Abstract
Premature ovarian failure (POF) has become one of the main causes of infertility in women of childbearing age and the incidence of POF is increasing year by year, seriously affecting the physical and mental health of patients and increasing the economic burden on families and society as a whole. The etiology and pathogenesis of POF are complex and not very clear at present. Currently, hormone replacement therapy is mainly used to improve the symptoms of low estrogen, but cannot fundamentally solve the fertility problem. In recent years, stem cell (SC) transplantation has become one of the research hotspots in the treatment of POF. The results from animal experiments bring hope for the recovery of ovarian function and fertility in patients with POF. In this article, we searched the published literature between 2000 and 2020 from the PubMed database (https://pubmed.ncbi.nlm.nih.gov), and summarized the preclinical research data and possible therapeutic mechanism of mesenchymal stem cells (MSCs) in the treatment of POF. Our aim is to provide useful information for understanding POF and reference for follow-up research and treatment of POF.
Keywords: mesenchymal stem cells, fertility, premature ovarian failure (POF), ovarian dysfunction, reproductive medicine
Introduction
POF is a kind of ovarian dysfunction characterized by menstrual disorder, ovarian atrophy, decreased sexual life and decreased fertility in women between puberty and 40 years old, which seriously affects female reproductive health and endocrine balance and is one of the main causes of female infertility (Sheikhansari et al., 2018). Approximately 1% of women under the age of 40 suffer from premature ovarian failure (Huhtaniemi et al., 2018). Under the influence of high pressure and a fast paced life, the incidence of POF is increasing and manifesting at younger ages, and it has affected more than 10% of women in recent years (Thakur et al., 2018).
POF treatment is extremely difficult. Although assisted reproductive technology has become an effective treatment, it is not ideal, and fertility loss and low estrogen status have become a great threat to female reproductive health (Laven, 2016). POF has become one of the most severe problems threatening the reproductive health of women of normal childbearing age. Its occurrence may be related to an insufficient reserve of primordial follicular cistern, accelerated follicular atresia, changes of dominant follicular recruitment, follicular maturation disorders and so on (Xiang et al., 2019). In view of the limitations of conventional treatment, clinical and scientific research work has focused on improving ovarian function and restoring fertility in patients with POF. In recent years, MSC transplantation has opened up a new direction for the treatment of POF, but this is still in the stage of preclinical research (Lai et al., 2015; Sun et al., 2017; Zhang et al., 2018; Liu S. et al., 2019; Zheng et al., 2019), and there are few clinical studies so far. The mechanism by which MSCs improve ovarian function has also not been completely elucidated. At present, there is no clear and effective treatment to restore the reproductive function of ovaries. In this paper, we reviewed the preclinical research data of the treatment of POF using MSCs and the possible therapeutic mechanisms to provide a reference for follow-up research and treatment of POF.
The Current Situation of POF Treatment
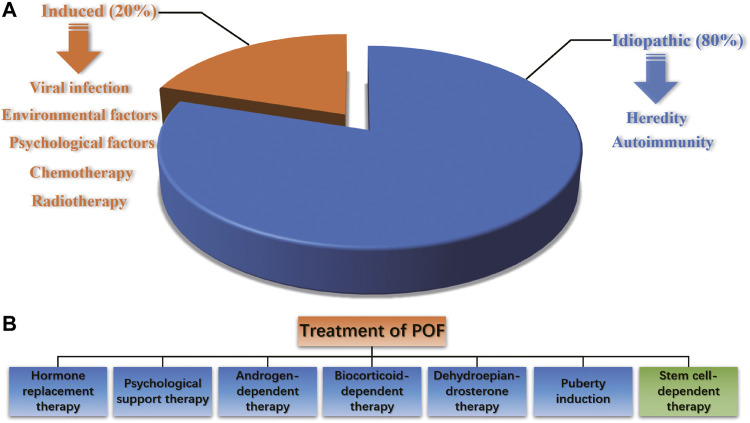
POF is a reproductive endocrine disease that occurs before the age of 40 and is characterized by increased gonadotropin levels and decreased estrogen levels, accompanied by primary or secondary amenorrhea. It is also one of the common diseases leading to female infertility. POF is a highly heterogeneous condition. Abnormal follicular development in all stages can lead to POF, and such damage to ovarian function is irreversible. The pathogenic factors of POF include heredity, autoimmunity, viral infection, iatrogenic factors, and environmental and psychological factors, and approximately eighty percent of POF cases are idiopathic (Webber et al., 2016) (Figure 1A). It has been reported that radiotherapy, chemotherapy and bone marrow transplantation of cancer can result in POF (Dolmans and Donnez, 2021; Imai et al., 2008). The traditional treatment of POF includes hormone replacement therapy (HRT), psychological support therapy, androgen-dependent therapy, biocorticoid-dependent therapy, dehydroepiandrosterone therapy and puberty induction (Figure 1B). However, HRT can only relieve low estrogen symptoms such as vaginal dryness, hot flashes and genitourinary tract atrophy, but has no essential effect on improving ovarian reproductive function. Long-term use of HRT is controversial because it increases the risk of endometrial and ovarian cancer (Ali, 2013; Lee et al., 2020). Since the etiology of POF infertility is complex, the current treatment efficacy is unsatisfactory, and the pregnancy rate and carrying to term rates are still quite low after treatments. Therefore, for women with fertility requirements, it is necessary to strengthen early prevention, early detection and early treatment to delay the development of POF and improve the live birth rate.
FIGURE 1.
The pathogenic factors and treatment options of POF.
The results from animal experiments of MSC transplantation has brought hope to the recovery of ovarian function and fertility in patients with POF. In the following, we will introduce advances in the treatment of POF with MSCs.
MSCs and Fertility Protection
MSCs were the first type of adult stem cell discovered in bone marrow. They originate from mesoderm and are distributed in almost all connective tissue and organ stroma of the entire body. They have the potential for multidirectional differentiation of stem cells and also have a strong migration ability to damaged tissues. Since MSCs have low immunogenicity and fewer disputes in bioethics than fetal-derived stem cells, they are widely applied in clinical research and medical bioengineering (Pers et al., 2016; Badawy et al., 2017; Mu et al., 2018). Currently, MSCs have been used to treat diseases related to the blood, nervous, motor, cardiovascular and skin systems, showing good curative effects (Zaher et al., 2014).
The reproductive capacity of most female mammals is mainly affected by the primordial follicular pool. Under normal circumstances, to avoid depletion of the follicular pool, most primordial follicles in the ovary are maintained in a resting phase. Primordial follicles undergo follicular activation and a series of developmental processes and finally develop into mature follicles. Various molecules are involved in regulating follicular activation, growth and atresia. Ovarian function recovery is based on oocyte production and follicular quantity/quality recovery (Woods and Tilly, 2012; Truman et al., 2017). Several studies have shown that MSCs can directly differentiate into oocyte-like cells, and transplantation of MSCs is conducive to restoring ovarian function and reproductive capacity (Bahrehbar et al., 2020; Yoon et al., 2020; Taheri et al., 2021). Therefore, MSCs are considered a new choice for the treatment of POF.
The effectiveness of MSCs in the treatment of reproductive system diseases has been confirmed by preclinical and clinical research, which has brought great hope to POF infertility and improved female reproductive health (Herraiz et al., 2019; Fu et al., 2021; Li et al., 2021). MSCs used for the treatment of POF include BMSCs, UCMSCs, PMSCs, AMSCs, AFMSCs, MenSCs and ADMSCs. MSCs originating from different sources have some common characteristics, which make them an ideal treatment choice for POF. A number of animal experiments and clinical trials have confirmed that ovarian function can be improved by MSC homing, inhibiting the apoptosis of OGC and promoting ovarian angiogenesis (Esfandyari et al., 2020). For example, Yan et al. transplanted MSCs to 61 patients with POF and found that the number of follicles in each developmental stage, including antral follicles, dominant follicles and mature follicles, increased significantly (Yan et al., 2020). Other researchers have found that autologous MSC transplantation can trigger menstruation to resume, relieve menopausal symptoms, improve ovarian function and help patients become pregnant (Bukovsky and Caudle, 2012; Igboeli et al., 2020; Mashayekhi et al., 2021; Ulin et al., 2021). Ling et al. treated POF mice with MSCs and found that MSC transplantation could significantly restore their hormone secretion ability, improve follicular growth and GC survival, and recover the ovarian function that was destroyed by chemotherapy used to create the POF mice (Ling et al., 2019). A meta-analysis of POF indicated that MSCs could decrease the level of FSH, increase the level of E2 and promote the proliferation of follicles, thus improving the quality of ovaries in POF animals and humans (Chen et al., 2018). Interestingly, Bahrehbar et al. proved that MSC-transplanted POF mice can produce offspring (Bahrehbar et al., 2020).
Table 1 summarizes the preclinical and clinical trials that indicate the validity of treating POF with MSCs. However, the underlying molecular and cellular mechanisms are still controversial and need to be further clarified. Additionally, current clinical research is still insufficient, and there is still a long way to go before the large-scale clinical application of MSCs.
TABLE 1.
