Abstract
The coronavirus disease 19 (COVID-19) infection requires major efforts in healthcare systems, due to the high risk of mortality, particularly in subjects with significant comorbidity (≥ 2 pathologies) and polypharmacy (≥ 5 drugs). The treatment of COVID-19 needs a careful evaluation, to reduce the risk of potentially adverse drug reactions. The aim of the study was to examine the use of computerized prescription support in the management and treatment of the COVID-19 infection. We evaluated n.33 patients (51% females) admitted to the west COVID Low-Medium Intensity of Care of Sant’Andrea Hospital during the period March–April 2020 and n.42 subjects (50% females) admitted to the Internal Medicine ward (as control group), by INTERCheck® and Drug-PIN®. The comorbidity (n. pathologies), polypharmacy (n. drugs), and total INTERCheck score in COVID-19 patients and controls were, respectively (mean ± standard deviation): 5.8 ± 3.8, 7.9 ± 4.5, and 9.2 ± 7.1 and 6.8 ± 2.6, 8.0 ± 2.6, and 4.9 ± 3.8 (statistically significant for comorbidity p < 0.01 and INTERCheck score p < 0.01). The correlation between the scores obtained by the INTERCheck and Drug-PIN software was statistically significant, either at admission (p < 0.0000001) or during hospitalization (p < 0.00000001). Both the computerized prescription support systems, INTERCheck® and Drug-PIN®, are useful to better characterize the patients and to ameliorate the drugs prescriptions in COVID-19 infection, with particular attention to the elderly population.
Keywords: INTERCheck, Drug-PIN, Polypharmacy, Comorbidity, COVID-19
Introduction
The coronavirus disease 19 (COVID-19) infection has required major efforts in healthcare systems, mainly to protect elderly subjects at high risk of mortality [1, 2]. The elderly population is particularly vulnerable, due to the presence of comorbidity (two or more pathologies) and polypharmacy (five or more drugs, the most commonly reported definition of polypharmacy) [3, 4]. Recent experimental therapies for the treatment of COVID-19 need a careful evaluation, to reduce the risk of potentially adverse drug reactions (ADRs) [5–7]. In a case–control study conducted in a Brazilian hospital from March to April 2020, the presence of any adverse drugs reaction involved about half group of the hospitalized patients with COVID-19 [8]. Adherence to the local/national/international guidelines and training programs to enhance the skill of healthcare professionals has been suggested, in order to reduce the risk of ADRs [9, 10].
The aim of the study was to evaluate the use of computerized prescription support in the management of the complex comorbidity and polypharmacy in COVID-19 infection.
Materials and Methods
The study was conducted as a case–control observational study, in which the exposed subjects are not randomized (the sample size calculation was not suitable). The inclusion/exclusion criteria for the case/control group were the presence/absence of COVID-19 infection. Thirty-three patients (mean ± standard deviation, m ± SD, age 72 ± 17 years, range 35–97; 51% females) were consecutively admitted to the west COVID Low-Medium Intensity of Care of Sant’Andrea Hospital on March–April 2020, with infection of COVID-19, as an unselected sample. After an informed consent, every subject underwent a complete clinical assessment, including the evaluation of the polypathology (by the means of the Cumulative Illness Rating Scale, with the Severity Index and Comorbidity Index, -SI and -CI) [11], pharmacological history (drug number and dosage), hemodynamic parameters (systolic and diastolic blood pressure, heart rate, electrocardiography with QTc interval measurements), and blood chemistry tests (with estimation of glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation) [12]. According to our local guideline and ethical committee (DS n.48, 23rd of March, 2020; Prot. n. 52 SA_2020, CE 5773_2020) [13, 14], we used the computerized prescription support systems to examine the drug-drug interactions and physiological suitability in polypharmacy [15]. The drug-drug interaction risk was analyzed using the INTERCheck® software and the Drug-PIN® (Personalized Interactions Network) tool. By using the INTERCheck® computerized support, the potential DDIs according to their clinical relevance are divided in contraindicated (class D, drug combinations that should be avoided); major (C, drug combinations that need close monitoring for potentially serious clinical consequences); moderate (B, drug combinations requiring dose adjustment); and minor (A, drug combinations with no known clinical relevance) [16]. The INTERCheck® total score was the sum of all obtained interactions.
