Abstract
Background and Purpose:
Cervical spinal cord injury (CSCI) can cause severe respiratory impairment. Although mechanical ventilation (MV) is a life-saving standard of care for these patients, it is associated with diaphragm atrophy and dysfunction. Diaphragm pacing (DP) is a strategy now used acutely to promote MV weaning and to combat the associated negative effects. Initial reports indicate that DP also may promote neuromuscular plasticity and lead to improvements in spontaneous diaphragm activation and respiratory function. These outcomes suggest the need for re-evaluation of respiratory rehabilitation for patients with CSCI using DP and consideration of new rehabilitation models for these patients and their unique care needs.
Summary of Key Points:
This article discusses the rationale for consideration of DP as a rehabilitative strategy, particularly when used in combination with established respiratory interventions. In addition, a model of Respiratory Rehabilitation and Recovery (RRR) is presented, providing a framework for rehabilitation and consideration of DP as an adjuvant rehabilitation approach. The model promotes goals such as respiratory recovery and independence, and life-long respiratory health, via interdisciplinary care, respiratory training, quantitative measurement, and use of adjuvant strategies such as DP. Application of the model is demonstrated through a description of an inpatient rehabilitation program that applies model components to CSCI patients who require DP.
Recommendations for Clinical Practice:
As DP use increases for patients with acute CSCI, so does the need and opportunity to advance rehabilitation approaches for these patients. This perspective paper is a critical step in addressing this need and motivating the advancement of rehabilitation strategies for CSCI patients. (See Video Abstract, Supplemental Digital Content)
INTRODUCTION
Respiratory dysfunction is a leading cause of illness and death following SCI.1–4 Individuals with cervical spinal cord injury (CSCI) have the greatest risk of developing respiratory dysfunction and associated complications such as pneumonia, atelectasis, and respiratory failure.3,5 Nearly 40% of SCI cases involve the upper cervical segments (C1-C5)1 resulting in neurological impairment of the phrenic system and weakened diaphragm activation.6–9 Therefore consideration of strategies for respiratory rehabilitation that can be implemented into rehabilitation care settings is necessary. This perspective paper discusses use of intramuscular diaphragm stimulation, or diaphragm pacing (DP), for individuals with CSCI. We also introduce a conceptual model focused on respiratory rehabilitation and recovery that incorporates strategies for DP use
The current standard of care for managing acute respiratory dysfunction following CSCI is mechanical ventilation (MV).1,10 While this is a life-saving approach, it can be associated with severe negative consequences, such as rapid and profound diaphragm atrophy11–14 increased infection rates,15 and increased reliance on long-term MV.16,17 The impact of MV on the diaphragm has been termed ventilator-induced diaphragm dysfunction (VIDD), and this results at least in part from oxidative stress in the diaphragm.18 Importantly, VIDD appears to be greater after CSCI. That is, conditions created by SCI (e.g., disuse, inflammation, etc.) exacerbate the impact of MV.13 Collectively, these factors associated with MV can prolong hospitalization, degrade health, and lead to higher healthcare utilization and costs.19
Intramuscular diaphragm stimulation, or diaphragm pacing (DP), is a strategy to promote improved respiratory function and MV weaning - thereby reducing related complications.20,21 Briefly, this approach involves laparoscopic placement of intramuscular electrodes in each hemidiaphragm, near the insertion of the phrenic nerve.22,23 The electrode wires are externalized and connected to a pulse generator to deliver stimulation at an intensity and rate individualized for each patient. Historically DP has been used in chronic SCI cases to enable liberation from MV.24 However, there is now increasing use of DP acutely after CSCI to facilitate weaning from MV, and promote earlier transitions from acute hospitalization to subacute rehabilitation care settings.20,25 Recent reports indicate that some trauma centers now routinely evaluate all patients admitted with acute traumatic CSCI for DP implantation.20 Increased use and earlier placement of DP systems have led to emerging evidence that DP promotes diaphragm muscle health as well as improved recovery of independent breathing function.20,24,25 The potential benefits of DP are perhaps not surprising since prevention of disuse atrophy in skeletal myofibers via electrical stimulation is well established.26–28
As the understanding and use of DP is growing, consideration should be given to advancement of rehabilitation models specific to the unique circumstances and needs of the relevant patient population. Rehabilitation models provide a framework for goal establishment, development of care guidelines, and research priorities.29 While many rehabilitation models exist for SCI care and weaning from MV,30–32 the use of DP has not yet been considered as a component of SCI care and rehabilitation.
