Abstract
EFSA received a mandate from the European Commission to assess the effectiveness of some of the control measures against diseases included in the Category A list according to Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’). This opinion belongs to a series of opinions where these control measures will be assessed, with this opinion covering the assessment of control measures for sheep and goat pox. In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of: (i) clinical and laboratory sampling procedures, (ii) monitoring period and (iii) the minimum radii of the protection and surveillance zones, and the minimum length of time the measures should be applied in these zones. The general methodology used for this series of opinions has been published elsewhere; nonetheless, the transmission kernels used for the assessment of the minimum radii of the protection and surveillance zones are shown. Several scenarios for which these control measures had to be assessed were designed and agreed prior to the start of the assessment. Different risk‐based sampling procedures based on clinical visits and laboratory testing are assessed in case of outbreak suspicion, granting animal movements and for repopulation purposes. The monitoring period of 21 days was assessed as effective. The estimated probability of transmission beyond the protection zone of 3 km radius from an infectious establishment is 9.6% (95% CI: 3.1–25.8%) and 2.3% (95% CI: 1–5.5%) for the surveillance zone of 10 km radius. This may be considered sufficient to contain the disease spread (95% probability of containing transmission corresponds to 5.3 Km). To contain 99% of the spread, the radius should be increased to 19.4 km (95% CI: 9.8–26.8). This may increase the number of farms in the surveillance zone, since the area would increase fourfold.
Keywords: SPP/GTP , sampling procedures, monitoring period, protection zone, surveillance zone
Summary
This opinion is part of a series of opinions, in which the three first Terms of Reference (ToRs) of a mandate received from the European Commission have been considered. The background and specific details of this mandate can be found in the opinion. The ToRs in this mandate request an assessment of the effectiveness of:
the clinical and laboratory examination in their capacity to detect disease (or estimate the disease prevalence within an establishment), either in suspect or confirmed animals in a single establishment or in establishments within restriction zones (ToR 1);
the effectiveness of the duration of the monitoring period (for different scenarios) in the control of suspected and confirmed outbreaks (ToR 2);
the size (ToR 3.1) and duration (ToR 3.2) of the restriction zones, in their capacity for mitigating disease spread.
In order to harmonise the approach to these assessments, the methodology used in this series of opinions, covering all Category A diseases, was agreed on and published in a separate technical report.
Specific clinical and laboratory procedures for sheep and goat pox (SPP/GTP) for each scenario of ToR 1 have been assessed. For assessing the effectiveness of detecting SPP/GTP in a flock, a model to study the within‐herd transmission of SPP/GTP was designed. This allowed the calculation of infection and seroprevalence at different points in time from virus introduction in a herd, so to calculate the sample size needed for early detection of suspected animals in an infected flock.
With a suspicion of SPP/GTP in an establishment, the purpose of the clinical examination based on detection of clinical signs related to SPP/GTP is to identify potentially infected animals in order to target the sampling correctly. In a period between 14 and 21 days after disease introduction, at least three animals are expected to be clinically affected, and these should be tested for confirmation. The confirmation of a clinical suspicion is based on laboratory testing, by confirming the presence of the virus, i.e. its nucleic acids (the test of choice is real‐time polymerase‐chain reaction (PCR)) or of antibodies (ELISA). In case of suspicion because of contact, import, etc., if clinical signs are not so evident, the sampling of randomly selected asymptomatic animals can be performed based on the expected infection prevalence from time of introduction. In this case, ELISA can be performed from day 21 post virus introduction. When SPP/GTP is officially confirmed in an establishment, further sampling procedures will be targeted to obtain information on the origin of the disease, the length of time that the disease is present, i.e. analysis of age of skin lesion and phylogenetic analysis of virus isolates.
In the scenarios to grant derogation for animal movement, clinical visits and sampling and testing should be conducted according to the risk level of such movements. The same risk‐based approach is valid for sampling procedures for repopulation purposes.
To answer ToR 2, the assessment of the length of the monitoring period, and to assess the minimum duration of measures to be implemented in the protection and surveillance zones (ToR 3.2), an extensive literature search (ELS) was carried out. This ELS aimed to assess the average, shortest and longest period between the earliest point of infection of small ruminants with SPP/GTP virus and the time of reporting of a suspicion by the competent authority. Twenty‐one days as defined in the Delegated Regulation is considered effective for all scenarios mentioned in ToR 2.
Based on the assessment, the minimum period of 21 days indicated in the Delegated Regulation for the restriction measures being in place in the protection zone and 30 days for the restriction measures in the surveillance zone are considered effective to detect infected establishments and to prevent the movement of infected animals from the protection and surveillance zone, respectively.
To assess the effectiveness of the minimum radius to be implemented in the protection and surveillance zones (ToR 3.1), transmission kernels were used. These kernels were estimated using data on outbreaks of SPP/GTP reported in the Evros region of Greece from 2013 to 2014, because it was considered that these outbreaks were not linked to animal movements, in agreement with the assumption made, i.e. exclusion of the spread due to animal movements. The estimated probability of transmission beyond the protection zone of 3 km radius from an infectious establishment is 9.6% (95% CI: 3.1–25.8%) and 2.3% (95% CI: 1–5.5%) for the surveillance zone of 10 km radius (), which may be considered sufficient to contain disease spread. The 95% probability of containing transmission would correspond to 5.3 Km of protection zone (CI: 1.8–10.6 km).To reduce the probability of transmission beyond the surveillance zone to 1%, its radius should be increased from 10 to 19 km (95% CI: 9.8–26.8). This, nonetheless, might increase the number of farms in the surveillance zone, since the area would increase fourfold.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’), hereinafter referred to as AHL, requires the Commission to lay down detailed rules on the disease control measures against listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). The Commission is empowered to adopt delegated acts supplementing the rules laid down in Part III of Regulation (EU) 2016/429 on transmissible animal diseases (Animal Health Law) on disease control measures for listed diseases as referred to in point (a), (b) and (c) of its Article 9 (category A, B and C diseases). Therefore, the Commission has developed and adopted a Delegated Regulation laying down rules for the prevention and control of certain diseases (‘the Delegated Regulation’). The rules laid down in the Delegated Regulation are in respect of terrestrial animals largely replicating the rules currently in force concerning the disease control measures in the event of animal diseases with serious effects on the livestock as they have proven to be effective in preventing the spread of those diseases within the Union. Consequently, many animal disease control measures laid down in existing Directives will be, to the extent that not already done by the Animal Health Law, replaced by the rules provided in the Delegated Regulation. At the same time, these rules have been aligned with the international standards from the World Organisation for Animal Health (OIE), wherever these existed. However, certain disease control measures proposed in the Delegated Regulation, in particular in its Annexes, were considered as outdated i.e. possibly not based on most recent scientific evidence at the time of development. Their review is considered as necessary. Moreover, for those category A diseases for which rules were not established before or were not detailed enough, certain disease control and risk mitigating measures are, due to the lack of scientific basis, extrapolated from other diseases, for which rules existed in the past. Finally, for some other diseases the evidence and scientific knowledge, was not available to the Commission and to the Member States at the time of developing the Delegated Regulation due to the time constraints. The following diseases are examples of the later: infection with Rift Valley fever (RVF), infection with Mycoplasma mycoides subsp. Mycoides SC (Contagious bovine pleuropneumonia) (CBPP), Contagious caprine pleuropneumonia (CCPP), Sheep pox and goat pox, infection with peste des petits ruminants virus (PPR), African horse sickness (AHS), Glanders. In this regard, the existing rules will cease to apply as from the date of application of the Animal Health Law and its complementing legislation including the Delegated Regulation, i.e. from 21 April 2021. Certain of the proposed measures for the prevention and control of category A diseases of terrestrial animals should therefore be assessed in order to ensure that they are effective and updated based on the latest scientific knowledge in this new set of legislation. This is particularly important in the case of those diseases that are less common or have been never reported in the Union.
1.1.1. ToR 1: Sampling of animals and establishments for the detection of category A diseases in terrestrial animals
Based on available scientific information, assess the effectiveness of existing sampling procedures to detect or rule out the presence of each category A disease of terrestrial animals and, in case of absence of effective procedures, develop them, in order to complete the rules provided for in Annex I to the Delegated Regulation. In particular, provide for disease‐specific procedures for the sampling of:
ToR 1.1 Animals for clinical examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 13(3)(c), 14(1) and 26(2) of the Delegated Regulation.
ToR 1.2 Animals for laboratory examinations to ensure the detection of the relevant category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 12(3), 13(3)(c), 14(1), 26(2) of the Delegated Regulation.
ToR 1.3 Establishments to ensure the detection of the relevant category A disease for the performance of visits in establishments located in protection zones larger than 3 km and establishments located in the surveillance zone in accordance with Articles 26(5) and 41 of the Delegated Regulation.
ToR 1.4 Animals for clinical and laboratory examinations to ensure the detection of the relevant category A disease for the movement of animals from restricted zones in accordance with Articles 28(5), 43(5), 56(1)(c) of the Delegated Regulation.
ToR 1.5 Animals for laboratory examinations to ensure the detection of the relevant category A disease before and after being introduced in the affected establishment for repopulation, in accordance with Article 59(2), (3) and (9) of the Delegated Regulation.
1.1.2. ToR 2: Monitoring period
ToR 2.1 Assess the effectiveness of the length of the monitoring periods set out in Annex II of the Delegated Regulation for each category A disease of terrestrial animals. In this regard, it is important to take into consideration that the monitoring period was introduced as a management tool, which represents a time frame of reference assigned to each category A disease for the competent authority to apply certain control measures and to carry out investigations in the event of suspicion and confirmation of category A diseases in terrestrial animals.
This assessment should be carried out with respect to the following situations:
the records analysis carried out by the competent authority in the framework of the epidemiological enquiry referred to in Article 57 of Regulation (EU) 2016/429, in the event of suspicion of a category A disease (Article 8(4) of the Delegated Regulation);
the derogation from killing in the event of an outbreak of a category A disease in establishments keeping animals of listed species in two or more epidemiological units (Article 13(1) of the Delegated Regulation);
the tracing carried out by the competent authority to identify establishments and other locations epidemiologically linked to an establishment affected by a category A disease (Article 17(2) of the Delegated Regulation);
the exemption applied to certain products from the prohibitions laid down in Annex VI taking into account the date they were produced (Article 27(3)(c) of the Delegated Regulation);
the specific conditions for authorising movements of semen from approved germinal product establishments in the protection and surveillance zones (Article 32(c) and 48(c) of the Delegated Regulation);
the repopulation of establishments affected by a category A disease (Article 57(1)(b) and 59(4)(b) of the Delegated Regulation).
ToR 2.2 Propose the length of what should be the monitoring period in those diseases for which the time is assessed as not effective.
1.1.3. ToR 3: Minimum radius of restricted zones and duration of the disease control measures in restricted zones
ToR 3.1 Assess the effectiveness to control the spread of the disease of the minimum radius of the protection and surveillance zones set out in Annex V of the Delegated Regulation for each category A disease of terrestrial animals.
ToR 3.2 Assess the effectiveness to control the spread of the disease of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI for each category A disease of terrestrial animals.
1.1.4. ToR 4: Prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials
ToR 4.1 Assess the effectiveness to control the spread of disease of prohibitions set out in Annex VI of the Delegated Regulation with respect to the risk associated for each category A disease, to the listed activities and commodities.
ToR 4.2 Review the available scientific information on risk‐mitigating treatments that are effective to control the presence of category A disease agents in products of animal origin and other relevant materials. Based on this:
provide an opinion on the effectiveness of the risk‐mitigating treatments for products of animal origin and other materials produced or processed in the restricted zone set out in Annex VII and VIII, and
if relevant, suggest new treatments or procedures that can be effective to mitigate or to eliminate such risk.
1.2. Interpretation of the Terms of Reference
To address the ToRs of the mandate, EFSA proposed and agreed with the European Commission the following:
The publication of fourteen individual opinions, one per each of the diseases included in the list of category A diseases for terrestrial animals, with each of these opinions providing the answer to ToRs 1, 2 and 3. The current manuscript is one of the 14 opinions covering ToRs 1, 2 and 3 for sheep and goat pox (SPP/GTP).
The publication of a unique opinion covering ToR 4 for all diseases listed (i.e. ToR 4 is not covered in this opinion).
To address ToR 1 (effectiveness of sampling procedures), EFSA agreed with the European Commission on 21 scenarios based on different articles of the Delegated Regulation (EC) 2020/687 (hereinafter referred to as Delegated Regulation), for which the effectiveness of the sampling procedures will be assessed (Annex B). Although these scenarios will be assessed independently, some of these scenarios may be merged if the assessment processes are the same.
To address ToR 2 (effectiveness of the monitoring period), seven scenarios previously agreed with the contractor were defined (Annex C). The assessment of the effectiveness of the monitoring period will be done by assessing its ability to ensure that specific actions can be carried out without posing a risk of disease spread, if the monitoring period is calculated backwards or forwards from a specific date. If the length of the monitoring period estimated by EFSA is longer than the existing monitoring periods, the existing monitoring period will be considered non effective. If the length of the monitoring period estimated by EFSA is shorter than the existing monitoring period, this existing monitoring period will be considered effective from a disease control point of view. No assessment of the plausible unnecessary economic burden that may be placed on the stakeholders as a result of an excessive length of the monitoring periods will be done by EFSA.
The assessment of the minimum duration and the length of the radius of the protection and surveillance zones (ToR 3) will be done independently. The setting of these two zones (protection and surveillance zones) surrounding an affected establishment and the control measures implemented in each one of the zones are based on the general principle that the probability of disease spread is larger the closer the establishment is to an affected establishment. The validity of this statement will not be assessed in this manuscript; nonetheless, the limitations that this assumption may have in the control of certain diseases will, when relevant, be discussed.
-
The following scenarios of the ToR 1 of the of the Annex B are not relevant for the SPP/GTP, and therefore not included in the assessment of the current Opinion:
scenario 6 because the minimum radius of the protection zone for SPP/GTP is 3 km,
scenarios 10, 11, 16 and 17 because they are referring to poultry.
The duration of the monitoring period for SPP/GTP as described in Annex II of the Delegated Regulation is 21 days.
The minimum length of the radius of the protection zone (PZ) and surveillance zone (SZ) for SPP/GTP as described in Annex V of the Delegated regulation are 3 and 10 km, respectively.
The minimum duration of the measures in the PZ and SZ for SPP/GTP as described in Annex X and XI of the Delegated Regulation are 21 and 30 days, respectively.
Vaccination against SPP/GTP has not been taken into consideration in the assessment of ToRs 2 and 3 as agreed with the requestor. For ToR 1, some relevant aspects related to vaccination were discussed (a deep review was not requested or maybe needed).
2. Disease characterisation and geographical distribution of sheep and goat pox
2.1. Aetiology
Sheep pox (SPP) and goat pox (GTP) are two contagious viral diseases affecting, respectively, sheep and goats. The causative agent are the sheep pox virus (SPPV) and goat pox virus (GTPV), which are two DNA viruses, closely related members of the family Poxviridae, and belong to the genus Capripoxvirus, which also includes Lumpy skin disease virus (LSDV), affecting cattle. Despite that SPPV and GTPV cannot be differentiated by conventional diagnostic tests (apart from some species‐specific PCRs) including serological tests, they differ slightly in their genetic sequence (OIE, 2013; EFSA AHAW Panel, 2014; CFSPH, 2017; Haegeman et al., 2019). They were originally considered strains of the same virus, but genetic analyses have demonstrated that they are separate species within the genus Capripoxvirus. In addition, recombination can occur between sheep and goat pox viruses, which may lead to different strains with intermediate host preference and virulence (OIE, 2013).
2.2. Epidemiology
SPPV infects mostly sheep and GTPV mostly goats, but there are some strains of both viruses that can infect both species, although the disease is usually more severe in the homologous host species. The level of virulence is strain dependent (OIE, 2013; EFSA AHAW Panel, 2014). Neither SPPV nor GTPV are zoonotic (EFSA AHAW Panel, 2014). Wild ruminant species (other than wild caprine and ovine belonging to the subfamily Caprinae) and cattle are not affected by these viruses (EFSA AHAW Panel, 2014; CFSPH, 2017). The first outbreak of GTP in wildlife was reported in wild red serow (Capricornis rubidus), in India in 2015 (Dutta et al., 2019). In the same region, GTP spillover from domestic goats to wild Himalayan goral (Naemorhedus goral) has been described (Bora et al., 2021). It is known that European sheep and goat breeds are more susceptible to capripoxvirus infection than indigenous African and Asian breeds (Bhanuprakash et al., 2010). Regarding European wildlife, feral goats are present in several regions, particularly in Mediterranean Europe, and are susceptible, as well as wild sheep such as European mouflon (Ovis aries), but it is unclear if wild caprinae such as chamois (Rupicapra spp.), Alpine ibex (Capra ibex) or Iberian ibex (Capra pyrenaica) might be susceptible, too (Gortázar et al., 2021).
SPPV and GTPV are transmitted mainly through direct contact with infected animals in pens or gatherings (pasture, markets, etc.) or through inhalation of aerosol in an environment contaminated by infectious particles present in saliva, nasal and conjunctival secretions. Virus is also abundant in skin lesions and scabs and can be detected in milk, urine, faeces and semen. Indirect transmission through fomites (including hair and wool) in which virus can persist several months or mechanical transmission by insects (biting flies) is possible but less frequent than direct transmission (Fassi‐Fehri and Lefèvre, 2003, EFSA AHAW Panel, 2014; CFSPH, 2017).
Currently, the two diseases are endemic in north and central Africa, the Middle East, Turkey, and a large part of Asia including China and India. They have been eradicated from most countries of Western Europe; occasional outbreaks occurred in Greece and Bulgaria in the last 10 years. America and Australia are considered free from the infection (OIE, 2013; EFSA AHAW Panel, 2014; CFSPH, 2017).
In case of outbreaks in a non‐endemic area, it is recommended to control the disease by depopulation of infected and exposed flocks, movement restriction and disinfection of farms and equipment.
2.3. Clinical signs and diagnosis
The clinical course is similar for SPP and GTP. The severity and the morbidity and mortality rates are higher in naive populations and in exotic breeds imported to endemic areas, in young animals above 2–3 months of age that are no longer protected anymore by maternal antibodies, and in animals being in unfavourable living conditions (underfeeding, parasitism, co‐infection, dry or cold season). Morbidity and mortality (case‐fatality) rates are 70–90% in previously disease‐free areas and 5–10% in endemic areas but can reach 80–100% of morbidity and case‐fatality in non‐immune animals or kids and lambs (Fassi‐Fehri and Lefèvre, 2003; OIE, 2013; CFSPH, 2017).
The incubation period is usually 1–2 weeks but can be shorter (2–4 days) after mechanical inoculation by a biting insect or in an experimental infection (OIE, 2010). In a review of experimental infections, the median incubation period was 4 days and the minimum was 1 day in sheep (Dórea et al., 2021).
In the EU context, typically SPP/GTP‐free area, low‐virulent virus strains that cause subclinical infection without clinical signs have not been reported neither described in the literature.
Though it should be remarked that much less research effort has been put into understanding the potential subclinical pathogenesis for SPPV/GTPV compared to LSDV. And while it is not possible to rule out the possibility of subclinical form of SPP/GTP, the literature lacks evidence for it. However, experts from EU and OIE reference laboratories consider that under experimental infections, 100% of inoculated fully susceptible animals will develop disease (personal communications from Kris De Clercq, former Head of EURL for capripox viruses and Pip Beard, capripox expert at the Pirbright Institute – OIE Reference Laboratory, 2021).
The first clinical signs of SPP/GTP are nasal and ocular discharge, hypersalivation, palpebral oedema, hyperthermia (40–42°C), difficulty in breathing, depression and loss of appetite. Within 2–5 days, skin lesions first erupt on hairless zones: face (lips, muzzle, eyelids, ears), udder, inguinal area, perineum and at the base or under the tail. They appear as erythematous macules and evolve into 0.5–1.5 cm circular hard swellings (papules). Rarely, papules become vesicles filled with fluid or even pustules. Within 4–5 days, lesions desiccate and become necrotic, evolving into yellowish crusts and then persistent scabs leaving small scars in surviving animals. In more severe forms, lesions can cover the entire body and even become coalescent. Ulcerative mucosal lesions can appear in the mouth, eyes, nasal cavities, vagina, anus and prepuce, and invade the respiratory and digestive tracts leading to inappetence, drooling, blepharoconjunctivitis, mucopurulent nasal discharge, coughing, dyspnoea and diarrhoea. Abortion and enlargement of the udder may occur. Pneumonia due to bacterial secondary infection (pasteurellosis) is common. Lymphadenopathy of superficial and internal lymph nodes is frequent. Sick animals may die at any stage of the disease or recover within 3–4 weeks (Fassi‐Fehri and Lefèvre, 2003; OIE, 2013; EFSA AHAW Panel, 2014; CFSPH, 2017). Importantly, animals are already shedding infectious virus when the first clinical signs of SPP/GTP are detectable in the flock. Animals with mild clinical signs, with only a few pox lesions on the skin and mucous membranes, do not spread the virus as effectively as animals with severe signs, although they are still infectious (EFSA AHAW Panel, 2014).
Direct detection of SPPV and GTPV or their nucleic acids can be made from skin lesions (scrapings, scabs), secretions (oral, nasal, ocular) and blood and tissue samples collected at necropsy. Except for PCR tests, samples should preferably be taken during the first week of the disease to avoid interference of neutralising antibodies. Commonly used direct tests are gel‐based and real‐time PCR, LAMP (loop‐mediated isothermal amplification), antigen capture ELISAs and AGID (agar gel diffusion). Most of these tests detect all capripoxviruses but cannot discriminate SPPV from GTPV or from LSDV apart from some species‐specific PCRs. Some PCRs are also able to differentiate wild type from vaccine strains.
The preferred direct tests are PCR assays, compared to virus isolation, since they are quicker, SPPV and GTPV grow slowly on cell cultures and several passages may be required to grow the virus (EFSA AHAW Panel, 2014). In a systematic literature review performed by EFSA (EFSA AHAW Panel, 2014), the generic real‐time capripoxvirus PCR displayed higher sensitivity than conventional gel‐based PCR assays, with values of 100% and specificity ranging from 94.7% to 100%.1 Species‐specific real‐time PCR methods for the differentiation between SPPV, GTPV and LSDV have been published (EFSA AHAW Panel, 2014). Species‐specific PCR assays detect differences in the melting point temperatures for SPPV, GTPV and LSDV, obtained after fluorescence melting curve analysis.
For antibody detection, virus neutralisation test (VNT) is the gold standard. Both in house and a commercial ELISAs (e.g. ID Screen® Capripox by IDVet2) are available, but these tests cannot discriminate among capripoxvirus antibodies (LSD or SPP/GTP). The sensitivity of the commercial test has been validated only for LSD in cattle so far, while the producer declares that the specificity for small ruminants is 99.7%.3 The sensitivity of neutralisation tests for SPP/GTP varies between 70% and 96% and specificity can reach 100%, while an experimental ELISA developed for the detection of antibodies against SPP/GTP (Babiuk et al., 2009) has a sensitivity of 96.3% and a specificity of 95.4% (EFSA AHAW Panel, 2014).
