalence of vitamin D deficiency was higher in critically ill patients than in community participants. One associated factor of vitamin D deficiency of this study was a high PTH level indicates that some critically ill patients had chronic vitamin D deficiency before ICU admission (30–32). These two studies shared two associated factors of vitamin D deficiency, namely young age and female gender. Younger and female adults might use more sun protection and have more indoor activities than elderly patients (33, 34). A similar finding in an urban Korean hospital showed that vitamin D levels were higher in older critically ill surgical patients (35). Moreover, the type and severity of critical illness between young and elderly patients may be different, and further studies are warranted to investigate the differences and figure out the complicated effects of age and sex on Vitamin D deficiency in critically ill patients. Two other associated factors for vitamin D deficiency in this study were low albumin level (hypoalbuminemia) and high SOFA score. Hypoalbuminemia as an associated factor for vitamin D deficiency was revealed in a previous study (14), and their multivariable analysis revealed that serum albumin level was associated with vitamin D deficiency with an OR of 0.92 (95% CI 0.87-0.97). We suggest that albumin and vitamin D deficiency are associated with several clinical conditions, including chronic intake insufficiency and acute consumption. SOFA score has been reported to be associated with vitamin D deficiency in several previous studies (14, 32), and it may represent acute consumption or decreased production of vitamin D in patients with a high SOFA score.
In this study, vitamin D deficiency was associated with a longer duration of ventilator use and longer length of ICU stay but was not associated with the length of hospital stay or mortality. To avoid the influence of non-survived critically ill patients with short ICU stay, we also compared the ICU-free days to day 28 among patients with different vitamin D levels and confirmed that vitamin D deficiency was associated with less ICU-free days. This finding is compatible with a previous study (32). Although a systemic review and meta-analysis suggested that vitamin D deficiency increases the mortality risk of critically ill patients (11), our study and other recent studies have reported an uncertain relationship with mortality (16). We suggest that vitamin D deficiency is affected by multiple factors and may have different contributions to mortality in different diseases or in different populations of critically ill patients. Further studies are warranted to investigate the effect of vitamin D deficiency on clinical outcomes in different populations of critically ill patients.
This study has several limitations. First, the number of patients in this study was not sufficient to analyze the effect of vitamin D deficiency on clinical outcomes in the subgroups of specific diseases or populations. Second, this observational study did not limit the use of higher daily supplement of vitamin D (>2,000 IU/day) or high-dose vitamin D3 (>540,000 IU) in these patients during their ICU stay (29, 36). In total, 10 patients received high-dose vitamin D3 during their ICU stay. Third, information regarding the outdoor activity of patients and the ICU environment of sunlight exposure was not included and analyzed in this study. Forth, the effects of age, obesity, and seasons on the vitamin D levels might be confounded by different diseases or diagnoses at ICU admission, and careful subgroup analysis with more patients is recommended in further investigations. This study has two strengths. First, this was a multicenter study that included different areas of Northern Taiwan. Second, a serial follow-up of vitamin D levels was performed for group 2, and it indicated that normal daily supplement of vitamin D were not sufficient to increase the vitamin D level to >30 ng/ml. Further studies are warranted to investigate the effects of daily supplement or high-dose vitamin D on serum vitamin D level and clinical outcomes in critically ill patients.
Conclusions
Our results demonstrated that the prevalence of vitamin D deficiency is high in critically ill patients in Northern Taiwan. Young age, female gender, low albumin level, high PTH level, and high SOFA score are significantly associated risk factors for vitamin D deficiency. Further studies in different countries or areas are warranted and crucial for investigating the prevalence, associated factors, and clinical impacts of vitamin D deficiency in different regions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of National Taiwan University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
K-WC, C-WC, K-CY, L-KK, and Y-CY: concept and design. C-WC, K-CY, I-TW, F-MH, A-YW, Y-CW, Y-CK, S-YR, C-TC, AC, Y-YH, L-KK, and Y-CY: patient enrollment and data collection. K-WC, Y-TK, Y-CL, and Y-CY: statistics and data interpretation. K-WC, L-KK, and Y-CY: drafting manuscript. L-KK and Y-CY: study supervision. All authors: critical revision of the manuscript.
