Abstract
Sodium glucose cotransporter‐2 inhibitors (SGLT2is) are now widely used to treat diabetes, but their effects on nonalcoholic fatty liver disease (NAFLD) remain to be determined. We aimed to evaluate the effects of SGLT2is on the pathogenesis of NAFLD. A multicenter, randomized, controlled trial was conducted in patients with type 2 diabetes with NAFLD. The changes in glycemic control, obesity, and liver pathology were compared between participants taking ipragliflozin (50 mg/day for 72 weeks; IPR group) and participants being managed without SGLT2is, pioglitazone, glucagon‐like peptide‐1 analogs, or insulin (CTR group). In the IPR group (n = 25), there were significant decreases in hemoglobin A1c (HbA1c) and body mass index (BMI) during the study (HbA1c, −0.41%, P < 0.01; BMI, −1.06 kg/m2, P < 0.01), whereas these did not change in the CTR group (n = 26). Liver pathology was evaluated in 21/25 participants in the IPR/CTR groups, and hepatic fibrosis was found in 17 (81%) and 18 (72%) participants in the IPR and CTR groups at baseline. This was ameliorated in 70.6% (12 of 17) of participants in the IPR group and 22.2 % (4 of 18) of those in the CTR group (P < 0.01). Nonalcoholic steatohepatitis (NASH) resolved in 66.7% of IPR‐treated participants and 27.3% of CTR participants. None of the participants in the IPR group developed NASH, whereas 33.3% of the CTR group developed NASH. Conclusion: Long‐term ipragliflozin treatment ameliorates hepatic fibrosis in patients with NAFLD. Thus, ipragliflozin might be effective for the treatment and prevention of NASH in patients with diabetes, as well as improving glycemic control and obesity. Therefore, SGLT2is may represent a therapeutic choice for patients with diabetes with NAFLD, but further larger studies are required to confirm these effects.
In the randomized controlled trial, long‐term ipragliflozin treatment ameliorates hepatic outcomes, including fibrosis, in patients with type 2 diabetes with NAFLD. Ipragliflozin also ameliorates obesity and glycemic control in patients with type 2 diabetes with NAFLD.
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CT
computed tomography
- CTR
control group
- CVD
cardiovascular disease
- FIB‐4 index
fibrosis 4 index
- GLP‐1
glucagon‐like peptide‐1
- GGT
γ‐glutamyl transpeptidase
- HbA1c
hemoglobin A1c
- IPR
ipragliflozin group
- M2BPGi
Mac2‐binding protein glycan isomer
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- RCT
randomized controlled trial
- SGLT2is
sodium glucose cotransporter‐2 inhibitors
The global prevalences of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) in the general population have been estimated to be about 25% and 5%, and are increasing.( 1 ) Furthermore, the economic burden of NAFLD is predicted to increase two‐fold by 2030,( 2 , 3 ) but effective pharmacological therapies have not been identified. There is a close interaction between NAFLD and diabetes. The prevalence of diabetes is 23% in patients with NAFLD and 47% in those with NASH.( 4 ) A recent meta‐analysis showed that the risk of type 2 diabetes is two‐fold higher in patients with NAFLD than in those without.( 5 ) Furthermore, according to previous epidemiological studies, the presence of abnormal glucose metabolism and/or diabetes in NAFLD is associated with the progression of liver fibrosis and the development of hepatocellular carcinoma.( 6 ) The coexistence of diabetes and NAFLD accelerates the progression of both, and worsens hepatic outcomes and overall mortality, including from cardiovascular disease (CVD).( 7 , 8 )
Recent studies have shown that the severity of hepatic fibrosis is the most significant independent risk factor for mortality and liver‐related complications in NAFLD.( 8 , 9 ) Therefore, liver fibrosis should be assessed as a primary endpoint in clinical trials of candidate therapeutic agents. Sodium‐glucose cotransporter 2 inhibitors (SGLT2is) are hypoglycemic agents that lower blood glucose concentrations by inhibiting renal glucose reabsorption. The recent clinical trials, EMPA‐REG OUTCOME and CANVAS Program, and their related subanalyses have demonstrated that the SGLT2is empagliflozin and canagliflozin reduce the risk of CVD and improve renal outcomes in diabetes.( 10 , 11 , 12 ) This has led to an American and European consensus to prioritize the use of SGLT2is in patients with diabetes with insufficient glycemic control following lifestyle modification and metformin therapy.( 13 ) In addition, effects of SGLT2is on NAFLD have also been reported. Reductions in alanine aminotransferase (ALT), hepatic steatosis and liver fibrosis, assessed using the fibrosis 4 (FIB‐4) index, have been demonstrated,( 14 , 15 ) but liver pathology has not previously been well evaluated, and randomized controlled trials have not been conducted to date. In particular, it is important to determine whether SGLT2is ameliorate liver fibrosis, which is the pathological change that carries the most significant risk of mortality in patients with NAFLD. Therefore, we conducted a randomized controlled trial (RCT) that aimed to evaluate the effect of ipragliflozin on hepatic pathology, as well as glycemic control and obesity, in patients with diabetes with NAFLD.
Materials and Methods
Patients
Patients with type 2 diabetic NAFLD who had been clinically diagnosed by liver biopsy (first liver biopsy) within the preceding 6 months were recruited at seven sites in Japan between August 2015 and September 2017. The inclusion criteria were as follows: (1) age 20‐80 years; (2) histological diagnosis of NAFLD by the nominated study pathologists; (3) hemoglobin A1c (HbA1c) ≥ 6.0%; (4) no existing medication with an SGLT2i, pioglitazone, glucagon‐like peptide‐1 (GLP‐1) analog, or insulin; and (5) steatosis involving ≥5% of the hepatic parenchyma of a liver biopsy performed at baseline. The exclusion criteria were as follows: (1) presence of a severe complication of diabetes, including severe diabetic nephropathy (estimated glomerular filtration rate <30 mL/min/1.73 m2)( 16 ) and/or diabetic retinopathy at a more severe stage than simple diabetic retinopathy; (2) diagnosis of type 1 diabetes; (3) history of severe CVD, including ischemic heart disease, chronic heart failure, cerebral infarction, and/or peripheral vascular disorders; (4) presence of etiological factors suggesting a diagnosis of a different liver disease, including habitual alcoholic intake (ethanol consumption >30 g/day and >210 g/week in men and >20 g/day and >140 g/week in women), positivity for hepatitis B surface antigen, positivity for hepatitis C antibody, and abnormal serum thyroid hormone concentration; and (5) diagnosis of autoimmune liver disease, drug‐induced hepatotoxicity, hemochromatosis, or Wilson disease.
