Abstract
Objective:
The objectives of this study were (1) to describe the current use of etomidate and other induction agents in patients with sepsis and (2) to compare adverse events between etomidate and ketamine in sepsis.
Methods:
Observational cohort study of the prospective National Emergency Airway Registry (NEAR) data set. Descriptive statistics were used to report the distribution of induction agents used in patients with sepsis. Adverse events were compared using bivariate analysis, and a sensitivity analysis was conducted using a propensity score-adjusted analysis of etomidate vs. ketamine.
Results:
A total of 531 patients were intubated for sepsis, and the majority (71%) were intubated with etomidate as the initial induction agent. Etomidate was less frequently used in sepsis patients than non-sepsis patients (71 vs. 85%, OR 0.4, 95%CI 0.4–0.5). Sepsis patients had a greater risk of adverse events, and vasopressor therapy was required for 25% of patients after intubation. Post-procedure hypotension was higher between those intubated for sepsis with ketamine vs. etomidate (74 vs. 50%, OR 2.9, 95%CI 1.9–4.5). After accounting for confounding by indication in the propensity score-adjusted analysis, ketamine was associated with more post-procedure hypotension (OR=2.7, 95%CI 1.1–6.7). No difference in ED deaths were observed.
Conclusions:
Etomidate is used less frequently in sepsis patients than non-sepsis patients, with ketamine being the most frequently used alternative. Ketamine was associated with more post-procedural hypotension than etomidate. Future clinical trials are needed to determine the optimal induction agent in patients with sepsis.
Keywords: sepsis, intubation, endotracheal, ketamine, etomidate
Introduction:
Etomidate is commonly used as an induction agent for rapid sequence induction (RSI) in critically ill patients in the emergency department (ED) because of its reliable sedation effect and stable hemodynamic profile.1 Etomidate also inhibits adrenal steroidogenesis by its transient inhibition of 11 β-hydroxylase2–4, which has raised safety concerns in patients with sepsis.3, 5–8 Based on cohort studies and post-hoc analyses, etomidate use for patients with sepsis was challenged, 3, 9–16 but subsequent studies have suggested minimal impact on long-term outcomes.17 Some providers, however, continued to avoid etomidate in sepsis patients.
Ketamine is an excellent alternative induction agent in sepsis because of its stable hemodynamic profile and its lack of adrenal suppression.1, 9, 18–22 Recent studies, however, have suggested that ketamine may be associated with a significant risk of hypotension, especially in patients with catecholamine depletion.13, 23, 24 Randomized and observational studies comparing etomidate vs. ketamine have shown mixed results in short-term adverse events such as hypotension.21, 25
With an estimated 1.7 million sepsis cases in the U.S. per year, and 26% of those cases requiring endotracheal intubation, optimizing intubation safety is important to improve sepsis outcomes.26 Prior work has focused on the relationship between induction agent selection and long-term survival, but there has been little focus on how these data have influenced induction agent selection or ED complications from endotracheal intubation in this population. The objective of this study is to describe the induction agents used for sepsis patients requiring intubation in the ED, and to compare peri-intubation adverse events (e.g., hypotension, first-pass success) between etomidate and ketamine.
Methods:
Study Design, Setting, and Population
This study is a multicenter observational cohort study of sepsis patients in the National Emergency Airway Registry (NEAR), a prospective ED-based airway registry of consecutive ED intubations in an international network of 25 academic hospitals between January 1, 2016 through December 31, 2017. Sepsis was defined by the intubating clinician using clinical criteria. We compare patients intubated using etomidate as an induction agent to those intubated using other agents.
We expected that most sepsis cases being intubated without etomidate would use ketamine, and we planned an a priori etomidate vs. ketamine propensity score-adjusted analysis to measure the relationship between the induction agent and short-term adverse events. This study was conducted with approval of the local institutional review boards for all participating centers for registry data collection under waiver of informed consent and is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.27
Measurements
The primary intubating clinician entered data about the procedure and ED outcomes into a structured secure, web-based data collection tool (StudyTRAX; version 3.47.0011; ScienceTRAX, Macon, GA). Study investigators reviewed all data in aggregate, using quality assurance algorithms to identify and validate data entry elements. Each participating center had a designated site investigator to track local intubations and ensure complete data entry. All sites had a run-in period to demonstrate reporting of greater than 90% of total ED intubations before sites were included in the registry. The coordinating center monitored data integrity and ensured that a registry-wide average of greater than 90% of intubations were maintained at all participating sites.
Data included patient demographics, peri-intubation vital signs, medications used, route of intubation, training of intubating clinician, disposition, and peri-intubation outcomes. Data were collected for each intubation attempt separately. Outcomes included intubation success, peri-intubation adverse events, desaturation, lowest oxygen saturation, post-intubation hypotension, lowest systolic blood pressure (SBP), treatment for hypotension, and whether the patient received vasopressors. Methods for NEAR data collection, quality control, and analysis have been previously described.28, 29
Outcomes
Induction Agent Selection (Primary Outcome).
The primary outcome was induction agent choice for sepsis intubations, and induction agent selection was compared between sepsis and non-sepsis control patients. Induction agents included etomidate, ketamine, midazolam, propofol, and no medications.
Adverse Events (Secondary Outcome).
Our secondary analysis was conducted only among sepsis intubations. Secondary outcomes included first-pass intubation success, overall intubation success, and occurrence of adverse events. Adverse events included cardiac arrest (during or immediately after intubation), dental trauma, direct airway injury, dysrhythmias, epistaxis, esophageal intubation, post-intubation hypotension (SBP<100 mm Hg), treatment required for hypotension, use of a vasopressor medication, desaturation (oxygen saturation <90%), iatrogenic bleeding, equipment failure, laryngospasm, lip laceration, bronchial intubation, medication error, pharyngeal laceration, pneumothorax, endotracheal tube cuff failure, and vomiting.29
Analysis
Patient demographics, baseline characteristics, and outcomes were compared after stratifying all patients according to sepsis status and clustering on hospital. Comparisons were made with Student’s t-test, Wilcoxon rank-sum, Chi-squared test, and Fisher’s exact test, as appropriate. When comparing continuous variables, the Mann-Whitney U test and the Hodges Lehman Estimator were used to describe median differences with the 95% CI. The proportion of induction agents selected was compared between the sepsis and non-sepsis cohorts using bivariate analysis.
