Abstract
We propose a new conceptual framework (computational validity) for translation across species and populations based on the computational similarity between the information processing underlying parallel tasks. Translating between species depends not on the superficial similarity of the tasks presented, but rather on the computational similarity of the strategies and mechanisms that underlie those behaviours. Computational validity goes beyond construct validity by directly addressing questions of information processing. Computational validity interacts with circuit validity as computation depends on circuits, but similar computations could be accomplished by different circuits. Because different individuals may use different computations to accomplish a given task, computational validity suggests that behaviour should be understood through the subject's point of view; thus, behaviour should be characterized on an individual level rather than a task level. Tasks can constrain the computational algorithms available to a subject and the observed subtleties of that behaviour can provide information about the computations used by each individual. Computational validity has especially high relevance for the study of psychiatric disorders, given the new views of psychiatry as identifying and mediating information processing dysfunctions that may show high inter-individual variability, as well as for animal models investigating aspects of human psychiatric disorders.
This article is part of the theme issue ‘Systems neuroscience through the lens of evolutionary theory’.
Keywords: computational psychiatry, construct validity, circuit validity, cross-species translation, face validity
1. The challenges of cross-species translation
Humans share extensive similarities with other species in their interactions with the environment and in the behaviours they use to achieve goals within those environments. As such, researchers trying to understand human behaviour can use non-human animal models to investigate mechanisms of behaviour. Importantly, some experiments are differentially feasible in different species. We currently have powerful genetic and circuit access in rodents, allowing microcircuit manipulation of their nervous systems. Mice, rats and monkeys allow for the study of large neural ensembles and direct observation of neural representations, as well as manipulations of neural circuits through lesion, pharmacological, chemogenetic and optogenetic technologies. Human experiments are generally limited to non-invasive imaging methods, except in certain clinical cases. Further, behaviours may not be comparable across species. In practice, the paths to those behaviours are often different. Humans are typically provided linguistic instructions and very limited experience (training and testing) on a given task, while monkeys are often provided months or years of training, and rats and mice are provided days or weeks of training to accomplish their behaviours. Thus, if our ultimate goal is to understand how brains interact with environments to produce behaviour, we must integrate our knowledge across these different types of experiments, and, thus, we need a process to compare experiments across species.
How do we then translate mechanistic knowledge across species? And, for that matter, how can we combine and integrate knowledge across different levels of analysis in non-human animal species? We propose that an understanding of computational processes can help answer these questions, which requires us to move beyond traditional comparisons of validity to establish a new conceptual framework—that of computational validity.
For example, let us assume that our goal is to understand why someone would spend a large portion of their weekly paycheck on cigarettes, even though they found their first experience with cigarettes severely unpleasant, and they continue to state linguistically that they know it is ruining their health and wish to quit. To achieve our goal of understanding what drives their addictive behaviour, researchers must delineate the underlying molecular, neurophysiological and computational processes that are fostering the continued action-selection of smoking. We could examine the neuronal circuits underlying addiction in mice, but mice do not smoke cigarettes, even though they will self-administer nicotine. More importantly, a mouse cannot linguistically tell us whether it wishes to quit smoking, or whether it knows it should quit smoking. However, mice can show hesitation or caution, and can re-evaluate behaviours after taking an action, even in the absence of new information. These behaviours suggest that they may experience motivational conflict. This underlying cognitive process of motivational conflict, therefore, may be more fruitfully tested across species, provided it is appropriately operationalized. Importantly, operationalizing the process requires understanding the computations that go into the recognition of and resolution of motivational conflict. Comparing those computations across species brings us to computational validity.
2. Translation and validity
When comparing experimental studies across species, theoreticians talk of at least four kinds of validity [1–6]. (i) Predictive, treatment or criterion validity asks whether an instrument or task reliably predicts a similar measure or outcome across conditions [7–9]; (ii) face validity asks whether two behaviours appear intuitively similar [5,10]; (iii) mechanistic validity (in neuroscience, often identified as circuit validity) asks whether identical neurophysiological or other mechanisms align between experiments or observations [11]; and (iv) construct validity seeks to align a theoretical description of an abstraction with the experimental observations seen in different conditions [1,12–16].
We argue here that these concepts of validity are incomplete, and that they miss the important question of computational validity—whether the information processing used during a given behavioural task generalizes across different experiments (species, behaviours, scenarios). Computational validity interacts with these other concepts of validity. We discuss this in more detail below.
(a) . Predictive validity
Predictive validity [4–9,16] assesses whether a task or other measure is effective in predicting an outcome. Sometimes referred to as criterion validity [7–9,16], predictive validity includes the concept of treatment validity [7–9,17], which asks to what extent an experimental paradigm (task, measurement) is effective in predicting response to treatment. Although, in a sense, predictive validity is the ultimate goal of preclinical experiments, it has had limited success [4,5,16]. We argue that this comes in part because predictive validity makes no actual claims as to the mechanism of the action, only that it produces similar outward results.
Achieving predictive validity is particularly challenging in neuroscience due to the brain's complexity and the further complexity of the brain's interaction with its environment [18,19]. Additionally, due to the vast social, ethological and environmental differences between species, comparisons of the treatments themselves can be particularly difficult. For instance, successful human treatments for addiction often include methods to substitute another human's judgement during moments of temptation, such as calling one's Alcoholics Anonymous sponsor before drinking [20]. These types of complex social–interactional interventions are difficult if not impossible to model in non-human animals. However, there are examples of predictive success, even within the social realm, such as the social attachment work of Harlow and Bowlby [21–23] or the clear evidence that social isolation increases the susceptibility to addictive drugs in rodents as well as humans [24,25], and that the presence of an alternate option, such as interacting with a conspecific, can reduce self-administration of addictive drugs in rodents [26].
Predictive validity defined on its own has limitations in that it does not attempt to assess the underlying hypothesized mechanisms or constructs of a phenomenon. Given the multifaceted nature of causality, predictive validity depends on more nuanced measures of similarity.
(b) . Face validity
Face validity [1,5,10] is generally evaluated based on superficial behavioural similarities and has been successfully applied to basic behaviours that span species, such as a freezing response to an acute threat. Most mammals, including both human and non-human animals, will freeze in response to sudden danger in similar ways [27–29]. As another example, non-human mammals tend to self-administer the same chemicals that humans do, when given the opportunity, even if the methods of delivery differ (smoking a crack pipe versus lever-controlled intra-jugular infusions) [30]. However, taken to its extreme, the invocation of face validity can lead to absurd conclusions, such as the suggestion that humans should poke their nose into ports or that rats cannot be addicted if they cannot indicate their desire to quit, or can only show emotional conflict if they can linguistically describe it to the experimenter.
In addition to these obvious dissimilarities across species, two behaviours that on the surface appear superficially similar could be driven by substantially different mechanisms across species or conditions. As an example, the activity-based anorexia rodent model of anorexia nervosa is predicated on the assumption that the specific mechanisms that underlie the excess weight loss of rodents' wheel-running to the exclusion of food consumption are the same as the mechanisms that underlie the excess weight loss seen in individuals with anorexia nervosa [31]. Humans, however, experience a host of different social and environmental drivers for weight loss goals [32], while there is no evidence for the same social environment precipitants for weight loss in rats.
To get around this difficulty of superficial relationships, one must consider other types of validity, including mechanistic, construct, and, we argue here, computational validities, that address the underlying components of the behaviour.
(c) . Mechanistic validity
Mechanistic validity [11], often referred to in neuroscience as circuit validity, measures the degree to which underlying mechanisms or homologous neural circuits across species are involved in similar ways during a given behaviour. For example, the identification of dopaminergic dysfunction as a critical mechanistic step in Parkinsonian behavioural dysfunctions supports experimental dopamine depletion in monkeys or rats as a means to understand the underlying neurophysiological dysfunction contributing to Parkinson's disease [33–35]. Mechanistic validity asks whether dopaminergic manipulations have similar effects on downstream structures across species, even though the mechanism by which dopaminergic dysfunction is induced is different in animal models as compared to humans with Parkinson's disease. Experimental comparisons based on mechanistic (circuit) validity will be more likely to produce good predictive (treatment) validity.
Circuit validity works particularly well for conditions in which there is a strong homology between species. For example, the amygdala plays a central role in defensive responses across species, such as freezing in rodents and increased galvanic skin responses in humans to cues predicting punishment [36]. However, circuit validity becomes difficult when there are disagreements about homologies and when there are clear differences in circuit anatomy between species. For example, the homologies between rodent and primate prefrontal cortices are deeply controversial, making circuit validity difficult if not impossible for some questions [37–41]. For instance, whether rodents have a homologue of the primate dorsolateral prefrontal cortex, a key structure for executive control in humans, is very much under dispute. Primates (including humans) may be more cortically dependent than rodents in general [42], which likely changes the underlying functionality of many circuits. To circumvent these issues, experimentalists often focus on abstractions of the overall construct they are attempting to study, in order to provide translation across species.
(d) . Construct validity
Construct validity [1,12–16] assesses the degree to which an experimental design will provide observations that align with a theoretical model of a specific behavioural, psychological or cognitive process.1 It builds on the understanding that different behaviours may reflect the same underlying construct. For example, one experiment might ask a human to remember a number told to them linguistically and then repeat it back after a few minutes [44–46], while another experiment might show a monkey a pair of objects, hide them, and then reveal them again [47,48], asking them to move the object to reveal a reward, and another experiment might ask a rat to return to a location previously experienced for reward [49,50]. These experiments access three entirely different behaviours across the three species, but all require the subject to remember a piece of concrete information across a time gap and thus theoretically access the construct of working memory [51–53].
(e) . Computational (or algorithmic) validity
Our contention is that all of these validity considerations are important and that animal models should address all of them. However, we argue that there is a critical, but often missing, validity comparison that exists alongside these other validities and potentially integrates them in an important way: that of computational validity.
We define ‘computation’ here as a formal process addressing how information is stored and processed within an agent performing a task. We include within our term ‘computation’ both a description of the task-relevant information that must be represented in order to achieve a task goal and also a description of the algorithmic processes by which this information is encoded and manipulated. These form a continuum of formal description that can be used to compare questions across tasks and species. One of the most important discoveries in the computational sciences over the last 50 years is the observation that how one represents data shapes how one can efficiently process it, and furthermore that how one processes that data can change the behavioural consequences of a task [18]. As such, the question of computation is one of what information is represented within the system, how that information is transformed and made available to other processes within the system, and how that information is used to guide behaviour.
A given task can be described at multiple levels of abstraction that provide different predictions with different granularities and specificities. While distinctions are often made between ‘computational’ and ‘algorithmic’ descriptions [54], we include measures of similarity and dissimilarity of both of these in the term ‘computational validity’.
In addition to the behavioural neuroscience examples used in this manuscript, computational analyses can be applied to multiple levels of abstraction within a neural system (subcellular, single cellular, network, cognitive). Questions of information processing can be applied at all of these levels. Our focus here is on behaviour and our examples are high-level, but lower-level computational analyses also have behavioural consequences. For example, retinal receptors responding to specific wavelengths of light, colour being measured through an opponency process, and visual cortex cells normalizing firing to ambient levels, are all low-level computational processes that have been important to our understanding of visual perception and critical in our ability to translate discoveries across species. The question of levels of abstraction is beyond the scope of this paper, but has recently been discussed in detail elsewhere [55] and warrants further consideration in the investigation of computational validity.