The goals of this perspective paper are to 1) discuss advances in DP use, potential effects of diaphragm stimulation, and consideration of DP as adjunctive rehabilitation strategy; 2) introduce a conceptual model for respiratory rehabilitation and recovery focused on individuals with CSCI who may require DP due to severe respiratory impairment, and; 3) describe application of the model in an inpatient SCI rehabilitation program.
Advancements in Diaphragm Pacing post-SCI
In recent years, DP has begun to be implemented acutely after CSCI to promote MV weaning and minimize MV related complications. Another benefit of early use of MV is to enable earlier care transitions, such as from intensive care settings to less acute settings, and from acute to subacute care settings where intense rehabilitation can be a focus.20,25,33 Acute DP use is associated with gains in respiratory function, recovery of voluntary diaphragm activation (recorded via indwelling wires), and recovery of independent breathing without reliance on DP.20,25,33 These indications that DP can be beneficial are not unique to cases of SCI. For example, after DP, increased respiratory function, reduced ventilator reliance, and increased voluntary diaphragm activation was described in patients with Pompe disease, a rare but severe neuromuscular disorder where respiratory failure often necessitates ventilator dependence.34 The reported gains in function and patient outcomes associated with DP use suggest that diaphragm stimulation may be a beneficial rehabilitation strategy and have benefits beyond maintenance of respiratory rhythm and use as a replacement for MV. 20,25,33,34
Effects of Stimulation on Diaphragm Function and Respiratory Pathways
The use of DP has potential as a rehabilitation tool by directly impacting diaphragm myofibers, as well as inducing neuroplasticity via activation of sensory afferents. Since VIDD is associated with diaphragm atrophy,12,13 it follows that preventing diaphragm inactivity through the use of DP may be beneficial. In support of this concept, studies conducted during acute surgical procedures confirm that direct electrical stimulation of the diaphragm improves contractile force and mitochondrial function.35,36 Thus, at least part of the physiological benefit of DP is likely to be improved diaphragm muscle health.
In regards to a possible neural impact of DP and subsequent neuroplastic changes, there are several possibilities. First, the vigorous diaphragm contractions induced during DP are certain to activate diaphragm sensory afferents. Indeed, the phrenic nerve contains a large number of sensory afferents including large diameter myelinated (group Ia, Ib, II), small diameter myelinated (group III) and unmyelinated axons (group IV). Smaller diameter fibers are numerous and discharge across the respiratory cycle (for review, see Nair37). Activation of group III-IV phrenic afferents results in increased diaphragm activity, and group I and II afferents can excite or inhibit diaphragm output. Therefore, DP, through repeated activation of sensory afferents, has the potential to evoke neuroplastic changes in the neurons and networks that control the diaphragm. A second consideration is the trophic relationship between motoneurons and the muscle fibers they innervate (reviewed in Mantilla38). Because of this relationship, phrenic motoneuron activation during DP (e.g., antidromically or via sensory afferent feedback) may impact the overall health of diaphragm motor units.
Further work is needed regarding the physiology of DP effects after SCI, but the reported successes in weaning from DP to independent breathing20,25,33 suggest that some of the aforementioned mechanisms may be activated during DP and highlight its potential as a rehabilitation strategy.