The Drug-PIN® software analyzed the whole therapy of each patient after inclusion of available data (current drugs, morbidities, age, gender, habits, laboratory data, and eventually genetic data), calculating the risk score for the current therapy and considering physiological suitability in polypharmacy (like kidney and liver function in general as well as metabolic compatibility of drugs among each other). The Drug-PIN® software permits a fine-tuning of the therapy, selecting drugs in the ranked list of alternative medications to achieve the optimal therapy [17–20, 21].
Forty-two patients (m ± SD age 81 ± 8 years, range 68–101; 50% females), consecutively admitted in the same period to the Internal Medicine ward (without COVID-19 infection), were considered as control group.
The statistical analyses were performed using Primer of Biostatistics (version 7, SE Glantz, 2011). The one-way analysis of variance (ANOVA) was used to compare data (age, comorbidity, CIRS-SI and -CI scores, number of drugs, QTc intervals, CKD-EPI rates, INTERCheck total scores) between the groups (cases and controls). The z-test was used to compare the proportion of the mortality (cases and controls). The association between the two variables (INTERCheck® software and the Drug-PIN®) was assessed using by simple linear regression models. A value p < 0.05 was considered statistically significant.
Results
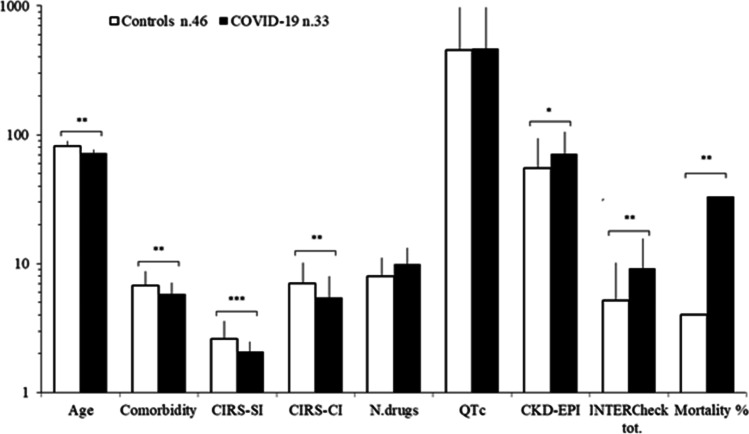
The patients with COVID-19 infection had a high comorbidity (≥ 2 comorbidity in 91% of cases, 5.8 ± 3.8 pathologies per patient) and polypharmacy (≥ 2 drugs in 91% of cases, 7.9 ± 4.5 drugs per patient); 85% of the patients were exposed to at least one potential DDI, and 73% were exposed to at least one potentially severe DDI (mean severe DDI class C + D for patient = 99/33 = 3.0) that increased to 94% during hospitalization and antiviral treatment, as evaluated by INTERCheck. The control group (n.42) presented ≥ 2 comorbidity in 71% of cases (6.8 ± 2.6 pathologies per patient) and ≥ 2 drugs in 100% of cases (8.0 ± 2.6 drugs for patient); 88% of the patients exposed to at least one potential DDI (at least one potentially severe DDI in 64% of cases, mean severe DDI class C + D for patient = 99/33 = 1.9) (Fig. 1). The differences of age, comorbidity, CIRS-SI and -CI, glomerular filtration rate by CKD-EPI, INTERCheck total score, and intra-hospital mortality between controls and patients with COVID infection were statistically significant (Fig. 1) (controls versus COVID patients age p < 0.01, comorbidity p < 0.01, CIRS-SI p < 0.001 and -CI p < 0.01, glomerular filtration rate p < 0.025, INTERCheck total score p < 0.01, and intra-hospital mortality p < 0.01).
Fig. 1.
Clinical features of controls (n.46) and patients with COVID-19 (n.33). CIRS-SI and -CI, Cumulative Illness Rating Scale-Severity and -Comorbidity Index; QTc, corrected QT; CKD-EPI, glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration. *p < 0.025, **p < 0.01, ***p < 0.001
The correlation between the scores obtained by the INTERCheck and Drug-PIN software was statistically significant, either at admission or during hospitalization (Fig. 2) (p < 0.0000001 and p < 0.00000001).
Fig. 2.