Fundamentally, if behaviour depends on information processing in the nervous system, then the key to translating between observed behaviours across different species is to align the computational and algorithmic processes that underlie the behaviours, asking (i) what information is being represented and maintained (versus discarded/ignored), (ii) how is that information being processed (algorithm) and (iii) how do specific behaviours (output) arise from that information processing cascade. We argue that the key to computational validity is to first operationalize behavioural or cognitive processes as a computational process. That is, rather than trying to define a cognitive process such as ‘working memory’ without defining its underlying computations, we need to identify the computational steps that we commonly describe as working memory operations [50,51,56,57]: what are the inputs that enable or trigger working memory computations? What are the potential outputs (stored information—complete or partial or contaminated by distractors)? What are the steps in the computations? And what are common failure modes that correspond to the different steps in the computations? These questions differentiate storing information across a time gap and processing that information. Moreover, if we take neural populations as performing a computation, then that computation can resolve differently in different behavioural tasks [45,50,58–60], and various hypothesized computational processes will predict contrasting patterns of behaviour and neural activity across those tasks. Dissociating the relevant computations becomes critically important for resolving the underlying neural circuit mechanisms.
For example, one can define deliberation and planning as entailing an explicit imagination of a potential outcome, an evaluation of that outcome, potentially in the light of other remembered options, and then a decision based on those deliberations [18,61–64]. This process is fundamentally different from that of procedural, cached action chains, in which one recognizes a situation and releases a well-practiced action chain [18,65,66]. Neural studies of the hippocampus in both rodents and humans have provided evidence for the construction of those imagined outcomes in deliberation/planning, most likely instigated by inputs from the medial prefrontal cortex [67–71], while dorsolateral striatal neural circuits learn to represent situation–action pairs useful for procedural decisions, but do not contain information about those future outcomes [50,72–74].
3. The complexity of behaviour (getting the ethology right)
Asking what underlying algorithm is being used is particularly important because animals (including humans) are not general information processing machines, but rather carry out their behaviours within their species-specific ethological limitations. This means that it is critical to ‘get the ethology right’. For example, it is often easier to ask primates (humans, monkeys) to categorize visual signals, but easier to ask rodents to categorize olfactory, auditory or spatial signals. Thus perceptual decision-making has been studied through the categorization of random dot motion (are most of the dots moving left or right?) in primates [75–77], but by using clicks (frequency or side) or through running past spatial cues in a virtual environment (number of ‘posts’ on the left or the right) in rodents [78–81]. While these signals can arrive through different sensory modalities in the different species, homologous cognitive structures and information processing operations are evoked [82–87].
In general, species have evolved ethological processes that make some behavioural domains easier to access than others. Thus, when asking questions about computationally similar processes, we may need to reveal those processes through ethologically designed tasks. For example, on lever-press experimental paradigms, rats tend to perseverate (defaulting to win-stay algorithms), while on spatial experimental paradigms, rats tend to alternate (defaulting to win-shift algorithms) [50,88–90]. If we want to study economic decision-making processes in rats, we need to provide them with environments in which they will reveal those processes [91–94]. Importantly, these observations are not limited to non-human animals. Humans also find it easier to identify the counter-positive in logical puzzles if framed in a cultural manner that they have experience with [95].
(a) . The problem of equifinality (similar behaviours can arise from multiple algorithmic processes)
One of the key challenges for behavioural experiments is that a given behaviour can arise from multiple algorithmic processes. To see how multiple decision-making algorithms can produce a given action, one can look at the classic plus-maze task. In this task, rats are exposed to a plus-shaped maze and then trained to run from the south arm to the west arm. There are two computational processes that rats could be using to solve this task—they could use a representation of the spatial relationship between start and goal to plan a path, using a cognitive map, or they could learn to associate being put on the maze with turning left [50,96–99]. As will be laid out in depth below, these two representations engender very different computational processes: knowing the spatial relationship between start and goal enables a search process that hypothesizes the consequence of one's actions, allowing the evaluation of that consequence, which enables flexible but slow action decisions [18,60,66,100–102]. By contrast, an association between the maze and the action requires only a recognition of the situation and the release of the associated action, enabling fast but inflexible action decisions [18,60,66,97,102,103]. Turning from the south arm to the west arm on the plus-maze cannot differentiate these computational processes, but a probe trial in which the rat is placed on the north arm can. The cognitive map strategy from the north arm will reach the west arm by turning right, but the situation–action association will turn left, taking the rat to the east arm. On this task, rats normally transition from cognitive map to situation–association strategies with experience [99,104].
A similar decision-making transition has been seen in other tasks, particularly sequential tasks wherein a subject can get into a flow, but that flow can be disrupted. For example, in the left–right-alternation task, a rat learns that there are three potential contingencies to achieve reward (make a left lap, make a right lap or alternate sides). When contingencies change, cognitive map processes drive behaviour, but when an animal runs a single contingency for a number of laps, procedural systems begin to drive behaviour [72,105–107]. Primates show similar effects in the telephone task, in which subjects have to enter a sequence on a grid of numbers [108–111], and humans show similar effects in the serial reaction-time task, in which the subject is given a keypad of buttons and told to push the button that lights up. Unbeknownst to the subject, the buttons light up in a sequence. With extensive experience with the sequence, behavioural control transitions to a smoother procedural process [108–115].
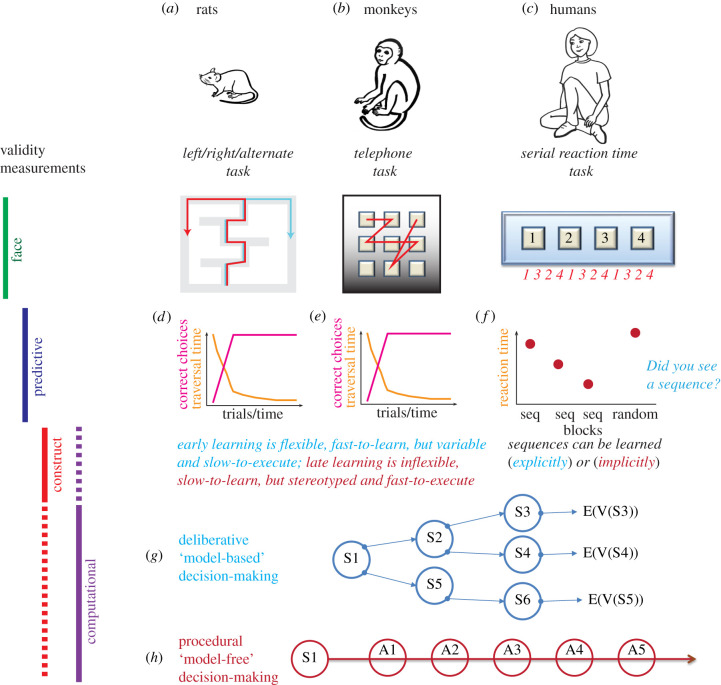
The equifinality seen in these tasks demonstrates how computational validity can be used to ascertain circuit validity [18,63,65,66,72,104,110,112]: early actions on these tasks are driven by explicit, deliberative decision processes that neurophysiologically include representations of goals, paths and outcomes, but create variable paths in action execution. By contrast, after repeated exposure, late actions on these tasks are driven by implicit, procedural action-chain processes that neurophysiologically include representations of the situation–action relationships. Early actions are more flexible, but also slower and more variable in their execution. The different decision-making models (a forward-looking planning process versus a backward-chained reinforcement learning process) produce subtle variations in these tasks that change as animals transition between these two decision-making processes. Moreover, early learning produces flexible behaviour, while late actions are less flexible, but also faster and more reliable in their execution. Early processes depend on interactions between the hippocampus, prefrontal cortex and medial striatum, while late processes depend on the motor cortex, cerebellum and dorsolateral striatum. Even though the plus-maze, the left–right-alternate task, the telephone task and the serial reaction-time task are superficially different, they access similar computational processes, involve parallel circuits and show similar predictive validity (figure 1).
Figure 1.
An example of different kinds of validity measures applied to cross-species tasks. (a) Rats run the left–right-alternate (LRA) task, in which they learn to run left for food, right for food, or alternate, and then change contingencies one or more times in a session [106,107,116,117]. (b) Monkeys learn to type a sequence of keys on a telephone keypad [108–111]. (c) Humans are told to push a button that lights up; unbeknownst to them, the buttons light up in a sequence [113,115]. (d,e) Both the LRA and telephone task show an early fast learning followed by a slower automation of behaviour that does not increase the correct choices, but is characterized by an increase in anticipatory movements, particularly in monkeys. Early learning is dependent on and reflected in the information processing of prefrontal, hippocampal and ventral striatal components, while the later automation is dependent on and reflected in the information processing of dorsolateral striatal components [64,72,104,106,110,118]. (f) Similarly, humans show both decreases in reaction time (and increases in anticipatory movements) and a dissociable declarative recognition of the sequence. The motor changes are reduced in patients with Parkinson's or Huntington's disease, while the declarative sequence recognition is disrupted in patients with Alzheimer's disease [113–115]. Theories explain the early learning as deliberative, declarative and explicit and the late learning as procedural and implicit [46,50,60,72,102,119]. Computationally, theories describe early learning as dependent on ‘model-based’ search processes that include explicit representations of outcomes (g) and late learning as dependent on ‘model-free’ action chains (h) [18,65,72,112]. Face validity measures direct task similarity. Predictive validity measures whether similar manipulations appear across the tasks, such as, for example, the effects of different neural disruptions. Construct validity measures the theoretical components of the task. Computational validity measures the extent to which the tasks are solved by similar computations. (Online version in colour.)
The long history of literature exploring these issues has found that because some behavioural measures show similarity (e.g. error rates can be low whichever system is driving behaviour), it is critical to measure multiple aspects of behaviour simultaneously and apply manipulations to constrain the algorithms. For example, probe trials in which the rat starts from the north arm produces different outcomes under deliberative/planning strategies and procedural/habit strategies [98,99,104]. Furthermore, the quantitative assessment of behavioural execution can reveal subtle differences characteristic of distinct algorithms. Deliberative processes show more variability in the paths taken, including pause and re-orientation behaviours at choices, and hesitation at components [63,109,120,121]. Procedural processes permit anticipatory motor preparation, which leads to smoother navigation paths in rats and smoother finger paths in monkeys [72,106,108,111,121].
Importantly, because these computational hypotheses depend specifically on information processing, neurophysiological measurements that directly measure the information within neural systems (such as the decoding of neural signals and changing tuning curves) can test those predictions [122]. For example, in the tasks shown in figure 1, one can directly observe different information processes in the hippocampus, ventral striatum and dorsolateral striatum [63,72]. Hippocampal ensembles sweep representations of location from the current position of the rat to the goal [67,123], also seen in similar tasks [68,69,124–126], consistent with a hypothesized role in planning. Ventral striatal signals show transient representations of goal outcomes during early learning [127] and ramps of increasing firing during late learning [128], consistent with a hypothesized role in evaluation. By contrast, dorsolateral striatal ensembles develop bursts of firing at the start and end of the journey as the behaviour automates [105,129–131], and cells that encode different actions to be taken at different points of the maze [105,129,132–134]. Similarly, one can find quantitative signals in parietal cortices that integrate information as predicted by drift–diffusion and race-to-threshold models [87,135], and confidence-related signals in the lateral orbitofrontal cortex [136,137].
