Abstract
In Schizosaccharomyces pombe, the catalytic subunit of DNA polymerase epsilon (Pol ɛ) is encoded by cdc20+ and is essential for chromosomal DNA replication. Here we demonstrate that the N-terminal half of Pol ɛ that includes the highly conserved polymerase and exonuclease domains is dispensable for cell viability, similar to observations made with regard to Saccharomyces cerevisiae. However, unlike budding yeast, we find that fission yeast cells lacking the N terminus of Pol ɛ (cdc20ΔN-term) are hypersensitive to DNA-damaging agents and have a cell cycle delay. Moreover, the viability of cdc20ΔN-term cells is dependent on expression of rad3+, hus1+, and chk1+, three genes essential for the DNA damage checkpoint control. These data suggest that in the absence of the N terminus of Pol ɛ, cells accumulate DNA damage that must be repaired prior to mitosis. Our observation that S phase occurs more slowly for cdc20ΔN-term cells suggests that DNA damage might result from defects in DNA synthesis. We hypothesize that the C-terminal half of Pol ɛ is required for assembly of the replicative complex at the onset of S phase. This unique and essential function of the C terminus is preserved in the absence of the N-terminal catalytic domains, suggesting that the C terminus can interact with and recruit other DNA polymerases to the site of initiation.
Genetic analysis of yeast has demonstrated that Pol ɛ is required for chromosomal DNA replication (4, 7, 9, 14, 32, 40). However, its precise function at the replication fork has remained elusive. Based on our earlier observations that cdc20 mutants (cdc20+ encodes the catalytic subunit of Pol ɛ in fission yeast) show a cell cycle arrest with a 1C DNA content, we proposed that Pol ɛ is necessary during the initiation of DNA replication (14). Consistent with this hypothesis, a chromatin immunoprecipitation assay has demonstrated that Pol ɛ associates with replication origins in budding yeast (3, 27). Moreover, the observation that Pol ɛ remains associated with replication forks following initiation suggests that it also participates directly in chain elongation (3).
In addition to its role in DNA replication, Pol ɛ has also been implicated in DNA repair. Pol ɛ from human cells has been shown to function in nucleotide excision repair in vitro (39) and has been identified as a component of a high-molecular-weight complex that catalyzes recombinational repair of DNA double-strand breaks in vitro (18). Genetic analysis of Saccharomyces cerevisiae also supports a role for Pol ɛ in DNA double-strand break repair (17) and base-excision repair (44).
Pol ɛ purified from S. cerevisiae consists of at least four subunits, including the 256-kDa catalytic subunit encoded by POL2 and three additional subunits of approximately 80, 34, and 29 kDa encoded by DPB2, DPB3, and DPB4, respectively (4, 5, 7, 15). In human cells, Pol ɛ is also composed of at least four subunits, all of which display significant homology to their yeast counterparts. These include the large catalytic subunit encoded by POLE (21), the second-largest subunit homologous to DPB2, called DPE2 (23), and two smaller subunits of approximately 17 and 12 kDa which share homology with DPB4 and DPB3, respectively (24).
Other proteins that interact with and might regulate Pol ɛ activity include Dpb11p, a multicopy suppressor of both pol2 and dpb2 temperature-sensitive mutants (6), and Drc1p (Sld2p), which physically interacts with Dpb11p (19, 43). Dpb11p is required for normal S-phase progression (6, 27) and interacts genetically with Cdc45p, a protein implicated in the assembly of the initiation complex (35, 46). DPB11 shares homology with cut5+, a gene required for DNA replication initiation and G2-M checkpoint control in fission yeast (29, 37, 38, 41). However, it is not known whether Cut5p interacts directly with Pol ɛ or other components of the replicative complex.
Recently, it has been reported that the C-terminal half of Pol ɛ lacking the conserved polymerase or exonuclease domains is sufficient to rescue a pol2 null mutant in S. cerevisiae (12, 22). Surprisingly, these cells displayed only marginal defects in either DNA replication or DNA repair and did not require the checkpoint gene MEC1 for viability (12, 22). Here we demonstrate that Schizosaccharomyces pombe cells lacking the N terminus of Pol ɛ (cdc20ΔN-term) are also viable. However, these cells display increased sensitivity to DNA-damaging agents and have a cell cycle delay. Moreover, cell viability is dependent on the DNA damage checkpoint control. These data demonstrate that the C terminus of Pol ɛ has a critical role in DNA replication that does not rely on its ability to synthesize DNA. Considering that these two yeasts are evolutionarily distant, our results suggest that the N terminus of Pol ɛ may be dispensable in all eukaryotic cells. Based on our earlier observation that cdc20 temperature-sensitive mutants show cell cycle arrest early in S phase, we propose that the function of the C terminus of Pol ɛ is to ensure proper assembly of the DNA replicative complex.
MATERIALS AND METHODS
Yeast strains and methods.
All fission yeast strains used for this study were derived from S. pombe strains 972 and 975 and are listed in Table 1. All media, growth conditions, and genetic manipulations were used as previously described (31).
TABLE 1.
S. pombe strains used in this study
| Strain or mutation | Genotype | Source |
|---|---|---|
| 972 | h− | P. Nurse |
| cdc20-M10 | h− cdc20-M10 leu1-32 ura4-D18 | P. Nurse |
| cdc20-P7 | h− cdc20-P7 leu1-32 | P. Nurse |
| Δcdc20/cdc20+ | h−/h+ cdc20+/cdc20::ura4+ ade6-M216/ade6-M210 leu1-32/leu1-32 ura4-D18/ura4-D18 | P. Nurse |
| cdc20ΔN-term | h− cdc20::ura4+ ade6-704 leu1-32 ura4-D18 int pJK148-nmt41-3hacdc20C1 int pRep6X | This study |
| cdc20N-term+C-term | h− cdc20::ura4+ ade6-704 leu1-32 ura4-D18 int pJK148-nmt41-3hacdc20C1 int pRep6X-cdc20N | This study |
| cdc203hacdc20+ | h− cdc20::ura4+ ade6-704 leu1-32 ura4-D18 int pJK148-nmt41-3hacdc20+ | This study |
| cdc20tsN-term+C-term | h− cdc20::ura4+ ade6-704 leu1-32 ura4-D18 int pJK148-nmt41-3hacdc20C1 int pRep6X-cdc20N(M10) | This study |
| Δhus1 | h− hus1::leu2+ ade6-704 leu1-32 ura4-D18 | T. Enoch |
| Δrad3 | h− rad3::ura4+ ade6-704 leu1-32 ura4-D18 | A. Carr |
| Δcds1 | h− cds1::ura4+ ade6-704 leu1-32 ura4-D18 | P. Nurse |
| Δchk1 | h− chk1::ura4+ ade6-704 leu1-32 ura4-D18 | P. Nurse |
| cdc20N-term+C-term Δchk1 | h+ cdc20::ura4+ chk1::ura4+ ade6-704 leu1-32 ura4-D18 int pJK148-nmt41-3hacdc20C1 int pRep6X-cdc20N | This study |
| cdc20tsN-term+C-term Δchk1 | h+ cdc20::ura4+ chk1::ura4+ ade6-704 leu1-32 ura4-D18 int pJK148-nmt41-3hacdc20C1 int pRep6X-cdc20N-M10 | This study |
Molecular cloning and the construction of cdc20ΔN-term, cdc20N-term+C-term, and cdc203hacdc20+strains.
