Abstract
Background
Beta blockers prolong life in patients with cardiovascular diseases. Negative chronotropic and inotropic effects carry the potential to adversely effect peripheral skeletal and airway smooth muscle contributing to further fatigue, dyspnea and exercise intolerance.
Research questions
Do beta-blockers reduce maximal power output (MPO), VO2 max, cardiorespiratory responses, increase the perceived effort required to cycle and breath during cardiopulmonary exercise tests (CPET) and limit the capacity to exercise?
Methods
Retrospective observational study of subjects performing CPET to capacity from 1988 to 2012. Subjects with and without beta-blockers were compared: baseline physiological characteristics, MPO, VO2 max, heart rate max, ventilation responses and perceived exertion required to cycle and breathe (modified Borg scale). Forward stepwise linear additive regression was performed with MPO as the dependent factor with height, age, gender, muscle strength, FEV1 and DLCO as independent contributors.
Results
42,771 subjects were included 7,787 were receiving beta-blocker [mean age 61 yrs, BMI 28.40 kg/m2, 9% airflow obstruction (FEV1/FVC<0.7)] and 34,984 were not [mean age 51yrs, BMI 27.40 kg/m2, 11% airflow obstruction]. Heart rate was lower by 18.2% (95% C.I. 18.15–18.38) (p<0.0001) while Oxygen pulse (VO2/HR) was higher by 19.5% (95% C.I. 19.3–19.7) in those receiving beta blockers. Maximum power output (MPO) was 3.3% lower in those taking beta-blockers. The perceived effort required to cycle and breathe (mBorg) was 8% lower in those taking beta-blockers.
Interpretation
Increases in oxygen pulse minimize the reduction in exercise intolerance and symptom handicap associated with beta-blockers.
Keywords: Beta-blocker, Cardio-pulmonary exercise testing, Respiratory physiology
Highlights
-
•
Comprehensive set of exercise physiology measurements in a large cohort, to delineate any hazardous effects of beta blockade.
-
•
Beta-blockers attenuate the heart rate at rest and during exercise.
-
•
Beta-blockade has no meaningful effects on muscle strength, breathing capacity or exercise induced bronchoconstriction.
-
•
Beta Blockers were not associated with a reduction in Maximal power output.
-
•
Increases in Oxygen pulse minimize the reduction in exercise intolerance and symptom handicap associated with beta-blockers.
Abbreviations
- AL
Airflow Limitation
- BB
Beta Blocker
- BMI
Body Mass Index
- BPM
Beats per minute
- BSA
Body Surface Area
- CPET
cardiopulmonary exercise test
- CI
Confidence interval
- DLCO
diffusion capacity for carbon monoxide;
- ECG
electrocardiogram
- EIBc
exercise induced bronchoconstriction
- EIBd
exercise induced bronchodilation
- ETCO2
End tidal CO2
- FEV1
Forced Expired Volume over one Second
- FIF
Forced Inspiratory Flow
- FVC
Forced Vital Capacity
- Hb
Haemoglobin
- HR
Heart rate
- KCO
The carbon monoxide transfer coefficient
- Kpm
kilopond meters
- MI
Myocardial Infarction
- MIP
Maximal Inspiratory Pressure
- MEP
Maximal Expiratory Pressure
- MPO
maximal power output
- PaO2
partial pressure of Oxygen
- PECO2
mixed expired CO2
- PEFR
Peak Expiratory Flow Rate
- PIF
Peak Inspiratory Flow
- RQ
Respiratory exchange ratio
- RR
Respiratory rate
- RV
residual volume
- SaO2
Oxygen Saturation
- SD
standard deviation
- TLC
otal Lung Capacity
- VA
Single breath lung volume
- FVC
Forced vital capacity
- VO2
oxygen consumption
- VCO2
Carbon dioxide production
1. Introduction
Adrenalin, originally extracted from the adrenal medulla and later chemically synthesised, is unusual in that it has both excitatory and inhibitory effects (Bozler, 1940). Demethylated adrenalin is the neurotransmitter of the sympathetic autonomic system and shares the excitatory properties of adrenalin (Dahlstroem and Fuxe, 1964). Adrenalin molecules are found in intracellular vesicles and when released bind to beta-adrenergic receptors. The beta-adrenergic receptor was the first G protein coupled receptor reported. In 1948, Alquist suggested that the excitatory and inhibitory effects of adrenalin is due to attachment to different receptors; alpha and beta receptors (Ahlquist, 1948). Sir James Black began his collaboration with Imperial Chemical Industries (ICI) pharmaceuticals in 1958 which led to the development of propranolol “to find a way of reducing myocardial demand for oxygen in hearts whose oxygen supply was restricted by arterial disease” (Black, 1976).
Further modifications to the molecule led to selective beta blockers without the bronchoconstriction induced by propranolol in asthmatics(Chen et al., 2001; Sheppard et al., 1986; Yamakage et al., 2009) Selective beta blockers are used for their anti-arrhythmic properties, to reduce the workload on the heart (Waagstein et al., 2003), reduce blood pressure, inhibit the renin angiotensin system (Holmer et al., 1998). Beta blockers prolong life in patients with cardiovascular diseases (Yancy et al., 2013). However, the reduction in heart rate together with the potential for a negative inotropic effect on peripheral skeletal and airway smooth muscle may contribute to fatigue, dyspnea and exercise intolerance and may be disabling in some (Lynch and Ryall, 2008).
