Abstract
Nonalcoholic fatty liver disease (NAFLD) is a common but complex chronic liver disease, driven by environmental and genetic factors. We assessed metabolic and dietary risk factor associations with NAFLD liver mortality using the Global Burden of Disease (GBD) 2017 data. NAFLD liver deaths were calculated (per 100,000) as age‐standardized rates (ASRs) from 195 countries and territories (21 GBD regions; 7 GBD superregions). Dietary risks included low intake of fruits, vegetables, legumes, whole grains, nuts/seeds, milk, fiber, calcium, seafood omega‐3 fatty acids, and polyunsaturated fatty acids, and high intake of red meat, processed meat, sugar‐sweetened beverages, trans fatty acids, and sodium. Metabolic risks included high low‐density lipoprotein cholesterol, systolic blood pressure (BP), fasting glucose (FG), body mass index (BMI), as well as low bone mineral density and impaired kidney function (IKF). Socio‐demographic index (SDI)–adjusted partial Spearman correlation coefficients and multivariable generalized linear regression models/bidirectional stepwise selection (significance level for entry, 0.2; for stay, 0.05) determined the associations. The ASR for NAFLD liver deaths was 2.3 per 100,000 (2017) and correlated with dietary risk factors (0.131, −0.010‐0.267) and metabolic risk factors (SDI‐adjusted = 0.225, 95% CI 0.086‐0.354). High intake of sugar‐sweetened beverages and red meat (0.358, 0.229‐0.475; 0.162, 0.022‐0.296), and low intake of nuts/seed and milk (0.154, 0.014‐0.289; 0.145, 0.004‐0.280) was significant for NAFLD liver deaths. Other risk factors for liver death included IKF (0.402, 0.276‐0.514), increased BMI (0.353, 0.223‐0.407), FG (0.248, 0.111‐0.376), and BP (0.163, 0.022‐0.297). High intake of trans fatty acids (2.84% increase [1.65%‐4.03%]) was the largest associated risk of NAFLD liver deaths. In addition to metabolic risks, dietary risks independently drive the global burden of NAFLD‐related liver mortality. Conclusion: These data provide additional support for policies to improve dietary environment for NAFLD burden reduction.
Abbreviations
- ASR
age‐standardized rate
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- COD
cause of death
- GBD
Global Burden of Disease
- IHME
Institute for Health Metrics and Evaluation
- IKF
impaired kidney function
- LDL
low‐density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- PAF
population attributable fraction
- SDI
socio‐demographic index
- UI
uncertainty interval
In 2016, the World Health Organization reported that more people around the world were obese or overweight than those who were malnourished and underweight.( 1 ) Obesity is now affecting 1 in 5 children. In this context, data from the United Kingdom suggest that the rate of obesity among children and adolescents (aged 5 to 19 years) has risen 10‐fold over 4 decades.( 2 ) This alarming rise in the rate of obesity in both adults and children is being observed across the world. Although this may partly be related to some genetic predisposition, the substantial increases in the rates of worldwide obesity is most likely attributed to unhealthy eating environments where the availability and affordability of healthy food is limited.( 1 , 2 ) In this context, the current evidence points at the widespread availability of cheap, ultraprocessed, calorie‐dense, nutrient‐poor foods that appear to be important contributors to the rising prevalence of obesity.( 1 , 2 )
These increases in obesity are responsible for the rise in obesity‐related complications such a type 2 diabetes and nonalcoholic fatty liver disease (NAFLD).( 3 , 4 ) In this context, NAFLD is now recognized as a major global cause of cirrhosis and hepatocellular carcinoma, and the second‐most‐common indication for liver transplantation in the United States.( 5 , 6 ) In addition, recent data from Global Burden of Disease (GBD) suggest that the incidence, mortality, and disability‐adjusted life years from NAFLD are increasing across the world.( 7 , 8 ) Currently, weight loss through diet and exercise is the cornerstone of the management of NAFLD, where weight loss of at least 5%‐10% can be associated with improvement of hepatic fibrosis, which is the most important independent predictor of mortality among those with NAFLD.( 3 , 9 , 10 ) Given the increasing burden of NAFLD across the world, our aim was to assess the contribution of dietary risk factors to the mortality of NAFLD using GBD.
Materials and Methods
Data Sources
This study was based on data obtained from the 2017 GBD study, which is presently being coordinated by the Institute for Health Metrics and Evaluation (IHME). As a continuous quality improvement, IHME annually updates each GBD study by re‐estimating the entire time series through the inclusion of all known advances in data, modeling, estimation methods, and health knowledge, to ensure that each GBD study contains the most up‐to‐date estimates. The GBD 2017 study was published in 2018 with epidemiologic assessments of 359 diseases and injuries and 84 risk factors from 195 countries and territories as well as subnational estimates for certain countries. For this study, we obtained the publication estimates of deaths for liver cancer and cirrhosis due to NAFLD as well as all‐cause death attributable to metabolic and dietary risk factors.( 11 ) Methodologies for GBD estimates have been published previously.( 7 , 8 , 12 , 13 ) Herein, we briefly present the GBD estimation process for liver cancer and cirrhosis deaths due to their etiology and all metabolic and dietary‐related deaths for 2017.