Adverse events (both individual risks and aggregate) were compared among sepsis patients between etomidate and ketamine induction groups. To account for confounding by indication, we calculated a propensity score using logistic regression with an outcome of whether ketamine was used for induction. The logistic regression model used predictors identified by the authors and prior literature as possibly associated with agent selection. In generating the propensity score, we focused on theory in inclusion, and the score was based on the following predictors: body habitus, need for immediate intubation, baseline oxygen saturation, pre-intubation vasopressors, initial assessment of airway difficulty, and intubating clinician level of training. Using the logistic regression model, the predicted probability of receiving ketamine was calculated for each sepsis case in the data set. Then separate regression models were built using generalized estimating equations (GEE) clustered on hospital, with an outcome of adverse events, and with predictors of drug choice dose and the propensity score to account for factors that might make ketamine use more likely. Odds ratios with 95% CI comparing outcomes of patients receiving etomidate vs. ketamine were then calculated, as described above, and the Hosmer-Lemeshow goodness of fit statistic was used to verify model fit.
Sample Size Estimate
Because the primary objective of this study was to estimate the prevalence of induction agent selection in sepsis patients, our power was determined by the total number of cases in the NEAR registry at the time of the analysis. We determined that with 500 sepsis cases and a previously reported prevalence of etomidate use of 91%28, we would be able to estimate a confidence interval with a width of ±1.5%. All statistical analysis was conducted using SAS v. 9.4 (SAS Institute, Cary, NC). Statistical testing was considered significant if p<0.05 with 2-sided hypothesis testing. As a secondary analysis of an existing dataset, no adjustments for multiple testing were performed and results should be considered hypothesis-generating.
Results:
Characteristics of Study Subjects
Induction Agent Selection.
During the study period, 12,722 intubations were recorded at 25 participating sites of which 531 (4.2%) patients were intubated for sepsis (Figure 1). Sepsis patients were predominantly female (58%), and 36% were obese or morbidly obese. One-quarter of intubations were classified by the intubating clinician as emergent, and 95% were intubated by resident or fellow physicians. Vasopressor therapy was used prior to intubation more frequently for sepsis patients than for non-sepsis patients. (13.9% vs. 1.6%, OR=10.2, 95%CI 7.5–13.8).
Figure 1.
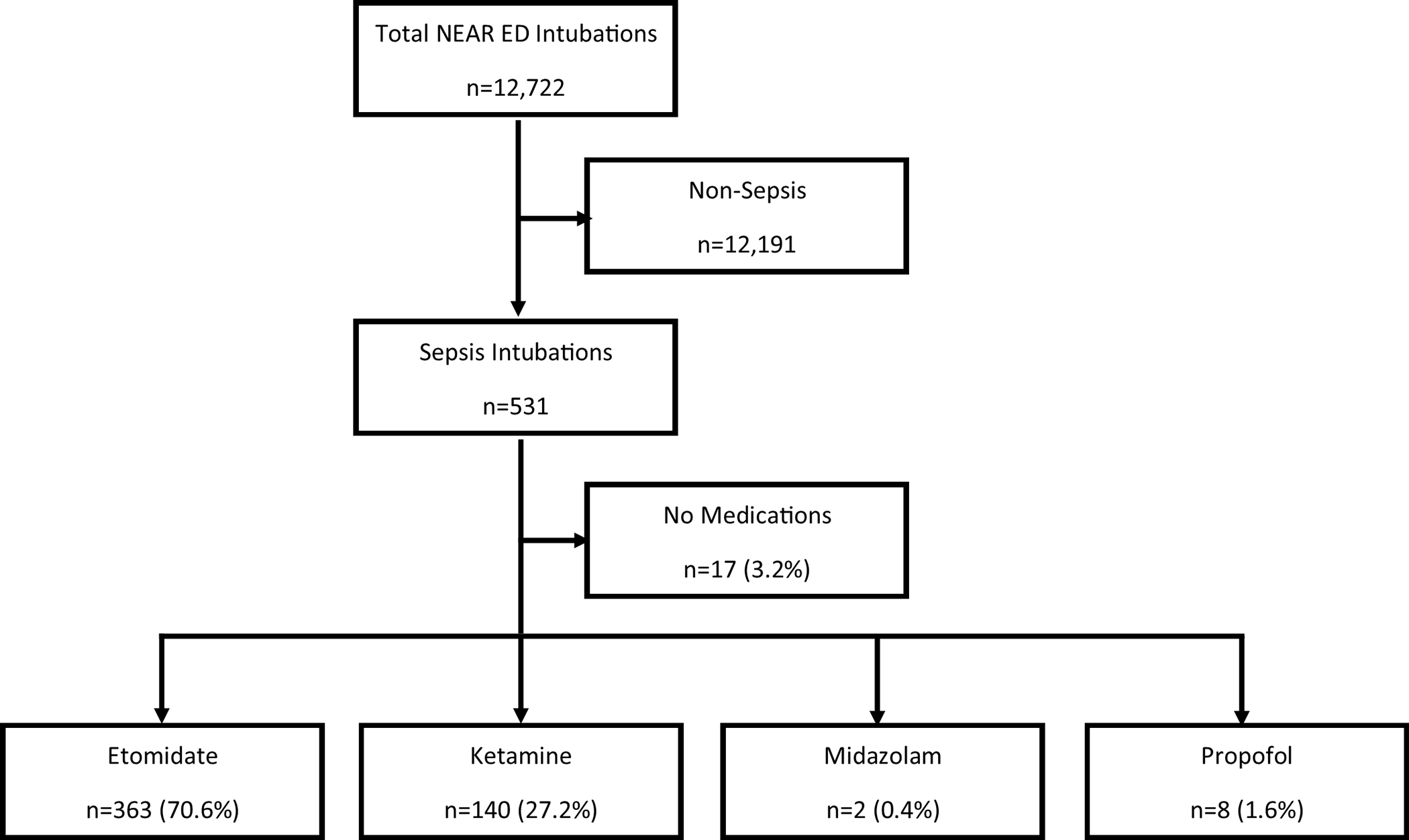
Flow chart of study subjects
Adverse Events.