Advances in the treatment of POF with MSCs.
| Research category | Type of MSCs | Method | Outcome of MSC treatment | Molecular mechanism | Biological effect | References |
|---|---|---|---|---|---|---|
| Preclinical research/animal experiment | Mouse menSCs | Injection by the tail vein | Repairing ovarian injury, improving ovarian function and stimulating regeneration | MenSCs produce high level of FGF2, which is essential for angiogenesis and the proliferation and remodeling of endometrial cells that plays important roles in repairing and regenerating the damaged tissues | MenSCs increase the follicular numbers, return sex hormone level, repair oocyte function and protect ovary damage | Wang et al. (2017) |
| Preclinical research/animal experiment | Human PMSCs | Injected subcutaneously | Restoring ovarian function | PMSCs activate the PI3K/Akt pathway, reduce Th17 cells percentage and increase Treg cells percentage | PMSCs increase serum levels of E2 and AMH and decrease FSH, LH and AZPAb levels | Yin et al. (2018b) |
| Preclinical research/animal experiment | Human AMSCs | Intraperitoneal injection and intragastric administration | Improving injured ovarian tissue structure and function | AMSC transplantation elevate serum oestrogen level and decrease FSH secretions | AMSCs promote follicular development, granulosa cell proliferation and secretion function by improving the local microenvironment of POF mouse ovary | Liu et al. (2019b) |
| Preclinical research/animal experiment | Mouse ADSCs | Intravenous injection | Improving ovarian function | Expression levels of ZCCHC11, ANGPTL and ONECUT2 are upregulated | ADSCs increase follicle number, ovulation and inhibit cell apoptosis in POF ovaries | Sun et al. (2013) |
| Preclinical research/laboratory research | Human BMSCs | Collection of MSC conditioned media | — | BMSCs conditioned media increase angiogenesis marker including VEGF, VEGFR, Endoglin, Tie-2 and VE-Cadherin through the PI3K/ALK pathway | MSC conditioned media stimulates the proliferation of HOVEC cells | Park et al. (2019) |
| Preclinical research/animal experiment | Human BMSCs | Intraovarian injection | Restoring ovarian hormone production and reactivating folliculogenesis | BMSCs decrease FSH level and increase AMH level | BMSCs induce follicle growth and increase the pregnancy rate | Mohamed et al. (2018) |
| Preclinical research/animal experiment | Human UCMSC | Intraovarian injection | UCMSC transplantation preserved ovarian function of POF mice | UCMSC transplantation increase estrogen (E2) and AMH levels, and increase the expression of CD31 | UCMSCs increase ovarian volume and the number of antral follicles, and promote granulosa cell proliferation and ovarian angiogenesis | Yang et al. (2019b) |
| Clinical research | Human UCMSC | Intraovarian injection | Two POF patients conceived naturally within 1 year after UCMSC transplantation | UCMSCs activate primordial follicles via phosphorylation of FOXO3a and FOXO1 | UCMSCs rescue ovarian function, elevate estradiol concentrations, improve follicular development and increase the number of antral follicles | Ding et al. (2018) |
| Clinical research | Human autologousBMSC | Laparoscopic intraovarian injection | BMSC treatment revealed promising improvement of POF. | — | BMSCs elevate serum estrogen level, increase volume of the treated ovaries and improve menopausal symptoms | Igboeli et al. (2020) |
| Clinical research | Human autologous BMSC | Intraovarian instillation | Perimenopausal woman delivered a healthy baby | BMSCs increase AMH level | BMSCs improve follicular development | Gupta et al. (2018) |
| Clinical research | Human autologous BMSC | Intraarterial catheterization to ovarian artery | 5/15 poor responders conceived and 3 healthy babies were born after the stem cell administration | BMSCs increase AMH level and antral follicular count | BMSCs increase the number of antral follicles and retrieve oocytes | Herraiz et al. (2018) |
| Clinical research | Human autologous ADSCs | Intraovarian injection | Menstruation resumption | BMSCs decreased FSH level | — | Mashayekhi et al. (2021) |
| Clinical research | Human UCMSC | Intraovarian injection | UCMSC transplantation improved the injured ovarian function, and 4/61 POI patients obtained clinical delivery | — | UCMSCs increase follicular development and improve egg collection | Yan, et al. (2020) |
The Mechanism of Treating POF With MSCs
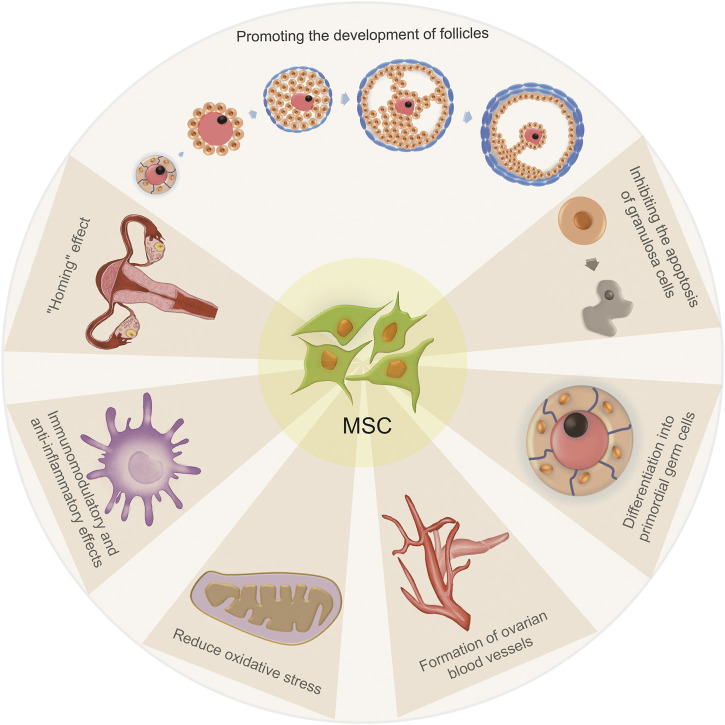
The mechanism of treating POF with MSCs can be summarized as follows (Figure 2): 1) MSCs have a “homing” effect; 2) MSCs can promote the growth and development of follicles at all developmental stages; 3) MSCs may induce and differentiate into primordial germ cells (uncertain); 4) MSCs can directly differentiate into GCs or inhibit the apoptosis of GCs; 5) MSCs can promote the formation of ovarian blood vessels; 6) MSCs have immunomodulatory and anti-inflammatory effects and 7) MSCs can reduce oxidative stress.
FIGURE 2.
The mechanisms of treating POF with MSCs.
Homing Effect of MSCs
The homing capacity of MSCs is an important determinant of effective MSC-based therapy (Li et al., 2017; Lin et al., 2017). Homing refers to the process by which MSCs migrate to damaged tissues and promote their recovery. Therefore, enhancing the homing efficiency of MSCs is essential for optimizing the therapeutic outcome of POF. Noory et al. reported the application of MenSC transplantation as a treatment modality in a rat model of POF and observed that MSCs can survive in ovarian stroma at 2 months after MSC transplantation and directly differentiated into GCs (Noory et al., 2019). Experiments from Liu et al., Jalalie, et al., Lai et al., Song et al. and Park et al. also demonstrated that after transplantation, MSCs home to damaged tissue and reach the site of injured ovaries (Liu et al., 2014; Lai et al., 2015; Jalalie et al., 2019; Park et al., 2021). However, studies have also shown that although MSCs have a homing effect, they cannot directly differentiate into oocytes but do localize in the ovarian matrix, secrete various cytokines and improve ovarian reserve function through the paracrine pathway (Takehara et al., 2013; Gabr et al., 2016; Li et al., 2017). A study by Taheri et al. demonstrated that MSC isolated from follicular fluid cultured in human recombinant BMP15 medium may differentiate into oocyte-like cells in vitro, but they did not investigate whether such MSCs can differentiate into oocytes in vivo (Taheri et al., 2021). Therefore, whether MSCs can directly differentiate into oocytes remains unclear, and more in-depth laboratory experiments are still necessary to solve this scientific problem.
Effects of MSCs on Follicular Development
Folliculogenesis is an important part of ovarian function, as it provides oocytes for reproduction (Hua et al., 2015). A large number of genes/proteins have been identified to be associated with follicular development, growth, ovulation and atresia processes. It has been reported that PMSC transplantation can increase the secretion of growth factors, angiogenic factors, pleiotropic cytokines, chemotactic cytokines and extracellular matrix proteins, which are all essential for folliculogenesis (Kupcova Skalnikova, 2013). In POF treatment, the widely discussed follicular development related genes are Nanos3, Nobox and Lhx8. Lai et al. proved that SMSC transplantation could reactivate injured mouse ovaries, with increased expression of the folliculogenesis marker genes Nobox, Nanos3, and Lhx8 in the ovaries of SMSC-treated mice (Lai et al., 2014). Kim et al. showed that three-dimensional cultured PDMSC spheres could upregulate the expression level of Nanos3, Nobox and Lhx8, and resume ovulation through regulation of the follicular microenvironment and stimulation of follicular development (Kim et al., 2018). Peng et al. also showed that the mRNA levels of these three genes in POF mice treated with BMSCs were significantly higher than those in the untreated group (Peng et al., 2018). Other follicular development-related genes include Foxo3a and Foxo1. Ding et al. found that UCMSCs on a collagen scaffold can activate primordial follicles in vitro via phosphorylation of FOXO3a and FOXO1, and transplantation of collagen/UCMSCs to the ovaries of POF patients can elevate estradiol concentrations, improve follicular development and increase the number of antral follicles (Ding et al., 2018).
Cytokines are critical regulators of folliculogenesis and ovulation. They contribute to creating an environment supporting follicle selection and growth, regulating cellular proliferation/differentiation, follicular survival/atresia and oocyte maturation (Field et al., 2014). The most important cytokines in POF treatments are TGF-β and IFN-γ. TGF-β superfamily members, including TGF-βs, AMH, activins, inhibins, BMPs and GDFs, impact several stages of follicular development (Trombly et al., 2009; Sanfins et al., 2018). According to Knight et al., the positive TGF-β regulators of preantral follicle growth, include GDF-9 and BMP-15 of oocyte origin, activins of granulosal origin, BMP-4 and BMP-7 of thecal origin and TGF-β from theca and GCs; in contrast, AMH plays a negative role in preantral follicle development (Knight and Glister, 2006). However, the existing research conclusions are not consistent with each other. El-Derany et al. transplanted BMSCs to a γ-ray induced POF rats model and reported that BMSCs recovered the folliculogenesis process, upregulating Foxo1, Gdf-9 and Fst gene expression accompanied by downregulating TGF-β (El-Derany et al., 2021), whereas Song et al. and Yin et al. found that MSC transplantation could increase the level of TGF-β and decrease the level of IFN-γ in POF models (Yin et al., 2018a; Song et al., 2018). Additionally, Ling et al. reported that amnion-derived mesenchymal stem cell transplantation can inhibit granulosa cell apoptosis and that the expression levels of AMH were significantly increased in the treatment group compared to the POF group (Ling et al., 2017). Zhang et al. and Mohamed et al. also found that after MSC transplantation, AMH expression in ovarian tissue was significantly higher than that in the POF group (Mohamed et al., 2018; Zhang et al., 2018).