The study protocol was approved by the Clinical Research Ethics Review Committee in each facility and the Certified Review Board of Saga University Hospital, in accordance with the principles of the 1975 Declaration of Helsinki, revised in 2013, the CONSORT 2010 Statement, and the Japanese Clinical Trials Act. An outline of the study protocol and outcomes was registered with the UMIN Clinical Trials Registry (UMIN000015727) and the Japan Registry of Clinical Trials (jRCTs071180069). All participants gave their written, informed consent.
Study Design
This multicenter, open‐label RCT compared the use of ipragliflozin at a dose of 50 mg once a day (the IPR group) and a control (CTR) group who performed lifestyle modifications, including diet and exercise therapy, and/or took antidiabetic drugs, with the exception of SGLT2is, pioglitazone, or GLP‐1 analogs, for 72 weeks (Fig. 1). Randomization was performed such that HbA1c level and body mass index (BMI) were matched between the groups. After the first liver biopsy and during the study period, the antidiabetic therapy was maintained, unless the patient demonstrated poor glycemic control, with an HbA1c > 7.0%, when treatment with an additional antidiabetic agent, excluding SGLT2is, pioglitazone and GLP‐1 analogs, was allowed. In the IPR group, diet and exercise consultations and therapy were maintained throughout the study period.
FIG. 1.
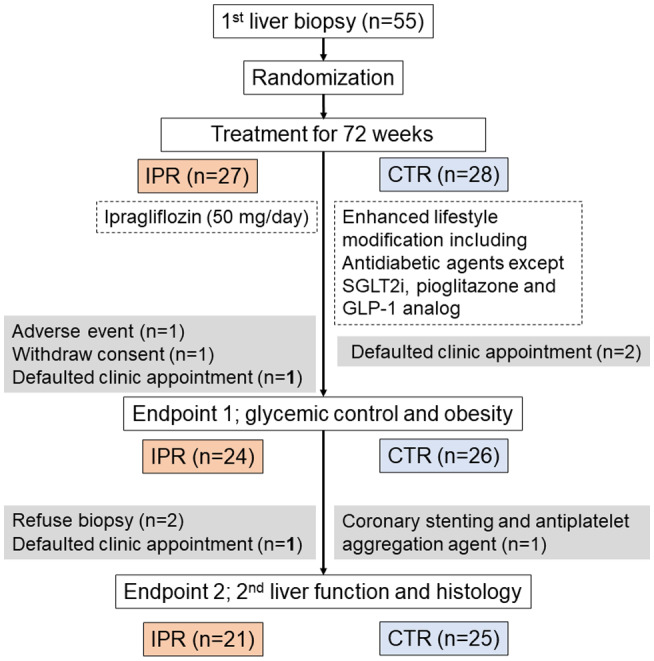
Flow diagram of the study participants.
Glycemic control and obesity were evaluated as endpoint 1, and hepatic outcomes (including changes in pathological findings between the first and second liver biopsies) were evaluated as endpoint 2. For the assessment of endpoint 1, 3 participants in the IPR group were excluded because of gastric cancer, which was reported as an adverse event (AE) (see “Safety” subsection), a withdrawal of informed consent, and a missed clinic appointment; and 2 participants in the CTR group were excluded because of missed clinic appointments. For the assessment of endpoint 2, 3 patients in the IPR group were excluded because they refused a liver biopsy or missed a clinic appointment, and 1 patient in the CTR group was excluded because they started taking an antiplatelet aggregation agent after undergoing coronary stenting (see “Safety” subsection). Therefore, 24 participants in the IPR group and 26 participants in the CTR group were evaluated for endpoint 1, and 21 participants in the IPR group and 25 participants in the CTR group were evaluated for endpoint 2. The safety of the interventions was evaluated in all of the participants.
