Summary
Yield in rice is determined mainly by panicle architecture. Using map‐based cloning, we identified an R2R3 MYB transcription factor REGULATOR OF GRAIN NUMBER1 (RGN1) affecting grain number and panicle architecture. Mutation of RGN1 caused an absence of lateral grains on secondary branches. We demonstrated that RGN1 controls lateral grain formation by regulation of LONELY GUY (LOG) expression, thus controlling grain number and shaping panicle architecture. A novel favourable allele, RGN1 C, derived from the Or‐I group in wild rice affected panicle architecture by means longer panicles. Identification of RGN1 provides a theoretical basis for understanding the molecular mechanism of lateral grain formation in rice; RGN1 will be an important gene resource for molecular breeding for higher yield.
Keywords: germplasm, Regulator of Grain Number1, grain number per panicle, MYB transcription factor
Introduction
Rice is a staple food for almost half of the world population (Sasaki and Burr, 2000). Grain yield is determined by plant architecture which includes plant height, tiller number and angle, and panicle architecture (Jiao et al., 2010), which in turn comprises grain number, grain size and panicle size including branches (Tabuchi et al., 2011).
Many genes affecting panicle development in rice and other grasses have been identified (Ikeda et al., 2007; Ikeda‐Kawakatsu et al., 2012; Ishikawa et al., 2005; Jiang et al., 2013; Koumoto et al., 2013; Kwon et al., 2012; Li et al., 2013; McSteen, 2009; McSteen et al., 2007; Raman et al., 2008; Spinelli et al., 2011; Takeda et al., 2003; Tanaka et al., 2015; Wu et al., 2016; Yao et al., 2019; Yoshida et al., 2013; Zhou et al., 2013). Most of them are transcription factors or transcriptional regulators. For example, FRIZZY PANICLE (FZP) encodes an ERF transcription factor containing an AP2 domain, which can prevent axillary meristem (AM) formation, but can also promote the formation of flower meristems (Komatsu et al., 2003b). Researchers have found that an 18‐bp insertion at 5.3 kilobase (kb) upstream of FZP leads to decreased expression of FZP and a consequent increase in yield (Bai et al., 2017). LAX PANICLE1 (LAX1) encodes a bHLH transcription factor that regulates initiation of AM (Komatsu et al., 2003a). LAX1 mRNA is specifically expressed in the boundary region between the point of AM initiation and shoot apical meristem; LAX1 protein is subsequently trafficked towards the AM where it enhances cell proliferation (Oikawa and Kyozuka, 2009). Mutation of LAX1 caused the absence of lateral grains from secondary branches (SBs). Mutation of LAX1 homologs BARREN STALK1 (BA1) in maize (Gallavotti et al., 2004), and REGULATOR OF AXILLARY MERISTEM FORMATION (ROX) in Arabidopsis showed similar defects in panicle and shoot branching respectively (Yang et al., 2012). Recent studies in maize showed that BA1 was involved in auxin signalling during reproductive AM initiation and was directly regulated by BARREN INFLORESCENCE1 (BIF1) and BARREN INFLORESCENCE4 (BIF4) (Galli et al., 2015). LAX1 in rice can also function along with MONOCULM1 (MOC1) which encodes a transcriptional regulator of the GRAS family. Disruption of MOC1 in rice, or homologous genes LATERAL SUPPRESSOR (LAS) in Arabidopsis and Lateral suppressor (Ls) in tomato (Solanum lycopersicum), leads to a lack of shoot branching (Greb et al., 2003; Li et al., 2003; Schumacher et al., 1999). In rice, LAX2/GNP4 genetically interacts with both LAX1 and MOC1 which encodes a nuclear protein with a conserved RAWUL domain. Loss of function of LAX2/GNP4 affects both vegetative and reproductive branching (Tabuchi et al., 2011; Zhang et al., 2011).
Plant hormones also play important role in regulating branch and spikelet number in rice. GRAIN NUMBER 1a (Gn1a), the first major QTL implicated in to grain number per panicle explained 44% of the phenotypic variation in a biparental cross (Ashikari et al., 2005). Gn1a encodes cytokinin oxidase OsCKX2 that degrades cytokinin. LONELY GUY (LOG) encoding a cytokinin activating enzyme with opposite effect to Gn1a plays an important role in the final step of cytokinin biosynthesis (Kurakawa et al., 2007). Meristematic activity in a log mutant could not be maintained and led to early termination of panicle development, causing a shorter panicle and reduced number of panicle branches.
Gain‐of‐function mutant exb1‐D in Arabidopsis displays a dramatically increased number of branches that resulted from over‐expression of EXCESSIVE BRANCHES1 (EXB1) (Guo et al., 2015). EXB1, encoding WRKY transcription factor WRKY71 with transactivation activity, positively regulates REGULATOR OF AXILLARY MERISTEMS (RAX) genes at the transcriptional level. RAX genes encode R2R3 MYB family transcription factors that are also involved in shoot branching (Keller et al., 2006; Muller et al., 2006; Schmitz et al., 2002). Double and triple mutants show that RAX1 functions partially redundantly with ROX and LAS in regulating AM formation (Yang et al., 2012). Orthologous RAX genes in other dicots species such as BLIND (BL) in tomato and CaBLIND (CaBL) in pepper (Capsicum annuum) also have a role in regulating AM initiation (Jeifetz et al., 2011; Schmitz et al., 2002), but the homolog in monocots has not been identified (McSteen, 2009). Hence, the role of MYB transcription factors in regulating branching in monocots remains to be elucidated.
In this study, REGULATOR OF GRAIN NUMBER1 (RGN1) was identified by map‐based cloning using segregating population constructed by rice germplasm resources with different grain number per panicle. RGN1 encodes an R2R3 MYB transcription factor, with high similarity to RAX in Arabidopsis. RGN1 plays a significant role in regulating panicle architecture and grain number together with LOG. Plants with the loss‐of‐function mutant allele rgn1 displayed highly decreased grain number. Elite haplotype RGN1 C for panicle length identified in natural germplasm has potential to improve the yield of rice.
Results
Phenotypic characterization of NIL‐RGN1 and NIL‐rgn1
We undertook a germplasm screening program to explore and dissect genetic networks involved in regulation of panicle architecture in rice. A japonica variety named BS208 showed a highly abnormal panicle branching pattern, with reduced numbers of lateral grains on SBs (Figure 1a–c and Figure S1). To determine the genetic mechanism underlying the abnormal phenotype, we crossed BS208 with indicia variety Teqing (TQ). F1 plants had normal panicle architecture, and the F2 population segregated 338 normal: 117 abnormal panicle phenotype indicative of segregation at a single locus (χ2 = 0.124; P 1d.f . > 0.9), which was named RGN1. To detail the effects of RGN1 on panicle development, a pair of near‐isogenic lines (NIL) was developed with the RGN1 allele from TQ and rgn1 allele from BS208, namely NIL‐RGN1 and NIL‐rgn1 (Figure S2). The NILs had significant differences in panicle phenotype (Figure 1d–f). The numbers of panicles and primary branches (PBs) in the NILs were similar (Figure 1g,h), but the numbers of SBs, number of lateral grains on the SBs and panicle length in NIL‐rgn1 were greatly reduced (Figure 1i–k). Thus, the grain number per panicle in NIL‐rgn1 was decreased (Figure 1l). Although the grain size and grain weight were increased in NIL‐rgn1, the yield per plant was significantly decreased compared with NIL‐RGN1 (Figure S3). These overall results indicated that RGN1 was involved in panicle architecture and the formation of lateral grains on secondary branches.