The generation of sustained power during exercise is tightly coupled to aerobically generated ATP requiring oxygen consumption (VO2): Power ≈ VO2 = HR x stroke volume (SV) x arterio-venous oxygen (a-vO2) difference. Thus, to preserve the capacity to sustain power, any reduction in heart rate must be mathematically accommodated by increasing the product of SV x a-vO2 difference, commonly known as the oxygen pulse. The oxygen pulse (VO2/HR) = stroke volume x a-vO2 difference and is easily measured using CPET. With beta-blockade, failure of the compensatory increase in oxygen pulse would potentially reduce the capacity for oxygen transport to the muscles and limits the capacity to exercise by inducing fatigue in either the respiratory or limb muscles.
When the limb or respiratory muscles fatigue, the central motor (efferent) output command must increase to support a given level of power production or ventilation, respectively. It is this (compensatory) increase in central motor drive (and the attendant central corollary discharge) that is perceived as an increase in limb effort or respiratory effort. These symptoms are critically important to patients and data to demonstrate the effects of beta-blockage on symptoms during exercise are lacking.
Therefore, the aim of this study was to identify substantial and meaningful consequences of beta-blockade on cardio-respiratory physiology and symptoms. he objective of this study was to investigate differences in muscle strength, spirometry, gas transfer capacity, VO2 max, oxygen consumption, carbon dioxide (CO2) production, heart rate (HR), ventilation, blood pressure (BP) and symptoms measured at rest and during increasing increments of power to maximum power output on a cycle ergometer in those prescribed beta blockers compared to those without. The hypothesis that beta blockade is associated with reduced heart rate- and therefore reduced VO2 max and maximal power output was examined.
2. Methods
2.1. Study design and subjects
The study was retrospective based on data collected from sequential patients referred for clinical exercise testing prescribed or not prescribed beta blocking drugs at McMaster University Medical Center from 1988 to 2012. The indication for exercise testing was predominantly for the assessment of exercise induced chest pain, dyspnea, and fatigue. No subject was excluded. This study was approved by the Hamilton integrated research ethics board (13188-C).
2.2. Study procedures
Clinical exercise testing done in McMaster University Medical center includes a full screening pulmonary function testing, skeletal muscle strength assessment and capillary blood gas, regardless of indication. After the risks of exercise were explained, informed consent for exercise testing was obtained. Before exercise, muscle strength was measured using maximum volitional contraction of the inspiratory and expiratory muscles against an occluded airway at residual volume (RV) and total lung capacity (TLC), maximal inspiratory pressure, maximal expiratory pressure (MIP & MEP), seated bench press and row, knee extension (quadriceps) and flexion (hamstrings) using maximum contraction against hydraulic resistance with quasi-isokinetic characteristics. Spirometry was measured with a maximum inspiratory and expiratory maneuver from RV to TLC yielding forced vital capacity (FVC) and forced expired volume over 1 s (FEV1), peak expiratory flow rates (PEFR), forced expired volume at 25, 50 and 75% of the expired vital capacity. The peak inspirator flow rate and FIF 25, 50 and 75% were also measured. Single breath lung volume (communicating lung volume), diffusion capacity for carbon monoxide (DLCO) and KCO were measured. Haemoglobin Hb, Hb Co SaO2, and arterialized capillary blood gases were also measured. The extent of arterialization was assessed by comparing the ScO2 with the SaO2 measured using pulse oximetry.
CPET was conducted on a servo-controlled cycle ergometer in accordance with institutional guidelines; the workload is independent of cycling frequency from 45 to 95 rpm. When the rpm drops braking increases and when the rpm increases breaking decreases in a compensatory fashion. These properties and the work loads were validated using torsion balance (Jones and Kane, 1979).
The stepwise increase in power output was 100 kpm/min (16 Watts) every minute until symptom limited capacity. At each workload, the effort required to breath and cycle was measured on a mBorg scale. During exercise, oxygen uptake, carbon dioxide output, respiratory exchange ratio (RQ), end tidal O2, CO2 mixed expired O2 and CO2, ventilation, tidal volume, respiratory frequency, heart rate, blood pressure, and electrocardiogram were monitored. All patients wore a mouthpiece and nose clip throughout exercise to ensure accurate measurement of the metabolic demand. After exercise, ECG monitoring continued for 10 min and spirometry was repeated 10 min post exercise. Maximum Power Output was defined by the maximum power achieved and sustained for >30 s. The physiological data was averaged over the total period of each increment of work; the VO2 was measured as single breaths with variability, and this was averaged over the final 30 s of each increment. Exercise induced bronchoconstriction and exercise induced bronchodilation were identified. The prevalence of exercise induced bronchoconstriction (FEV1 > 10% drop) in those with and without beta-blockade was also recorded.
2.3. Statistical analyses
Subjects with and without beta-blockers were compared. Demographic data are shown as mean and standard deviation (SD). Differences were calculated based on univariate ANOVA. These were only considered relevant when the differences were substantial with a p<0.0001.
During exercise, the physiological and perceptual responses were dependent on the power and the maximum power output (MPO) achieved by the subject. Multivariate linear additive, non-linear interactive regression, and both combined were used dependent on the pattern of responses. All models included 3 independent contributors, power, the maximum power output achieved by each subject and taking a beta-blocker (Yes/No). All statistical analyses were conducted on the complete data set with MPO from 0 to 2400 kpm/min and powers from 0 to 2400 kpm/min. The values shown in the figures were confined to MPO from 400 to 1200 kpm/min and power outputs from 0 to 1200 kpm/min. This was done for clarity.
The model equations used are shown with the respective figures with mean95% confidence intervals together with the derived pearson r value for each equation.(Statistica version 13.2).
In order to determine the contributors to maximal power output with and without beta-blockade, forward stepwise linear additive regression was performed with MPO as the dependent factor with height, age, gender, muscle strength, FEV1 and DLCO as independent contributors.