Although the mechanism of MSCs on follicular development is not completely clear, most research agrees that MSC transplantation can promote the development and formation of primordial follicles, eggs and reduce the apoptosis of GCs. All of the involved genes and their correlated mechanisms are listed in Table 2.
TABLE 2.
The effects of MSCs on follicular development.
| Related gene/hormones/cytokines | Regulation of expression | Outcome of MSC treatment | References |
|---|---|---|---|
| Nanos3 | Up | Reducing atretic follicle and increasing antral follicle and secondary follicle | Lai et al. (2014) |
| Nobox | Up | ||
| Lhx8 | Up | ||
| Nanos3 | Up | Stimulating follicular development and resuming ovulation | Kim et al. (2018) |
| Nobox | Up | ||
| Lhx8 | Up | ||
| TGF-β | Up | Inhibiting follicular atresia and reducing the apoptosis of GCs in secondary follicles and cystic follicles | Knight and Glister (2006) |
| GDF-9 | Up | ||
| BMP-15 | Up | ||
| BMP-4 | Up | ||
| BMP-7 | Up | ||
| Foxo1 | Up | Recovering the suppressed folliculogenesis process and promoting egg formation | El-Derany et al. (2021) |
| Gdf-9 | Up | ||
| Fst | Up | ||
| TGF-β | Up | Promoting follicular growth | Song et al. (2018) |
| IFN-γ | Down | Inhibiting granulosa cell apoptosis | Zhao et al. (2018a) |
| AMH | Up | Increasing the number of follicles | Ling et al. (2017) |
| AMH | Up | Promoting follicular growth | Mohamed et al. (2018) |
| FOXO3a | Up | Promoting follicular development and maturation | Ding et al. (2018) |
| FOXO1 | Up |
MSCs and PGCs
Multiple studies have shown that MSCs can be induced and differentiate into PGCs. Fang et al. and Li et al. proved that CD61 could promote the differentiation of ADMSC into PGC-like cells through activation of the TGF-β pathway (Li et al., 2016; Fang et al., 2017). Wei et al. found that AMSC can be induced into PGC-like cells by BMP4 (Wei et al., 2016). Ge et al. found that when hfSDSCs were cultured in porcine follicle fluid, they may differentiate into both male and female germ cell-like cells (Ge et al., 2015). Park et al. proved that female mouse skin-derived stem cells could differentiate into ovarian-cell-like cells that are consistent with female germ, and ovarian follicle somatic cells. When ovarian cell-like cells are transplanted into ovariectomized mice, they restore the estrus cycle and serum estradiol levels (Park et al., 2014). Unfortunately, no in vivo research has reported whether MSC-differentiated germ cells can be fertilized and form embryos, and studies in this area are still lacking.
MSCs Can Promote the Proliferation of GCs
OGCs are the most important stromal cells in the ovary, providing necessary nutrition for oocyte development and follicle maturation, participating in the regulation of gonadotropins that modulate oocyte development and maintaining the microenvironment of oocyte maturation through autocrine and paracrine mechanisms. GCs play an important role in all developmental stages of follicles. GCs abnormalities can lead to abnormal hormone secretion, follicular development disorders and even follicular atresia (Lai et al., 2014). Chemotherapy induces GC apoptosis by damaging DNA and activating apoptosis pathways, thus leading POF. Therefore, enhancing GC function and inhibiting GC apoptosis may effectively prevent POF (Bedoschi et al., 2016). Studies have shown that GCs and MSCs express some similar surface markers (Dzafic et al., 2014; Maleki et al., 2014). Transplanted MSCs are mainly located in the GC layer around follicles, suggesting that MSCs have a significant effect on follicle formation and ovulation (Manshadi et al., 2019).
MSCs can inhibit GC apoptosis and promote GC proliferation by releasing cytokines and hormones, upregulating proliferation-related genes and inhibiting apoptosis-related genes (He et al., 2018; Wang et al., 2020). Zhang et al. showed that PMSC transplantation could upregulate the expression of AMH and FSHR in GCs of POF mice, inhibit GC apoptosis and follicular atresia, and thus restore ovarian function (Zhang et al., 2018). Fu et al. also found that BMSC transplantation may reduce GC apoptosis and improve ovarian function by releasing VEGF, HGF, and IGF-1 and upregulating Bcl-2 expression (Fu et al., 2008). Ding et al. showed that coculturing of AMSCs and GCs might inhibit the apoptosis of GCs, and transplantation of AMSCs may improve ovarian function during natural aging by secreting HGF and EGF (Ding et al., 2018).
The underlying mechanism of MSC treatment of POF may be related to exosome-mediated microRNA modulation. Multiple studies have highlighted the potential therapeutic advantages of using exosomal miRNAs from MSCs for the treatment of various diseases and injuries, including POF. Yang et al. demonstrated that BMSC-derived exosomes prevent ovarian follicular atresia in POF rats via the delivery of miR-144-5p, which can decrease GC apoptosis by targeting the PTEN pathway (Yang et al., 2020). Xiao et al. found that miR-146a and miR-10a are rich in exosomes secreted by AFSCs. miR-146a can restore ovarian function by downregulating IRAK1 and TRAF632 expression and miR-10a can inhibit GC apoptosis and prevent follicular atresia by suppressing Bim and caspase-9 expression (Xiao et al., 2016). Sun et al. found that exosomes derived from UCMSCs may prevent and treat chemotherapy-induced OGC apoptosis in vitro by upregulating the expression level of Bcl-2 and downregulating the expression levels of caspase-3, Bax, cleaved caspase-3 and cleaved PARP (Sun et al., 2017). miR-21 is related to apoptosis. Studies have shown that MSC treatment suppresses the expression of PTEN and PDCD4 through upregulation of miR-21 and inhibiting the apoptosis of GCs (Fu et al., 2017). Sun et al. reported that miR-644-5p carried by MSC exosomes could regulate p53 signaling and inhibit GC apoptosis (Sun et al., 2019).
MSCs Promote Angiogenesis
The establishment and remodeling of the ovarian vascular system is the basis of ovarian development and functional recovery. The follicles and corpus luteum can obtain nutritional support through ovarian blood vessels and transport hormones to target organs. Some researchers observed the distribution of BMSCs in ovaries by labeling specific markers of BMSCs and found that BMSCs were mainly distributed in the blood vessels of damaged ovaries (Liu et al., 2014), implying that BMSCs may play a role in ovarian blood vessels construction. Angiogenesis-related factors secreted by MSCs, such as VEGF, HGF, IGF and FGF, are increased in MSC-transplanted POF ovaries. VEGF and HGF have a synergistic effect and synergistically promote angiogenesis (Golocheikine et al., 2010). The combination of VEGF and HGF leads to an increased vascular diameter (Beilmann et al., 2004); VEGF promotes the length, area and branch point number of the induced vessels, while HGF contributes to vascular area growth (He et al., 2018). Wang et al. showed that MSCs could promote ovarian angiogenesis and reduce interstitial fibrosis by secreting VEGF, IGF-1, GCSF and HGF (Wang et al., 2017). Xia et al. demonstrated that MSC transplantation could enhance the expression levels of VEGF, FGF2 and angiogenin, significantly stimulate neovascularization and increase blood perfusion of the grafts in ovarian tissue (Xia et al., 2015). Zhang et al., Cho et al. and Park et al., also proved that MSC transplantation could repair damaged POF ovaries and promote ovarian development and function through angiogenesis (Zhang et al., 2017; Park et al., 2019; Cho et al., 2021).
Microvesicles are cell-derived membrane and cytoplasmic components. There are three subtypes of EVs: exosomes, microvesicles and apoptotic bodies. Exosomes and microvesicles can transfer mRNA, protein and lipids to target cells through surface-expressed ligands and surface receptors, thus affecting the phenotype and function of the target cells (Bidarimath et al., 2017). EVs have a therapeutic effect on female reproductive disorders, such as repairing injured endometrium, suppressing fibrosis of the endometrium, regulating immunity and anti-inflammation, and repressing the apoptosis of GCs in ovaries (Liao et al., 2021). Several studies have shown that MSC-derived microvesicles contain multiple pro-angiogenic proteins, such as VEGF and HGF (Merino-Gonzalez et al., 2016; Pakravan et al., 2017; Han et al., 2019; Shi et al., 2019). Yang et al. showed that UCMSC microvesicles transplantation in POI mice could induce angiogenesis by activating the PI3K/Akt signaling pathway and improve ovarian function (Yang Z. et al., 2019). Sun et al. found that miR-644-5p carried by BMSC-derived exosomes inhibited the apoptosis of ovarian GCs by targeting the p53 pathway (Sun et al., 2019); Zhang et al. also found that UCMSC-derived microvesicles can inhibit the apoptosis of GSs by downregulating the expression level of caspase-3 and upregulating the ratio of Bcl-2/Bax (Zhang J. et al., 2020).
Anti-inflammatory and Immunomodulatory Effect of MSCs
POF is an autoimmune disease. Autoimmune dysfunction is one of the most important pathogeneses of POF, causing inflammatory reactions of the ovary, destroying the ultrastructure of follicular cells (such as zona pellucida damage, gap link rupture and mitochondrial swelling), causing apoptosis of ovarian cells, affecting the maturation and atresia of follicles and inducing a decline in ovarian function (Nelson, 2001; Luo et al., 2017). It has been reported that certain types of immune cells will expand in ovaries with POF and infiltrate into the ovarian tissue, indicating that they are involved in the inflammation associated with POF (van Kasteren et al., 2000; Chernyshov et al., 2001; La Marca et al., 2010; Wang et al., 2018).