The sequence corresponding to the C terminus of the product of cdc20+ was amplified by PCR using the forward primer 5′GGAATTCCATATGCGTCTAGGATCAGTAGTAC3′ and the reverse primer 5′CCCCCCGGGGGGGCATGAGTGGAAAAATGG3′, tagged with NdeI and SmaI as underlined. The resulting 3.4-kb fragment encoding the C terminus of Pol ɛ (amino acids 1141 to 2199) was cloned into pRep1, generating pRep1-cdc20C1. Further truncations of cdc20C1 yielded cdc20C2 and cdc20C3. To generate pRep1-nlscdc20C3, a PCR fragment containing the putative nuclear localization signal (NLS) was amplified and cloned into pRep1-cdc20C3 at the NdeI and BamHI sites.
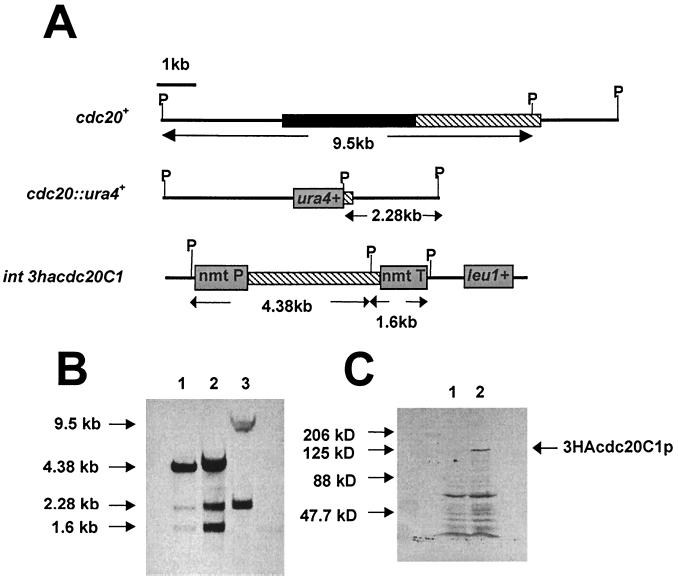
To construct the cdc20ΔN-term strain, the cdc20C1 gene was first cloned into pARC613, tagging the gene with three tandem copies of the hemagglutinin (HA) epitope. A 5.6-kb PstI/SacI fragment from pARC613-cdc20C1 was then cloned into pJK148 (20). The plasmid pJK148-cdc20C1 was linearized at the Bsu36I site within the leu1+ gene and transformed into the Δcdc20/cdc20+ diploid strain (cdc20+/cdc20::ura4+ ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 h+/h−). Stable integrants were isolated and induced to sporulate under low-nitrogen conditions. Spores were then germinated on minimal medium lacking uracil and leucine. The ade6-M210 marker was then removed by backcrossing to leu1-32 ura4-D18 and selecting for leucine and uracil prototrophs, yielding cdc20ΔN-term. Integration of the cdc20C1 gene at the leu1 site was confirmed by Southern blot hybridization (see Fig. 3B).
FIG. 3.
Construction of the cdc20ΔN-term mutant. (A) Expected genomic structure of the cdc20 and leu1 loci following integration of pJK148-cdc20C1. The solid and cross-hatched bars indicate the regions of cdc20 corresponding to the N terminus and the C terminus, respectively. P indicates the location of the PstI restriction sites used for the Southern blot analysis. (B) Southern blot of genomic DNA prepared from the diploid Δcdc20/cdc20+ strain (lane 3) and from two independent cdc20ΔN-term isolates (lanes 1 and 2), probed with a PCR fragment corresponding to the C-terminal half encoded by cdc20. (C) Western blot of a protein extract prepared from wild-type cells (lane 1) and cdc20ΔN-term cells (lane 2), using anti-HA monoclonal antibodies. The apparent molecular mass of 3HACdc20C1p is approximately 122 kDa.
To create the cdc20N-term+C-term strain, the sequence corresponding to the N terminus of the cdc20+ product (from amino acid 1 to 1281) was amplified using the forward primer 5′CGGCGGTCGACTATGCCCTTAAAAACAGCTCG3′ and reverse primer 5′GCCGAACCCGGGGAATTGCCTTGATTGAAACC3′, tagged with SalI and SmaI, respectively. The 3.8-kb fragment was cloned into pRep6X that contains the sup3-5 allele, a suppressor of the ade6-704 mutant allele, thus creating pRep6X-cdc20N. This plasmid was transformed into a cdc20ΔN-term ade6-704 mutant. Stable integrants were selected on minimal medium containing a low level of adenine. The cdc20tsN-term+C-term strain was generated in a similar manner, except that the sequence corresponding to the N terminus of the product of cdc20+ was PCR amplified from genomic DNA derived from cdc20-M10.
To generate the control (cdc203hacdc20+) strain, the sequence corresponding to the N terminus of the cdc20+ product was amplified by PCR using the forward primer 5′CGGCGGAGATCTATGCCCTTAAAAACAGCTCG3′ (tagged with BglII as underlined) and the reverse primer 5′CGATTTCATCAACATTGACG3′. The 1.7-kb PCR product was digested with BglII and BamHI and cloned into the BamHI site of pARC613. The 2.9-kb PstI/SmaI fragment from the resulting plasmid, pARC613-cdc20N, was then cloned into pJK148. This plasmid was digested with ApaI and SmaI and ligated to a 4.0-kb ApaI/PstI fragment and a 1.8-kb PstI/SmaI fragment from pIRT2-cdc20+ and pRep1-cdc20C1 plasmids, respectively, in a three-way ligation. The resulting plasmid, pJK148-3hacdc20+, was linearized with Bsu36I in the leu1+ marker and transformed into the Δcdc20 strain. All additional steps are identical to those used for the generation of cdc20ΔN-term.
Determination of cell generation time.
Cells were grown for at least eight generations in minimal medium at 32°C prior to analysis. Samples were collected every hour and counted using a hemacytometer. The cell generation time, T, was calculated as the log (2t2−t1)/log (y/x), where y is the number of cells/ml at time t2 and x is the number of cells/ml at time t1.
Cell synchronization using cdc10-129.