4. Examples of uses of computational validity
As noted above, while humans and other animals use computational abilities to guide adaptive behaviour, they did not evolve as general computational machines. This means that when asking questions about abstract computational abilities, it is important to design tasks that access the inputs and abilities of a given species. It is also important to provide each species with an appropriate output that can be used to reveal the computational process. For example, if one defines addiction as continued costly behaviours despite a stated preference to stop the behaviour, then non-linguistic animals (such as mice or rats) can never be addicted. However, it is possible to identify motivational changes, for example, an increased willingness to pay a cost for drug delivery, and to measure motivational conflict within mice and rats through hesitation and re-orientation behaviours, both of which are increased in subsets of animals willing to pay high costs for drug delivery.
(a) . Perceptual evidence accumulation
An early example of the utility of focusing on computational validity has been accumulation-of-evidence models [82,87,138,139]. This class of models proposed an explanation for how observed patterns of choice accuracy, reaction times and evidence are related through a simple computational process: evidence is accumulated over time until a threshold is reached, releasing a response. With the earliest models, it was possible to separate when the individual's accumulation process began, how rapidly they accumulated evidence, what their evidence threshold was and whether they had a bias towards a type of response [140], each of which have provided measurable targets in neurophysiological recordings in non-human animals. Precisely defining the exact noise process, how evidence is integrated, and other necessary components resulted in the ability to parse unique differences that could match a range of behavioural patterns in humans [141,142].
These models imply that evidence accumulation should depend on the information available at each moment—for example, in the random dots task, accumulation should depend on the coherence of the stimulus [143]. In this task, participants are shown a large number of dots on the screen that move in a random direction except for a proportion of these that move in a particular direction. The dots that are cohesively moving in a particular direction indicate which response the participant should give. By manipulating the proportion of dots moving in the same direction (i.e. the overall coherence of the representation) the experimenter can manipulate the rate of information provided. Typically, individuals show slow reaction times in trials that have low stimulus coherence and faster reaction times to trials with high stimulus coherence, which can be quantitatively predicted based on evidence accumulation [76]. There are a number of related algorithms for evidence accumulation, from drift–diffusion to race models [144], and identifying which specific variant can explain specific behavioural patterns remains a challenge [136,145,146].
Neurophysiological recordings in non-human primates have identified neural signals that encode the evidence as it accumulates, and which accumulates from a starting point reflecting the subject's experience (the bias), accumulating faster for more coherent signals, and reaching a consistent threshold before the choice is initiated [75,87,146,147]. Rodents have poorer visual ability compared to the primates, so researchers have either significantly reduced the visual complexity of perceptual evidence accumulation tasks or have changed the sensory modality to use auditory, olfactory or tactile cues [78,79,136,148,149]. But after making these changes to sensory modalities, neural and behavioural correlates of the drift–diffusion process were found in rodents [150]. Careful analysis of the behavioural data within a computational framework has enabled these models of perceptual evidence accumulation to relate task performance neurophysiology across species.
(b) . Fear conditioning and anxiety
Classical fear conditioning experiments are actually built primarily on face and circuit validity rather than construct or computational validity. For instance, these experiments largely capitalized on cross-species similarities in biobehavioural responses to acute (and perceived) threats that were tractable in laboratory settings (face validity) [29,151–154]. Neurophysiologically, the likelihood of observing the response is both causally and correlationally related to the synaptic efficacy across the lateral and basolateral to central amygdala connection (circuit validity) [36]. Behavioural experiments that create conditioned responses also lead to increased synaptic efficacy across the amygdala [155]. Manipulations of the strength of that connection change the performance of that response [156].
While these models were often justified as a means to address questions of post-traumatic stress disorder or anxiety, they have not been particularly successful as such [152,157,158]. The development of cross-species tasks that capture the human experience of anxiety has proven challenging [5,159,160]. Early theories of anxiety (such as [161]) included underlying computational hypotheses—that anxiety entailed imagined representations of future threat and underlying conflicts between approach and avoidance motivational goals [162], but these computations are not easily accessed through classical fear conditioning experiments [152,157,158]. Computational analyses of threat assessment that take into account distance between predator and prey and the available action strategies may provide a more translatable story [154,163,164], but have not been as well explored computationally. However, new tasks that directly access approach–avoid conflict, hesitation and representations of potentially dangerous future outcomes may provide more direct access to these computations [63,164–167]. Direct manipulation of outcome uncertainty may provide additional opportunities for identifying specific computations [168–170].
Experiments in which rodents hesitate before taking actions that may lead to threat have been suggested as a more computationally valid measure of worry and anxiety [154,164,165], including classic observed behaviours such as the stretch-attend posture, seen before progressing out from safe to dangerous zones [63,171–173]. Neurophysiological studies suggest that these moments may include imagined representations of future threat [174–176], suggesting computational validity, while pharmacological manipulations producing similar outcomes may suggest predictive validity as well [166,173]. We argue that applying computational validity measures to the underlying processes within these tasks is likely to improve translatability beyond the simpler paradigms that have dominated the classical literature.
(c) . Restaurant Row/WebSurf
In the Restaurant Row task, mice or rats forage for differently flavoured food in sequential encounters with four ‘restaurants’ [91,92]. In the WebSurf task, humans forage for videos in sequential encounters with four ‘galleries’ [177–179]. Although the modalities are different, both rewards are consumed in task, and it has been possible to align the computational decision flow between the two tasks, revealing computational similarities between the species [93]. In general, on encountering a restaurant/gallery, a delay is revealed, and the subject is given the option to wait out the delay for reward or to skip the reward and proceed on to the next restaurant/gallery. Subjects are time-limited and thus are spending time from a budget to maximize their reward intake. Mice, rats and humans all typically exhibit thresholds for each restaurant/gallery, such that if the delay is lower than that threshold, they wait out the delay, but if higher, then they forgo the reward. For mice and rats, receiving many days of experience, these thresholds are stable—differing from animal to animal and from flavour to flavour, but remaining constant over days [91,92]. For humans, these thresholds are consistent with stated preferences, including rankings of the four galleries, and average ratings of individual videos [177–179]. These observations are consistent with the concept that each restaurant or gallery provides a reward of a given subjective value and subjects wait out the delay if the cost is lower than that value.
In versions of the task with separate offer and wait zones, where delay is revealed, but does not count down in the offer zone, and only counts down on entry into a wait zone, the normative behaviour would be to proceed through the offer zone to the wait zone, make the decision in the wait zone and quit if necessary. Neither mice, rats, nor humans behave in this manner. All take time to make decisions in the offer zone, and show a resistance to quitting out of the wait zone [93]. Other computational similarities can be seen between these two tasks. For example, reaction times in the offer zone are increased not at the threshold itself (as would be expected from a simple perceptual model), but rather just above threshold, suggesting that subjects find it easier to stay than to reject a given offer where the cost and value are close [91–93]. From this, we conclude that human and non-human animals are likely using similar decision-making processes in these two tasks, even though the two tasks access different perceptual–action modalities. Interestingly, a subset of humans do show reaction times peaked at thresholds, suggesting that this subset of humans may be using a different decision-making process [180].
(d) . Measuring decision confidence across species
Historically, confidence judgements have been taken to be a prime example of a uniquely human cognitive capacity, metacognition, which would make confidence unsuitable for translational studies [181–183]. While intuitively the sense of confidence reflects a process of apparent self-reflection, it can be tremendously useful for survival in an uncertain world. Determining how much time or effort to invest, whether in the stock market or a rich food patch, requires accurate estimates of confidence about each option. Indeed, confidence can be also defined as a statistical quantity, the likelihood that a belief is correct [184,185]. This definition lends itself to a computational operationalization with the potential to connect behavioural observations across species. The key insight is that we can create behavioural tasks that incentivize the use of decision confidence so that making confidence-guided choices somehow benefits the subject. Incentivized subjects can demonstrate that these confidence-related computations drive behavioural strategies.
Using this approach, confidence-guided behaviours have been shown in rats, mice and non-human primates [136,186–190]. For instance, rats were trained to first decide between two options based on noisy sensory information and then to wait for uncertain delayed rewards. The rats' time investments from trial to trial quantitatively matched the statistically appropriate use of confidence information, which can be inferred based on their choice behaviour [191,192]. In this case, the use of a confidence computation can be determined based on a normative statistical theory. Similarly, experiments that have asked monkeys to wager rewards or opt out of choices [186–188,193], or have allowed preverbal infants to ask for help or persist in choices [194] have found that they behave in proportion to statistical confidence. There are also numerous algorithmic models that have been used to explain confidence-guided behavioural strategies and learning across species, including reinforcement learning, statistical classifiers and evidence accumulation models [136,186,195–198]. Importantly, explicit self-reports of subjective confidence in people have also been found to reflect these statistical computations [199,200]. Based on these approaches, there is increasing neurobiological understanding about the brain regions supporting confidence, including single neuron and inactivation studies in orbitofrontal, frontopolar, anterior cingulate and parietal cortices, as well as the pulvinar and supplementary eye field regions [137,189,201,202].
Dysfunctions of confidence appear to contribute to a range of psychiatric disorders and have been found in numerous clinical patient populations [203,204]. For instance, underconfidence is associated with pathological doubt in anxiety disorders including obsessive–compulsive disorder [205–207]. Overconfidence is a characteristic of narcissistic personality disorder [208], while patients with major depression tend to exhibit attenuated and biased confidence reports [209,210]. These studies are increasingly yielding quantitative metrics in behavioural tasks that can be assessed in both human patients and in non-human animal models.
5. Models of computational change
Computational validity is a particularly useful construct when we examine treatments and their effects.
An interesting example lies in the common addiction treatment of Contingency Management, in which subjects are rewarded (monetarily) for not succumbing to their addiction in a recent time frame (for example, not using their drug of abuse for the previous week) [211,212]. Early theories of Contingency Management were based on hypotheses of alternate reward and increased economic opportunity costs, but Contingency Management works better than would be expected given this theory [213,214]. If one measures the expected effect of the rewards offered in Contingency Management given the elasticity of drug use as a function of cost on the street, Contingency Management works much better than expected [215,216].
Rats make different valuation decisions when faced with breakpoint experiments (how much effort is an animal willing to expend to receive a drug?) than when faced with choice experiments (which of two options would an animal prefer?) [217]. Computational theories suggest that these two experiments access different decision-making system processes, consistent with human economic studies suggesting a difference between willing-to-pay experiments and choose-between experiments [18,218]. Regier & Redish [216] suggested that Contingency Management may be computationally akin to these changes in experimental paradigms, providing the addict with a deliberative choose-between option which interferes with the willing-to-pay decisions usually made.
Computational theories suggest that decisions about particular futures arise from deliberative decision processes which entail imagination and evaluation of those future outcomes [18,61–63]. This process is referred to as episodic future thinking. Rats, monkeys and humans have all been found to neurophysiologically imagine future outcomes using similar processes, including explicit representations of those future outcomes, and through similar neurophysiological circuits (involving ventromedial prefrontal cortex in humans, the homologous medial prefrontal cortex in rats, as well as hippocampus, nucleus accumbens and orbitofrontal cortex in all three species) [63,67,71,219,220]. The explicit nature of the representation necessary for future evaluation suggests that concrete futures are easier to imagine and evaluate than abstract futures. This may be one reason that Contingency Management works so well—it provides a concrete option to look forward to. These hypotheses suggest that Contingency Management will work best with concrete options (rather than simple monetary rewards), that it will depend on prefrontal–hippocampal–accumbens circuits and on an intact orbitofrontal cortex, and that it could be improved with episodic future thinking training [221,222] and motivational interviewing [223,224].