Funding
This work was supported, in part, by grant from the National Taiwan University Hospital (108-S4130).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the ICU staff of the participated hospitals.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. (2007) 357:266–81. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. (2004) 80:1689S–96S. 10.1093/ajcn/80.6.1689S [DOI] [PubMed] [Google Scholar]
- 3.Divakar U, Sathish T, Soljak M, Bajpai R, Dunleavy G, Visvalingam N, et al. Prevalence of vitamin D deficiency and its associated work-related factors among indoor workers in a multi-ethnic southeast asian country. Int J Environ Res Public Health. (2019) 17:164. 10.3390/ijerph17010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. (2011) 31:48–54. 10.1016/j.nutres.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Al Zarooni AAR, Al Marzouqi FI, Al Darmaki SH, Prinsloo EAM, Nagelkerke N. Prevalence of vitamin D deficiency and associated comorbidities among Abu Dhabi Emirates population. BMC Res Notes. (2019) 12:503. 10.1186/s13104-019-4536-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koekkoek WA, van Zanten RA. Vitamin D deficiency in the critically ill. Ann Med. (2016) 48:301–4. 10.3109/07853890.2016.1162910 [DOI] [PubMed] [Google Scholar]
- 7.Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. (2009) 35:2028–32. 10.1007/s00134-009-1642-x [DOI] [PubMed] [Google Scholar]
- 8.Krishnan A, Ochola J, Mundy J, Jones M, Kruger P, Duncan E, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care. (2010) 14:R216. 10.1186/cc9341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J Crit Care. (2017) 38:109–14. 10.1016/j.jcrc.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 10.Zhang YP, Wan YD, Sun TW, Kan QC, Wang LX. Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Crit Care. (2014) 18:684. 10.1186/s13054-014-0684-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Haan K, Groeneveld AB, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. (2014) 18:660. 10.1186/s13054-014-0660-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. (2012) 204:37–43. 10.1016/j.amjsurg.2011.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. (2011) 15:R292. 10.1186/cc10585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucidarme O, Messai E, Mazzoni T, Arcade M, du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. (2010) 36:1609–11. 10.1007/s00134-010-1875-8 [DOI] [PubMed] [Google Scholar]
- 15.Sankar J, Lotha W, Ismail J, Anubhuti C, Meena RS, Sankar MJ. Vitamin D deficiency and length of pediatric intensive care unit stay: a prospective observational study. Ann Intensive Care. (2016) 6:3. 10.1186/s13613-015-0102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchtele N, Lobmeyr E, Cserna J, Zauner C, Heinz G, Sengolge G, et al. Prevalence and impact of vitamin D deficiency in critically ill cancer patients admitted to the intensive care unit. Nutrients. (2020) 13:22. 10.3390/nu13010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ala-Kokko TI, Mutt SJ, Nisula S, Koskenkari J, Liisanantti J, Ohtonen P, et al. Vitamin D deficiency at admission is not associated with 90-day mortality in patients with severe sepsis or septic shock: observational FINNAKI cohort study. Ann Med. (2016) 48:67–75. 10.3109/07853890.2015.1134807 [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Hsu HJ, Wu IW, Sun CY, Ting MK, Lee CC. Vitamin D deficiency in northern Taiwan: a community-based cohort study. BMC Public Health. (2019) 19:337. 10.1186/s12889-019-6657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrea L, Colao A, Savastano S, Muscogiuri G. Specific cut-off for the 25-OH vitamin D levels to predict the highest Body Mass Index and fat mass: a sex-related analysis in obese patients. Minerva Endocrinol. (2020) 45:266–8. 10.23736/S0391-1977.20.03177-6 [DOI] [PubMed] [Google Scholar]
- 20.Barrea L, Frias-Toral E, Pugliese G, Garcia-Velasquez E, DE LAC M, Savastano S, et al. Vitamin D in obesity and obesity-related diseases: an overview. Minerva Endocrinol. (2021) 46:177–92. 10.23736/S2724-6507.20.03299-X [DOI] [PubMed] [Google Scholar]
- 21.Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. (2020) 84:111106. 10.1016/j.nut.2020.111106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zapatero A, Dot I, Diaz Y, Gracia MP, Perez-Teran P, Climent C, et al. Severe vitamin D deficiency upon admission in critically ill patients is related to acute kidney injury and a poor prognosis. Med Intensiva. (2018) 42:216–24. 10.1016/j.medine.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Brook K, Camargo CA, Christopher KB, Quraishi SA. Admission vitamin D status is associated with discharge destination in critically ill surgical patients. Ann Intensive Care. (2015) 5:23. 10.1186/s13613-015-0065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnett N, Zhao Z, Koyama T, Janz DR, Wang CY, May AK, et al. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: a case-control study. Ann Intensive Care. (2014) 4:5. 10.1186/2110-5820-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 28.Remmelts HH, van de Garde EM, Meijvis SC, Peelen EL, Damoiseaux JG, Grutters JC, et al. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis. (2012) 55:1488–94. 10.1093/cid/cis751 [DOI] [PubMed] [Google Scholar]
- 29.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. (2014) 312:1520–30. 10.1001/jama.2014.13204 [DOI] [PubMed] [Google Scholar]
- 30.Sattar S, Adnan F, Waheed S. Frequency of Vitamin D deficiency and its association with serum PTH levels in end stage renal disease patients. J Pak Med Assoc. (2020) 70:2190–4. 10.47391/JPMA.479 [DOI] [PubMed] [Google Scholar]
- 31.Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol. (2016) 6:561–601. 10.1002/cphy.c140071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ardehali SH, Dehghan S, Baghestani AR, Velayati A, Vahdat Shariatpanahi Z. Association of admission serum levels of vitamin calcium D. Phosphate, magnesium and parathormone with clinical outcomes in neurosurgical ICU patients. Sci Rep. (2018) 8:2965. 10.1038/s41598-018-21177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea–a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. (2011) 96:643–51. 10.1210/jc.2010-2133 [DOI] [PubMed] [Google Scholar]
- 34.Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health. (2011) 11:853. 10.1186/1471-2458-11-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Doo SR, Kim D, Park YK, Park EJ, Lee JM. Vitamin D deficiency and mortality among critically ill surgical patients in an urban Korean hospital. Int J Vitam Nutr Res. (2020) 2020:1–8. 10.1024/0300-9831/a000639 [DOI] [PubMed] [Google Scholar]
- 36.National Heart Lung Blood Institute PCTN. Ginde AA, Brower RG, Caterino JM, Finck L. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N Engl J Med. (2019) 381:2529–40. 10.1056/NEJMoa1911124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.