Within the sepsis cohort (n=531), 122 (23.1%) of patients had adverse events, with the most common adverse event being hypotension (46.7%). In sepsis patients, the median dose of etomidate was 20 mg (IQR 15–20 mg), and the median dose of ketamine was 100 mg (IQR 72.2–150 mg).
Main Results
The most common induction agents used in patients with sepsis were etomidate (n=363, 71%) and ketamine (n=140, 27%), with few patients being intubated with midazolam or propofol. Patients with sepsis were more likely to be undergo rapid sequence intubation as the first method chosen (OR=1.8, 95%CI 1.4–2.4) and were more likely to receive an induction agent than those without sepsis (97% vs. 82%, OR=2.8, 95%CI 1.7–4.6). Etomidate was selected as the initial induction agent less frequently in sepsis cases than in non-sepsis cases (71% vs 85%, OR=0.4, 95%CI 0.4–0.5) and ketamine use was much more prevalent (27% vs.12%, OR=2.8, 95%CI 2.3–3.5). No other important differences between patient groups were observed (Table 1). When sepsis patients were stratified by site, the facility-specific proportion of patients being intubated with ketamine in sepsis varied widely (0% to 92%) (Figure 2).
Table 1.
Baseline characteristics of patients, stratified by sepsis diagnosis
| Factor | All Cases (n=12,722) | Sepsis (n=531) |
Non-Sepsis (n=12191) |
OR (95%CI) or Difference (95%CI) |
|---|---|---|---|---|
| Male, n (%) | 4,272 (33.6) | 224 (42) | 4048 (33) | 1.5 (1.2–1.7) |
| Weight (kg), median (IQR) | 75.2 (65–90) | 75 (60–90) | 77 (70–90) | −2 (−4 – 0) |
| Habitus, n (%) | ||||
| Very Thin | 518 (4.1) | 54 (10) | 464 (4) | 2.9 (2.1–3.8) |
| Thin | 1,958 (15.4) | 97 (19) | 1861 (16) | 1.2 (0.9–1.6) |
| Normal | 6,093 (47.9) | 183 (35) | 5910 (50) | 0.4 (0.4–0.5) |
| Obese | 3,352 (26.3) | 143 (27) | 3209 (27) | 1.0 (0.8–1.3 |
| Morbidly Obese | 645 (5.1) | 48 (9) | 597 (5) | 1.9 (1.4–2.6) |
| Emergency intubation (e.g., unable to pre-oxygenate), n (%) | 4,500 (35.4) | 130 (25) | 4370 (36) | 0.6 (0.5–0.7 |
| Oxygen saturation at start of intubation, median (IQR) | 100 (97–100) | 99 (95–100) | 100 (98–100) | 0 (0–0) |
| Pharmacologic method of Intubation, n (%) | ||||
| Sedation and Paralysis | 10,308 (81.0) | 496 (94) | 9812 (81) | 1.8 (1.4–2.4) |
| Sedation only | 159 (1.2) | 17 (3.2) | 142 (1.2) | 2.8 (1.7–4.6) |
| Paralysis only | 381 (3.0) | 5 (0.9) | 376 (3.1) | 0.3 (0.1–0.7) |
| Topical Anesthesia | 29 (0.2) | 1 (0.2) | 28 (0.2) | 0.8 (0–4.8) |
| Topical with Sedation | 29 (0.2) | 0 (0) | 29 (0.2) | 0 (0–3.0) |
| No Medications | 1,771 (13.) | 11 (2.1) | 1760 (14.5) | 0.1 (0.1–0.2) |
| Route of Intubation, n (%) | ||||
| Nasal | 111 (0.9) | 5 (0.9) | 106 (0.9) | 1.1 (0.5–2.6) |
| Oral | 12,421 (97.6) | 517 (97.5) | 11904 (98.1) | 0.8 (0.4–1.3) |
| Surgical | 132 (1.0) | 8 (1.5) | 124 (1.0) | 1.5 (0.7–3.0) |
| Pre-treatment, n (%) | ||||
| Atropine | 77 (0.6) | 5 (0.9) | 72 (0.6) | 1.6 (0.6–3.9) |
| Epinephrine | 55 (0.4) | 8 (1.5) | 47 (0.4) | 4.0 (1.9–8.4) |
| Fentanyl | 399 (3.1) | 9 (1.7) | 390 (3.2) | 0.5 (0.3–1.0) |
| Lidocaine | 94 (0.7) | 1 (0.2) | 93 (0.8) | 0.2 (0.0–1.8) |
| Norepinephrine | 80 (0.6) | 33 (6.2) | 47 (0.4) | 17.1 (10.9–27.0) |
| Phenylephrine | 87 (0.7) | 28 (5.3) | 59 (0.5) | 11.4 (7.2–18.1) |
| Topical Anesthesia | 99 (0.8) | 1 (0.2) | 98 (0.8) | 0.2 (0–1.7) |
| No pre-treatment used | 9,931 (78.1) | 367 (69.1) | 9564 (78.5) | 0.6 (0.5–0.7) |
| Primary Induction agenta, n (%) | ||||
| Etomidate | 8,809 (69.2) | 363 (70.8) | 8446 (84.6) | 0.4 (0.4–0.5) |
| Ketamine | 1,310 (10.3) | 140 (27.3) | 1170 (11.7) | 2.8 (2.3–3.5) |
| Midazolam | 111 (0.9) | 2 (0.4) | 109 (1.1) | 0.4 (0–1.3) |
| Propofol | 271 (2.1) | 8 (1.6) | 263 (2.6) | 0.6 (0.3–1.2) |
| No Induction used | 2,182 (17.2) | 17 (3.2) | 2165 (17.8) | 0.2 (0.1–0.2) |
| Training Level, n (%) | ||||
| PGY1 | 1,337 (10.5) | 67 (12.9) | 1270 (10.7) | 1.2 (0.9–1.6) |
| PGY2 | 4,029 (31.7) | 174 (33.6) | 3855 (32.4) | 1.1 (0.9–1.3) |
| PGY3 | 4,997 (39.3) | 182 (35.1) | 4815 (40.5) | 0.8 (0.7–1.0) |
| PGY4 | 1,131 (8.9) | 43 (8.3) | 1088 (9.2) | 0.9 (0.7–1.2) |
| PGY5 >= or fellow | 410 (3.2) | 25 (4.8) | 385 (3.2) | 1.5 (1.0–2.5) |
| Attending | 495 (3.9) | 27 (5.2) | 468 (3.9) | 1.3 (0.9–2.0) |
Proportions of patients receiving various induction agents are reported among those who received any induction agent, rather than among all patients.