GBD Estimation Framework
In this GBD study, the cause of death (COD) database is a compilation of data that were assembled from a variety of primary source documents (vital registration, sample vital registration, and verbal autopsy). The incidence and mortality data are derived from a multiple‐step process with adjustments by age group and an aggregation of implausible and unspecified COD codes.( 14 ) Because mortality data from these sources were sparse in most countries, incidence data from cancer registries were converted to mortality data by modeling the mortality‐to‐incidence ratio. In the COD database, liver cancer and cirrhosis mortality were estimated separately using the COD ensemble model (CODEm). The CODEm is an approach that incorporates a wide variety of individual models (linear mixed‐effects regression and spatiotemporal Gaussian process regression) and covariates to create a predictive model for CODs. All individual and ensemble models were evaluated using out‐of‐sample predictive validity tests, were vetted by experts in each disease, and then validated by IHME and their collaborators from around the world. Mortality estimates were then scaled with other causes of deaths to sum to 100% of all‐cause mortality estimates within each age, sex, year, and location.( 15 )
Because International Classification of Diseases (10th Revision) coding is valid for defining liver cancer and cirrhosis and not for etiological estimates, the GBD study used the models to split the parent‐cause “liver cancer and cirrhosis” mortality and morbidity into the five causes, including hepatitis B virus, hepatitis C virus, alcohol, NAFLD or steatohepatitis, as well as other causes such as haemochromatosis, autoimmune hepatitis, Wilson disease, cryptogenic, idiopathic, or unknown. The proportions of liver cancer and cirrhosis cases and deaths due to different liver diseases were identified by the systematic literature review and modeled in the DisMod‐MR model, an integrative meta‐regression method to obtain age/sex/location and year‐specific estimates.( 15 , 16 ) A complete list of predictive covariates used in the models can be found in Supporting Table S1.
A potential limitation of the GBD estimates is lack of primary data, as the data are collected from cancer registries, vital registration systems, or other similar sources. To remedy this limitation, GBD uses an integrative meta‐regression approach that comprehensively incorporates all dimensions of health data, accounting for spatial heterogeneity and heterogeneity in data sources and biases. This approach is designed to correct inconsistencies and allow for analyzing diseases and risk factors within the same computational framework, facilitating comparison across geographies and disease categories. It is also important to note that GBD has developed a simple star‐rating system from 0 to 5, to give an assessment of the quality of the data available in a given country over the entire time series used for their estimates. A country list of GBD region with data‐quality rating for the causes of death data is available in Supporting Table S2.
Dietary and Metabolic Risk Factors Defined
Dietary risks included diets low in intake of fruits, vegetables, legumes, whole grains, nuts/seeds, milk, fiber, calcium, seafood omega‐3 fatty acids, and polyunsaturated fatty acids, as well as high intake of red meat, processed meat, sugar‐sweetened beverages, trans fatty acids, and sodium. GBD collected consumption of each dietary factor across nations through a systematic review of the scientific literature, nationally representative nutrition surveys, and household budget surveys. The optimal level of intake was defined as the level of risk exposure minimizing the risk from all‐cause death. High intake of a dietary component was defined as an intake level ≥ the midpoint of the optimal range of intake, whereas low intake was defined as an intake level ≤ the midpoint of the optimal range of intake.
Metabolic risk factors included high fasting plasma glucose, high low‐density lipoprotein (LDL) cholesterol, high systolic BP, high BMI, but low bone mineral density and impaired kidney function (IKF). Like a dietary risk factor, high/low metabolic risk factors were defined by comparing the theoretical minimum risk exposure level.
Deaths Attributable to Defined NAFLD/Nonalcoholic Steatohepatitis, Dietary, and Metabolic Risk Factors
The number of deaths attributable to each risk factor was estimated by multiplying the population attributable fraction (PAF) for the dietary and metabolic risks pair by the total number of deaths for each age, sex, location, and year. The PAF for a reduction in death risk is defined as the proportion in which the death rate would be reduced in a given population if the exposure to a risk factor was reduced to the optimal level of intake or theoretical minimum risk exposure level. The PAF reduction was estimated by the GBD comparative risk assessment approach.( 17 ) For example, using the metabolic and dietary risk factors, the GBD definition for each risk factor, the optimal level of intake, and the data representativeness index (defined as the fraction of countries where data were identified for the risk‐factor exposure, such that the closer to 100% the more representative the data are of the risk factor) (Table 1), we calculated the number of deaths attributed to risk factor.( 18 )
TABLE 1.