3. Results
3.1. Study population
A total of 42,771 subjects (Age range 7–92) performed cycle ergometry to symptom limitation from 1988 to 2012. Of these, 7,787 (18%) were receiving beta blockers and 34,984 were not. Subjects on beta blockers were predominantly male (74% vs. 54%), were older (61 yrs. vs. 51), with similar BMI (28.4 vs 27.4). The proportion of subjects with a previous MI was 56% (54.2–57.0) vs. 10% (9.4–10.2%) (Odds ratio 11.5 (10.7–12.4)) in the subjects taking beta-blockers. The proportion of subjects with airflow obstruction (FEV1/FVC< 0.7) was lower in those taking beta-blockers [11.4%) vs. 8.7% Odds ratio 0.74 (0.7–0.8)]. There were no clinically meaningful differences in muscle strength (Table 1).
Table 1.
Demographics and Baseline Muscle Strength. Mean, Standard Deviation and total numbers shown. P-value calculated using ANOVA. BSA: body surface area; BMI: Body mass index; MI Myocardial infarction; MIP Maximal inspiratory pressure; MEP maximal expiratory pressure.
| VARIABLE | Not on Beta Blocker |
On Beta-Blocker |
p-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean/N | S.D./% | N | Mean/N | S.D./% | N | ||||
| Age (years) | 50.7 | 17.8 | 34,984 | 60.6 | 12.1 | 7,787 | <0.001 | ||
| %Male | 54% | 74% | |||||||
| Height (m) | 1.68 | 0.10 | 34,984 | 1.70 | 0.09 | 7,787 | <0.0001 | ||
| Weight (kg) | 77.89 | 19.37 | 34,983 | 82.68 | 16.97 | 7,785 | <0.0001 | ||
| BSA m2 | 1.87 | 0.25 | 34,983 | 1.94 | 0.22 | 7,785 | <0.0001 | ||
| BMI (kg/m2) | 27.41 | 5.93 | 34,983 | 28.40 | 4.85 | 7,785 | <0.0001 | ||
| Previous MI (n, %) | 1847 | 10% | 19,132 | 2722 | 56% | 4,898 | |||
| FEV1/FVC <0.7, % | 3840 | 11.4% | 34,563 | 674 | 8.7% | 7,775 | <0.0001 | ||
| BASELINE MUSCLE STRENGTH | |||||||||
| Quadriceps (kg) | 45.74 | 20.52 | 29,009 | 47.22 | 19.70 | 7,414 | <0.0001 | ||
| Hamstrings (kg) | 24.29 | 11.93 | 10,547 | 26.88 | 12.18 | 2,595 | <0.0001 | ||
| Row (kg) | 43.42 | 18.23 | 29,312 | 46.60 | 18.03 | 7,499 | <0.0001 | ||
| Bench (kg) | 55.54 | 25.85 | 29,305 | 57.66 | 23.76 | 7,497 | <0.0001 | ||
| MIP (cmH20) | 74.77 | 30.83 | 32,430 | 74.50 | 29.71 | 7,781 | 0.4851 | ||
| MEP (cmH20) | 104.50 | 37.72 | 32,414 | 111.44 | 38.02 | 7,779 | <0.0001 | ||
Baseline cardiorespiratory physiological measurements between the groups is described in Table 2. Resting heart rate was 14 bpm lower in the group on beta-blockers (18.27% lower (18.15–18.38 95% CI) (p<0.0001). There were no clinically meaningful differences in FEV1, FVC, DLCO, KCO, Hb, blood pressure, ventilation, end-tidal CO2 or mixed-expired CO2 at rest (Table 2).
Table 2.
Baseline Cardio-Respiratory Physiology. Mean, Standard Deviation and total numbers shown. P-value calculated using ANOVA. FEV1 Forced Expired Volume over one Second; FVC forced vital capacity; DLCO diffusion capacity for carbon monoxide; VA Single breath lung volume; KCO carbon monoxide transfer coefficient; ; HB- Haemoglobin; BP blood pressure; ; PETCO2 end tidal carbon dioxide; PECO2- mixed expired carbon dioxide.
| VARIABLE |
Not on Beta Blocker |
On Beta-Blocker |
p-value |
||||
|---|---|---|---|---|---|---|---|
| BASELINE CARDIO-RESPIRATORY PHYSIOLOGY | |||||||
| Mean | S.D. | N | Mean | S.D. | N | ||
| FEV1 (L) | 2.76 | 0.91 | 32,925 | 2.70 | 0.78 | 7,784 | <0.0001 |
| FEV1 (%predicted) | 91.55 | 19.98 | 32,925 | 91.05 | 17.40 | 7,784 | 0.0329 |
| FVC (L) | 3.46 | 1.05 | 32,909 | 3.39 | 0.95 | 7,783 | 0.0037 |
| FVC (%predicted) | 102.90 | 20.62 | 32,909 | 103.89 | 19.63 | 7,784 | <0.0001 |
| FEV1/VC | 79.53 | 9.40 | 32,881 | 79.41 | 7.22 | 7,775 | 0.0023 |
| DLCO (ml/mmHg/min) | 22.66 | 6.92 | 30,453 | 21.79 | 6.11 | 7,396 | <0.0001 |
| DLCO % Predicted | 94.20 | 20.90 | 30,453 | 90.64 | 18.63 | 7396 | <0.0001 |
| VA(L) | 5.15 | 1.36 | 30,430 | 5.33 | 1.26 | 7,388 | <0.0001 |
| KCO (ml/mmHg/min/L) | 4.47 | 1.02 | 30,423 | 4.13 | 0.87 | 7,388 | <0.0001 |
| HB (g/dl) | 13.77 | 1.49 | 29,609 | 13.82 | 1.42 | 7,271 | 0.0166 |
| HR | 80.91 | 14.43 | 32,459 | 66.34 | 12.06 | 7,771 | <0.0001 |
| BP Systolic (mmHg) | 129.97 | 20.97 | 32,447 | 131.95 | 20.70 | 7,780 | <0.0001 |
| BP Diastolic (mmHg) | 76.46 | 9.80 | 32,426 | 76.19 | 9.10 | 7,776 | 0.0267 |
| Ventilation at Rest (L) | 12.75 | 3.84 | 32,956 | 12.87 | 3.37 | 7,782 | <0.0001 |
| PETCO2 (mmHg) | 34.25 | 3.79 | 18,813 | 34.34 | 3.53 | 4,894 | 0.5575 |
| PECO2 (mmHg) | 20.12 | 4.49 | 32,685 | 20.32 | 4.68 | 7,724 | 0.0188 |
Exercise induced bronchoconstriction (FEV1 fell >10% compared with pre-exercise) was experienced by 1.7% (95 C.I. 1.4–2.0) in subjects with beta blockers compared to 2.9% (2.7–3.1) in subjects without beta blockers yielding an odds ratio of 0.57 (0.5–0.7 95% CI). There were no differences in the proportion of patients developing exercise induced bronchodilation (FEV1 improved by >10% post-exercise, 6.1% without beta-blockers, 6.4% on beta-blockers).