The anti-inflammatory effect is a critical mechanism by which MSCs restore ovarian function. MSCs may inhibit the activation and proliferation of lymphocytes, inhibit the secretion of proinflammatory cytokines, inhibit the function of antigen-presenting cells, and convey regulatory messages to immune cells (Zhou et al., 2019). In contrast, since the ovaries of most POF patients are in inflammatory conditions, the presence of inflammatory cytokines is also crucial for the regulation of MSC immunological and regenerative functions. Beldi et al. proved that the tumor TNF-α-TNFR2 axis is necessary for MSCs to produce anti-inflammatory mediators (such as IL-10, TGFβ and NO) and sustain regenerative functions such as wound healing, complex tube formation and endothelial pro-angiogenic support (Beldi et al., 2020a; Beldi et al., 2020b). IFN-γ and MSCs have a synergistic effect on immunosuppression. They upregulate PGE2, HGF, IL-6 and TGF-1 in MSCs and induce MSCs to express IDO, promoting GC proliferation and increasing the number of follicles (Najar et al., 2016; Liang et al., 2018). Yin et al. showed that the level of proinflammatory IFN-γ increased and the level of anti-inflammatory TGF-β decreased in POF mice, whereas PMSC transplantation reversed this situation and improved ovarian function (Yin et al., 2018a). A study also showed that PMSCs increase the secretion of IL-10 by inhibiting NF-κB-mediated pro-inflammatory reactions and thus promote tissue repair (Wang et al., 2016).
Immune cells (Treg cells, NK cells, Th cells, etc.) are important pathogenic factors in several models of autoimmune diseases (Alvarez Arias et al., 2014; Gianchecchi et al., 2018; Zhang X.-M. et al., 2020; Sakaguchi et al., 2020). These results indicate that the interaction of MSCs and immune cells plays a critical role in regulating the inflammatory microenvironment of POF. Yin et al. showed that PMSC transplantation might restore the ovarian function of POF mice by balancing the ratios of Th17/Tc17 and Th17/Treg cells (Yin et al., 2018b). Lu et al. reported that the serum levels of IL-2 and IFN-γ secreted by Th1 cells increased, while IL-4 secreted by Th2 cells decreased in POF mice; however, after UMSC transplantation, the amounts of these cytokines were reversed (Lu et al., 2019). Yin et al. showed that UCMSC transplantation into POF mice upregulates the ratio of CD8+ Treg cells, which have a typical immunosuppressive function and can reduce immune rejection (Su et al., 2014; Yin et al., 2020).
The Effect of MSCs on Oxidative Stress
Oxidative stress is a phenomenon of imbalance between the oxidative system and the antioxidant system caused by excessive ROS produced in cells. Reduction of ROS can protect the structure and function of ovarian mitochondria, increase the levels of antioxidant and antiapoptotic enzymes, and reduce apoptosis and oxidative damage of the ovary (He et al., 2018). Abumaree et al. indicated that cocultured PMSCs could reverse the destructive effect of OS on H2O2-treated endothelial cells and increase cell proliferation and migration (Abumaree et al., 2017). One study showed that ROS inhibit the expression and activity of TERT and induce POF (Jiang et al., 2018). MSCs can increase the production of antioxidant enzymes and inhibit ROS production through secretion of HGF, IL-6, IL-8, VEGF, BDNF and LIF and activation of the FOXO, NOQ1/MAPK, PI3K/Akt and Nrf2-ARE pathways (Amoroso et al., 2017). One study indicated that fMSCs upregulate MT1, JNK1, PCNA and AMPK levels and enhance antioxidant effects (Huang et al., 2019). Recently, it has been found that PMSC transplantation can reduce the levels of UCP-2, SOD1, reactive oxygen species and 8-hydroxydeoxyguanosine in POF rats, improving mitochondrial function in vivo, inhibiting oxidative stress and improving ovarian function (Zhang et al., 2016).
Using MSCs to treat POF is a sophisticated project. To better understand the mechanism by which MSCs improves ovarian functions, we summarized the cytokines and regulatory factors involved in the homing effect, follicular development, cell proliferation/apoptosis, angiogenesis, immunomodulation and oxidative stress processes, as shown in Table 3.
TABLE 3.
Factors involved in the process of MSC treatment of POF.
| Issues | Factors | Function | References |
|---|---|---|---|
| Follicular development | TGF-βs, AMH, BMPs, GDFs | Promoting follicular development. | Sanfins et al. (2018) |
| TGF-β, GDF-9, BMP-15, BMP-4, BMP-7, AMH | Reducing GC apoptosis and promoting GC proliferation. | Knight and Glister (2006) | |
| TGF-β | Recovering the suppressed folliculogenesis process. | El-Derany et al. (2021) | |
| TGF-β, IFN-γ | Promoting follicular growth. | Song et al. (2018) | |
| AMH | Inhibiting GC apoptosis and promoting follicular growth. | Ling et al. (2017) | |
| Primordial germ cells | CD61, TGF-β | Promoting MSCs different into PGC-like cells. | Fang et al. (2017) |
| BMP4 | Inducing MSC into PGC-like cells | Wei et al. (2016) | |
| Proliferation of GC | AMH | Inhibiting GC apoptosis. | Zhang et al. (2018) |
| VEGF, HGF, IGF-1, Bcl-2 | Reducing GC apoptosis and improving ovarian function. | Fu et al. (2008) | |
| HGF, EGF | Reducing apoptosis of ovarian GC. | Ding et al. (2018) | |
| PARP | Inhibiting ovarian follicular atresia and reducing GC apoptosis. | Sun et al. (2017) | |
| Bcl-2, AMH, FSHR, caspase-3 | Promoting GC proliferation and inhibiting GC apoptosis. | Wang et al. (2020) | |
| Angiogenesis | VEGF, HGF | Promoting ovarian angiogenesis. | Golocheikine et al. (2010) |
| VEGF, HGF | Increasing vascular diameter. | Beilmann et al. (2004) | |
| VEGF, IGF-1, GCSF, HGF | Promoting ovarian angiogenesis and reducing interstitial fibrosis. | Wang et al. (2017) | |
| VEGF, FGF2 | Stimulating neovascularization and increasing blood perfusion of the grafts. | Xia et al. (2015) | |
| Immunomodulatory effect | IL-2, IFN-γ, IL-4 | Reducing GC apoptosis. | Lu et al. (2019) |
| Anti-inflammatory effect | PGE2, HGF, IL-6, TGF-1 | Promoting GC proliferation. | Liang et al. (2018) |
| IFN-γ, TGF-β | Improving ovarian function | Yin et al. (2018a) | |
| Oxidative stress | HGF, IL-6, IL-8, VEGF, BDNF, LIF | Increasing the production of antioxidant enzymes and inhibiting ROS production. | Amoroso et al. (2017) |
Perspective
MSCs possess multiple differentiation potentials and homing and immunomodulatory functions. They can be used as seed cells to participate in the regeneration and reconstruction of tissues and organs in various diseases, such as rheumatoid arthritis, amyotrophic lateral sclerosis, systemic lupus erythematosus and other degenerative diseases (spinal cord injury, Parkinson’s disease, Alzheimer’s disease). At present, more than ten kinds of stem cell preparations have been used to treat graft-versus-host disease (Zhao et al., 2019), acute myocardial infarction (Cho et al., 2017), osteoarthritis (Matas et al., 2019), etc. The clinical application of MSCs has brought great hope to the treatment of POF infertility and the improvement of female reproductive health, and a large number of clinical studies are actively being carried out. However, with increasing age, the number and function of MSCs decrease accordingly. The senescence of MSCs may be related to telomere shortening, DNA damage, epigenetics and immunological characteristics (Trachana et al., 2017; Wagner, 2019). At present, senescence of MSC is still the bottleneck of stem cell tissue engineering and clinical applications. Therefore, how to deeply understand the molecular mechanism of MSC senescence and delay or prevent MSC senescence efficiently through reasonable gene manipulation or drug intervention, has crucial practical significance and important economic value.
Conclusion
MSCs derived from different sources have similar curative effects in the treatment of POF through multiple mechanisms. MSCs have attractive clinical transformation and application prospects in the restoration of reproductive function in POF patients, even in older women with POF. Therefore, understanding the molecular mechanism of POF is still a key scientific problem for comprehensively and deeply evaluating the safety and effectiveness of MSC transplantation, especially the long-term impact on parents and offspring.
Author Contributions
XS and JW contributed to the conception and design of the article. JW and WL drafted the article. ZY, DY, and SL drafted the figures. All authors have read and approved the final manuscript.