Definition, Optimal Level, and Data Representativeness Index for Dietary Risk Factor in 2017
| Definition | Optimal Level of Intake (Optimal Range of Intake)/Theoretical Minimum Risk Exposure Level | Data Representativeness Index (%)* | |
|---|---|---|---|
| Diet low in fruits | Mean daily consumption of fruits (fresh, frozen, cooked, canned, or dried fruits, excluding fruit juices and salted or pickled fruits) | 250 g (200‐300) per day | 94.9 |
| Diet low in vegetables | Mean daily consumption of vegetables (fresh, frozen, cooked, canned, or dried vegetables, excluding legumes and salted or pickled vegetables, juices, nuts, seeds, and starchy vegetables such as potatoes or corn) | 360 g (290‐430) per day | 94.9 |
| Diet low in legumes | Mean daily consumption of legumes (fresh, frozen, cooked, canned, or dried legumes) | 60 g (50‐70) per day | 94.9 |
| Diet low in whole grains | Mean daily consumption of whole grains (bran, germ, and endosperm in their natural proportion) from breakfast cereals, bread, rice, pasta, biscuits, muffins, tortillas, pancakes, and other sources | 125 g (100‐150) per day | 94.9 |
| Diet low in nuts and seeds | Mean daily consumption of nut and seed foods | 21 g (16‐25) per day | 94·9 |
| Diet low in milk | Mean daily consumption of milk including nonfat, low‐fat, and full‐fat milk, excluding soy milk and other plant derivatives | 435 g (350‐520) per day | 94.9 |
| Diet high in red meat | Mean daily consumption of red meat (beef, pork, lamb, and goat, but excluding poultry, fish, eggs, and all processed meats) | 23 g (18‐27) per day | 94.9 |
| Diet high in processed meat | Mean daily consumption of meat preserved by smoking, curing, salting, or addition of chemical preservatives | 2 g (0‐4) per day | 36.9 |
| Diet high in sugar‐sweetened beverages | Mean daily consumption of beverages with ≥ 50 kcal per 226·8 serving, including carbonated beverages, sodas, energy drinks, fruit drinks, but excluding 100% fruit and vegetable juices | 3 g (0‐5) per day | 36.9 |
| Diet low in fiber | Mean daily intake of fiber from all sources including fruits, vegetables, grains, legumes, and pulses | 24 g (19‐28) per day | 94.9 |
| Diet low in calcium | Mean daily intake of calcium from all sources, including milk, yogurt, and cheese | 1.25 g (1.00‐1.50) per day | 94.9 |
| Diet low in seafood omega‐3 fatty acids | Mean daily intake of eicosatetraenoic acid and docosahexaenoic acid | 250 mg (200‐300) per day | 94.9 |
| Diet low in polyunsaturated fatty acids | Mean daily intake of omega‐6 fatty acids from all sources, primarily liquid vegetable oils, including soybean oil, corn oil, and safflower oil | 11% (9‐13) of total daily energy | 94.9 |
| Diet high in trans fatty acids | Mean daily intake of trans fat from all sources, primarily partially hydrogenated vegetable oils and ruminant products | 0.5% (0.0‐1.0) of total daily energy | 36.9 |
| Diet high in sodium | 24‐hour urinary sodium (g/day) | 3 g (1‐5) per day* | 26.2 |
| High fasting plasma glucose | Serum fasting plasma glucose (mmol/L) | 4.8‐5.4 mmol/L | 67.9 |
| High LDL cholesterol | Serum LDL (mmol/L) | 0.7‐1.3 mmol/L | 71.5 |
| High systolic BP | Systolic BP (mm Hg) | 110‐115 mm Hg | 81.4 |
| High BMI | BMI (kg/m2) | 20‐25 kg/m2 | 100.0 |
| Low bone mineral density | Standardized mean bone mineral density values measured by dual X‐ray absorptiometry at the femoral neck (g/cm2) | 99th percentile of NHANES 1988‐2014 by age and sex | 25.9 |
| IKF | Proportion of the population with ACR > 30 mg/g or GFR < 60 mL/min/1.73 m2, excluding end‐stage renal disease | GFR > 60 mL/min/1.73 m2 and ACR < 30 mg/g | 31.1 |
Data representativeness index is defined as the fraction of countries where data were identified for the risk factor exposure such that the closer to 100%, the more representative the data are of the risk factor. Source: GBD 2017 Risk Factor Collaborators, 2018.
Abbreviations: GFR, glomerular filtration rate; and NHANES, National Health and Nutrition Examination Survey.
Relevant metadata can be retrieved through the publicly available Data Input Sources Tool (http://ghdx.healthdata.org/gbd‐2017/data‐input‐sources).
Risk Adjustments for Global/Regional Socio‐demographic Profiles and Age
The world is split, for administrative and data analysis purposes, into 21 GBD regions according to epidemiological similarities and geographical proximity. The SDI, which is a measure of average income per capita, educational attainment, and total fertility rate at the state level, is also available in the GBD study. The value of SDI is between 0 and 1, with a higher index indicating greater socio‐demographic development. Countries were also categorized into quintiles of the SDI (high, high‐medium, medium, medium‐low, and low level of development) (Supporting Table S2).
ASRs were based on the world standard population developed for the GBD study. GBD estimates for a disease burden are reported with the 95% uncertainty intervals (UIs), including the true value of a parameter with 95% probability. UIs account for not only variance in parameter estimation but also uncertainty from data collection, model selection, and other sources of uncertainty under the parameter‐estimation process.
Data Analysis
Results and findings of GBD 2017 can be explored interactively through the GBD visualization hub.( 19 ) To understand the burden of NAFLD, liver cancer and cirrhosis deaths due to NAFLD were combined to define liver deaths due to NAFLD. Because there are no published UIs for liver deaths due to NAFLD, we calculated 95% confidence intervals (CIs) based on the corresponding standard errors, obtained by the width of 95% UI divided by 1.96*2.
Associations at the country level were determined using unadjusted and SDI‐adjusted partial Spearman’s correlation coefficients (ρ) and multivariable generalized linear regression with gamma distribution. Independent risk factors were identified by bidirectional stepwise selection (significance level for entry, 0.2; for stay, 0.05) on a multivariable general linear model (GLM).
All analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). Microsoft Excel (Redmond, WA) was used for data visualization. This study was approved as exempt by the Inova Health Systems Internal Review Board.
Results
Globally, in 2017, there were 184,905 NAFLD‐related liver deaths, accounting for 8.6% of liver death, with the ASR for NAFLD liver deaths being 2.32 per 100,000. Dietary and metabolic factors accounted for 10.9 million deaths (19.5% of all deaths) and 17.6 million deaths (31.4% of all deaths), respectively, with an ASR of about 140.24 per 100,000 for all diet‐related deaths, and about 228.99 per 100,000 for those all metabolic‐related deaths (Fig. 1 and Supporting Tables S3 and S4).
FIG. 1.
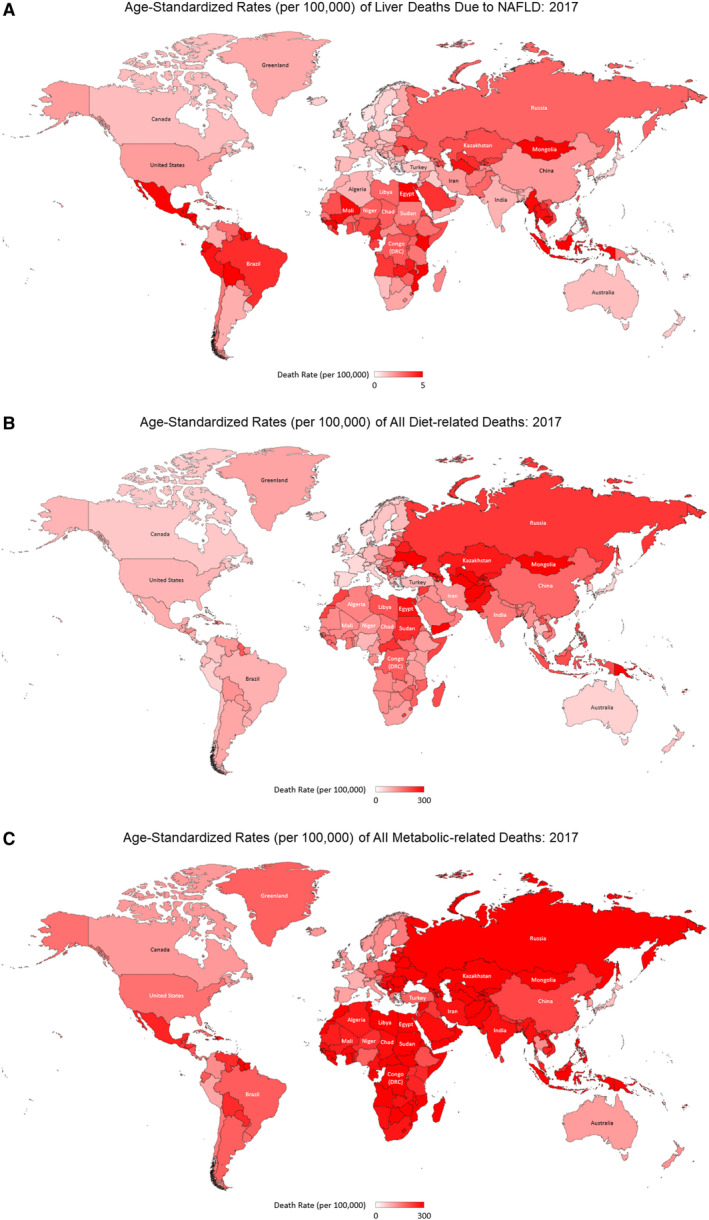
Age‐Standardized Rates (per 100,000) of Liver Deaths due to NAFLD: 2017 (A), All Diet‐related Deaths: 2017 (B) and All Metabolic‐related Deaths: 2017 (C).
NAFLD, Diet‐Related Risks, and Metabolic Risk with Mortality Across the 21 GBD Regions
GLM modeling was used to compare the ASR of liver deaths due to NAFLD, and all metabolic‐related and diet‐related deaths across the 21 GBD regions (Table 2) and superregions (Supporting Tables S5‐S7A‐C).
TABLE 2.