Stimulation as an Adjunctive Approach
If diaphragm stimulation has beneficial effects similar to other forms of functional electrical stimulation following SCI,36,39–41 then it may be particularly beneficial when used in combination with respiratory rehabilitation as an adjunctive, or supportive, therapy. The rationale for this is accumulating evidence that use of electrical stimulation in combination with task-specific physical rehabilitation increases task-related motor output and enhances functional gains in persons with SCI.40,42–45 Specific to functional electrical stimulation via surface or implanted electrodes, improvements in grasp46 and walking following SCI43,47,48 have been demonstrated when stimulation is combined with task-specific training. This accumulating evidence suggests that use of stimulation in combination with physical rehabilitation may be particularly beneficial in this population. Therefore, diaphragm stimulation could be considered an adjunctive rehabilitation tool that can be used in combination with other strategies such as respiratory strength training to promote respiratory health and recovery.
Why is a model that considers the use of DP necessary?
Use of DP early for individuals with CSCI, as well as facilitation of more rapid transitions from acute care to rehabilitation, suggests that these complex patients will begin inpatient rehabilitation sooner after injury and will also require support for DP use.20 Therefore, comprehensive rehabilitation programs should not only consider the unique needs of these patients but also prioritize respiratory rehabilitation and recovery as primary rehabilitation goals. Development of a model that conveys concepts, goals, and relationships may help facilitate adoption of these emerging ideas and development of new rehabilitation strategies. Specifically, a model that incorporates DP and is focused on respiratory health after CSCI should be considered. Although models are widely used in rehabilitation, few focus on respiratory rehabilitation post-SCI. Instead, most respiratory models focus on protocols to decrease reliance on acute respiratory interventions such as MV or tracheostomy, rather than rehabilitation based approaches.10,30,31,49 Moreover, there is a lack of guidance on rehabilitation of patients with CSCI who require DP. In light of these gaps, a model that considers respiratory rehabilitation of patients with CSCI using devices such as DP is needed.
To address this gap we have developed a model of Respiratory Rehabilitation and Recovery (RRR) to provide a framework for rehabilitation of individuals with CSCI that considers DP or other adjunctive approaches. The goals and components of the model are described below, followed by a brief description of an inpatient rehabilitation program that applies this model.