Physical Examination and Serum Biochemical Measurements
Venous blood samples were obtained after an overnight fast and used to measure platelet count, fasting plasma glucose concentration, liver enzyme activities, total bilirubin, total cholesterol, high‐density lipoprotein–cholesterol (HDL‐C), low‐density lipoprotein–cholesterol (LDL‐C), triglyceride, creatinine, C‐peptide, ferritin concentrations and HbA1c, using conventional laboratory techniques. Insulin resistance was evaluated using the homeostasis model assessment of insulin resistance (fasting insulin [IU/mL] × fasting blood sugar [mg/dL]/405).( 17 ) Type IV collagen 7s was measured using a radioisotopic immunoassay,( 18 ) and Mac2‐binding protein glycan isomer (M2BPGi) was measured using a glycan‐based immunoassay( 19 ) at SRL Co. Ltd. (Tokyo, Japan). Subcutaneous fat area (cm2) and visceral fat area (cm2) were measured at the umbilical level on computed tomography (CT) images using Fat Scan software (N2 System Co., Osaka, Japan).( 20 )
Pathological Assessment
Ultrasonographically guided liver biopsy was performed using a 16‐gauge biopsy needle. All liver biopsies were ≥20 mm in length. Liver biopsy sections, stained with hematoxylin and eosin and Azan stain, were evaluated by two experienced pathologists (M.K. and S.A.) who specialize in liver pathology and were blinded to the clinical data. The assignment of the participants was known by the pathologists. The two pathologists evaluated a sample simultaneously, discussed the scoring, and agreed on a diagnosis. The pathologists examined all of the sections at baseline for inclusion in the study, and re‐examined all of the sections, without reference to baseline or outcome data, at the end of the trial. Hepatic steatosis, lobular inflammation, and hepatocyte ballooning were evaluated using the NAFLD activity score (NAS).( 21 ) Liver fibrosis was classified according to Kleiner et al.( 21 ) and Brunt et al.( 22 ) Steatosis was scored as 0, 1, 2, or 3 (score 0, <5%; score 1, 5%‐33%; score 2, 34%‐66%; and score 3, >66% of the biopsy), and fibrosis was scored as 0, 1, 2, 3, or 4 (stage 0, no fibrosis; stage 1, perisinusoidal or periportal fibrosis; stage 2, perisinusoidal and portal/periportal fibrosis; stage 3, bridging fibrosis; and stage 4, cirrhosis). NAFLD was diagnosed if steatosis was scored as 1 or higher. NAFLD of types 3 or 4 was defined as NASH, according to Matteoni’s classification,( 23 ) and the resolution of NASH was defined according to the Food and Drug Administration guidance (https://www.fda.gov/media/119044/download) as an absence of steatohepatitis and a NAS score of 0‐1 for inflammation, 0 for ballooning, and any value for steatosis, without worsening fibrosis. Types 1 and 2 NAFLD were defined as non‐NASH, and on this basis 10 participants in the IPR group and 12 participants in the CTR group were diagnosed as not having NASH at baseline and included in endpoint 2. Samples with fibrosis but no ballooning were defined as “unclassified NAFLD.” Two participants were diagnosed as “unclassified” at baseline (1 in the IPR group, with scores for steatosis of 1, inflammation 0, ballooning 0, and fibrosis stage 1; and 1 in the CTR group, with scores for steatosis of 1, inflammation 0, ballooning 0, and fibrosis stage 2) and included in endpoint 2. There were 2 patients with cirrhosis who had a steatosis score of 0 (so‐called “burn‐out NASH”), who had been previously diagnosed with NAFLD by liver biopsy and had been followed up at their local institutions. After diagnoses had been made by the study pathologists, and the study director (K.A.), the physician in charge, and the patients themselves had agreed, the patients were included in the study.
Safety Assessments
Safety data were collected throughout the study period, from randomization to 30 days after the end of treatment. These consisted of the results of laboratory tests and physical examinations, and AEs. AEs and their severity were reported according to the Common Terminology Criteria for Adverse Events (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Statistical Analyses
Sample size was calculated for endpoint 1 on the basis of the findings of a previous study of ipragliflozin( 24 ): The change in HbA1c from baseline was predicted to be −0.76 ± 0.69% and 0 ± 0.98% for the IPR and CTR groups, respectively. With a power of 80% and a two‐sided significance level of 0.05, n = 21 patients per group were calculated to be required. The baseline characteristics of the two groups were compared using the chi‐square test for categorical data and Welch’s t‐test for continuous data. Welch’s t‐test was used to compare changes in continuous data from baseline between the groups and to compare liver biopsy findings in endpoint 2. The single‐sample t‐test was used to compare paired continuous data. A mixed‐effect model was used to test the HbA1c response during the study period, and was adjusted for basal HbA1c, basal BMI, and basal use of antidiabetic agents. The analyses were conducted using R 3.6.1 or library lmerTest 3.1‐1 for the mixed‐effect model. A two‐sided P value of 0.05 was regarded as statistically significant.
Results
Demographics and Baseline Characteristics
A summary of the baseline characteristics of the participants for endpoint 1 is shown in Table 1, and all of the data are shown in Supporting Table S1. There were no significant differences in age, sex, BMI, or the prevalence of concomitant lifestyle‐related diseases between the groups. The total cholesterol concentration was higher in the CTR group than in the IPR group, but there were no significant differences in HDL‐C or LDL‐C. There were also no significant differences in fasting glucose or HbA1c. Patients with various stages of liver fibrosis were included, but there were no significant differences between the groups. The visceral and abdominal subcutaneous fat areas did not differ between the groups. The baseline characteristics for endpoint 2 are summarized in Supporting Table S2, and were similar to those for endpoint 1. The agents being used to treat concomitant lifestyle‐related diseases, including diabetes, dyslipidemia and hypertension, are summarized in Supporting Table S3.
Table 1.
Characteristics of the Patients for Endpoint 1
| Characteristics | IPR | CTR | P Value |
|---|---|---|---|
| n = 24 | n = 26 | ||
| Age, years | 59.0 (46.8‐64.3) | 50.0 (48.0‐68.8) | 0.82 |
| Male, n (%) | 15 (62.5) | 14 (53.8) | 0.54 |
| BMI, kg/m2 | 29.9 (27.2‐32.3) | 28.8 (25.7‐32.9) | 0.85 |
| Hypertension, n (%) | 9 (37.5) | 7 (26.9) | 0.42 |
| Dyslipidemia, n (%) | 13 (54.2) | 19 (73.1) | 0.16 |
| Platelet count, ×103/µL | 19.6 (16.2‐23.5) | 22.0 (18.6‐27.1) | 0.29 |
| AST, U/L | 43.5 (35.3‐57.0) | 41.5 (33.0‐75.5) | 0.17 |
| ALT, U/L | 57.0 (43.8‐70.3) | 52.0 (38.3‐99.0) | 0.60 |
| GGT, U/L | 69.0 (39.5‐112.5) | 55.5 (36.3‐93.0) | 0.70 |
| Fasting glucose, mg/dL | 115 (102‐133) | 123(113‐142) | 0.10 |
| HbA1c, % | 6.5 (6.1‐7.1) | 6.8 (6.3‐7.0) | 0.27 |
| Ferritin, ng/mL | 256 (149‐311) | 177 (100‐346) | 0.57 |
| Visceral fat area, cm2 | 181(162‐234) | 176 (141.5‐226) | 0.70 |
| Subcutaneous fat area, cm2 | 238 (187‐289) | 237 (169‐311) | 0.78 |
Continuous valuables are shown as median (lower and upper quartile). The complete list of characteristics of the patients for endpoint 2 is available in Supporting Table S1. P values were calculated using the chi‐square test or Welch’s t‐test.