SDI‐Adjusted Comparisons in ASRs Related to Liver Deaths, Metabolic‐Related Deaths, and Diet‐Related Deaths Across 21 GBD regions in 2017
| Liver Deaths due to NAFLD | Metabolic Related Risks | Diet‐related Deaths | ||||
|---|---|---|---|---|---|---|
| SDI‐Adjusted Percent Change % (95% CI) | P | SSDI‐Adjusted Percent Change % (95% CI) | P | SDI‐Adjusted Percent Change % (95% CI) | P | |
| High‐income North America | Reference | Reference | Reference | |||
| Australasia | −27.72 (−63.3‐42.36) | 0.3480 | −19.14 (−44.87‐18.6) | 0.2769 | −23.53 (−50.21‐17.45) | 0.2204 |
| High‐income Asia Pacific | −33.34 (−62.2‐17.55) | 0.1611 | −12.08 (−36.2‐21.14) | 0.4310 | −5 (−33.67‐36.04) | 0.7794 |
| Southern Latin America | 4.76 (−43.08‐92.82) | 0.8813 | −4.35 (−32.25‐35.02) | 0.8003 | −12.27 (−40.38‐29.09) | 0.5064 |
| Western Europe | −29.73 (−55.52‐11.02) | 0.1306 | −10.65 (−31.01‐15.7) | 0.3930 | −20.69 (−40.63‐5.95) | 0.1167 |
| Central Europe | −9.29 (−43.66‐46.03) | 0.6881 | 73.94 (32.9‐127.65) | 0.0001 | 76.59 (30.64‐138.71) | 0.0002 |
| Eastern Europe | 82.67 (9.19‐205.6) | 0.0218 | 110.33 (57.38‐181.08) | 0.0000 | 143.92 (76.26‐237.56) | 0.0000 |
| Central Asia | 144.91 (47.53‐306.55) | 0.0005 | 127.99 (71.31‐203.42) | 0.0000 | 160.04 (88.81‐258.15) | 0.0000 |
| Southeast Asia | 98.51 (20.59‐226.77) | 0.0070 | 38.41 (4.79‐82.8) | 0.0220 | 24.96 (−8.47‐70.61) | 0.1606 |
| East Asia | 52.04 (−17.33‐179.6) | 0.1777 | 7.18 (−24.18‐51.5) | 0.6947 | 23.8 (−16.03‐82.53) | 0.2811 |
| Oceania | 94.47 (17.38‐222.19) | 0.0098 | 119.74 (65.18‐192.31) | 0.0000 | 92.52 (39.85‐165.03) | 0.0001 |
| South Asia | −7.19 (−48.21‐66.34) | 0.8021 | 16.77 (−15.9‐62.14) | 0.3545 | 11.97 (−22.48‐61.72) | 0.5467 |
| Andean Latin America | 244.25 (84.76‐541.42) | 0.0001 | −12.94 (−38.72‐23.69) | 0.4393 | −31.53 (−53.8‐1.48) | 0.0592 |
| Caribbean | 99.31 (23.86‐220.7) | 0.0045 | 38.06 (5.61‐80.47) | 0.0183 | −0.97 (−26.64‐33.69) | 0.9494 |
| Central Latin America | 145.91 (46‐314.19) | 0.0007 | −1.85 (−26.67‐31.37) | 0.9000 | −26.83 (−47.21‐1.4) | 0.0606 |
| Tropical Latin America | 89.12 (−5.03‐276.59) | 0.0698 | 8.02 (−26.8‐59.4) | 0.6976 | −14.11 (−44.45‐32.81) | 0.4941 |
| North Africa and Middle East | 47.89 (−7.82‐137.28) | 0.1047 | 60.78 (23.1‐110) | 0.0005 | 37.33 (1.79‐85.3) | 0.0379 |
| Central Sub‐Saharan Africa | 62.25 (−7.39‐184.25) | 0.0907 | 46.66 (6.67‐101.65) | 0.0184 | −0.8 (−30.56‐41.7) | 0.9647 |
| Eastern Sub‐Saharan Africa | 57.28 (−9.41‐173.07) | 0.1077 | 4.25 (−23.59‐42.21) | 0.7930 | −26.72 (−48.22‐3.69) | 0.0792 |
| Southern Sub‐Saharan Africa | 18.97 (−31.52‐106.71) | 0.5377 | 66.3 (21.93‐126.82) | 0.0013 | 15.01 (−18.74‐62.78) | 0.4302 |
| Western Sub‐Saharan Africa | 72.56 (0.26‐197) | 0.0489 | 2.34 (−24.47‐38.67) | 0.8811 | −24.91 (−46.53‐5.45) | 0.0982 |
| SDI | −49.27 (−73.4 to −3.25) | 0.0394 | −74.02 (−81.81 to −62.88) | 0.0000 | −84.93 (−89.88 to −77.57) | 0.0000 |
Note: Pink means that the trend is negative (and when it turns red the trend is even worse).
In the SDI‐adjusted model, compared with high‐income North America, Andean Latin America, Central Latin America, Central Asia, Caribbean, South East Asia, Oceana, and Eastern Europe experience higher NAFLD liver deaths (Table 2). Of these countries, all except Andean Latin America and Central Latin America experienced an increase in SDI‐adjusted metabolic deaths. Furthermore, of the regions with increase in liver deaths, only Eastern Europe, Eastern Asia, and Oceana also experienced higher diet‐related deaths.
Association of NAFLD With Diet Risks and Metabolic Risk at the Country Level
The pattern of ASR for NAFLD liver deaths versus all‐cause death attributable to metabolic and dietary risks in 2017 is shown in Fig. 2A,B.
FIG. 2.