Figure 1.
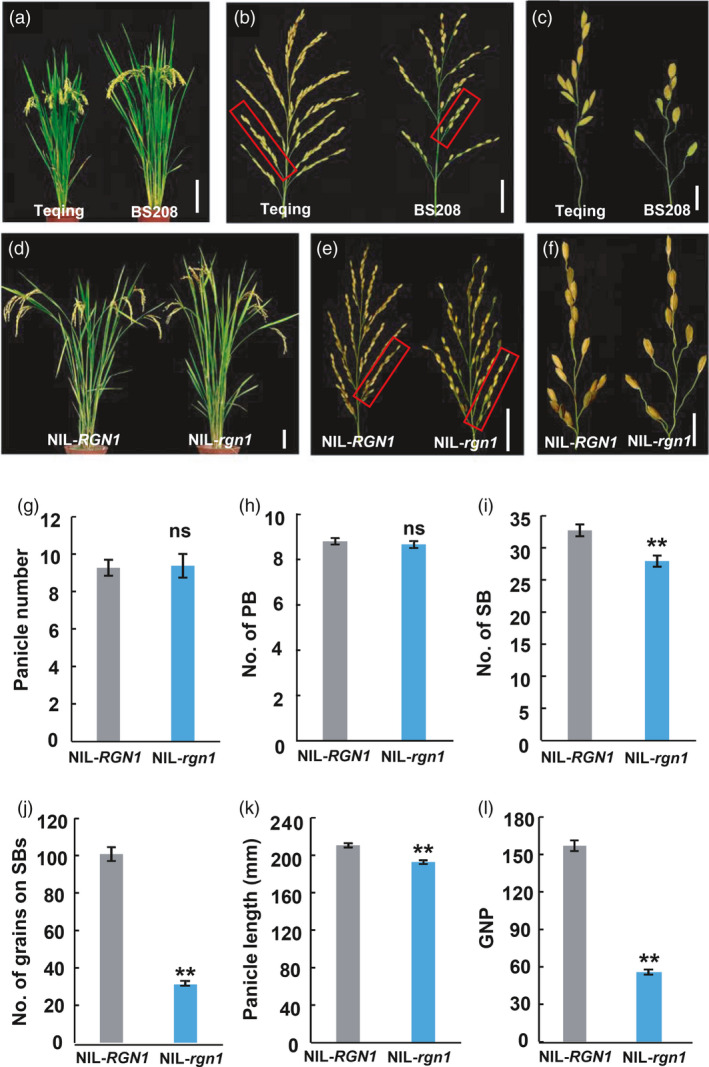
Phenotypic characterization of Teqing, BS208 and RGN1/rgn1 NILs. (a–c) Plant architecture (a), panicles (b) and primary branches (c) from (b) within red boxes of Teqing and BS208. (d–f) Plant architecture (d), panicles (e), primary branches (f) from (e) within red boxes of NIL‐RGN1 and NIL‐rgn1. (g–l) Statistical comparisons of panicle number (g), primary branches number (h), secondary branches number (i), grain number on secondary branches (j), panicle length (k) and grain number per panicle (l) of NIL‐RGN1 and NIL‐rgn1. PB, primary branch; SB, secondary branch; GNP, grain number per panicle. Values are means ± SEM (n = 12). **, P < 0.01, ns, no significant difference. Two‐tailed student’s t‐tests. Scale bars, 20 cm for (a, d); 5 cm for (b, e); 2 cm for (c, f).
Map‐based cloning of RGN1
Heterozygous lines were selected to isolate the candidate gene for RGN1 by map‐based cloning. The RGN1 locus was initially mapped to the long arm of chromosome 1 flanked by the SSR markers RM7341 and RM3285 by analysis of F2 plants with the recessive phenotype from cross BS208 ×TQ. The locus was then fine mapped to a 10.7 kb region flanked by SSR markers MM4411 and RM11529 using an F3:4 population (Figure 2a). According to the Rice Genome Annotation Project (RGAP) only one open reading frame, namely LOC_Os01g49160, was predicted within this genomic region (Figure 2a). LOC_Os01g49160 encodes an R2R3 MYB transcription factor containing three exons and two introns. Comparison of the genomic DNA sequences showed three synonymous SNP and one indel (GAG 58–60) difference between TQ and BS208, and the indel (GAG 58–60) caused a single amino acid deletion in the conserved R2R3 domain (Figure 2b). We also noted this indel (GAG 58–60) difference between BS208 and Nipponbare (NIP) in the 10.7 kb region (Figure 2b). These findings indicated that LOC_Os01g49160 was a strong candidate for RGN1.
Figure 2.
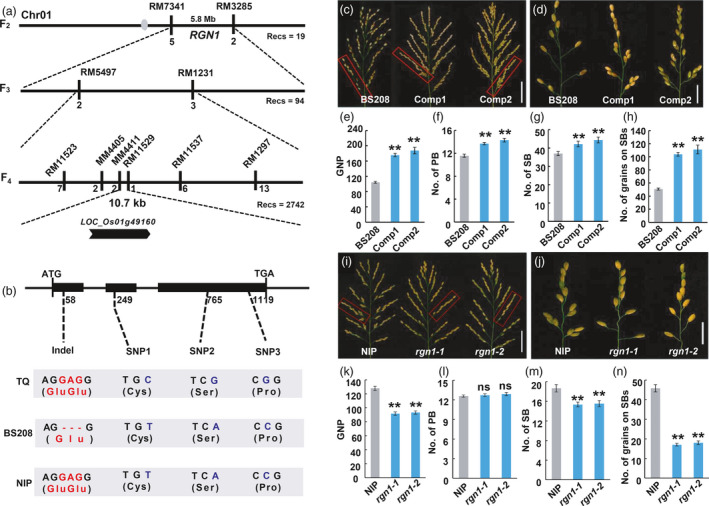
Mapping and validation of RGN1. (a) Summary of RGN1 mapping. Recs, numbers of plants with the recessive phenotype; numbers below the markers indicate numbers of recombinant individuals. (b) Comparison of DNA sequence of LOC_Os01g49160 for TQ, BS208 and NIP. The numbers below the schematic of the gene structure indicate the positions of the start codon (ATG), the stop codon (TGA) and polymorphic nucleotide(s). The first nucleotide of start codon is as No. 1. (c,d) Panicle architectures (c), primary branches (d) from (c) within red boxes of BS208 and complementation lines Comp1 and Comp2. (e–h) Statistical comparisons of grain number per‐panicle (e), primary branch number (f), secondary branch number (g) and number of grains on secondary branches (h) of BS208, Comp1 and Comp2 plants. (i–j) Panicle architectures (i), primary branches (j) from (i) within red boxes of NIP, rgn1‐1 and rgn1‐2 plants. (k–n) Statistical results for grain number per panicle (k), primary branch number (l), second branches number (m) and number of grains on secondary branch (n) of NIP, rgn1‐1 and rgn1‐2 plants. Values are means ± SEM, (n = 12). **P < 0.01, ns, no significant difference. Two‐tailed student’s t‐tests. Scale bars, 5 cm for (c, i); 2 cm for (d,j).