MODEL OF RESPIRATORY REHABILITATION AND RECOVERY
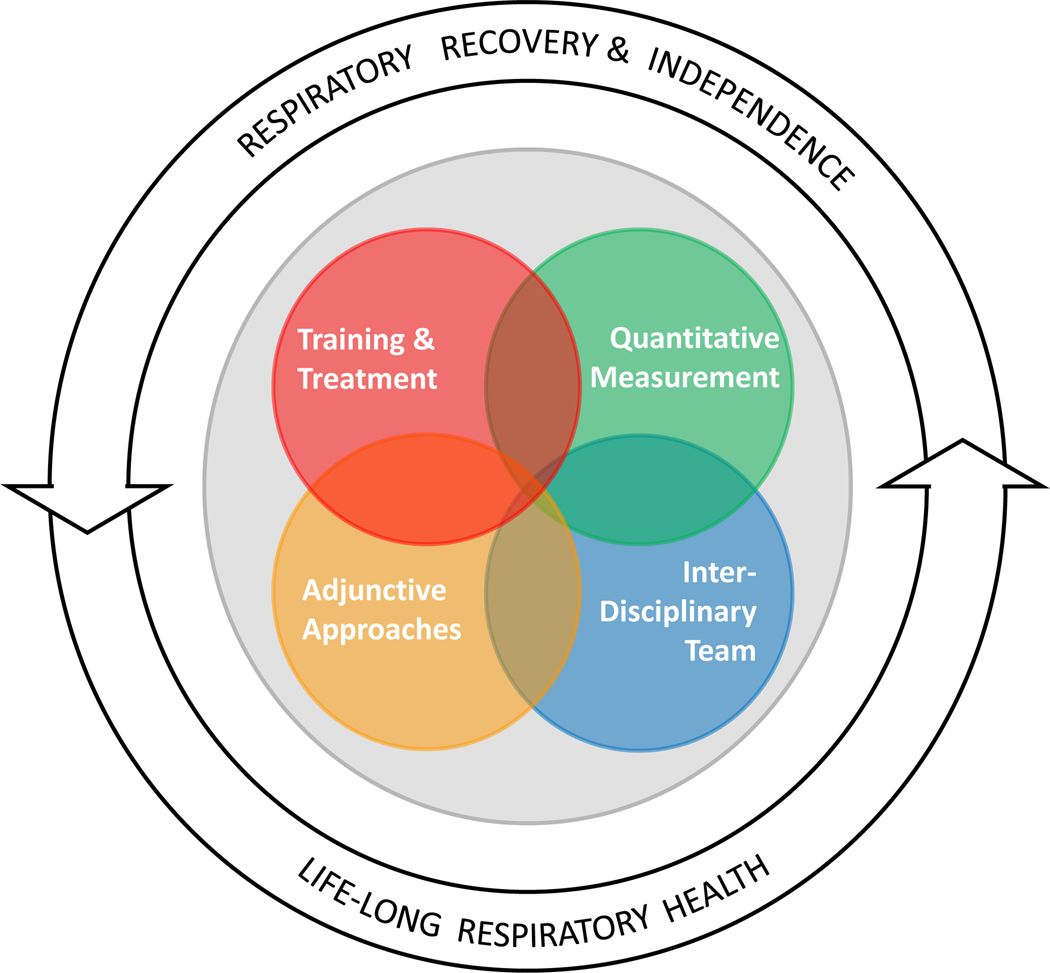
Our proposed model for RRR (Figure. 1) focuses on two overarching and related goals—a) Recovery of respiratory function and breathing independence and b) promotion of lifelong respiratory health. The two goals provide the outer frame of the model and are connected by bidirectional arrows to depict their interdependence. The model components that contribute to the attainment of these goals are represented by overlapping circles at the model core. These components are a) an interdisciplinary team; b) quantitative assessment; c) training and treatment; and d) adjunctive approaches (i.e., diaphragm pacing). The model depicts these components as overlapping since they should work synergistically such that, for instance, interdisciplinary team management enhances outcomes enabling engagement of the patient in intensive rehabilitation interventions, guided by quantitative measurement and further enhanced by adjunctive rehabilitative approaches such as DP. This is in line with the American Thoracic Society/European Respiratory Society which defines pulmonary rehabilitation, in part, as a comprehensive intervention inclusive of exercise training, delivered by an interdisciplinary team to improve function, quality of life, and long term adherence to health enhancing behaviors.50
Figure 1.
The Model for Respiratory Rehabilitation and Recovery for Individuals with Cervical spinal cord injury. Model components are synergistic, depicted by overlapping circles (Training & Treatment, Quantitative Measurement, Interdisciplinary Team, Adjunctive Approaches). These four core components serve two interdependent goals, represented by outer rings with bidirectional arrows.
Respiratory Rehabilitation and Recovery Model Goals
The model goals are aligned with SCI rehabilitation models for other body systems or functions.42,51 The rationale for these goals is based on the critical need to address the high prevalence of infection, decreased respiratory function, high rehospitalization rates, and high healthcare costs after CSCI 52. In addition to these issues, individuals with severe respiratory dysfunction often require home device use (i.e. MV, DP, positive airway pressure machines) and caregiver burden is also particularly high while quality of life after CSCI is low.53,54 Thus the model goals are broadly meant to address these issues and encourage focus on respiratory rehabilitation, as well as interventions and resources to address lifelong respiratory health.
