Abstract
Redox regulation in phytoplankton is critical to monitor and stabilize metabolic pathways under changing environmental conditions1. In plastids, the thioredoxin (TRX) system is linked to photosynthetic electron transport and fine tuning of metabolic pathways to fluctuating light levels. Expansion of the number of redox signal transmitters and their protein targets, as seen in plants, is believed to increase cell robustness2. In this study, we searched for genes related to redox regulation in the genome of the photosynthetic amoeba Paulinella micropora KR01 (hereafter, KR01). The genus Paulinella includes testate filose amoebae, in which a single clade acquired a photosynthetic organelle, the chromatophore, from an alpha cyanobacterial donor3. This independent primary endosymbiosis occurred relatively recently (~ 124 Ma), when compared to Archaeplastida (> 1 Ga), making photosynthetic Paulinella a valuable model for studying the earlier stages of primary endosymbiosis4. Our comparative analysis demonstrates that this lineage has evolved a thioredoxin system similar to other algae, relying however on genes with diverse phylogenetic origins (i.e., the endosymbiont, host, bacteria, red algae). One TRX of eukaryotic provenance is targeted to the chromatophore, implicating host-endosymbiont coordination of redox regulation. A chromatophore targeted glucose-6-phosphate dehydrogenase of red algal origin suggests that Paulinella exploited the existing redox regulation system in Archaeplastida to foster integration. Our study elucidates the independent evolution of the thioredoxin system in photosynthetic Paulinella, whose parts derive from the existing genetic toolkit in diverse organisms.
eTOC blurb
In plastids, the thioredoxin system is linked to photosynthetic electron transport and fine tuning the response to fluctuating light levels. Photosynthetic Paulinella, the only other known case of primary photosynthetic organelle origin, have a similar system using genes with diverse phylogenetic origins.
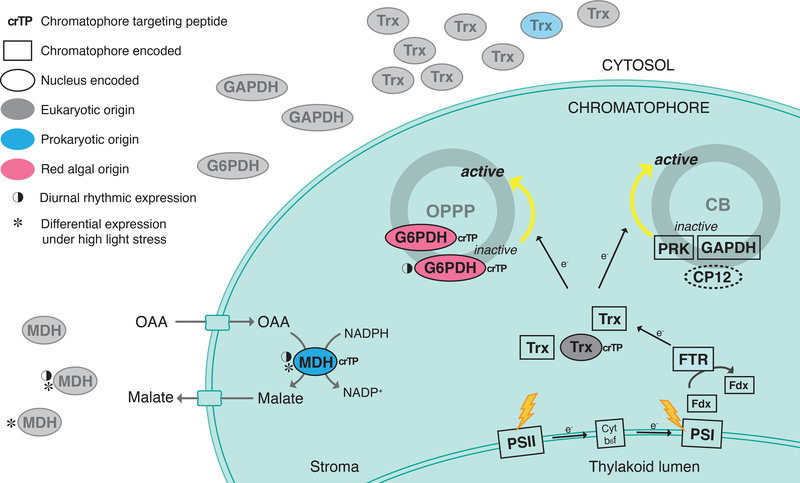
TRXs are thiol-disulfide oxidoreductases that participate in the regulation of metabolism and defense against oxidative stress and respond to environmental signals5. In the KR01 nuclear genome, there are 9 gene candidates, each of which contains a single TRX domain with a redox-active cysteine pair1. Apart from the TRXs encoded in the nucleus, the chromatophore genome encodes two such genes, whose products remain in the chromatophore, based on proteomic data from P. chromatophora6 (Fig. 1). Phylogenetic analysis of TRX genes shows that the nuclear encoded copies are of eukaryotic derivation except for g38191.t1, which groups with bacteria (Data S2A). One (g35100.t1) of the eukaryotic TRX proteins is likely to be chromatophore targeted because it contains a distinctive chromatophore transit peptide (crTP). Proteomic data from P. chromatophora demonstrates that the protein derived from the ortholog of this gene (i.e., g35100.t1) is present in the chromatophore of this species6. In Arabidopsis thaliana, there are ~20 TRXs, all of which are nuclear-encoded, and five types (f, m, x, y, and z) are imported into the plastid after translation. Among these five types, four have originated from cyanobacteria via endosymbiotic gene transfer (EGT)5. In red algae, one TRX encoding gene is present in the plastid genome that shares highest sequence similarity to type-m TRXs of A. thaliana7. In the case of photosynthetic Paulinella, when compared to A. thaliana, the amoeba appears to have jettisoned more of the endosymbiont derived TRX genes, perhaps indicating a lower level of integration between the nucleus and chromatophore. However, it is interesting to note that chromatophore origin precipitated the targeting of an eukaryote derived TRX to the novel organelle, likely reflecting selection pressure to expand the number of redox signal transmitters to respond to environmental cues2. Photosynthetic Paulinella relies on thioredoxin reductases (TRs) that are encoded in the chromatophore, whereas in green plants, TRs are nuclear encoded (Table S1).