To further confirm that LOC_Os01g49160 was the correct gene, we performed a genetic complementation test in which the coding sequence (CDS) of LOC_Os01g49160 from NIP driven by its native promoter was introduced into BS208 by Agrobacterium tumefaciens‐mediated transformation. Positive complementary lines (Comp1 and Comp2) regenerated lateral grains on the SBs (Figure 2c,d and Figure S4). Quantitative analysis of Comp1 and Comp2 showed that the numbers of primary and secondary branches, panicle length and grain number per panicle were significantly increased compared with BS208 (Figure 2e–h). In addition, we produced two homozygous mutants of LOC_Os01g49160 named rgn1‐1 and rgn1‐2 in NIP background by a CRISPR/Cas9 approach. Lines rgn1‐1 and rgn1‐2 harboured 2 bp and 5 bp deletions, respectively, in LOC_Os01g49160 CDS (Figure S5). The absence of lateral grains at SBs in both mutants caused a panicle phenotype that was similar to of BS208 (Figure 2i–n). We concluded that LOC_Os01g49160 was responsible for shaping panicle architecture in rice, and the locus is hereafter referred to as RGN1.
Next, an over‐expression construct was generated and introduced into NIP. Positive transgenic lines showed no significant differences in branch number and grain number compared to the wild type (Figure S6). We also obtained a T‐DNA insertion mutant, rgn1‐D, with an activation tag inserted into the promoter region in which allowing the expression level of the RGN1 allele to increase (Figure S7a–d). There was no obvious difference in panicle architecture between the rgn1‐D mutant and wild type cultivar Dongjin (Figure S7e–i). These results indicated that the increased expression level of RGN1 had no positive influence on panicle development.
RGN1 encodes an R2R3‐MYB transcription factor
Although the rice annotation database predicted that RGN1 encoded an R2R3 MYB transcription factor, its function is unclear. From protein blast results, we found that the RGN1 protein was a homolog of RAX in Arabidopsis (Figure S8) that functioned redundantly in control of shoot branching (Keller et al., 2006; Muller et al., 2006). The functions of RAX genes were known to be conserved in dicots, but until now homologs of RAX genes in monocots had not been identified. A phylogenetic analysis showed that protein homologs of RGN1 in the Poaceae were highly conserved compared with other species (Figure S9).
We generated transgenic plants with a 35S:RGN1‐green fluorescent protein (GFP) signal to determine the subcellular localization of RGN1. Green fluorescence was detected in the nuclei of root tip cells (Figure 3a). Transient expression experiments in rice protoplasts showed similar results (Figure 3b). These results were consistent with RGN1 having a role as a transcription factor. We also noted that the mutant allele in BS208 did not change the subcellular location of rgn1 protein (Figure 3b). To explore the expression pattern of RGN1, we generated ProRGN1: GUS transgenic plants and GUS activity was detected in various organs, especially in young panicles (Figure 3c–g), and consistent with its function in regulating panicle development. We also found similar results by quantitative RT‐PCR analyses. Quantitative RT‐PCR analyses showed that RGN1 was expressed in roots, stems, leaves, leaf sheaths and young panicles at different developmental stages (Figure S10).
Figure 3.
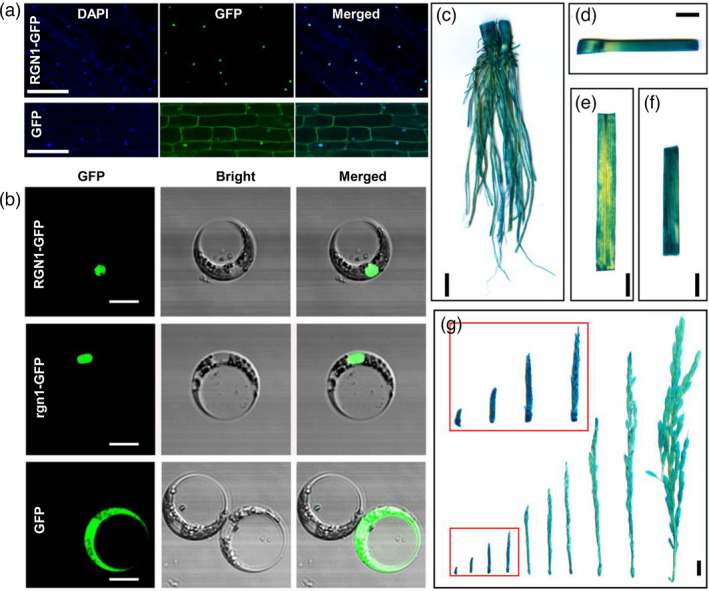
Expression pattern of RGN1. (a) Subcellular localization of RGN1‐GFP in root tips of transgenic plants. (b) Subcellular localization of RGN1‐GFP and rgn1‐GFP fusion protein in rice protoplast cells. Empty vector pSuper1300‐GFP was used as control in (a) and (b). (c–g) GUS staining of tissues from ProRGN1:GUS transgenic plants. Root (c), stem (d), leaf (e), leaf sheath (f), panicles at different stages (g). Scale bars, 10 μm for (a); 20 μm for (b); 1 cm for (c–g).
RGN1 activates expression of LOG to affect panicle architecture
Previous studies showed that cytokinin (CK) was an essential regulators of inflorescence architecture (Han et al., 2014). Two cytokinin related genes in rice Gn1a (OsCKX2) and LOG were reported to be involved in panicle development (Ashikari et al., 2005; Kurakawa et al., 2007). To determine the relationship of Gn1a and LOG with RGN1, we first examined the expression levels of Gn1a and LOG in NIP and rgn1‐1, NIL‐RGN1 and NIL‐rgn1 plants. LOG expression was down‐regulated in rgn1‐1 and NIL‐rgn1 compared with NIP and NIL‐RGN1 respectively (Figure 4a and Figure S11a), whereas Gn1a expression was up‐regulated (Figure S11b,c). Additionally, yeast one‐hybrid assays (Y1H) showed that RGN1 could not bind to the promoter of Gn1a (Figure S11d). We next checked LOG expression in RGN1‐OE3 plants and found that it was up‐regulated (Figure 4a). These results suggested that RGN1 might participate in cytokinin metabolism by regulating LOG expression. Yeast one‐hybrid assays showed that RGN1 bound directly to the promoter region of LOG (Figure 4b). As the binding sites of RGN1 and its homologs in rice had not been identified, we searched the Plant Transcription Factor Database for RGN1 homologs in Arabidopsis and found that the binding motif of AtMYB103/MS188 had been identified by DNA affinity purification sequencing (DAP‐seq) (Jin et al., 2017; O'Malley et al., 2016) (Figure S11e). Then, we screened the LOG promoter and found one MS188‐binding motif. EMSA showed that RGN1 could bind to the MS188‐binding motif of the LOG promoter, whereas rgn1 could not. Quantitative chromatin immunoprecipitation PCR (qChIP‐PCR) assays indicated that the DNA fragment containing the MS188‐binding site from the LOG promoter was enriched by affinity‐purified anti‐Flag antibodies (Figure 4c–d). These experiments thus showed that RGN1 could regulate LOG both in vitro and in vivo.
Figure 4.