To block cells in pre-Start G1 phase using the cdc10-129 mutation (34), cells were incubated in minimal media at 36°C for 4 h. Cells were released from the G1 block by rapidly cooling cultures to 25°C. Samples were collected every 15 minutes and fixed in 70% ethanol for fluorescence-activated cell sorter (FACS) analysis and microscopic examination.
Flow cytometry analysis and microscopic examination.
For DNA content measurements, cells were stained with propidium iodide and analyzed by FACS as described previously (31). For microscopic examination, cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) and examined with a Zeiss fluorescence microscope.
Cell survival rate measurements.
Cells were grown to mid-log phase (optical density at 595 nm, 0.3 to 0.5) in minimal media prior to treatment with either hydroxyurea (HU) (12 mM) or methylmethane sulfonate (MMS) (0.2%). Cell samples were collected, diluted, plated on minimal medium, and incubated for 4 days at 32°C. The number of colonies was determined and plotted as the percent viability relative to the untreated control. For the UV sensitivity assay, cells were irradiated with increasing doses of UV light (254 nm) in a GS Gene Linker (Bio-Rad, Hercules, Calif.). Total output energy (in millijoules) was measured by an internally mounted photodetector. The gene linker was programmed to release a specific amount of total energy from 1 to 5 mJ in 1-mJ increments. Following irradiation, equal numbers of cells (approximately 500) were plated on minimal agar plates and incubated for 4 days at 32°C. The number of colonies was determined, and data were analyzed using SigmaPlot software.
RESULTS
Expression of the C-terminal half of Pol ɛ complements cdc20ts mutants.
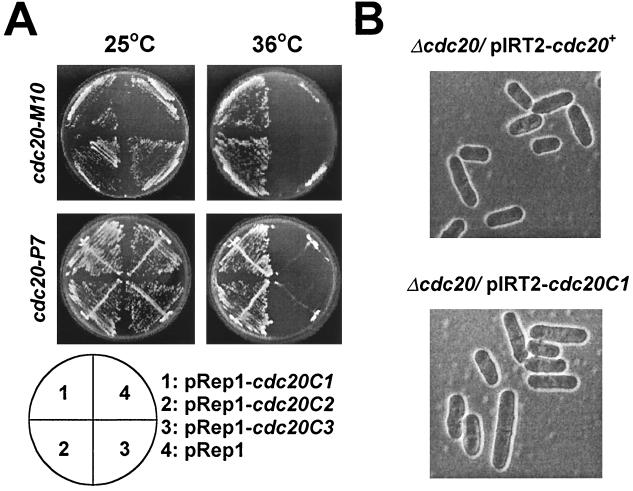
To determine if the C-terminal half of Pol ɛ can complement cdc20 mutants, we transformed two different alleles, cdc20-M10 and cdc20-P7, with the plasmid pRep1-cdc20C1 (Fig. 1). This plasmid contains a gene encoding the C terminus of Pol ɛ from amino acid 1141 to 2199 expressed under the control of the thiamine-repressible nmt1 promoter (28). This deletion removes all the conserved polymerase and exonuclease domains of Pol ɛ. Transformed colonies were selected at the permissive temperature of 25°C and then streaked out on minimal agar at both 25°C and the nonpermissive temperature of 36°C. Both cdc20-M10 and cdc20-P7 transformed with pRep1-cdc20C1 but not with pRep1 alone were able to grow at the restrictive temperature, demonstrating that expression of the C terminus of Pol ɛ can rescue the temperature sensitivity of the cdc20 mutants (Fig. 2A).
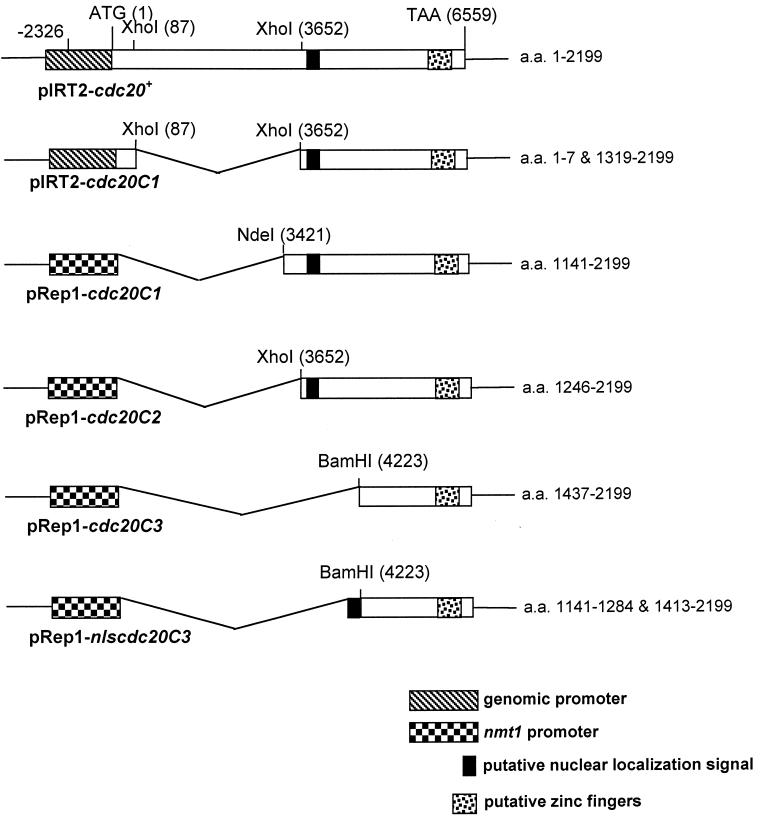
FIG. 1.
Schematic representation of N-terminally truncated forms of cdc20 that were used for the complementation analysis shown in Fig. 2 (the gene structure is not drawn in scale). All plasmids were derived from pIRT2-cdc20+, which contains a 10,054-bp genomic fragment of the cdc20+ gene. The numbers in parentheses indicate nucleotide positions. The first nucleotide of the start codon was numbered 1, and all other sequences were designated accordingly. The numbers on the right of each plasmid indicate the numbers of amino acids of Pol ɛ being included in each construct. The series of pRep1 plasmids with which the C-terminal fragments of the cdc20 product were expressed under the nmt1 promoter were generated by cloning restriction fragments of pIRT2-cdc20+ into the pRep1 vector. Initiation of translation is presumed to take place at the first internal methionine. The putative NLS and zinc finger motifs are as indicated.
FIG. 2.
Expression of the C-terminal half of Pol ɛ rescues both cdc20ts mutants and cells with the complete cdc20+ gene deleted. (A) Complementation of the temperature-sensitive cdc20-M10 (top) and cdc20-P7 (bottom) strains by transformation with plasmids expressing the C-terminally truncated forms of Pol ɛ. Transformants were streaked on minimal agar and incubated at 25°C (left) and 36°C (right). (B) Expression of the C-terminal half of the cdc20 product can rescue the Δcdc20 strain. The cdc20+/Δcdc20 diploid strain was transformed with pIRT2-cdc20+ or pIRT2-cdc20C1. Following sporulation and germination of positive transformants, haploid cells containing the deletion of cdc20+ and either the plasmid pIRT2-cdc20+ (top) or pIRT2-cdc20C1 (bottom) were selected and visualized by phase contrast microscopy.