Interdisciplinary Team
An interdisciplinary approach involves delivery of patient-centric care through collaborative treatment planning in pursuit of common goals.55 Programs that utilize interdisciplinary teams generate better results compared to those that lack them.56,57 Therefore, an interdisciplinary team approach is valuable for management of the health and medical needs of individuals with CSCI who may use DP. These individuals’ care needs are high due to factors such as previous prolonged MV use,1,10 tracheostomy,10 care of externalized pacing wires,58 management of the pacing device,58 and high rates of anxiety, fear, and depression.59,60 Coordination of the interdisciplinary team requires a high degree of reciprocal communication,61 which helps ensure all members remain informed. Table 1 outlines members of an interdisciplinary team and their roles regarding patients with CSCI who use DP. Team member involvement will depend on patient care requirements, characteristics of the care setting, and available resources.62
Table 1.
| Discipline | Role |
|---|---|
| Respiratory specialist (Pulmonologist, RT) | Perform and assess respiratory outcomes (i.e., Pulmonary function testing) Labs (ABGs, ETCO2, etc.) |
| Therapy team (PT/OT/SLP/RT) | Respiratory Strength Training Assisted cough, draining/positioning, chest therapy Respiratory-focused education |
| Physician/Physiatrist | DP stimulation parameter adjustment and weaning (as appropriate) Medical management/oversight Initiate order for DP weaning (as appropriate) |
| Nurse | 24 hour patient monitoring DP wire site wound management and battery changes Secretion management/suctioning |
| Psychologist | Coping strategies Adjustment to illness/disability Patient and family support |
| Social Worker/Case Manager | Identifying discharge needs with emphasis on SCI and respiratory health needs Discharge planning Psychosocial support |
| Dietician | Oversee nutritional status and dietary needs Ensure sufficient caloric intake to address respiratory demands |
Abbreviations: RT, respiratory therapist; ABGs, arterial blood gases; ETCO2, end-tidal CO2; PT, physical therapist; OT, occupational therapist; SLP, speech language pathologist; RT, respiratory therapist; DP, diaphragm pacer; SCI, spinal cord injury. Disciplines and roles should be adapted as needed based on site-specific interdisciplinary team make-up and individual member knowledge/skill.
Training and Treatment
An effective, well-established rehabilitation treatment for respiratory impairment after SCI is respiratory strength training (RST).63–66 RST increases inspiratory and expiratory pressure-generating capacity, vital capacity, and maximal voluntary ventilation in adults with SCI.66–68 These gains in respiratory function help protect against potentially life-threatening complications like pneumonia.69 Examples of RST include resistive training or threshold training for both inspiration and expiration (IMST/EMST)67 and can successfully be implemented in rehabilitation settings.64 RST can be performed similar to limb muscle training with considerations of exercise frequency, duration, and intensity (Table 2).66 Training must be sufficiently intense, such as 15 out of 20 or ‘hard’ on a self-rating of perceived exertion and parameters should be progressed to maintain the intensity.70 RST should be started as early as possible after SCI and ongoing training should be a part of lifelong respiratory health management.70 This is critical since respiratory function has potential to decline when these programs are not continued. In fact, experts suggest that “respiratory muscle training may be a central aspect of maintaining health and quality of life in this group of individuals with SCI”. 68
Table 2.