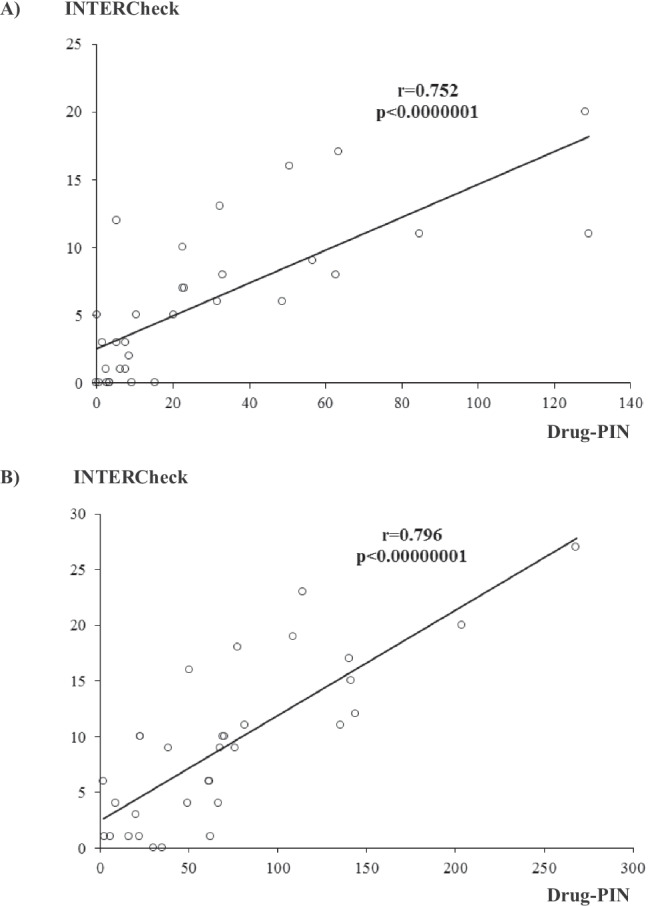
The correlation between the INTERCheck (total score) and Drug-PIN score at admission (A) and during hospitalization (B) in the patients with COVID-19 infection
Discussion
Recent evidence in the literature described the clinical characteristics of COVID-19 hospitalized patients (n.23, m ± SD age 76.1 ± 14.4, 45.5% females) and their related ADRs (evaluated by Drug Interaction Checker and IBM Micromedex®) of the COVID-19 Units of Careggi University Hospital, Florence (Italy), between January and May 2020 [22]. The comorbidity (79% of patients with ≥ 2 comorbidity), the polypharmacy (100% of patients treated with ≥ 2 drugs), and the treatments for COVID-19 were all risk factors for drug-drug interactions (DDIs).
Cattaneo D et al. [23] also presented the clinical features of the patients (n.502, m ± SD age 61 ± 16 years, range 15–99; 33% females) with COVID-19 infection hospitalized between February and April 2020 in the Department of Infectious Diseases of Luigi Sacco Hospital (Milan, Italy). The comorbidity (89% of patients with ≥ 2 comorbidity, mean per patient = 1.7) and the polypharmacy (79% of patients treated with ≥ 2 drugs) were significant. Overall, 68% of the patients with COVID-19 infection were exposed to at least one potential DDI, and 55% were exposed to at least one potentially severe DDI (mean severe DDI class C + D for patient = 1329/399 = 3.3), as revealed by INTERCheck [17, 23]. The proportion of patients experiencing potentially severe DDIs increased to 80% during hospitalization, mainly due to the antiviral treatment.
A high prevalence of ADRs among patients (n.188) with COVID-19 (48.5% versus 28.8% in controls n.66, p = 0.008) was recently described in a Brazilian study [8]. They also evaluated the ADRs and drug-drug interactions by the means of IBM Micromedex ®, Up To Date electronic databases, and the Credible Meds website (http://www.crediblemeds.org, for the potential risk for QT interval prolongation) [8].
In our preliminary study, the control group presented higher mean age and comorbidity (with higher CIRS-SI and -CI) and decreased renal function (with reduced glomerular filtration rate by CKD-EPI equation), as previously described [24]. Besides, the patients with COVID-19 infection presented a higher risk of polypharmacy (with higher total INTERCheck score) and intra-hospital mortality. Both the computerized prescription support systems described the risk associated to the polypharmacy, as demonstrated by the linear regression analysis.
The severity of the COVID-19 infection has been recently reported, describing cardiovascular disorders (like myocarditis, pericarditis, and acute hearth failure), cytokine release syndrome, central sympathetic hyperactivation (with Takotsubo syndrome and atrial fibrillation), acute kidney or liver injury, brain damage (acute confusion or delirium), in addition to the severe pulmonary insufficiency with involvement of the coagulation pathway and superinfections [25–28].