6. When computations do not translate
All of these validities (face validity, predictive [treatment] validity, mechanistic [circuit] validity, and construct and computational validity) are, in actuality, measurements; that is, they ask the question: to what extent are these two experiments similar or different? We can learn useful information both from when we find close validity—a strong similarity between the experiments—and from when we find disruptions in validity, when an expected similarity is found instead to be dissimilar. These concepts of validity are particularly important when translation fails. Moreover, these measures interact in important ways. Recognizing differences in circuit validity can be important when treatment validity fails. Recognizing differences in computation can explain differences in how different species (or different subjects) process different constructs.
Early models of navigation assumed that cognitive maps were built by stringing routes (chains of cues) together, but studies found, instead, that representations of allocentric spatial information entailed an internal representation of a coordinate system which cues were then associated with [50,60]. These two different theories make very different predictions when faced with mismatches between external cues and internal coordinate frames. For example, early descriptions of place cells (hippocampal cells which encode location within an environment [225]), and head direction cells (cells in the postsubiculum and anterior thalamus that encode orientation within an environment [226,227]), assumed that these cells were derived from cues (see [50] for a historical review). However, attractor network computational models of the head direction system suggested that the internal coordinate structure of a one-dimensional circular ring (orientation) came first and external cues could be associated with the representation on the ring to reset the representation if the animal became disoriented [228–230]. These models suggested that if the internal coordinate frame changed on each experience, cues would never become associated with a given spatial signal because they would appear as unstable to the rat, because the models suggested that the rat prioritized the internal representation over the external cues. Testing this theory directly, Knierim et al. [231] found that the place and head direction cells in a disoriented rat never became tied to external cues. Gallistel and colleagues [50,232] tested this in a simple behavioural experiment in which rats were placed in a rectangular environment and highly salient cues were provided at the corners. Non-disoriented rats (whose head direction representations were thus consistent on each entry) were capable of learning the cues and identifying one unique corner to gain food reward [233]; however, rats who were disoriented on each entry (thus with head direction representations different on each entry) were unable to differentiate the opposite corners (which were geometrically equivalent) [234].
When Hermer & Spelke [235,236] first tested this in humans by asking people to find an object placed in a coloured box, they found that humans did not show this disorientation effect. But, of course, the humans were able to remember the location of the object across the disorientation by linguistically repeating the description. ‘It's in the blue box. Blue box. Blue box…’ If they gave the humans a linguistic blocking task (counting backwards by sevens from 1000, for example), then even adult humans reverted to showing the similar disorientation effects that rats did. Similarly, children who did not yet use linguistic orientation words (‘to the left of’) were unable to differentiate a box close to a black wall from a box far from it after disorientation, but children who had those linguistic orientation words could.
Because the navigation literature had applied computational analyses to the various processes underlying maps, orientation, cues, and how cues reset those cognitive map representations, it became possible to identify how language changed human memory signals, and were able to reveal that hidden under those linguistic mechanisms were similar computational processes in both human and non-human animals.
7. Individual differences
Designing tasks for computational validity might also address a major open problem in clinical neuroscience—reliable measurement of between- and within-individual differences. A common goal of behavioural neuroscience is to understand sources of behavioural variability, and particularly sources of extreme/outlier behaviour that manifest as mental disorders. These disorders are internally heterogeneous—patients with a common diagnostic label such as ‘addiction’ or ‘depression’ can report very different symptom patterns [237,238]. There is also remarkable comorbidity between disorders, to the point that multiple diagnoses are more common than ‘pure’ syndromes [239]. A prominent view, exemplified by the US National Institute of Mental Health's Research Domain Criteria (RDoC) project, argues that the solution to heterogeneity and comorbidity lies in a new, quantitative taxonomy of mental illness [240]. In this framework, it is commonly assumed that patients can be reliably phenotyped by comparing their performance on psychophysical tasks to an appropriate set of norms [237,241,242]. Unfortunately, emerging evidence suggests that standard psychophysical performance metrics (response times, correct responses, number of trials to criterion) are poor measures of inter-individual variability. In fact, they were designed to be poor measures of inter-individual variability, because they were designed to increase the contrast between groups [243–245]. Because we normally want to analyse a task in terms of the contrast between two or more conditions/trial types, tasks and stimuli are generally designed and psychometrically validated to consistently produce differences between conditions.
A computational perspective might recover viable individual-difference metrics even from tasks that are designed to suppress those differences. As noted above, a participant might arrive at a correct response or a series of economic choices by many different algorithms. In the presence of significant circuit dysfunction, some of those algorithms may be inaccessible or disfavoured, and computationally informed analyses might be able to detect these algorithmic biases. For instance, recent studies identified deficits in construction/use of reward contingency models in patients with compulsive disorders, even when those patients showed no outward deficits on a reward learning task [242,246]. While most common decision models have not yet been validated for test–retest reliability, if that work is done, computational analyses might also track changes in response to treatment or might provide biomarkers of successful clinical target engagement [237,247]. For instance, a validated measurement of episodic future thinking might help identify cases where a contingency management intervention was failing to boost that thought pattern.
8. Conclusion
In sum, it is important to always be asking what computations the individual subject may be performing to accomplish a given task. These computations will have consequences that constrain the animal's behavioural responses (perhaps with subtle changes under probing conditions), will make predictions about what information is encoded within different neural circuits (an important step towards measuring circuit validity), can be linked to specific manipulable components (important for finding treatment validity), and can reveal critical inter-individual differences relevant for the understanding of psychopathology.
Monkeys are not small humans. Rats and mice are certainly not. No rat has built a spaceship to the moon (although monkeys were in space before humans and there have been rats navigating mazes in space). It would be ludicrous to argue that all of these species are performing the same computations in all behaviourally similar situations. Rather, we argue that by delineating the computations being performed in a given task, we can identify the similarities and differences underlying the information processing and the behaviour both across species and between individuals of the same species. This should improve our ability to translate knowledge and understanding across species.
Acknowledgements
We thank the NeuroPlasticity Research in Support of Mental Health (NeuroPRSMH) group at the University of Minnesota for helpful discussions.
Endnote
Construct validity has also been used in recent publications as a means of testing the validity of a specific manipulation [43]. This is usually used as a justification for the idea that one is testing the construct of a gene variant, and thus asking whether an animal model is ‘valid’. It is our contention that this is a misuse of the term ‘construct validity’ (and a misuse of models in general). Models are a tool through which one can explore effects and consequences. Our contention is that validity is a form of measurement, thus the question should not be one of whether a model is valid or not, but rather, how valid the model is under different measurements. Moreover, the validity of a model depends on the specific questions it is addressing and the specific context in which it is applied. Asking a question of the consequences of a gene variant is a question about underlying physiology, and thus a question of ‘mechanistic validity’.
Data accessibility
This article has no additional data.
Authors' contributions
All authors co-wrote the paper together.
Competing interests
We declare we have no competing interests.
Funding
P50 MH119569 (general). Minnesota Medical Discovery Team - Addictions (general). T32 DA037183 (O.L.C.). T32 DA007234 (C.J.W.). R21 MH120785 (A.S.W., A.D.R.). K23 MH112867 (A.F.H.). K23 MH123910 (L.M.A.). R01 MH097061 and R01 DA038209 (A.K.).
References
- 1.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HCW. 2010. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J. Clin. Epidemiol. 63, 737-745. ( 10.1016/j.jclinepi.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 2.Monteggia LM, Heimer H, Nestler EJ.. 2018. Meeting report: can we make animal models of human mental illness? Biol. Psychiatry 84, 542-545. ( 10.1016/j.biopsych.2018.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coolidge FL, Segal DL. 2010. Validity. In The Corsini Encyclopedia of Psychology (eds I Weiner, WE Craghead), pp. 1448–1449. New York, NY: Wiley. ( 10.1002/9780470479216.corpsy1019) [DOI]
- 4.Keifer J, Summers CH. 2016. Putting the ‘biology’ back into ‘neurobiology’: the strength of diversity in animal model systems for neuroscience research. Front. Syst. Neurosci. 10, 69. ( 10.3389/fnsys.2016.00069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard DC, Summers CH, Blanchard RJ. 2013. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neurosci. Biobehav. Rev. 37, 1567-1577. ( 10.1016/j.neubiorev.2013.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young JW, Amitai N, Geyer MA. 2012. Behavioral animal models to assess pro-cognitive treatments for schizophrenia. In Novel antischizophrenia treatments (eds Geyer MA, Gross G), pp. 39-79. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 7.Anastasi A. 1950. The concept of validity in the interpretation of test scores. Educ. Psychol. Meas. 10, 67-78. ( 10.1177/001316445001000105) [DOI] [Google Scholar]
- 8.Cureton EE. 1965. Reliability and validity: basic assumptions and experimental designs. Educ. Psychol. Meas. 25, 327-346. ( 10.1177/001316446502500204) [DOI] [Google Scholar]
- 9.O'Connor EC, Chapman K, Butler P, Mead AN. 2010. The predictive validity of the rat self-administration model for abuse liability. Neurosci. Biobehav. Rev. 35, 912-938. ( 10.1016/j.neubiorev.2010.10.012) [DOI] [PubMed] [Google Scholar]