An immunoperoxidase monolayer assay (IPMA) was recently validated for SPP and GTP (Haegeman et al., 2020). Other serological tests (AGID, immunofluorescence test) also cross‐react with other poxviruses such as Orf virus, the agent of contagious ecthyma, mainly in small ruminants (OIE, 2013; EFSA AHAW Panel, 2014; CFSPH, 2017; Haegeman et al., 2019).
2.4. Vaccines
All the commercially available vaccines for SPP and GTP are live‐attenuated vaccines, prepared with a limited number of strains. General requirements set up for SPPV vaccines are described in the European Pharmacopoeia and in the OIE Manual of Diagnostic Tests and Vaccines (OIE, 2010).
There are several examples of live SPPV/GTPV vaccines produced locally from pathogenic strains by attenuation in cell culture, including the following strains: A1 strain, Algerian, Bakirkoy, Bucharest, Cairo, Chinese, Chitinsk, Hyderabad, Indo‐China (India), Jaipur, K Strain, Karnal, Kazakhstan, Kenyan, Mathura, Mauritanian, Mongolian, Mysore, Nairobi, Niski and SP6 (Bhanuprakash et al., 2006), Pakistan, Pendic, Pendik Kedong, Perego M, Persian, Ranipet, RM‐65, Romanian, Romanian Fanar (RF), Russian, Soba, SP8, SPPV/RH, Stavropol, Turkey. The vaccines currently available do not support the DIVA concept. The RM65, KSPGP 0240 and RF strains are the most used SPPV/GTPV vaccine strains and are reported to produce high levels of protection (Kitching, 1986; Chaudhary et al., 2009; Yogisharadhya et al., 2011).
Vaccination is used to control the disease in endemic areas, or in a free zone to prevent the spread in case of multiple outbreaks (ring vaccination), but its use can be limited by country regulations and trade restrictions.
None of the existing live SPPV/GTPV vaccines are licensed for use within the EU and use of these vaccines would inflict immediate restrictions on the international trade of live sheep and goats and their products. The use of inactivated vaccines could be an option to protect areas at risk (EFSA AHAW Panel, 2014). Several efficient live‐attenuated vaccines are available worldwide, providing at least 2 years protective immunity while current inactivated vaccines give only a 6‐month protection. Infection or vaccination with any capripoxvirus gives cross‐protection against all capripoxviruses, but the use of homologous vaccine is recommended since it is more protective (OIE, 2013, 2018; EFSA AHAW Panel, 2014; CFSPH, 2017).
Certain live‐attenuated vaccines are sufficiently safe and effective, and can be used in pregnant animals, providing lambs with a 3‐month immunity. However, some live‐attenuated vaccines may have unacceptably high levels of residual pathogenicity (EFSA AHAW Panel, 2014; Tuppurainen et al., 2017), and there is lack of evidence of studies showing safety of SPP/GTP vaccines in EU breeds.
2.5. Geographical distribution of SPP/GTP
Historically SPP/GTP has been present in almost the whole African and Asian continents (Figure 1), where cases regularly occur (Tuppurainen et al., 2017). The diseases are also endemic in Turkey and, in the last decade, outbreaks were reported also in the EU, in Bulgaria and in Greece. OIE reported outbreaks of SPP/GTP since 2010 are displayed in Figure 2 and Table 1.
Figure 1.
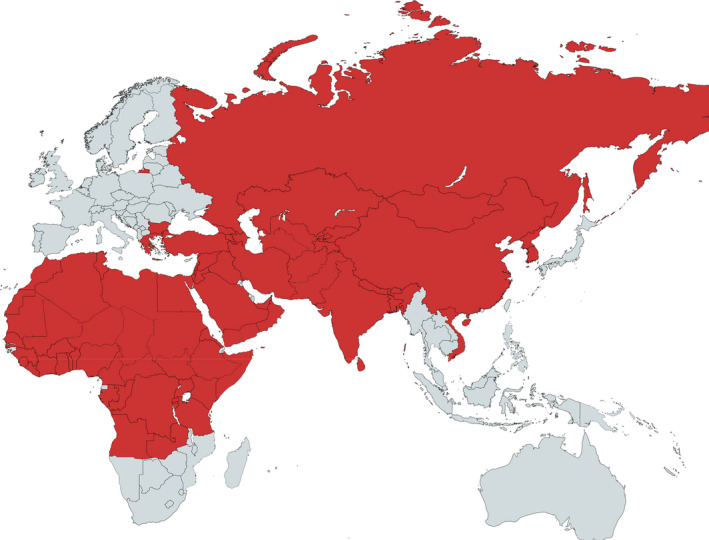
Historical geographic distribution of SPP/GTP (modified from Tuppurainen et al., 2017)
Figure 2.
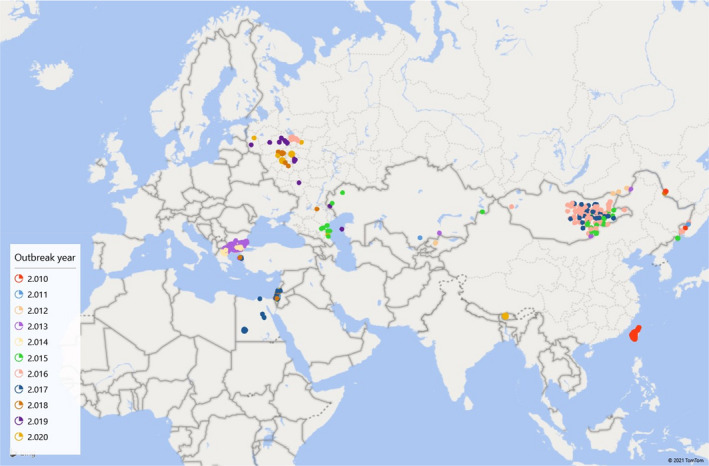
Map of countries with reported outbreaks of SPP/GTP between 2010 and 2020 (Data sources: OIE)
Table 1.
OIE reported outbreaks of SPP/GTP since 2010
| Country | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Azerbaijan | |||||||||||
| Bhutan | 2 | ||||||||||
| Bulgaria | 7 | ||||||||||
| Chinese Taipei | 298 | ||||||||||
| Egypt | 5 | ||||||||||
| Greece | 108 | 119 | 2 | 29 | 4 | ||||||
| Israel | 2 | 2 | 1 | 1 | 14 | 4 | 1 | ||||
| Kazakhstan | 1 | 1 | 3 | 2 | |||||||
| Kyrgyz Republic | 2 | ||||||||||
| Mongolia | 1 | 17 | 69 | 31 | |||||||
| Russian Federation | 3 | 1 | 3 | 1 | 9 | 18 | 12 | 13 | 8 | ||
| Vietnam |
3. Data and methodologies
3.1. Methodology used in ToR 1
Although the general methodology applied to all opinions covering the assessment of control measures for the Category A diseases produced under this mandate has been published elsewhere (EFSA AHAW Panel, 2020), specific details of the methodology related to the SPP/GTP opinion are presented below.
3.1.1. Mathematical model for within‐herd dynamics of SPP/GTP and transmission scenarios considered
3.1.1.1. Model description
The within‐herd dynamics of sheep and goat pox virus (SPPV/GTPV) in small ruminants were modelled using a stochastic SEIR epidemic model (Keeling and Rohani, 2011). The small ruminant population was divided into four classes: susceptible (i.e. uninfected), S; exposed (i.e. infected, but not yet infectious), E; infectious, I; and recovered, R. Disease‐associated mortality was assumed to occur at a constant rate during the infectious period. No distinction was made between sheep and goats.
The force of infection is given by,
where β is the transmission rate, I(t) is the number of infectious animals and N(t) is the total number of animals at time t. This formulation assumes homogeneous mixing (i.e. individuals uniformly and randomly contact each other) and frequency‐dependent transmission (i.e. the number of contacts is independent of the population size) (Keeling and Rohani 2011). The durations of the latent and infectious periods were assumed to follow gamma distributions with means μE and μI and shape parameters kE and kI, respectively (i.e. with variances μE 2/kE and μI 2/kI). This was incorporated in the model by subdividing the latent and infectious classes into kE and kI stages each of mean duration μE/kE and μI/kI, respectively (Anderson and Watson, 1980).
The number of animals in each class takes an integer value, while transitions between classes are stochastic processes. The number of transitions of each type during a small time interval δt was drawn from a binomial distribution with number of animals in the class, n; and transition probability, q (the appropriate per capita rate multiplied by δt), as parameters.
The initial herd size was assumed to be 50, 100, 500 or 1,000 small ruminants. Transmission parameters were estimated from a small transmission experiment (Wolff et al., 2020) using Bayesian methods (Hu et al., 2017), (Table 2 and Annex H for more details). This was the only study that could be found on SPPV/GTPV transmission. The experiment used a strain of GTPV that was identical to one from India isolated in 1983.
Table 2.
Parameters in the model for the transmission of sheep and goat pox virus in small ruminants
| Scenario | R0 | β† | μE | kE | μI | kI | Case fatality (%) |
|---|---|---|---|---|---|---|---|
| Low mortality | 5.8 | 0.24 | 4.9 | 2 | 29.0 | 2 | 20 |
| High mortality | 0.62 | 80 |
The transmission rate was calculated so that R0 is the same in the two scenarios for case fatality.
The within‐herd dynamics is shown in Figure 3.
Figure 3.
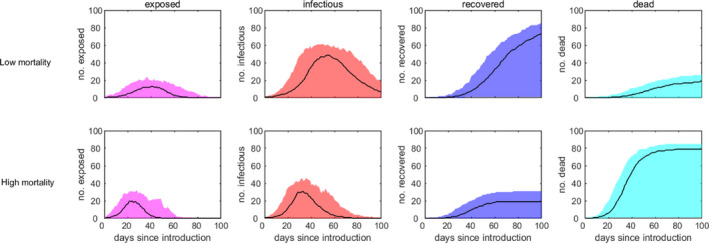
- The plots show the median (solid line) and 95% prediction interval (shading) for the number of exposed animals (first column; magenta), infectious animals (second column; red), recovered animals (third column; blue) and cumulative number of dead animals (fourth column; cyan) for four scenarios under different mortality rates in a flock of 100 animals (rows; see Table 2 for details).
The accuracy of the transmission scenarios (in a situation of natural spread, without control measures) mentioned above (30% and 50% infected animals after 1 month post introduction in the low and high mortality scenarios, respectively; see curves of exposed and infectious animals in Figure 3) could not be supported using evidence from the epidemics occurred in Bulgaria and Greece in 2014 since, in Bulgaria, there were only seven outbreaks reported (not enough to infer on SPPV/GTPV transmission) and, in Greece, the natural spread could not be extrapolated, since control was massive and immediate. Therefore, field evidence was gathered from the large SPP/GTP epidemic in Taiwan in 2010 with 300 notified outbreaks (data reported to OIE). The median value of morbidity (infected divided by susceptible animals) in that epidemic was 12% (lower and upper quartile 5–33%). The strategy used initially to control the spread of GTP during the epidemic was the immediate culling of infected animals, which afterwards was changed to vaccination with Kenyan vaccine strain of GTPV (Chan et al., 2014). The fact that control measures, including culling and vaccination, were implemented at an early stage in Taiwan can be assumed to have reduced the morbidity caused by the outbreak. With this in mind, the output from the simulated scenarios (30 and 50% infected animals after 1 month post introduction in the low and high mortality scenario, respectively) can be considered realistic.
3.1.1.2. Detection of sheep and goat pox virus
The prevalence of virus‐positive sheep and goats was assumed to correspond to the prevalence of infectious animals. At least 80% of infectious animals were assumed to show clinical signs, based on evidence provided in previous EFSA opinion (EFSA AHAW Panel, 2014). The proportion of animals seroconverting during the infectious period was estimated to be 80%. This is based on:
the latent and infectious period distributions in the model (Table 2);
a gamma distribution for the time to seroconversion with a shape parameter of 7 and a mean of 10.9 days (based on the data in Table 9); and
the latent and infectious periods and time to seroconversion are independent of one another.
Table 9.
Range of days for seroconversion and latest detected day of antibody presence in sheep and goats after experimental inoculation with sheep pox virus and goat pox virus
| Animals in the study | Laboratory method | Infection | Range of days for seroconversion (days post infection) | Latest day of antibodies detection/end of experiment | Total number of references | Reference ID | |
|---|---|---|---|---|---|---|---|
| Earliest day of seroconversion | Latest day of seroconversion | ||||||
| Sheep | VNT | Shaded/scarified skin | 14 (Afshar et al., 1986) | – | 18 months (Davies and Otema, 1978) | 2 | Davies and Otema (1978), Afshar et al. (1986) |
| ID | 4 (Boshra et al., 2015) | 21 (Bowden et al., 2009) | 63 (Bowden et al., 2009) | 2 | Bowden et al. (2009), Boshra et al. (2015) | ||
| IV + SC | 15 | – | 28 | 1 | Wolff et al. (2020) | ||
| In‐contact | 7 | – | 14 | 1 | Ayalet et al. (2012) | ||
| IgG‐ELISA | ID | 4 (Boshra et al., 2015) | 21 (Bowden et al., 2009) | 63 (Bowden et al., 2009) | 2 | Bowden et al. (2009), Boshra et al. (2015) | |
| DA‐ELISA | IV + SC | 21 | – | 28 | 1 | Wolff et al. (2020) | |
| In‐contact | No seroconversion | 1 | Wolff et al. (2020) | ||||
| Goats | VNT | ID | 10 (Boshra et al., 2015) | 14 (Bowden et al., 2009) | 63 (Bowden et al., 2009) | 2 | Bowden et al. (2009), Boshra et al. (2015) |
| IN | 15 | – | 28 | 1 | Wolff et al. (2020) | ||
| IV + SC | 10 | – | – | 1 | Wolff et al. (2020) | ||
| In‐contact | 7 (Ayalet et al., 2012) | 23 (Wolff et al., 2020) | – | 2 | Ayalet et al. (2012), Wolff et al. (2020) | ||
| IgG‐ELISA | ID | 4 (Boshra et al., 2015) | 21 (Bowden et al., 2009) | 63 (Bowden et al., 2009) | 2 | Bowden et al. (2009), Boshra et al. (2015) | |
| DA‐ELISA | IN | 21 | 28 | 29 | 1 | Wolff et al. (2020) | |
| IV + SC | 10 | – | – | 1 | Wolff et al. (2020) | ||
| In‐contact | No seroconversion | 1 | Wolff et al. (2020) | ||||
ID: intradermal; IN: intranasal; IV: intravenously; SC: subcutaneously; DA: Double‐antigen ELISA.
Using a similar reasoning, all recovered sheep and goats were assumed to have seroconverted.
The prevalence of infection or seropositivity is the proportion of live sheep and goats either virus positive or seropositive, so the denominator in the calculations is the initial herd size minus the cumulative number of animals that have died of SPP/GTP.
The infection prevalence of SPPV/GTPV reached at 7, 14, 21, 28 days post introduction of one infected animal is displayed in Table 3, while seroprevalence of SPPV/GTPV reached at 21, 28, 35, 42 days post introduction (longer time is needed for seroconversion, up to 21 dpi, see Section 4.2.3) is shown in Table 4. These values are useful to calculate the sample size needed for detection of suspect animals in an infected flock at different moments in time from introduction into the herd (see Section 4.1.1.1). Mortality rate due to SPP/GTP reached at 7, 14, 21, 28 post introduction is shown in Table 5.
Table 3.
Median (M), lower (L) and upper (U) 95% prediction intervals for the infection prevalence (%) of sheep and goat pox virus in sheep and goats at different days post introduction (dpi) into the herd
| dpi | Scenario | Herd size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 1,000 | ||||||||
| M | L | U | M | L | U | M | L | U | ||
| 7 | Low mortality | 2.0 | 2.0 | 6.1 | 1.0 | 0 | 4.0 | 0.1 | 0 | 0.4 |
| High mortality | 4.0 | 0 | 12.0 | 1.0 | 0 | 5.1 | 0.2 | 0 | 0.6 | |
| 14 | Low mortality | 6.0 | 0 | 16.0 | 4.0 | 0 | 11.0 | 0.3 | 0 | 0.8 |
| High mortality | 10.6 | 0 | 31.9 | 4.2 | 0 | 18.9 | 0.7 | 0 | 2.4 | |
| 21 | Low mortality | 12.0 | 0 | 36.0 | 7.0 | 0 | 22.0 | 0.7 | 0 | 1.9 |
| High mortality | 30.1 | 0 | 62.9 | 15.2 | 0 | 45.1 | 2.8 | 0 | 8.3 | |
| 28 | Low mortality | 20.6 | 0 | 54.0 | 13.3 | 0 | 44.8 | 1.6 | 0 | 4.1 |
| High mortality | 51.4 | 0 | 78.1 | 37.7 | 0 | 64.8 | 9.4 | 0 | 25.7 | |
Table 4.
Median (M), lower (L) and upper (U) 95% prediction intervals for the seroprevalence (%) of sheep and goat pox virus in sheep and goats at different days post introduction (dpi) into the herd4
| dpi | Scenario | Herd size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 1,000 | ||||||||
| M | L | U | M | L | U | M | L | U | ||
| 21 | Low mortality | 11.3 | 1.6 | 30.8 | 6.7 | 0.8 | 21.4 | 0.6 | 0 | 1.8 |
| High mortality | 26.3 | 0 | 60.5 | 13 | 0 | 38.2 | 2.4 | 0 | 7.3 | |
| 28 | Low mortality | 22.1 | 2.0 | 53.5 | 12.7 | 0.8 | 38.8 | 1.5 | 0 | 3.8 |
| High mortality | 51.7 | 0 | 80 | 32.1 | 0 | 64 | 8.4 | 0 | 22.2 | |
| 35 | Low mortality | 37.2 | 2.0 | 68.9 | 24.3 | 1.0 | 60 | 3.5 | 0 | 8.0 |
| High mortality | 71.4 | 0 | 90 | 59.2 | 0 | 83.1 | 23.5 | 0 | 47.3 | |
| 42 | Low mortality | 56.4 | 2.0 | 77.4 | 42.8 | 1.0 | 75.7 | 7.4 | 0 | 17.9 |
| High mortality | 82.8 | 0 | 96.4 | 75.5 | 0 | 91.2 | 49.5 | 0 | 69.5 | |
Table 5.
Median (M), lower (L) and upper (U) 95% prediction intervals for the cumulative number of sheep and goats in a herd dying due to infection with sheep and goat pox virus at different days post introduction (dpi) into the herd
| dpi | Scenario | Herd size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 1,000 | ||||||||
| M | L | U | M | L | U | M | L | U | ||
| 7 | Low mortality | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| High mortality | 1 | 0 | 2 | 1 | 0 | 2 | 1 | 0 | 2 | |
| 14 | Low mortality | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 |
| High mortality | 2 | 0 | 8 | 2 | 0 | 8 | 3 | 0 | 10 | |
| 21 | Low mortality | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 0 | 2 |
| High mortality | 8 | 1 | 18 | 7 | 1 | 22 | 11.5 | 0 | 32 | |
| 28 | Low mortality | 1 | 0 | 4 | 1 | 0 | 5 | 1 | 0 | 4 |
| High mortality | 17 | 1 | 29 | 20.5 | 1 | 43 | 45.5 | 0 | 115 | |
3.2. Methodology used in ToR 2
3.2.1. Time lag between infection and reporting
To estimate the time lag between infection and reporting of an SPP/GTP suspicion (ToR 2), an extensive literature search (ELS) was outsourced by EFSA (OC/EFSA/ALPHA/2020/02 – LOT 2). The aim of this ELS was to answer the epidemiological question of: ‘what is the average, shortest and longest period of time for an outbreak of SPP/GTP to be reported (measured as the number of days from the earliest point of infection with SPPV/GTPV to the time of declaration of a suspicion by the competent authority after the clinical investigation by an official veterinarian)?’. To answer this question, an ELS on case reports, papers describing outbreaks or epidemics of SPP/GTP, and any other relevant grey literature or data was carried out. For the inclusion criteria in the ELS, the earliest point of infection had to have been estimated by carrying out an epidemiological investigation. Papers and other sources of data where the earliest point of infection was determined purely by subtracting a known incubation period from the date of the suspicion of the outbreak were excluded. The ELS was restricted to studies conducted in Europe or describing results obtained in Europe. If none or very few articles were retrieved (less or equal to 5) in the first search, the search was extended to the rest of the world. An ELS protocol similar to that shown in Annex 5 of the Methodology report (EFSA AHAW Panel, 2020) was followed.
3.2.2. Seroconversion period
Considering scenario 5 of the second ToR ‘the earliest day of the seroconversion after the infection, detected by different serological methods in different animal species is necessary to be identified for each disease of concern. In addition, the time interval between the earliest day of antibodies detection and the latest day of antibodies detection by different laboratory methods would be useful’, a scientific literature review on the earliest day of seroconversion and the latest day of antibodies detection after infection for SPP/GTP and the relevant target population (listed species) of the disease, was considered appropriate to successfully address this scenario.
3.2.2.1. Objectives
The objectives of the literature review were to identify:
the earliest date when antibodies are detected after infection/inoculation for each serological test used, for different animal species,
the duration of serological positivity after infection/inoculation for each serological test used, for each animal species,
the target population (listed species) for the disease.
The methodology used to perform the literature search is described in Annex J.
3.3. Methodology used in ToR 3
3.3.1. Methodology for assessing the effectiveness of the minimum radius of the protection and surveillance zones
The assessment of radius size of restricted zones (ToR 3), to prevent further disease spread at a given probability, was performed by using disease transmission kernels (EFSA AHAW Panel, 2020).
In Annex I, details on the modelling approach used to estimate kernels for the spread of sheep and goat pox between farms are presented.
3.3.2. Methodology for assessing the effectiveness of the duration of the protection and surveillance zones
To estimate the duration of measures in the protection and surveillance zones, the outputs obtained from the ELS described in Section 3.2 were used. Further details can be found in the Methodology report (EFSA AHAW Panel, 2020).
3.4. Uncertainty
A description of the methodology followed to deal with uncertainty is provided in a Methodology report published by EFSA AHAW Panel (2020).
4. Assessment
4.1. Assessment of sampling procedures (ToR 1)
4.1.1. Assessment of sampling procedures in the event of suspicion or confirmation of SPP/GTP
4.1.1.1. In the event of a suspicion of SPP/GTP in an establishment where animals of the listed species are kept
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect SPPV/GTPV in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). For further details, see Annexes B and C.
1.
1st Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 6(2) of the Delegated Regulation (EU) 2020/687
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It concerns an event of suspicion of SPP/GTP in an establishment with kept animals of the listed species;
The listed species for SPP/GTP as provided in Commission Implemented Regulation 2018/1882 are those belonging to the Bovidae family;
Subsequent to the suspicion, the competent authority shall immediately conduct an investigation to confirm or rule out the presence of the disease;
The official veterinarian must perform a clinical examination and collect samples for further laboratory examination (see Annex C for details on guidelines on how the clinical and laboratory examination must be carried out).