Effect of Ipragliflozin on Obesity and Glycemic Control in Patients With NAFLD
In the analysis of endpoint 1, the changes in glycemic control, BMI, and fat area from baseline were compared between the groups (Fig. 2). The IPR group showed a significant decrease in HbA1c during the study, whereas HbA1c did not change from its baseline level in the CTR group (Fig. 2A). The mixed‐effect model showed that ipragliflozin was a significant factor in the change in HbA1c (log odds ratio = −0.311, P = 0.01), and the only significant adjustment factor was basal HbA1c (log odds ratio = 0.992, P < 0.01). The IPR group also showed a significant reduction in fasting glucose concentration after 12 weeks, but there was no significant difference between the groups at this time point (Fig. 2B). The IPR group showed significant reductions in BMI, visceral fat area, and subcutaneous fat area from baseline (Fig. 2C‐E). Moreover, the reductions in BMI (at 24, 48, and 72 weeks) and visceral fat area (at 48 and 72 weeks) were significantly larger in the IPR group than in the CTR group. Interestingly, serum ferritin concentration also decreased in the IPR group to a greater extent than in the CTR group during the first 12, 24, and 48 weeks of the study. These effects of ipragliflozin on glycemic control, obesity, and ferritin concentration were also shown in the analysis of endpoint 2 (Supporting Fig. S1).
FIG. 2.
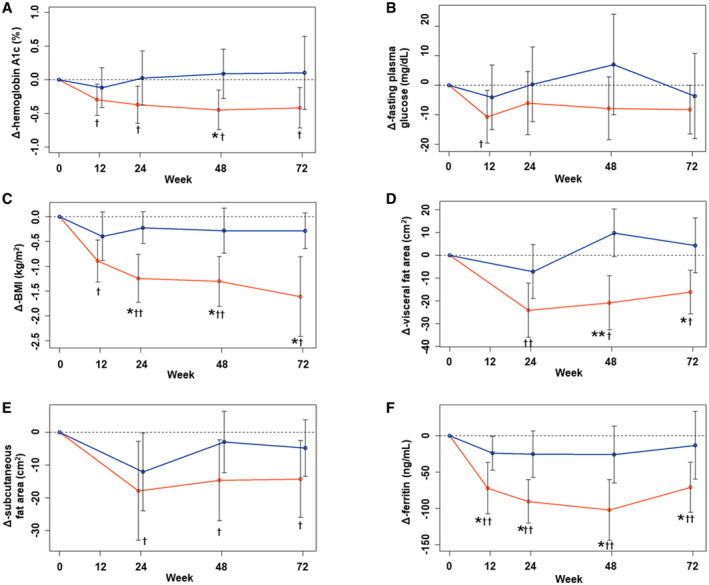
Changes in parameters from baseline for endpoint 1. Mean changes in HbA1c (A), fasting plasma glucose concentration (B), BMI (C), visceral fat area (D), subcutaneous fat area (E), and serum ferritin concentration (F) in the IPR (red line) and CTR (blue line) groups from baseline are shown. Data are presented as the mean and SD. *P < 0.05 and **P < 0.01 between the groups, according to Welch’s two‐sample t‐test. † P < 0.05 and †† P < 0.01 versus baseline, according to the single‐sample t‐test.
Effect of Ipragliflozin on the Pathogenesis of NAFLD
In the analysis of endpoint 2, changes in liver enzyme activities, markers of serum fibrosis, and pathological findings were analyzed. The aspartate aminotransferase (AST), ALT, and gamma‐glutamyltransferase (GGT) activities were significantly lower than at baseline in the IPR group, whereas the changes in the CTR group were not significant (Fig. 3A‐C). There were significant differences in the changes in GGT activity at 24, 48, and 72 weeks of the study between the groups. However, there was no significant difference in the changes in total bilirubin concentration (Fig. 3D). With regard to the markers of serum fibrosis, there was no significant difference in the changes in M2BPGi (Fig. 3E), but there were significant reductions in type IV collagen 7s in the IPR group after 24 weeks and 72 weeks of the study, and in the CTR group after 48 weeks, although this latter change had disappeared by 72 weeks (Fig. 3F).
FIG. 3.
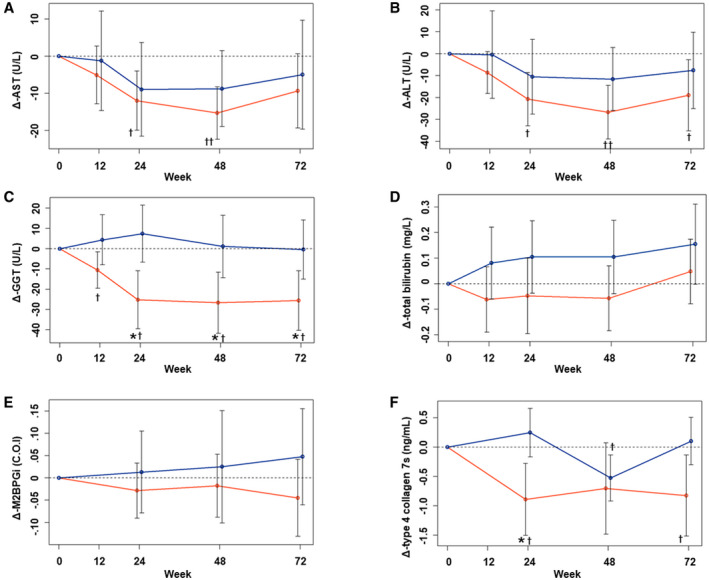
Changes in parameters from baseline for endpoint 2. Mean changes in AST (A), ALT (B), GGT (C), total bilirubin (D), M2BPGi (E), and type 4 collagen 7s (F) in the IPR (red line) and CTR (blue line) groups from baseline are shown. Data are presented as the mean and SD. *P < 0.05 and **P < 0.01 between the groups, according to Welch’s two‐sample t‐test. † P < 0.05 and †† P < 0.01 versus baseline, according to the single‐sample t‐test.