To identify the minimal C-terminal sequences required for complementation, we transformed the cdc20 mutant strains with plasmids containing further truncations of the cdc20C1 gene. Since we previously demonstrated that the extreme C terminus of cdc20+ is essential for cell viability (14), we deleted sequences from the N-terminal end only. Two additional plasmids were generated, pRep1-cdc20C2 and pRep1-cdc20C3, encoding Pol ɛ from amino acid 1246 to 2199 and from amino acid 1437 to 2199, respectively (Fig. 1). We observed that pRep1-cdc20C2 but not pRep1-cdc20C3 was able to complement both cdc20-M10 and cdc20-P7 (Fig. 2A). Sequence analysis using ProfileScan revealed a putative bipartite NLS located at amino acids 1257 to 1274. To test whether the inability of pRep1-cdc20C3 to rescue the cdc20 mutants was due to deletion of the NLS, this sequence was fused to cdc20C3 to create the plasmid pRep1-nlscdc20C3 (Fig. 1). However, expression of this fusion protein failed to rescue either cdc20-M10 or cdc20-P7 (data not shown). We conclude that the minimal Pol ɛ sequences required for complementation of the cdc20 mutants include amino acids 1246 to 2199.
Expression of the C-terminal half of Pol ɛ rescues a deletion of the cdc20+ gene.
Our observation that the C-terminal half of Pol ɛ is capable of rescuing cdc20 mutants was surprising considering that the mutations in both the cdc20-M10 and cdc20-P7 strains map to the N-terminal half of the protein (unpublished observations). Therefore, we tested whether expression of the C terminus of Pol ɛ alone can rescue a strain with the entire cdc20+ gene deleted. We transformed pRep1-cdc20C1 into the Δcdc20/cdc20+ diploid strain, in which a single copy of cdc20+ has been replaced by ura4+ (14). In addition, the Δcdc20/cdc20+ strain was transformed with pIRT2-cdc20+ and pIRT2-cdc20C1, expressing either the cdc20+ gene or cdc20-C1 under the control of the endogenous cdc20 promoter. Diploids transformed with these plasmids were induced to sporulate, and haploid cells prototrophic for uracil and leucine were selected. Cell growth was observed only following transformation with pRep1-cdc20C1, pIRT2-cdc20+, and pIRT2-cdc20C1 (Fig. 2B, cells transformed with pIRT2-cdc20+ and pIRT2-cdc20C1) but not with the vector alone or with a nonrelevant gene. Consistent with our earlier results, cdc20C2, but not cdc20C3, was able to rescue the deletion of cdc20+. We then generated a cdc20ΔN-term strain, in which cdc20+ is deleted but which contains an integrated copy of 3hacdc20C1 under the control of the nmt41 promoter. Southern blot analysis confirmed the absence of wild-type cdc20+ and the presence of cdc20C1 near the leu1 locus in this strain (Fig. 3A and B). Western blot analysis of cellular extracts prepared from the cdc20ΔN-term strain identified a polypeptide with a molecular weight consistent with that of 3HACdc20C1p that was not present in extracts prepared from wild-type cells (Fig. 3C).
The cdc20ΔN-term strain shows a delay in cell cycle progression and is sensitive to DNA-damaging agents.
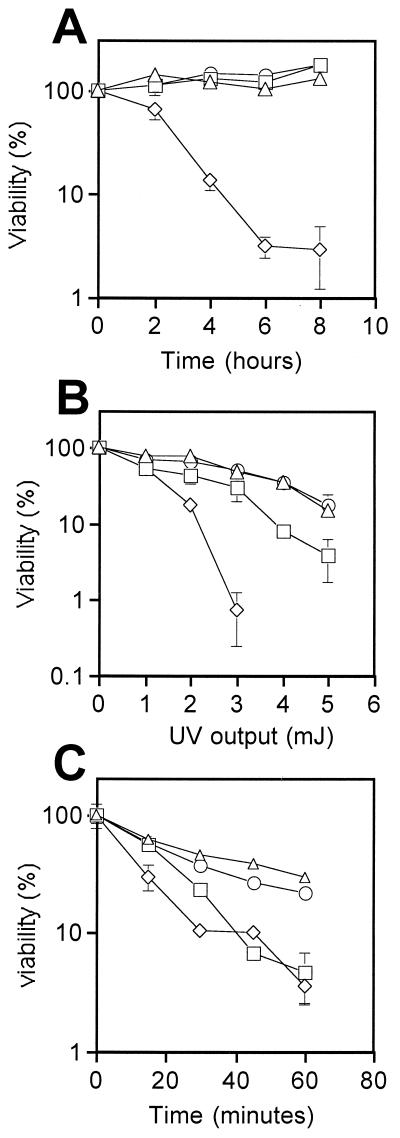
So far, we have shown that the N-terminal catalytic domains of Pol ɛ are dispensable for cell viability in fission yeast. To address whether the absence of the N terminus of Pol ɛ has any effect on DNA replication or DNA repair, we tested whether the cdc20ΔN-term strain is sensitive to either replication blocks or DNA-damaging agents. In these experiments, cell viability was monitored following treatment of the cdc20ΔN-term strain with HU, MMS, and UV irradiation. Although our results demonstrate that the cdc20ΔN-term strain displays normal sensitivity to HU (Fig. 4A), these cells show increased sensitivity to both UV irradiation and MMS (Fig. 4B and C). These data suggest that cells lacking the N-terminal domains of Pol ɛ are defective in DNA repair.
FIG. 4.
Cells lacking the N-terminal half of Pol ɛ display increased sensitivity to DNA damage. Survival rates of cdc203hacdc20+ (triangle), cdc20ΔN-term (square), cdc20N-term+C-term (circle), hus1-14 (diamond in panel A), and rad2-44 (diamonds in panels B and C) cells. Following treatment with 11 mM HU (A), increasing doses of UV irradiation (B), and 0.2% MMS (C), cells were plated at 32°C for 3 days, colonies were counted, and the survival rate was determined by SigmaPlot. Error bars indicate standard deviations.