Funding
This work was supported in part by the National Natural Science Foundation of China Grant (No. #81801227 to XS), the Subject Arrangement Program from Science and Technology Department of Jilin Province (Nos. #20200201123JC to DY and #20190201209JC to SL), and Clinical-Translational Medicine Project from the First Hospital of Jilin University (No. #JDYYJCHX2020013 and #2020-ZL-13 to XS and DY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- ADMSC
adipose-derived stem cells
- AFMSC
amniotic fluid mesenchymal stem cells
- akt
protein kinase B
- AMH
anti-mullerian hormone
- AMPK
adenosine 5‘-monophosphate (AMP)-activated protein kinase
- AMSC
amniotic mesenchymal stem cells
- ARE
antioxidant response element
- AZPAb
anti-Zona pellucida antibody
- Bax
Bcl-2 associated X protein
- Bcl-2
B-cell lymphoma-2
- b-FGF
basic fibroblast growth factor
- BDNF
brain-derived neurotrophic factor
- Bim
Bcl-2 interacting mediator of cell death
- BMP
bone morphogenetic protein
- BMSC
bone marrow stem cells
- CD
cluster of differentiation
- EGF
epidermal growth factor
epidermal growth factor
- E2
estrogen
- EGF
epidermal growth factor
epidermal growth factor
- FGF
fibroblast growth factor
- FOXO
forkhead box O
- FSH
follicle stimulating hormone
- FSHR
follicle stimulating hormone receptor
- fst
homo sapiens follistatin
- GC
ovarian granulosa cells
granulosa cells
- GC
ovarian granulosa cells
granulosa cells
- GCSF
granulocyte colony stimulating factor
- Gdf
growth differentiation factor
- HGF
hepatocyte growth factor
- HOVEC
human ovarian endothelial cell
- HRT
hormone replacement therapy
- IDO
indoleamine 2,3-dioxygenase
- IGF-1
insulin-like growth factors-1
- IFN-γ
interferon γ
- IRAK1
interleukin 1 receptor associated Kinase 1
- IL
interleukin-10
- JNK1
jun n-terminal kinase1
- LH
luteinizing hormone
- LIF
interleukin 6 family cytokine
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemotactic protein
- Mensc
menstrual-derived stem cell
- MSC
mesenchymal stem cells
- MVs
microvesicles
- MT1
melatonine receptor1
- Nanos3
nanos C2HC-type Zinc finger 3
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
natural killer cell
- Nobox
NOBOX oogenesis homeobox
- NO
nitric oxide
- NOQ1
NAD(P)H quinone dehydrogenase 1
- Nrf2
NF-E2-related factor 2
- OGCs
ovarian granulosa cells
- PARP
poly ADP-ribose polymerase
- PCNA
proliferating cell nuclear antigen
- PDCD4
programmed cell death 4
- PDMSC
placenta-derived mesenchymal stem cells
- PGF
placental growth factor
- PGE2
prostaglandin E2
- PGC
primordial germ cell
- PMSC
placenta-derived mesenchymal stem cell
- POF
premature ovarian failure
- PTEN
phosphatase and tensin homolog
- PI3K
phosphatidylinositol-3-kinase
- ROS
reactive oxygen species
- SC
stem cell
- SOD1
superoxide dismutase 1
- TERT
telomerase reverse transcriptase
- TGF
transforming growth factor
- th
helper T cell
- TNF-α
tumor necrosis factor-α
- TRAF632
receptor associated factor 632
- UCMSC
umbilical cord mesenchymal stem cells
- UCP-2
uncoupling protein-2
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor
References
- Abumaree M. H., Hakami M., Abomaray F. M., Alshabibi M. A., Kalionis B., Al Jumah M. A., et al. (2017). Human Chorionic Villous Mesenchymal Stem/Stromal Cells Modify the Effects of Oxidative Stress on Endothelial Cell Functions. Placenta 59, 74–86. 10.1016/j.placenta.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Ali A. T. (2013). Risk Factors for Endometrial Cancer. Ceska Gynekol 78, 448–459. [PubMed] [Google Scholar]
- Alvarez Arias D. A., Kim H.-J., Zhou P., Holderried T. A. W., Wang X., Dranoff G., et al. (2014). Disruption of CD8+ Treg Activity Results in Expansion of T Follicular Helper Cells and Enhanced Antitumor Immunity. Cancer Immunol. Res. 2, 207–216. 10.1158/2326-6066.cir-13-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso M. R., Matassa D. S., Agliarulo I., Avolio R., Maddalena F., Condelli V., et al. (2017). Stress-Adaptive Response in Ovarian Cancer Drug Resistance. Adv. Protein Chem. Struct. Biol. 108, 163–198. 10.1016/bs.apcsb.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Badawy A., Sobh M., Ahdy M., Abdelhafez M. (2017). Bone Marrow Mesenchymal Stem Cell Repair of Cyclophosphamide-Induced Ovarian Insufficiency in a Mouse Model. Int. J. women's Health 9, 441–447. 10.2147/ijwh.s134074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrehbar K., Valojerdi M. R., Esfandiari F., Fathi R., Hassani S.-N., Baharvand H. (2020). Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells Improved Premature Ovarian Failure. World J. Stem Cell 12, 857–878. 10.4252/wjsc.v12.i8.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoschi G., Navarro P. A., Oktay K. (2016). Chemotherapy-Induced Damage to Ovary: Mechanisms and Clinical Impact. Future Oncol. 12, 2333–2344. 10.2217/fon-2016-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilmann M., Birk G., Lenter M. C. (2004). Human Primary Co-Culture Angiogenesis Assay Reveals Additive Stimulation and Different Angiogenic Properties of VEGF and HGF. Cytokine 26, 178–185. 10.1016/j.cyto.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Beldi G., Bahiraii S., Lezin C., Nouri Barkestani M., Abdelgawad M. E., Uzan G., et al. (2020a). TNFR2 Is a Crucial Hub Controlling Mesenchymal Stem Cell Biological and Functional Properties. Front. Cel Dev. Biol. 8, 596831. 10.3389/fcell.2020.596831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldi G., Khosravi M., Abdelgawad M. E., Salomon B. L., Uzan G., Haouas H., et al. (2020b). TNFα/TNFR2 Signaling Pathway: An Active Immune Checkpoint for Mesenchymal Stem Cell Immunoregulatory Function. Stem Cel Res Ther 11, 281. 10.1186/s13287-020-01740-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidarimath M., Khalaj K., Kridli R. T., Kan F. W., Koti M., Tayade C. (2017). Extracellular Vesicle Mediated Intercellular Communication at the Porcine Maternal-Fetal Interface: A New Paradigm for Conceptus-Endometrial Cross-Talk. Sci. Rep. 7, 40476. 10.1038/srep40476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A., Caudle M. R. (2012). Immunoregulation of Follicular Renewal, Selection, POF, and Menopause In Vivo, vs. Neo-Oogenesis In Vitro, POF and Ovarian Infertility Treatment, and a Clinical Trial. Reprod. Biol. Endocrinol. 10, 97. 10.1186/1477-7827-10-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Guo S., Wei C., Li H., Wang H., Xu Y. (2018). Effect of Stem Cell Transplantation of Premature Ovarian Failure in Animal Models and Patients: A Meta-Analysis and Case Report. Exp. Ther. Med. 15, 4105–4118. 10.3892/etm.2018.5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshov V. P., Radysh T. V., Gura I. V., Tatarchuk T. P., Khominskaya Z. B. (2001). Immune Disorders in Women with Premature Ovarian Failure in Initial Period. Am. J. Reprod. Immunol. 46, 220–225. 10.1034/j.1600-0897.2001.d01-5.x [DOI] [PubMed] [Google Scholar]
- Cho D. I., Kang W. S., Hong M. H., Kang H. J., Kim M. R., Kim M. C., et al. (2017). The Optimization of Cell Therapy by Combinational Application with Apicidin-Treated Mesenchymal Stem Cells after Myocardial Infarction. Oncotarget 8, 44281–44294. 10.18632/oncotarget.17471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Kim T.-H., Seok J., Jun J. H., Park H., Kweon M., et al. (2021). Vascular Remodeling by Placenta-Derived Mesenchymal Stem Cells Restores Ovarian Function in Ovariectomized Rat Model via the VEGF Pathway. Lab. Invest. 101, 304–317. 10.1038/s41374-020-00513-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Zou Q., Wang F., Wu H., Chen R., Lv J., et al. (2018). Human Amniotic Mesenchymal Stem Cells Improve Ovarian Function in Natural Aging through Secreting Hepatocyte Growth Factor and Epidermal Growth Factor. Stem Cel Res Ther 9, 55. 10.1186/s13287-018-0781-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Yan G., Wang B., Xu L., Gu Y., Ru T., et al. (2018). Transplantation of UC-MSCs on Collagen Scaffold Activates Follicles in Dormant Ovaries of POF Patients with Long History of Infertility. Sci. China Life Sci. 61, 1554–1565. 10.1007/s11427-017-9272-2 [DOI] [PubMed] [Google Scholar]
- Dolmans M.-M., Donnez J. (2021). Fertility Preservation in Women for Medical and Social Reasons: Oocytes vs Ovarian Tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 70, 63–80. 10.1016/j.bpobgyn.2020.06.011 [DOI] [PubMed] [Google Scholar]
- Dzafic E., Stimpfel M., Novakovic S., Cerkovnik P., Virant-Klun I. (2014). Expression of Mesenchymal Stem Cells-Related Genes and Plasticity of Aspirated Follicular Cells Obtained from Infertile Women. Biomed. Res. Int. 2014, 508216. 10.1155/2014/508216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Derany M. O., Said R. S., El-Demerdash E. (2021). Bone Marrow-Derived Mesenchymal Stem Cells Reverse Radiotherapy-Induced Premature Ovarian Failure: Emphasis on Signal Integration of TGF-β, Wnt/β-Catenin and Hippo Pathways. Stem Cel Rev Rep 17, 1429–1445. 10.1007/s12015-021-10135-9 [DOI] [PubMed] [Google Scholar]
- Esfandyari S., Chugh R. M., Park H. S., Hobeika E., Ulin M., Al-Hendy A. (2020). Mesenchymal Stem Cells as a Bio Organ for Treatment of Female Infertility. Cells 9, 2253. 10.3390/cells9102253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Wei Y., Lv C., Peng S., Zhao S., Hua J. (2017). CD61 Promotes the Differentiation of Canine ADMSCs into PGC-like Cells through Modulation of TGF-β Signaling. Sci. Rep. 7, 43851. 10.1038/srep43851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S. L., Dasgupta T., Cummings M., Orsi N. M. (2014). Cytokines in Ovarian Folliculogenesis, Oocyte Maturation and Luteinisation. Mol. Reprod. Dev. 81, 284–314. 10.1002/mrd.22285 [DOI] [PubMed] [Google Scholar]