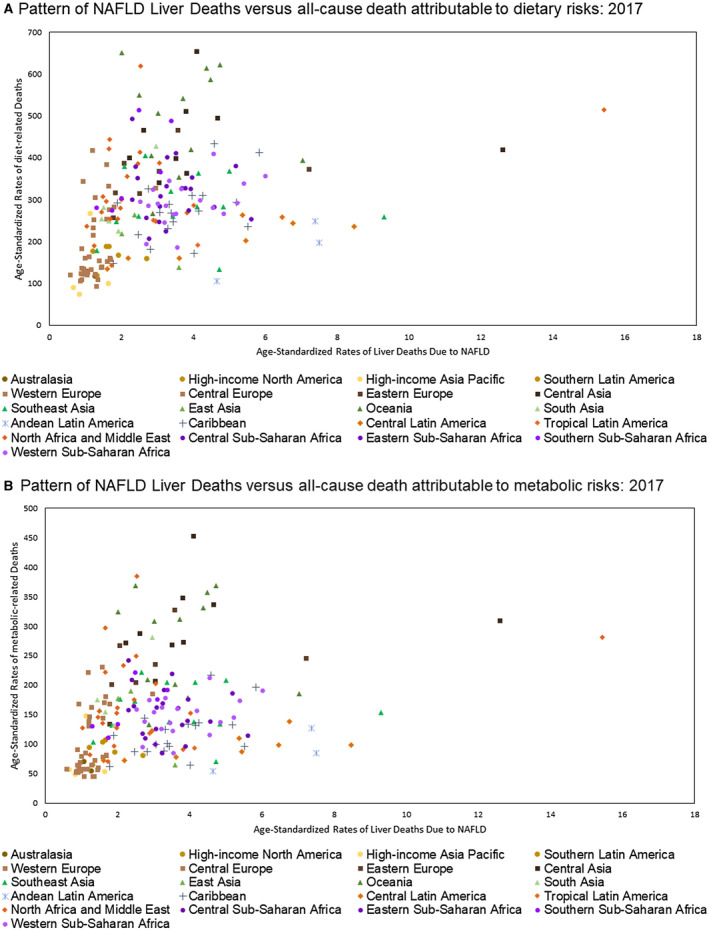
Pattern of Age Standardized Rate for NAFLD Liver Deaths by Dietary (A) and Metabolic (B) Risk Factors.
Globally, NAFLD liver deaths were associated with metabolic risk factors (SDI‐adjusted ρ = 0.225 [0.086‐0.354, P = 0.002]) and dietary risks (SDI‐adjusted ρ = 0.131 [−0.01‐0.267, P = 0.069]) (Table 3). Across super GBD regions, the highest SDI‐adjusted correlations for dietary and metabolic risk factors and NAFLD liver deaths were observed in Central and Eastern Europe (ρ = 0.643 [0.354‐0.819, P < 0.001]) and Central Asia (P = 0.384 [0.013‐0.662, P = 0.043]), as well as North Africa (ρ = 0.631 [0.262‐0.839, P = 0.002]) and the Middle East (ρ = 0.623 [0.25‐0.835, P = 0.003]) (Supporting Table S8).
TABLE 3.
Spearman’s Partial Correlation Coefficients for Association Between Liver Deaths due to NAFLD, With Death Attributable to Risk Factors (2017)
| Risk | SDI‐Adjusted ρ (95% CI) | P |
|---|---|---|
| Dietary risks | 0.131 (−0.010‐0.267) | 0.0688 |
| Diet low in fruits | 0.005 (−0.136‐0.146) | 0.9409 |
| Diet low in vegetables | 0.128 (−0.013‐0.264) | 0.0752 |
| Diet low in nuts and seeds | 0.154 (0.014‐0.289) | 0.0318 |
| Diet low in whole grains | 0.111 (−0.03‐0.248) | 0.1230 |
| Diet low in milk | 0.145 (0.004‐0.28) | 0.0441 |
| Diet high in red meat | 0.162 (0.022‐0.296) | 0.0238 |
| Diet high in processed meat | 0.07 (−0.072‐0.209) | 0.3337 |
| Diet high in sugar‐sweetened beverages | 0.358 (0.229‐0.475) | 0.0000 |
| Diet low in fiber | 0.084 (−0.057‐0.223) | 0.2423 |
| Diet low in seafood omega‐3 fatty acids | 0.021 (−0.12‐0.162) | 0.7685 |
| Diet low in polyunsaturated fatty acids | 0.022 (−0.119‐0.162) | 0.7612 |
| Diet high in trans fatty acids | 0.103 (−0.039‐0.24) | 0.1538 |
| Diet high in sodium | 0.147 (0.006‐0.282) | 0.0409 |
| Diet low in calcium | 0.072 (−0.069‐0.211) | 0.3157 |
| Diet low in legumes | 0.04 (−0.102‐0.179) | 0.5839 |
| Metabolic risks | 0.225 (0.086‐0.354) | 0.0019 |
| High fasting plasma glucose | 0.248 (0.111‐0.376) | 0.0005 |
| High BP | 0.163 (0.022‐0.297) | 0.0232 |
| High BMI | 0.353 (0.223‐0.47) | 0.0000 |
| Low bone mineral density | −0.05 (−0.19‐0.091) | 0.4865 |
| IKF | 0.402 (0.276‐0.514) | 0.0000 |
| High LDL cholesterol | 0.106 (−0.036‐0.243) | 0.1432 |
Bold indicates statistically significant percent changes.
Dietary risk factors associated with NAFLD liver deaths included high sugar‐sweetened beverages (ρ = 0.358 [0.229‐0.475]), low intake of nuts and seeds (ρ = 0.154 [0.014‐0.289]), and low intake of milk (ρ = 0.145 [0.004‐0.280]), as well as IKF (ρ = 0.402 [0.276‐0.514]), higher BMI (ρ = 0.353 [0.223‐0.407]), high fasting plasma glucose (ρ = 0.248 [0.111‐0.376]), and high systolic BP (ρ = 0.163 [0.022‐0.297]) (Table 3). However, there was wide geographic variation of these findings across the superregions, which is summarized in Supporting Table S8. After adjustments for regions and SDI, high intake of trans fatty acids (2.84% increase [95% CI 1.65%‐4.03%]) and IKF (0.71% increase [0.25%‐1.18%]) were associated with a high risk of NAFLD liver deaths. Comparisons across GBD region and GBD superregion data are summarized in Supporting Table S9.