Summary of sampling procedures
No specific guidelines on sampling procedures for clinical or laboratory examination in the event of a suspicion of SPP/GTP are available in the EU legislation.
Severe forms of SPP/GTP show highly characteristic clinical signs and lesions that can be recognised by experienced veterinarians and farmers, with many infected animals exhibiting all the symptoms of infection. In general, clinical diagnosis is effective, particularly in naive populations where clinical signs are fully expressed and subsequently confirmed by laboratory diagnosis with high agreement as described in EFSA AHAW Panel (2014). On the other hand, mild forms of SPP/GTP may be confused with contagious ecthyma (contagious pustular dermatitis, orf), bluetongue, peste des petits ruminants, etc.
Guidelines for sampling procedures in case of SPP/GTP suspicion are reported in other available documents. They refer more particularly to the optimal period of sampling. The manual by CFSPH (2017) as well as Department of Agriculture and CSIRO (2019) suggest that samples for virus isolation and for PCR should be collected during the first week of clinical illness, at the viraemic stage of infection before neutralising antibodies develop. After development of neutralising antibodies, the virus isolation and the PCR are unlikely to be successful, due to short viraemia and neutralised virus being cleared by the immune system.
Lamien et al. (2006) suggest collecting serum samples for serology from acute and chronic cases and at least 2–3 weeks after the appearance of skin lesions.
In relation to the number of samples to be taken and submitted for laboratory diagnosis in the event of a suspicion, Department of Agriculture and CSIRO (2019) suggests to collect serum samples from at least 10 live, clinically affected animals, and from convalescent and/or recovered animals. In CABI (2015), it is suggested that biopsy specimens on live animals should include samples from two or three lesions at the papular or vesicular stage and concerning specimens collected at post‐mortem, one or two severely affected acute cases are preferable.
Assessment
The collection of specimens for PCR testing can be performed either on dead or live animals. On live animals, samples should be collected within the first week of clinical signs, before neutralising antibodies develop, to maximise the probability of detecting the viral genome (see above). The recommended specimens from live animals are fresh tissue from characteristic pox skin lesions (e.g. biopsies, scrapings, vesicular fluid, scabs); oral, nasal and ocular secretions; anticoagulant‐treated blood collected aseptically from early febrile cases (7–10 mL/animal). As a complement to molecular detection, in order to make a definitive diagnosis, SPPV and GTPV can be isolated in lamb testis cell culture, sheep or goat kidney cell cultures and sheep, goat or bovine cell lines.
In dead or euthanised animals, the best samples for PCR examination are pox lesions, i.e. nodules/scabs, either on skin or in the respiratory (nasal turbinates, trachea, lungs) and gastrointestinal tract, or on enlarged lymph nodes. All samples must be refrigerated and quickly dispatched to the laboratory (CIRAD, 2019).
SPPV or GTPV are readily distinguishable in the laboratory from other poxviruses that cause similar clinical signs in ruminants by confirming the presence of the virus nucleic acids using PCR in combination with a clinical history consistent with generalised SPPV/GTPV infection (Haegeman et al., 2013).
Complementing the PCR testing, indirect ELISA can confirm infection with SPPV/GTPV in a disease‐free country by detection of specific antibodies if no vaccination is implemented. To determine the progression of antibody response against GTPV and SPPV in sheep and goats, the collection of paired blood samples, 3 weeks apart, starting from 10 to 14 days after infection is required. Sampling of the same animals would be required, which is not always feasible in the field. Serological surveys are useful to determine the presence or absence of capripoxvirus infection in a defined country or area and its extent in a population but not by specific virus species.
SPPV, GTPV and LSDV cannot be differentiated from each other by serological tests (ELISA, VNT). Similarly, immunoperoxidase staining (IPMA) or immunofluorescence fluorescent antibody test (IFAT), AGID; histopathology and electron microscopy can distinguish the agent as a capripoxvirus, but not by specific species.
Development of new procedures
Suspicion of SPP/GTP will normally be raised based on clinical signs and lesions. Clinical signs and lesions of SPP/GTP are usually fully expressed in naive populations, such as in EU, with many infected animals exhibiting most symptoms of infection (high fever, pox lesions in the skin and mucous membranes, eye and nasal discharge and enlarged lymph nodes) at the same time, allowing effective clinical surveillance and suspicion. Animals with clinical disease should be sampled for confirmation and pox lesions are the most suitable matrix sample for PCR, being the test of choice for confirmation. Post‐mortem examination should be carried out on euthanised or recently dead susceptible animals for the collection of organs and tissues on which PCR could be performed.
It must be considered that, according to the model simulation as displayed in Table 3, in a period between 14 and 21 days after disease introduction in, e.g. a 100‐head flock, the infection prevalence would range between 4% and 30% and, if it is assumed that at least 80% infected animal in previously disease‐free areas can show clinical signs (EFSA AHAW Panel, 2014), at least three animals are expected to be clinically affected and show clinical signs, that could be easily detected. Given this, considering the high sensitivity of PCR (100%, see Section 2.3), the probability of not detecting the infection after testing three affected animals is almost nil.
In case of suspicion because of previous contact, import, etc., if clinical signs are not so evident, the sampling of randomly selected asymptomatic animals can be performed based on the expected infection prevalence from time of introduction. ELISA for antibody detection can also be performed from day 21 after the suspected introduction of the virus. Calculated sample sizes to detect SPP/GTP with a 95% confidence using PCR and ELISA test in a 50‐, 100‐ or 1,000‐head flock at different times post virus introduction are displayed in Tables 6 and 7, respectively.
Table 6.
Sample size for random sampling to detect sheep and goat pox virus infection with 95% confidence based on different values of infection prevalence (median values) at 7, 14, 21, 28 days after virus introduction into the herd for testing by PCR (Se: 100%%, Sp: 100%), for different scenarios of mortality and herd sizes
| Days post introduction | Scenario | Herd size | ||
|---|---|---|---|---|
| 50 | 100 | 1,000 | ||
| 7 | Low mortality | 48 | 96 | 951 |
| High mortality | 39 | 96 | 777 | |
| 14 | Low mortality | 32 | 53 | 632 |
| High mortality | 22 | 53 | 348 | |
| 21 | Low mortality | 19 | 35 | 348 |
| High mortality | 9 | 18 | 101 | |
| 28 | Low mortality | 13 | 20 | 170 |
| High mortality | 5 | 7 | 31 | |
Table 7.
Sample size for random sampling to detect sheep and goat pox virus serological positivity with 95% confidence based on different values of seroprevalence (median values) at 21, 28, 35, 42 days after virus introduction into the herd for testing by ELISA (Se: 96.3%, Sp: 95.4%), for different scenarios of mortality and herd sizes
| Days post introduction | Scenario | Herd size | ||
|---|---|---|---|---|
| 50 | 100 | 1,000 | ||
| 21 | Low mortality | 20 | 36 | 408 |
| High mortality | 10 | 21 | 121 | |
| 28 | Low mortality | 12 | 21 | 188 |
| High mortality | 5 | 9 | 36 | |
| 35 | Low mortality | 7 | 12 | 85 |
| High mortality | 4 | 5 | 13 | |
| 42 | Low mortality | 5 | 7 | 41 |
| High mortality | 3 | 4 | 6 | |
In case of very recent virus introduction (e.g. 7 days post introduction), to carry out a large number of PCR tests may not be feasible; in that case it would be more advisable to put animals in quarantine, check possible development of clinical signs and then test by ELISA (which is more feasible) 21 days post suspicion.
4.1.1.2. For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an establishment affected and officially confirmed with SPP/GTP
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. For further details, see Annexes B and C.
1.
2nd Scenario of sampling procedures
ToR 1.2 in accordance with Mandate
Article 12(3) and the Art. 7 (4) (Preventive killing) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
The following elements of the scenario were taken into consideration for the assessment:
It concerns an establishment officially confirmed as affected by SPP/GTP;
Kept animals of listed species found dead or before/when they are killed are sampled;
Competent authority collects samples for laboratory examination;
-
The purposes of the sampling are:
-
supporting the epidemiological enquiry to:
identify the likely origin of the disease;
calculate the likely length of time that the disease is present;
identify establishments where the animals could have contracted the disease and movements from the affected establishment that could have led to the spread of the disease; and
obtain information on the likely spread of the listed disease in the surrounding environment, including the presence and distribution of disease vectors
confirming/ruling out disease in the event of preventive killing.
-
Summary of sampling procedures
There are no sampling procedures defined for the purposes of the epidemiological enquiry in an establishment affected and officially confirmed with SPP/GTP.
Assessment
When SPP/GTP has been officially confirmed in an establishment, further sampling procedures will support the needs of the epidemiological enquiry to obtain information on the origin of the disease, and the length of time that the disease is present. In addition, in case preventive killing is decided, suppling procedures will confirm or rule out the disease.
Development of new procedures
Estimate the prevalence of animals with clinical signs within the affected establishment
For this purpose, all live animals should be subjected to individual clinical examination and dead animals should be examined. In an establishment where the number of animals is large, and therefore, the individual clinical examination of all the animals is not feasible, a minimum sample of animals should be taken, to detect or rule out the presence of animals with clinical signs with at least a 95% confidence, as described in Section 4.1.1.1. The clinical signs and lesions (skin nodules, lacrimation, nasal and oral discharges, lesions at the mucous membranes of eyes, nose and mouth, etc.) should be described and recorded per animal.
To estimate the length of time that the disease is present in the establishment and its possible origin
An approximate estimation of the length of the presence of SPP/GTP in the establishment can be based on the age of the pox lesions identified (taking into account the incubation period 4–14 days), by thorough individual clinical examination of the animals that are still alive, or necropsy of those that are found dead. Skin lesions are usually first noticed on the face, around the lips and nares and on the eyelids and start to develop at the onset of initial fever reaction. Skin lesions progress through macular, papular, vesicular and pustular stages until scabs form: The initial rise in body temperature to above 40°C is followed in 2–5 days by the development of macules and then papules. Within 24 h of generalised papules, the animals develop rhinitis, conjunctivitis and enlarged lymph nodes. In the following 6–10 days, the papules form scabs, which persist for 6 weeks, leaving small scars (OIE, 2010). The lesions may cover the entire body but are more easily detected on the hairless parts of the skin and mammary glands.
Virus is preferably isolated from skin lesions (see Section 4.1.1.1). Sequencing of these isolates followed by phylogenetic analysis may help to identify the possible origin of the virus by comparison with the genetic profile of other isolates (e.g. incursion of infection from neighbouring countries/areas).
Antibodies are detectable, respectively, in sheep and goats within 4–21 days and 10–14 days post‐infection (see Section 4.2.3) and it can be assumed that they remain detectable for the whole productive life of the animals. Consequently, detection of antibodies suggests that infection occurred more than 4 days prior to sampling, but no other inferences can be made upon the time of exposure on the basis of serological results. No commercial tests are available for the detection of IgM and other more transient antibody classes.
Confirm or rule out SPP/GTP when preventive killing is implemented
In the Delegated Regulation, preventive killing may be applied for the animals of species listed for SPP/GTP (Ovis spp., Capra spp.) to reduce the likelihood of undetected spread in three cases: (i) in an establishment where SPP/GTP is suspected, (ii) in the establishments in temporary restricted zones and (iii) in the establishments of the restricted zones (i.e. the protection and surveillance zones and further restricted zones).
Before preventive killing is undertaken, all animals in the establishment should be subjected to individual clinical examination and if animals with clinical signs are identified, there is no need to continue the individual clinical examination; the establishment should be considered as suspected and the procedures as described in Section 4.1.1.1 should be followed.
In an establishment where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals should be clinically examined to ensure a confidence level of at least 95% to detect or rule out the disease.
4.1.1.3. For granting a specific derogation from killing animals of the categories described in article 13.2 of the Delegated Regulation in an SPP/GTP affected establishment
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. For further details, see Annexes B and C.
1.
3rd Scenario of sampling procedure
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 13(3)c of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration during for the assessment:
It concerns an establishment officially confirmed as affected by SPP/GTP;
-
In the establishment where there are kept animals of listed species of the following specific categories animal categories based on article 13(2):
animals kept in a confined establishment;
animals kept for scientific purposes or purposes related to conservation of protected or endangered species;
animals officially registered in advance as rare breeds;
animals with a duly justified high genetic, cultural or educational value;
the competent authority may grant specific derogation from killing all the animals of listed species belonging to any of the above categories in an affected establishment, provided that specific conditions are fulfilled;
The animals should be subjected to clinical surveillance, including laboratory examinations;
Sampling procedures should ensure that the animals do not pose a risk of transmission of the category A disease if left alive.
Summary of sampling procedures
There are no sampling procedures to grant a derogation from killing of animals in an affected establishment.
Assessment
Animals in an affected establishment and for which a specific derogation from killing has been granted, should be subjected to clinical and laboratory examination. Sampling procedures should ensure that the animals do not pose a risk of transmission if left alive.
Animals of the holding that are negative for antibodies and virus at least after a monitoring period after virus introduction, do not pose a risk of transmission of SPPV/GTPV.
Development of new procedures
The number of animals to ask for derogation from killing in a herd is generally small because derogation may deal with endangered species, rare breeds, high genetic, cultural or educational value animals (see above). Therefore, given the risk of not killing animals from affected establishment, all of them should be checked thoroughly clinically, preferably every day or at least at weekly interval for a period of at least the monitoring period of 21 days calculated forwards from the day of confirmation of the latest case. In case of clinical signs, samples should be taken for direct diagnosis (see Section 4.1.1.1 for details).
Sampling all animals for laboratory examination (both for virus detection and antibodies is feasible since it can be assumed to concern a limited number of animals, see above), as soon as the derogation from killing is granted and irrespective of the presence of clinical signs, will enable to identify additional infected animals without clinical signs. Sampling for laboratory examination can be repeated at any time, but the last sampling should be carried out not earlier than 21 days calculated forwards from the day of confirmation of the latest case.
Sampling procedures for laboratory examinations in order to detect or rule out the presence of SPP/GTP virus should follow the procedures described in Section 4.1.1.1.
4.1.1.4. For the animals of non‐listed species kept in an SPP/GTP affected establishment
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. For further details, see Annex B.
1.
4th scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Article 14(1) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario should be taken into consideration during for the assessment:
It concerns an establishment officially confirmed as affected by SPP/GTP;
In the affected establishment there are kept animals of non‐listed species of epidemiological relevance for the control of the disease;
Animals of non‐listed species are those animals that are not listed in Commission Implementing Regulation (EU) 2018/1882 for each of the category A diseases;
The animal species acting purely as mechanical carriers of the virus will not be covered;
The competent authority is not obliged to carry out the sampling of non‐listed species, but they may establish it in addition to other measures;
The purpose of the sampling procedures is to ensure detection of the virus in these species.
Summary of sampling procedures
There are no sampling procedures defined for of non‐listed species kept in an affected establishment by SPP/GTP.
Assessment
The listed species for SPP/GTP according to Commission Implementing Regulation (EU) 2018/18825 are Ovis spp. and Capra spp. Thus, all susceptible animals belonging to these genera, domestic or wild, should undergo the same sampling procedures. No other genera are known to have any epidemiological relevance for the control of SPP/GTP.
4.1.1.5. For wild animals of the listed species within a SPP/GTP affected establishment and its surroundings
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. For further details, see Annex B.
1.
5th scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Article 14(1) of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Commission Implemented Regulation 2018/1882 on listed species
The following elements of the scenario were taken into consideration for the assessment:
It concerns an establishment officially confirmed as affected by SPP/GTP;
It refers to wild animals of listed species within the establishment and in the surroundings of the establishment;
As listed in Commission Implementing Regulation (EU) 2018/1882 for SGP; the wild animals of listed species animals are those wild species belonging to Ovis and Capra genera;
The competent authority may establish these sampling procedures in addition to other measures;
The purpose of the sampling procedures in wild animals of listed species is to ensure the detection of the virus, if the virus is present in these wild animals.
Summary of sampling procedures
There are no sampling procedures defined for wild animals of the listed species within the SPP/GTP‐affected establishment and its surroundings.
Assessment
In the scenario where wild sheep or goats belonging to genus Ovis spp. and Capra spp. are kept or living in the surrounding area of the affected establishment, these may acquire the infection by direct or indirect contact with affected animals, if no or low biosecurity measures are in place to keep animal species separated.
Development of new procedures
The surveillance of wildlife around the affected establishment may include the visual inspection of these animals from distance and clinical and laboratory examination of fallen stock and hunted animals both by PCR and serology. Unexpected mortality events in susceptible wildlife should be investigated the same way.
Samples from animals with clinical signs from dead or hunted animals should be collected for laboratory analysis, following the procedures of Section 4.1.1.1. Wildlife population health experts would be able to provide additional advice in these circumstances, about what kind of wild animals should be sampled, under which epidemiological suspicions, which type of sample matrix, etc.
4.1.1.6. For animals of listed species in the non‐affected establishments located in a protection zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. For further details, see Annexes B and C.
1.
6th Scenario of sampling procedures
ToR 1.1 and ToR 1.2 in accordance with Mandate
Article 26(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario should be taken into consideration during for the assessment:
It concerns the protection zone with radius up to 3 km;
Official veterinarians must visit at least once all the non‐affected establishments with kept animals of listed species located in the protection zone;
Among others, they must perform a clinical examination of kept animals of listed species and if necessary, collection of samples for laboratory examination;
The purpose of sampling procedures is to confirm or rule out the presence of SGP.
Summary of sampling procedures
There are no sampling procedures defined for animals of listed species in the non‐affected establishments located in a protection zone for SPP/GTP.
Assessment
All establishments located in the protection zone should be visited and the animals should be subjected to clinical surveillance.
Development of new procedures
In an establishment where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined, to detect or rule out the presence of animals with clinical signs with at least a 95% confidence, as described in Section 4.1.1.1.
In case of suspicion, the sampling procedures as described in Section 4.1.1.1 would apply.
For the purpose of this scenario, the guidelines provided in Section 4.1.1.1 can be followed based on whether clinical signs are observed or not at the clinical examination.
Active surveillance via serological or PCR testing of randomly selected animals (i.e. in absence of clinical signs) should be conducted only if this could be considered necessary due to epidemiological considerations in the affected establishment in the protection zone (for active surveillance approach a risk‐based sampling approach can be used, e.g. a two‐stage sampling, cluster sampling or); for example due to anamnesis of an epidemiological link with the affected establishment (e.g. movement of animals from the affected establishment, prior to outbreak). Raised awareness and enhanced passive surveillance should also be recommended.
4.1.1.7. For non‐affected establishments located in a protection zone with a radius larger than 3 km
This scenario is not applicable, since, for SPP/GTP, it is not foreseen that the protection zone is larger than 3 km radius.
4.1.1.8. For non‐affected establishments located in a surveillance zone
1.
8th scenario of sampling procedures:
ToR 1.3 in accordance with Article 41 of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
Ιt concerns the surveillance zone;
Sample of the establishments of kept animals of listed species in the surveillance zone;
Official veterinarians carry out visits to a sample of the establishments among others perform clinical examination of kept animals of listed species and if necessary, collection of samples for laboratory examination;
The purpose of sampling procedure is to ensure the detection of the disease if the disease is present in any of the establishments.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone. For further details, see Annex B.
Summary of sampling procedures
There are no sampling procedures defined for animals of listed species in the non‐affected establishments located in a surveillance zone for SPP/GTP.
Assessment
It is very unlikely (1–10%) (EFSA Scientific Committee, 2018) that establishments in the surveillance zone (see Section 4.3.1), not epidemiologically linked with an outbreak, will become infected with SPPV/GTPV without having additional outbreaks in the protection zone.
Consequently, for the surveillance zone, it is recommended that the efforts will be allocated to enhance passive surveillance by increasing awareness in all establishments, industry and public, to be able to detect any clinical signs typical of SPP/GTP.
Development of new procedures
Any establishment where typical clinical signs of SPP/GTP such as fever, pox lesions on the skin and mucous membranes, eye and nasal discharge and enlarged lymph nodes and even changes in the individual animal behaviour and /or in the feed intake are reported should be visited, the animals should be clinically examined and samples should be collected following the procedures described in Sections 4.1.1.1.
4.1.2. Assessment of sampling procedures to grant derogations for animal movements
4.1.2.1. From non‐affected establishments located in the protection zone to slaughterhouses located within the protection zone or in the surveillance zone or outside the restricted zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29). For further details, see Annex B.
1.
9th Scenario of sampling procedures
ToR 1.4 in accordance with Article 28(5) of the Delegated Regulation (EU) 2020/687
Article 29 of the Delegated Regulation
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
Grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
Animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No sampling procedures are defined for SPP/GTP for this scenario.
Development of new procedures
All the animals in the establishment of origin should be clinically examined 24 h before their movement following the procedures described in Section 4.1.1.1.
In an establishment where the number of animals is large, and therefore, the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined to detect or rule out the presence of animals with clinical signs with at least 95% confidence, as described in Section 4.1.1.1.
Where clinical signs compatible with SPP/GTP are identified, the establishment is considered as suspect, the procedures described in Section 4.1.1.1 are followed and no movements are allowed in this situation.
In addition to clinical examination, if animals are moved to a slaughterhouse located outside the restricted zone, laboratory examination of samples from the animals intended to be moved is necessary to rule out the presence of SPP/GTP, with a confidence level of 95%. The procedures of Section 4.1.1.1 for sampling in the absence of clinical signs should be followed.
4.1.2.2. From non‐affected establishments located in the protection zone to a plant approved for processing or disposal of animal by‐products in which the animals are immediately killed
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art. 37). For further details, see Annexes B and C.
1.
12th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 28(5) and article 37 of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the protection zone;
To grant derogation for movement of kept animals of listed species from a non‐affected establishment in the protection zone;
The animals to be moved to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed;
Clinical examinations and laboratory examinations of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No specific guidelines on sampling procedures for clinical or laboratory examination were found for the 12th Scenario in EU legislation.
Assessment
This scenario is very similar to the 9th scenario of Section 4.1.2.1 and therefore the assessment is the same.
Development of new procedures
This scenario is very similar to the 9th scenario of Section 4.1.2.1; therefore, the same new procedures are suggested.
4.1.2.3. From an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone and from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: (a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, (b) from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone. For further details, see Annexes B and C.
1.
13th Scenario of sampling procedures
ToR 1.4 in accordance with Mandate
Article 43(5) and article 44 of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the:
It concerns kept animals of listed species of the establishments in the surveillance zone;
To grant derogation for movement from an establishment in the surveillance zone to be moved to a slaughterhouse within the restricted zone or outside the restricted zone;
To grant derogation for movement from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No sampling procedures are defined for SPP/GTP for this scenario.
Assessment
This scenario is very similar to the scenario of Section 4.1.2.1, and therefore, the assessment is the same.
Development of new procedures
To grant derogations for animal movements from an establishment in a surveillance zone to a slaughterhouse located outside the restricted zone, clinical examination and sample collection for laboratory investigation for should be performed as described in Section 4.1.1.1.