3.2. Impact on maximum power and heart rate responses
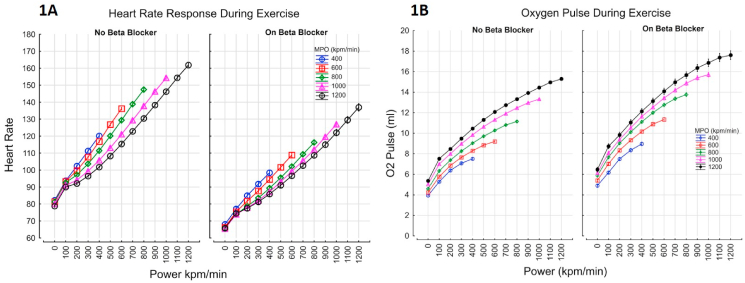
Maximal power output was modestly lower in the subjects taking beta-blocker [751 kpm/min (745–758) vs. 788 (784–791), p<0.0001]. The MPO expressed as a percentage of predicted normal based on the contributions of height, age and sex was 80.9% (95% C.I. 80.7–81.2) in those taking beta-blockers vs 77.6% (95 C.I. 71.1–78.1) (p<0.0001). Maximal heart rate was 19% lower in the subjects taking beta-blocker [116bpm (95 C.I. 115.9–117.0) vs 145bpm (144.6–145.1), p<0.0001, Table 3, and Fig. 1A]. Oxygen pulse (VO2/HR) was greater by 19.5% (19.3–19.7 95% CI) in those taking beta-blocker (Fig. 1B).
Table 3.
Physiological Assessment at Peak Exercise During Incremental Cardio-Pulmonary Exercise Testing. MPO- maximal power output; VO2- oxygen consumption; VCO2- Carbon dioxide production; HR-heart ratel; VE-ventilation; FEV1- Forced Expired Volume over one Second; EIBc – exercise induced bronchoconstriction.
| VARIABLE | Not on Beta Blocker |
On Beta-Blocker |
p-value |
||||
|---|---|---|---|---|---|---|---|
| Mean | S.D./% | N | Mean | S.D./% | N | ||
| CARDIO-RESPIRATORY PHYSIOLOGY AT PEAK EXERCISE | |||||||
| MPO | 787.52 | 349.37 | 32,989 | 751.45 | 298.23 | 7,787 | 0.0572 |
| MPO % predicted | 80.94 | 24.80 | 32,877 | 77.61 | 22.76 | 7,787 | <0.0001 |
| VO2 at Maximum | 1.63 | 0.73 | 32,712 | 1.52 | 0.61 | 7,709 | 0.0001 |
| VO2% predicted at Max | 95.42 | 15.69 | 32,712 | 90.53 | 14.55 | 7,709 | 0.0001 |
| VCO2 at Maximum | 1.77 | 0.82 | 32,712 | 1.67 | 0.71 | 7,734 | 0.1705 |
| RQ at Maximum | 1.07 | 0.12 | 32,712 | 1.07 | 0.12 | 7,734 | 0.0000 |
| HR at Maximum | 144 | 26 | 32,511 | 116 | 23. | 7,785 | <0.0001 |
| VE at Maximum | 57.14 | 23.76 | 32,833 | 54.48 | 21.00 | 7,785 | 0.8808 |
| FEV1 %Post Exercise | 2.77 | 0.91 | 28,185 | 2.73 | 0.80 | 7,171 | 0.0013 |
| % EIBc | 2.91% | 28,185 | 1.67% | 7,171 | <0.0001 | ||
| SYMPTOMS AT PEAK EXERCISE (mBorg Scale) | |||||||
| Dyspnea | 5.04 | 2.64 | 32,987 | 4.64 | 2.51 | 7,787 | 0.0111 |
| Leg Effort | 6.12 | 2.55 | 32,988 | 5.76 | 2.36 | 7,787 | ns |
| Chest Pain | 0.43 | 1.27 | 32,987 | 0.46 | 1.28 | 7,787 | 0.0002 |
Fig. 1.