To test whether expression of the N terminus of Pol ɛ in trans can complement the defects in cdc20ΔN-term cells, we generated a plasmid Rep6X-cdc20N expressing the N-terminal half of Pol ɛ (from amino acid 1 to 1281). This clone was then integrated into the cdc20ΔN-term strain, generating the cdc20N-term+C-term strain. Thus, in cdc20N-term+C-term cells, the N- and C-terminal domains of Pol ɛ are expressed from two independent genes. First, we measured the cell generation time for each strain; the results are summarized in Table 2. As expected, the cdc203hacdc20+ control strain has a generation time of approximately 2.3 h in minimal medium at 32°C, identical to that of wild-type (972) cells. In contrast, the cdc20ΔN-term strain is delayed approximately 80 min. Interestingly, the generation time of the cdc20N-term+C-term strain is similar to that of the wild type, suggesting that expression of the N terminus in trans can rescue the slow-growth phenotype. We then measured the survival rate of each strain after exposure to HU and DNA-damaging agents. As mentioned above, cdc20ΔN-term cells are resistant to HU but are sensitive to both UV irradiation and MMS. Interestingly, expression of the N-terminal half of Pol ɛ in cdc20ΔN-term cells is able to restore the survival rate after exposure to UV and MMS to levels comparable to those for wild-type cells (Fig. 4B and C). These results suggest that not only is the N terminus of Pol ɛ important for DNA repair, but it can still function when physically separated from the C-terminal half of the enzyme.
TABLE 2.
Summary of physiological characterizations of cdc20 mutants
| Strain or mutation | Generation time (h)b | Resistancec to:
|
||
|---|---|---|---|---|
| HU | UV | MMS | ||
| 972 (WT)a | 2.32 | +++ | +++ | +++ |
| cdc203hacdc20+ | 2.30 | +++ | +++ | +++ |
| cdc20ΔN-term | 3.63 | +++ | ++ | + |
| cdc20N-term+C-term | 2.36 | +++ | +++ | ++ |
WT, wild type.
Generation time was measured in minimal medium at 32°C.
+, poor resistance, +++, normal resistance.
cdc20ΔN-term cells are delayed during S phase.
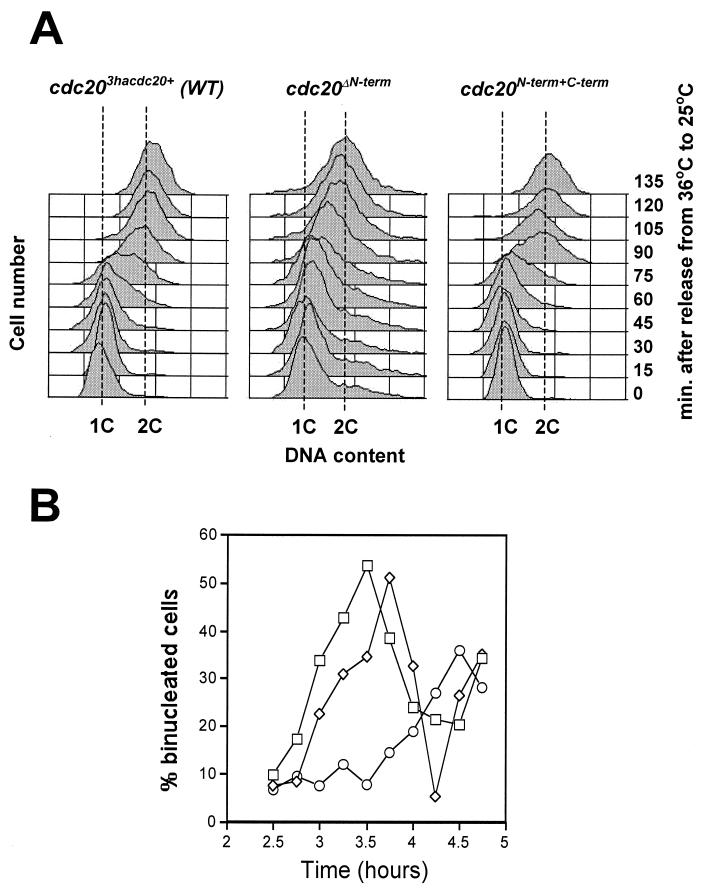
To determine if the longer generation time of the cdc20ΔN-term strain is due to a delay during specific phases of the cell cycle, we monitored the timing of both S phase and mitosis by monitoring DNA content and the appearance of binucleate cells following release from a G1 block. To do this, we constructed the cdc10- 129 cdc20ΔN-term double mutant and shifted these cells to the restrictive temperature for cdc10-129 (36°C), causing cell cycle arrest in G1. Upon return to the permissive temperature of 25°C, cells enter S phase synchronously (Fig. 5A). In three independent experiments, we observed that mitosis is delayed approximately 1 h in cdc20ΔN-term cells compared to results with either cdc203hacdc20+ or cdc20N-term+C-term cells (Fig. 5B). Analysis of DNA content by FACS shows that S phase is approximately 30 to 60 min slower in the cdc20ΔN-term strain compared to results with cells containing an intact Pol ɛ (Fig. 5A, compare cdc20ΔN-term, 90 min, to cdc203hacdc20+, 90 min). It is not known whether this delay reflects inefficient DNA replication initiation or elongation. However, unlike when DNA replication is inhibited by treatment with HU, cdc20ΔN-term cells do not require the checkpoint gene cds1+ for viability (see below) (Table 3). Consistent with the results of the DNA damage sensitivity assays and the cell cycle generation time measurements, expression of the N terminus in trans was able to rescue the S phase delay (Fig. 5, see results for cdc20N-term+C-term). These experiments suggest that cells lacking the N terminus of Pol ɛ undergo a defective round of DNA synthesis. To test the possibility that DNA damage accumulates in these cells and contributes to the cell cycle delay, we examined whether the DNA damage checkpoint is required for cell viability in the cdc20ΔN-term strain.
FIG. 5.
Cells lacking the N-terminal half of Pol ɛ show delayed S and G2 phases of the cell cycle. (A) FACS analysis of the DNA content of cells released from the cdc10-129 cell cycle arrest. Samples were collected every 15 min for approximately 2 h. The 1C and 2C DNA control peaks are indicated. (B) Percentage of binucleate cells for cdc10-129 cdc203hacdc20+ (square), cdc10-129 cdc20ΔN-term (circle), and cdc10-129 cdc20N-term+C-term (diamond) strains at the indicated times following release from the G1 block.
TABLE 3.
Summary of genetic interactions of cdc20 mutants
| Mutation | Viability of cellsa
|
||
|---|---|---|---|
| cdc203hacdc20+ (WT) | cdc20ΔN-term | cdc20N-term+C-term | |
| Δchk1 | + | − | + |
| Δcds1 | + | + | + |
| Δchk1 Δcds1 | + | N/A | + |
| Δrad3 | + | − | + |
| Δhus1 | + | − | + |
| cdc6-23 | + | − | + |
+, viable; −, synthetically lethal; N/A, not analyzed; WT, wild type.
The viability of the cdc20ΔN-term mutant is dependent on the DNA damage checkpoint control.