For animals intended to be moved from an establishment located outside the surveillance zone to a slaughterhouse situated in the surveillance zone, there is no need for laboratory examination, if there are no other reasons based on the national risk assessment to recommend it (e.g. epidemiological link with affected establishment or with affected or high‐risk area). Only clinical examination as described above would be enough.
4.1.2.4. From an establishment in a surveillance zone to pastures situated within the surveillance zone
1.
14th scenario of sampling procedures
ToR 1.4 in accordance with article 43(5) and article 45(1) of the Delegated Regulation(EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns kept animals of listed species from establishments located in the surveillance zone;
To grant derogation for movement from the surveillance zone;
To be moved to pastures situated within the surveillance zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for these animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone. For further details, see Annex B.
Summary of sampling procedures
No sampling procedures are defined for SPP/GTP for this scenario.
Development of new procedures
All the animals in the establishment of origin should be clinically examined 24 h before their movement following the procedures described in Section 4.1.1.1.
In an establishment where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined to detect or rule out the presence of animals with clinical signs with at least 95% confidence as described in Section 4.1.1.1.
If animals with clinical signs are identified, the establishment is considered suspect and the procedures for the laboratory confirmation described in Section 4.1.1.1 should be followed. There is a need for increased vigilance on the part of farmers as the grazing animals are usually less supervised and the observation of clinical signs may delay.
4.1.2.5. From an establishment in a surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter. For further details, see Annex B.
1.
15th scenario of sampling procedures
ToR 1.4 in accordance with article 43(5) and article 45(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment
It concerns the surveillance zone;
Grant derogation for movement of kept animals of listed species;
from the surveillance zone;
To be moved to an establishment belonging to the same supply chain, located in or outside the surveillance zone, to complete the production cycle before slaughter;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
Summary of sampling procedures
No specific guidelines on sampling procedures for clinical or laboratory examinations were found for the 15th Scenario in EU legislation.
Assessment
Animals in a surveillance zone for which a specific derogation has been granted to be moved to an establishment of the same supply chain located in or outside the surveillance zone should be subjected to clinical and, when relevant, laboratory examinations.
Development of new procedures
All the animals in the establishment of origin should be clinically examined before their movement to an establishment belonging to the same supply chain within the surveillance zone, following the procedures described in Section 4.1.1.1. Visual inspection of the herd would be helpful to identify animals with signs compatible with SPP/GTP.
In an establishment, where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined to detect or rule out the presence of animals with clinical signs with at least a 95% confidence, as described in Section 4.1.1.1.
If animals with clinical signs are identified, the establishment is considered as suspect and the procedures for the laboratory confirmation that are described in Section 4.1.1.1 should be followed, and movement prohibited until confirmation of being negative.
If the animals from an establishment in the surveillance zone are moved to an establishment belonging to the same supply chain, located outside the surveillance zone, in addition to the clinical examination, laboratory examination for presence of virus and antibodies of samples from the animals intended to be moved is necessary to rule out the presence of SPP/GTP, with a confidence level of 95%. The procedures of Section 4.1.1.1 for sampling in the absence of clinical signs should be followed.
4.1.2.6. From an establishment located in the restricted zone to move within the restricted zone when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation
1.
18th scenario of sampling procedures
ToR 1.4 in accordance with article 56(1) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the restricted zone when restriction measures are maintained beyond the period set out in Annex XI;
To grant derogation for movement of kept animals of listed species from an establishment within the restricted zone;
Clinical examinations and laboratory examination of animals kept in the establishment, including those animals to be moved.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation. For further details, see Annex B.
Summary of sampling procedures as described in the diagnostic manual
No specific guidelines on sampling procedures for clinical or laboratory examination were found for the 18th Scenario.
Assessment
Animals in the restricted zone, for which a specific derogation has been granted for movement within the restricted zone, should be subjected to clinical examination.
In case of suspicion, then the scenario as in Section 4.1.1.1 applies.
Development of new procedures
Sampling procedures should be implemented as described in Sections 4.1.2.1, 4.1.2.3, 4.1.2.4 and 4.1.2.5.
4.1.3. Assessment of sampling procedures for repopulation purposes
4.1.3.1. For the animals that are kept for the repopulation prior to their introduction
1.
19th scenario of sampling procedures
ToR 1.5 in accordance with article 59(2) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulation of a previously affected establishment;
Animals intended to repopulation shall be sampled prior to their introduction into the establishment of destination;
The samples shall be collected from a representative number of animals to be introduced of each consignment from each establishment or from a representative number of animals of each consignment (if animals are all to be introduced at different times or from different establishments of origin);
Laboratory examinations;
The purpose sampling procedures is to rule out the presence of the disease.
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. For further details, see Annex B.
Summary of sampling procedures as described in the diagnostic manual
No specific guidelines on sampling procedures for laboratory examination were found for the 19th scenario.
Assessment
For animals kept for repopulation, clinical examination and sampling should be used as standard procedures to ensure that the animals do not pose a risk of SPPV/GTPV transmission/re‐introduction. For animals that are introduced from disease‐free areas outside the restricted zone, laboratory testing can be omitted because they have not been exposed to SPPV/GTPV before entry and, consequently, can only produce a negative test result.
Development of new procedures
Animals intended for repopulation should be subjected to clinical examinations, to guarantee healthy animals are used for repopulation.
In case clinical signs compatible with SPP/GTP are identified, the establishment is considered as suspect and the procedures for the laboratory confirmation as described in Section 4.1.1.1 should be followed.
If animals are sourced from restricted areas, all the animals in the establishment of origin should be clinically checked and samples tested in laboratory, since they all must be negative. In the case at least one animal is positive (to clinical or laboratory examinations), no animal from the same establishment of origin should be introduced. In an establishment where the number of animals is large, sampling procedures for laboratory examination should ensure that the animals do not pose a risk of transmission at a 95% confidence level. Laboratory examinations should be in accordance with the procedures described in Section 4.1.1.1.
In case the animals originate from establishments located in disease‐free areas, there is no need for laboratory examination if there are no other reasons based on the authorities’ risk assessment to recommend it (e.g. epidemiological link with an affected establishment or with an affected or high risk area). Clinical examination as described above would be enough.
4.1.3.2. In the event of unusual mortalities or clinical signs being notified during the repopulation
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. For further details, see Annex B.
1.
20th scenario of sampling procedures
ToR 1.5 in accordance with article 59(9) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulated establishment;
Unusual mortalities or clinical signs during the repopulation;
The official veterinarians shall without delay collect samples for laboratory examination;
The purpose of sampling procedures is to rule out the presence of the disease.
Summary of sampling procedures as described in the diagnostic manual
No specific guidelines on sampling procedures for laboratory examination were found for the 20th scenario.
Assessment
In the case of unusual mortalities or clinical signs compatible with SPP/GTP are notified during the repopulation, it is important to rule out the presence of the disease.
Development of new procedures
In the event of unusual mortalities during repopulation, the establishment is considered as suspect. The repopulation should be stopped and the procedures for clinical and the laboratory confirmation to rule out the presence of SPP/GTP as described in Section 4.1.1.1 should be followed.
In addition, the establishments from where the suspect animals are coming from, should be considered as suspect; the procedures described in Section 4.1.1.1 should be followed as well.
4.1.3.3. For animals that have been repopulated
The purpose of this section is to assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. For further details, see Annex B.
1.
21st scenario of sampling procedures
ToR 1.5 in accordance with article 59(5) of the Delegated Regulation (EU) 2020/687
The following elements of the scenario were taken into consideration for the assessment:
It concerns the repopulated establishment;
Animals that have been used for repopulation;
Laboratory examinations;
Sampling procedures to rule out the presence of the disease.
Summary of sampling procedures
No specific guidelines on sampling procedures for laboratory examination were found for the 21st scenario.
Assessment
During the repopulation of an establishment previously affected by SPP/GTP, there is still a risk of re‐introduction of the disease with the new animals being infected either at the establishment of origin or during their transport, and a risk of re‐emergence of the disease if the new animals are infected after their arrival at the establishment of destination, or due to the virus being still present in the environment in the repopulated establishment, since SPPV/GTPV has a strong resistance in shaded environments, when protected from UV light. The animals that have been used for the repopulation should be submitted to thorough clinical and, if showing clinical signs, laboratory examination in order to rule out the presence of the disease.
Development of new procedures
Animals must be subjected to clinical inspection at least every 3 days for the first 14 days following the introduction, and weekly from 15 to at least 21 days (monitoring period as defined in the Commission Delegated Regulation (EU) 2020/687) after re‐introduction. The last day of the monitoring period following the latest day of animals’ introduction, all the animals should be subjected to thorough clinical examination as described in Section 4.1.1.1.
In an establishment where the number of animals is large, the individual clinical examination of all the animals may not be feasible; in this case a minimum sample of animals (including all animals moved) should be clinically examined, to detect or rule out the presence of animals with clinical signs with at least 95% confidence, as described in Section 4.1.1.1.
If clinical signs are identified, then the procedures for the laboratory confirmation that are described in Section 4.1.1.1 should be followed.
It is superfluous to remark that proper disinfection of the barns and structures of the repopulated establishment should be carried out before introducing new animals.
4.2. Assessment of the length of the monitoring period
The concept of the monitoring period was introduced as a management tool for the investigation and control of suspected and confirmed outbreaks of Category A diseases in terrestrial animals. This tool aimed to standardise the methodology by which relevant authorities responded to suspected and confirmed cases of these diseases. In this regard, a disease‐specific monitoring period was set for each of the 14 diseases included in the Category A list. Throughout the EU legislation, the monitoring period is used as an aid in the control of these diseases, although the specific purpose in which the monitoring period is used varies depending on the articles of the legislation.
The length of the monitoring period for each disease is set out in Annex II of the Commission Delegated Regulation (EU) 2020/687 supplementing the rules laid down in Part III of Regulation (EU) 2016/429 (Animal Health Law).
The table in Annex D describes the seven scenarios for which an assessment of the length of the monitoring period for SPP/GTP had been requested.
The details of the review protocol are in Annex E. A database search was carried out on 19/3/2021, identifying 114 unique references. As only a limited number of references were available for outbreak data (n = 2) and from the EU/EEA (n = 2), the search was extended to simulation data and to data from the rest of the world. Among the 114 references, six were selected to be included in the qualitative review. The full selection process is displayed in Figure 4 .
Figure 4.
PRISMA diagram SPP/GTP Monitoring period
4.2.1. Extracted data
The final extraction table is available in Annex E. Almost all references (5 out of 6) reported dates instead of periods; therefore, the dates were used to calculate the different periods of interest (as described in Annex E).
Table 8 provides an overview of the data that were extracted for the main outcome of interest, i.e. the period between the earliest point of infection and the suspicion report, for which two references were retrieved:
Table 8.
Summary of the SPP/GTP data extraction for the period between earliest point of infection and suspicion report outbreak data from Greece and Bulgaria in 2013–2014 (238 and 4 outbreaks reported to ADIS, respectively)
| Reference | Country | Year | Species | Period (days) |
|---|---|---|---|---|
| SCoFCAH (2013a,b) | Greece | 2013 | Sheep | 141 |
| SCoFCAH (2014) | Bulgaria | 2013 | Sheep | 22 |
NA: information not available.
Primary outbreak; Based on the estimated age of lesions at suspicion report and adding 10.5 days, the average incubation period (OIE, 2013).
Fourth outbreak detected in Bulgaria during the 2013 epidemic; Primary outbreak; no indication on the estimation method. Probable source of infection: illegal animal movement.
4.2.2. Discussion and conclusions
As described in Table 8 (outbreak data), the shortest period between the earliest point of infection and the suspicion report found in the selected references was 2 days. The latter concerned an outbreak, which occurred in 2013 in a sheep farm in Bulgaria (SCoFCAH, 2014). Although this was the primary outbreak in the Plovdiv region, it was the fourth reported outbreak in 2013 in Bulgaria.
The longest period found in the selected references (Table 8 ) concerned the primary outbreak in 2013 in the Evros prefecture in Greece (SCoFCAH, 2013a,b). The age of the lesions at suspicion was estimated at less than or equal to 3–4 days. Adding to this the average incubation period (OIE, 2013), the period between the earliest point of infection and the suspicion report was estimated at 14 days.
We propose to consider the following set of values for the period between the earliest point of infection and the suspicion report:
Based on the extracted values for sheep (Table 8 ):
Shortest period = 2 days
Longest period = 14 days
As no data were available for the period between the first suspicion and suspicion report, we did not attempt to reconstruct the period between the earliest point of infection and the suspicion report for SPP/GTP. In addition, no data were retrieved on outbreaks in goats.
In conclusion, the currently proposed monitoring period (21 days) for SPP/GTP is 1 week longer than the longest period estimated from the literature (14 days), from the evidence gathered in outbreaks in two countries (Bulgaria and Greece). Nevertheless, a 1‐week longer monitoring period than what estimated is considered adequate, given the following disease patterns:
the incubation period can be up to 2 weeks;
the majority of infected animals show clinical signs, but the first signs appearing are lacrimation and nasal discharge, which are not disease‐specific and may require some time for the differential diagnosis.
small ruminants are not so easily and closely checked as cattle.
limited data from the primary outbreaks in Greece and Bulgaria are available, thus providing a certain degree of uncertainty.
Greece and Bulgaria were at risk of introduction of SPP/GTP since this was already present in the neighbouring country: This had raised awareness. In case of other MS not at risk of incursion, it could possibly take more time to identify a primary outbreak.
Therefore, a 21‐day monitoring period for SPP/GTP appears adequate.
4.2.3. Seroconversion in animals
The results regarding the range of days for seroconversion and the latest date of detectable antibody presence in listed species of SPP/GTP after experimental infection with the SPPV/GTPV is presented in Table 9.
In experimentally infected animals, the seroconversion date depends on the severity of infection. In severely infected animals, seroconversion started approximately 1 week after the onset of clinical disease, which equals 14 dpi in experimentally infected animals (Afshar et al., 1986; EFSA AHAW Panel, 2014). In experimentally infected animals with mild clinical disease, seroconversion may occur up to 21 dpi and these animals may show only low levels of antibodies, not detectable using a neutralisation assay (Kitching, 2003; EFSA AHAW Panel, 2014). Moreover, in general, antibodies are detectable for a period of 6–18 months post infection by means of indirect fluorescent antibody test and a micro‐serum/virus neutralisation test (Davies and Otema, 1978; EFSA AHAW Panel, 2014).
4.2.3.1. Sheep
The detection of SPPV/GTPV antibodies varied depending on the laboratory method of antibody detection and the method of inoculation used.
When detection was performed with the use of virus neutralisation test and animals were challenged intradermally (ID), the range for seroconversion was 4–21 days post infection (Bowden et al., 2009; Boshra et al., 2015) and the latest day of antibody detection was 63 dpi (Bowden et al., 2009). When animals were scarified with wire brush and the virus suspension was applied on the shaded skin, animals seroconverted at 14 dpi (Afshar et al., 1986) and the latest day of antibody detection was 18 months after inoculation (Davies and Otema, 1978); when challenged intravenously and subcutaneously (IV + SC), they seroconverted at 15 dpi and the latest day of antibody detection was 28 dpi (Wolff et al., 2020); when animals were in‐contact with infected ones, they seroconverted at 7 dpi and the latest day of antibody detection was 14 dpi (Ayalet et al., 2012). In all experimental studies, the latest day of antibody detection was the end of the experiment (Bowden et al., 2009; Boshra et al., 2015).
When detection of antibodies was performed with IgG‐ELISA, the range of days for seroconversion for sheep that were challenged ID was 4–21 dpi and the latest day of antibody detection was 63 dpi.
When detection of antibodies was performed with a double‐antigen ELISA (DA‐ELISA), sheep that were challenged IV + SC seroconverted at 21 dpi and the latest day of antibody detection was 28 dpi. However, the in‐contact animals had not seroconverted by the end of experiment at 28 dpi (Wolff et al., 2020).
Overall, irrespectively of the laboratory method used for antibody detection and the method of inoculation or the strain used for challenge, animals seroconverted between 4 and 21 dpi and antibodies were detectable until the end of the experiment.
4.2.3.2. Goats
The detection of capripoxvirus‐specific antibodies varied depending on the laboratory method of antibody detection and the method of inoculation used. In general, results were comparable with those of sheep.
With the use of VNT and ID challenge, animals seroconverted between 10 and 14 dpi and the latest day of antibody detection was 63 dpi (Bowden et al., 2009; Boshra et al., 2015); intranasally (IN) challenged animals seroconverted at 15 dpi and the latest day of antibody detection was 28 dpi (Wolff et al., 2020); at IV + SC challenge, only one animal out of three seroconverted at 10 dpi, with the remaining being seronegative until the end of the study (Wolff et al., 2020). When animals were in‐contact with infected ones, they seroconverted between 7 and 23 dpi (Ayalet et al., 2012; Wolff et al., 2020). However, one out of two in‐contact animals remained seronegative until 28 dpi (Wolff et al., 2020). In all experimental studies, the latest day of antibody detection was the end of the experiment.
When detection of antibodies was performed with IgG‐ELISA, the range of days for seroconversion for animals that were challenged ID was 4 and 21 dpi and the latest day of antibody detection was 63 dpi (Bowden et al., 2009; Boshra et al., 2015).
When the detection of antibodies was performed with DA‐ELISA, goats that were challenged IN seroconverted between 21 and 28 dpi and the latest day of antibody detection was 28 dpi as well; with IV + SC challenge, goats seroconverted at 10 dpi (end of experiment). However, the two in‐contact goats had not seroconverted by the end of experiment (Wolff et al., 2020).
4.2.4. Assessment
Considering the results presented above, an assessment of the effectiveness of the current monitoring period for SPP/GTP, depending on the purpose of that period in the different scenarios shown in Annex C, was carried out. For SPP/GTP, the length of the monitoring period as defined in Annex II of the Delegated Regulation is 21 days.
Scenarios 1, 2 and 3
1.
1st scenario of monitoring period
ToR 2 in accordance with article 8 and Annex II of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion of a SPP/GTP outbreak.
1.
2nd scenario of monitoring period
ToR 2 in accordance with article 17(2) and Annex II of the Delegated Regulation (EU) 2020/687
Article 57 of the Regulation (EU) 2016/429
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of a SPP/GTP outbreak.
1.
3rd scenario of monitoring period
ToR 2 in accordance with article 13(b) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a SPP/GTP outbreak in an epidemiological unit in which the disease has not been confirmed, in order to provide derogations from killing the animals in this unit, if this unit has been completely separated, and handled by different personnel during this monitoring period.
For the first three scenarios, the main purpose of the monitoring period is to carry a full epidemiological investigation (i.e. in Scenarios 1 and 2, at the time of the suspicion and confirmation, respectively), or part of the epidemiological investigation (i.e. Scenario 3, where the aim is to identify any possible epidemiological links between the affected establishment and any separated non‐affected epidemiological units).
The length of the monitoring period should then dictate how far backward or forward the activities related to tracing (and other activities needed during an epidemiological investigation) should go (checks for production records, animal movement records, etc.). This monitoring period is the time when the infection could have been present unknowingly in an establishment, and due to the regular activities carried out in this establishment, could have spread to other epidemiological units.
In the case of Scenario 3, if no epidemiological links between the establishment that has been confirmed positive and the other epidemiological units are found during the investigation (and only if other conditions described in the legislation are met), a derogation from killing the animals in the separated non‐affected epidemiological units could be granted.
The period of time the disease could have been present, unknowingly, in an establishment, equates then to the time period between the entry of SPPV/GTPV into the establishment and the reporting of the suspicion. Once the suspicion has been officially reported, control measures are implemented and further spread should in this way be prevented.
Based on the ELS carried out and presented above, the length of the time between the earliest point of infection and the suspicion report, described above, was estimated at 14 days, as the longest period estimated from the evidence gathered in outbreaks in Greece in 2013–2014. The monitoring period as defined in Annex II of the Delegated Regulation of 21 days comprises this range and could thus be considered effective, even with an additional week, as safety margin.
Scenario 4
1.
4th scenario of monitoring period
ToR 2 in accordance with article 27(3)c and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the SPP/GTP outbreak in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements.
The main purpose of the monitoring period in scenario 4 is to ensure that certain products or materials, likely to spread the disease, that have been produced in a non‐affected establishment located in the protection zone of an affected establishment, can be moved safely and without posing a risk of disease spread. In this scenario, and in contrast with the previous three scenarios, the establishment of concern is neither a suspect establishment nor an affected establishment. For the assessment of this scenario, we assume that the earliest plausible point of infection of these products or materials in the establishment of concern would be the earliest plausible point of infection of the establishment that originated the protection zone. If these products have been obtained or produced before the earliest point of infection of the affected establishment, then they could be exempted from prohibitions to be moved, as long as other conditions specified in the legislation are met (e.g. the products must have been clearly separated during the production process, storage and transport, from products not eligible for dispatch outside the restricted zone).
As the disease has already been detected in the area, and high awareness is expected, the length of monitoring period foreseen by the Regulation for SPP/GTP, i.e. 21 days, is considered effective in this scenario.
Scenario 5
1.
5th scenario of monitoring period
ToR 2 in accordance with article 32(c), article 48(c) and Annex II of the Delegated Regulation (EU) 2020/687
The purpose of this section is to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period.
The aim of the monitoring period is to ensure that semen from animals in a non‐affected establishment (located in a protection or surveillance zone) that has been collected and frozen after the earliest time of infection of the affected establishment that originated the protection zone, is safe to be moved without posing a risk of disease spread. In this scenario, EFSA is requested to assess the length of time, after the semen was taken, when the animal should be tested in order to allow that semen to be moved. Here, it is assumed that the earliest point of infection of the animal would be on, or after the earliest point of infection of the affected establishment that originated the protection zone, and the latest date the semen could have become contaminated would be the date the semen was collected.
In the case of an SPP/GTP outbreak, based on the existing legislation, the animals would have to be tested not earlier than the time in days of the monitoring period plus 7 days (21 + 7 = 28 days) counted after the semen was taken.
There is, however, uncertainty regarding detection of SPP/GTP virus in semen, since transmission of the virus in semen has not yet been established (OIE, 2013). Despite this, and assuming that missing an infected establishment as described above would be plausible, below we summarise the assessment in the case sheep or goats need to be sampled via serology in order to assess the infection status of the animal at the time the semen was taken (indicating whether the semen was infected or not). A negative serological test, if carried out at the right time, would indicate that the animal has never been exposed to the agent, and therefore, that the semen is free of the agent too.
Based on the results presented in Section 4.2.1 in relation to the seroconversion in non‐vaccinated naive animals, the latest date of seroconversion was identified as 21 days for sheep and 28 days for goats. The latter was a very late scenario; it was detected in only one animal (out of eight) in one experiment (Wolff et al., 2020), while the other animals in the same study were already positive at 10–21 days; another study showed seroconversion at 2–3 weeks at the latest (Bowden et al., 2009).