Heart rate (Fig. 1A) and Oxygen pulse (VO2/HR, Fig. 1B) response during exercise; Fig. 1A: Maximum heart rate was lower by 19% in subjects taking beta blockers, for every maximal power output category, at every given power generated. HR=(93+(0.09*Power))*(1-0.00015*MPO)*(1 + 0.18268*BB) r=0.8168; Fig. 1B: Oxygen Pulse (VO2/HR) increased by 19% in subjects taking beta blockers, for every maximal power output category, at every given power generated. VO2/HR=(3.3+(0.08*Power0.62))*(1 + 0.00047*MPO)*(1 + 0.19*BB) r=0.8443.
3.3. Impact on blood pressure responses
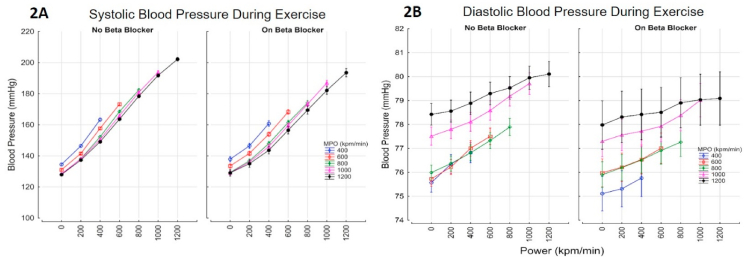
Overall, during the whole period of exercise, beta blockade was associated with a lower systolic [2.88 mmHg lower (95% C.I 2.62–3.14)] and lower diastolic blood pressure [0.35 mmHg (0.24–0.45)] (Fig. 2A–B). The increase in BP with power and MPO for those with and without beta-blockers are shown in Fig. 2A–B.
Fig. 2.
Blood pressure response during exercise; systolic (Fig. 2A) and Diastolic (Fig. 2B) BP increased with power and maximal power output. Beta blockade was associated with a lower systolic and diastolic blood pressure; 2.88 mmHg systolic [2.62–3.14], 0.35 mmHg Diastolic [0.24–0.45]. BP sys = ((139 + 0.06*(Power)) - (0.01*MPO)) - 2.88 *BB r=0.69 SEE 22.1; BP diastolic = ((75 + 0.002*(MPO)) + (0.0017*Power))-0.35*BB r=0.13 SEE 9.6.
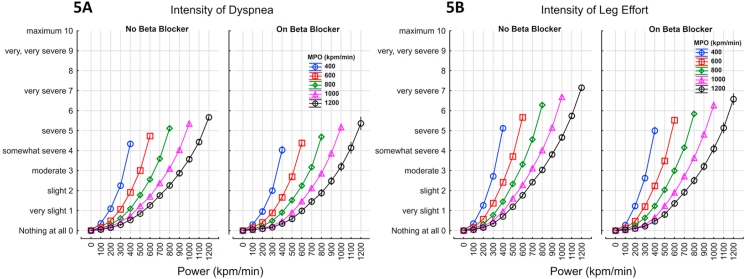
Fig. 5.
The effort required to breath (Fig. 5A) and cycle (Fig. 5B) (modified Borg scale) - the perceived effort was higher as the maximal power output was decreased. Beta blockade was not associated with a significant change in effort; Dyspnea = 0.0004*Power1.45*(1-(0.00046*MPO))*(1-(0.085*BB)) r=0.6919; Leg Effort = 0.0005*Power1.45*(1-(0.00046*MPO))*(1-(0.08*BB)) r=0.7673.
3.4. Impact on ventilation, gas consumption and exchange
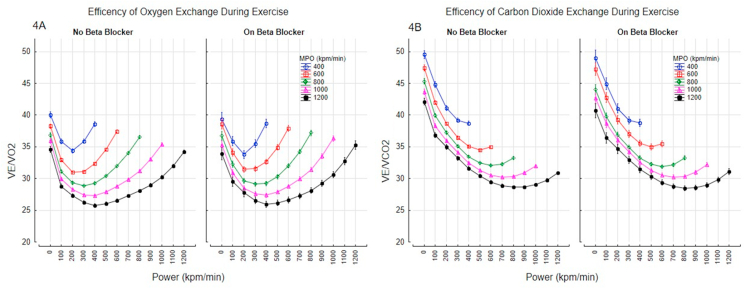
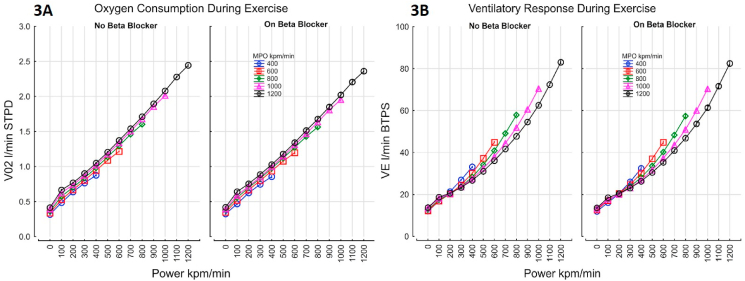
Maximal oxygen uptake at peak exercise was lower in those taking a beta-blocker [1.52 L/min (95% C.I 1.51–1.53) vs. 1.65L/min (1.64–1.65), p=0.0001]. The increase in oxygen consumption with power and MPO for those with and without beta-blockade is shown Fig. 3A. Oxygen uptake over the whole exercise period was lower by 21.2 ml (95%C.I. 19.6–22.9) in those taking beta-blockers at any given power. The higher in ventilation with power for those with and without beta-blocker are shown Fig. 3B. Overall, ventilation was lower by 0.72% (95% CI 0.6–0.9) in those taking beta-blocker (Fig. 3B). In patients taking a beta-blocker there was a was a lower VCO2 output at peak exercise [1.67L/min (1.65–1.68) vs 1.78L/min (1.77–1.79)]. The efficiency of gas exchange improved at low intensity exercise and deteriorated towards maximal exercise, but beta blockers did not impact the efficiency of oxygen exchange (VE/VO2) or carbon dioxide exchange (VE/VCO2) during exercise (Fig. 4A–B).