- 10.Fleming EG, Fleming CW.. 1929. The validity of the Matthews' revision of the Woodworth personal data questionnaire. J. Abnorm. Soc. Psychol. 23, 500-506. ( 10.1037/h0075316) [DOI] [Google Scholar]
- 11.Belzung C, Lemoine M. 2011. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 1, 9. ( 10.1186/2045-5380-1-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacCorquodale K, Meehl PE. 1948. On a distinction between hypothetical constructs and intervening variables. Psychol. Rev. 55, 95-107. ( 10.1037/h0056029) [DOI] [PubMed] [Google Scholar]
- 13.Cronbach LJ, Meehl PE. 1955. Construct validity in psychological tests. Psychol. Bull. 52, 281-302. ( 10.1037/h0040957) [DOI] [PubMed] [Google Scholar]
- 14.Loevinger J. 1957. Objective tests as instruments of psychological theory. Psychol. Rep. 3, 635-694. ( 10.2466/pr0.1957.3.3.635) [DOI] [Google Scholar]
- 15.Campbell DT, Fiske DW. 1959. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol. Bull. 56, 81-105. ( 10.1037/h0046016) [DOI] [PubMed] [Google Scholar]
- 16.Strauss ME, Smith GT. 2009. Construct validity: advances in theory and methodology. Annu. Rev. Clin. Psychol. 5, 1-25. ( 10.1146/annurev.clinpsy.032408.153639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallery ME, Hofmeister A. 1978. A method for assessing the treatment validity of tests in special education. Except. Child. 25, 105-113. ( 10.1080/0156655780250203) [DOI] [Google Scholar]
- 18.Redish AD. 2013. The mind within the brain: how we make decisions and how those decisions go wrong. New York, NY: Oxford University Press. [Google Scholar]
- 19.Redish AD, Kazinka R, Herman AB. 2019. Taking an engineer's view: implications of network analysis for computational psychiatry. Behav. Brain Sci. 42, e24. ( 10.1017/S0140525X18001152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonigan JS, Rice SL. 2010. Is it beneficial to have an alcoholics anonymous sponsor? Psychol. Addict. Behav. 24, 397-403. ( 10.1037/a0019013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlow HF. 1971. Learning to love. San Francisco, CA: Albion Publishing Co. [Google Scholar]
- 22.Bowlby J. 1973. Separation: anxiety and anger. Attachment and loss: volume II. London, UK: The Hogarth Press and the Institute of Psycho-Aalysis. [Google Scholar]
- 23.Blum D. 2002. Love at Goon Park: Harry Harlow and the science of affection. New York, NY: Perseus Books. [Google Scholar]
- 24.Stockdale SE, Wells KB, Tang L, Belin TR, Zhang L, Sherbourne CD. 2007. The importance of social context: neighborhood stressors, stress-buffering mechanisms, and alcohol, drug, and mental health disorders. Soc. Sci. Med. 65, 1867-1881. ( 10.1016/j.socscimed.2007.05.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitaker LR, Degoulet M, Morikawa H. 2013. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron 77, 335-345. ( 10.1016/j.neuron.2012.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y. 2018. Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520-1529. ( 10.1038/s41593-018-0246-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers K, Davis M. 2007. Mechanisms of fear extinction. Mol. Psychiatry 12, 120-150. ( 10.1038/sj.mp.4001939) [DOI] [PubMed] [Google Scholar]
- 28.Shansky RM, Woolley CS. 2016. Considering sex as a biological variable will be valuable for neuroscience research. J. Neurosci. 36, 11 817-11 822. ( 10.1523/JNEUROSCI.1390-16.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ly V, Huys QJM, Stins JF, Roelofs K, Cools R. 2014. Individual differences in bodily freezing predict emotional biases in decision making. Front. Behav. Neurosci. 8, 237. ( 10.3389/fnbeh.2014.00237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koob GF, Le Moal M. 2006. Neurobiology of addiction. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 31.Casper RC, Sullivan EL, Tecott L. 2008. Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl.) 199, 313-329. ( 10.1007/s00213-008-1102-2) [DOI] [PubMed] [Google Scholar]
- 32.Stice E. 2002. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol. Bull. 128, 825-848. ( 10.1037/0033-2909.128.5.825) [DOI] [PubMed] [Google Scholar]
- 33.Smeyne RJ, Jackson-Lewis V. 2005. The MPTP model of Parkinson's disease. Brain Res. Mol. Brain Res. 134, 57-66. ( 10.1016/j.molbrainres.2004.09.017) [DOI] [PubMed] [Google Scholar]
- 34.Langston JW. 1996. The etiology of Parkinson's disease with emphasis on the MPTP story. Neurology 47, S153-S160. ( 10.1212/WNL.47.6_Suppl_3.153S) [DOI] [PubMed] [Google Scholar]
- 35.Dorval AD, Grill WM. 2014. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J. Neurophysiol. 111, 1949-1959. ( 10.1152/jn.00713.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeDoux J. 2007. The amygdala. Curr. Biol. 17, R868-R874. ( 10.1016/j.cub.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 37.Kolb B. 1990. Prefrontal cortex. In The cerebral cortex of the rat (eds Kolb B, Tees RC), pp. 437-458. Cambridge, MA: MIT Press. [Google Scholar]
- 38.Uylings HBM, Groenewegen HJ, Kolb B. 2003. Do rats have a prefrontal cortex? Behav. Brain Res. 146, 3-17. ( 10.1016/j.bbr.2003.09.028) [DOI] [PubMed] [Google Scholar]
- 39.Wise SP. 2008. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 31, 599-608. ( 10.1016/j.tins.2008.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. 2016. Circuit based cortico-striatal homologies between rat and primate. Biol. Psychiatry 80, 509-521. ( 10.1016/j.biopsych.2016.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widge AS, Heilbronner SR, Hayden BY. 2019. Prefrontal cortex and cognitive control: new insights from human electrophysiology. F1000Res 8, 1696. ( 10.12688/f1000research.20044.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Striedter GF. 2006. Précis of principles of brain evolution. Behav. Brain Sci. 29, 1-12; discussion 12–36. ( 10.1017/S0140525X06009010) [DOI] [PubMed] [Google Scholar]
- 43.Nestler EJ, Hyman SE. 2010. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161-1169. ( 10.1038/nn.2647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luria AR. 1976. The neuropsychology of memory. New York. NY: John Wiley and Sons. [Google Scholar]
- 45.Cohen NJ, Eichenbaum H. 1993. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press. [Google Scholar]
- 46.Squire LR. 1987. Memory and brain. New York, NY: Oxford University Press. [Google Scholar]
- 47.Murray EA, Mishkin M. 1998. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 18, 6568-6582. ( 10.1523/JNEUROSCI.18-16-06568.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. 2013. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat. Neurosci. 16, 1140-1145. ( 10.1038/nn.3440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681-683. ( 10.1038/297681a0) [DOI] [PubMed] [Google Scholar]
- 50.Redish AD. 1999. Beyond the cognitive map: from place cells to episodic memory. Cambridge, MA: MIT Press. [Google Scholar]
- 51.Goldman-Rakic PS. 1995. Cellular basis of working memory. Neuron 14, 477-485. ( 10.1016/0896-6273(95)90304-6) [DOI] [PubMed] [Google Scholar]
- 52.Fuster JM. 2008. The prefrontal cortex. 4th edn. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 53.Redish AD. 2001. The hippocampal debate: are we asking the right questions? Behav. Brain Res. 127, 81-98. ( 10.1016/S0166-4328(01)00356-4) [DOI] [PubMed] [Google Scholar]
- 54.Marr D. 1982. Vision. New York, NY: W. H. Freeman and Co. [Google Scholar]
- 55.Levenstein D, et al. 2020. On the role of theory and modeling in neuroscience. arXiv 2003.13825 [q-bio.NC].
- 56.Baddeley A. 1992. Working memory. Science 255, 556-559. ( 10.1126/science.1736359) [DOI] [PubMed] [Google Scholar]
- 57.Fuster JM. 1990. Prefrontal cortex and the bridging of temporal gaps in the perception-action cycle. Ann. NY Acad. Sci. 608, 318-329; discussion 330–336. ( 10.1111/j.1749-6632.1990.tb48901.x) [DOI] [PubMed] [Google Scholar]
- 58.Churchland P, Sejnowski TJ. 1994. The computational brain. Cambridge, MA: MIT Press. [Google Scholar]
- 59.Milner D, Goodale M. 2006. The visual brain in action. New York, NY: Oxford University Press. [Google Scholar]
- 60.O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive Map. Oxford, UK: Clarendon Press. [Google Scholar]
- 61.Gilbert DT, Wilson TD. 2007. Prospection: experiencing the future. Science 317, 1351-1354. ( 10.1126/science.1144161) [DOI] [PubMed] [Google Scholar]
- 62.Buckner RL, Carroll DC. 2007. Self-projection and the brain. Trends Cogn. Sci. 11, 49-57. ( 10.1016/j.tics.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 63.Redish AD. 2016. Vicarious trial and error. Nat. Rev. Neurosci. 17, 147-159. ( 10.1038/nrn.2015.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson A, van der Meer MAA, Redish AD.. 2007. Integrating hippocampus and striatum in decision-making. Curr. Opin. Neurobiol. 17, 692-697. ( 10.1016/j.conb.2008.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daw ND, Niv Y, Dayan P. 2005. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat. Neurosci. 8, 1704-1711. ( 10.1038/nn1560) [DOI] [PubMed] [Google Scholar]
- 66.Niv Y, Joel D, Dayan P. 2006. A normative perspective on motivation. Trends Cogn. Sci. 10, 375-381. ( 10.1016/j.tics.2006.06.010) [DOI] [PubMed] [Google Scholar]
- 67.Johnson A, Redish AD. 2007. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 27, 12 176-12 189. ( 10.1523/JNEUROSCI.3761-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kay K, Chung JE, Sosa M, Schor JS, Karlsson MP, Larkin MC, Liu DF, Frank LM. 2020. Constant sub-second cycling between representations of possible futures in the hippocampus. Cell 180, 552-567.e25. ( 10.1016/j.cell.2020.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt B, Duin AA, Redish AD. 2019. Disrupting the medial prefrontal cortex alters hippocampal sequences during deliberative decision making. J. Neurophysiol. 121, 1981-2000. ( 10.1152/jn.00793.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito HT, Zhang S-J, Witter MP, Moser EI, Moser M-B. 2015. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature 522, 50-55. ( 10.1038/nature14396) [DOI] [PubMed] [Google Scholar]
- 71.Wang JX, Cohen NJ, Voss JL. 2015. Covert rapid action-memory simulation (CRAMS): a hypothesis of hippocampal--prefrontal interactions for adaptive behavior. Neurobiol. Learn. Mem. 117, 22-33. ( 10.1016/j.nlm.2014.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van der Meer MAA, Johnson A, Schmitzer-Torbert NC, Redish AD.. 2010. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron 67, 25-32. ( 10.1016/j.neuron.2010.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abraham L, Potegal M, Miller S. 1983. Evidence for caudate nucleus involvement in an egocentric spatial task: return from passive transport. Physiol. Psychol. 11, 11-17. ( 10.3758/BF03326764) [DOI] [Google Scholar]
- 74.Yin HH, Knowlton B, Balleine BW. 2004. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci. 19, 181-189. ( 10.1111/j.1460-9568.2004.03095.x) [DOI] [PubMed] [Google Scholar]
- 75.Roitman JD, Shadlen MN. 2002. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 22, 9475-9489. ( 10.1523/JNEUROSCI.22-21-09475.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer J, Huk AC, Shadlen MN. 2005. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J. Vis. 5, 376-404. ( 10.1167/5.5.1) [DOI] [PubMed] [Google Scholar]
- 77.Britten KH, Shadlen MN, Newsome WT, Movshon JA. 1992. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 12, 4745-4765. ( 10.1523/JNEUROSCI.12-12-04745.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brunton BW, Botvinick MM, Brody CD. 2013. Rats and humans can optimally accumulate evidence for decision-making. Science 340, 95-98. ( 10.1126/science.1233912) [DOI] [PubMed] [Google Scholar]