Discussion
As the burden of NAFLD increases globally, understanding the contribution of the dietary factors to this rising global epidemic is extremely important. In this study, we used the GBD database to determine the associations of dietary and metabolic risks with mortality related to NAFLD. Our data show that in 2017, there were 184,905 deaths from liver complications due to NAFLD, which translated into an ASR of NAFLD liver deaths of 2.32 per 100,000 persons. As expected, the presence of metabolic risk factors was closely associated with NAFLD, such as high cholesterol, and high fasting blood glucose was associated with NAFLD liver deaths. More disturbingly, a number of dietary factors such as low intake of fruits, vegetables, seeds, and legumes were responsible for a large number of NAFLD liver deaths. As such, these dietary factors could be superimposed on metabolic risk factors, leading to more NAFLD‐related liver deaths than reported.( 20 , 21 ) In fact, our data show that NAFLD liver deaths were associated with metabolic risk factors even after adjustment for socio‐demographic factors. This finding corroborates a recent study’s results, which highlighted that for every metabolic component present in those with NAFLD, the higher the risk of mortality.( 5 ) In addition, we found that countries with the highest mortality from metabolic disease were also the countries with the highest prevalence of reported NAFLD among their adult population. For example, in this report we found that compared with North American, Oceania and Central Asia had the highest rates of metabolic disease–related mortality, and in the latest reports on the prevalence of NAFLD worldwide, both of these areas had the highest rates of obesity and type 2 diabetes mellitus, which suggests a high prevalence of NAFLD.( 20 ) Although the strong association of metabolic risks with NAFLD mortality has been previously reported from country‐specific data, this analysis provides a more global perspective.
More importantly, our analysis showed an important association between dietary risk factors and NAFLD‐related mortality. We noted that the highest dietary factor associated with mortality related to NAFLD was the consumption of fructose‐sweetened beverages in all regions of the world, even after adjusting for the SDI. Additionally, the dietary risk of NAFLD liver mortality included the low intake of nuts and seeds, fruits, whole grains, and omega‐3‐fatty seafood, which are all dietary components of the Mediterranean diet. In addition, our multivariable analysis showed that a high intake of trans fatty acids was associated with a 3% increase in a liver death due to NAFLD. In this context, our data provide indirect evidence that diets rich in components of the Mediterranean diet may positively impact mortality from NAFLD. In fact, a diet rich in fresh vegetables, fruit, legumes, minimally processed whole grains, omega 3 rich seafood, along with other omega 3 rich foods such as olive oil, as well as a reduction in the intake of processed red meat, dairy, and the avoidance of fructose, may have a beneficial impact on mortality from NAFLD.( 21 , 22 , 23 ) Despite these data, it is important to point out that the impact of a diet rich in omega 3–rich foods on the histologic features of NASH has recently been questioned.( 24 , 25 ) Nevertheless, the authors did conclude that a diet rich in omega 3 may be still be beneficial in certain regions of the world where the local diets are deficient of these nutrients. In this context, our study provides evidence that the use of omega 3, especially when obtained through natural sources, may be advantageous for patients with NAFLD. We believe additional research is needed to further clarify the impact of natural sources of omega 3 on the long‐term outcomes of NAFLD.
Additionally, it is important to note that the Mediterranean diet is not overly high in carbohydrates, and this may provide an additional benefit of this diet in NAFLD.( 26 ) Finally, the beneficial impact of the Mediterranean diet can be enhanced by moderate exercise related to a reduction in visceral and hepatic fat.( 27 ) Again, further research is needed to confirm the benefit of exercise in patients with NAFLD.
Interestingly, when comparing other world countries to North America using multivariable analysis, we found that Andean Latin America experienced the highest increase in the number of NAFLD liver deaths. This finding can partially be explained by the fact that this region has one of the highest ASRs of deaths related to ingestion of sugar (fructose) beverages, and provides additional evidence supporting the negative impact of sugar‐sweetened beverages, which may override the positive impact of another healthier dietary factor.( 28 , 29 ) In this context, the geographic variation suggests that the impact of dietary factors on NAFLD liver death may be influenced by other variables such as place of living, income, availability of food, education, affordability, convenience, promotion, and quality.( 30 , 31 , 32 , 33 , 34 , 35 ) These issues must be considered by policy makers for a national and regional strategy to help manage NAFLD and its complications.
The most important strength of the current study is that we used the data from GBD estimates. GBD estimates provide the only peer‐reviewed estimates of cause‐specific mortality available for each age, sex, year, and location throughout the world.
However, these data have some limitations. An important shortcoming is based on the GBD estimates themselves, which are dependent on the quality and availability of each country’s vital registration system for some locations. To overcome these limitations, a modeling process was used to determine trends, although it is important to realize that in the GBD, dietary and metabolic risk factors are not currently considered in their modeling strategies for NAFLD‐related deaths. Therefore, our analyses relied on all‐cause death attributable to NAFLD risk factors, not NAFLD‐related death. In this context, our reporting may show an underestimation or overestimation for the association. Additionally, the GBD framework of estimation tends to underestimate liver cancer mortality in the low‐income countries due to the lack of advanced diagnostic techniques. Nevertheless, we believe that our integrative methodology helped us to overcome these limitations and provided reasonable estimates to better understand the metabolic and dietary factors that could be driving liver mortality among those with NAFLD. However, we believe that further research is needed to confirm our results.