Therefore, the mortality of COVID-19 patients is proportionally higher with increasing age, especially in those with pre-existing comorbidities. Our results about the mortality in COVID-19 infection are consistent with the Chinese Center for Disease Control and Prevention report on a large sample (n.72314 cases) of COVID-19 infection in China, indicated an overall case-fatality rate (CFR) of 2.3%, that increases with age to 14.8% in patients ≥ 80 years, up to 49.0% among critical cases [29]. Preexisting comorbidity (such as cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer) significantly increased mortality.
In a systematic review and meta-analysis, older age was found to be significantly associated with the COVID-19 disease severity, as well as male sex, comorbidity (41% of cases, with RR 1.72 p < 0.001), chronic kidney disease (RR 7.10 p < 0.001), chronic obstructive pulmonary disease, diabetes, cardiovascular/cerebrovascular disease, and smoking [30].
Conclusions
In conclusion, we found a high comorbidity (mean ± standard deviation, 5.8 ± 3.8 associated diseases) and polypharmacy (m ± SD, 7.9 ± 4.5 drugs) that were involved in COVID-19 infection and related to the increased mortality. Both the described computerized prescription support systems, INTERCheck® and Drug-PIN®, were useful to better characterize the patients and to ameliorate the drug prescriptions in COVID-19 infection, with particular attention to the elderly population.
Author Contribution
Conceptualization, A.M., A.R., M.S., R.P., P.M.; methodology, A.M., A.R., C.B.; software, A.M., M.S., R.P.; validation, A.M., M.S., R.P. P.M..; formal analysis, A.M., C.B.; investigation, A.M., G.C., F.F., V.M., A.P., A.P., M.R.M., A.S., V.S., L.T., G.S., P.M.; resources, A.M., M.S., G.S, R.P., P.M.; data curation, A.M., C.B.; writing—original draft preparation, A.M., C.B., R.P., M.P.; writing—review and editing, A.M., C.B., M.S., R.P., P.M.; visualization, A.M., C.B., P.M.; supervision, P.M.
Data availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
The study is according to local guideline and Ethical Committee (DS n.48, 23rd of March, 2020; Prot. n. 52 SA_2020, CE 5773_2020).
Consent to Participate
An informed consent was obtained by every patient of the study.
Consent for Publication
All authors give consent for the publication of this article and associated tables and graphics in SN Comprehensive Clinical Medicine.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman S, Singh K, Dhingra S, Charan J, Sharma P, Islam S, et al. The double burden of the COVID-19 pandemic and polypharmacy on geriatric population – public health implications. Ther Clin Risk Manag. 2020;16:1007–1022. doi: 10.2147/TCRM.S272908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannucci PM, Nobili A, Pasina L, REPOSI Collaborators. (REPOSI is the acronym of REgistroPOliterapie SIMI, SocietàItaliana di MedicinaInterna) Polypharmacy in older people: lessons from 10 years of experience with the REPOSI register. Intern Emerg Med. 2018;13:1191–200. doi: 10.1007/s11739-018-1941-8. [DOI] [PubMed] [Google Scholar]
- 4.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S. Important steps to control COVID-19/SARS-CoV-2 infection. SN Compr Clin Med. 2020:1–2. 10.1007/s42399-020-00271-7. [DOI] [PMC free article] [PubMed]
- 7.Panati K, Narala VR. COVID-19 outbreak: an update on therapeutic options. SN Compr Clin Med 2020:1–2. 10.1007/s42399-020-00264-6. [DOI] [PMC free article] [PubMed]
- 8.Marins TA, Marra AR, Edmond MB, Prystaj Colombo LR, Favalli VS, de Oliveira Xavier F, Gomes Chauvin A, Rebello Pinho JR, de Almeida SM, Souza DM. Adverse drug reactions and drug interactions in the treatment of hospitalized patients with coronavirus disease 2019 (COVID-19) Antimicrob Stewardship Healthc Epidemiol. 2021;1:1–8. doi: 10.1017/ash.2021.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Therapeutics and COVID-19: living guideline. 2021 WHO reference number: WHO/2019-nCoV/therapeutics/2021.1. https://apps.who.int/iris/handle/10665/340374
- 10.Mair A, Fernandez-Llimos F, Alonso A, the SIMPATHY consortium. Polypharmacy management by 2030: a patient safety challenge. Coimbra: SIMPATHY Consortium 2017:1–64. www.simpathy.eu. Accessed 09 21 2021