Exercise Prescription Considerations for Respiratory Strength Training 67
| Definition | |
|---|---|
| Training Type | Method of strengthening via increasing load on respiratory muscles or minute ventilation |
| Resistive Training | Involves breathing through a small diameter hole (resistor), which limits available flow and increases ventilatory (training) load |
| Threshold Training | Involves breathing with sufficient force to overcome a spring-loaded valve to enable airflow |
| Normocapnic hyperpnea | Involves simultaneous training of inspiratory and expiratory muscles via re-breathing bag (at 30% to 40% of the participant’s forced ventilatory capacity) connected to a tube system and mouthpiece |
| Intensity | Set at a percentage of maximal effort (via respiratory strength, pressure or ventilatory capacity) |
| Time/Duration | Number of repetitions performed or amount of time spent performing training in a single session |
| Frequency | Number of weekly training sessions or intersession time interval (i.e., 7 days per week or daily) |
DP as an Adjunctive Approach
While RST is an established rehabilitation approach for individuals with SCI,66 there are no established adjunctive interventions to enhance or maximize the benefits of RST. Combinatorial therapeutic approaches to amplify the effects of single interventions may synergistically augment spinal plasticity, inducing greater functional improvement. Since combinatorial approaches that pair adjunctive therapies with task-specific training are beneficial in other aspects of SCI rehabilitation,42–45,71 it is reasoned that they should be considered for use in respiratory rehabilitation. Diaphragm pacing may provide a means to deliver this type of combinatorial approach to individuals requiring its use after CSCI. DP works by rhythmically stimulating the diaphragm according to a pre-established set of stimulation parameters. Current stimulation approaches are open-loop and do not adjust to user needs (i.e. increased ventilation, blood gas changes). Therefore, a closed-loop delivery system could be particularly effective in meeting patient needs and is currently under development.72 One aspect that has yet to be evaluated is stimulation parameters to maximize individualized responses. While these are important steps in the consideration of how DP can be harnessed as a rehabilitation tool, further research is necessary to evaluate DP effects, especially when used acutely after injury. Despite the need for further research, it is not uncommon for rehabilitation strategies to be used, often to the benefit of patients, before definitive evidence is available. The incorporation of DP into respiratory rehabilitation paradigms may help emphasize the importance of understanding the effects of diaphragm stimulation and stimulate research and advancements in clinical practice.
Quantitative Measurement
Quantitative measures of respiratory function are essential to assess pulmonary function, monitor patient progress, and set patient-specific goals. Measures of pressure generation such as maximal inspiratory and expiratory pressures generation are indirect measures of respiratory muscle strength.73 Improvements in these measures are associated with improved cough capacity and secretion clearance.74 Additionally, thresholds for maximal inspiratory pressure generation have been established to identify individuals who are at risk of developing pneumonia after SCI.69 Measures of spirometry, such as forced vital capacity, forced expiratory volume in 1 second, and peak expiratory flow provide metrics of respiratory function75 and are moderately accurate predictors of respiratory infection in the first year after discharge from inpatient rehabilitation in SCI.76 These measures are reliable in the SCI population,77 easy to perform, are relatively low-cost measures, and are necessary to perform regularly to adapt RST goals (Table 3).68,78 Furthermore, quantitative measurement of respiratory function should be used to guide education needs, home exercise, monitoring frequency, and resource needs after discharge from inpatient rehabilitation. 68,78
Table 3.
| Type of assessment | Description | Equipment |
|---|---|---|
| MIP (also PImax) | Maximal inspired pressure generated at the mouth; reflects inspiratory muscle strength | Pressure manometer with flanged mouthpiece, filter, nose clip |
| SNIP | Short, sharp voluntary inspiratory maneuver performed through one or both unconcluded nostrils; reflects inspiratory muscle strength | Pressure manometer, nasal catheter |
| MEP (also PEmax) | Maximal expired pressure generated at the mouth; reflects expiratory muscle strength | Pressure manometer with flanged mouthpiece, filter, nose clip |
| FVC | Maximal volume of air exhaled with maximal forced effort from a maximal inspiration | Spirometer, filter, nose clip |
| FEV1 | Maximal volume of air exhaled in the first second of a forced expiration from a position of full inspiration | Spirometer, filter, nose clip |
| PEF | Highest flow achieved from a maximum forced expiratory maneuver started without hesitation from a position of maximal lung inflation | Peak flow meter or spirometer, filter, nose clip |
Abbreviations: MIP or PImax, maximal inspiratory pressure; SNIP, sniff nasal inspiratory pressure; MEP or PEmax, maximal expiratory pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in 1-second, PEF, peak expiratory flow
Application of the RRR Model and Program Development
The goal of the following section is to describe application of the RRR Model in an inpatient rehabilitation program that serves CSCI patients who require DP. The program was initiated in 2013 due to an increase in admissions of patients who had received DP during acute hospitalization. The initial program goal was to address unique rehabilitation needs and facilitate safe and healthy transitions from inpatient rehabilitation to home or another setting. Since program inception, 34 patients have participated, and the program has evolved and now includes a formalized program for weaning from DP (for those who meet criteria). All patients involved in this program had a CSCI and received DP during acute hospitalization. Nearly all individuals had injuries at or above C5 and had injuries classified by International Standards for the Neurologic Classification of Spinal Cord Injury79 as A, B, or C. Approximately half of the individuals had injuries classified as incomplete vs. complete. The demographic characteristics of the group were heterogeneous and varied based on cause of injury (i.e., violence or vehicular accident, fall), secondary health conditions, length of acute hospitalization, and prior health status. Most individuals received the implanted diaphragm pacer within 2 weeks after injury and were admitted to inpatient rehabilitation within ~3 to 8 weeks after injury.