- 79.Sanders JI, Kepecs A. 2012. Choice ball: a response interface for two-choice psychometric discrimination in head-fixed mice. J. Neurophysiol. 108, 3416-3423. ( 10.1152/jn.00669.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinto L, Koay SA, Engelhard B, Yoon AM, Deverett B, Thiberge SY, Witten IB, Tank DW, Brody CD. 2018. An accumulation-of-evidence task using visual pulses for mice navigating in virtual reality. Front. Behav. Neurosci. 12, 36. ( 10.3389/fnbeh.2018.00036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Constantinople CM, Piet AT, Brody CD. 2019. An analysis of decision under risk in rats. Curr. Biol. 29, 2066-2074.e5. ( 10.1016/j.cub.2019.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. 2003. A role for neural integrators in perceptual decision making. Cereb. Cortex 13, 1257-1269. ( 10.1093/cercor/bhg097) [DOI] [PubMed] [Google Scholar]
- 83.Ding L, Gold JI. 2010. Caudate encodes multiple computations for perceptual decisions. J. Neurosci. 30, 15 747-15 759. ( 10.1523/JNEUROSCI.2894-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanks TD, Kopec CD, Brunton BW, Duan CA, Erlich JC, Brody CD. 2015. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. Nature 520, 220-223. ( 10.1038/nature14066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erlich JC, Brunton BW, Duan CA, Hanks TD, Brody CD. 2015. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. Elife 4, e05457. ( 10.7554/elife.05457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katz LN, Yates JL, Pillow JW, Huk AC. 2016. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature 535, 285-288. ( 10.1038/nature18617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gold JI, Shadlen MN. 2001. Neural computations that underlie decisions about sensory stimuli. Trends Cogn. Sci. 5, 10-16. ( 10.1016/S1364-6613(00)01567-9) [DOI] [PubMed] [Google Scholar]
- 88.Papale AE, Stott JJ, Powell NJ, Regier PS, Redish AD. 2012. Interactions between deliberation and delay discounting in rats. Cogn. Affect. Behav. Neurosci. 12, 513-526. ( 10.3758/s13415-012-0097-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cardinal RN, Daw N, Robbins T, Everitt BJ. 2002. Local analysis of behaviour in the adjusting-delay task for choice of delayed reinforcement. Neural Netw. 15, 617-634. ( 10.1016/S0893-6080(02)00053-9) [DOI] [PubMed] [Google Scholar]
- 90.McDonald RJ, White NM. 1994. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural. Biol. 61, 260-270. ( 10.1016/S0163-1047(05)80009-3) [DOI] [PubMed] [Google Scholar]
- 91.Sweis BM, Thomas MJ, Redish AD. 2018. Mice learn to avoid regret. PLoS Biol. 16, e2005853. ( 10.1371/journal.pbio.2005853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steiner AP, Redish AD. 2014. Behavioral and neurophysiological correlates of regret in rat decision-making on a neuroeconomic task. Nat. Neurosci. 17, 995-1002. ( 10.1038/nn.3740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sweis BM, Abram SV, Schmidt BJ, Seeland KD, MacDonald AW, Thomas MJ, Redish AD. 2018. Sensitivity to ‘sunk costs’ in mice, rats, and humans. Science 361, 178-181. ( 10.1126/science.aar8644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalenscher T, Wingerden MV. 2011. Why we should use animals to study economic decision making? A perspective. Front. Neurosci. 5, 82. ( 10.3389/fnins.2011.00082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fiddick L, Cosmides L, Tooby J. 2000. No interpretation without representation: the role of domain-specific representations and inferences in the Wason selection task. Cognition 77, 1-79. ( 10.1016/S0010-0277(00)00085-8) [DOI] [PubMed] [Google Scholar]
- 96.Tolman EC, Ritchie BF, Kalish D.. 1946. Studies in spatial learning. II. Place learning versus response learning. J. Exp. Psychol. 36, 221-229. ( 10.1037/h0060262) [DOI] [PubMed] [Google Scholar]
- 97.Hull CL. 1943. Principles of behavior. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- 98.Barnes CA, Nadel L, Honig WK. 1980. Spatial memory deficit in senescent rats. Can. J. Psychol. 34, 29-39. ( 10.1037/h0081022) [DOI] [PubMed] [Google Scholar]
- 99.Packard MG, McGaugh JL. 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 65, 65-72. ( 10.1006/nlme.1996.0007) [DOI] [PubMed] [Google Scholar]
- 100.Tolman EC. 1939. Prediction of vicarious trial and error by means of the schematic sowbug. Psychol. Rev. 46, 318-336. ( 10.1037/h0057054) [DOI] [Google Scholar]
- 101.Tolman EC. 1948. Cognitive maps in rats and men. Psychol. Rev. 55, 189-208. ( 10.1037/h0061626) [DOI] [PubMed] [Google Scholar]
- 102.van der Meer MAA, Kurth-Nelson Z, Redish AD.. 2012. Information processing in decision-making systems. Neuroscientist 18, 342-359. ( 10.1177/1073858411435128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Redish AD, Jensen S, Johnson A, Kurth-Nelson Z. 2007. Reconciling reinforcement learning models with behavioral extinction and renewal: implications for addiction, relapse, and problem gambling. Psychol. Rev. 114, 784-805. ( 10.1037/0033-295X.114.3.784) [DOI] [PubMed] [Google Scholar]
- 104.Yin HH, Knowlton BJ. 2004. Contributions of striatal subregions to place and response learning. Learn. Mem. 11, 459-463. ( 10.1101/lm.81004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Regier PS, Amemiya S, Redish AD. 2015. Hippocampus and subregions of the dorsal striatum respond differently to a behavioral strategy change on a spatial navigation task. J. Neurophysiol. 114, 1399-1416. ( 10.1152/jn.00189.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hasz BM, Redish AD. 2020. Dorsomedial prefrontal cortex and hippocampus represent strategic context even while simultaneously changing representation throughout a task session. Neurobiol. Learn. Mem. 171, 107215. ( 10.1016/j.nlm.2020.107215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Powell NJ, Redish AD. 2016. Representational changes of latent strategies in rat medial prefrontal cortex precede changes in behaviour. Nat. Commun. 7, 12830. ( 10.1038/ncomms12830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rand MK, Hikosaka O, Miyachi S, Lu X, Miyashita K. 1998. Characteristics of a long-term procedural skill in the monkey. Exp. Brain Res. 118, 293-297. ( 10.1007/s002210050284) [DOI] [PubMed] [Google Scholar]
- 109.Rand MK, Hikosaka O, Miyachi S, Lu X, Nakamura K, Kitaguchi K, Shimo Y. 2000. Characteristics of sequential movements during early learning period in monkeys. Exp. Brain Res. 131, 293-304. ( 10.1007/s002219900283) [DOI] [PubMed] [Google Scholar]
- 110.Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. 1999. Parallel neural networks for learning sequential procedures. Trends Neurosci. 22, 464-471. ( 10.1016/S0166-2236(99)01439-3) [DOI] [PubMed] [Google Scholar]
- 111.Desrochers TM, Amemori K-I, Graybiel AM. 2015. Habit learning by naive macaques is marked by response sharpening of striatal neurons representing the cost and outcome of acquired action sequences. Neuron 87, 853-868. ( 10.1016/j.neuron.2015.07.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Graybiel AM. 1998. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 70, 119-136. ( 10.1006/nlme.1998.3843) [DOI] [PubMed] [Google Scholar]
- 113.Knopman D, Nissen MJ. 1991. Procedural learning is impaired in Huntington's disease: evidence from the serial reaction time task. Neuropsychologia 29, 245-254. ( 10.1016/0028-3932(91)90085-M) [DOI] [PubMed] [Google Scholar]
- 114.Jackson GM, Jackson SR, Harrison J, Henderson L, Kennard C. 1995. Serial reaction time learning and Parkinson's disease: evidence for a procedural learning deficit. Neuropsychologia 33, 577-593. ( 10.1016/0028-3932(95)00010-Z) [DOI] [PubMed] [Google Scholar]
- 115.Jackson GM, Jackson SR. 1995. Do measures of explicit learning actually measure what is being learnt in the serial reaction time task? A critique of current methods. Psyche 2, 20. [Google Scholar]
- 116.Steiner A, Redish AD. 2012. Orbitofrontal cortical ensembles during deliberation and learning on a spatial decision-making task. Front. Decis. Neurosci. 6, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blumenthal A, Steiner A, Seeland KD, Redish AD. 2011. Effects of pharmacological manipulations of NMDA-receptors on deliberation in the multiple-T task. Neurobiol. Learn. Mem. 95, 376-384. ( 10.1016/j.nlm.2011.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith AC, Frank LM, Wirth S, Yanike M, Hu D, Kubota Y, Graybiel AM, Suzuki WA, Brown EN. 2004. Dynamic analysis of learning in behavioral experiments. J. Neurosci. 24, 447-461. ( 10.1523/JNEUROSCI.2908-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cohen NJ, Squire LR. 1980. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science 210, 207-210. ( 10.1126/science.7414331) [DOI] [PubMed] [Google Scholar]
- 120.Gardner RS, Uttaro MR, Fleming SE, Suarez DF, Ascoli GA, Dumas TC. 2013. A secondary working memory challenge preserves primary place strategies despite over-training. Learn. Mem. 20, 648-656. ( 10.1101/lm.031336.113) [DOI] [PubMed] [Google Scholar]
- 121.Schmidt BJ, Papale AE, Redish AD, Markus EJ. 2013. Conflict between place and response navigation strategies: effects on vicarious trial and error (VTE) behaviors. Learn. Mem. 20, 130-138. ( 10.1101/lm.028753.112) [DOI] [PubMed] [Google Scholar]
- 122.Johnson A, Fenton AA, Kentros C, Redish AD. 2009. Looking for cognition in the structure in the noise. Trends Cogn. Sci. 13, 55-64. ( 10.1016/j.tics.2008.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amemiya S, Redish AD. 2016. Manipulating decisiveness in decision making—effects of clonidine on hippocampal search strategies. J. Neurosci. 36, 814-827. ( 10.1523/JNEUROSCI.2595-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wikenheiser AM, Redish AD. 2015. Hippocampal theta sequences reflect current goals. Nat. Neurosci. 18, 289-294. ( 10.1038/nn.3909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papale AE, Zielinski MC, Frank LM, Jadhav SP, Redish AD. 2016. Interplay between hippocampal sharp-wave-ripple events and vicarious trial and error behaviors in decision making. Neuron 92, 975-982. ( 10.1016/j.neuron.2016.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allen TA, Salz DM, McKenzie S, Fortin NJ. 2016. Nonspatial sequence coding in CA1 neurons. J. Neurosci. 36, 1547-1563. ( 10.1523/JNEUROSCI.2874-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Meer MAA, Redish AD.. 2009. Covert expectation-of-reward in rat ventral striatum at decision points. Front. Integr. Neurosci. 3, 1-15. ( 10.3389/neuro.07.001.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van der Meer MAA, Redish AD.. 2011. Theta phase precession in rat ventral striatum links place and reward information. J. Neurosci. 31, 2843-2854. ( 10.1523/JNEUROSCI.4869-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. 1999. Building neural representations of habits. Science 286, 1746-1749. [DOI] [PubMed] [Google Scholar]
- 130.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. 2005. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437, 1158-1161. ( 10.1038/nature04053) [DOI] [PubMed] [Google Scholar]
- 131.Smith KS, Graybiel AM. 2013. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron 79, 361-374. ( 10.1016/j.neuron.2013.05.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmitzer-Torbert NC, Redish AD. 2004. Neuronal activity in the rodent dorsal striatum in sequential navigation: separation of spatial and reward responses on the multiple-T task. J. Neurophysiol. 91, 2259-2272. ( 10.1152/jn.00687.2003) [DOI] [PubMed] [Google Scholar]
- 133.Schmitzer-Torbert NC, Redish AD. 2008. Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience 153, 349-360. ( 10.1016/j.neuroscience.2008.01.081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thorn CA, Atallah H, Howe M, Graybiel AM. 2010. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66, 781-795. ( 10.1016/j.neuron.2010.04.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang T, Shadlen MN. 2007. Probabilistic reasoning by neurons. Nature 447, 1075-1080. ( 10.1038/nature05852) [DOI] [PubMed] [Google Scholar]