Figure 2.
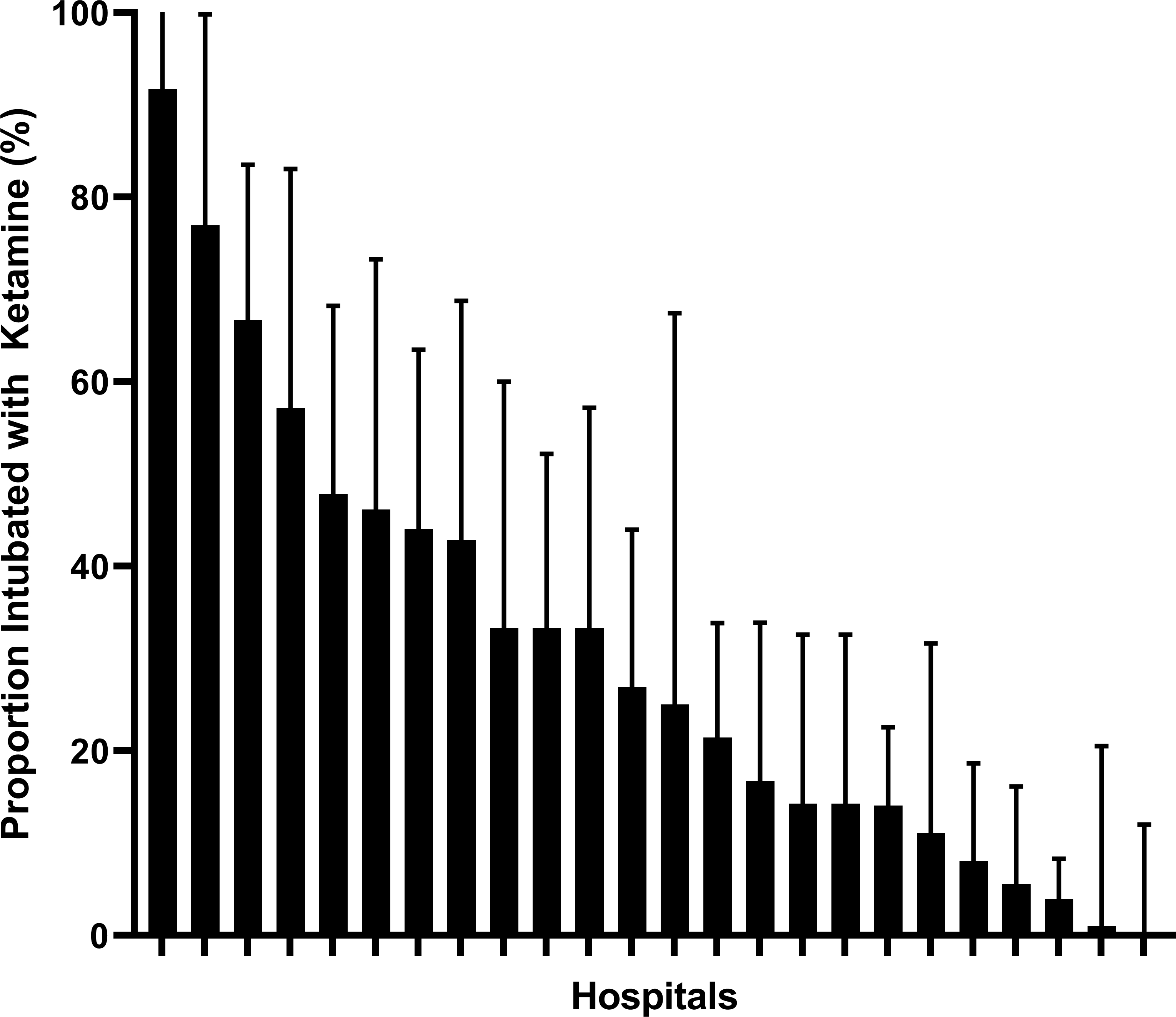
Proportion of sepsis patients intubated with ketamine, stratified by facility. The thick black bars reflect the proportion of intubations that used ketamine, and the error bars reflect the upper limit of the 95% confidence interval.