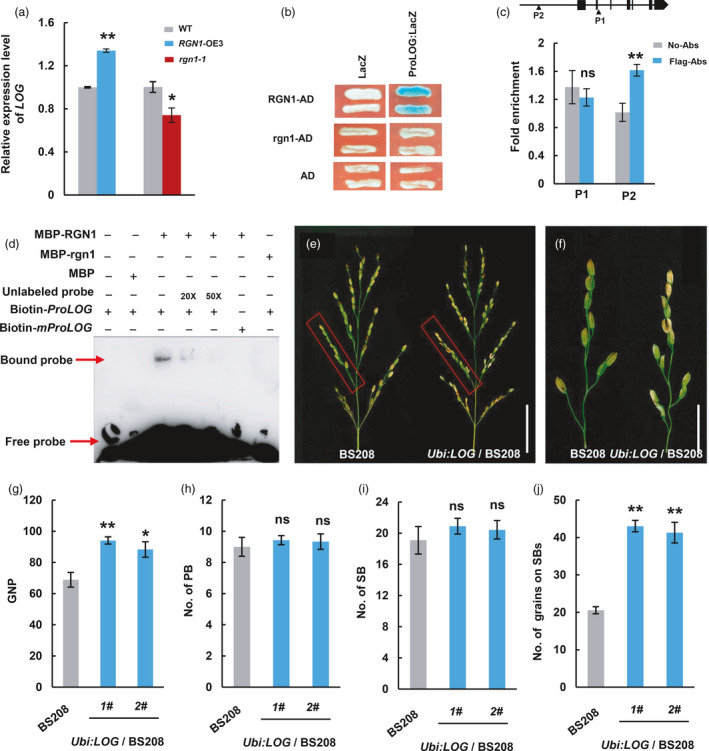
RGN1 controls LOG expression to regulate the formation of lateral grains on secondary branches. (a) Expression levels of LOG in NIP compared to rgn1‐1 and RGN1‐OE3 plants. Values are means ± SEM, (n = 3, each with three technical repeats). (b) Yeast one‐hybrid assays demonstrate that RGN1 binds to the LOG promoter (ProLOG). (c) Quantitative ChIP‐PCR indicates that RGN1 binds to the MS188‐binding motif (P2 region) from the LOG promoter in vivo. Values are means ± SEM, (n = 3). (d) Electrophoretic mobility shift assay shows that RGN1 binds to the MS188‐binding motif from the LOG promoter in vitro. mProLOG, LOG promoter containing mutated MS188 binding motif. (e,f) Phenotype of BS208 was partially rescued by LOG overexpression: panicles architecture (e), primary branches (f) from (e) within red boxes of BS208 and Ubi:LOG/BS208 transgenic plant. Scale bars, 5 cm for (e); 2 cm for (f). (g–j) Comparisons of grain number per panicle (g), primary branch number (h), secondary branches number (i), number of grains on secondary branches (j) between BS208 and Ubi:LOG/BS208 transgenic plants. Values are means ± SEM (n = 10). *P < 0.05, **P < 0.01, ns, no significant difference. Two‐tailed student’s t‐tests.
Next, the contents of bioactive CKs in young panicles of NIL‐RGN1 and NIL‐rgn1 were compared to determine the effect of RGN1 on endogenous CK contents. Compared with NIL‐RGN1, the bioactive CKs (IP and cZ) contents in NIL‐rgn1 were significantly lower (Figure S11f). In addition, over‐expression of LOG in BS208 partially rescued the absence of lateral grains in secondary branches (Figure 4e–j and Figure S11g). These findings indicated that RGN1 regulates LOG to control lateral grain development thus determining panicle architecture.
Elite allele RGN1 C for panicle length was identified from natural germplasm of O. sativa and O. rufipogon
An investigation of sequence variation at the RGN1 locus in 2858 cultivated and 446 wild rice accessions encompassing taxonomic groups admix, aromatic (aro), aus, indica (ind), temperate japonica (tej), tropical japonica (trj) and three types O. rufipogon species (Or‐I, Or‐II and Or‐III) identified three SNPs (S28250218, S28250398 and S28251268) (Data S1). Only S28250218 was non‐synonymous and resulted in a single amino acid change (Glu vs. Asp) in the conserved R2R3 DNA‐binding domain of RGN1 (Figure 5a, Data S1 and Figure S8). However, the GAG 58‐60 difference Indel between TQ and BS208 was not identified, indicating that the rare RGN1 BS208 allele could be a spontaneous mutant unique to BS208. Based on S28250218, the accessions were divided into two haplotypes namely Hap‐RGN1 C and Hap‐RGN1 G. Accessions containing the RGN1 C allele exhibited longer panicles than accessions containing the RGN1 G allele (Figure 5b). Expression of LOG was higher in accessions containing RGN1 C than accessions containing RGN1 G (Figure S12a). Transient expression of LOG was also higher in rice protoplasts expressing RGN1C than in protoplasts expressing RGN1G or rgn1 (Figure S12b). The phylogenetic tree of RGN1 base on 13 SNPs in the genomic region including a 2 kb promoter indicated that all wild and cultivated accessions with RGN1 C had high similarity (Figure 5c). The RGN1 C allele in wild rice was very frequent in Or‐I wild rice accessions (61 of 64, 95.3%) and was the most common allele in the overall Or‐I group (61 of 154 accessions, 39.6%). It was also common in aro (63 of 73 accessions, 86.3%) and aus (155 of 195 accessions, 79.5%), but was a minor allele in ind (34 of 1705, 2.0%) and trj (34 of 453, 7.5%) and an extremely rare allele in tej (1 of 272, 0.4%) (Figure 5a). These results suggest that the RGN1 C allele in both wild and cultivated rice might originate in wild rice Or‐I prior to domestication.
Figure 5.
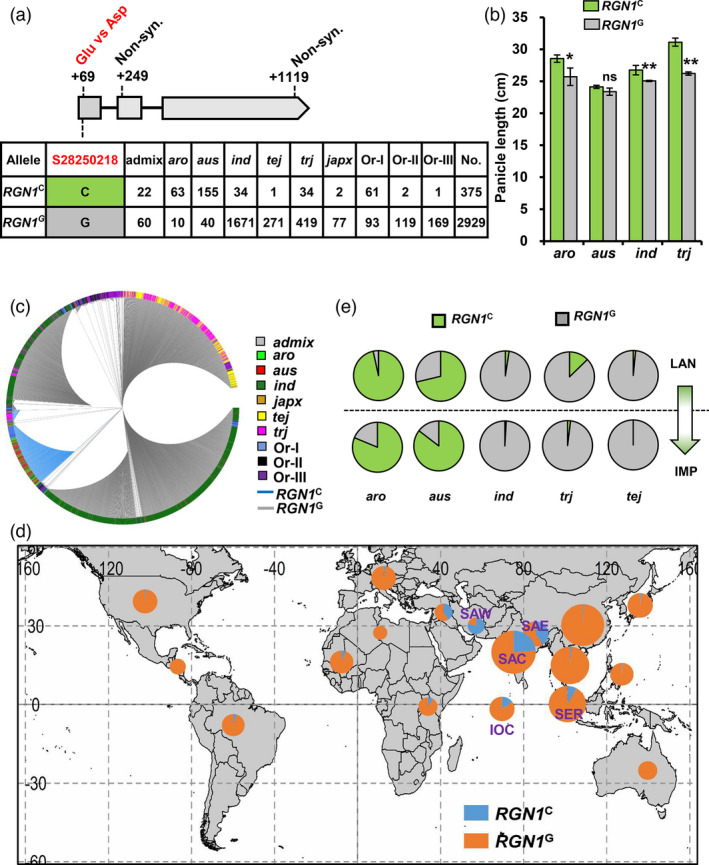
Variation of RGN1 in germplasm. (a) Haplotypes of RGN1 based on SNP S28250218. (b) Statistical comparison for panicle length in accessions containing the RGN1 C and RGN1 G alleles. Data are means ± SEM, n = 44 and 7 (aro), 132 and 30 (aus), 23 and 969 (ind), 29 and 247 (trj) for RGN1 C and RGN1 G alleles respectively. *P < 0.05; **P < 0.01; Two‐tailed student’s t‐tests. (c) Phylogram of RGN1 generated from 2848 cultivated and 446 wild rice accessions. (d) Geographical distribution of the accessions containing RGN1 C or RGN1 G. SAW (South Asia‐West, including Afghanistan and Pakistan), SAE (South Asia‐East, including Bangladesh and Bhutan), SAC (South Asia‐Central, including India and Nepal), SER (SEA islands, including Brunei, Indonesia, Malaysia and the Philippines), IOC (Indian Ocean, including Madagascar and Sri Lanka). (e) Frequencies of RGN1 C and RGN1 G in landraces (LAN) and improved varieties (IMP).