Consequently, and based on the results of the publications, sampling the animals at least 28 (21 + 7) days after semen collection for antibody testing, as it is foreseen in the Delegated Regulation, with negative results, is considered effective to ensure that semen is safe to be moved without posing a risk of disease spread.
Scenarios 6 and 7
1.
6th scenario of monitoring period
ToR 2 in accordance with article 57 (1) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date of the final cleaning and disinfection in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority (assuming relevant control of insects and rodents was carried out).
1.
7th scenario of monitoring period
ToR 2 in accordance with article 59 (4) and Annex II of the Delegated Regulation (EU) 2020/687
Aim: to assess the effectiveness of the length of the Monitoring Period, as the time period calculated forward from the date the first animal was introduced for the purpose of repopulation, during this monitoring period, all animals of the listed species intended for repopulation should be introduced.
In scenarios 6 and 7, the monitoring period is used in the context of repopulation. In scenario 6, the monitoring period is used to ensure that the repopulation process is not put at risk due to the disease still being present unknowingly in establishments within the surrounding area of the establishment to be repopulated (if an establishment tested positive to SPPV/GTPV within a distance equal to or lower than the radius of the surveillance zone, the repopulation process could not take place). Repopulation can only take place after a number of days equal to the monitoring period has elapsed since the final cleaning, disinfection and disinfestation of the affected establishment.
In this regard, the number of days of the monitoring period for SPP/GTP, counted from the day of the final cleaning and disinfection must ensure enough time for any potentially infected surrounding establishment to be reported as a suspicion. Considering the results presented above, the monitoring period as defined in Annex II of the Delegated Regulation of 21 days is considered effective for this scenario.
In Scenario 7, the monitoring period must be counted forwards from the date in which the first animal is introduced into the establishment to be repopulated, with all the animals intended for repopulation of this establishment being introduced within the length of time of this monitoring period.
The aim of the monitoring period in this scenario is to ensure the early detection of any potentially recently infected animal intended for repopulation once it has been moved into the repopulated establishment. Although the preferred option is that all animals are introduced into the establishment to be repopulated at the same time, this is not always feasible. The first clinical and laboratory sampling of the repopulated animals takes place once all the animals are in situ. By restricting the period of time during which animals may be introduced into the establishment, the period of time during which the disease could be unknowingly spreading within the establishment is reduced. Assuming that the latest point of infection of the first animal or batch of animals introduced into the repopulated establishment is the day when all animals have been moved, clinically ill animals would be observed at the first visit, if this visit is carried out a number of days equal to the incubation period. For SPP/GTP, the incubation period is typically 1–2 weeks. The EFSA AHAW Panel thus considers the existing length of the monitoring period as defined in Annex II of the Delegated Regulation (21 days) effective as it would allow for early detection of potentially infected animals at the first visit following re‐stocking.
4.3. Assessment of the minimum radius and time periods of the protection and surveillance zones set in place subsequent to a disease outbreak
4.3.1. Assessment of the minimum radius
The purpose of this section is to assess the effectiveness to control the spread of SPPV/GTPV by implementing a protection and surveillance zones of a minimum radius, as set out in Annex V of the Delegated Regulation, surrounding the establishment where the disease has been confirmed. Based on this regulation, the minimum radius of the protection and surveillance zone for SPP/GTP should be of 3 and 10 km, respectively (see Annex F).
To assess this, transmission kernels6 have been used to estimate the minimum radius for SPPV/GTPV spread, under the assumption of excluding the spread due to animal movements. Transmission kernels were estimated using data on outbreaks of SPP/GTP reported in the east of Evros region of Greece from 2013 to 2015 (extracted from ADNS) (Annex I for details). Those outbreaks were most likely not linked to animal movements. Four functional forms were fitted to the data (Table 10 and Figure 5), with the alternative fat‐tailed kernel yielding the best fit to the data based on the Akaike information criterion (AIC). Furthermore, the difference in AIC is > 6 for the other kernels (see Annex I), so it is reasonable to base the assessment only on the alternative fat‐tailed kernel.
Table 10.
Kernels for the transmission of sheep and goat pox
| Epidemic | Kernel | Function | Parameters* | ||
|---|---|---|---|---|---|
| d0 (km) | α | ||||
| Evros region, Greece 2013–2015 | Fat‐tailed |
|
2.28 (1.40, 5.05) | – | |
| Gaussian |
|
10.04 (7.23, 14.49) | – | ||
| Exponential |
|
5.67 (3.36, 10.05) | – | ||
| Alternative fat‐tailed |
|
0.61 (0.08, 3.26) | 1.32 (0.98, 2.07) | ||
Maximum likelihood estimate (95% confidence interval).
Figure 5.
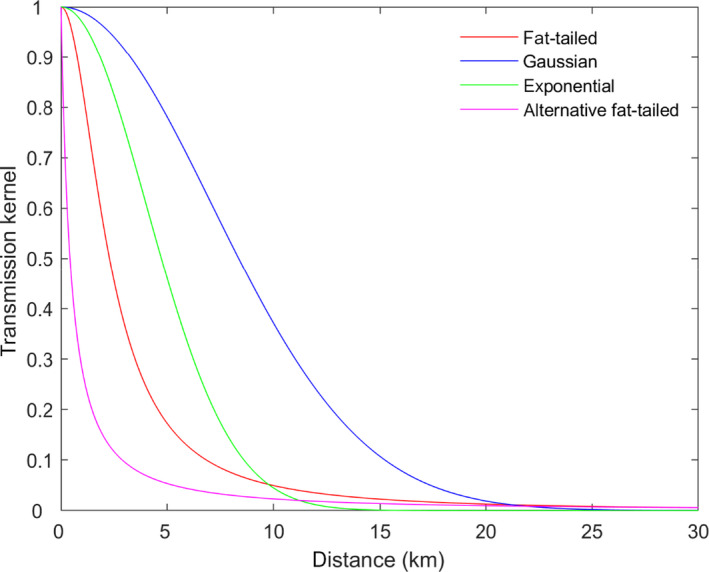
- Parameters were estimated by fitting the models to data on a sheep pox epidemic in the Evros region of Greece, 2013–2015.
The reason why only the outbreaks reported in Evros region were used for the analysis (and not all those reported in Greece during the 2014 epidemic) lies on the fact that outbreaks that occurred outside this region were most likely linked to animal movements, which would be in contrast with the assumption made above, i.e. exclusion of the spread due to animal movements (EFSA AHAW Panel, 2021).
For the alternative fat‐tailed kernel in Table 10 (i.e. the best‐fitting one), the probability of transmission beyond given distances (if transmission were to occur from an infected establishment) was computed using the estimates, lower 95% confidence limits and upper 95% confidence limits, including beyond the proposed radius for the protection and surveillance zones (3 and 10 km, respectively) (Table 11). In addition, the distances at which a threshold probability of transmission beyond that distance is reached were also calculated for the kernel using the estimates, lower 95% confidence limits and upper 95% confidence limits (Table 12).
Table 11.
Probability of transmission of sheep and goat pox virus beyond different distances
| Distance (km) | |||||||
|---|---|---|---|---|---|---|---|
| 3 | 5 | 10 | 15 | 20 | 25 | 50 | |
| Estimate (median) | 0.096 | 0.054 | 0.023 | 0.014 | 0.010 | 0.007 | 0.003 |
| Lower 95% CI | 0.031 | 0.019 | 0.010 | 0.007 | 0.005 | 0.004 | 0.002 |
| Upper 95% CI | 0.258 | 0.145 | 0.055 | 0.028 | 0.017 | 0.011 | 0.003 |
Table 12.
Distances (km) at which the probability of transmission of sheep and goat pox virus beyond that distance reaches a threshold level
| Threshold probability of transmission | |||||||
|---|---|---|---|---|---|---|---|
| 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.2 | 0.5 | |
| Estimate (median) | 103.5 | 33.2 | 19.4 | 5.3 | 2.9 | 1.5 | 0.4 |
| Lower 95% CI | 88.2 | 20.0 | 9.8 | 1.8 | 0.9 | 0.4 | 0.1 |
| Upper 95% CI | 113.7 | 38.8 | 26.8 | 10.6 | 6.6 | 3.8 | 1.3 |
From Table 11, the estimated probability of transmission beyond a protection zone with 3 km radius, if transmission occurred, is 9.6% (CI: 3.1–25.8%) and 2.3% (1–5.5%) for a zone with 10 km radius, which may be considered sufficient to contain disease spread. If a 95% probability of containing transmission in the protection zone is desired, then the zone should be extended to 5.3 Km (CI: 1.8–10.6; Table 12).
If the aim is to reduce the probability of transmission beyond the surveillance zone to 1% (and not 2.3% as estimated above for a 10‐km zone), the radius should be increased to 19.4 km (9.8–26.8). This, nonetheless, may increase the number of farms in the surveillance zone (which would then be affected by movement restrictions), since the area would increase approximately fourfold.
4.3.2. Assessment of the minimum period
The purpose of this section is to assess the effectiveness to control the spread of SPP/GTP of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones. The length of the minimum period of duration of measures for SPP/GTP in protection zone is 21 days, while for the surveillance zone is 30 days (Annex X of the Delegated Regulation).
To assess the minimum length of time the protection and the surveillance zones should be kept in place, the average (for the protection zones) and the longest (for the surveillance zones) period between the earliest point of infection and the notification of a suspicion will be used (EFSA AHAW Panel, 2020).
Based on the results of the ELS as presented in Table 8 in Section 4.2.2, the maximum time between the earliest point of infection and the notification of the suspicion for SPP/GTP is 14 days. This is shorter than the minimum period of 21 days indicated in the Delegated Regulation for the restriction measures in the protection zone; therefore, the latter is considered effective to detect infected establishments and to prevent the movement of infected animals from the protection zone.
In addition, the maximum period between the earliest point of infection and the suspicion report has been reconstructed as 28 days, by adding the maximum incubation period (14 days) to the maximum period between first suspicion and suspicion report (14 days). Consequently, the minimum period of 30 days indicated in the Delegated Regulation for the restriction measures in the surveillance zone is considered adequate to detect infected establishments and to prevent the movement of infected animals from the surveillance zone.
4.3.3. Uncertainty analysis
Although several sources of uncertainty were identified during the scientific assessment (see Annex G), their impact on the outputs of the assessment could not be quantified.
5. Conclusions and recommendations
| Sampling procedure | Conclusions | Recommendations |
|---|---|---|
| ToR 1: In the event of suspicion or confirmation | ||
| 1st scenario In the event of a suspicion of SPP/GTP in an establishment where animals of the listed species are kept | Suspicion of SPP/GTP would be raised based on clinical signs and lesions (high fever, pox lesions in the skin and mucous membranes, eye and nasal discharge and enlarged lymph nodes). Clinical signs and lesions are usually fully expressed in naive populations, such as in EU, no subclinical infection is expected.The collection of specimens for PCR testing can be performed either on dead or live animals.The recommended specimens are fresh tissue from characteristic pox skin lesions.Given the sensitivity of PCR, and the expected prevalence, in a period between 14 and 21 days after disease introduction in a, e.g. 100‐head flock, at least three animals are expected to be clinically affected and show clinical signs, and the probability of not detecting the infection after testing three affected animals is almost nil.ELISA can confirm infection with SPPV/GTPV in a free country by detection of antibodies, if no vaccination is implemented.In case of suspicion because of contact, import, etc., if clinical signs are not so evident, the sampling of randomly selected asymptomatic animals can be performed based on the expected infection prevalence from time of introduction. In this case, ELISA can be performed from day 21 post virus introduction. | On live animals, samples should be collected from at least 3 clinically affected animals within the first week of clinical signs, before neutralising antibodies develop, to maximise the probability of detecting the viral genome.To determine the progression of antibody response against GTPV and SPPV in sheep and goats, the collection of paired blood samples, three weeks apart, from 10 to 14 days after infection is required. |
| 2nd scenario For the purposes of the epidemiological enquiry as referred to Article 57 of Regulation (EU)2016/429 in an SPP/GTP officially confirmed establishment | When SPP/GTP has been officially confirmed in an establishment, further sampling procedures will support the needs of the epidemiological enquiry to obtain information on the origin of the disease, the length of time that the disease is present.To estimate the prevalence of animals with clinical signs within the affected establishment, all live animals (or a sample taken to detect or rule out the presence of animals with clinical signs with at least a 95% confidence) should be subjected to individual clinical examination and dead animals should be examined.An approximate estimation of the length of the presence of SPPV/GTPV in the establishment can be based on the age of the pox lesions identified (taking into account the incubation period of 4–14 days), by thorough individual clinical examination of the animals that are still alive, or necropsy of those that are found dead. Approximately it takes 6–10 days for the papules to form scabs, which may persist for 6 weeks.Phylogenetic analysis of virus isolates, preferably from skin lesions, may help to identify the possible origin of the virus.Antibodies are detectable starting from 4 to 21 days in sheep and from 10 to 14 days in goats from the infection. Consequently, detection of antibodies suggests that infection occurred more than 4–10 days prior to detection of antibodies. | Samples from animals with clinical signs from dead or hunted animals should be collected for laboratory analysis as in 1st scenario. |
| 3rd scenario For granting a specific derogation from killing animals of the categories of article 13.2 of the Delegated Regulation in an SPP/GTP affected establishment | The number of animals to ask for derogation from killing in a herd is generally small, because it may deal with endangered species, rare breeds, high genetic, cultural or educational value, so, given the risk of not killing animals from affected establishment, all of them should be clinically checked thoroughly, preferably every day or at least at weekly interval for a period of at least the monitoring period of 21 days calculated forwards from the day of confirmation of the latest case. In case of clinical signs, samples should be taken for PCR. | |
| 4th scenario For the animals of non‐listed species kept in an SPP/GTP affected establishment. | The listed species for SPP/GTP according to Commission Implementing Regulation (EU) 2018/18825 are Ovis spp., Capra spp., thus all susceptible animals belonging to these genera, domestic or wild, should undergo the same sampling procedures. No other genera are known to have any epidemiological relevance for the control of SPP/GTP. | |
| 5th scenario For wild animals of the listed species within the SPP/GTP affected establishment and its surroundings. | The surveillance of wildlife around the affected establishment may include the visual inspection of these animals from distance and the testing of fallen stock and hunted animals both by PCR and serology. Unexpected mortality events in susceptible wildlife should be investigated | Samples from animals with clinical signs from dead or hunted animals should be collected for laboratory analysis as in 1st scenario. |
| 6th scenario For animals of listed species in the non‐affected establishments located in a protection zone | All establishments located in the protection zone should be visited and the animals should be subjected to clinical surveillance.In case of suspicion, the sampling procedures as described in 1st scenario would apply.Active surveillance via serological or PCR of randomly selected animals (i.e. in the absence of clinical signs) should be conducted only if this could be considered necessary due to epidemiological considerations. | In an establishment where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined with at least 95% confidence. |
| 7th scenario For non‐affected establishments located in a protection zone with a radius larger than 3 km | This scenario is not applicable, since, for SPP/GTP, it is neither foreseen nor assessed that the protection zone is larger than 3 km radius. | |
| 8th scenario For non‐affected establishments located in a surveillance zone | It is very unlikely that establishments in the surveillance zone, not epidemiologically linked with an outbreak, will become infected with SPPV/GTPV without having additional outbreaks in the protection zone, if movements are excluded.Consequently, for the surveillance zone, it is recommended that the efforts will be allocated to enhance passive surveillance by increasing awareness in all establishments, industry and public, to be able to detect any clinical signs typical of SPP/GTP. | If suspicion is raised, the procedures described in 1st scenario should be followed. |
| ToR 1: To grant derogations for animal movements | ||
| 9th scenario From non‐affected establishments located in the protection zone to slaughterhouses located within the protection zone or in the surveillance zone or outside the restricted zone | All the animals in the establishment of origin should be clinically examined 24 h before their movement following the procedures described in the first scenario. | If animals are moved to slaughterhouse outside the restricted zone, laboratory examination of samples from the animals intended to be moved is necessary to rule out the presence of SPP/GTP, with a confidence level of 95%. |
| 12th scenario From non‐affected establishments located in the protection zone to a plant approved for processing or disposal of animal by‐products in which the animals are immediately killed | This scenario is very similar to the 11th scenario, therefore the assessment and new procedures are the same. | |
| 13th scenario From an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone and from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone | To grant derogations for animal movements from an establishment in a surveillance zone to a slaughterhouse located outside the restricted zone, clinical examination and sample collection for laboratory investigation should be performed as described in the 1st scenario.For animals intended to be moved from an establishment located outside the surveillance zone to a slaughterhouse situated in the surveillance zone, there is no need for laboratory examination, if there are no other reasons based on the national risk assessment to recommend it; clinical examination would be enough. | |
| 14th scenario From an establishment in a surveillance zone to pastures situated within the surveillance zone | All the animals in the establishment of origin should be clinically examined 24 h before their movement.In an establishment where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined with at least 95% confidence level. In case of suspicion, the procedures as in 1st scenario apply. | There is a need for increased vigilance on the part of farmers as the grazing animals are usually less supervised and the detection of clinical signs may delay. |
| 15th scenario From an establishment in a surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone | All the animals in the establishment of origin should be clinically examined before their movement to an establishment belonging to the same supply chain within the surveillance zone. In an establishment, where the number of animals is large and therefore the individual clinical examination of all the animals is not feasible, a minimum sample of animals (including all animals to be moved) should be clinically examined with at least a 95% confidence level. | If the animals from an establishment in the surveillance zone are moved to an establishment belonging to the same supply chain, located outside the surveillance zone, in addition to the clinical examination, laboratory examination for presence of virus and antibodies of samples from the animals intended to be moved is necessary to rule out the presence of SPP/GTP, with a confidence level of 95%. |
| 18th scenario From an establishment located in the restricted zone to move within the restricted zone when restriction measures are maintained beyond the period set out in Annex XI of the Delegated Regulation | Animals in the restricted zone, for which a specific derogation has been granted for movement within the restricted zone, should be subjected to clinical examination.In case of suspicion, then the procedures as in 1st scenario apply. | Sampling procedures should be implemented as described in 9th scenario. |
| ToR 1: For repopulation purposes | ||
| 19th scenario For the animals that are kept for the repopulation prior to their introduction | For animals kept for repopulation, clinical examination and sampling should be used as standard procedures to ensure that the animals do not pose a risk of SPPV/GTPV transmission/re‐introduction. | For animals that are introduced from disease‐free areas outside the restricted zone, laboratory testing can be omitted because they have not been exposed to virus before entry. If animals are sourced from restricted areas, all the animals in the establishment of origin should be clinically checked and samples tested in laboratory, since it is needed they are negative. In an establishment where the number of animals is large, sampling procedures for laboratory examination should ensure that the animals do not pose a risk of transmission at a confidence level of 95%. |
| 20th scenario In the event of unusual mortalities or clinical signs being notified during the repopulation | In the event of unusual mortalities during repopulation, the establishment is considered suspect, as well as the establishments from where the suspect animals are coming from. | The repopulation should be stopped and the procedures for clinical and the laboratory confirmation to rule out presence of SPP/GTP as described in scenario 1 should be followed. |
| 21st scenario For animals that have been repopulated | Animals must be subjected to clinical inspection at least every three days for the first 14 days following the introduction, and weekly from 15 to at least 21 days (monitoring period) after re‐introduction. The last day of the monitoring period following the latest day of animals’ introduction, all the animals should be subjected to thorough clinical examination. | In an establishment where the number of animals is large, a minimum sample of animals should be clinically examined, to detect or rule out the presence of animals with clinical signs with at least 95% confidence.If clinical signs are identified, then the animals are considered suspected and the procedures described in scenario 1 should be followed. |
| ToR 2 | ||
|---|---|---|
| Description | Conclusions | Recommendations |
| Assessment of the length of the monitoring period of SPP/GTP | The monitoring period (21 days) for SPP/GTP currently proposed in the Delegated Regulation is considered adequate, since it is one week longer than the longest period (14 days) estimated from the evidence gathered from the epidemics in two countries in EU (Bulgaria and Greece). Since this estimation is affected by some uncertainty (limited number of primary outbreaks in the epidemics considered), one week longer than this period provides some safety margin. | |
| ToR 3 | ||
| Description | Conclusions | Recommendations |
| Assessment of the minimum radius | The estimated probability of transmission beyond the protection zone of 3 km radius from an infectious establishment is 9.6% (95% CI: 3.1–25.8%) and 2.3% (95% CI: 1–5.5%) for the surveillance zone of 10 km radius, which may be considered sufficient to contain disease spread. | In order to have the 95% probability of containing disease transmission, the protection zone should be increased to 5.3 km (CI: 1.8–10.6 km).To reduce the probability of transmission beyond the surveillance zone to 1%, its radius should be increased from 10 km to 19 km (95% CI: 9.8–26.8). This, nonetheless, may increase the number of farms in the surveillance zone (which would then be affected by movement restrictions), since the area would increase approximately fourfold. |
| Assessment of the minimum period for the restriction measures | The minimum period of 21 days indicated in the Delegated Regulation for the restriction measures in the protection zone is considered effective to detect infected establishments and to prevent the movement of infected animals from the protection zone. In addition, the maximum period between the earliest point of infection and the suspicion report has been reconstructed as 28 days, consequently, the minimum period of 30 days indicated in the Delegated Regulation for the restriction measures in the surveillance zone is considered adequate to detect infected establishments and to prevent the movement of infected animals from the surveillance zone | |
Abbreviations
- ASF
African swine fever
- AHS
African horse sickness
- CSF
Classical swine fever
- CBPP
Contagious bovine pleuropneumonia
- CCPP
Contagious caprine pleuropneumonia
- dpi
days post inoculation
- ELISA
enzyme‐linked immunosorbent assay
- ELS
extensive literature search
- FMD(V)
Foot and mouth disease (virus)
- GTPV
Goat pox virus
- HPAI
Highly Pathogenic Avian Influenza
- LSD(V)
Lumpy skin disease (virus)
- NCD(V)
Newcastle disease (virus)
- OIE
World Organisation for Animal Health
- PCR
polymerase chain reaction
- PZ
protection zone
- RP
rinderpest virus
- RVF(V)
Rift Valley fever (virus)
- SPGP
Sheep pox and goat pox
- SPPV
Sheep pox virus
- SZ
surveillance zone
- ToR
Terms of Reference
Annex A – Definitions in EU legislation
1.