Our data have shown that S phase is delayed in the cdc20ΔN-term strain. Eukaryotic cells respond to DNA replication blocks or DNA damage by activating checkpoint controls that delay the onset of mitosis until DNA replication and repair are completed (2, 13, 16, 33, 45). A number of S. pombe genes have been identified that are essential to activate the checkpoint control. These include the rad genes (rad 1, 3, 9, 17, and 26), hus1, chk1, and cds1. The rad and hus genes are thought to be involved in the recognition of DNA damage and generation of checkpoint signals that ultimately block the onset of mitosis by inhibiting the mitotic kinase Cdc2p (10). Cds1p and Chk1p are two protein kinases that function downstream in the checkpoint control pathway during S and G2 phases, respectively (1, 25, 32, 36, 42). In addition to its proposed role in the checkpoint control, Cds1p has an additional role during recovery from replication blocks imposed by HU (8, 26). To test whether any of the checkpoint genes are required for viability in cdc20ΔN-term cells, we crossed the cdc20ΔN-term strain with various checkpoint mutants, including the Δrad3, Δhus1, Δchk1, and Δcds1 mutants, and the viability of the double mutants was examined by tetrad analysis. We found that hus1, rad3, and chk1 are all essential for viability in the cdc20ΔN-term strain, demonstrating that cdc20ΔN-term requires the DNA damage checkpoint control.
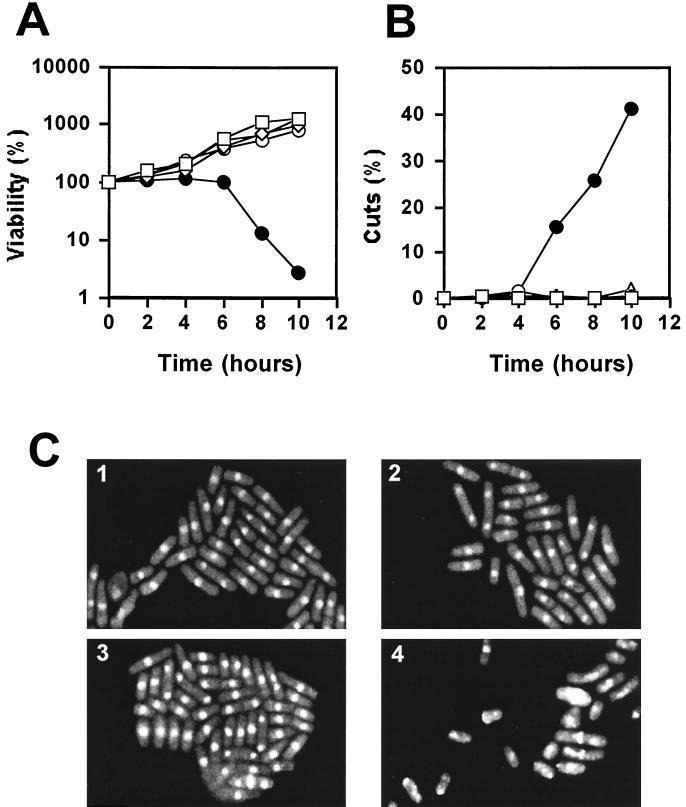
To confirm that the lethality of the cdc20ΔN-term cells when combined with DNA damage checkpoint mutations is indeed due to a checkpoint failure, we examined more closely the terminal phenotype of the cdc20ΔN-term Δchk1 double mutant. To do this, we first constructed the cdc20tsN-term+C-term strain, which is identical to the cdc20N-term+C-term strain except that the N terminus was derived from the temperature-sensitive cdc20-M10 strain. We then showed that the cdc20tsN-term+C-term strain is viable at 36°C, demonstrating that inactivation of the N terminus of Pol ɛ, when physically separated from the C terminus, does not interfere with normal C-terminal function. However, we found that the cdc20tsN-term+C-term Δchk1 double mutant rapidly lost viability upon a shift to the restrictive temperature (Fig. 6A). Microscopic examination of these cells after staining them with the DNA-binding dye DAPI revealed a high incidence of aberrant mitoses typical of the cut (cell untimely torn) phenotype (Fig. 6C). The appearance of mitotic abnormalities correlated with the observed decrease in cell viability (Fig. 6B). As a control, cdc20N-term+C-term Δchk1 cells displayed no aberrant mitoses at 36°C. These results confirmed that in the absence of the N terminus of Pol ɛ, cells are dependent on the DNA damage checkpoint control. Considering that Chk1p is required only for the DNA damage checkpoint operating in G2 (1, 41), these data provide evidence that G2 is also delayed in these cells. Interestingly, we found that none of the checkpoint genes are required for viability of cdc20N-term+C-term cells, providing further support that the N terminus of Pol ɛ is functional when expressed independently from the C-terminal half of the enzyme.
FIG. 6.
Cell viability for the cdc20ΔN-term strain is dependent on the DNA damage checkpoint control. The cdc20tsN-term+C-term Δchk1 double mutant is inviable at 36°C. (A) Exponentially growing cultures of 972+ (square), cdc20N-term+C-term (diamond), cdc20tsN-term+C-term (circle), cdc20N-term+C-term Δchk1 (triangle), and cdc20tsN-term+C-term Δchk1 (closed circle) strains were shifted from 25 to 36°C for 10 h. Samples were collected every hour and plated to determine cell viability. (B) The loss of viability of cdc20tsN-term+C-termΔchk1 cells at 36°C correlates with an increase in the number of cells undergoing an aberrant mitosis. These events are plotted as the percentage of “cut” cells and cells with abnormal nuclear morphology. (C) Visualization of the cut phenotype by DAPI staining of nuclei. Panels 1 and 2, cdc20N-term+C-term Δchk1 cells at 25 and 36°C, respectively. Panels 3 and 4, cdc20tsN-term+C-term Δchk1 cells at 25 and 36°C, respectively.
DISCUSSION
The precise role of Pol ɛ in eukaryotic DNA replication remains to be resolved. Recently, the N-terminal half of Pol2p, which contains all the conserved catalytic domains of Pol ɛ, has been shown to be dispensable for cell viability in S. cerevisiae (12, 22). This suggests that the essential function of Pol ɛ does not rely on its ability to synthesize DNA. Consistent with this observation, we demonstrate that fission yeast cells with the N-terminal half of Pol ɛ deleted are also viable. The fact that these two organisms are evolutionarily distant suggests that this is a conserved feature of Pol ɛ in all eukaryotic cells.
However, in contrast to S. cerevisiae, S. pombe cells lacking the N-terminal domains are sensitive to DNA-damaging agents, have a cell cycle delay, and require the DNA damage checkpoint to maintain cell viability. These results suggest that the N terminus, although dispensable for cell viability, normally participates in both DNA replication and repair. Expression of the N-terminal half of Pol ɛ in trans in cdc20ΔN-term cells rescues the DNA damage sensitivity and alleviates the dependency on the DNA damage checkpoint control, suggesting that the N terminus of Pol ɛ is active when expressed independently of the C-terminal half of the enzyme.