Although RGN1 C was found in wild and cultivated accessions distributed worldwide, it was more frequent among accessions from the South Asia‐Central (SAC), South Asia‐East (SAE), SEA Islands (SER), South Asia‐West (SAW) and Indian Ocean (IOC) region (Figure 5d). As the proportions of accessions containing RGN1 C were lower in both landraces (LAN) and improved varieties (IMP) within the ind, trj and tej groups, there is considerable potential for molecular breeding to improve panicle architecture in these groups (Figure 5e). Further study of the function of RGN1 C may provide clearer evidences for its utilization in yield improvement.
Discussion
Branching is an iconic characteristic that plant obtained through body plan evolution (Graham et al., 2000). Branching in rice causes the formation of tillers and panicle branches. The first step in branching is AM initiation (Stirnberg et al., 2002). Initiation of AM in Arabidopsis requires the participation of at least three groups of transcription factors: ROX, LAS and RAX1 (Yang et al., 2012). The ROX homolog in rice is LAX1, and the LAS homolog is MOC1. The RAX homolog was previously unknown. Here, we demonstrated that RGN1 encodes an R2R3 MYB transcription factor that is homologous to RAX1 and controls lateral grains formation.
Considering all results from this study, we proposed a working model explaining the difference in panicle development between NIL‐RGN1 and NIL‐rgn1 (Figure 6). The RGN1 protein in NIL‐RGN1 directly binds to the LOG promoter to positively regulate LOG expression. LOG transforms inactive CKs to active forms that amplify the CK signalling pathway (Kurakawa et al., 2007). The amplified CK signalling causes NIL‐RGN1 to develop a normal panicle. The contrasting situation in NIL‐rgn1 is that the rgn1 protein fails to bind to the LOG promoter, thus preventing amplification of CK signalling. Unamplified CK signalling causes NIL‐rgn1 to develop a lax panicle.
Figure 6.
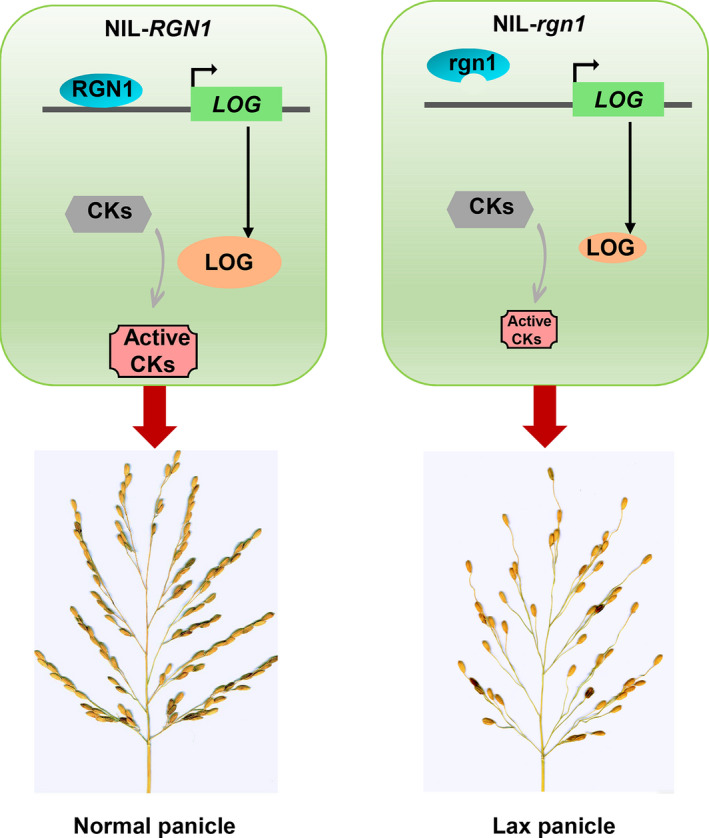
Model showing the difference in AM formation between NIL‐RGN1 and NIL‐rgn1. Grey arrows are previously known pathways; black arrows indicate direct regulations demonstrated in the current work.
Elevated expression of RGN1 had no obvious effect on panicle branch formation (Figures S6 and S7). When LAX1 or LAX2/GNP4 was over‐expressed, the effect on panicle branching was similar to that of RGN1 (Komatsu et al., 2003a; Zhang et al., 2018). However, mutations in RGN1, LAX1 or LAX2/GNP4 strongly suppress lateral grain formation, implying that RGN1, LAX1 and LAX2/GNP4 are essential for determination of panicle architecture. These indicated that the vital function of these genes in regulating panicle architecture. Coincidently, we found the RGN1 BS208 allele only in accession BS208 despite and search of a large panel of wild and cultivated rice accessions, providing evidence that RGN1 has an indispensable role in maintaining basic panicle architecture. It could be considered an indispensable house‐keeping or core‐genes that are essential for plant growth, development and reproduction (Wang et al., 2018).
We also isolated a novel RGN1 C allele, in which SNP S28250218 caused an amino acid change (Glu vs. Asp) in the conserved DNA‐binding domain. The increased panicle size in accessions with RGN1 C may be caused by the enhanced regulation of genes downstream of RGN1C. This variation (from Glu in RGN1G to Asp in RGN1C) might provide a mimic continuous phosphorylation site in RGN1, thus affecting its post‐transcriptional modification or might change its protein conformation for DNA binding. Although RGN1 C is a favourable allele mainly present in aro and aus varieties, it apparently was not selected during domestication and improvement of rice. It is possible that RGN1 has adverse effects on other unidentified traits, or that linkage drag caused by undesirable genes in its vicinity might have constrained its selection. For example, we found that OsETT2 affecting awn development (Toriba and Hirano, 2014) is located about 750 kb upstream of RGN1 and elimination of the undesirable allele(s) controlling awn formation could have prevented selection of the RGN1 C allele during domestication.
We are currently facing a severe constraint in yield potential in rice (Wei et al., 2015). A more detailed understanding of the mechanism of grain initiation could provide new approaches for breeders to increase yield. From this study, we have a preliminary understanding of the mechanism of how lateral grains are produced on SBs. The mechanism could also serve as a reference for maize, wheat and other cereal crops.
Methods
Plant materials and growth conditions
A cross between rice (Oryza sativa) varieties BS208 and Teqing was used to map the RGN1 locus. NIL‐RGN1 and NIL‐rgn1 were homozygous sister lines with contrasting phenotypes selected from an F7 population derived from an F2 RGN1/rgn1 heterozygote. T‐DNA insertion mutant rgn1‐D (PFG_3A‐03714.R) and its wild type (variety Dongjin) were purchased from the Rice T‐DNA Insertion Sequence Database. Nipponbare (NIP) and BS208 were used for transformation. All rice plants were grown under natural paddy conditions at Beijing or at Sanya in Hainan province.