Fig. 4.
Efficiency of gas exchange with and without beta-blockers; Efficiency of oxygen exchange (Fig. 4A), and carbon dioxide exchange (Fig. 4B). Data points shown as mean and 95% confidence intervals.
Fig. 3.
Increases in oxygen consumption (Fig. 3A) and ventilatory response during exercise (3 B); Figure 3A- Oxygen uptake was lower by 21.2 ml (19.6–22.9 95%C.I.) in those with Beta blockers; VO2 = 0.211 + 0.0016*Power +0.00019*MPO – 0.21* BB r=0.9525 SEE 0.193; Figure 3B- Ventilation was lower by 0.72% (0.56–0.88 95% CI) in those with Beta blocker; VE = (16.8 + 0.0185*Power1.45) * (1+(0.002*MPO)) *(1-(0.0072*BB) r=0.9329.
3.5. Impact on perceived exertion
The perceived effort required to breathe and cycle accelerated with power (kpm/min) and was higher at any given workload as the maximum power output achieved decreased (Fig. 5A and B). The intensity of dyspnea and leg effort at any given power and MPO was decreased in those taking beta-blockers [8.5% (8.0–9.1% 95%CI) for dyspnea and 8.1% (7.6–8.6%) for leg effort].
Overall, 51.4% were limited by the leg effort required to cycle (51.8% on beta-blocker vs 51.32% off); 34.1% were limited equally by the effort required to cycle and breathe (35.2% on beta-blocker vs 33.8% off); 13.3% were limited by the effort required to breath (11.4% on beta-blockers vs 13.8% off). Overall, 1.2% were limited by chest pain alone or in combination with the effort required to breathe or cycle (1.6% on beta-blocker vs 1.2% off).
3.6. Contributors to maximal power output
As patients taking a beta-blocker were 10 years older, and greater proportion being males, a forward stepwise linear regression was adopted to investigate the independent contributions of each of the physiological and anthropomorphic variables along with taking a beta-blocker. Quadriceps strength(kg), FEV1 and the DLCO respectively were the most important contributors to the variability in maximum power output achieved (Table 4). Age, sex, beta-blockade and height had a minimal influence (Std Beta<0.1).
Table 4.
Forward Stepwise multi-variate regression model for Maximum Power Output (MPO). Data shown as standardized beta (Std b), beta (b) and t statistic (t). Gender ns r=0.8286 SEE 187. DLCO- diffusion capacity for carbon monoxide; FEV1- Forced Expired Volume over one Second.
| VARIABLE | Std b | S.E Std b | b | S.E b | t(34,686) | P-value |
|---|---|---|---|---|---|---|
| Intercept | −29.44 | 22.99 | −1.28 | 0.0000 | ||
| Quadriceps Strength (kg) | 0.41 | 0.00 | 6.69 | 0.08 | 88.93 | 0.0000 |
| FEV1 (L) | 0.25 | 0.01 | 94.56 | 1.93 | 48.91 | 0.0000 |
| DLCO (ml/mmHg/min) | 0.21 | 0.00 | 10.38 | 0.24 | 43.79 | 0.0000 |
| Age (years) | −0.10 | 0.00 | −1.90 | 0.07 | −27.07 | 0.0000 |
| Sex | 0.05 | 0.00 | 34.62 | 2.77 | 12.50 | 0.0000 |
| Beta-Blockade | −0.04 | 0.00 | −31.86 | 2.59 | −12.31 | 0.0000 |
| Weight (m) | −0.05 | 0.00 | −0.90 | 0.07 | −12.79 | 0.0000 |
| Height (m) | 0.03 | 0.01 | 108.32 | 16.53 | 6.55 | 0.0000 |
4. Discussion
Over the 60 years since the introduction of beta blocking drugs there have been a very large number of studies, which have anticipated but failed to find meaningful impairment in maximal exercise performance, in different groups e.g. healthy subjects (Mitchell et al., 2019) , athletes (Fikenzer et al., 2020), patients with coronary artery disease (Eynon et al., 2008) and patients with hypertension (Reybrouck et al., 1977). All these and others have shown that oxygen uptake was maintained. This study failed to find any major negative consequences on exercise capacity and symptoms by taking beta-blockers, thus confirming previous findings. The novelty of this study is that the reduction in heart rate was accompanied by an increase in oxygen pulse. This study is unique in the number of subjects, the wide number of variables addressed and the addition of the perceptual responses to exercise in all subjects which are the primary concern to patients.
Physiological measurements were made in over 40,000 consecutive patients undergoing CPET testing over a 25-year period. The aim of the study was to address negative consequences in the performance of muscular exercise due to beta blockers. The heart rate response was 18–19% lower due to the physiological effect of beta-blockers. This was accompanied by a higher oxygen delivery per heartbeat of 19.5% (VO2/HR: Oxygen Pulse). Whether this was due to an increase in the stroke volume and/or arterio-venous oxygen difference is of interest but could not be definitively answered. The maximal arterio-venous oxygen difference is the arterial oxygen content with 70% extraction being a reasonable limiting value. Over half the subjects taking a beta-blocker had a previous myocardial infarct and were 10 years older; impaired ability to generate power would be expected. Despite this, in a multi-variate analysis, there were only very minor differences in the maximum power output between those receiving or not receiving beta-blockers. At any given power output up to the maximum power achieved the oxygen uptake was the same. Impairment in muscle strength, spirometry, gas exchange capacity, exercise induced bronchoconstriction were either absent or of questionable clinical importance.