Analysis of cell cycle progression in cdc20ΔN-term cells demonstrates that S phase is delayed at least 30 min at 25°C. Furthermore, cell viability is dependent on expression of the checkpoint genes rad3, hus1, and chk1, suggesting that the cell cycle is also delayed in G2 in response to DNA damage. We have considered two possible models to explain the checkpoint dependency of these cells. In the first model, we propose that in the absence of the N terminus of Pol ɛ, DNA synthesis occurs inefficiently, as suggested by the slower S phase, and is error prone. Under these conditions, accumulation of excess DNA damage leads to activation of the checkpoint control. DNA damage might result from inefficient chain elongation and subsequent DNA strand breaks or from nucleotide misincorporations due to inefficient proofreading. In this model, the primary function of the N terminus of Pol ɛ is in DNA replication, with a secondary role in DNA repair. In our second model, DNA damage that normally occurs during S phase is inefficiently repaired in cells lacking the N terminus of Pol ɛ. This leads to a checkpoint-dependent cell cycle delay. In this model, the primary role of the N terminus is in DNA repair. Currently, it is difficult to distinguish between these two possibilities.
The precise role of the C terminus of Pol ɛ in DNA replication remains unclear. Based on our results that temperature-sensitive mutants in Pol ɛ show cell cycle arrest early in S phase (14) and that the N-terminal catalytic domains are not essential for DNA synthesis (this study), we propose that Pol ɛ, through its C-terminal domain, is required for assembly of the replicative complex. Interestingly, in S. cerevisiae, Pol ɛ is found associated with ARS elements during initiation of DNA replication (3), consistent with our hypothesis that Pol ɛ functions early in S phase. Moreover, Pol ɛ was found to associate with replication origins prior to Pol α, a striking result considering that Pol α has been generally believed to be the first polymerase that binds to origins during initiation (27, 30). Whether this reflects a requirement for Pol ɛ for the assembly of the Pol α-primase complex to DNA is not yet known. In the absence of the N-terminal domain of Pol ɛ, we predict that the C terminus can interact with other DNA polymerases, such as Pol δ, which can then substitute for the N terminus of Pol ɛ in DNA synthesis. Consistent with this hypothesis, we have found that cdc20ΔN-term is synthetically lethal with cdc6-23, a temperature-sensitive mutant defective in the catalytic subunit of DNA Pol δ (Table 3).
Analysis of the amino acid sequence of the C-terminal half of Pol ɛ has not revealed any obvious protein function(s). The most striking feature consists of a series of zinc finger DNA binding motifs at the extreme end of the protein. Site-specific mutagenesis of some of the key cysteine residues within the zinc finger domains has shown that this region of the protein is essential for cell viability in S. cerevisiae (11, 12). In S. pombe, short C-terminal truncations of Pol ɛ near the zinc finger motifs are lethal (14). Comparison of the C-terminal sequences of Pol ɛ from different organisms reveals substantial sequence similarity (>30% identity); however, this is significantly less than what is observed within the N-terminal catalytic domain (>60% identity). This suggests that the polymerase function of Pol ɛ is much less tolerant of genetic change than is the C-terminal domain, which has clearly undergone considerable species-dependent modifications throughout evolution. It has been reported that S. pombe Pol ɛ is unable to complement mutations in POL2 in S. cerevisiae (40). This is likely to reflect differences within the C-terminal domain. It will be interesting to determine if the N terminus of S. cerevisiae or human Pol ɛ can complement the defects in the S. pombe cdc20ΔN-term strain. We suspect that the genetic diversity observed within the C terminus reflects species-specific protein-protein interactions that are critical during the early stages of initiation of DNA replication.
Our studies clearly demonstrate that the role of Pol ɛ in eukaryotic DNA replication is more complex than previously anticipated. Along with its polymerase and exonuclease activities, Pol ɛ has an additional essential function(s) that resides in the C-terminal half of the enzyme. Future experiments will be focused on providing a better understanding of the structure and function of Pol ɛ, particularly within the C-terminal domain, and how this important enzyme interacts with other components of the replication machinery.
ACKNOWLEDGMENTS
We thank Kathleen Downey and Antero So for critically reviewing the manuscript. We are also grateful to Tony Carr and Tamar Enoch for providing S. pombe strains.
W. Feng is supported by a predoctoral research fellowship from the American Heart Association. G. D'Urso is supported by research project grant RPG-00-262-01-GMC from the American Cancer Society.
REFERENCES
- 1.al-Khodairy F, Fotou E, Sheldrick K S, Griffiths D J, Lehmann A R, Carr A M. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.al-Khodairy F, Carr A M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aparicio O M, Weinstein D M, Bell S P. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 4.Araki H, Hamatake R K, Johnston L H, Sugino A. DPB2, the gene encoding DNA polymerase II subunit B, is required for chromosome replication in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:4601–4605. doi: 10.1073/pnas.88.11.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki H, Hamatake R K, Morrison A, Johnson A L, Johnston L H, Sugino A. Cloning DPB3, the gene encoding the third subunit of DNA polymerase II of Saccharomyces cerevisiae. Nucleic Acids Res. 1991;19:4867–4872. doi: 10.1093/nar/19.18.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki H, Leem S H, Phongdara A, Sugino A. Dpb11, which interacts with DNA polymerase II(epsilon) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc Natl Acad Sci USA. 1995;92:11791–11795. doi: 10.1073/pnas.92.25.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araki H, Ropp P A, Johnson A L, Johnston L H, Morrison A, Sugino A. DNA polymerase II, the probable homolog of mammalian DNA polymerase epsilon, replicates chromosomal DNA in the yeast Saccharomyces cerevisiae. EMBO J. 1992;11:733–740. doi: 10.1002/j.1460-2075.1992.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddy M N, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- 9.Budd M E, Campbell J L. DNA polymerases delta and epsilon are required for chromosomal replication in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:496–505. doi: 10.1128/mcb.13.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caspari T, Carr A M. DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie. 1999;81:173–181. doi: 10.1016/s0300-9084(99)80050-9. [DOI] [PubMed] [Google Scholar]
- 11.Dua R, Levy D L, Campbell J L. Role of the putative zinc finger domain of Saccharomyces cerevisiae DNA polymerase ɛ in DNA replication and the S/M checkpoint pathway. J Biol Chem. 1998;273:30046–30055. doi: 10.1074/jbc.273.45.30046. [DOI] [PubMed] [Google Scholar]