Adverse Events
Patients intubated for sepsis had a greater risk of peri-intubation adverse events than those without sepsis (23% vs 12%, OR=2.3, 95%CI 1.8–2.8), with the most common adverse event being hypotension. Hypotension was much more common in patients with sepsis than those without (47% vs. 18%, OR=4.1, 95%CI 3.5–5.0) with the lowest systolic blood pressure lower in the sepsis group than the non-sepsis group [72 vs. 78 mmHg, median difference=−2.5, 95%CI (−5.0) – 0]. More sepsis patients required therapy for hypotension (87% vs. 79%, OR=1.8, 95%CI 1.2–2.6). Sepsis patients were also more likely to require vasopressor therapy within 15 minutes after induction medications were given (25% vs. 8%, OR=4.0, 95%CI 3.5–5.0). The proportion of patients receiving no additional sedative agent within 15 minutes after induction was higher in sepsis patients than non-sepsis patients (16% vs. 9%, OR 2.0, 95%CI 1.4–2.5). First-pass success was no different between sepsis and non-sepsis patients (87.4% vs. 86.9%, OR 1.0, 95%CI 0.8–1.3).
Among sepsis patients, ketamine and etomidate had similar first-pass intubation success (89% vs. 84%, OR 1.5, 95%CI 0.8–2.6), similar probability of oxygen desaturation (11% vs. 11%, OR 1.0, 95%CI 0.5–1.8), but higher post-procedure hypotension (74% vs. 50%, OR 2.9, 95%CI 1.9–4.5). Ketamine-induced patients were not more likely to receive vasopressor therapy before intubation (27% vs. 23%, OR 1.2, 95%CI 0.8–2.0). There was no difference in cardiac arrest or serious adverse events between the two drugs (Table 3).
Table 3.
Adverse events in sepsis intubations, stratified by induction agent. Only sepsis cases (n=531) are reported in this table.
| Outcome | Induction agent | ||||
|---|---|---|---|---|---|
| Etomidate (n=363) | Ketamine (n=140) |
Midazolam (n=2) |
Propofol (n=8) |
No meds (n=17) |
|
| First-Pass Intubation Success, n (%) | 322 (88.7) | 118 (84.3) | 1 (50.0) | 8 (100.0) | 14 (82.4) |
| Peri-Intubation Adverse Events, n (%) | 81 (15.8) | 38 (27.1) | 1 (50.0) | 1 (12.5) | 1 (5.9) |
| Oxygen Desaturation (SpO2<90%), n (%) | 41 (11.3) | 16 (11.4) | 2 (100.0) | 1 (12.5) | 1 (5.9) |
| Hypotension (SBP < 100 mmHg) within 15 minutes after medications, n (%) | 182 (49.5) | 100 (74.1) | 1 (50.0) | 1 (12.5) | 3 (17.6) |
| Lowest SBP (mmHg), median (IQR) | 72 (60–84) | 74.5 (65–84) | 95 (95–95) | 70 (70–70) | 60 (53–70) |
| Medications given within 15 minutes after induction | |||||
| Any treatment for hypotension, n (%) | 136 (37.5) | 76 (54.3) | 2 (100.0) | 1 (12.5) | 3 (17.6) |
| Vasopressor, n (%) | 85 (23.4) | 42 (30.0) | 2 (100.0) | 0 (0.0) | 5 (29.4) |
| Any sedation (including propofol, ketamine, morphine, midazolam, diazepam, fentanyl, etomidate, or morphine) n (%) | 274 (75.5) | 105 (75.0) | 2 (100.0) | 6 (75.0) | 9 (52.9) |
| Peri-Intubation adverse events OR post-intubation hypotension (<100mmHg), n (%) | 82 (22.6) | 41 (29.3) | 2 (100.0) | 1 (12.5) | 1 (5.9) |
Sensitivity Analysis: Propensity-Adjusted Cohort Study
In the propensity-adjusted analysis (Supplemental Table 1, Supplemental Figure 1), ketamine was not associated with greater incidence of overall adverse events than etomidate (aOR=2.3, 95%CI 0.5–11.5), hypoxia (aOR=2.1, 95%CI 0.4–10.6), or the need to administer a vasopressor after intubation (aOR=1.2, 95%CI 0.5–2.8, Supplemental Table 2). Ketamine was, however, independently associated with a greater incidence of post-intubation hypotension than etomidate (aOR=2.7, 95%CI 1.1–6.7).
Discussion
Etomidate is known to impair cortisol production in response to exogenous corticotropin.2, 3, 5–8, 10, 17 Ketamine’s reputed hemodynamic stability has led to recommendations that it might be preferable to etomidate for induction during rapid sequence intubation of sepsis patients.1, 18–22 Others have questioned whether ketamine is hemodynamically neutral in sepsis patients.13, 23, 24 Our study demonstrates that etomidate is used as the induction agent for patients with sepsis in EDs 16% less frequently than for non-sepsis intubations.
We identified significant variation in induction agent selection for patients with sepsis between different institutions. This variation suggests that induction agents are selected based on institution-specific criteria, local preferences, or influenced by the impact of local thought leaders on beliefs regarding the safety of etomidate. Some facilities almost never use ketamine in sepsis intubations while others nearly always use ketamine. This degree of variation is unusual in clinical observations, and it may reflect ongoing controversy regarding the safety of etomidate in sepsis.
Ketamine induction of sepsis patients is associated with an increased rate of hypotension, when compared with patients receiving etomidate. This effect was the single difference in post-intubation care observed in our propensity-adjusted analysis. In the only large randomized trial of ketamine vs. etomidate for emergency intubation, no significant difference in hypotension was observed, but only 17% of enrolled patients had sepsis.25 Other observational studies have reported hypotension with ketamine use13, 23, 24, and a case series reported two cases of ketamine-associated cardiac arrest in critically ill patients.30 These data call into question the widely held belief that ketamine is a hemodynamically stable choice among induction agents, specifically when compared with etomidate. Ketamine’s hemodynamic effects are predominantly mediated through catecholamine release, so catecholamine-depleted, critically ill patients may be relatively unprotected from the unbalanced sedative effects of ketamine—an effect seen in a previous trial.31 While many episodes of hypotension we observed did not require intervention, post-intubation hypotension has been associated with increased hospital mortality and hospital length-of-stay.32 Previous authors have been concerned that avoiding etomidate in sepsis may put patients at risk of peri-intubation complications and patient harm.33
How should these results be applied? First, these findings confirm something many clinicians already know—patients with sepsis are at risk for adverse events, particularly hemodynamic deterioration in the peri-intubation period. Adverse events were more than twice as prevalent in sepsis patients than non-sepsis patients requiring intubation. Septic shock patients have a combination of preload dependent, vasodilatory, and cardiogenic shock, and this complex physiology can lead to decompensation at the time of intubation. Hemodynamic collapse may be caused by vasodilation effects of induction agents, mechanical effects of positive pressure ventilation decreasing venous return, or hypoxia leading to right heart dysfunction and poor myocardial oxygen delivery. While endogenous catecholamine release associated with ketamine administration makes it a relatively hemodynamically stable induction agent, this benefit may be lost in patients who are catecholamine depleted. This includes many sepsis patients who, therefore, are at greater risk for post-intubation hypotension caused by ketamine than their non-sepsis counterparts.