- 136.Kepecs A, Uchida N, Zariwala HA, Mainen ZF. 2008. Neural correlates, computation and behavioural impact of decision confidence. Nature 455, 227-231. ( 10.1038/nature07200) [DOI] [PubMed] [Google Scholar]
- 137.Ott T, Masset P, Kepecs A. 2018. The neurobiology of confidence: from beliefs to neurons. Cold Spring Harb. Symp. Quant. Biol. 83, 9-16. ( 10.1101/sqb.2018.83.038794) [DOI] [PubMed] [Google Scholar]
- 138.Link SW, Heath RA. 1975. A sequential theory of psychological discrimination. Psychometrika 40, 77-105. ( 10.1007/BF02291481) [DOI] [Google Scholar]
- 139.Ratcliff R. 1988. Continuous versus discrete information processing modeling accumulation of partial information. Psychol. Rev. 95, 238-255. ( 10.1037/0033-295X.95.2.238) [DOI] [PubMed] [Google Scholar]
- 140.Ratcliff R, Rouder JN. 1998. Modeling response times for two-choice decisions. Psychol. Sci. 9, 347-356. ( 10.1111/1467-9280.00067) [DOI] [Google Scholar]
- 141.Glaze CM, Kable JW, Gold JI. 2015. Normative evidence accumulation in unpredictable environments. Elife 4, e08825. ( 10.7554/eLife.08825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ratcliff R, Smith PL, Brown SD, McKoon G. 2016. Diffusion decision model: current issues and history. Trends Cogn. Sci. 20, 260-281. ( 10.1016/j.tics.2016.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Downing CJ, Movshon JA. 1989. Spatial and temporal summation in the detection of motion in stochastic random dot displays. Invest. Ophthalmol. Vis. Sci. 30, 72. [Google Scholar]
- 144.Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. 2006. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 113, 700-765. ( 10.1037/0033-295X.113.4.700) [DOI] [PubMed] [Google Scholar]
- 145.Forstmann BU, Ratcliff R, Wagenmakers E-J. 2016. Sequential sampling models in cognitive neuroscience: advantages, applications, and extensions . Annu. Rev. Psychol. 67, 641-666. ( 10.1146/annurev-psych-122414-033645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cisek P, Puskas GA, El-Murr S. 2009. Decisions in changing conditions: the urgency-gating model. J. Neurosci. 29, 11 560-11 571. ( 10.1523/JNEUROSCI.1844-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hanks T, Kiani R, Shadlen MN. 2014. A neural mechanism of speed-accuracy tradeoff in macaque area LIP. Elife 3, e02260. ( 10.7554/eLife.02260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morita T, Kang H, Wolfe J, Jadhav SP, Feldman DE. 2011. Psychometric curve and behavioral strategies for whisker-based texture discrimination in rats. PLoS ONE 6, e20437. ( 10.1371/journal.pone.0020437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Raposo D, Sheppard JP, Schrater PR, Churchland AK. 2012. Multisensory decision-making in rats and humans. J. Neurosci. 32, 3726-3735. ( 10.1523/JNEUROSCI.4998-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brody CD, Hanks TD. 2016. Neural underpinnings of the evidence accumulator. Curr. Opin. Neurobiol. 37, 149-157. ( 10.1016/j.conb.2016.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davis M, Falls WA, Campeau S, Kim M. 1993. Fear-potentiated startle: a neural and pharmacological analysis. Behav. Brain Res. 58, 175-198. ( 10.1016/0166-4328(93)90102-V) [DOI] [PubMed] [Google Scholar]
- 152.LeDoux JE. 2015. Anxious: using the brain to understand and treat fear and anxiety. New York, NY: Penguin. [Google Scholar]
- 153.Pellman BA, Kim JJ. 2016. What can ethobehavioral studies tell us about the brain's fear system? Trends Neurosci. 39, 420-431. ( 10.1016/j.tins.2016.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim JJ, Jung MW. 2018. Fear paradigms: the times they are a-changin’. Curr. Opin. Behav. Sci. 24, 38-43. ( 10.1016/j.cobeha.2018.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rogan MT, Stäubli UV, LeDoux JE. 1997. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604-607. ( 10.1038/37601) [DOI] [PubMed] [Google Scholar]
- 156.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. 2014. Engineering a memory with LTD and LTP. Nature 511, 348-352. ( 10.1038/nature13294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. 2009. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. 29, 12 236-12 243. ( 10.1523/JNEUROSCI.2378-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mobbs D, Hagan CC, Dalgleish T, Silston B, Prévost C. 2015. The ecology of human fear: survival optimization and the nervous system. Front. Neurosci. 9, 55. ( 10.3389/fnins.2015.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu JQ, Szpunar KK, Godovich SA, Schacter DL, Hofmann SG. 2015. Episodic future thinking in generalized anxiety disorder. J. Anxiety Disord. 36, 1-8. ( 10.1016/j.janxdis.2015.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mobbs D, Headley DB, Ding W, Dayan P. 2020. Space, time, and fear: survival computations along defensive circuits. Trends Cogn. Sci. 24, 228-241. ( 10.1016/j.tics.2019.12.016) [DOI] [PubMed] [Google Scholar]
- 161.Beck AT, Emery G, Greenberg RL. 2005. Anxiety disorders and phobias: a cognitive perspective. New York, NY: Basic Books.
- 162.McNally GP. 2021. Motivational competition and the paraventricular thalamus. Neurosci. Biobehav. Rev. 125, 193-207. ( 10.1016/j.neubiorev.2021.02.021) [DOI] [PubMed] [Google Scholar]
- 163.Fanselow MS, Lester LS. 1988. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In Evolution and learning (ed. Bolles RC), pp. 185-212. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 164.Mobbs D, Kim JJ. 2015. Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Curr. Opin. Behav. Sci. 5, 8-15. ( 10.1016/j.cobeha.2015.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Choi J-S, Kim JJ.. 2010. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc. Natl Acad. Sci. USA 107, 21 773-21 777. ( 10.1073/pnas.1010079108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Walters CJ, Jubran J, Sheehan A, Erickson MT, Redish AD. 2019. Avoid-approach conflict behaviors differentially affected by anxiolytics: implications for a computational model of risky decision-making. Psychopharmacology (Berl.) 236, 2513-2525. ( 10.1007/s00213-019-05197-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amir A, Lee S-C, Headley DB, Herzallah MM, Pare D. 2015. Amygdala signaling during foraging in a hazardous environment. J. Neurosci. 35, 12 994-13 005. ( 10.1523/JNEUROSCI.0407-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morriss J, Christakou A, van Reekum CM.. 2016. Nothing is safe: intolerance of uncertainty is associated with compromised fear extinction learning. Biol. Psychol. 121, 187-193. ( 10.1016/j.biopsycho.2016.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koenig S, Uengoer M, Lachnit H. 2017. Attentional bias for uncertain cues of shock in human fear conditioning: evidence for attentional learning theory. Front. Hum. Neurosci. 11, 266. ( 10.3389/fnhum.2017.00266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kepecs A, Mensh BD. 2015. Emotor control: computations underlying bodily resource allocation, emotions, and confidence. Dialogues Clin. Neurosci. 17, 391-401. ( 10.31887/DCNS.2015.17.4/akepecs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Molewijk HE, van der Poel AM, Oliver B. 1995. The ambivalent behaviour ‘stretched approach posture’ in the rat as a paradigm to characterize anxiolytic drugs. Psychopharmacology (Berl.) 121, 81-90. ( 10.1007/BF02245594) [DOI] [PubMed] [Google Scholar]
- 172.Grewal SS, Shepherd JK, Bill DJ, Fletcher A, Dourish CT. 1997. Behavioural and pharmacological characterisation of the canopy stretched attend posture test as a model of anxiety in mice and rats. Psychopharmacology (Berl.) 133, 29-38. ( 10.1007/s002130050367) [DOI] [PubMed] [Google Scholar]
- 173.Gray J, McNaughton N. 2000. The neuropsychology of anxiety. New York, NY: Oxford University Press. [Google Scholar]
- 174.Wu C-T, Haggerty D, Kemere C, Ji D. 2017. Hippocampal awake replay in fear memory retrieval. Nat. Neurosci. 20, 571-580. ( 10.1038/nn.4507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Heller AS, Bagot RC. 2020. Is hippocampal replay a mechanism for anxiety and depression? JAMA Psychiatry 77, 431-432. ( 10.1001/jamapsychiatry.2019.4788) [DOI] [PubMed] [Google Scholar]
- 176.Walters CJ. 2021. Neural computations underpinning anxiety in health and disease. PhD thesis, University of Minnesota, MN. [Google Scholar]
- 177.Abram SV, Breton Y-A, Schmidt B, Redish AD, MacDonald AW III. 2016. The web-surf task: a translational model of human decision-making. Cogn. Affect. Behav. Neurosci. 16, 37-50. ( 10.3758/s13415-015-0379-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kazinka R, MacDonald AW III, Redish AD. 2021. Sensitivity to sunk costs depends on attention to the delay. Front. Psychol. 12, 604843. ( 10.3389/fpsyg.2021.604843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huynh T, Alstatt K, Abram SV, Schmitzer-Torbert N. 2021. Vicarious trial-and-error is enhanced during deliberation in human virtual navigation in a translational foraging task. Front. Behav. Neurosci. 15, 586159. ( 10.3389/fnbeh.2021.586159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haynos A, Abram S, Sweis B, MacDonald AW III, Redish AD, Crow S. 2019. S74. Identifying valuation disturbances in anorexia nervosa using a translational decision-making paradigm. Biol. Psychiatry 85, S325. ( 10.1016/j.biopsych.2019.03.825) [DOI] [Google Scholar]
- 181.Flavell JH. 1979. Metacognition and cognitive monitoring: a new area of cognitive-developmental inquiry. Am. Psychol. 34, 906-911. ( 10.1037/0003-066x.34.10.906) [DOI] [Google Scholar]
- 182.Metcalfe J. 2008. Evolution of metacognition. Handb. Metamemory Mem. 29, 46. ( 10.4324/9780203805503.ch3) [DOI] [Google Scholar]
- 183.Fleming SM, Dolan RJ. 2012. The neural basis of metacognitive ability. Phil. Trans. R. Soc. B 367, 1338-1349. ( 10.1098/rstb.2011.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hangya B, Sanders JI, Kepecs A. 2016. A mathematical framework for statistical decision confidence. Neural Comput. 28, 1840-1858. ( 10.1162/NECO_a_00864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pouget A, Drugowitsch J, Kepecs A. 2016. Confidence and certainty: distinct probabilistic quantities for different goals. Nat. Neurosci. 19, 366-374. ( 10.1038/nn.4240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kiani R, Shadlen MN. 2009. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324, 759-764. ( 10.1126/science.1169405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Middlebrooks PG, Sommer MA. 2012. Neuronal correlates of metacognition in primate frontal cortex. Neuron 75, 517-530. ( 10.1016/j.neuron.2012.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Komura Y, Nikkuni A, Hirashima N, Uetake T, Miyamoto A. 2013. Responses of pulvinar neurons reflect a subject's confidence in visual categorization. Nat. Neurosci. 16, 749-755. ( 10.1038/nn.3393) [DOI] [PubMed] [Google Scholar]
- 189.Stolyarova A, Rakhshan M, Hart EE, O'Dell TJ, Peters MAK, Lau H, Soltani A, Izquierdo A. 2019. Contributions of anterior cingulate cortex and basolateral amygdala to decision confidence and learning under uncertainty. Nat. Commun. 10, 1-4. ( 10.1038/s41467-019-12725-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schmack K, Bosc M, Ott T, Sturgill JF, Kepecs A. 2021. Striatal dopamine mediates hallucination-like perception in mice. Science 372, eabf4740. ( 10.1126/science.abf4740) [DOI] [PubMed] [Google Scholar]