Of 34 patients, 31 recovered independent respiration without reliance on the pacing device. Anecdotally, individuals with high cervical complete injuries, as well as those with comorbidities or advanced age had greater difficulty weaning from DP, had more setbacks, or continued to require device use to maintain adequate ventilation.
While overall outcomes are encouraging, individuals continue to have respiratory impairment and remain at high risk for illness and infection, necessitating ongoing rehabilitation, support, and resources. Program details aligned with the RRR Model components are summarized below.
Immediately following patient admission into the program, the interdisciplinary care team is established. Interdisciplinary team members address typical SCI care needs but also are focused on unique requirements such as wound care at the site of the externalized DP wires, DP device management, tracheostomy care and secretion management, support for depression and anxiety, peer mentorship, dietary requirements, caregiver training, discharge preparation, equipment needs, home modifications, and rehabilitation care transitions. To ensure clear and consistent communication amongst the team members a variety of communication methods were developed, many by trial and error, to ensure timeliness and efficiency. Methods include clinical tracking of patient respiratory status and function, daily 10 min ‘huddles’, weekly team conferences that may include the patient and/or caregiver, as well as modifications to the electronic documentation system to include respiratory specific information.
Quantitative measurements of respiratory function and RST are performed by trained members of the multidisciplinary team. Upon program inception, physical therapists and speech-language pathologists shared this responsibility. The program was expanded to include greater involvement of respiratory therapy, and currently, a respiratory therapist with experience in CSCI oversees quantitative respiratory assessments (i.e. forced vital capacity, maximal pressure generation). The respiratory therapist also is responsible for conducting daily RST. Additionally, measures of perceived exertion, dyspnea, oxygen saturation, and respiratory rate are monitored regularly during self-care, treatment sessions, and throughout the day by members of the care team. Tracking of daily RST session parameters, quantitative respiratory assessments, and other aspects of respiratory functioning help ensure progression of RST, monitoring of respiratory and health status, and determination of DP needs.
A key development of our program was the formalization of a weaning process to reduce reliance on DP and in many cases, promote liberation from the device. This program aspect initially was handled on a patient by patient basis without a consistent process. Over time and with experience, the program evolved to include clearer, more consistent guidelines for DP weaning initiation, weaning progression and a formal process for patient monitoring, suspending weaning, as well as enhanced communication during the weaning process. Decisions regarding DP weaning or diminishing DP use are based on patient progression, medical status, quantitative respiratory assessments indicating progressive gains in respiratory function, and demonstration of independent respiration, such as when the pacer is off (i.e. battery changes) or a demonstrated volitional ability to alter respiratory volumes and rate.
While the program is now formalized and based on the RRR Model, further development of DP use and respiratory rehabilitation is needed. Better understanding and development of approaches to adjust DP stimulation parameters is an immediate consideration since the device is in use and appears to have beneficial effects. Advancing combinatorial approaches for respiratory rehabilitation also is necessary. Additionally, better understanding of long-term outcomes following participation in respiratory rehabilitation should be evaluated, especially for individuals who were able to successfully wean from DP during rehabilitation. While DP liberation is a desired outcome, the overall goal is improved respiratory function and recovery to promote lifelong health. To promote ongoing recovery and health, our program places high emphasis on patient and caregiver education and referral to community-based resources that specialize in programs for individuals with CSCI. Long term follow-up is critical to ensure patient and caregiver needs such as early detection of problems, referral to appropriate health resources, addressing equipment needs, and ensuring adequate access to health and community resources are met.80 Our program currently has several community programs for wellness and adaptive sports, as well as peer mentoring. Our focus is respiratory health awareness and development of respiratory rehabilitation approaches that can be integrated into existing programs.