This adaptive physiological response required an increase in stroke volume and/or arteriovenous oxygen extraction. The average 20% reduction in heart rate could be accommodated by a 10% increase in stroke volume and a 10% increase in arteriovenous oxygen difference. Any combination meeting the 20% decrease in heart rate would suffice. In those generating maximum power outputs exceeding 1000 kpm/min, the proportionate increase in arteriovenous oxygen extraction is likely to exceed the increase in stroke volume as the end diastolic cardiac volume does not change during exercise. In addition, the proportion of the total cardiac output going to the exercising muscle increases with increasing MPO resulting in a higher oxygen extraction.
Previous studies investigating the effects of acute beta-blockade on cardiac output response during exercise have shown conflicting results. Three studies in the 1960's with acute beta-blockade found no significant effect on cardiac output, implying sympathetic stimulation of the heart is not needed for exercise. However, Epstein et al. studied 7 healthy males and 9 patients with heart disease to show that compared with placebo, intravenous propranolol did reduce cardiac output and exercise endurance time (Epstein et al., 1965). In contrast, chronic beta-blockade is a fundamental pillar of treatment of heart failure, improving ejection fraction (Packer et al., 1996), quality of life, and mortality (Packer et al., 1996; The Cardiac Insufficiency, 1999; Effect of metoprolol/X, 1999). But, this has not always translated to improvement in exercise capacity (Wolfel et al., 1997) , despite studies showing no changes in peak HR and VO2 max (Maldonado-Martín et al., 2020; Conraads et al., 2012; Zheng et al., 2018; Metra et al., 2000; Magrì et al., 2012). Chronic metoprolol and carvedilol treatment in patients with heart failure showed increase in 6-min walk distance and increased exercise duration, although maximum power output was not shown (Metra et al., 2000).
There are limitations to this study. First, this was a retrospective study in a single-center. Second, there is a risk of selection bias, as patients who did not tolerate beta-blockers were likely discontinued by physicians and not referred for exercise testing. Third, we do not have data on the different beta-blockers which were prescribed. Fourth, patients were recruited over a period of 25 years, over which there have been improvements in the management of primary and secondary prevention of acute and chronic cardiac disease. Fifth, we do not have reliable information on other co-morbidities which can be accurately verified and hence are unable to perform sub-group analyses with other cardio-respiratory diseases. Sixth, the maximum heart rate increases with the maximum power achieved in both those on and off beta blockers, although there is a blunted response on beta-blockers. As the maximum heart rate contributes to the capacity for oxygen delivery, although there were no substantial group average differences in MPO, this may not be the case at the extremely high work loads in an individual where the oxygen pulse may not be able to compensate with increased stroke volume and/or oxygen extraction. Further research is needed to elucidate the predictors of these limitations at the elite levels of exercise.
The implications of these findings are, that the current practice of using beta-blockade to modulate cardiovascular risk does not negatively impact the symptoms associated with exercise performance or the capacity , even in the presence of cardio-respiratory diseases. Previous studies have described the safety in the presence of airflow limitation (Bhatt et al., 2016; Etminan et al., 2012; Salpeter et al., 2002). Regardless of a diagnostic label, these findings derived from a broad, “real life” cohort with no exclusion criteria, should be interpreted with caution-in an individual, the inability to increase stroke volume may cause respiratory distress and decreased capacity to exercise.
5. Conclusions
Beta-blockers attenuate the heart rate at rest and during exercise. In clinical practice, beta-blockade has no meaningful negative consequences effects on muscle strength, breathing capacity, exercise induced bronchoconstriction or gas transfer capacity. Beta-blockers were not associated with exercise limitation, likely due to a combination of improvements in stroke volume and/or arterio-venous oxygen difference. If this adaptive mechanism is impaired within an individual, a reduction in heart rate will likely result in exercise limitation.
Author contributions
EP: All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript, MW: All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript, TP: All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript, AF: All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript, PMO: All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript, KJK: takes responsibility for the content of the manuscript, including the data and analysis. All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript, IS: All authors conceptualized and designed the study. All authors had full access to all the data, contributed data analysis, interpretation and writing of the manuscript.
Author disclosure
EP, MW, TM, AF, KJK have no disclosures to report. IS reports grants from ERS Respire 3 Marie Curie Fellowship, grants and personal fees from Merck Canada, personal fees from GSK, AstraZeneca, Genentech outside the submitted work; POB reports grants and personal fees from AstraZeneca, personal fees from GSK, grants from Novartis, grants and personal fees from Medimmune, personal fees from Chiesi, outside the submitted work.
Funding support
I.S. is supported by the E.J. Moran Campbell Early Career Award, Department of Medicine, McMaster University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Guarantor: KJK takes responsibility for the content of the manuscript, including the data and analyses.