Second, patients with sepsis were much less likely to receive post-intubation sedation than non-sepsis patients. This observation is likely related to hypotension and clinical instability described above, but hypotension should not preclude appropriate, carefully titrated sedation and analgesia. With most sepsis patients receiving a neuromuscular blocking agent at the time of intubation and the increasing use of rocuronium, delaying or deferring sedation puts patients at risk for awareness during paralysis.34 While awareness and inadequate pain control may increase blood pressure, choosing a strategy of appropriate pre-intubation resuscitation and stabilization, peri-intubation vasoactive medications, and post-intubation sedation and pain control is preferred.
The purpose of this analysis was not to elucidate the safety or appropriateness of individual induction agents for patients with sepsis. Meta-analyses have drawn different conclusions on this point, and they vary on data included, study designs, and outcomes analyzed.10, 17 Our purpose was strictly to understand the current use of ED induction agents for patients with sepsis and to compare the adverse effects between etomidate and ketamine.
Limitations
While this multicenter observational study design permits the prospective acquisition of data for a very large number of intubations, the data are observational and causality cannot be proven. We attempted to control for confounding by using an adjusted analysis, but residual confounding may exist. Second, our data do not include long-term outcomes. If adrenal suppression were to lead to complications after ICU admission, for example, those outcomes are not captured in our data set. Other studies previously have sought to answer questions about long-term survival 3, 6, 7, 9, 10, 17, 35, so our study focused on decision-making regarding induction agents and short-term adverse events. Third, the NEAR registry enrolls primarily in large academic medical centers so results may not be generalizable to lower-volume or nonacademic centers. Finally, registry data are self-reported, which may be susceptible to recall bias or selective inclusion. The compliance program, requiring verification of entry of data for ≥90% of intubations mitigates any selective inclusion. While self-reporting may contribute to bias in selecting which patients have sepsis, these data are the best available evidence of what the provider knew at the time of the intubation.
Conclusions
In conclusion, our study showed etomidate to be the most commonly used induction agent among sepsis patients in the ED. Not surprisingly, sepsis patients have a greater risk of adverse events in the peri-intubation period, and hypotension requiring post-intubation vasopressor therapy was common. Ketamine was used more frequently among sepsis patients than patients without sepsis, but there was wide variation across centers in ketamine use. When compared with etomidate, ketamine use is associated with a higher incidence of hypotension, but the clinical importance of that finding is unclear. Further well-designed randomized trials to elucidate the causal relationship between induction agent and outcomes in sepsis patients are needed.
Supplementary Material
Supplemental Figure 1. Distribution of propensity scores, stratified by induction agent.
Table 2.
Outcomes
| Outcome | All Cases (n=12,722) | Sepsis (n=531) |
Non-Sepsis (n=12191) | OR (95%CI) or Difference (95%CI) |
|---|---|---|---|---|
| First-Pass Intubation Success, n (%) | 11,007 (86.5) | 463 (87.4) | 10544 (86.9) | 1.0 (0.8–1.3) |
| Peri-Intubation Adverse Events? n (%) | 1,535 (12.1) | 122 (23.1) | 1413 (11.7) | 2.3 (1.8–2.8) |
| Oxygen Desaturation (SpO2<90%), n (%) | 966 (7.6) | 61 (12.4) | 905 (9.1) | 1.4 (1.1–1.9) |
| Lowest oxygen saturation, median (IQR) | 78 (65–85) | 76 (65–84) | 76 (57.5–80) | −1 (−4 – 0) |
| Hypotension (SBP < 100 mmHg) within 15 minutes after medications, n (%) | 2,051 (16.1) | 241 (46.7) | 1810 (17.5) | 4.1 (3.5–5.0) |
| Lowest SBP (mmHg), median (IQR) | 77 (63–87) | 72 (60–84) | 85.5 (70–91) | −2.5 (−5 – 0) |
| Treatment for hypotension required within 15 min after medications, n (%) | 1,624 (12.8) | 208 (86.7) | 1416 (78.6) | 1.8 (1.2–2.6) |
| Disposition, n (%) | ||||
| ICU | 10,214 (80.2) | 487 (92.1) | 9727 (80.3) | 2.8 (2.1–3.9) |
| Died in ED – failed airway | 1 (0.0) | 1 (0.2) | 0 (0) | NA |
| Died in ED – other cause | 1,288 (10.1) | 16 (3.0) | 1272 (10.5) | 0.3 (0.2–0.4) |
| Operating Room | 909 (7.1) | 20 (3.8) | 889 (7.3) | 0.5 (0.3–0.8) |
| Extubated in ED | 54 (0.0) | 1 (0.2) | 53 (0.4) | 0.4 (0–2.5) |
| Transferred to another facility | 172 (1.4) | 4 (0.8) | 168 (1.4) | 0.5 (0.2–1.4) |
| Medications within 15 min after intubation, n (%) | ||||
| None | 1,959 (15.4) | 46 (8.7) | 1913 (15.7) | 0.5 (0.4–0.7) |
| Propofol | 6002 (47.2) | 165 (31.1) | 5837 (47.9) | 0.5 (0.4–0.6) |
| Midazolam | 2,069 (16.3) | 116 (21.9) | 1953 (16.0) | 1.5 (1.2–1.8) |
| Diazepam | 96 (0.8) | 5 (0.9) | 91 (0.8) | 1.3 (0.5–3.1) |
| Ketamine | 663 (5.2) | 47 (8.9) | 616 (5.1) | 1.8 (1.3–2.5) |
| Fentanyl | 3,874 (30.5) | 205 (38.6) | 3669 (30.1) | 1.5 (1.2–1.7) |
| Long acting paralytic | 686 (5.4) | 27 (5.1) | 659 (5.4) | 0.9 (0.6–1.4) |
| Vasopressor agent | 1,044 (8.2) | 132 (24.9) | 912 (7.5) | 4.0 (3.3–5.0) |
| Etomidate | 46 (0.4) | 2 (0.4) | 44 (0.4) | 1.0 (0.3–4.3) |
| Morphine | 20 (0.2) | 1 (0.2) | 19 (0.2) | 1.2 (0.2–9.0) |
Acknowledgements
The authors would like to acknowledge Andrea Fantegrossi, MPH for her assistance with data collection and data set access. The authors would also like to acknowledge Karisa Harland, PhD, Morgan Swanson, BS, and Priyanka Vakkalanka, MS, for their assistance with statistical analysis and interpretation.