- Fu X., He Y., Xie C., Liu W. (2008). Bone Marrow Mesenchymal Stem Cell Transplantation Improves Ovarian Function and Structure in Rats with Chemotherapy-Induced Ovarian Damage. Cytotherapy 10, 353–363. 10.1080/14653240802035926 [DOI] [PubMed] [Google Scholar]
- Fu X., He Y., Wang X., Peng D., Chen X., Li X., et al. (2017). Overexpression of miR-21 in Stem Cells Improves Ovarian Structure and Function in Rats with Chemotherapy-Induced Ovarian Damage by Targeting PDCD4 and PTEN to Inhibit Granulosa Cell Apoptosis. Stem Cel Res Ther 8, 187. 10.1186/s13287-017-0641-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. X., Ji J., Shan F., Li J., Hu R. (2021). Human Mesenchymal Stem Cell Treatment of Premature Ovarian Failure: New Challenges and Opportunities. Stem Cel Res. Ther. 12, 161. 10.1186/s13287-021-02212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabr H., Rateb M. A., El Sissy M. H., Ahmed Seddiek H., Ali Abdelhameed Gouda S. (2016). The Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Chemotherapy Induced Ovarian Failure in Albino Rats. Microsc. Res. Tech. 79, 938–947. 10.1002/jemt.22725 [DOI] [PubMed] [Google Scholar]
- Ge W., Ma H. G., Cheng S. F., Sun Y. C., Sun L. L., Sun X. F., et al. (2015). Differentiation of Early Germ Cells from Human Skin-Derived Stem Cells without Exogenous Gene Integration. Sci. Rep. 5, 13822. 10.1038/srep13822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianchecchi E., Delfino D. V., Fierabracci A. (2018). NK Cells in Autoimmune Diseases: Linking Innate and Adaptive Immune Responses. Autoimmun. Rev. 17, 142–154. 10.1016/j.autrev.2017.11.018 [DOI] [PubMed] [Google Scholar]
- Golocheikine A., Tiriveedhi V., Angaswamy N., Benshoff N., Sabarinathan R., Mohanakumar T. (2010). Cooperative Signaling for Angiogenesis and Neovascularization by VEGF and HGF Following Islet Transplantation. Transplantation 90, 725–731. 10.1097/tp.0b013e3181ef8a63 [DOI] [PubMed] [Google Scholar]
- Gupta S., Lodha P., Karthick M. S., Tandulwadkar S. R. (2018). Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World's First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age 45 Year. J. Hum. Reprod. Sci. 11, 125–130. 10.4103/jhrs.JHRS_57_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Ren J., Bai Y., Pei X., Han Y. (2019). Exosomes from Hypoxia-Treated Human Adipose-Derived Mesenchymal Stem Cells Enhance Angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cel Biol. 109, 59–68. 10.1016/j.biocel.2019.01.017 [DOI] [PubMed] [Google Scholar]
- He Y., Chen D., Yang L., Hou Q., Ma H., Xu X. (2018). The Therapeutic Potential of Bone Marrow Mesenchymal Stem Cells in Premature Ovarian Failure. Stem Cel Res Ther 9, 263. 10.1186/s13287-018-1008-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz S., Romeu M., Buigues A., Martínez S., Díaz-García C., Gómez-Seguí I., et al. (2018). Autologous Stem Cell Ovarian Transplantation to Increase Reproductive Potential in Patients Who Are Poor Responders. Fertil. sterility 110, 496–505. 10.1016/j.fertnstert.2018.04.025 [DOI] [PubMed] [Google Scholar]
- Herraiz S., Pellicer N., Romeu M., Pellicer A. (2019). Treatment Potential of Bone Marrow-Derived Stem Cells in Women with Diminished Ovarian Reserves and Premature Ovarian Failure. Curr. Opin. Obstet. Gynecol. 31, 156–162. 10.1097/gco.0000000000000531 [DOI] [PubMed] [Google Scholar]
- Hua J., Xu B., Yang Y., Ban R., Iqbal F., Cooke H. J., et al. (2015). Follicle Online: an Integrated Database of Follicle Assembly, Development and Ovulation. Database 2015, bav036. 10.1093/database/bav036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Qian C., Ding C., Meng Q., Zou Q., Li H. (2019). Fetal Liver Mesenchymal Stem Cells Restore Ovarian Function in Premature Ovarian Insufficiency by Targeting MT1. Stem Cel Res Ther 10, 362. 10.1186/s13287-019-1490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I., Hovatta O., La Marca A., Livera G., Monniaux D., Persani L., et al. (2018). Advances in the Molecular Pathophysiology, Genetics, and Treatment of Primary Ovarian Insufficiency. Trends Endocrinol. Metab. 29, 400–419. 10.1016/j.tem.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Igboeli P., El Andaloussi A., Sheikh U., Takala H., ElSharoud A., McHugh A., et al. (2020). Intraovarian Injection of Autologous Human Mesenchymal Stem Cells Increases Estrogen Production and Reduces Menopausal Symptoms in Women with Premature Ovarian Failure: Two Case Reports and a Review of the Literature. J. Med. Case Rep. 14, 108. 10.1186/s13256-020-02426-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Furui T., Yamamoto A. (2008). Preservation of Female Fertility during Cancer Treatment. Reprod. Med. Biol. 7, 17–27. 10.1111/j.1447-0578.2007.00197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalalie L., Rezaie M. J., Jalili A., Rezaee M. A., Vahabzadeh Z., Rahmani M. R., et al. (2019). Distribution of the CM-Dil-Labeled Human Umbilical Cord Vein Mesenchymal Stem Cells Migrated to the Cyclophosphamide-Injured Ovaries in C57BL/6 Mice. Iranian Biomed. J. 23, 200–208. 10.29252/ibj.23.3.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. L., Cao L. Q., Chen H. Y. (2018). Protective Effects ROS Up-Regulation on Premature Ovarian Failure by Suppressing ROS-TERT Signal Pathway. Eur. Rev. Med. Pharmacol. Sci. 22, 6198–6204. 10.26355/eurrev_201810_16025 [DOI] [PubMed] [Google Scholar]
- Kim T. H., Choi J. H., Jun Y., Lim S. M., Park S., Paek J. Y., et al. (2018). 3D-Cultured Human Placenta-Derived Mesenchymal Stem Cell Spheroids Enhance Ovary Function by Inducing Folliculogenesis. Sci. Rep. 8, 15313. 10.1038/s41598-018-33575-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P. G., Glister C. (2006). TGF-β Superfamily Members and Ovarian Follicle Development. Reproduction 132, 191–206. 10.1530/rep.1.01074 [DOI] [PubMed] [Google Scholar]
- Kupcova Skalnikova H. (2013). Proteomic Techniques for Characterisation of Mesenchymal Stem Cell Secretome. Biochimie 95, 2196–2211. 10.1016/j.biochi.2013.07.015 [DOI] [PubMed] [Google Scholar]
- La Marca A., Brozzetti A., Sighinolfi G., Marzotti S., Volpe A., Falorni A. (2010). Primary Ovarian Insufficiency: Autoimmune Causes. Curr. Opin. Obstet. Gynecol. 22, 277–282. 10.1097/gco.0b013e32833b6c70 [DOI] [PubMed] [Google Scholar]
- Lai D., Wang F., Dong Z., Zhang Q. (2014). Skin-Derived Mesenchymal Stem Cells Help Restore Function to Ovaries in a Premature Ovarian Failure Mouse Model. PloS one 9, e98749. 10.1371/journal.pone.0098749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D., Wang F., Yao X., Zhang Q., Wu X., Xiang C. (2015). Human Endometrial Mesenchymal Stem Cells Restore Ovarian Function through Improving the Renewal of Germline Stem Cells in a Mouse Model of Premature Ovarian Failure. J. Transl Med. 13, 155. 10.1186/s12967-015-0516-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven J. (2016). Primary Ovarian Insufficiency. Semin. Reprod. Med. 34, 230–234. 10.1055/s-0036-1585402 [DOI] [PubMed] [Google Scholar]
- Lee A. W., Wu A. H., Wiensch A., Mukherjee B., Terry K. L., Harris H. R., et al. (2020). Estrogen Plus Progestin Hormone Therapy and Ovarian Cancer. Epidemiology 31, 402–408. 10.1097/ede.0000000000001175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Liu W., Zhuang M., Li N., Wu S., Pan S., et al. (2016). Overexpression of CD61 Promotes hUC-MSC Differentiation into Male Germ-like Cells. Cell Prolif. 49, 36–47. 10.1111/cpr.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Mao Q., He J., She H., Zhang Z., Yin C. (2017). Human Umbilical Cord Mesenchymal Stem Cells Improve the Reserve Function of Perimenopausal Ovary via a Paracrine Mechanism. Stem Cel. Res. Ther. 8 (1), 55. 10.1186/s13287-017-0514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang M., Tian Y., Li Q., Huang X. (2021). Mesenchymal Stem Cells in Premature Ovarian Insufficiency: Mechanisms and Prospects. Front. Cel Dev. Biol. 9, 718192. 10.3389/fcell.2021.718192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Jiang E., Yao J., Wang M., Chen S., Zhou Z., et al. (2018). Interferon-γ Mediates the Immunosuppression of Bone Marrow Mesenchymal Stem Cells on T-Lymphocytes In Vitro . Hematology 23, 44–49. 10.1080/10245332.2017.1333245 [DOI] [PubMed] [Google Scholar]