- 191.Lak A, Costa GM, Romberg E, Koulakov AA, Mainen ZF, Kepecs A. 2014. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron 84, 190-201. ( 10.1016/j.neuron.2014.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Masset P, Ott T, Lak A, Hirokawa J, Kepecs A. 2020. Behavior- and modality-general representation of confidence in orbitofrontal cortex. Cell 182, 112-126; e18. ( 10.1016/j.cell.2020.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Odegaard B, Grimaldi P, Cho SH, Peters MAK, Lau H, Basso MA.. 2018. Superior colliculus neuronal ensemble activity signals optimal rather than subjective confidence. Proc. Natl Acad. Sci. USA 115, E1588-E1597. ( 10.1073/pnas.1711628115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goupil L, Kouider S. 2016. Behavioral and neural indices of metacognitive sensitivity in preverbal infants. Curr. Biol. 26, 3038-3045. ( 10.1016/j.cub.2016.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Drugowitsch J, Mendonça AG, Mainen ZF, Pouget A.. 2019. Learning optimal decisions with confidence. Proc. Natl Acad. Sci. USA 116, 24 872-24 880. ( 10.1073/pnas.1906787116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Meyniel F, Schlunegger D, Dehaene S. 2015. The sense of confidence during probabilistic learning: a normative account. PLoS Comput. Biol. 11, e1004305. ( 10.1371/journal.pcbi.1004305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wei Z, Wang X-J. 2015. Confidence estimation as a stochastic process in a neurodynamical system of decision making. J. Neurophysiol. 114, 99-113. ( 10.1152/jn.00793.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lak A, et al. 2020. Reinforcement biases subsequent perceptual decisions when confidence is low, a widespread behavioral phenomenon. Elife 9, e49834. ( 10.7554/eLife.49834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sanders JI, Hangya B, Kepecs A. 2016. Signatures of a statistical computation in the human sense of confidence. Neuron 90, 499-506. ( 10.1016/j.neuron.2016.03.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fleming SM, Daw ND. 2017. Self-evaluation of decision-making: a general Bayesian framework for metacognitive computation. Psychol. Rev. 124, 91-114. ( 10.1037/rev0000045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rutishauser U, Aflalo T, Rosario ER, Pouratian N, Andersen RA. 2018. Single-neuron representation of memory strength and recognition confidence in left human posterior parietal cortex. Neuron 97, 209-220.e3. ( 10.1016/j.neuron.2017.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Miyamoto K, Setsuie R, Osada T, Miyashita Y. 2018. Reversible silencing of the frontopolar cortex selectively impairs metacognitive judgment on non-experience in primates. Neuron 97, 980-989.e6. ( 10.1016/j.neuron.2017.12.040) [DOI] [PubMed] [Google Scholar]
- 203.Hoven M, Lebreton M, Engelmann JB, Denys D, Luigjes J, van Holst RJ.. 2019. Abnormalities of confidence in psychiatry: an overview and future perspectives. Transl. Psychiatry 9, 268. ( 10.1038/s41398-019-0602-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rouault M, Seow T, Gillan CM, Fleming SM. 2018. Psychiatric symptom dimensions are associated with dissociable shifts in metacognition but not task performance. Biol. Psychiatry 84, 443-451. ( 10.1016/j.biopsych.2017.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Seow TXF, Gillan CM. 2020. Transdiagnostic phenotyping reveals a host of metacognitive deficits implicated in compulsivity. Sci. Rep. 10, 2883. ( 10.1038/s41598-020-59646-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vaghi MM, Luyckx F, Sule A, Fineberg NA, Robbins TW, De Martino B.. 2017. Compulsivity reveals a novel dissociation between action and confidence. Neuron 96, 348-354; e4. ( 10.1016/j.neuron.2017.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Banca P, Vestergaard MD, Rankov V, Baek K, Mitchell S, Lapa T, Castelo-Branco M, Voon V. 2015. Evidence accumulation in obsessive-compulsive disorder: the role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology 40, 1192-1202. ( 10.1038/npp.2014.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Campbell WK, Goodie AS, Foster JD. 2004. Narcissism, confidence, and risk attitude. J. Behav. Decis. Mak. 17, 297-311. ( 10.1002/bdm.475) [DOI] [Google Scholar]
- 209.Dunning D, Story AL. 1991. Depression, realism, and the overconfidence effect: are the sadder wiser when predicting future actions and events? J. Pers. Soc. Psychol. 61, 521-532. ( 10.1037/0022-3514.61.4.521) [DOI] [PubMed] [Google Scholar]
- 210.Korn CW, Sharot T, Walter H, Heekeren HR, Dolan RJ. 2014. Depression is related to an absence of optimistically biased belief updating about future life events. Psychol. Med. 44, 579-592. ( 10.1017/S0033291713001074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. 1991. A behavioral approach to achieving initial cocaine abstinence. Am. J. Psychiatry 148, 1218-1224. ( 10.1176/ajp.148.9.1218) [DOI] [PubMed] [Google Scholar]
- 212.Petry NM. 2012. Contingency management: for substance abuse treatment. New York, NY: Routledge. [Google Scholar]
- 213.Hursh SR, Galuska CM, Winger G, Woods JH. 2005. The economics of drug abuse: a quantitative assessment of drug demand. Mol. Interv. 5, 20-28. ( 10.1124/mi.5.1.6) [DOI] [PubMed] [Google Scholar]
- 214.Higgins ST, Heil SH, Lussier JP. 2004. Clinical implications of reinforcement as a determinant of substance use disorderse. Annu. Rev. Psychol. 55, 431-461. ( 10.1146/annurev.psych.55.090902.142033) [DOI] [PubMed] [Google Scholar]
- 215.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. 2006. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 101, 192-203. ( 10.1111/j.1360-0443.2006.01311.x) [DOI] [PubMed] [Google Scholar]
- 216.Regier PS, Redish AD. 2015. Contingency management and deliberative decision-making processes. Front. Psychiatry 6, 0076. ( 10.3389/fpsyt.2015.00076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ahmed SH. 2010. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci. Biobehav. Rev. 35, 172-184. ( 10.1016/j.neubiorev.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 218.Redish AD, Schultheiss NW, Carter EC. 2016. The computational complexity of valuation and motivational forces in decision-making processes. Curr. Top. Behav. Neurosci. 27, 313-333. ( 10.1007/7854_2015_375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schacter DL, Addis DR, Buckner RL. 2007. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 8, 657-661. ( 10.1038/nrn2213) [DOI] [PubMed] [Google Scholar]
- 220.Hassabis D, Maguire EA. 2011. The construction system in the brain. In Predictions in the brain: using our past to generate a future (ed. Bar M), pp. 70-82. New York, NY: Oxford University Press. [Google Scholar]
- 221.Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. 2011. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol. Psychiatry 69, 260-265. ( 10.1016/j.biopsych.2010.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Radu PT, Yi R, Bickel WK, Gross JJ, McClure SM. 2011. A mechanism for reducing delay discounting by altering temporal attention. J. Exp. Anal. Behav. 96, 363-385. ( 10.1901/jeab.2011.96-363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sayegh CS, Huey SJ, Zara EJ, Jhaveri K. 2017. Follow-up treatment effects of contingency management and motivational interviewing on substance use: a meta-analysis. Psychol. Addict. Behav. 31, 403-414. ( 10.1037/adb0000277) [DOI] [PubMed] [Google Scholar]
- 224.DiClemente CC, Corno CM, Graydon MM, Wiprovnick AE, Knoblach DJ. 2017. Motivational interviewing, enhancement, and brief interventions over the last decade: a review of reviews of efficacy and effectiveness. Psychol. Addict. Behav. 31, 862-887. ( 10.1037/adb0000318) [DOI] [PubMed] [Google Scholar]
- 225.O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely moving rat. Brain Res. 34, 171-175. ( 10.1016/0006-8993(71)90358-1) [DOI] [PubMed] [Google Scholar]
- 226.Taube JS, Muller RU, Ranck JB Jr. 1990. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420-435. ( 10.1523/JNEUROSCI.10-02-00420.1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Taube JS. 1995. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci. 15, 70-86. ( 10.1523/JNEUROSCI.15-01-00070.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Skaggs WE, Knierim JJ, Kudrimoti HS, McNaughton BL. 1995. A model of the neural basis of the rat's sense of direction. In Advances in neural information processing systems 7 (eds Tesauro G, Touretzky DS, Leen TK), pp. 173-180. Cambridge, MA: MIT Press. [PubMed] [Google Scholar]
- 229.Redish AD, Elga AN, Touretzky DS. 1996. A coupled attractor model of the rodent head direction system. Netw. Comput. Neural Syst. 7, 671-685. ( 10.1088/0954-898X_7_4_004) [DOI] [Google Scholar]
- 230.Zhang K. 1996. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J. Neurosci. 16, 2112-2126. ( 10.1523/JNEUROSCI.16-06-02112.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Knierim JJ, Kudrimoti HS, McNaughton BL. 1998. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J. Neurophysiol. 80, 425-446. ( 10.1152/jn.1998.80.1.425) [DOI] [PubMed] [Google Scholar]
- 232.Gallistel CR. 1990. The organization of learning. Cambridge, MA: MIT Press. [Google Scholar]
- 233.Margules J, Gallistel CR. 1988. Heading in the rat: determination by environmental shape. Anim. Learn. Behav. 16, 404-410. ( 10.3758/BF03209379) [DOI] [Google Scholar]
- 234.Cheng K. 1986. A purely geometric module in the rat's spatial representation. Cognition 23, 149-178. ( 10.1016/0010-0277(86)90041-7) [DOI] [PubMed] [Google Scholar]
- 235.Hermer L, Spelke ES. 1994. A geometric process for spatial reorientation in young children. Nature 370, 57-59. ( 10.1038/370057a0) [DOI] [PubMed] [Google Scholar]
- 236.Wang RF, Hermer L, Spelke ES. 1999. Mechanisms of reorientation and object localization by children: a comparison with rats. Behav. Neurosci. 113, 475-485. ( 10.1037/0735-7044.113.3.475) [DOI] [PubMed] [Google Scholar]
- 237.Widge AS, et al. 2017. Treating refractory mental illness with closed-loop brain stimulation: progress towards a patient-specific transdiagnostic approach. Exp. Neurol. 287, 461-472. ( 10.1016/j.expneurol.2016.07.021) [DOI] [PubMed] [Google Scholar]
- 238.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 239.Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, Kupfer DJ. 2013. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry 170, 59-70. ( 10.1176/appi.ajp.2012.12070999) [DOI] [PubMed] [Google Scholar]
- 240.Cuthbert BN, Insel TR. 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126. ( 10.1186/1741-7015-11-126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Rashed Ahmed AP, Samara Z, Williams LM. 2018. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry 75, 201. ( 10.1001/jamapsychiatry.2017.3951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND. 2016. Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. Elife 5, e11305. ( 10.7554/elife.11305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dang J, King KM, Inzlicht M.. 2020. Why are self-report and behavioral measures weakly correlated? Trends Cognit. Sci. 24, 267-269. ( 10.31234/osf.io/v796c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Enkavi AZ, Eisenberg IW, Bissett P, Mazza GL, MacKinnon D, Marsch L, Poldrack R. 2019. A large-scale analysis of test-retest reliabilities of self-regulation measures. PsyArXiv. ( 10.31234/osf.io/x5pm4) [DOI]
- 245.Poldrack RA, Yarkoni T. 2016. From brain maps to cognitive ontologies: informatics and the search for mental structure. Annu. Rev. Psychol. 67, 587-612. ( 10.1146/annurev-psych-122414-033729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gillan CM, et al. 2020. Comparison of the association between goal-directed planning and self-reported compulsivity vs obsessive-compulsive disorder diagnosis. JAMA Psychiatry 77, 77-85. ( 10.1001/jamapsychiatry.2019.2998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Basu I, et al. 2020. Closed loop enhancement and neural decoding of human cognitive control. bioRxiv, 2020.04.24.059964. ( 10.1101/2020.04.24.059964) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.