Conflicts of Interest and Source of Funding:
None of the authors report financial conflicts of interest. The National Emergency Airway Registry is funded by the Brigham and Women’s Hospital Department of Emergency Medicine. Dr. Mohr additionally received support from the Agency for Healthcare Research and Quality (K08HS025753). These contents are solely the responsibility of the authors and do not necessarily reflect the views of the Agency for Healthcare Research and Quality.
Footnotes
Reprints will not be available.
References:
- 1.Edwin SB, Walker PL. Controversies Surrounding the Use of Etomidate for Rapid Sequence Intubation in Patients with Suspected Sepsis. Ann Pharmacother. 2010;44:1307–1313. [DOI] [PubMed] [Google Scholar]
- 2.Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of Adrenal Steroidogenesis by the Anesthetic Etomidate. N Engl J Med. 1984;310:1415–1421. [DOI] [PubMed] [Google Scholar]
- 3.Albert SG, Ariyan S, Rather A. The Effect of Etomidate on Adrenal Function in Critical Illness: A Systematic Review. Intensive Care Med. 2011;37:901–910. [DOI] [PubMed] [Google Scholar]
- 4.Chan CM, Mitchell AL, Shorr AF. Etomidate Is Associated with Mortality and Adrenal Insufficiency in Sepsis: A Meta-Analysis*. Crit Care Med. 2012;40:2945–2953. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Udwadia ZF, Lawler PG. Cortisol Response to Corticotropin and Survival in Septic Shock. Lancet. 1991;337:582–583. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Maxime V, Ibrahim F, Alvarez JC, Abe E, Boudou P. Diagnosis of Adrenal Insufficiency in Severe Sepsis and Septic Shock. Am J Respir Crit Care Med. 2006;174:1319–1326. [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-Level Prognostic Classification in Septic Shock Based on Cortisol Levels and Cortisol Response to Corticotropin. Jama. 2000;283:1038–1045. [DOI] [PubMed] [Google Scholar]
- 8.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J. Hydrocortisone Therapy for Patients with Septic Shock. N Engl J Med. 2008;358:111–124. [DOI] [PubMed] [Google Scholar]
- 9.Cuthbertson BH, Sprung CL, Annane D, Chevret S, Garfield M, Goodman S, Laterre PF, Vincent JL, Freivogel K, Reinhart K, Singer M, Payen D, Weiss YG. The Effects of Etomidate on Adrenal Responsiveness and Mortality in Patients with Septic Shock. Intensive Care Med. 2009;35:1868–1876. [DOI] [PubMed] [Google Scholar]
- 10.Jung B, Clavieras N, Nougaret S, Molinari N, Roquilly A, Cisse M, Carr J, Chanques G, Asehnoune K, Jaber S. Effects of Etomidate on Complications Related to Intubation and on Mortality in Septic Shock Patients Treated with Hydrocortisone: A Propensity Score Analysis. Crit Care. 2012;16:R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu WJ, Wang F, Tang L, Liu JC. Single-Dose Etomidate Does Not Increase Mortality in Patients with Sepsis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies. Chest. 2015;147:335–346. [DOI] [PubMed] [Google Scholar]
- 12.Dmello D, Taylor S, O’Brien J, Matuschak GM. Outcomes of Etomidate in Severe Sepsis and Septic Shock. Chest. 2010;138:1327–1332. [DOI] [PubMed] [Google Scholar]
- 13.Groth CM, Acquisto NM, Khadem T. Current Practices and Safety of Medication Use During Rapid Sequence Intubation. J Crit Care. 2018;45:65–70. [DOI] [PubMed] [Google Scholar]
- 14.McPhee LC, Badawi O, Fraser GL, Lerwick PA, Riker RR, Zuckerman IH, Franey C, Seder DB. Single-Dose Etomidate Is Not Associated with Increased Mortality in Icu Patients with Sepsis: Analysis of a Large Electronic Icu Database. Crit Care Med. 2013;41:774–783. [DOI] [PubMed] [Google Scholar]
- 15.Tekwani KL, Watts HF, Rzechula KH, Sweis RT, Kulstad EB. A Prospective Observational Study of the Effect of Etomidate on Septic Patient Mortality and Length of Stay. Acad Emerg Med. 2009;16:11–14. [DOI] [PubMed] [Google Scholar]
- 16.Tekwani KL, Watts HF, Sweis RT, Rzechula KH, Kulstad EB. A Comparison of the Effects of Etomidate and Midazolam on Hospital Length of Stay in Patients with Suspected Sepsis: A Prospective, Randomized Study. Ann Emerg Med. 2010;56:481–489. [DOI] [PubMed] [Google Scholar]
- 17.Bruder EA, Ball IM, Ridi S, Pickett W, Hohl C. Single Induction Dose of Etomidate Versus Other Induction Agents for Endotracheal Intubation in Critically Ill Patients. Cochrane Database Syst Rev. 2015;1:Cd010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JM, Shin TG, Hwang SY, Yoon H, Cha WC, Sim MS, Jo IJ, Song KJ, Rhee JE, Jeong YK. Sedative Dose and Patient Variable Impacts on Postintubation Hypotension in Emergency Airway Management. Am J Emerg Med. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Smischney NJ, Seisa MO, Heise KJ, Busack KD, Loftsgard TO, Schroeder DR, Diedrich DA. Practice of Intubation of the Critically Ill at Mayo Clinic. J Intensive Care Med. 2017:885066617691495. [DOI] [PubMed] [Google Scholar]