- Liao Z., Liu C., Wang L., Sui C., Zhang H. (2021). Therapeutic Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Female Reproductive Diseases. Front. Endocrinol. 12, 665645. 10.3389/fendo.2021.665645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Xu L., Zwingenberger S., Gibon E., Goodman S. B., Li G. (2017). Mesenchymal Stem Cells Homing to Improve Bone Healing. J. orthopaedic translation 9, 19–27. 10.1016/j.jot.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L., Feng X., Wei T., Wang Y., Wang Y., Zhang W., et al. (2017). Effects of Low-Intensity Pulsed Ultrasound (LIPUS)-pretreated Human Amnion-Derived Mesenchymal Stem Cell (hAD-MSC) Transplantation on Primary Ovarian Insufficiency in Rats. Stem Cel Res Ther 8, 283. 10.1186/s13287-017-0739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L., Feng X., Wei T., Wang Y., Wang Y., Wang Z., et al. (2019). Human Amnion-Derived Mesenchymal Stem Cell (hAD-MSC) Transplantation Improves Ovarian Function in Rats with Premature Ovarian Insufficiency (POI) at Least Partly through a Paracrine Mechanism. Stem Cel Res Ther 10, 46. 10.1186/s13287-019-1136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang H., Zhang Y., Li N., Wen Y., Cao F., et al. (2014). Homing and Restorative Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Cisplatin Injured Ovaries in Rats. Mol. Cell 37, 865–872. 10.14348/molcells.2014.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang J., Han R., Meng M., Wang W., Zhao Y., et al. (2019a). Therapeutic Effect of Transplanted Umbilical Cord Mesenchymal Stem Cells in a Cynomolgus Monkey Model of Multiple Sclerosis. Am. J. Transl Res. 11, 2516–2531. [PMC free article] [PubMed] [Google Scholar]
- Liu R., Zhang X., Fan Z., Wang Y., Yao G., Wan X., et al. (2019b). Human Amniotic Mesenchymal Stem Cells Improve the Follicular Microenvironment to Recover Ovarian Function in Premature Ovarian Failure Mice. Stem Cel Res Ther 10, 299. 10.1186/s13287-019-1315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Cui J., Cui L., Luo Q., Cao Q., Yuan W., et al. (2019). The Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation on Endometrial Receptivity Are Associated with Th1/Th2 Balance Change and uNK Cell Expression of Uterine in Autoimmune Premature Ovarian Failure Mice. Stem Cel Res Ther 10, 214. 10.1186/s13287-019-1313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q., Yin N., Zhang L., Yuan W., Zhao W., Luan X., et al. (2017). Role of SDF-1/CXCR4 and Cytokines in the Development of Ovary Injury in Chemotherapy Drug Induced Premature Ovarian Failure Mice. Life Sci. 179, 103–109. 10.1016/j.lfs.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Maleki M., Ghanbarvand F., Behvarz M. R., Ejtemaei M., Ghadirkhomi E. (2014). Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cell 7, 118–126. 10.15283/ijsc.2014.7.2.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manshadi M. D., Navid S., Hoshino Y., Daneshi E., Noory P., Abbasi M. (2019). The Effects of Human Menstrual Blood Stem Cells‐derived Granulosa Cells on Ovarian Follicle Formation in a Rat Model of Premature Ovarian Failure. Microsc. Res. Tech. 82, 635–642. 10.1002/jemt.23120 [DOI] [PubMed] [Google Scholar]
- Mashayekhi M., Mirzadeh E., Chekini Z., Ahmadi F., Eftekhari-Yazdi P., Vesali S., et al. (2021). Evaluation of Safety, Feasibility and Efficacy of Intra-ovarian Transplantation of Autologous Adipose Derived Mesenchymal Stromal Cells in Idiopathic Premature Ovarian Failure Patients: Non-Randomized Clinical Trial, Phase I, First in Human. J. ovarian Res. 14, 5. 10.1186/s13048-020-00743-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M. I., et al. (2019). Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. STEM CELLS Translational Med. 8, 215–224. 10.1002/sctm.18-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino-González C., Zuñiga F. A., Escudero C., Ormazabal V., Reyes C., Nova-Lamperti E., et al. (2016). Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 7, 24. 10.3389/fphys.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S. A., Shalaby S. M., Abdelaziz M., Brakta S., Hill W. D., Ismail N., et al. (2018). Human Mesenchymal Stem Cells Partially Reverse Infertility in Chemotherapy-Induced Ovarian Failure. Reprod. Sci. 25, 51–63. 10.1177/1933719117699705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Wu X., Hao Z. (2018). Comparative Evaluation of Mesenchymal Stromal Cells from Umbilical Cord and Amniotic Membrane in Xeno-free Conditions. BMC Cel Biol 19, 27. 10.1186/s12860-018-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar M., Raicevic G., Fayyad-Kazan H., Bron D., Toungouz M., Lagneaux L. (2016). Mesenchymal Stromal Cells and Immunomodulation: A Gathering of Regulatory Immune Cells. Cytotherapy 18, 160–171. 10.1016/j.jcyt.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Nelson L. (2001). Autoimmune Ovarian Failure: Comparing the Mouse Model and the Human Disease. J. Soc. Gynecol. Investig. 8, S55–S57. 10.1016/s1071-5576(00)00110-6 [DOI] [PubMed] [Google Scholar]
- Noory P., Navid S., Zanganeh B. M., Talebi A., Borhani-Haghighi M., Gholami K., et al. (2019). Human Menstrual Blood Stem Cell-Derived Granulosa Cells Participate in Ovarian Follicle Formation in a Rat Model of Premature Ovarian Failure In Vivo . Cell Reprogramming 21, 249–259. 10.1089/cell.2019.0020 [DOI] [PubMed] [Google Scholar]
- Pakravan K., Babashah S., Sadeghizadeh M., Mowla S. J., Mossahebi-Mohammadi M., Ataei F., et al. (2017). MicroRNA-100 Shuttled by Mesenchymal Stem Cell-Derived Exosomes Suppresses In Vitro Angiogenesis through Modulating the mTOR/HIF-1α/VEGF Signaling axis in Breast Cancer Cells. Cell Oncol. 40, 457–470. 10.1007/s13402-017-0335-7 [DOI] [PubMed] [Google Scholar]
- Park B.-W., Pan B., Toms D., Huynh E., Byun J.-H., Lee Y.-M., et al. (2014). Ovarian-Cell-Like Cells from Skin Stem Cells Restored Estradiol Production and Estrus Cycling in Ovariectomized Mice. Stem Cell Dev. 23, 1647–1658. 10.1089/scd.2014.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S., Ashour D., Elsharoud A., Chugh R. M., Ismail N., El Andaloussi A., et al. (2019). Towards Cell Free Therapy of Premature Ovarian Insufficiency: Human Bone Marrow Mesenchymal Stem Cells Secretome Enhances Angiogenesis in Human Ovarian Microvascular Endothelial Cells. HSOA J. Stem Cell Res Dev Ther 5, 19. 10.24966/srdt-2060/100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S., Chugh R. M., Elsharoud A., Ulin M., Esfandyari S., Aboalsoud A., et al. (2021). Safety of Intraovarian Injection of Human Mesenchymal Stem Cells in a Premature Ovarian Insufficiency Mouse Model. Cel Transplant. 30, 963689720988502. 10.1177/0963689720988502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Xiao N., Cheng L. (2018). Therapeutic Potential of BMSCs for Premature Ovarian Failure in Mice. Zhong nan da Xue Xue Bao Yi Xue ban = J. Cent. South Univ. Med. Sci. 43, 7–13. 10.11817/j.issn.1672-7347.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Pers Y.-M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F., et al. (2016). Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cell translational Med. 5, 847–856. 10.5966/sctm.2015-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Mikami N., Wing J. B., Tanaka A., Ichiyama K., Ohkura N. (2020). Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 38, 541–566. 10.1146/annurev-immunol-042718-041717 [DOI] [PubMed] [Google Scholar]
- Sanfins A., Rodrigues P., Albertini D. F. (2018). GDF-9 and BMP-15 Direct the Follicle Symphony. J. Assist. Reprod. Genet. 35, 1741–1750. 10.1007/s10815-018-1268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhansari G., Aghebati-Maleki L., Nouri M., Jadidi-Niaragh F., Yousefi M. (2018). Current Approaches for the Treatment of Premature Ovarian Failure with Stem Cell Therapy. Biomed. Pharmacother. 102, 254–262. 10.1016/j.biopha.2018.03.056 [DOI] [PubMed] [Google Scholar]
- Shi Y., Shi H., Nomi A., Lei-Lei Z., Zhang B., Qian H. (2019). Mesenchymal Stem Cell-Derived Extracellular Vesicles: A New Impetus of Promoting Angiogenesis in Tissue Regeneration. Cytotherapy 21, 497–508. 10.1016/j.jcyt.2018.11.012 [DOI] [PubMed] [Google Scholar]
- Song K., Cai H., Zhang D., Huang R., Sun D., He Y. (2018). Effects of Human Adipose-Derived Mesenchymal Stem Cells Combined with Estrogen on Regulatory T Cells in Patients with Premature Ovarian Insufficiency. Int. Immunopharmacology 55, 257–262. 10.1016/j.intimp.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Su J., Xie Q., Xu Y., Li X. C., Dai Z. (2014). Role of CD8(+) Regulatory T Cells in Organ Transplantation. Burns Trauma 2, 18–23. 10.4103/2321-3868.126086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Wang S., Li Y., Yu L., Gu F., Wang C., et al. (2013). Adipose-Derived Stem Cells Improved Mouse Ovary Function after Chemotherapy-Induced Ovary Failure. Stem Cel Res Ther 4, 80. 10.1186/scrt231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Li D., Song K., Wei J., Yao S., Li Z., et al. (2017). Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Protect against Cisplatin-Induced Ovarian Granulosa Cell Stress and Apoptosis In Vitro . Sci. Rep. 7, 2552. 10.1038/s41598-017-02786-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Ma Y., Wang F., Hu L., Sun Y. (2019). miR-644-5p Carried by Bone Mesenchymal Stem Cell-Derived Exosomes Targets Regulation of P53 to Inhibit Ovarian Granulosa Cell Apoptosis. Stem Cel Res Ther 10, 360. 10.1186/s13287-019-1442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri M., Saki G., Nikbakht R., Eftekhari A. R. (2021). Bone Morphogenetic Protein 15 Induces Differentiation of Mesenchymal Stem Cells Derived from Human Follicular Fluid to Oocyte‐Like Cell. Cell Biol Int 45, 127–139. 10.1002/cbin.11475 [DOI] [PubMed] [Google Scholar]