| Terms | Definitions |
|---|---|
| Clinical examination | The clinical examination comprises: (i) an initial general evaluation of the animal health status of the establishment which comprises all the animals of listed species kept in the establishment; and (ii) an individual examination of the animals included in the sample referred to in point (a). The sampling of animals for clinical examination is carried out in accordance with point A.1 of Annex I for terrestrial animals (Delegated Regulation article 3) |
| Confined establishment | Means any permanent, geographically limited establishment, created on a voluntary basis and approved for the purpose of movements, where the animals are: (a) kept or bred for the purposes of exhibitions, education, the conservation of species or research; (b) confined and separated from the surrounding environment; and (c) subject to animal health surveillance and biosecurity measures; (AHL: Regulation 2016/429 article 4(48)) |
| Epidemiological unit | Means a group of animals with the same likelihood of exposure to a disease agent; (AHL: Regulation 2016/429 article 4(39)) |
| Establishment | Means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics; (AHL: Regulation 2016/429 article 4(27)) |
| Health status | Means the disease status as regards the listed diseases relevant for a particular listed species with respect to: (a) an animal; (b) animals within: (i) an epidemiological unit; (ii) an establishment; (iii) a zone; (iv) a compartment; (v) a Member State; (vi) a third country or territory; (AHL: Regulation 2016/429 article 4(34)) |
| Infected zone | Means a zone in which restrictions on the movements of kept and wild animals or products and other disease control and biosecurity measures may be applied with the view to preventing the spread of a category A disease in the event of official confirmation of the disease in wild animals. (Delegated Regulation article 2(15)) |
| Kept animals | Means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals; (AHL: Regulation 2016/429 article 4(5)) |
| Outbreak | Means the officially confirmed occurrence of a listed disease or an emerging disease in one or more animals in an establishment or other place where animals are kept or located; (AHL: Regulation 2016/429 article 4 (40) |
| Protection zone | Means a zone around and including the location of an outbreak, where disease control measures are applied in order to prevent the spread of the disease from that zone; (AHL: Regulation 2016/429 article 4(42)) |
| Listed diseases | Means diseases listed in accordance with Article 5(1); (AHL: Regulation 2016/429 article 4 (18))List of the diseases (AHL: Regulation 2016/429, Annex II) |
| Listed species | Means an animal species or group of animal species listed in accordance with Article 8(2), or, in the case of emerging diseases, an animal species or group of animal species which meets the criteria for listed species laid down in Article 8(2); (AHL: Regulation 2016/429 article 4(20))List of species and groups of species (Commission Implemented Regulation 2018/1882) |
| Monitoring periods | It is appropriate to follow a single approach for the measures to apply in the event of a category A disease. However, the epidemiology of diseases should be taken into account to establish the appropriate moment for the competent authority to apply control measures and to carry out investigations if there is suspicion or confirmation of those diseases. Therefore ‘monitoring periods’ should be provided, as reference time frames for each category A disease affecting terrestrial animals based on incubation periods and other relevant elements that may affect the spread of the disease. (Delegated Regulation whereas 10). |
| Restricted zone | Means a zone in which restrictions on the movements of certain animals or products and other disease control measures are applied, with a view to preventing the spread of a particular disease into areas where no restrictions are applied; a restricted zone may, when relevant, include protection and surveillance zones; (AHL: Regulation 2016/429 article 4(41)) |
| Surveillance zone | Means a zone which is established around the protection zone, and where disease control measures are applied in order to prevent the spread of the disease from the protection zone; (AHL: Regulation 2016/429 article 4(43)) |
| Wild animals | Means animals which are not kept animals; (AHL: Regulation 2016/429 article 4(8)) |
| Zone | Means: (a) for terrestrial animals, an area of a Member State, third country or territory with a precise geographical delimitation, containing an animal subpopulation with a distinct health status with respect to a specific disease or specific diseases subject to appropriate surveillance, disease control and biosecurity measures; (AHL: Regulation 2016/429 article 4 (35)) |
Annex B – Scenarios of ToR 1
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenario |
|---|---|---|---|---|
| In the event of suspicion or confirmation | ||||
| ToR 1.1ToR 1.2 | 6(2) of the Delegated Regulation | 1st scenario | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). |
|
| ToR 1.2 | Art. 12(3), Art. 7 (4) (Preventive killing) of the Delegated Regulation, and Art. 57 Reg.2016/429 | 2nd scenario | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. |
|
| ToR 1.1ToR 1.2 | Article 13(3)c of the Delegated Regulation | 3rd scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. |
|
| ToR 1.1ToR 1.2 | Article 14(1) of the Delegated RegulationArt. 57 Reg.2016/429 | 4th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. |
|
| ToR 1.1ToR 1.2 | Article 14(1) of the Delegated RegulationArt. 57 Reg.2016/429 | 5th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species |
|
| ToR 1.1ToR 1.2 | Article 26(2) of the Delegated Regulation | 6th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. |
|
| ToR 1.3 | Article 26(5) of the Delegated Regulation point A.3 of Annex I | 7th scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone |
|
| ToR 1.3 | Article 41 of the Delegated Regulation | 8th scenario | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone |
|
| Derogations to allow animal movements | ||||
| ToR 1.4 | Article 28(5) of the Delegated RegulationArticle 29 of the Delegated Regulation | 9th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29) |
|
| ToR 1.4 | Article 28(5) and Article 30(1) of the Delegated Regulation | 10th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of day‐old‐chicks located in the protection zone and hatched from eggs originating in the restricted zone or outside the restricted zone. The sampling procedures should ensure that the movement of these day‐old‐chicks to an establishment located in the same Member State but if possible, outside the restricted zone |
|
| ToR 1.4 | Article 28(5) and Article 30(2) of the Delegated Regulation | 11th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the protection zone to establishments located in the same MS and if possible within the restricted zone. |
|
| ToR 1.4 | Article 28(5) and Article 37 of the Delegated Regulation | 12th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37) |
|
| ToR 1.4 | Article 43(5) and Article 44 of the Delegated Regulation | 13th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved: (a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, (b) from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone |
|
| ToR 1.4 | Article 43(5) and Article 45(1) of the Delegated Regulation | 14th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for the animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone |
|
| ToR 1.4 | Article 43(5) and Article 45(2) of the Delegated Regulation | 15th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter |
|
| ToR 1.4 | Article 43(5) and Article 46(1) of the Delegated Regulation | 16th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations to grant derogation of movements of day‐old‐chicks hatched from establishment located in the surveillance zone, from eggs originating within the surveillance zone and eggs originating outside the restricted zone, to an establishment located in the same Member State where they were hatched |
|
| ToR 1.4 | Article 43(5) and Article 46(2) of the Delegated Regulation | 17th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to‐lay poultry located in the surveillance zone to establishments located in the same MS. |
|
| ToR 1.4 | Article 56(1)c of the Delegated Regulation | 18th scenario | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI |
|
| Repopulation | ||||
| ToR 1.5 | Article 59(2),(3) of the Delegated Regulation | 19th scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(9) of the Delegated Regulation | 20th scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. |
|
| ToR 1.5 | Article 59(5) of the Delegated Regulation | 21st scenario | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. |
|
Annex C –Existing sampling procedures for SPP/GTP
1.
Sampling scenarios for SPP/GTP – Based on Council Directive 2003/85/EC if not stated otherwise
| Scenario | Description of the Scenario | Clinical guidelines | Laboratory guidelines |
|---|---|---|---|
| 1st | To assess the effectiveness of disease‐specific sampling procedures of animals of listed species in a suspected establishment, based on clinical examination (TOR 1.1) and laboratory examination (TOR 1.2), in their ability to detect a category A disease in kept animals if the disease is present in that establishment, or to rule it out if not present (Art. 6 (2)). | Article 4: 1. When animals on a holding are suspected of being infected or contaminated with SPP/GTP, Member States shall ensure that the official veterinarian immediately activates official investigation arrangements to confirm or rule out the presence of the disease in question2. As soon as the suspected presence of the disease is notified, the competent authority shall have the holding placed under official surveillance and shall in particular require that: (a) a census be made of all categories of animals of susceptible species and that, in respect of each of these categories, the number of animals already dead, infected or liable to be infected or contaminated be recorded; the census must be kept up to date to take account of animals born or dying during the period of suspicion; the information in the census must be kept up to date and produced on request and may be checked at each visit. Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): Susceptible animals on suspect premises will be physically examined on a daily basis for the first 14 days and weekly thereafter, as will all susceptible animals in the RA (or a statistical sample if large numbers of susceptible animals are involved). Note: Scientific opinion on sheep and goat pox (EFSA AHAW Panel, 2014 ): SPGP shows highly characteristic clinical signs and lesions easily recognised by experienced veterinarians and farmers. Clinical signs and lesions are fully expressed in naive populations, with many infected animals exhibiting all the symptoms of infection (high fever, pox lesions in the skin and mucous membranes, eye and nasal discharge and enlarged lymph nodes) at the same time, allowing effective clinical surveillance programmes.The effectiveness of clinical diagnosis was demonstrated during the outbreaks in Greece in 2013–2014, when culling was implemented in 52 outbreaks on the basis of clinical signs before laboratory confirmation. In all of these outbreaks, clinical diagnosis was subsequently confirmed by laboratory results, with 100% agreement. In Bulgaria, all four outbreaks in 2013 were detected based on clinical signs and then confirmed by laboratory diagnosis. It is well understood that the effectiveness of clinical diagnosis depends on the experience of veterinarians in correctly identifying the disease, as well as on disease awareness, particularly in areas where no previous outbreaks have been detected. | Article 4: 1. When animals on a holding are suspected of being infected or contaminated with SPP/GTP, Member States shall ensure that the official veterinarian immediately activates official investigation arrangements to confirm or rule out the presence of the disease in question and, in particular, must take or have taken the samples necessary for laboratory examination. To that end the animals in question may be transported to the laboratories under the supervision of the competent authority, which shall take appropriate steps to prevent the disease from spreading. EU Reference Laboratory for Capripox Viruses: Sheep and goat pox (EURL Capripox, 2021): Laboratory confirmation of SPPV/GTPV is most rapid using the polymerase chain reaction (PCR) method in combination with a clinical history consistent with generalised SPPV/GTPV infection. Isolation of the virus is possible as capripoxviruses will grow on tissue culture of ovine, caprine or bovine origin, although field isolates may require up to 14 days to grow or require one or more additional tissue culture passage(s). OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, heading ‘B.Diagnostic techniques’ (OIE, 2019): 1. Identification of the agent: 1.1. Specimen collection and submission: Material for virus isolation and antigen detection should be collected by biopsy or at post‐mortem from skin papules, lung lesions or lymph nodes. Samples for virus isolation and antigen‐detection enzyme‐linked immunosorbent assay (ELISA) should be collected within the first week of the occurrence of clinical signs, before the development of neutralising antibodies. Samples for genome detection by polymerase chain reaction (PCR) may be collected before or after the development of neutralising antibody responses. Buffy coat from blood collected into EDTA (ethylenediaminetetraacetic acid) during the viraemic stage of capripox infection (before generalisation of lesions or within 4 days of generalisation), can also be used for virus isolation. Laboratory confirmation of SPPV/GTPV is most rapid using the polymerase chain reaction (PCR) method in combination with a clinical history consistent with generalised capripox infection. Diagnostic tools to detect capripoxvirus infections and diva strategies; Joint FAO/IAEA Division (Lamien, 2017): Specimens for the laboratory: –Skin biopsies, swab samples for virus isolation, histopathology and electron microscopy and molecular detection. –Serum samples for serology: from acute and chronic cases and 2 to 3 weeks after the first appearance of skin lesions. Sheep & Goat Pox (CFSPH, Iowa State University, 2008): Samples for virus isolation and for some antigen‐detection tests should be collected during the first week of illness, before neutralising antibodies develop. Blood samples should be taken as early as possible; virus isolation is unlikely to be successful after generalised lesions have been present for more than a few days. Sheep and Goat Pox. (Centre for Agriculture and Biosciences International, 2019): A tentative clinical diagnosis can be made in the field. Specimens to submit for laboratory diagnosis (virus isolation) can include biopsy tissue material, but post‐mortem specimens collected from one or two severely affected acute cases are preferable. Biopsy specimens should include samples from two or three lesions at the papular or vesicular stage. Blood (in EDTA for PCR test and in heparin for virus isolation) should be collected aseptically from early febrile cases. Post‐mortem specimens should include lesions from skin, turbinates, trachea, lungs and enlarged lymph nodes. Emergency animal diseases: A field guide for Australian veterinarians (Breed et al. , 2019 ): You will be able to isolate the virus within the first week of clinical signs developing, before neutralising antibodies develop. Collect: – serum from at least 10 live, clinically affected animals, and from exposed animals (particularly those that are convalescent). – EDTA blood from live, clinically affected animals (7–10 ml/animal) fresh tissue‐characteristic pox lesions from skin as well as respiratory and gastrointestinal tracts (2 g of each tissue). – nasal swabs from live, clinically affected animals. – fixed tissue—characteristic pox lesions from skin as well as respiratory and gastrointestinal tracts (in neutral‐buffered formalin). The most rapid, sensitive and specific diagnostic procedure is the detection of viral DNA in characteristic poxvirus lesions or nasal swabs by real‐time or conventional PCR. A positive result can be obtained within 1 day of the laboratory receiving samples. Emergency Animal Disease Bulletin No. 118 (Department of Agriculture, Water and the Environment, 2017): Sheep pox and goat pox should be considered in cases where there is high morbidity and mortality in sheep and goats, and where typical pox lesions are seen on the skin and respiratory and digestive mucosa. Confirmatory diagnosis is made by specifically identifying the virus in tissues by virus isolation, in addition to detecting viral DNA in tissue samples using PCR techniques. Specimens required for testing include biopsies of skin, respiratory and gastrointestinal lesions (fresh and fixed), nasal swabs and whole blood collected in EDTA tubes. |
| 2nd | To assess the effectiveness of disease‐specific sampling procedures, based on laboratory examination (ToR 1.2), in their ability to detect the disease in the event of preventive killing, and in their ability to support with the epidemiological investigation (disease detection, prevalence estimation, virus identification, etc.) in kept animals of listed species in an affected establishment, before or when they are killed or found dead. The purposes of the epidemiological enquiry are described in Article 57 of Regulation (EU)2016/429. | NA | No specific guidelines described in legislation Article 8: 1. The epizootiological enquiry shall deal with: (a) the length of time during which the disease may have existed on the holding before being notified or suspected; (b) the possible origin of the disease on the holding and the identification of other holdings on which there are animals of susceptible species which may have become infected or contaminated; (c) the movement of persons, animals, carcasses, vehicles, equipment or any other substances likely to have carried the agent of the disease to or from the holdings in question; 2. A crisis unit shall be established in order to provide full coordination of all measures necessary to ensure eradication of the disease as quickly as possible and for the purpose of carrying out the epizootiological enquiry. |
| 3rd | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species belonging to the categories described in article 13(2)) of an affected establishment, in order to grant a specific derogation from killing these animals, while ensuring that they do not pose a risk for the transmission of the disease. | NA Article 5: 1. Once it has been officially confirmed that SPP/GTPis present on a holding, Member States shall ensurethat, in addition to the measures laid down in Article 4 (2), thecompetent authority requires application of the following measures: (a) all animals of susceptible species on the holding shall be killed on the spot, without delay. The animals which have died or been killed shall either be burnt or buried on the spot, if possible, or destroyed in a carcase disposal plant. | NA |
| 4th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of non‐listed species kept in an affected establishment, in their ability to ensure the detection of the virus if the virus is present in these species. | No specific guidelines described in legislation Note: Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): Although experience overseas is that cattle are unlikely to be significant in the course of an SPP/GTP outbreak, any cattle in nose‐to‐nose contact with infected sheep or goats may need to be included in the stamping‐out program. | No specific guidelines described in legislation |
| 5th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the wild animals of listed species within the affected establishment and in its surroundings. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these wild species. | No specific guidelines described in legislation Article 6: Where animals living in the wild are infected or suspected of being infected, Member States shall ensure that appropriate action is taken . Note: Scientific opinion on sheep and goat pox (EFSA AHAW Panel, 2014 ): There is, to date, no evidence of SPPV and GTPV viruses in wildlife, and it is assumed that wildlife do not play a relevant role in the epidemiology of SPP and GTP (Babiuk et al., 2008), although it cannot be excluded that wild sheep and wild goats can be infected with SPPV. In support of this fact, the lumpy skin disease virus, closely related to SPPV/GTPV, has been isolated from wild ruminants. Note: Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): If the disease occurs in an area where there are feral goat populations, a goat culling or control program, combined with surveillance, will be established to determine whether the infection has entered the population. | No specific guidelines described in legislation |
| 6th | To assess the effectiveness of disease‐specific sampling procedures based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species in establishments located in the protection zone. The purpose of the sampling procedures is to ensure the detection of the virus, if the virus is present in these animals. | Article 11: 1. Member States shall ensure that the following measures are applied in the protection zone: (a) all holdings within the zone having animals of susceptible species shall be identified; (b) there shall be periodic visits to holdings having animals of susceptible species, a clinical examination of those animals; a record of visits and findings must be kept, with the frequency of visits being proportional to the seriousness of the epizootic on those holdings at greatest risk. Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): On properties in the Restricted Area (RA) (at least 5 km from the Infected Premise (IP)), physical inspection surveillance visits should be made as soon as possible after the first IP is declared in the RA and then 1, 2, 3 and 6 weeks later. At physical inspection surveillance visits, every mob of susceptible animals must be inspected and numbers accounted for. In extensive grazing areas, where the degree of contact between groups of animals in a flock may be low, care must be taken to ensure that all groups of animals are present and healthy. A final inspection may be needed 6 months after the last case. | No specific guidelines described in legislation Article 11: 1. Member States shall ensure that the following measures are applied in the protection zone: (a) all holdings within the zone having animals of susceptible species shall be identified; (b) there shall be periodic visits to holdings having animals of susceptible species, a clinical examination of those animals including, if necessary, the collection of samples for laboratory examination; a record of visits and findings must be kept, with the frequency of visits being proportional to the seriousness of the epizootic on those holdings at greatest risk. |
| 7th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of establishments located in a protection zone when the radius is larger than 3 km. The purpose of the sampling procedure is to ensure disease detection of the virus if the virus is present in establishments within the protection zone. | Article 10: 1. Once the diagnosis of one of the diseases in question has beenofficially confirmed, Member States shall ensure that the competent authority establishes around the infected holding a protection zone with a minimum radius of three kilometres, itself contained in a surveillance zone with a minimum radius of 10 kilometres. The establishment of the zones must take account of geographical, administrative, ecological and epizootiological factors relating to the disease in question, and of monitoring facilities. →See 6th scenario | See 6th scenario |
| 8th | To assess the effectiveness of disease‐specific sampling procedures, based on clinical (ToR 1.1) and laboratory (ToR 1.2) examinations of the animals of listed species, for the sampling of the establishments located within the surveillance zone. The purpose of the sampling procedure is to ensure disease detection if the virus is present in establishments within the surveillance zone. | No specific guidelines described in legislation Article 12: 1. Member States shall ensure that the following measures are applied in the surveillance zone: (a) all holdings having animals of susceptible species shall be identified; (b) the movement of animals of susceptible species on public roads shall be prohibited except for the purpose of leading them to pasture or animal buildings; the competent authority may, however, grant a derogation from that prohibition for the transit of animals by road or rail without unloading or stopping; (c) the transport of animals of susceptible species within the surveillance zone shall be subject to authorisation by the competent authority; (d) animals of susceptible species must remain inside the surveillance zone for a maximum incubation period after the most recent recorded case of disease. | No specific guidelines described in legislation |
| Derogations to allow animal movements | |||
| 9th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant a derogation from prohibitions in the movement of animals, and allow for the animals to be moved to a slaughterhouse located within the protection zone or in the surveillance zone or outside the restricted zone (Art29). | Article 11: 1. Member States shall ensure that the following measures areapplied in the protection zone: (d) animals of susceptible species must remain on the holding on which they are being kept, except to be transported under official supervision directly to a slaughterhouse located in that zone foremergency slaughter or, if that zone has no slaughterhouse underveterinary supervision, to a slaughterhouse in the surveillance zone designated by the competent authority. Such transport may be authorised by the competent authority only after the official veterinarian has carried out an examination of all the animals of susceptible species on the holding and confirmed that none of the animals is suspected of being infected. The competent authority responsible for the slaughterhouse shall be informed of the intention to send animals to it. Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): All movement of susceptible animals within the Restricted Are (RA) will be prohibited for an initial period of at least 21 days so that the animals within the area can be observed by direct physical examination and appropriate diagnostic tests. Animals on Dangerous Contact Premises (DCPs) and Suspect Premises (SPs) will be examined daily for the first 2 weeks and then at weekly intervals. Other properties in the RA will be examined weekly. In the absence of any signs of disease during this 21‐day period of observation, animals from the RA may be sent for slaughter, under permit, at approved abattoirs. | No specific guidelines described in legislation |
| 10th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of day-old‐chicks located in the protection zone and hatched from eggs originating in the restricted zone or outside the restricted zone. The sampling procedures should ensure that the movement of these day-old‐chicks to an establishment located in the same Member State but if possible, outside the restricted zone. | NA | NA |
| 11th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to-lay poultry located in the protection zone, to establishments located in the same Member State and if possible within the restricted zone. | NA | NA |
| 12th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment in a protection zone, in order to grant derogation from prohibitions in the movement of these animals to a plant approved for processing or disposal of animal by‐products in which the kept animals are immediately killed (Art37). | No specific guidelines described in legislation | No specific guidelines described in legislation |
| 13th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of listed species in order to grant derogation from prohibitions and allow for these animals to be moved : a) from an establishment in a surveillance zone to a slaughterhouse located within or outside the restricted zone, b)from an establishment outside the surveillance zone to a slaughterhouse situated in the surveillance zone. | Article 12: 1. Member States shall ensure that the following measures are applied in the surveillance zone: (d) animals of susceptible species must remain inside the surveillance zone for a maximum incubation period after the most recent recorded case of disease. Thereafter, animals may be removed from that zone to be transported under official supervision directly to a slaughterhouse designated by the competent authority for emergency slaughter. Such transport may be authorised by the competent authority only after the official veterinarian has carried out an examination of all the animals of the susceptible species on the holding and confirmed that none of the animals is suspected of being infected. The competent authority responsible for the slaughterhouse shall be informed of the intention to send animals to it. | No specific guidelines described in legislation |
| 14th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant a derogation and allow for the animals to be moved from an establishment in the surveillance zone to pastures situated within the surveillance zone. | NA Article 12: 1. Member States shall ensure that the following measures are applied in the surveillance zone: (b) the movement of animals of susceptible species on public roads shall be prohibited except for the purpose of leading them to pasture or animal buildings. | NA |
| 15th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of kept ungulates of listed species in order to grant derogation and allow for them to be moved from an establishment in the surveillance zone to an establishment belonging to the same supply chain, located in or outside the surveillance zone, in order to complete the production cycle before slaughter. | NA Article 12: 1. Member States shall ensure that the following measures are applied in the surveillance zone: (b) the movement of animals of susceptible species on public roads shall be prohibited except for the purpose of leading them to pasture or animal buildings. | NA |
| 16th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations to grant derogation of movements of day-old‐chicks hatched from establishment located in the surveillance zone, from eggs originating within the surveillance zone and eggs originating outside the restricted zone, to an establishment located in the same Member State where they were hatched. | NA | NA |
| 17th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations, to grant a derogation from prohibitions in the movement of ready‐to-lay poultry located in the surveillance zone to establishments located in the same Member State. | NA | NA |
| 18th | To assess the effectiveness of disease‐specific sampling procedures based on clinical and/or laboratory examinations of the animals of an establishment located in the restricted zone of an outbreak in order to allow their move within the restricted zone, when restriction measures are maintained beyond the period set out in Annex XI. | Article 13: Where the prohibitions provided for in Articles 11 (1) (d) and 12 (1) (d) are maintained beyond 30 days because of the occurrence of further cases of the disease and as a result problems arise in keeping the animals, the competent authority may, following an application by the owner explaining the rounds for such application, by the owner explaining the grounds for such applications authorise the removal of the animals from a holding within the protection zone or the surveillance zone, provided that: (a) the official veterinarian has verified the facts; (b) an inspection of all animals on the holding has been carried out; (c) the animals to be transported have undergone a clinical examination, with negative result; (d) each animal has been marked by ear marking or has been identified by any other approved method; (e) the holding of destination is located either in the protection zone or within the surveillance zone. | No specific guidelines described in legislation |
| Repopulation | |||
| 19th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that are kept for the repopulation prior to their introduction to rule out the presence of the disease. | NA | No specific guidelines described in legislation Article 5: The restocking of the holding shall be authorised by the competent authority, following the satisfactory inspection by the official veterinarian of the cleaning and disinfection operations carried out in accordance with Article 16. Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): On infected premises (IPs), and on dangerous contact premises (DCPs) that have been destocked, sentinel animals may be introduced after decontamination is completed. These animals should undergo weekly physical inspection with appropriate testing for 6 weeks, when restocking may occur. The flock should be inspected at 1‐month intervals for 3 months. |
| 20th | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, in the event of unusual mortalities or clinical signs being notified during the repopulation; to rule out the presence of the disease. | NA | No specific guidelines described in legislation Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): Animals dying within 12 months after repopulation of IPs must be autopsied and appropriate samples taken for virus testing. |
| 21st | To assess the effectiveness of disease‐specific sampling procedures based on laboratory examinations of the animals that have been repopulated, on the last day of the monitoring period calculated forward from the date on which the animals were placed in the repopulated establishment. In case the repopulation takes place in several days, the monitoring period will be calculated forward from the last day in which the last animal is introduced in the establishment. | NA | No specific guidelines described in legislation Disease Strategy Sheep pox and goat pox (Animal Health Australia, 2011): On infected premises (IPs), and on dangerous contact premises (DCPs) that have been destocked, sentinel animals may be introduced after decontamination is completed. These animals should undergo weekly physical inspection with appropriate testing for 6 weeks, when restocking may occur. The flock should be inspected at 1‐month intervals for 3 months. |
References
Animal Health Australia, 2011. Disease Strategy: Sheep pox and goat pox. (Version 3.1). AHA, Canberra, ACT. Available online: https://www.animalhealthaustralia.com.au/our-publications/ausvetplan-manuals-and-documents/ [Accessed: 31 March 2021].