Pathological Outcomes
The pathological outcomes are summarized in Fig. 4. In the IPR group, the proportions of patients with at least a one‐score or one‐stage reduction after 72 weeks of treatment were 52.4% (11 of 21) with respect to ballooning and 57.1% (12 of 21) with respect to fibrosis, which were significantly larger proportions than in the CTR group (24% [6 of 25] for ballooning, P = 0.02; and 16% [4 of 25] for fibrosis, P = 0.01) (Fig. 4C,D). There were no significant differences in the changes in steatosis or inflammation between the groups (Fig. 4A,B). When individual NAS components and fibrosis stage were analyzed after the exclusion of the patients with score 0 or stage 0 (Supporting Fig. S2), ballooning in all of the patients (11 of 11) in the IPR group was at least one score lower, whereas 46.2% (6 of 13) of participants in the CTR group showed this reduction (P = 0.02) (Supporting Fig. S2C). In the IPR group, 70.6% (12 of 17) of the participants with fibrosis stage ≥1 showed at least a one‐stage reduction, whereas only 22.2% (4 of 18) showed this in the CTR group (P = 0.01) (Supporting Fig. S2D). All of the changes in individual NAS components and fibrosis stage are shown in Supporting Table S4. The relationships between the changes in glycemic control or BMI and ballooning or fibrosis are shown in Supporting Fig. S3. None of the participants who showed improvements in glycemic control or BMI demonstrated a worsening of ballooning or fibrosis in the IPR group, whereas several participants showed a worsening of these pathological lesions in the CTR group, regardless of whether glycemic control or BMI improved.
FIG. 4.
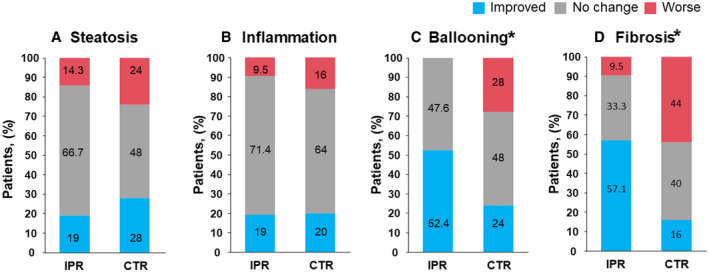
Evaluation of liver biopsies. (A‐D) Results of the pathological evaluation of biopsies from all of the participants for endpoint 2 (IPR, n = 21 vs. CTR, n = 25). *P < 0.05, according to Welch’s two‐sample t‐test.
The pathological outcomes, stratified according to the presence or absence of NASH at baseline, are summarized in Fig. 5. In the IPR group, none of the participants developed NASH, whereas 33.3% of the participants in the CTR group developed NASH. Furthermore, in the IPR group, 66.7% of the participants who had NASH at baseline showed NASH resolution, without a worsening of fibrosis.
FIG. 5.
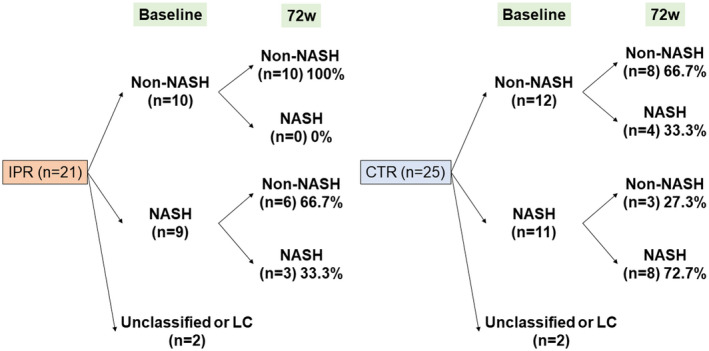
Pathological outcomes in the IPR and CTR groups. Abbreviation: LC, liver cirrhosis.
Safety
Table 2 provides information regarding the safety of the interventions. An AE was reported by 22.2% of the participants in the IPR group and 46.4% of the patients in the CTR group, most of which were of mild‐to‐moderate severity. Gastric cancer was diagnosed in a patient in the IPR group when they underwent upper gastrointestinal endoscopy during a health check, and this was reported as a serious AE. One participant in the CTR who underwent coronary artery CT was diagnosed with coronary artery stenosis, and therefore underwent a percutaneous coronary intervention. None of the participants showed hypoglycemia, evidence of lactic acidosis, congestive heart failure, or renal dysfunction. Two participants showed a higher frequency of urination in the IPR group, whereas none of the participants showed other symptoms consistent with urinary tract infection in either group. Laboratory abnormalities more severe than grade 2 were reported only in the CTR group: 3 participants showed an increase in HbA1c, and 2 of them were prescribed an additional antidiabetic drug.
Table 2.
Reported AEs
| IPR (n = 27) | CTR (n = 28) | |
|---|---|---|
| n (%) | n (%) | |
| Serious AEs | ||
| Gastric cancer (grade 3) | 1 (3.7) | 0 (0) |
| Asymptomatic coronary stenosis (grade 2) | 0 (0) | 1 (3.6) |
| Common AEs | ||
| Constipation | 1 (3.7) | 1 (3.6) |
| Abdominal distension | 0 (0) | 1 (3.6) |
| Abdominal discomfort | 0 (0) | 2 (7.1) |
| Frequency urination | 2 (8) | 0 (0) |
| Muscle cramp | 0 (0) | 1 (3.6) |
| Knee osteoarthritis | 1 (3.7) | 0 (0) |
| Perineum pruritus | 1 (3.7) | 0 (0) |
| Back pruritus | 0 (0) | 1 (3.6) |
| Patients with laboratory abnormalities severer than grade 2 | ||
| Elevation of HbA1c | 0 (0) | 3 (10.7)* |
| Elevation of uric acid | 0 (0) | 1 (3.6) |
| Total | ||
| Serious AEs | 1 (3.7) | 1 (3.6) |
| Common AEs | 5 (18.5) | 8 (30.8) |
| Patients with laboratory abnormalities severer than grade 2 | 0 (0) | 4 (14.3) |
| Any AEs | 6 (22.2) | 13 (46.4) |
Reported as an exacerbation of the primary disease.