- 12.Dua R, Levy D L, Campbell J L. Analysis of the essential functions of the C-terminal protein/protein interaction domain of Saccharomyces cerevisiae pol epsilon and its unexpected ability to support growth in the absence of the DNA polymerase domain. J Biol Chem. 1999;274:22283–22288. doi: 10.1074/jbc.274.32.22283. [DOI] [PubMed] [Google Scholar]
- 13.D'Urso G, Nurse P. Checkpoints in the cell cycle of fission yeast. Curr Opin Genet Dev. 1995;5:12–16. doi: 10.1016/s0959-437x(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 14.D'Urso G, Nurse P. Schizosaccharomyces pombe cdc20+ encodes DNA polymerase epsilon and is required for chromosomal replication but not for the S phase checkpoint. Proc Natl Acad Sci USA. 1997;94:12491–12496. doi: 10.1073/pnas.94.23.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamatake R K, Hasegawa H, Clark A B, Bebenek K, Kunkel T A, Sugino A. Purification and characterization of DNA polymerase II from the yeast Saccharomyces cerevisiae. Identification of the catalytic core and a possible holoenzyme form of the enzyme. J Biol Chem. 1990;265:4072–4083. [PubMed] [Google Scholar]
- 16.Hartwell L, Weinert T. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 17.Holmes A M, Haber J E. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell. 1999;96:415–424. doi: 10.1016/s0092-8674(00)80554-1. [DOI] [PubMed] [Google Scholar]
- 18.Jessberger R, Podust V, Hubscher U, Berg P. A mammalian protein complex that repairs double-strand breaks and deletions by recombination. J Biol Chem. 1993;268:15070–15079. [PubMed] [Google Scholar]
- 19.Kamimura Y, Masumoto H, Sugino A, Araki H. Sld2, which interacts with Dpb11 in Saccharomyces cerevisiae, is required for chromosomal DNA replication. Mol Cell Biol. 1998;18:6102–6109. doi: 10.1128/mcb.18.10.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keeney J B, Boeke J D. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics. 1994;136:849–856. doi: 10.1093/genetics/136.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesti T, Frantti H, Syvaoja J E. Molecular cloning of the cDNA for the catalytic subunit of human DNA polymerase epsilon. J Biol Chem. 1993;268:10238–10245. [PubMed] [Google Scholar]
- 22.Kesti T, Flick K, Keranen S, Syvaoja J E, Wittenberg C. DNA polymerase epsilon catalytic domains are dispensable for DNA replication, DNA repair, and cell viability. Mol Cell. 1999;3:679–685. doi: 10.1016/s1097-2765(00)80361-5. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Asahara H, Patel V S, Zhou S, Linn S. Purification, cDNA cloning, and gene mapping of the small subunit of human DNA polymerase epsilon. J Biol Chem. 1997;272:32337–32344. doi: 10.1074/jbc.272.51.32337. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Pursell Z F, Linn S. Identification and cloning of two histone fold motif-containing subunits of HeLa DNA polymerase epsilon. J Biol Chem. 2000;275:23247–23552. doi: 10.1074/jbc.M002548200. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay H D, Griffiths D J, Edwards R J, Christensen P U, Murray J M, Osman F, Walworth N, Carr A M. S-phase-specific activation of Cds1 kinase defines a subpathway of checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu V F, Bhaumik D, Wang T S. Mutator phenotype induced by aberrant replication. Mol Cell Biol. 1999;19:1126–1135. doi: 10.1128/mcb.19.2.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masumoto H, Sugino A, Araki H. Dpb11 controls the association between DNA polymerases alpha and epsilon and the autonomously replicating sequence region of budding yeast. Mol Cell Biol. 2000;20:2809–2817. doi: 10.1128/mcb.20.8.2809-2817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 29.McFarlane R J, Carr A M, Price C. Characterisation of the Schizosaccharomyces pombe rad4/cut5 mutant phenotypes: dissection of DNA replication and G2 checkpoint control function. Mol Gen Genet. 1997;255:332–340. doi: 10.1007/s004380050504. [DOI] [PubMed] [Google Scholar]
- 30.Mimura M, Masuda T, Matsui T, Takisawa H. Central role for Cdc45 in establishing an initiation complex of DNA replication in Xenopus egg extracts. Genes Cells. 2000;5:469–452. doi: 10.1046/j.1365-2443.2000.00340.x. [DOI] [PubMed] [Google Scholar]
- 31.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 32.Morrison A, Araki H, Clark A B, Hamatake R K, Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990;62:1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- 33.Murakami H, Nurse P. DNA replication and damage checkpoints and meiotic cell cycle controls in the fission and budding yeasts. Biochem J. 2000;349:1–12. doi: 10.1042/0264-6021:3490001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- 35.Reid R J D, Fiorani P, Sugawara M, Bjornsti M. CDC45 and DPB11 are required for processive DNA replication and resistance to DNA topoisomerase I-mediated DNA damage. Proc Natl Acad Sci USA. 1999;96:11440–11445. doi: 10.1073/pnas.96.20.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhind N, Russell P. The Schizosaccharomyces pombe S-phase checkpoint differentiates between different types of DNA damage. Genetics. 1998;149:1729–1737. doi: 10.1093/genetics/149.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saka Y, Fantes P, Sutani T, McInerny C, Creanor J, Yanagida M. Fission yeast cut5 links nuclear chromatin and M phase regulator in the replication checkpoint control. EMBO J. 1994;13:5319–5329. doi: 10.1002/j.1460-2075.1994.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saka Y, Yanagida M. Fission yeast cut5+, required for S phase onset and M phase restraint, is identical to the radiation-damage repair gene rad4+ Cell. 1993;74:383–393. doi: 10.1016/0092-8674(93)90428-s. [DOI] [PubMed] [Google Scholar]
- 39.Shivji M K, Podust V N, Hübscher U, Wood R D. Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry. 1995;34:5011–5017. doi: 10.1021/bi00015a012. [DOI] [PubMed] [Google Scholar]
- 40.Sugino A, Ohara T, Sebastian J, Nakashima N, Araki H. DNA polymerase epsilon encoded by cdc20+ is required for chromosomal DNA replication in the fission yeast Schizosaccharomyces pombe. Genes Cells. 1998;3:99–110. doi: 10.1046/j.1365-2443.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- 41.Verkade H M, O'Connell M J. Cut5 is a component of the UV-responsive DNA damage checkpoint in fission yeast. Mol Gen Genet. 1998;260:426–433. doi: 10.1007/s004380050913. [DOI] [PubMed] [Google Scholar]
- 42.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Elledge S J. DRC1, DNA replication and checkpoint protein 1, functions with DPB11 to control DNA replication and the S-phase checkpoint in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;90:3824–3829. doi: 10.1073/pnas.96.7.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Wu X, Freidberg E C. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinert T. DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell. 1998;94:555–558. doi: 10.1016/s0092-8674(00)81597-4. [DOI] [PubMed] [Google Scholar]
- 46.Zou L, Stillman B. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science. 1998;280:593–596. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]