Babiuk S, Bowden TR, Boyle DB, Wallace DB and Kitching RP, 2008. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transboundary and Emerging Diseases, 55, 263–272.
Breed et al., 2019. Emergency animal diseases: a field guide for Australian veterinarians; Chapter 3.23. Sheep pox and goat pox. Available online: https://www.outbreak.gov.au/for-vets-and-scientists/emergency-animal-diseases-guide
CABI (Centre for Agriculture and Biosciences International), 2019. Sheep and Goat Pox. Available online: http://www.cabi.org/isc/datasheet/81537 [Accessed: 6 April 2021].
CFSPH, Iowa State University, 2008. Sheep & Goat Pox. Last Updated: August 2017. Available online: http://www.cfsph.iastate.edu/Factsheets/pdfs/sheep_and_goat_pox.pdf [Accessed: 2 April 2021].
Department of Agriculture, Water, and the Environment, 2017. Emergency Animal Disease Bulletin No. 118. Available online: https://www.agriculture.gov.au/pests-diseases-weeds/animal/ead-bulletin [Accessed: 1 April 2021].
EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Scientific opinion on sheep and goat pox. EFSA Journal 2014;12.11:3885.
EURL Capripox, 2021. Sheep and goatpox. Available online: https://www.eurl-capripox.be/sheep-and-goat-pox [Accessed: 1 April 2021].
Lamien CE, 2017. LAMIEN. Animal Production and Health Laboratory, Joint FAO/IAEA Division. Diagnostic tools to detect capripoxvirus infections and diva strategies. Available online: http://www.fao.org/fileadmin/user_upload/reu/europe/documents/events2017/LSD_bp/d2/DIVA.pdf [Accessed: 1 April 2021].
OIE, 2019. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.7.12. Sheep pox and goat pox; heading ‘B.Diagnostic techniques. Available online: https://www.oie.int/international-standard-setting/terrestrial-manual [Accessed: 1 April 2021].
Annex D –Scenarios of length of monitoring period (ToR 2)
1.
| ToRs | Legislation | Scenario | Description of the Scenario | Elements of the Scenarios |
|---|---|---|---|---|
| ToR 2 | Article 8 of the Delegated Regulation Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation | 1st scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of the notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of a suspicion. |
|
| ToR 2 | Article 17(2) and Article 57 of 2016/429 Regulation Annex II of the Delegated Regulation | 2nd scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of a category A disease in an establishment with kept animals of listed species, for the purposes of the epidemiological enquiry in the event of confirmation of the disease. |
|
| ToR 2 | Article 13(b) of the Delegated RegulationAnnex II of the Delegated Regulation | 3rd scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of confirmation of a category A disease in an establishment with kept animals of listed species, during which the epidemiological units in which the disease has not been confirmed were kept completely separated and handled by different personnel, in order to provide derogations from killing. |
|
| ToR 2 | Article 27(3)c of the Delegated RegulationAnnex II of the Delegated Regulation | 4th scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated backwards from the date of notification of the suspicion of the latest outbreak of a category A disease in the protection zone. Products or other materials likely to spread the disease, must had been obtained or produced, before this time period in order to be exempted from prohibitions of movements. |
|
| ToR 2 | Article 32(c) of the Delegated RegulationArticle 48(c) of the Delegated RegulationAnnex II of the Delegated Regulation | 5th scenario | To assess the effectiveness of the length of the Monitoring Period, as the time period calculated forwards from the date of semen collection from animals of listed species kept in approved germinal product establishments in the protection or in the surveillance zone, to prove that the donor animal has tested favourable on a sample taken not earlier than 7 days after the monitoring period. |
|
| ToR 2 | Article 57(1)b of the Delegated RegulationAnnex II of the Delegated Regulation | 6th scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards from the date after the final cleaning and disinfection and when relevant control of insects and rodents was carried out in an affected establishment, after which the repopulation of the establishment may be allowed by the competent authority. |
|
| ToR 2 | Article 59(4)b of the Delegated RegulationAnnex II of the Delegated Regulation | 7th scenario | To assess the effectiveness of the length of the Monitoring Period, as the appropriate time period calculated forwards the date when the first animal was introduced, during which all the animals of listed species intended for repopulation should be introduced. |
|
Annex E –Literature search protocol for length of monitoring period
1.
Review question
The specific objective of this review will be to answer the epidemiological question of: ‘what is the average, shortest and longest period of time (measured as the number of days from the earliest point of infection with the agent, to the time of reporting of a suspicion by the competent authority after the clinical investigation by an official veterinarian) for an outbreak of each of the 9 diseases of concern to be reported’.
Criteria for including studies
Starting with the objectives of the review stated above, the study inclusion criteria are based on the PICOS strategy:
| Population | Domestic animal species |
| Intervention | PPR, CSF, ND, SPP/GTP, RVF, Glanders, CCPP, CBPP, Rinderpest |
| Comparison | Not applicable |
| Outcome | Number of days between the earliest point of infection and the suspicion reportNumber of days between the earliest point of infection and the first suspicion1Number of days between the earliest point of infection and the confirmation reportNumber of days between the first suspicion and the suspicion reportNumber of days between the first suspicion and the confirmation reportNumber of days between the suspicion report and the confirmation report |
| Study design | Outbreak investigation, case report, surveillance data, modelling studies |
The suspicion based on the first observed clinical signs.
Exclusion criteria
The references will be excluded from the ELR if they meet one or more of following criteria:
References in another language than English, Spanish, German, Dutch, Portuguese and French.
Review papers. However, original studies included in the review papers complying with the inclusion/exclusion criteria will be included.
References published before 1/1/2000.
References pertaining exclusively to diagnostics/vaccine development, entomology, in vitro/vivo studies.
References where the earliest point of infection is determined only by subtracting a known incubation period from the date of the suspicion of the outbreak. However, after discussion with EFSA and comment from experts, outbreaks investigations that do not determine the true date of infection but report about the time between the onset of clinical signs and date of suspicion of the disease could be included.
References presenting simulation exercises. However, if none or very few articles are retrieved (less or equal to 5) in the first search, these studies may be included but their data should be presented in a separate table in the report together with a description of the methodology.
References from outside the EU/EEA countries. However, If none or very few articles are retrieved (less or equal to 5) in the first search, the search should be extended to the rest of the world.
References related to outbreaks that took place in a slaughterhouse. Nonetheless, references referring to outbreaks that occurred elsewhere, and are detected in a slaughterhouse may be included if all other conditions for inclusion are met.
Information sources
Electronic databases
We will conduct a literature search in MEDLINE (via PubMed) and EMBASE to obtain peer‐reviewed, scientific publications related to the ELR.
Reference checking and hand searching
The reference list of relevant studies retrieved from the electronic database search will be hand searched to identify additional studies.
Grey literature selection
Data in the public domain pertaining to the objective of this study, outbreak investigation reports or surveillance data will be obtained via PAFF, OIE, EFSA, FAO, EuFMD websites, Google Scholar and websites of EU veterinary reference laboratories for to the nine investigated diseases as well as websites of national veterinary/animal health institutes, reference veterinary laboratories or ministry of livestock from EU countries that previously experienced outbreaks of any of the five investigated diseases.
Search strategy
The following search strategy will be used in PubMed:
| # | Search string | # of results |
|---|---|---|
| 1 | (((((((((((((((((((“first infection”[All Fields] OR “index case”[All Fields]) OR (“introduction”[All Fields] OR “introductions”[All Fields])) OR “source of infection”[All Fields]) OR “clinical signs”[All Fields]) OR “clinical symptoms”[All Fields]) OR “case studies”[All Fields]) OR (“suspicion”[All Fields] OR “suspicions”[All Fields])) OR (((“suspect”[All Fields] OR “suspected”[All Fields]) OR “suspecting”[All Fields]) OR “suspects”[All Fields])) OR ((((((“confirm”[All Fields] OR “confirmation”[All Fields]) OR “confirmations”[All Fields]) OR “confirmative”[All Fields]) OR “confirmed”[All Fields]) OR “confirming”[All Fields]) OR “confirms”[All Fields])) OR ((((((“confirm”[All Fields] OR “confirmation”[All Fields]) OR “confirmations”[All Fields]) OR “confirmative”[All Fields]) OR “confirmed”[All Fields]) OR “confirming”[All Fields]) OR “confirms”[All Fields])) OR ((((((((“reportable”[All Fields] OR “reporting”[All Fields]) OR “reportings”[All Fields]) OR “research report”[MeSH Terms]) OR (“research”[All Fields] AND “report”[All Fields])) OR “research report”[All Fields]) OR “report”[All Fields]) OR “reported”[All Fields]) OR “reports”[All Fields])) OR ((((((((“reportable”[All Fields] OR “reporting”[All Fields]) OR “reportings”[All Fields]) OR “research report”[MeSH Terms]) OR (“research”[All Fields] AND “report”[All Fields])) OR “research report”[All Fields]) OR “report”[All Fields]) OR “reported”[All Fields]) OR “reports”[All Fields])) OR ((((((((“reportable”[All Fields] OR “reporting”[All Fields]) OR “reportings”[All Fields]) OR “research report”[MeSH Terms]) OR (“research”[All Fields] AND “report”[All Fields])) OR “research report”[All Fields]) OR “report”[All Fields]) OR “reported”[All Fields]) OR “reports”[All Fields])) OR (“notification”[All Fields] OR “notifications”[All Fields])) OR ((((((“notifiable”[All Fields] OR “notified”[All Fields]) OR “notifier”[All Fields]) OR “notifiers”[All Fields]) OR “notifies”[All Fields]) OR “notify”[All Fields]) OR “notifying”[All Fields])) OR ((((((“declaration”[All Fields] OR “declaration s”[All Fields]) OR “declarations”[All Fields]) OR “declare”[All Fields]) OR “declared”[All Fields]) OR “declares”[All Fields]) OR “declaring”[All Fields])) OR ((((((“declaration”[All Fields] OR “declaration s”[All Fields]) OR “declarations”[All Fields]) OR “declare”[All Fields]) OR “declared”[All Fields]) OR “declares”[All Fields]) OR “declaring”[All Fields])) OR (((((((((((“detect”[All Fields] OR “detectabilities”[All Fields]) OR “detectability”[All Fields]) OR “detectable”[All Fields]) OR “detectables”[All Fields]) OR “detectably”[All Fields]) OR “detected”[All Fields]) OR “detectible”[All Fields]) OR “detecting”[All Fields]) OR “detection”[All Fields]) OR “detections”[All Fields]) OR “detects”[All Fields])) OR ((((“trace”[All Fields] OR “traced”[All Fields]) OR “traces”[All Fields]) OR “tracing”[All Fields]) OR “tracings”[All Fields])) OR (((((((((((“investigated”[All Fields] OR “investigates”[All Fields]) OR “investigating”[All Fields]) OR “investigation”[All Fields]) OR “investigations”[All Fields]) OR “investigative”[All Fields]) OR “investigator s”[All Fields]) OR “research personnel”[MeSH Terms]) OR (“research”[All Fields] AND “personnel”[All Fields])) OR “research personnel”[All Fields]) OR “investigator”[All Fields]) OR “investigators”[All Fields]) | 11,433,545 |
| 2 | #1 AND“time”[MeSH Terms] OR “time”[All Fields] OR “delay”[All Fields] OR “delayed”[All Fields] OR “delaying”[All Fields] OR “delays”[All Fields] OR “length”[All Fields] OR “lengths”[All Fields] OR “period”[All Fields] OR “periodic”[All Fields] OR “periodical”[All Fields] OR “periodically”[All Fields] OR “periodicals”[All Fields] OR “periodicity”[MeSH Terms] OR “periodicity”[All Fields] OR “periodicities”[All Fields] OR “periods”[All Fields] OR “duration”[All Fields] OR “durations”[All Fields] OR “days”[All Fields] OR “date”[All Fields] OR “timelier”[All Fields] OR “timeliness”[All Fields] OR “timelier”[All Fields] OR “timeliness”[All Fields] OR “timely”[All Fields] OR “timing”[All Fields] OR “timings”[All Fields] | 3,497,425 |
| 3a | #2 AND (“peste des petits ruminants”) Filters: from 2000 to 2021 | 168 |
| 3b | #2 AND (“classical swine fever”) Filters: from 2000 to 2021 | 530 |
| 3c | #2 AND (“newcastle disease”) Filters: from 2000 to 2021 | 821 |
| 3d | #2 AND (“sheep pox and goat pox”) Filters: from 2000 to 2021 | 31 |
| 3e | #2 AND (“rift valley fever”) Filters: from 2000 to 2021 | 360 |
| 3f | #2 AND (“glanders”) Filters: from 2000 to 2021 | 57 |
| 3g | #2 AND (“contagious caprine pleuropneumonia”) Filters: from 2000 to 2021 | 18 |
| 3h | #2 AND (“contagious bovine pleuropneumonia”) Filters: from 2000 to 2021 | 47 |
| 3i | #2 AND (“rinderpest”) Filters: from 2000 to 2021 | 66 |
Review methods
Selection of the studies
The list of studies identified from the different databases will be appended into a single file using Endnote and de‐duplicated. The resulting list will be exported to Rayyan7 to proceed with the title, abstract and keywords screening and study selection.
To decrease the risk of selection bias, two P95 reviewers will independently review the list of references obtained by screening key words in title/abstract to identify studies that fulfil the above‐mentioned selection criteria. Discrepancies will be discussed, and if not resolved, a third reviewer will take the final decision.
All identified reviews and studies conducted outside EU/EEA will be classified in specific folders for subsequent use.
Data extraction
In the second phase, full papers will be assessed for eligibility by a single reviewer. Data from the eligible full‐text papers identified will be extracted by two reviewers using a standardised extraction form in MS Excel (see below) to ensure that all relevant data are collected systematically.
Table: Data extraction format
| Ref ID |
| Ref |
| Source |
| Author |
| Disease |
| Objective |
| Country |
| Region |
| Outbreak ID |
| Year |
| Species |
| Farm type |
| Level |
| Sample size |
| Parameter_type |
| Parameter_unit |
| Parameter_value |
| Comment |
| Calculation_description |
| Calculation_output |
| Disease | Objective | Country | Region | Outbreak ID | Year | Species | Farm type | Level | Sample size | Parameter_type | Parameter_unit | Parameter_value | Calculation_description | Calculation_output | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGP | describe the outbreak | Greece | Evros | Index | 2013 | Sheep | NA | Farm | 10 | Date suspicion report | Date | 12/8/2013 | SCoFCAH (2013a,b) | ||
| SGP | describe the outbreak | Greece | Evros | Index | 2013 | Sheep | NA | Farm | 10 | Date confirmation | Date | 14/8/2013 | Period between suspicion report and confirmation | 2 | SCoFCAH (2013a,b) |
| SGP | describe the outbreak | Greece | Evros | Index | 2013 | Sheep | NA | Farm | 10 | Estimated age of lesions | Date | 3.5 | Period between earliest point of infection and suspicion report | 14 | SCoFCAH (2013a,b) |
| SGP | describe the outbreak | Bulgaria | Burgas | Index | 2013 | Sheep | NA | Farm | 7 | Date suspicion report | Date | 19/9/2013 | SCoFCAH (2013a,b) | ||
| SGP | describe the outbreak | Bulgaria | Burgas | Index | 2013 | Sheep | NA | Farm | 7 | Date confirmation | Date | 20/9/2013 | Period between suspicion report and confirmation | 1 | SCoFCAH (2013a,b) |
| SGP | describe the outbreak | Burgas | Plovdiv | Index | 2013 | Sheep | NA | Farm | 15 | Date earliest point of infection | Date | 2/12/2013 | SCoFCAH (2014) | ||
| SGP | describe the outbreak | Burgas | Plovdiv | Index | 2013 | Sheep | NA | Farm | 15 | Date suspicion report | Date | 4/12/2013 | Period between earliest point of infection and suspicion report | 2 | SCoFCAH (2014) |
| SGP | describe the outbreak | Burgas | Plovdiv | Index | 2013 | Sheep | NA | Farm | 15 | Date confirmation | Date | 5/12/2013 | Period between suspicion report and confirmation | 1 | SCoFCAH (2014) |
| SGP | describe the outbreak | Greece | Lesvos island | Index | 2016 | Sheep and goats | NA | Farm | 114 | Date suspicion report | Date | 7/12/2017 | PAFF (2017) | ||
| SGP | describe the outbreak | Greece | Lesvos island | Index | 2016 | Sheep and goats | NA | Farm | 114 | Date confirmation | Date | 9/12/2017 | Period between suspicion report and confirmation | 2 | PAFF (2017) |
| SGP | describe the outbreak | Greece | Lesvos island | Index | 2017 | Sheep and goats | NA | Farm | 42 | Date suspicion report | Date | 5/9/2017 | IZSAM. (2017) | ||
| SGP | describe the outbreak | Greece | Lesvos island | Index | 2017 | Sheep and goats | NA | Farm | 42 | Date confirmation | Date | 7/9/2017 | Period between suspicion report and confirmation | 2 | IZSAM (2017) |
| SGP | provide an update on the characterisation of the diseases, to assess the risk of spread and to determine if further measures are justified. | Greece and Bulgaria | NA | NA | NA | NA | NA | Epidemic | NA | Period between earliest point of infection and suspicion report | Days | 8 | EFSA (2014) | ||
| SGP | provide an update on the characterisation of the diseases, to assess the risk of spread and to determine if further measures are justified. | EU‐Turkey | NA | NA | NA | NA | NA | Epidemic | NA | Period between earliest point of infection and confirmation | Days | 21 | EFSA (2014) |
In addition, the section of the pdf manuscript from where data will be collected will be noted and/or highlighted.
The complete selection process will be documented in an Endnote file, containing folders that reflect the selection criteria.
Analysis and reporting
During the selection process, the results of the literature search will be imported into Endnote where a clear track of the selection process will be maintained, and the flow of publications will be noted. Based on these numbers, a flowchart of the studies selected in accordance with the PRISMA guidelines will be prepared for use in the subsequent reports.
If needed, extracted dates of interest will be combined together in order to calculate the periods of interest. Extracted data on age of the lesions can also be used to estimate the earliest point of infection. All these calculations will be described in an assigned column of the extraction form.
Using the data collected, a qualitative data synthesis of results will be performed for each specific disease and parameter in terms of average, shortest and longest period of time. The different findings will be synthesised using tables providing sufficient details on the methodology used in the references.
Annex F –Minimum radius and minimum period of duration of protection and surveillance zones
1.
| Category A diseases | Minimum radius of Protection zone Annex V | Minimum radius of Surveillance zone Annex V | Minimum period of duration of measures in the protection zone (Article 39(1)) Annex X | Additional period of duration of surveillance measures in the protection zone (Article 39(3)) Annex X | Minimum period of duration of measures in the surveillance zone (as referred to in Articles 55 and 56 of this Regulation) Annex XI |
|---|---|---|---|---|---|
| Foot and mouth disease (FMD) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Infection with rinderpest virus (RP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with Rift Valley fever virus (RVFV) | 20 km | 50 km | 30 days | 15 days | 45 days |
| Infection with lumpy skin disease virus (LSD) | 20 km | 50 km | 28 days | 17 days | 45 days |
| Infection with Mycoplasma mycoides subsp. mycoides SC (Contagious bovine pleuropneumonia) (CBPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| Sheep pox and goat pox (SPGP) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Infection with peste des petits ruminant virus (PPR) | 3 km | 10 km | 21 days | 9 days | 30 days |
| Contagious caprine pleuropneumonia (CCPP) | Establishment | 3 km | 45 days | Not applicable | 45 days |
| African horse sickness (AHS) | 100 km | 150 km | 12 months | Not applicable | 12 months |
| Infection with Burkholderia mallei (Glanders) | Establishment | Establishment | 6 months | Not applicable | Not applicable |
| Classical swine fever (CSF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| African swine fever (ASF) | 3 km | 10 km | 15 days | 15 days | 30 days |
| Highly pathogenic avian influenza (HPAI) | 3 km | 10 km | 21 day | 9 days | 30 days |
| Infection with Newcastle disease virus (NCD) | 3 km | 10 km | 21 days | 9 days | 30 days |
Annex G –Uncertainty
1.
| Source or location of the uncertainty | # | Nature or cause of uncertainty as described by the experts | Impact of the uncertainty on the assessment |
|---|---|---|---|
| ToR 1 | 1 | Parameters governing transmission dynamics and mortality rates in the model used for answering scenarios under ToR 1 are based on one study and on epidemics in extra European context (e.g. Taiwan) since not enough data available from EU epidemics (not enough outbreaks in epidemic in Bulgaria and control intervention in epidemic in Greece which modified the natural spread of SPP/GTP) | The effectiveness of the sampling strategies could be over or underestimated |
| 2 | Lack of evidence in the literature about possible occurrence of low virulent strains and host susceptibility leading to few or none clinical signs. | The effectiveness of the sampling strategies could be over or underestimated | |
| 3 | The generic real‐time capripoxvirus PCR shows values of 100% and specificity ranging from 94.7% to 100%. For serology, commercial antibody ELISA cannot discriminate SPPV and GTPV antibodies and proper validation should be carried out. There may be instances in which test performance may be limited (particularly with regards to serological tests). | The effectiveness of the sampling strategies could be overestimated | |
| ToR 2 | 4 | The assessment of length of monitoring is based on data are available from limited evidence of primary outbreaks in Greece and Bulgaria affecting only sheep, where awareness was already raised due to the disease being present in the neighbouring country (Turkey). | The effectiveness of the proposed strategy could be over or underestimated |
| 5 | References used to estimate the time between infection and seroconversion are based on experimental inoculation of SPPV/GTPV (instead of natural infection), what may not mimic the situation in naturally infected animals (particularly with lower infectious doses) | The effectiveness of the proposed strategy could be over or underestimated | |
| 6 | Transmission of SPP/GTP virus in semen has not yet been established | ||
| ToR 3 | 6 | SPP/GTP kernels were fitted only on data from a single region in Greece (Evros) and thus may not be representative of transmission in different epidemiological situation | The effectiveness of the proposed zone size could be over or underestimated |
Annex H –Estimating transmission parameters for sheep and goat pox virus
1.