Discussion
In this multicenter, open‐label RCT, treatment of patients with diabetes with NAFLD with ipragliflozin for 72 weeks improved their glycemic control, obesity, and hepatic outcomes, including liver fibrosis. Moreover, ipragliflozin reduced the severity of ballooning, resulting in NASH resolving or not developing in many of the participants. Although one severe AE, gastric cancer, occurred during treatment with ipragliflozin, it was not considered as related to the ipragliflozin treatment, on the basis of the findings of previous studies of ipragliflozin and other SGLT2is,( 10 , 11 , 12 , 24 , 25 , 26 ) which implies that ipragliflozin is safe for use in patients with diabetes with NAFLD.
To date, although the effects of SGLT2is on the liver in NAFLD have been frequently reported, there have been no controlled studies that have evaluated liver pathology in patients. Furthermore, several of the previous studies were uncontrolled. Akuta et al. reported an uncontrolled, open‐label study of 9 patients with NAFLD, in whom treatment with canagliflozin for 24 weeks ameliorated liver steatosis (78%), inflammation (33%), hepatocyte ballooning (22%), and fibrosis (33%), as well as the abnormalities in liver function tests and glycemic control.( 15 ) Changes in liver enzyme activities were a major endpoint in most of the clinical studies of the use of SGLT2i in NAFLD. A recent meta‐analysis demonstrated reductions in AST, ALT, and GGT activities in 6,475 patients with NAFLD who had been treated with canagliflozin for 26‐52 weeks.( 27 ) In addition, treatment with other SGLT2is (dapagliflozin, empagliflozin, or ipragliflozin) was associated with reductions in liver enzyme activities.( 28 , 29 ) Imaging modalities, such as CT and proton magnetic resonance spectroscopy, have also shown effects of SGLT2is on hepatic steatosis in patients with NAFLD. Liver steatosis, defined using the liver‐to‐spleen ratio on CT images, was significantly ameliorated by treatment with dapagliflozin( 30 ) or luseogliflozin( 31 ) for 24 weeks. In addition, in a placebo‐controlled study that used proton magnetic resonance spectroscopy for the evaluation of liver steatosis, 24 weeks of treatment with canagliflozin reduced intrahepatic triglyceride storage in patients with NAFLD with type 2 diabetes.( 32 ) However, in the present study, there were significant decreases in liver enzyme activities in the IPR group, whereas the changes in hepatic steatosis did not significantly differ between the groups. A possible explanation for this is a recovery of the lipid storage during the study, which was longer than previous studies.
We also found that visceral fat area, measured by CT, tended to be higher after 24 weeks of treatment (Fig. 2D and Supporting Fig. S1D). It has been reported that SGLT2i treatment increases the appetite of rodents and changes their food preference, and that patients taking dapagliflozin for the treatment of type 2 diabetes eat more sugar.( 33 , 34 ) Therefore, participants in the IPR group might have eaten more food, causing them to regain visceral fat by the 24‐week time point. This might imply that we missed the best time point to identify an amelioration of liver steatosis, which was probably between 24 and 48 weeks. Although the present study demonstrated an amelioration of liver fibrosis as a result of SGLT2i treatment, which is the only pathological finding that is associated with the prognosis of NAFLD,( 8 , 9 ) it is unknown whether this amelioration is maintained beyond 72 weeks, and whether the use of an SGLT2i improves the prognosis of NAFLD. Therefore, further studies are required.
The mechanism of the improvement in liver fibrosis induced by ipragliflozin in the present study remains unclear. However, the improvements in glycemic control and BMI induced by SGLT2i in the present and previous studies( 14 , 15 , 32 ) could contribute to the amelioration of hepatic steatosis. Consistent with this, the reductions in HbA1c and BMI positively correlated with the reduction in intrahepatic triglyceride induced by the luseogliflozin or canagliflozin treatment of patients with NAFLD with type 2 diabetes.( 32 , 35 ) However, there is also evidence that SGLT2is ameliorate hepatic fibrosis in rodents and steatosis in rodents and humans, independent of a loss in body mass,( 36 , 37 ) which suggests that there might be a mechanism whereby the hepatic outcome of NAFLD is improved by SGLT2is, independent of their effect on obesity. In the present study, it is unclear whether there was an independent effect of SGLT2i on the pathological findings, because of the limited number of the participants, and especially of those who showed a worsening of HbA1c and BMI in the IPR group (Supporting Fig. S3). However, interestingly, in the IPR group, improvements in ballooning and fibrosis occurred in several patients who showed a worsening of glycemic control and BMI. These data might suggest that the effects of long‐term SGLT2i therapy on ballooning and fibrosis are more marked when reductions in HbA1c and body weight are achieved, but these metabolic improvements might not be necessary. SGLT2is increase hepatic glucose production as a result of the up‐regulation of gluconeogenesis and glycogenolysis,( 38 ) which is a response to the increase in renal glucose excretion and the direct effect of SGLT2is to increase the secretion of glucagon by pancreatic alpha cells.( 39 ) In addition, a recent study demonstrated that glucagon promotes hepatic lipolysis and activates hepatic mitochondria by stimulating inositol triphosphate receptor 1, which is a membrane glycoprotein complex that is responsible for mitochondrial calcium signaling in hepatocytes.( 40 ) The stimulation of hepatic β‐oxidation by SGLT2is has also been shown in rodents,( 41 ) and this might be a key glucagon‐mediated mechanism for the hepatic effects of SGLT2is. There are several clinical trials in progress that aim to evaluate the utility of a combination of a glucagon receptor agonist and a glucagon‐like peptide‐1 receptor agonist or co‐agonists of these receptors, and preclinical studies have shown positive effects on hepatic fibrosis.( 42 , 43 ) Thus, glucagon‐mediated effects of SGLT2is might contribute to their amelioration of hepatic fibrosis, as well as their beneficial effects on metabolic dyshomeostasis.