Vector constructions and plant transformation
To construct the complementation vector, RGN1‐Comp‐1F and RGN1‐Comp‐1R primers were employed to amplify the promoter of RGN1, and RGN1‐Comp‐2F and RGN1‐Comp‐2R primers were employed to amplify the CDS of RGN1. The two fragments were fused and cloned into the PmeI and AscI sites of binary plant expression vector pMDC162. To construct the CRISPR/Cas9 vector, a 20‐bp PAM sequence from the RGN1 CDS was selected for specific recognition and cloned into vector SK‐gRNA as previously described (Wang et al., 2015). To construct RGN1 and LOG over‐expression vectors, full‐length RGN1 and LOG cDNA without stop codons were amplified by PCR and cloned into the KpnI and HindⅢ sites of binary plant expression vector pC1305. To construct the 35S:Flag‐RGN1 vector, full‐length RGN1 cDNA without a stop codon was amplified and cloned into the SpeI and KpnI sites of binary plant expression vector pCM1307. To construct the ProRGN1:GUS vector, a genomic fragment of the RGN1 promoter region starting at 2.5 kb upstream of the ATG initiation codon was amplified and cloned into the PmeI and AscI sites of the binary plant expression vector pMDC162. For subcellular localization, RGN1 cDNA and rgn1 cDNA without stop codons were amplified by PCR with primers RGN1‐GFP‐F and RGN1‐GFP‐R and cloned into the KpnI and HindⅢ sites of the binary plant expression vector pSuper1300‐GFP. To produce transgenic plants, the corresponding constructs were introduced into Agrobacterium tumefaciens strain EHA105 and subsequently transformed into selected rice varieties through Agrobacterium‐mediated transformation (Toki et al., 2006).
Expression pattern analysis
qRT‐PCR was conducted to check the relative expression levels of RGN1 and other genes involved in this study. Total RNA was extracted from fresh tissues using Trizol reagent (Invitrogen, Carlsbad, CA). All experiments were conducted using a Takara Kit as previously reported (Zhang et al., 2018). The rice ubiqutin1 gene served as an internal control.
GUS‐staining assays were conducted to analyse the expression pattern of RGN1. Tissues from ProRGN1: GUS transgenic plants were collected and submerged in GUS‐staining solution as previously reported (Zhang et al., 2018).
Subcellular localization
Root tips from RGN1‐GFP transgenic plants stained with 1 µg/mL DAPI (4′, 6‐diamidino‐2‐phenylin‐dole, ~30 min) were used to check the subcellular location of RGN1. ProSuper1300:RGN1‐GFP and ProSuper1300:rgn1‐GFP vectors were transiently expressed in rice protoplasts to determine the subcellular locations of RGN1 and rgn1. Fluorescence signals of GFP fusion proteins were detected and photographed under a confocal microscope (Olympus FV1000,Tokyo, Japan).
Dual‐luciferase assay
The cDNA of rgn1, RGN1 G and RGN1 C without stop codon were amplified and cloned into the KpnI and PstI sites of the pGreenII 62‐SK. The resulting rgn1‐62‐SK, RGN1C‐62‐SK and RGN1G‐62‐SK constructs were used as effectors; empty pGreenII 62‐SK vector was used as control; pGreenII0800‐ProLOG:LUC vector was used as reporter which containing 35S:REN served as an internal reference.
Reporters and effectors or control were transformed into rice protoplasts. Firefly luciferase (LUC) and Renilla luciferase (REN) activity levels were quantified by a Dual‐Luciferase reporter assay system (Promega, Madison, Wisconsin, USA).
Yeast one‐hybrid assay
Full‐length RGN1 and rgn1 cDNA were amplified and cloned into the EcoRI and XhoI sites of the pB42AD vector to yield RGN1‐AD and rgn1‐AD constructs respectively. Genomic fragments of the LOG and Gn1a promoter regions starting at 2.0 kb upstream of the ATG start codons were amplified by PCR and cloned into the EcoRI and XhoI sites of pLacZi2µ vector to yield reporters. RGN1‐AD was co‐transformed with the reporter or control (empty pLacZi2µ vector) into the yeast strain EGY48 through the PEG/LiAc method. Positive clones were cultured on SD/‐Ura‐Trp plates containing β‐d‐galactopyranoside to check for possible interactions between RGN1 and the reporter.
qChIP‐PCR assays
Young panicles (1 cm in length) from 35S:Flag‐RGN1 transformants were collected and cross‐linked. ChIP assays were performed using a ChIP Assay Kit (P2078, Beyotime, Shanghai, China) following the manufacturer’s instructions. The ChIP products were used for qChIP‐PCR assays. Primers LOG‐ChIP‐qRT‐F and LOG‐ChIP‐qRT‐R were designed to amplify a 101 bp fragment from the LOG promoter containing the MS188 binding site in the ChIP enrichment test by qPCR.
Electrophoretic mobility shift assay
For expression and purification of the RGN1 and rgn1 proteins in Escherichia coli, the cDNA of RGN1 and rgn1 were amplified and cloned into the EcoRI and HindⅢ sites of vector pMAL‐c5X to yield MBP‐RGN1 and MBP‐rgn1 constructs. These recombinant constructs were introduced into an Escherichia coli Rosetta (DE3) cell line. A PurKine™ MBP‐Tag Protein Purification Kit (Dextrin) was used to purify the proteins. A fragment of the LOG promoter (723–742 bp upstream of the ATG codon) containing an MS188‐binding site (ACCAAA) was selected as the probe. The mutant probe was synthesized by replacing ACCAAA with TTTTTT. Biotin‐labelled primers were synthesized and purified by Invitrogen. EMSA reactions were conducted using a LightShift Chemiluminescent EMSA kit (Thermo Scientific, Waltham, MA) following the manufacturer’s instruction. Signals were detected by a Tanon‐5200 Chemiluminescent Imaging System (Shanghai,China).
Measurement of endogenous CKs levels
Young panicles (1 cm in length) of NIL‐RGN1 and NIL‐rgn1 plants were collected, ground into powder, and extracted with 1 mL methanol/water/formic acid (15 : 4 : 1, V/V/V), with three independent biological repeats per sample. The combined extracts were evaporated to dryness under a nitrogen gas stream and reconstituted in 100 μL 80% methanol (V/V). Then, CK (isopentenyladenine, tZ and cZ) contents were measured by MetWare (http://www.metware.cn/) based on the AB Sciex QTRAP4500 LC‐MS/MS platform.
Haplotype and phylogenetic tree analyses
SNPs in 2848 cultivated accessions and 446 wild rice accessions were obtained from the rice 3 K project and a previous publication (Huang et al., 2012; Wang et al., 2018) respectively. The neighbour‐joining phylogenetic tree was constructed using MEGA 5.0 (Tamura et al., 2011) and then visualized and annotated using EvolView (Zhang et al., 2012).
Primers
Primers used in the study are listed in Data S2.
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
G. Li designed the research and wrote the manuscript. G. Li and B. Xu performed most of experiments. Y. Xu and Z. Wu assisted with experiments. J. Xie, X. Sun, H. Guo and X. Wang assisted with data analysis. N.U. Khan assisted with revisions for the manuscript. H. Zhang, J. Li, J. Xu and W. Wang provided technical assistance. Z. Zhang designed the research, conducted origin and evolutionary analyses, and wrote and revised the manuscript. Z. Zhang and Z. Li conceived the project, designed the research and revised the manuscript.