Figure 1.
Putative thioredoxin system in the chromatophore of photosynthetic Paulinella species. When the chromatophore is exposed to light (yellow lightning bolt), induced reducing power is transferred from ferredoxin (Fdx) in the electron transport chain to specific target proteins via the ferredoxin-thioredoxin reductase (FTR)/thioredoxin (Trx) redox cascade. Thereafter, the reduced thioredoxin acts as a molecular on/off switch for redox regulated proteins by reducing the disulfide bond of enzymes involved in photosynthesis. The genes shown here are discussed in the main text. The localization of proteins to the chromatophore is supported by proteomic analysis of P. chromatophora, except for CP12, that did not have an identifiable ortholog in these data. Therefore, the location of this protein is unclear, and indicated with a dashed line.
Activity of a key enzyme in the oxidative pentose phosphate pathway (OPPP), plastid-targeted glucose-6-phosphate dehydrogenase (G6PDH), is regulated by light via TRX5. In the case of KR01, we found three G6PDHs encoded in the nucleus, all of which are of eukaryotic origin (Data S2B). However, two of these genes (g40784.t1, g9229.t1) are most closely related to red algal homologs, implying their origin via horizontal gene transfer (HGT). Interestingly, the two HGT-derived genes encode a crTP and contain the same redox-active cysteine pair as plastid encoded G6PDH. Moreover, one of these genes (g40784.t1) exhibits a diurnal expression pattern8. This result suggests that photosynthetic Paulinella acquired G6PDH genes that had already evolved redox-active cysteine residues during Archaeplastida evolution. Given sequence conservation over most of the protein sequence, it is less likely that convergent evolution targeted only the active sites or structural elements in these enzymes, after their provenance in Paulinella. It has been suggested that nuclear-encoded genes acquired from taxa that have previously undergone endosymbiosis may aid novel endosymbiont integration9. This is because the expression of these genes is already under host control and they contain import signals that can be more easily reused to target proteins to a new compartment10. The foreign genes encoding chromatophore-targeted G6PDH in Paulinella may have been acquired via phagotrophy involving red algal cells and likely played important roles in redox regulation prior to endosymbiosis.
In addition to G6PDH in plastids, six Calvin-Benson cycle enzymes are light-regulated through the ferredoxin-linked TRX system5. In the case of KR01, most of these enzymes are encoded in the chromatophore (organelle) genome, unlike other Archaeplastida (Table S1). Another well studied light-regulated enzyme is chloroplast NADP-dependent malate dehydrogenase1. We found four malate dehydrogenase genes (MDH) that are nuclear-encoded, of which, one (g8917.t1) is of bacterial origin and contains a crTP (Data S2C). Proteome data from P. chromatophora demonstrates that the protein derived from the ortholog of g8917.t1 in this species is targeted to the chromatophore. In A. thaliana and other members of the green lineage, plastid-targeted NADP-MDH is of prokaryote (likely chlamydial) origin. Irrespective of whether chromatophore targeted MDH is redox sensitive or not, this protein apparently plays an important role in primary endosymbiosis because the ancestor of the green lineage and Paulinella have acquired this gene from bacteria in two independent HGT events.