The amelioration of hepatocyte ballooning by ipragliflozin is another notable finding of the present study. Ballooning is a degenerative change that precedes hepatocyte apoptosis, and is caused by metabolic abnormalities, inflammation, or circulatory disturbances.( 44 ) Ballooning is considered to be a hallmark of NASH, which is frequently diagnosed in association with several other liver diseases.( 22 , 45 ) It has been reported that SGLT2is suppress oxidative stress, endoplasmic reticulum stress, and hypoxia‐related signaling, which reduces cell death and apoptosis in renal proximal tubular cells in vitro and in vivo.( 46 , 47 , 48 ) However, because SGLT2 is not expressed in hepatocytes, indirect effects of SGLT2i must have been responsible for the amelioration of hepatocyte ballooning. It has also been reported that hepatic iron overload and hyperferritinemia are frequently present in patients with NASH.( 49 ) Moreover, iron‐induced oxidative stress and inflammasome activation cause hepatocyte ballooning in the liver of leptin receptor–deficient mice that are overfed iron.( 50 ) In the current study, there was a significant decrease in serum ferritin concentration in the IPR group (Fig. 2F), which suggests that a reduction in iron overload may also be a mechanism whereby SGLT2is ameliorate or prevent hepatocyte ballooning in NAFLD.
The present study had several limitations. The sample size that was calculated for endpoint 1 was too small to identify a superior effect of ipragliflozin on NASH and fibrosis. Therefore, to confirm the effect of ipragliflozin or SGLT2is on the hepatic outcomes of patients with NAFLD, further studies are needed. Because the study was not placebo‐controlled, but instead used an active‐controlled design, including various control treatments, the outcomes of the CTR group were likely to be variable and affect comparisons with the IPR group. Despite the randomization, there was a significant difference between the groups with respect to serum total cholesterol for endpoints 1 and 2, and also in fasting glucose for endpoint 2 at baseline. These differences could have altered the treatment effects in both groups. Because the inclusion criteria of the present study did not limit the participants to those with advanced NASH, and patients with mild‐to‐moderate NASH were included, the effectiveness of ipragliflozin should be interpreted with caution. However, the inclusion of patients with various levels of severity of NAFLD in the present study has enabled us to demonstrate that ipragliflozin might be able to prevent the development of NASH, as well as resolve NASH and ameliorate liver fibrosis. Therefore, we believe that a beneficial effect of ipragliflozin could be expected in patients along the entire spectrum of NAFLD.
In conclusion, this RCT has provided evidence that ipragliflozin ameliorates obesity and improves hepatic outcomes, including fibrosis, in patients with diabetes with NAFLD. Therefore, SGLT2is could represent an appropriate therapy for patients with NAFLD. Larger phase 3 trials of SGLT2is are warranted to confirm its effects in patients at specific stages of NAFLD.
Supporting information
Supporting Material
Acknowledgment
The authors thank Ms. Maki Miyahara, Ms. Kiyoka Yasunaga, Ms. Saori Fuchigami, Ms. Chieko Ogawa, and all of the medical staff and processing personnel at the research facilities who collected the clinical data. They also thank Mark Cleasby, Ph.D., from Edanz Group (https://en‐author‐services.edanz.com/ac), for editing the drafts of this manuscript.
Supported by Astellas Pharma Inc.
Potential conflict of interest: Dr. Anzai is on the speakers’ bureau and received grants from Daiichi Sankyo, Sumitomo Dainippon Pharma, Mitsubishi Tanabe Pharma, and Novo Nordisk Pharma. He is on the speakers’ bureau for Kyowa Kirin, Sanofi K.K., and Takeda Pharmaceutical. He received grants from Astellas Pharma, the Japan IDDM network, Taisho Pharma, and Kracie Holdings. Dr. Nakajima is on the speakers’ bureau for and received grants from EA Pharma, Kowa, and Mylan EPD. He is on the speakers’ bureau for Astellas Pharma, MSD, and Sumitomo Dainippon Pharma. He received grants from Biofermin. Dr. Yoneda received grants from Kowa.
Clinical trial registration numbers: UMIN000015727 and jRCTs071180069.
Contributor Information
Hirokazu Takahashi, Email: akeizo@cc.saga-u.ac.jp, Email: takahas2@cc.saga-u.ac.jp.
Keizo Anzai, Email: akeizo@cc.saga-u.ac.jp, Email: takahas2@cc.saga-u.ac.jp.
References
Author names in bold designate shared co‐first authorship.