Supporting information
Figure S1 Comparison of yield related traits between TQ (Teqing) and BS208.
Figure S2 Genome constitution of NIL‐RGN1 and NIL‐rgn1.
Figure S3 Comparison of yield related traits between NIL‐RGN1 and NIL‐rgn1.
Figure S4 Characterization of complementation plants.
Figure S5 Characterization of rgn1‐1 and rgn1‐2 plants.
Figure S6 Characterization of RGN1 over‐expression plants.
Figure S7 Characterization of the T‐DNA insertion plant rgn1‐D.
Figure S8 Sequence alignment of RGN1 with RAX proteins from Arabidopsis.
Figure S9 Phylogenetic analysis of RGN1 protein and homologs from in rice and other angiosperm species.
Figure S10 The expression pattern of RGN1 in different tissues from NIP determined by qRT‐PCR.
Figure S11 RGN1 participates in cytokinin metabolism.
Figure S12 RGN1C causes higher expression of LOG.
Data S1 The information of SNPs used in in this study.
Data S2 The information of primers used in this study.
Acknowledgements
We thank Robert A. McIntosh (University of Sydney) for critical reading and suggested revisions for the manuscript. This study was supported by grants from Ministry of Science and Technology of China (2016YFD0100803‐2), the National Natural Science Foundation of China (32072036, 31801324 and 31171521) and the Fundamental Research Funds for the Central Universities, China Agricultural University (2018QC163, 2019TC0211, and 2020RC031).
Li, G. , Xu, B. , Zhang, Y. , Xu, Y. , Khan, N. U. , Xie, J. , Sun, X. , Guo, H. , Wu, Z. , Wang, X. , Zhang, H. , Li, J. , Xu, J. , Wang, W. , Zhang, Z. and Li, Z. (2022) RGN1 controls grain number and shapes panicle architecture in rice. Plant Biotechnol. J., 10.1111/pbi.13702
Contributor Information
Zhanying Zhang, Email: zhangzhanying@cau.edu.cn.
Zichao Li, Email: lizichao@cau.edu.cn.
References
- Ashikari, M. , Sakakibara, H. , Lin, S. , Yamamoto, T. , Takashi, T. , Nishimura, A. , Angeles, E.R. et al. (2005) Cytokinin oxidase regulates rice grain production. Science, 309, 741–745. [DOI] [PubMed] [Google Scholar]
- Bai, X. , Huang, Y. , Hu, Y. , Liu, H. , Zhang, B. , Smaczniak, C. , Hu, G. et al. (2017) Duplication of an upstream silencer of FZP increases grain yield in rice. Nat. Plants, 3, 885–893. [DOI] [PubMed] [Google Scholar]
- Gallavotti, A. , Zhao, Q. , Kyozuka, J. , Meeley, R.B. , Ritter, M.K. , Doebley, J.F. , Pe, M.E. et al. (2004) The role of barren stalk1 in the architecture of maize. Nature, 432, 630–635. [DOI] [PubMed] [Google Scholar]
- Galli, M. , Liu, Q. , Moss, B.L. , Malcomber, S. , Li, W. , Gaines, C. , Federici, S. et al. (2015) Auxin signaling modules regulate maize inflorescence architecture. Proc. Natl Acad. Sci. USA, 112, 13372–13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, L.E. , Cook, M.E. and Busse, J.S. (2000) The origin of plants: body plan changes contributing to a major evolutionary radiation. Proc. Natl Acad. Sci. USA, 97, 4535–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb, T. , Clarenz, O. , Schafer, E. , Muller, D. , Herrero, R. , Schmitz, G. and Theres, K. (2003) Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 17, 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D. , Zhang, J. , Wang, X. , Han, X. , Wei, B. , Wang, J. , Li, B. et al. (2015) The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in Arabidopsis . Plant Cell, 27, 3112–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , Yang, H. and Jiao, Y. (2014) Regulation of inflorescence architecture by cytokinins. Front. Plant Sci. 5, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X. , Kurata, N. , Wei, X. , Wang, Z.‐X. , Wang, A. , Zhao, Q. , Zhao, Y. et al. (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature, 490, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, K. , Ito, M. , NagasawaO, N. , Kyozuka, J. and Nagato, Y. (2007) Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F‐box protein, regulates meristem fate. Plant J. 51, 1030–1040. [DOI] [PubMed] [Google Scholar]
- Ikeda‐Kawakatsu, K. , Maekawa, M. , Izawa, T. , Itoh, J.I. and Nagato, Y. (2012) ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1. Plant J. 69, 168–180. [DOI] [PubMed] [Google Scholar]
- Ishikawa, S. , Maekawa, M. , Arite, T. , Onishi, K. , Takamure, I. and Kyozuka, J. (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 46, S192. [DOI] [PubMed] [Google Scholar]
- Jeifetz, D. , David‐Schwartz, R. , Borovsky, Y. and Paran, I. (2011) CaBLIND regulates axillary meristem initiation and transition to flowering in pepper. Planta, 234, 1227–1236. [DOI] [PubMed] [Google Scholar]
- Jiang, L. , Liu, X. , Xiong, G. , Liu, H. , Chen, F. , Wang, L. , Meng, X. et al. (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature, 504, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, Y. , Wang, Y. , Xue, D. , Wang, J. , Yan, M. , Liu, G. , Dong, G. et al. (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544. [DOI] [PubMed] [Google Scholar]
- Jin, J.P. , Tian, F. , Yang, D.C. , Meng, Y.Q. , Kong, L. , Luo, J.C. and Gao, G. (2017) PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 45, D1040–D1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, T. , Abbott, J. , Moritz, T. and Doerner, P. (2006) Arabidopsis REGULATOR OF AXILLARY MERISTEMS1 controls a leaf axil stem cell niche and modulates vegetative development. Plant Cell, 18, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu, K. , Maekawa, M. , Ujiie, S. , Satake, Y. , Furutani, I. , Okamoto, H. , Shimamoto, K. et al. (2003a) LAX and SPA: major regulators of shoot branching in rice. Proc. Natl Acad. Sci. USA, 100, 11765–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu, M. , Chujo, A. , Nagato, Y. , Shimamoto, K. and Kyozuka, J. (2003b) FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development, 130, 3841–3850. [DOI] [PubMed] [Google Scholar]
- Koumoto, T. , Shimada, H. , Kusano, H. , She, K.C. , Iwamoto, M. and Takano, M. (2013) Rice monoculm mutation moc2, which inhibits outgrowth of the second tillers, is ascribed to lack of a fructose‐1,6‐bisphosphatase. Plant Biotechnol. 30, 47–56. [Google Scholar]
- Kurakawa, T. , Ueda, N. , Maekawa, M. , Kobayashi, K. , Kojima, M. , Nagato, Y. , Sakakibara, H. et al. (2007) Direct control of shoot meristem activity by a cytokinin‐activating enzyme. Nature, 445, 652–655. [DOI] [PubMed] [Google Scholar]
- Kwon, Y. , Yu, S.I. , Park, J.H. , Li, Y. , Han, J.H. , Alavilli, H. , Cho, J.I. et al. (2012) OsREL2, a rice TOPLESS homolog functions in axillary meristem development in rice inflorescence. Plant Biotechnol. Rep. 6, 213–224. [Google Scholar]
- Li, S. , Zhao, B. , Yuan, D. , Duan, M. , Qian, Q. , Tang, L. , Wang, B. et al. (2013) Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl Acad. Sci. USA, 110, 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Qian, Q. , Fu, Z. , Wang, Y. , Xiong, G. , Zeng, D. , Wang, X. et al. (2003) Control of tillering in rice. Nature, 422, 618–621. [DOI] [PubMed] [Google Scholar]