Transmission parameters were estimated by applying the methods described in Hu et al. (2017) to data from a transmission experiment presented by Wolff et al. (2020). In this experiment three goats were infected by intravenous and subcutaneous inoculation, three goats were infected by intranasal inoculation and two goats were kept in‐contact with the inoculated animals. Virus was isolated from all eight goats and all showed clinical signs and seroconverted. When analysing the data, detection of viral DNA in oral or nasal swabs was used as the indicator of infectiousness.
Priors for the latent and infectious period parameters (Table H.1) were based on previously published estimates (EFSA, 2014). No information was available with which to construct a prior for the transmission rate. Accordingly, three priors were considered to assess the impact of prior assumptions on the posterior estimates.
Table H.1.
Prior distributions used when estimating transmission parameters for sheep and goat pox virus
| Parameter | Symbol | Prior distribution |
|---|---|---|
| Mean latent period | μE | Gamma(mean = 7, shape = 10) |
| Shape parameter for latent period | kE | Gamma(mean = 2, shape = 10) |
| Mean infectious period | μI | Gamma(mean = 21, shape = 10) |
| Shape parameter for infectious period | kI | Gamma(mean = 2, shape = 10) |
| Transmission rate | β | Exponential(mean = 0.01) |
| Exponential(mean = 0.1) | ||
| Exponential(mean = 1) | ||
| Uniform(0, 5) |
The posterior distributions for the latent and infectious period distribution parameters did not depend on the prior assumptions about the transmission parameter (Table H.2). However, the posterior distribution for the transmission parameter was strongly dependent on the prior assumptions made about it (Table H.2). The parameters values assuming an exponential prior with mean 0.1 were used in the assessment as this produced the most plausible value for R0.
Table H.2.
Transmission parameters* for sheep and goat pox virus and their dependence on prior assumptions about the transmission rate
| Parameter | Prior for β | |||
|---|---|---|---|---|
| Exponential (0.01) | Exponential (0.1) | Exponential (1) | Uniform (0, 5) | |
| μE | 4.8 (3.2, 7.3) | 4.9 (3.2, 7.6) | 5.1 (3.3, 7.8) | 5.2 (3.5, 7.9) |
| kE | 2.4 (1.3, 4.1) | 2.4 (1.3, 4.0) | 2.5 (1.4, 4.1) | 2.5 (1.4, 4.2) |
| μI | 28.9 (18.2, 44.8) | 29.0 (18.5, 44.7) | 29.0 (18.5, 44.3) | 29.1 (18.6, 44.2) |
| kI | 2.2 (1.2, 3.7) | 2.2 (1.2, 3.7) | 2.2 (1.2, 3.7) | 2.2 (1.2, 3.7) |
| β | 0.03 (0.006, 0.07) | 0.2 (0.05, 0.6) | 0.9 (0.2, 3.7) | 2.5 (0.4, 4.9) |
| R0 | 0.8 (0.2, 2.2) | 5.7 (1.2, 17.3) | 27.6 (4.9, 117.3) | 70.2 (9.8, 171.8) |
Posterior median (95% credible interval).
Annex I – Model to estimate kernels for the spread of sheep and goat pox between farms
1.
This appendix presents full details of the modelling approach used to estimate kernels for the spread of sheep and goat pox between farms.
Epidemiological data
The location and time of destruction for reported outbreaks of sheep and goat pox from the epidemic in Greece between 2013 and 2015 were extracted from ADNS. Because times of infection are not observed, farms were assumed to become infected (and infectious) 30 days prior to destruction (the sensitivity of the estimates to this assumption was assessed; see Table I.1).
Table I.1.
Transmission kernels for sheep and goat pox and the impact of assumed outbreak duration on kernel selection and estimates
| Kernel | Function | Outbreak duration | ||||
|---|---|---|---|---|---|---|
| 15 days | 30 days | 60 days | 90 days | |||
| Fat‐tailed |
|
AIC = 3,240 d0 = 6.37 | AIC = 3,214 d0 = 5.67 | AIC = 3,222 d0 = 5.02 | AIC = 3,287 d0 = 4.45 | |
| Gaussian |
|
AIC = 3,258 d0 = 18.5 | AIC = 3,237 d0 = 17.5 | AIC = 3,248 d0 = 10.8 | AIC = 3,313 d0 = 10.1 | |
| Exponential |
|
AIC = 3,243 d0 = 10.3 | AIC = 3,220 d0 = 10.1 | AIC = 3,230 d0 = 10.8 | AIC = 3,295 d0 = 11.4 | |
| Alternative fat‐tailed |
|
AIC = 3,229 d0 = 1.03 α = 1.20 | AIC = 3,208 d0 = 2.23 α = 1.47 | AIC = 3,217 d0 = 2.27 α = 1.60 | AIC = 3,280 d0 = 1.90 α = 1.60 | |
Modelling approach
The spread of sheep and goat pox was modelled at the farm level. Transmission between farms was modelled using a kernel‐based approach. In this case, the force of infection, λi(t), for farm i on day t is given by,
where hB is the background transmission rate (e.g. due to introductions of SPP/GTP from outside Greece, unobserved infected farms or unexpectedly long distance animal movements), hK is the kernel transmission rate (i.e. due to known infected farms), Ni is the number of small ruminants (sheep and goats) on farm i, K(dij) is the transmission kernel (see below), dij is the great circle distance between farms i and j and Ij(t) is a variable indicating whether farm j is infectious (1) or not (0) on day t.
Four functional forms were considered for the kernel (Table I.1), which differ in their shape and the rate at which they decay to zero. In each kernel the parameter d 0 is the distance scaling and in the alternative fat‐tailed kernel α controls how rapidly the kernel decays with distance.
Parameter estimation
Parameters in the model were estimated using maximum likelihood methods. Because locations are available only for affected farms, we used a conditional likelihood for the data (Szmaragd et al., 2009). In this case, the likelihood is given by,
where λi(t) is the force of infection defined above tinf is the time at which the farm became infected, t0 is the time at the start of the epidemic and tend is the time at the end of the epidemic.
Selection of the best‐fit kernel was based on the Akaike information criterion,
where Lmax is the maximum likelihood and k is the number of parameters in the model. The model with the smallest AIC is preferred. Confidence intervals for the kernel parameters (d0 and α) were computed using the profile likelihood.
The sensitivity of the best‐fit kernel and the kernel estimates to assumptions about the duration of a within‐farm outbreak was assessed by fitting each of the four kernels assuming four outbreak durations (15, 30, 60 or 90 days). For each assumed outbreak duration, the best‐fitting kernel was the alternative fat‐tailed, followed by the fat‐tailed, exponential and Gaussian (Table I.1). In addition, the kernel estimates did not differ greatly in each case (Table I.1). Finally, the lowest AICs were obtained when the assumed outbreak duration was 30 days. Kernel estimates from this analysis (i.e. 30 days) where used to assess the minimum size of the protection and surveillance zones.
References
Szmaragd C, Wilson A, Carpenter S, Wood JLN, Mellor PS and Gubbins S, 2009 A modeling framework to describe the transmission of bluetongue virus within and between farms in Great Britain. PLoS One, 4, e7741.
Annex J – Literature search for seroconversion period of SPP/GTP
1.
Methodology
For the assessment of scenario 5 of the 2nd ToR, the methodology described in Section 2.3 of the Technical Report published by EFSA (https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2020.EN-1988) and in EFSA (2014) scientific opinion for SPP/GTP was followed.
The framework to meet the objectives is depicted in Table J.1.
Table J.1.
Framework of methodology for literature search for seroconversion period of SPP/GTP
| Years | From 1975 onwards |
| Comments/Explanation: This will depend on the availability of the bibliographic databases | |
| Language | Only studies written in English will be reviewed |
| Comments/Explanation: | |
| Publication type | Only primary research studies will be reviewed |
Comments/Explanation:
| |
| Population | Ovis ssp., Capra ssp. |
| Comments/Explanation: | |
| Intervention | Serological diagnostic tests for Sheep Pox and Goat Pox |
| Comments/Explanation: | |
| Target | Sheep Pox Virus and Goat Pox Virus (Poxviridae, genus Capripoxvirus) will be the targeted pathogens |
| Comments/Explanation: |
Information sources
Search strategies included the use of electronic search engines for bibliographic databases. Two databases were searched, PubMed and Mendeley.
Due to the specificity of the objective and time constrains only clinical trials and randomised controlled trials were included. Moreover, recent OIE diagnostic manual and relevant previous EFSA scientific opinions were also included. Reviews were not included, but their reference lists were screened as sources of studies. Book chapters, theses and informally reported or unpublished data were not collated.
Search strategy
The following search strategy was followed:
Population: sheep* or goat*
Serological Tests: “diagnostic test” or serolog* or antibod* or “virus neutralisation” or *ELISA or VNT or “indirect fluorescent antibody test” or IFAT
Target: “Sheep Pox Virus” or “Goat Pox Virus” or SPPV or GPT or SPP/GTP or Poxviridae or Capripoxvirus
A scoping search identified:
115 papers in PubMed and
319 papers in Mendeley
A database of the electronic search results was created with Mendeley software. Duplicate citations were deleted automatically or manually when appropriated (n = 34).
Study selection
Study selection was based on the following pre‐defined inclusion criteria (questions):
Is the paper in English? Yes Unclear No
Is the paper an original clinical trial
or a randomised controlled trial for
the targeted listed species? Yes Unclear No
Is the paper describing a serological
diagnostic test for SPP/GTP? Yes Unclear No
Is the paper describing the earliest
day of seroconversion and the latest
day of antibodies detection after infection Yes Unclear No
Final decision: Include Review Exclude
All papers with a ‘no’ or ‘unclear’ for any question were excluded based on title/abstract.
All papers with a ‘yes’ for each question were reviewed based on title/abstract.
Screening of titles and abstracts after the application of the above inclusion criteria was conducted for 52 papers.
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Gubbins S, Aznar I and Broglia A, 2021. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: sheep and goat pox. EFSA Journal 2021;19(12):6933, 89 pp. 10.2903/j.efsa.2021.6933
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00799
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Helen Clare Roberts, Barbara Padalino, Paolo Pasquali, Liisa Helena Sihvonen, Hans Spoolder, Karl Ståhl, Antonio Velarde Calvo, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The EFSA Panel on Animal Health and Welfare wishes to thank Evangelos Tsiamadis for the support provided to this scientific output.
Adopted: 22 September 2021
This article was originally published on the EFSA website http://www.efsa.europa.eu on 18 October 2021 as part of EFSA's publication procedures.
Notes
In the assessment section, when PCR is mentioned, it refers to ‘real time PCR’.
Transmission is assumed to be frequency dependent, so the transmission rate is the same for all flock sizes. The number of infected animals in a flock at a given time point is similar regardless of herd size, so the prevalence is lower in larger flocks.
The transmission kernel, K(x), is the probability of transmission beyond the specified distance, x, given that transmission occurs.
References
- Afshar A, Bundza A, Myers DJ, Dulac GC and Thomas FC, 1986. Sheep pox: experimental studies with a west African isolate. The Canadian Veterinary Journal = La revue veterinaire canadienne, 27, 301–306. [PMC free article] [PubMed] [Google Scholar]
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198. [Google Scholar]
- Ayalet G, Fasil N, Jembere S, Mekonen G, Sori T and Negussie H, 2012. Study on immunogenicity of combined sheep and goat pox and peste des petitis ruminants vaccines in small ruminants in Ethiopia. African Journal of Microbiology Research, 6, 7212–7217. [Google Scholar]
- Babiuk S, Wallace D, Smith S, Bowden T, Dalman B, Parkyn G, Copps J and Boyle D, 2009. Detection of antibodies against capripoxviruses using an inactivated sheeppox virus ELISA. Transboundary and Emerging Diseases, 56, 132–141. [DOI] [PubMed] [Google Scholar]
- Bhanuprakash V, Indrani BK, Hosamani M and Singh RK, 2006. The current status of sheep pox disease. Comparative immunology, microbiology and infectious diseases, 29, 27–60. [DOI] [PubMed] [Google Scholar]
- Bhanuprakash V, Venkatesan G, Balamurugan V, Hosamani M, Yogisharadhya R, Chauhan R, Pande A, Mondal B and Singh R, 2010. Pox outbreaks in sheep and goats at Makhdoom (Uttar Pradesh), India: evidence of sheeppox virus infection in goats. Transboundary and Emerging Diseases, 57, 375–382. [DOI] [PubMed] [Google Scholar]
- Bora DP, Ahmed J, Tadap S, Pariat AO, Mech P, Panda SP, Tashi T, Kakati P, Ingtipi S and Qayum A, 2021. Evidence of transmission of goatpox between domestic goats and wild himalayan goral (Naemorhedus goral) in Arunachal Pradesh, India. The Journal of Wildlife Diseases, 57, 439–442. [DOI] [PubMed] [Google Scholar]
- Boshra H, Truong T, Nfon C, Bowden TR, Gerdts V, Tikoo S, Babiuk LA, Kara P, Mather A, Wallace DB and Babiuk S, 2015. A lumpy skin disease virus deficient of an IL‐10 gene homologue provides protective immunity against virulent capripoxvirus challenge in sheep and goats. Antiviral Research, 123, 39–49. [DOI] [PubMed] [Google Scholar]
- Bowden TR, Coupar BE, Babiuk SL, White JR, Boyd V, Duch CJ, Shiell BJ, Ueda N, Parkyn GR, Copps JS and Boyle DB, 2009. Detection of antibodies specific for sheeppox and goatpox viruses using recombinant capripoxvirus antigens in an indirect enzyme‐linked immunosorbent assay. Journal of Virological Methods, 161, 19–29. [DOI] [PubMed] [Google Scholar]
- CABI (Centre for Agriculture and Biosciences International), 2015. Sheep and Goat Pox.
- CFSPH (Center for food security and public health), 2017. Sheep Pox and Goat Pox. https://www.cfsph.iastate.edu/Factsheets/pdfs/sheep_and_goat_pox.pdf
- Chan K‐W, Lee M‐L, Yang W‐C, Wong M‐L, Hsu W‐L, Ho C‐F, Hsieh Y‐C and Wang C‐Y, 2014. Differential diagnosis of Goatpox virus in Taiwan by multiplex polymerase chain reaction assay and high‐resolution melt analysis. Journal of Veterinary Diagnostic Investigation, 26, 195–202. [DOI] [PubMed] [Google Scholar]
- Chaudhary SS, Pandey KD, Singh RP, Verma PC and Gupta PK, 2009. A vero cell derived combined vaccine against sheep pox and Peste des Petits ruminants for sheep. Vaccine, 27, 2548–2553. [DOI] [PubMed] [Google Scholar]
- CIRAD (French Agricultural Research Centre for International Development), 2019. Sampling instructions for diagnostic investigation of suspected cases of Peste des Petits Ruminants (PPR)
- Davies FG and Otema C, 1978. The antibody response in sheep infected with a Kenyan sheep and goat pox virus. Journal of Comparative Pathology, 88, 205–210. [DOI] [PubMed] [Google Scholar]
- Department of Agriculture and CSIRO , 2019. Emergency animal diseases: a field guide for Australian veterinarians. CC BY 4.0. Sheep and goat pox. https://www.outbreak.gov.au/sites/default/files/documents/emergency-animal-diseases-field-guide-aus-vets.pdf
- Dórea FC, Swanenburg M, Horigan V, Han S YB, de Freitas Costa E, de Souza Santos M.A., Evans D, Royall E, Aznar I and Dhollander S, 2021. Data collection for risk assessments on animal health: review protocol 2021. EFSA supporting publication 2021;EN‐NNNN.
- Dutta TK, Roychoudhury P, Kawlni L, Lalmuanpuia J, Dey A, Muthuchelvan D, Mandakini R, Sarkar A, Ramakrishnan MA and Subudhi PK, 2019. An outbreak of goatpox virus infection in wild red serow (Capricornis rubidus) in Mizoram, India. Transboundary and Emerging Diseases, 66, 181–185. [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Scientific Opinion on sheep and goat pox. EFSA Journal 2014;12(11):3885, 122 pp. 10.2903/j.efsa.2014.3885 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2020. Technical report on the methodological approach used for the assessment of the control measures for Category A diseases in the context of the new Animal Health Law. EFSA Supporting Publications 2020;17:2397‐8325, 1988E. 10.2903/j.efsa.2020.1988E [DOI]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar C, Herskin M, Michel V, Miranda Chueca M A, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Libeau G, Broglia A, Aznar I and Van der Stede Y, 2021. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: peste des petits ruminants. EFSA Journal 2021;19 (7):6708, 94 pp. 10.2903/j.efsa.2021.6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):e05123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassi‐Fehri M and Lefèvre P, 2003. Clavelée et variole caprine. Principales maladies infectieuses et parasitaires du bétail‐ Europe et régions chaudes. Co‐ édition TEC/DOC et Editions Médicales internationales, Lavoisier. pp. 415–427. [Google Scholar]
- Gortázar C, Barroso P, Nova R and Cáceres G, 2021. The role of wildlife in the epidemiology and control of Foot‐and‐mouth‐disease And Similar Transboundary (FAST) animal diseases: a review. Transboundary and Emerging Diseases. [DOI] [PubMed] [Google Scholar]
- Haegeman A, De Leeuw I, Mostin L, Van Campe W, Aerts L, Vastag M and De Clercq K, 2020. An Immunoperoxidase Monolayer Assay (IPMA) for the detection of lumpy skin disease antibodies. Journal of Virological Methods, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, De Vleeschauwer A, De Leeuw I, Vidanović D, Šekler M, Petrović T, Demarez C, Lefebvre D and De Clercq K, 2019. Overview of diagnostic tools for Capripox virus infections. Preventive Veterinary Medicine, 104704. [DOI] [PubMed] [Google Scholar]
- Haegeman A, Zro K, Vandenbussche F, Demeestere L, Van Campe W, Ennaji M and De Clercq K, 2013. Development and validation of three Capripoxvirus real‐time PCRs for parallel testing. Journal of Virological Methods, 193, 446–451. [DOI] [PubMed] [Google Scholar]
- Hu B, Gonzales JL and Gubbins S, 2017. Bayesian inference of epidemiological parameters from transmission experiments. Scientific Reports, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZSAM , 2017. Sheep pox and goat pox outbreaks in Greece. Available online: https://www.izs.it/IZS/Sheep_pox_and_goat_pox_outbreaks_in_Greece
- Keeling MJ and Rohani P, 2011. Modeling Infectious Diseases in Humans and Animals. Princeton University Press. [Google Scholar]
- Kitching RP, 1986. Passive protection of sheep against capripoxvirus. Research in Veterinary Science, 41, 247–250. [PubMed] [Google Scholar]
- Kitching RP, 2003. Vaccines for lumpy skin disease, sheep pox and goat pox. Developments in Biologicals, 114, 161–167. [PubMed] [Google Scholar]
- Lamien CE, Lelenta M, Goger W, Silber R, Tuppurainen E, Matijevic M, Luckins AG and Diallo A, 2011. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. Journal of virological methods, 171, 134–40. [DOI] [PubMed] [Google Scholar]
- OIE (Office International des Epizooties), 2010. Sheep and goat pox. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 7th Edition. 1,2, 1404 pp. [Google Scholar]
- OIE (World Organisation for Animal Health), 2013. Technical disease card. Sheep pox and goat pox.
- OIE , 2018. Sheep pox and goat pox. 1513–1524.
- PAFF (Standing Committee on Plants, Animals, Food and Feed), 2017. Presentations 17‐18 January 2017: Sheep and Goat Pox ‐ Greece. https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en. Accessed on 22/03/2021.
- SCoFCAH (Standing Committee on the Food Chain and Animal Health), 2013a. Sheep and Goat Pox in Greece (Evros). SCoFCAH, Brussels 10‐11 Sep 2013. Available online: http://cerca.ministerosalute.it/search?q=sheep+pox&client=defaultPORT_front-end&proxystylesheet=defaultPORT_front-end&site=default_collection&output=xml_no_dtd&filter=p. Accessed on 25/03/2021
- SCoFCAH (Standing Committee on the Food Chain and Animal Health), 2013b. Sheep pox and goat pox outbreaks in Bulgaria. SCoFCAH 07‐08 Nov 2013. Available online: http://cerca.ministerosalute.it/search?q=sheep+pox&client=defaultPORT_front-end&proxystylesheet=defaultPORT_front-end&site=default_collection&output=xml_no_dtd&filter=p [Accessed: 25 March 2021]
- SCoFCAH (Standing Committee on the Food Chain and Animal Health), 2014. Sheep Pox situation in Bulgaria; SCoFCAH, 6‐7 Feb 2014.
- Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, Lyons NA, De Clercq K, Upton C, Bowden TR, Babiuk S and Babiuk LA, 2017. Review: capripoxvirus diseases: current status and opportunities for control. Transboundary and Emerging Diseases, 64, 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J, King J, Moritz T, Pohlmann A, Hoffmann D, Beer M and Hoffmann B, 2020. Experimental infection and genetic characterization of two different capripox virus isolates in small ruminants. Viruses, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogisharadhya R, Bhanuprakash V, Hosamani M, Venkatesan G, Balamurugan V, Bora DP, Bhanot V, Prabhu M and Singh RK, 2011. Comparative efficacy of live replicating sheeppox vaccine strains in ovines. Biologicals, 39, 417–423. [DOI] [PubMed] [Google Scholar]