- 11.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Chen J, Horio M, Imai E, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martocchia A, Spuntarelli V, Aiello F, Meccariello AL, Proietta M, Del Porto F, et al. Using INTERCheck® to evaluate the incidence of adverse events and drug-drug interactions in out- and inpatients exposed to polypharmacy. Drugs Real World Outcomes. 2020;7:243–249. doi: 10.1007/s40801-020-00193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennica A, Conforti G, Falangone F, Martocchia A, Tafaro L, Sentimentale A, et al. Clinical management of adult coronavirus infection disease 2019 (COVID-19) positive in the setting of low and medium intensity of care: a short practical review. SN Compr Clin Med. 2020;29:1–6. doi: 10.1007/s42399-020-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcari L, Luciani M, Cacciotti L, Musumeci MB, Spuntarelli V, Pistella E, et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15:1467–1476. doi: 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Occhipinti M, Brambilla M, Galli G, Manglaviti S, Giammaruco M, Prelaj A, et al. Evaluation of drug-drug interactions in EGFR-mutated non-small-cell lung cancer patients during treatment with tyrosine-kinase inhibitors. J Pers Med. 2021;11:424–438. doi: 10.3390/jpm11050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoniazzi S, Chiarelli MT, Nobili A, Pasina L, Venturini F. The value of software that provides clinically relevant information on drug interactions. Eur J Intern Med. 2015;26:e52–e53. doi: 10.1016/j.ejim.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Preissner S, Kroll K, Dunkel M, Senger C, Goldsobel G, Kuzman D, et al. SuperCYP: a comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res. 2010;38(Database issue):D237–43. doi: 10.1093/nar/gkp970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann MF, Preissner SC, Nickel J, Dunkel M, Preissner R, Preissner S. The Transformer database: biotransformation of xenobiotics. Nucleic Acids Res. 2014;42(Database issue):D1113–7. doi: 10.1093/nar/gkt1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberto M, Rossi A, Panebianco M, Pomes LM, Arrivi G, Ierinò D, et al. Drug–drug interactions and pharmacogenomic evaluation in colorectal cancer patients: the new Drug-PIN® system comprehensive approach. Pharmaceuticals. 2021;14:67. doi: 10.3390/ph14010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preissner S, Dunkel M, Hoffmann MF, Preissner SC, Genov N, Rong WW, et al. Drug cocktail optimization in chemotherapy of cancer. PLoS ONE. 2012;7:e51020. doi: 10.1371/journal.pone.0051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crescioli G, Brilli V, Lanzi C, Burgalassi A, Ieri A, Bonaiuti R, et al. Adverse drug reactions in SARS-CoV-2 hospitalised patients: a case-series with a focus on drug–drug interactions. Intern Emerg Med. 2021;16:697–710. doi: 10.1007/s11739-020-02586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cattaneo D, Pasina L, Maggioni AP, Giacomelli A, Oreni L, Covizzi A, et al. Drug-drug interactions and prescription appropriateness in patients with COVID-19: a retrospective analysis from a reference hospital in Northern Italy. Drugs Aging. 2020;37:925–933. doi: 10.1007/s40266-020-00812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martocchia A, Indiano I, Tafaro L, Frugoni P, Amici A, Cacciafesta M, et al. The evaluation of the presence of comorbidity by the Marigliano-Cacciafesta polypathology scale (MCPS) and the cumulative illness rating scale (CIRS) in elderly subjects with disability. Arch Gerontol Geriat. 2008;49:150–152. doi: 10.1016/j.archger.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Spuntarelli V, Luciani M, Bentivegna E, Marini V, Falangone F, Conforti G, et al. COVID-19: is it just a lung disease? A case-based review. SN Compr Clin Med. 2020:1–6. 10.1007/s42399-020-00418-6. [DOI] [PMC free article] [PubMed]
- 26.Bentivegna E, Luciani M, Spuntarelli V, Speranza ML, Guerritore L, Sentimentale A, et al. Extremely severe case of COVID-19 pneumonia recovered despite bad prognostic indicators: a didactic report. SN Compr Clin Med. 2020:1–4. 10.1007/s42399-020-00383-0. [DOI] [PMC free article] [PubMed]
- 27.Sharma K, Desai HD, Patoliya JV, Jadeja DM, Gadhiya D. Takotsubo syndrome a rare entity in COVID-19: a systemic review-focus on biomarkers, imaging, treatment, and outcome. SN Compr Clin Med. 2021;3:62–72. doi: 10.1007/s42399-021-00743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emmerton D, Abdelhafiz AH. Care for older people with dementia during COVID-19 pandemic. SN Compr Clin Med. 2021;3:437–443. doi: 10.1007/s42399-020-00715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12:12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.