- Takehara Y., Yabuuchi A., Ezoe K., Kuroda T., Yamadera R., Sano C., et al. (2013). The Restorative Effects of Adipose-Derived Mesenchymal Stem Cells on Damaged Ovarian Function. Lab. Invest. 93, 181–193. 10.1038/labinvest.2012.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M., Feldman G., Puscheck E. E. (2018). Primary Ovarian Insufficiency in Classic Galactosemia: Current Understanding and Future Research Opportunities. J. Assist. Reprod. Genet. 35, 3–16. 10.1007/s10815-017-1039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachana V., Petrakis S., Fotiadis Z., Siska E. K., Balis V., Gonos E. S., et al. (2017). Human Mesenchymal Stem Cells with Enhanced Telomerase Activity Acquire Resistance against Oxidative Stress-Induced Genomic Damage. Cytotherapy 19, 808–820. 10.1016/j.jcyt.2017.03.078 [DOI] [PubMed] [Google Scholar]
- Trombly D. J., Woodruff T. K., Mayo K. E. (2009). Roles for Transforming Growth Factor Beta Superfamily Proteins in Early Folliculogenesis. Semin. Reprod. Med. 27, 14–23. 10.1055/s-0028-1108006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman A. M., Tilly J. L., Woods D. C. (2017). Ovarian Regeneration: The Potential for Stem Cell Contribution in the Postnatal Ovary to Sustained Endocrine Function. Mol. Cell. Endocrinol. 445, 74–84. 10.1016/j.mce.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulin M., Cetin E., Hobeika E., Chugh R. M., Park H.-S., Esfandyari S., et al. (2021). Human Mesenchymal Stem Cell Therapy and Other Novel Treatment Approaches for Premature Ovarian Insufficiency. Reprod. Sci. 28, 1688–1696. 10.1007/s43032-021-00528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren Y. M., von Blomberg M., De Koning C., Lambalk N., Van Montfrans J., Schoemaker J., et al. (2000). Incipient Ovarian Failure and Premature Ovarian Failure Show the Same Immunological Profile. Am. J. Reprod. Immunol. 43, 359–366. 10.1111/j.8755-8920.2000.430605.x [DOI] [PubMed] [Google Scholar]
- Wagner W. (2019). The Link Between Epigenetic Clocks for Aging and Senescence. Front. Genet. 10, 303. 10.3389/fgene.2019.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen L., Liu Y., Luo B., Xie N., Tan T., et al. (2016). Implantation of Placenta-Derived Mesenchymal Stem Cells Accelerates Murine Dermal Wound Closure through Immunomodulation. Am. J. Transl Res. 8, 4912–4921. [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang Y., Yang T., Li J., Yang X. (2017). Study of the Reparative Effects of Menstrual-Derived Stem Cells on Premature Ovarian Failure in Mice. Stem Cel Res Ther 8, 11. 10.1186/s13287-016-0458-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Lu Y., Chen S., Chen Y., Hu C., Zuo Y. (2018). Protective Function of Bu Shen Huo Xue Formula on the Immunity of B6AF1 Mice with Experimental Autoimmune Premature Ovarian Failure. Exp. Ther. Med. 15, 3302–3310. 10.3892/etm.2018.5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wei Q., Wang H., Han L., Dai H., Qian X., et al. (2020). Mesenchymal Stem Cell Therapy Using Human Umbilical Cord in a Rat Model of Autoimmune-Induced Premature Ovarian Failure. Stem Cell Int 2020, 3249495. 10.1155/2020/3249495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber L., Webber L., Davies M., Anderson R., Bartlett J., Braat D., et al. (2016). ESHRE Guideline: Management of Women with Premature Ovarian Insufficiency. Hum. Reprod. 31, 926–937. 10.1093/humrep/dew027 [DOI] [PubMed] [Google Scholar]
- Wei Y., Fang J., Cai S., Lv C., Zhang S., Hua J. (2016). Primordial Germ Cell-like Cells Derived from Canine Adipose Mesenchymal Stem Cells. Cel Prolif. 49, 503–511. 10.1111/cpr.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. C., Tilly J. L. (2012). The Next (Re)generation of Ovarian Biology and Fertility in Women: Is Current Science Tomorrow's Practice? Fertil. Sterility 98, 3–10. 10.1016/j.fertnstert.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Yin T., Yan J., Yan L., Jin C., Lu C., et al. (2015). Mesenchymal Stem Cells Enhance Angiogenesis and Follicle Survival in Human Cryopreserved Ovarian Cortex Transplantation. Cel Transpl. 24, 1999–2010. 10.3727/096368914x685267 [DOI] [PubMed] [Google Scholar]
- Xiang J., Jiang T., Zhang W., Xie W., Tang X., Zhang J. (2019). Human Umbilical Cord-Derived Mesenchymal Stem Cells Enhanced HK-2 Cell Autophagy through MicroRNA-145 by Inhibiting the PI3K/AKT/mTOR Signaling Pathway. Exp. Cel. Res. 378, 198–205. 10.1016/j.yexcr.2019.03.019 [DOI] [PubMed] [Google Scholar]
- Xiao G. Y., Cheng C. C., Chiang Y. S., Cheng W. T., Liu I. H., Wu S. C. (2016). Exosomal miR-10a Derived from Amniotic Fluid Stem Cells Preserves Ovarian Follicles after Chemotherapy. Sci. Rep. 6, 23120. 10.1038/srep23120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Wu Y., Li L., Wu J., Zhao F., Gao Z., et al. (2020). Clinical Analysis of Human Umbilical Cord Mesenchymal Stem Cell Allotransplantation in Patients with Premature Ovarian Insufficiency. Cell Prolif 53, e12938. 10.1111/cpr.12938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Du X., Wang C., Zhang J., Liu C., Li Y., et al. (2019a). Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cell-Derived Microvesicles on Premature Ovarian Insufficiency in Mice. Stem Cel Res Ther 10, 250. 10.1186/s13287-019-1327-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Lei L., Wang S., Sheng X., Yan G., Xu L., et al. (2019b). Transplantation of Umbilical Cord-Derived Mesenchymal Stem Cells on a Collagen Scaffold Improves Ovarian Function in a Premature Ovarian Failure Model of Mice. In Vitro Cell.Dev.Biol.-Animal 55, 302–311. 10.1007/s11626-019-00337-4 [DOI] [PubMed] [Google Scholar]
- Yang M., Lin L., Sha C., Li T., Zhao D., Wei H., et al. (2020). Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miR-144-5p Improves Rat Ovarian Function after Chemotherapy-Induced Ovarian Failure by Targeting PTEN. Lab. Invest. 100, 342–352. 10.1038/s41374-019-0321-y [DOI] [PubMed] [Google Scholar]
- Yin N., Zhao W., Luo Q., Yuan W., Luan X., Zhang H. (2018a). Restoring Ovarian Function with Human Placenta-Derived Mesenchymal Stem Cells in Autoimmune-Induced Premature Ovarian Failure Mice Mediated by Treg Cells and Associated Cytokines. Reprod. Sci. 25, 1073–1082. 10.1177/1933719117732156 [DOI] [PubMed] [Google Scholar]
- Yin N., Wang Y., Lu X., Liu R., Zhang L., Zhao W., et al. (2018b). hPMSC Transplantation Restoring Ovarian Function in Premature Ovarian Failure Mice Is Associated with Change of Th17/Tc17 and Th17/Treg Cell Ratios through the PI3K/Akt Signal Pathway. Stem Cel Res Ther 9, 37. 10.1186/s13287-018-0772-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yin N., Wu C., Qiu J., Zhang Y., Bo L., Xu Y., et al. (2020). Protective Properties of Heme Oxygenase-1 Expressed in Umbilical Cord Mesenchymal Stem Cells Help Restore the Ovarian Function of Premature Ovarian Failure Mice through Activating the JNK/Bcl-2 Signal Pathway-Regulated Autophagy and Upregulating the Circulating of CD8+CD28− T Cells. Stem Cel Res Ther 11, 49. 10.1186/s13287-019-1537-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. Y., Yoon J. A., Park M., Shin E.-Y., Jung S., Lee J. E., et al. (2020). Recovery of Ovarian Function by Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells in Cisplatin-Induced Premature Ovarian Failure in Mice. Stem Cel Res Ther 11, 255. 10.1186/s13287-020-01769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher W., Harkness L., Jafari A., Kassem M. (2014). An Update of Human Mesenchymal Stem Cell Biology and Their Clinical Uses. Arch. Toxicol. 88, 1069–1082. 10.1007/s00204-014-1232-8 [DOI] [PubMed] [Google Scholar]
- Zhang J., Xiong J., Fang L., Lu Z., Wu M., Shi L., et al. (2016). The Protective Effects of Human Umbilical Cord Mesenchymal Stem Cells on Damaged Ovarian Function: A Comparative Study. Biosci. Trends 10, 265–276. 10.5582/bst.2016.01125 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xia X., Yan J., Yan L., Lu C., Zhu X., et al. (2017). Mesenchymal Stem Cell-Derived Angiogenin Promotes Primodial Follicle Survival and Angiogenesis in Transplanted Human Ovarian Tissue. Reprod. Biol. Endocrinol. 15, 18. 10.1186/s12958-017-0235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Luo Q., Lu X., Yin N., Zhou D., Zhang L., et al. (2018). Effects of hPMSCs on Granulosa Cell Apoptosis and AMH Expression and Their Role in the Restoration of Ovary Function in Premature Ovarian Failure Mice. Stem Cel Res Ther 9, 20. 10.1186/s13287-017-0745-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J., Yin H., Jiang H., Du X., Yang Z. (2020a). The Protective Effects of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles on Cisplatin-Damaged Granulosa Cells. Taiwanese J. Obstet. Gynecol. 59, 527–533. 10.1016/j.tjog.2020.05.010 [DOI] [PubMed] [Google Scholar]
- Zhang X.-M., Liu C.-Y., Shao Z.-H. (2020b). Advances in the Role of Helper T Cells in Autoimmune Diseases. Chin. Med. J. 133, 968–974. 10.1097/cm9.0000000000000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Chen S., Yang P., Cao H., Li L. (2019). The Role of Mesenchymal Stem Cells in Hematopoietic Stem Cell Transplantation: Prevention and Treatment of Graft-Versus-Host Disease. Stem Cel Res Ther 10, 182. 10.1186/s13287-019-1287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Fu X., Jiang J., Zhang N., Zou L., Wang W., et al. (2019). Umbilical Cord Mesenchymal Stem Cell Transplantation Prevents Chemotherapy-Induced Ovarian Failure via the NGF/TrkA Pathway in Rats. Biomed. Res. Int. 2019, 6539294. 10.1155/2019/6539294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yamamoto Y., Xiao Z., Ochiya T. (2019). The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 8, 1025. 10.3390/jcm8071025 [DOI] [PMC free article] [PubMed] [Google Scholar]