Acquisition of the capacity for oxygenic photosynthesis comes with the issue of reactive oxygen species (ROS) production by the electron transport chain (as in mitochondria). In the presence of excessive light energy, electrons may exit the thylakoid membrane and generate ROS. Therefore, it is necessary for algae and plants to sense and regulate such imbalances. The thiol-based redox regulation is a response to this stressor in plastids. When we searched for components of the TRX system that are well characterized in plastids, we found that photosynthetic Paulinella species have cobbled together a similar system using genes with diverse phylogenetic origins (i.e., endosymbiont, host, bacteria, red algae). Compared to Archaeplastida, the chromatophore genome still retains many genes related to redox regulation (e.g., TRX, GRX, TRs, see Table S1). Interestingly, chromatophore targeted G6PDH originated from red algae suggesting that Paulinella likely “stole” this function that had developed earlier in Archaeplastida. Independent evolution of the thioredoxin system in the chromatophore underlines the importance of this response to redox stress associated with plastid origin. Because our results are based on comparative methods, it is necessary to validate redox sensitivity of gene functions using experimental approaches. Mass spectrometry-based proteomics using cysteine selective isobaric and isotopic tags offers one avenue of investigating redox regulation in this fascinating amoeba lineage.
Supplementary Material
Acknowledgments
This study was supported by the Collaborative Genome Program of the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) (20180430), the National Research Foundation of Korea (NRF-2017R1A2B3001923, 2020R1C1C1008173), the Next-generation BioGreen21 Program (PJ01389003) from the RDA (Rural Development Administration), Korea, the United States National Aeronautics and Space Administration (80NSSC19K0462), and the NIFA-USDA Hatch program (NJ01170).
Footnotes
Declaration of Interests
The authors declare no competing interests.
Supplemental Information
Supplemental Information can be found with this article online at Current Biology website.
References
- 1.Balsera M, and Buchanan BB (2019). Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 140, 28–35. [DOI] [PubMed] [Google Scholar]
- 2.Woehle C, Dagan T, Landan G, Vardi A, and Rosenwasser S (2017). Expansion of the redox-sensitive proteome coincides with the plastid endosymbiosis. Nat. Plants 3, 17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon HS, Reyes-Prieto A, Melkonian M, and Bhattacharya D (2006). Minimal plastid genome evolution in the Paulinella endosymbiont. Curr. Biol. 16, R670–R672. [DOI] [PubMed] [Google Scholar]
- 4.Lhee D, Ha J-S, Kim S, Park MG, Bhattacharya D, and Yoon HS (2019). Evolutionary dynamics of the chromatophore genome in three photosynthetic Paulinella species. Sci. Rep. 9, 2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balsera M, Uberegui E, Schurmann P, and Buchanan BB (2014). Evolutionary development of redox regulation in chloroplasts. Antioxid. Redox Signal. 21, 1327–1355. [DOI] [PubMed] [Google Scholar]
- 6.Nowack ECM, Price DC, Bhattacharya D, Singer A, Melkonian M, and Grossman AR (2016). Gene transfers from diverse bacteria compensate for reductive genome evolution in the chromatophore of Paulinella chromatophora. Proc. Natl. Acad. Sci. U.S.A. 113, 12214–12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds AE, Chesnick JM, Woolford J, and Cattolico RA (1994). Chloroplast encoded thioredoxin genes in the red algae Porphyra yezoensis and Griffithsia pacifica: evolutionary implications. Plant Mol. Biol. 25, 13–21. [DOI] [PubMed] [Google Scholar]
- 8.Lhee D, Lee J, Ettahi K, Cho CH, Ha J-S, Chan Y-F, Zelzion U, Stephens TG, Price DC, Gabr A, et al. (2020). Amoeba genome reveals dominant host contribution to plastid endosymbiosis. Mol. Biol. Evol. In press ( 10.1093/molbev/msaa206). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponce-Toledo RI, López-García P, and Moreira D (2019). Horizontal and endosymbiotic gene transfer in early plastid evolution. New Phytol. 224, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo E, and Inagaki Y (2018). Patterns in evolutionary origins of heme, chlorophyll a and isopentenyl diphosphate biosynthetic pathways suggest non-photosynthetic periods prior to plastid replacements in dinoflagellates. PeerJ 6, e5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.