The burden of NAFLD and its associated comorbidities is growing around the world. Despite this increasing burden, there is no effective pharmacotherapy for NAFLD. Therefore, dietary intervention remains an important cornerstone of managing patients with NAFLD. Our data provide regional and global data about dietary factors most associated with NAFLD deaths. These data can not only help policy makers in providing strategic programs to make a positive impact on NAFLD‐related deaths, but can also be used as a foundation for further study on the impact of these dietary risk factors and NAFLD‐related mortality.
Supporting information
Table S1‐S9
Potential conflict of interest: ZMY has received research funds or served as consultant to Gilead Sciences, Intercept, NovoNordisk, BMS, AbbVie, Merck, Madrigal, Genfit, Siemens, BMS, Terns, and Viking.
References
- 1. World Health Organization . Obesity facts. https://www.who.int/health‐topics/obesity#tab=tab_1. Accessed February 10, 2020. [Google Scholar]
- 2. Imperial College of London . Childhood obesity. https://www.who.int/news‐room/detail/11‐10‐2017‐tenfold‐increase‐in‐childhood‐and‐adolescent‐obesity‐in‐four‐decades‐new‐study‐by‐imperial‐college‐london‐and‐who. Accessed February 10, 2020. [Google Scholar]
- 3. Mishra A, Younossi ZM. Epidemiology and natural history of non‐alcoholic fatty liver disease. J Clin Exp Hepatol 2012;2:135‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Healthline . Type 2 diabetes mellitus. https://www.healthline.com/health/type‐2‐diabetes/statistics#Worldwide. Accessed February 10, 2020. [Google Scholar]
- 5. Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine. 2018;97:e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 7. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 2020;72:1605‐1616. [DOI] [PubMed] [Google Scholar]
- 8. Golabi P, Paik JM, Arshad T, Younossi Y, Mishra A, Younossi ZM. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatol Commun 2020;4:1136‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Younossi ZM, Stepanova M, Rafiq N, Henry L, Loomba R, Makhlouf H, et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol Commun 2017;1:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611‐1625.e12. [DOI] [PubMed] [Google Scholar]
- 11. GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Global Burden of Disease Liver Cancer Collaboration , Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Institute for Health Metrics and Evaluation . The power of models. http://www.healthdata.org/acting‐data/power‐models. Published October 26, 2018. Accessed July 30, 2019. [Google Scholar]
- 16. Flaxman AD, Vos T, Murray CJL, eds. An Integrative Metaregression Framework for Descriptive Epidemiology. Seattle, WA: University of Washington Press; 2015. [Google Scholar]
- 17. GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. Health effects of dietary risks in 195 countries, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;393:1958‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. University of Washington Institute of Health Metrics and Evaluation. 2015. https://vizhub.healthdata.org/gbd‐compare/. Accessed June 27, 2019. [Google Scholar]
- 20. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 21. Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of population‐based observational studies. PLoS Med 2020;17:e1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672‐2682. [DOI] [PubMed] [Google Scholar]
- 23. Tosti V, Bertozzi B, Fontana L. Health benefits of the mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci 2018;73:318‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al.; WELCOME Study . Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology 2014;60:1211‐1221. [DOI] [PubMed] [Google Scholar]
- 25. Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M.; EPE‐A Study Group . No significant effects of ethyl‐eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014;147:377‐384.e1. [DOI] [PubMed] [Google Scholar]
- 26. Goss AM, Dowla S, Pendergrass M, Ashraf A, Bolding M, Morrison S, et al. Effects of a carbohydrate‐restricted diet on hepatic lipid content in adolescents with non‐alcoholic fatty liver disease: a pilot, randomized trial. Pediatr Obes 2020;15:e12630. [DOI] [PubMed] [Google Scholar]
- 27. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non‐alcoholic fatty liver disease. J Hepatol 2013;59:138‐143. [DOI] [PubMed] [Google Scholar]
- 28. Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology 2018;155:107‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rafiq N, Younossi ZM. Effects of weight loss on nonalcoholic fatty liver disease. Semin Liver Dis 2008;28:427‐433. [DOI] [PubMed] [Google Scholar]
- 30. Downs SM, Ahmed S, Fanzo J, Herforth A. Food environment typology: advancing an expanded definition, framework, and methodological approach for improved characterization of wild, cultivated, and built food environments toward sustainable diets. Foods 2020;9:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci 2016;61:1282‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leslie T, Pawloski L, Kallman‐Price J, Escheik C, Hossain N, Fang Y, et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann Hepatol 2014;13:533‐540. [PubMed] [Google Scholar]
- 33. Zhang T, Huang B. Local retail food environment and consumption of fruit and vegetable among adults in Hong Kong. Int J Environ Res Public Health 2018;15:2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spence JC, Cutumisu N, Edwards J, Raine KD, Smoyer‐Tomic K. Relation between local food environments and obesity among adults. BMC Public Health 2009;18:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, et al.; US Burden of Disease Collaborators . The state of US health, 1990‐2016: burden of diseases, injuries, and risk factors among US states. JAMA 2018;319:1444‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S9