- 1. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Hepatology 2019;69:2672‐2682. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 3. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with nonalcoholic fatty liver disease. J Hepatol 2008;48:829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta‐analysis. Diabetes Care 2018;41:372‐382. [DOI] [PubMed] [Google Scholar]
- 6. Dyson J , Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014;60:110‐117. [DOI] [PubMed] [Google Scholar]
- 7. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol 2018;68:335‐352. [DOI] [PubMed] [Google Scholar]
- 8. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 9. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 11. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 12. Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018;137:323‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020;63:221‐228. [DOI] [PubMed] [Google Scholar]
- 14. Lai LL , Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci 2020;65:623‐631. [DOI] [PubMed] [Google Scholar]
- 15. Akuta N, Kawamura Y, Watanabe C, Nishimura A, Okubo M, Mori Y, et al. Impact of SGLT2 inhibitor to histological features and glucose metabolism of non‐alcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Res 2019;49:531‐539. [DOI] [PubMed] [Google Scholar]
- 16. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein‐Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864‐2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996;19:1138‐1141. [DOI] [PubMed] [Google Scholar]
- 18. Murawaki Y, Ikuta Y, Koda M, Yamada S, Kawasaki H. Comparison of serum 7S fragment of type IV collagen and serum central triple‐helix of type IV collagen for assessment of liver fibrosis in patients with chronic viral liver disease. J Hepatol 1996;24:148‐154. [DOI] [PubMed] [Google Scholar]
- 19. Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin‐positive Mac‐2 binding protein (WFA(+)‐M2BP), for assessing liver fibrosis. J Gastroenterol 2015;50:76‐84. [DOI] [PubMed] [Google Scholar]
- 20. Yoshizumi T, Nakamura T, Yamane M, Waliul Islam AHM, Menju M, Yamasaki K, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999;211:283‐286. [DOI] [PubMed] [Google Scholar]
- 21. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 22. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467‐2474. [DOI] [PubMed] [Google Scholar]
- 23. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 24. Maegawa H, Tobe K, Tabuchi H, Nakamura I. Baseline characteristics and interim (3‐month) efficacy and safety data from STELLA‐LONG TERM, a long‐term post‐marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real‐world clinical practice. Expert Opin Pharmacother 2016;17:1985‐1994. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz SL, Akinlade B, Klasen S, Kowalski D, Zhang W, Wilpshaar W. Safety, pharmacokinetic, and pharmacodynamic profiles of ipragliflozin (ASP1941), a novel and selective inhibitor of sodium‐dependent glucose co‐transporter 2, in patients with type 2 diabetes mellitus. Diabetes Technol Ther 2011;13:1219‐1227. [DOI] [PubMed] [Google Scholar]
- 26. Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2015;17:304‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li B, Wang Y, Ye Z, Yang H, Cui X, Wang Z, et al. Effects of canagliflozin on fatty liver indexes in patients with type 2 diabetes: a meta‐analysis of randomized controlled trials. J Pharm Pharm Sci 2018;21:222‐235. [DOI] [PubMed] [Google Scholar]
- 28. Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA‐REG OUTCOME® trial. Diabetologia 2018;61:2155‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee PCH , Gu Y, Yeung MY, Fong CHY, Woo YC, Chow WS, et al. Dapagliflozin and empagliflozin ameliorate hepatic dysfunction among Chinese subjects with diabetes in part through glycemic improvement: a single‐center, retrospective. Observ Study Diabetes Ther 2018;9:285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurinami N , Sugiyama S, Yoshida A, Hieshima K, Miyamoto F, Kajiwara K, et al. Dapagliflozin significantly reduced liver fat accumulation associated with a decrease in abdominal subcutaneous fat in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res Clin Pract 2018;142:254‐263. [DOI] [PubMed] [Google Scholar]
- 31. Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab 2018;20:438‐442. [DOI] [PubMed] [Google Scholar]
- 32. Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab 2018;21:812‐821. [DOI] [PubMed] [Google Scholar]
- 33. Tahara A, Kondo Y, Takasu T, Tomiyama H. Effects of the SGLT2 inhibitor ipragliflozin on food intake, appetite‐regulating hormones, and arteriovenous differences in postprandial glucose levels in type 2 diabetic rats. Biomed Pharmacother 2018;105:1033‐1041. [DOI] [PubMed] [Google Scholar]
- 34. Horie I, Abiru N, Hongo R, Nakamura T, Ito A, Haraguchi AI, et al. Increased sugar intake as a form of compensatory hyperphagia in patients with type 2 diabetes under dapagliflozin treatment. Diabetes Res Clin Pract 2018;135:178‐184. [DOI] [PubMed] [Google Scholar]
- 35. Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, et al. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective, single‐arm trial (LEAD trial). Hepatol Res 2019;49:64‐71. [DOI] [PubMed] [Google Scholar]
- 36. Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One 2016;11:e0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Honda Y, Imajo K, Kato T, Kessoku T, Ogawa Y, Tomeno W, et al. The selective SGLT2 inhibitor ipragliflozin has a therapeutic effect on nonalcoholic steatohepatitis in mice. PLoS One 2016;11:e0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonner C, Kerr‐Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512‐517. [DOI] [PubMed] [Google Scholar]
- 39. Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus: a 13C nuclear magnetic resonance study. J Clin Invest 1992;90:1323‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perry RJ, Zhang D, Guerra MT, Brill AL, Goedeke L, Nasiri AR, et al. Glucagon stimulates gluconeogenesis by INSP3R1‐mediated hepatic lipolysis. Nature 2020;579:279‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sawada Y, Izumida Y, Takeuchi Y, Aita Y, Wada N, Li E, et al. Effect of sodium‐glucose cotransporter 2 (SGLT2) inhibition on weight loss is partly mediated by liver‐brain‐adipose neurocircuitry. Biochem Biophys Res Commun 2017;493:40‐45. [DOI] [PubMed] [Google Scholar]
- 42. Patel V, Joharapurkar A, Kshirsagar S, Patel M, Sutariya B, Patel H, et al. Coagonist of glucagon‐like peptide‐1 and glucagon receptors ameliorates nonalcoholic fatty liver disease. Can J Physiol Pharmacol 2018;96:587‐596. [DOI] [PubMed] [Google Scholar]
- 43. Seghieri M, Christensen AS, Andersen A, Solini A, Knop FK, Vilsbøll T. Future perspectives on GLP‐1 receptor agonists and GLP‐1/glucagon receptor co‐agonists in the treatment of NAFLD. Front Endocrinol (Lausanne) 2018;9:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yip WW, Burt AD. Alcoholic liver disease. Semin Diagn Pathol 2006;23:149‐160. [DOI] [PubMed] [Google Scholar]
- 45. Lackner C, Gogg‐Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol 2008;48:821‐828. [DOI] [PubMed] [Google Scholar]
- 46. Maeda S, Matsui T, Takeuchi M, Yamagishi S. Sodium‐glucose cotransporter 2‐mediated oxidative stress augments advanced glycation end products‐induced tubular cell apoptosis. Diabetes Metab Res Rev 2013;29:406‐412. [DOI] [PubMed] [Google Scholar]
- 47. Hosokawa K , Takata T, Sugihara T, Matono T, Koda M, Kanda T, et al. Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipid deposition in renal tubules. Int J Mol Sci 2019;21:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, et al. Hypoxia‐inducible factor‐1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep 2019;9:14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp‐Arida A, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 2011;53:448‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Handa P, Morgan‐Stevenson V, Maliken BD, Nelson JE, Washington S, Westerman M, et al. Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. Am J Physiol Gastrointest Liver Physiol 2016;310:G117‐G127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material