- 20.Seisa MO, Gondhi V, Demirci O, Diedrich DA, Kashyap R, Smischney NJ. Survey on the Current State of Endotracheal Intubation among the Critically Ill: Hemair Investigators. J Intensive Care Med. 2018;33:354–360. [DOI] [PubMed] [Google Scholar]
- 21.Van Berkel MA, Exline MC, Cape KM, Ryder LP, Phillips G, Ali NA, Doepker BA. Increased Incidence of Clinical Hypotension with Etomidate Compared to Ketamine for Intubation in Septic Patients: A Propensity Matched Analysis. J Crit Care. 2017;38:209–214. [DOI] [PubMed] [Google Scholar]
- 22.Cherfan AJ, Arabi YM, Al-Dorzi HM, Kenny LP. Advantages and Disadvantages of Etomidate Use for Intubation of Patients with Sepsis. Pharmacotherapy. 2012;32:475–482. [DOI] [PubMed] [Google Scholar]
- 23.Tarquinio KM, Howell JD, Montgomery V, Turner DA, Hsing DD, Parker MM, Brown CA 3rd, Walls RM, Nadkarni VM, Nishisaki A. Current Medication Practice and Tracheal Intubation Safety Outcomes from a Prospective Multicenter Observational Cohort Study. Pediatr Crit Care Med. 2015;16:210–218. [DOI] [PubMed] [Google Scholar]
- 24.Upchurch CP, Grijalva CG, Russ S, Collins SP, Semler MW, Rice TW, Liu D, Ehrenfeld JM, High K, Barrett TW, McNaughton CD, Self WH. Comparison of Etomidate and Ketamine for Induction During Rapid Sequence Intubation of Adult Trauma Patients. Ann Emerg Med. 2017;69:24–33.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, Bertrand L, Beltramini A, Gamand P, Albizzati S, Perdrizet D, Lebail G, Chollet-Xemard C, Maxime V, Brun-Buisson C, Lefrant JY, Bollaert PE, Megarbane B, Ricard JD, Anguel N, Vicaut E, Adnet F. Etomidate Versus Ketamine for Rapid Sequence Intubation in Acutely Ill Patients: A Multicentre Randomised Controlled Trial. Lancet. 2009;374:293–300. [DOI] [PubMed] [Google Scholar]
- 26.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M, Program ftCPE. Incidence and Trends of Sepsis in Us Hospitals Using Clinical Vs Claims Data, 2009–2014. Jama. 2017;318:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (Strobe) Statement: Guidelines for Reporting Observational Studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 28.Brown CA 3rd, Bair AE, Pallin DJ, Walls RM. Techniques, Success, and Adverse Events of Emergency Department Adult Intubations. Ann Emerg Med. 2015;65:363–370.e361. [DOI] [PubMed] [Google Scholar]
- 29.April MD, Arana A, Pallin DJ, Schauer SG, Fantegrossi A, Fernandez J, Maddry JK, Summers SM, Antonacci MA, Brown CA 3rd. Emergency Department Intubation Success with Succinylcholine Versus Rocuronium: A National Emergency Airway Registry Study. Ann Emerg Med. 2018;72:645–653. [DOI] [PubMed] [Google Scholar]
- 30.Dewhirst E, Frazier WJ, Leder M, Fraser DD, Tobias JD. Cardiac Arrest Following Ketamine Administration for Rapid Sequence Intubation. J Intensive Care Med 2013;28:375–379. [DOI] [PubMed] [Google Scholar]
- 31.Miller M, Kruit N, Heldreich C, Ware S, Habig K, Reid C, Burns B. Hemodynamic Response after Rapid Sequence Induction with Ketamine in out-of-Hospital Patients at Risk of Shock as Defined by the Shock Index. Ann Emerg Med. 2016;68:181–188.e182. [DOI] [PubMed] [Google Scholar]
- 32.Heffner AC, Swords D, Kline JA, Jones AE. The Frequency and Significance of Postintubation Hypotension During Emergency Airway Management. J Crit Care. 2012;27:417.e419–413. [DOI] [PubMed] [Google Scholar]
- 33.Walls RM, Murphy MF. Clinical Controversies: Etomidate as an Induction Agent for Endotracheal Intubation in Patients with Sepsis: Continue to Use Etomidate for Intubation of Patients with Septic Shock. Ann Emerg Med. 2008;52:13–14. [DOI] [PubMed] [Google Scholar]
- 34.Chong ID, Sandefur BJ, Rimmelin DE, Arbelaez C, Brown CA 3rd, Walls RM, Pallin DJ. Long-Acting Neuromuscular Paralysis without Concurrent Sedation in Emergency Care. Am J Emerg Med. 2014;32:452–456. [DOI] [PubMed] [Google Scholar]
- 35.Alday NJ, Jones GM, Kimmons LA, Phillips GS, McCallister JW, Doepker BA. Effects of Etomidate on Vasopressor Use in Patients with Sepsis or Severe Sepsis: A Propensity-Matched Analysis. J Crit Care. 2014;29:517–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of propensity scores, stratified by induction agent.