References
- Ahlquist R.P. A study of the adrenotropic receptors. Am. J. Physiol. 1948;153(3):586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Bhatt S.P., Wells J.M., Kinney G.L., et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71(1):8–14. doi: 10.1136/thoraxjnl-2015-207251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J.W. Ahlquist and the development of beta-adrenoceptor antagonists. Postgrad. Med. 1976;52(Suppl. 4):11–13. [PubMed] [Google Scholar]
- Bozler E. An analysis of the excitatory and inhibitory effects of sympathetic nerve impulses and adrenaline on visceral smooth muscle. American Journal of Physiology-Legacy Content. 1940;130(4):627–634. doi: 10.1152/ajplegacy.1940.130.4.627. [DOI] [Google Scholar]
- Chen J., Radford M.J., Wang Y., Marciniak T.A., Krumholz H.M. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J. Am. Coll. Cardiol. 2001;37(7):1950–1956. doi: 10.1016/s0735-1097(01)01225-6. [DOI] [PubMed] [Google Scholar]
- Conraads V.M., Metra M., Kamp O., et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur. J. Heart Fail. 2012;14(2):219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- Dahlstroem A., Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. i. demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 1964;(Suppl. 232):1–55. [PubMed] [Google Scholar]
- Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. doi: 10.1016/S0140-6736(99)04440-2. [DOI] [PubMed] [Google Scholar]
- Epstein S., Robinson B.F., Kahler R.L., Braunwald E. Effects of beta-adrenergic blockade on the cardiac response to maximal and submaximal exercise in man. J. Clin. Invest. 1965;44(11):1745–1753. doi: 10.1172/JCI105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan M., Jafari S., Carleton B., FitzGerald J.M. Beta-blocker use and COPD mortality: a systematic review and meta-analysis. BMC Pulm. Med. 2012;12:48. doi: 10.1186/1471-2466-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eynon N., Sagiv M., Amir O., Ben-Sira D., Goldhammer E., Amir R. The effect of long-term beta-adrenergic receptor blockade on the oxygen delivery and extraction relationship in patients with coronary artery disease. J Cardiopulm Rehabil Prev. 2008;28(3):189–194. doi: 10.1097/01.HCR.0000320070.81470.75. [DOI] [PubMed] [Google Scholar]
- Fikenzer S., Fikenzer K., Laufs U., Falz R., Schulze A., Busse M. Effects of cardioselective beta-blockade on plasma catecholamines and performance during different forms of exercise. J. Sports Med. Phys. Fit. 2020;60(4):643–649. doi: 10.23736/S0022-4707.19.10225-3. [DOI] [PubMed] [Google Scholar]
- Holmer S.R., Hense H.W., Danser A.H., Mayer B., Riegger G.A., Schunkert H. Beta adrenergic blockers lower renin in patients treated with ACE inhibitors and diuretics. Heart. 1998;80(1):45–48. doi: 10.1136/hrt.80.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N.L., Kane J.W. Quality control of exercise test measurements. Med. Sci. Sports. 1979;11(4):368–372. [PubMed] [Google Scholar]
- Lynch G.S., Ryall J.G. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev. 2008;88(2):729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- Magrì D., Palermo P., Cauti F.M., et al. Chronotropic incompentence and functional capacity in chronic heart failure: no role of β-blockers and β-blocker dose. Cardiovasc Ther. 2012;30(2):100–108. doi: 10.1111/j.1755-5922.2010.00184.x. [DOI] [PubMed] [Google Scholar]
- Maldonado-Martín S., Brubaker P.H., Ozemek C., Jayo-Montoya J.A., Becton J.T., Kitzman D.W. Impact of β-blockers on heart rate and oxygen uptake during exercise and recovery in older patients with heart failure with preserved ejection fraction. J Cardiopulm Rehabil Prev. 2020;40(3):174–177. doi: 10.1097/HCR.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metra M., Giubbini R., Nodari S., Boldi E., Modena M.G., Dei Cas L. Differential effects of beta-blockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102(5):546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- Mitchell B.L., Davison K., Parfitt G., Spedding S., Eston R.G. Physiological and perceived exertion responses during exercise: effect of β-blockade. Med. Sci. Sports Exerc. 2019;51(4):782–791. doi: 10.1249/MSS.0000000000001845. [DOI] [PubMed] [Google Scholar]
- Packer M., Bristow M.R., Cohn J.N., et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Reybrouck T., Amery A., Billiet L. Hemodynamic response to graded exercise after chronic beta-adrenergic blockade. J. Appl. Physiol. 1977;42(2):133–138. doi: 10.1152/jappl.1977.42.2.133. [DOI] [PubMed] [Google Scholar]
- Salpeter S.S., Ormiston T., Salpeter E., Poole P., Cates C. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2002;2:CD003566. doi: 10.1002/14651858.CD003566. [DOI] [PubMed] [Google Scholar]
- Sheppard D., DiStefano S., Byrd R.C., et al. Effects of esmolol on airway function in patients with asthma. J. Clin. Pharmacol. 1986;26(3):169–174. doi: 10.1002/j.1552-4604.1986.tb02929.x. [DOI] [PubMed] [Google Scholar]
- The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. doi: 10.1016/S0140-6736(98)11181-9. [DOI] [PubMed] [Google Scholar]
- Waagstein F., Strömblad O., Andersson B., et al. Increased exercise ejection fraction and reversed remodeling after long-term treatment with metoprolol in congestive heart failure: a randomized, stratified, double-blind, placebo-controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. Eur. J. Heart Fail. 2003;5(5):679–691. doi: 10.1016/s1388-9842(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Wolfel E.E., Bristow M.R. In: Exercise and Heart Failure. American Heart Association Monograph Series. Balady G.J., Pina I.L., editors. Futura Publishing Company, Inc.; New York: 1997. Effects of long-term beta adrenergic blockade on exercise capacity in patients with chronic heart failure; pp. 141–170. [Google Scholar]
- Yamakage M., Iwasaki S., Jeong S.-W., Satoh J.-I., Namiki A. Beta-1 selective adrenergic antagonist landiolol and esmolol can be safely used in patients with airway hyperreactivity. Heart Lung. 2009;38(1):48–55. doi: 10.1016/j.hrtlng.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Yancy C.W., Jessup M., Bozkurt B., et al. ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. 2013. [DOI] [PubMed] [Google Scholar]
- Zheng S.L., Chan F.T., Nabeebaccus A.A., et al. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart. 2018;104(5):407–415. doi: 10.1136/heartjnl-2017-311652. [DOI] [PMC free article] [PubMed] [Google Scholar]