- McSteen, P. (2009) Hormonal regulation of branching in grasses. Plant Physiol. 149, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen, P. , Malcomber, S. , Skirpan, A. , Lunde, C. , Wu, X.T. , Kellogg, E. and Hake, S. (2007) barren inflorescence2 encodes a co‐ortholog of the PINOID serine/threonine kinase and is required for organogenesis during inflorescence and vegetative development in maize. Plant Physiol. 144, 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, D. , Schmitz, G. and Theres, K. (2006) Blind homologous R2R3 Myb genes control the pattern of lateral meristem initiation in Arabidopsis . Plant Cell, 18, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa, T. and Kyozuka, J. (2009) Two‐step regulation of LAX PANICLE1 protein accumulation in axillary meristem formation in rice. Plant Cell, 21, 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley, R.C. , Huang, S.C. , Song, L. , Lewsey, M.G. , Bartlett, A. , Nery, J.R. , Galli, M. et al. (2016) Cistrome and epicistrome features shape the regulatory DNA landscape. Cell, 165, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, S. , Greb, T. , Peaucelle, A. , Blein, T. , Laufs, P. and Theres, K. (2008) Interplay of miR164, CUP‐SHAPED COTYLEDON genes and LATERAL SUPPRESSOR controls axillary meristem formation in Arabidopsis thaliana . Plant J. 55, 65–76. [DOI] [PubMed] [Google Scholar]
- Sasaki, T. and Burr, B. (2000) International Rice Genome Sequencing Project: the effort to completely sequence the rice genome. Curr. Opin. Plant Biol. 3, 138–141. [DOI] [PubMed] [Google Scholar]
- Schmitz, G. , Tillmann, E. , Carriero, F. , Fiore, C. , Cellini, F. and Theres, K. (2002) The tomato Blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proc. Natl Acad. Sci. USA, 99, 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, K. , Schmitt, T. , Rossberg, M. , Schmitz, G. and Theres, K. (1999) The Lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc. Natl Acad. Sci. USA, 96, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, S.V. , Martin, A.P. , Viola, I.L. , Gonzalez, D.H. and Palatnik, J.F. (2011) A mechanistic link between STM and CUC1 during Arabidopsis development. Plant Physiol. 156, 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg, P. , van De Sande, K. and Leyser, H.M. (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis . Development, 129, 1131–1141. [DOI] [PubMed] [Google Scholar]
- Tabuchi, H. , Zhang, Y. , Hattori, S. , Omae, M. , Shimizu‐Sato, S. , Oikawa, T. , Qian, Q. et al. (2011) LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell, 23, 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, T. , Suwa, Y. , Suzuki, M. , Kitano, H. , Ueguchi‐Tanaka, M. , Ashikari, M. , Matsuoka, M. et al. (2003) The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 33, 513–520. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. and Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, W. , Ohmori, Y. , Ushijima, T. , Matsusaka, H. , Matsushita, T. , Kumamaru, T. , Kawano, S. et al. (2015) Axillary meristem formation in rice requires the WUSCHEL ortholog TILLERS ABSENT1. Plant Cell, 27, 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki, S. , Hara, N. , Ono, K. , Onodera, H. , Tagiri, A. , Oka, S. and Tanaka, H. (2006) Early infection of scutellum tissue with Agrobacterium allows high‐speed transformation of rice. Plant J. 47, 969–976. [DOI] [PubMed] [Google Scholar]
- Toriba, T. and Hirano, H.Y. (2014) The DROOPING LEAF and OsETTIN2 genes promote awn development in rice. Plant J. 77, 616–626. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Shen, L. , Fu, Y.P. , Yan, C.J. and Wang, K.J. (2015) A simple CRISPR/Cas9 system for multiplex genome editing in rice. J. Genet. Genomics, 42, 703–706. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Mauleon, R. , Hu, Z. , Chebotarov, D. , Tai, S. , Wu, Z. , Li, M. et al. (2018) Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature, 557, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X. , Zhang, Z. , Shi, P.J. , Wang, P. , Chen, Y. , Song, X. and Tao, F.L. (2015) Is yield increase sufficient to achieve food security in China? PLoS ONE, 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Wang, Y. , Mi, X.F. , Shan, J.X. , Li, X.M. , Xu, J.L. and Lin, H.X. (2016) The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Wang, Q. , Schmitz, G. , Muller, D. and Theres, K. (2012) The bHLH protein ROX acts in concert with RAX1 and LAS to modulate axillary meristem formation in Arabidopsis . Plant J. 71, 61–70. [DOI] [PubMed] [Google Scholar]
- Yao, H. , Skirpan, A. , Wardell, B. , Matthes, M.S. , Best, N.B. , McCubbin, T. , Durbak, A. et al. (2019) The barren stalk2 gene is required for axillary meristem development in maize. Mol. Plant, 12, 374–389. [DOI] [PubMed] [Google Scholar]
- Yoshida, A. , Sasao, M. , Yasuno, N. , Takagi, K. , Daimon, Y. , Chen, R. , Yamazaki, R. et al. (2013) TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc. Natl Acad. Sci. USA, 110, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Gao, S. , Lercher, M.J. , Hu, S. and Chen, W.H. (2012) EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 40, W569–W572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Li, J. , Yao, G. , Zhang, H. , Dou, H. , Shi, H. , Sun, X. et al. (2011) Fine mapping and cloning of the Grain Number Per‐Panicle Gene (Gnp4) on chromosome 4 in rice (Oryza sativa L.) . Agric. Sci. China, 10, 1825–1833. [Google Scholar]
- Zhang, Z. , Li, J. , Tang, Z. , Sun, X. , Zhang, H. , Yu, J. , Yao, G. et al. (2018) Gnp4/LAX2, a RAWUL protein, interferes with the OsIAA3‐OsARF25 interaction to regulate grain length via the auxin signaling pathway in rice. J. Exp. Bot. 69, 4723–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Lin, Q. , Zhu, L. , Ren, Y. , Zhou, K. , Shabek, N. , Wu, F. et al. (2013) D14‐SCF(D3)‐dependent degradation of D53 regulates strigolactone signalling. Nature, 504, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of yield related traits between TQ (Teqing) and BS208.
Figure S2 Genome constitution of NIL‐RGN1 and NIL‐rgn1.
Figure S3 Comparison of yield related traits between NIL‐RGN1 and NIL‐rgn1.
Figure S4 Characterization of complementation plants.
Figure S5 Characterization of rgn1‐1 and rgn1‐2 plants.
Figure S6 Characterization of RGN1 over‐expression plants.
Figure S7 Characterization of the T‐DNA insertion plant rgn1‐D.
Figure S8 Sequence alignment of RGN1 with RAX proteins from Arabidopsis.
Figure S9 Phylogenetic analysis of RGN1 protein and homologs from in rice and other angiosperm species.
Figure S10 The expression pattern of RGN1 in different tissues from NIP determined by qRT‐PCR.
Figure S11 RGN1 participates in cytokinin metabolism.
Figure S12 RGN1C causes higher expression of LOG.
Data S1 The information of SNPs used in in this study.
Data S2 The information of primers used in this study.