Conclusion
In summary, DP use for patients with acute CSCI is growing and therefore there is a need and opportunity to advance rehabilitation for these patients. Diaphragm stimulation, particularly early after injury, may promote diaphragm muscle health, enhance respiratory function, and even induce a rehabilitative or neuroplastic effect as the ‘task’ or rhythm of breathing is stimulated and facilitated (i.e. task-specific training and induction of activity-dependent plasticity). The use of combinatorial approaches is increasingly supported, especially when paired with rehabilitation. With the use of DP earlier after injury, patients are transitioning to rehabilitation care settings sooner while using this device. Although further understanding of how DP contributes to respiratory recovery, and continued development of respiratory rehabilitation strategies are still needed, there are indications that DP may be a valuable rehabilitation tool. Thus, we are at a juncture—the device can be ignored from a rehabilitation perspective or we can conceptualize how to maximize its use to promote rehabilitation outcomes and integrate ideas and evidence to enhance respiratory function after CSCI. We encourage that the RRR Model provides a foundation for advancing respiratory rehabilitation, recovery, and life-long respiratory health.
Supplementary Material
Acknowledgements
The authors would like to thank Kendra Milliron, Alison D’Alessandro, Alicia K. Vose, and Paul Freeborn for assistance in development and implementation of the Respiratory Rehabilitation and Recovery Model. In addition, we would like to thank all the clinicians and patients who have contributed or participated in our program.
Sources of funding:
• Emily J. Fox, DPT, PhD: Craig H. Neilsen Foundation, SCIRT Award; NIH Common Fund, SPARC Program, Award number OT2OD0023854; NIH NICHD K12 HD055929
• David D. Fuller: NIH 1R01HL139708-01A
Footnotes
Supplemental Digital Content. Video abstract. Mp4
Conflicts of Interest: None declared
References
- 1.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51(8):853–868;discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- 2.Fishburn MJ, Marino RJ, Ditunno JF. Atelectasis and pneumonia in acute spinal cord injury. Arch Phys Med Rehabil. 1990;71(3):197–200. [PubMed] [Google Scholar]
- 3.Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6(1):24–33. doi: 10.5312/wjo.v6.i1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82(10):803–814. doi: 10.1097/01.PHM.0000078184.08835.01 [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Apple DF, Hudson LM, Bode R. Medical complications during acute rehabilitation following spinal cord injury--current experience of the Model Systems. Arch Phys Med Rehabil. 1999;80(11):1397–1401. doi: 10.1016/s0003-9993(99)90250-2 [DOI] [PubMed] [Google Scholar]
- 6.Fuller DD, Golder FJ, Olson EB, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100(3):800–806. doi: 10.1152/japplphysiol.00960.2005 [DOI] [PubMed] [Google Scholar]
- 7.Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol. 2011;179(1):3–13. doi: 10.1016/j.resp.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 8.Ovechkin AV, Vitaz TW, Terson de Paleville DGL, McKay WB. Quality of residual neuromuscular control and functional deficits in patients with spinal cord injury. Front Neurol. 2013;4:174. doi: 10.3389/fneur.2013.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30(4):319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berney S, Bragge P, Granger C, Opdam H, Denehy L. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: a systematic review. Spinal Cord. 2011;49(1):17–29. doi: 10.1038/sc.2010.39 [DOI] [PubMed] [Google Scholar]
- 11.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]
- 12.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2013;305(5):R464–R477. doi: 10.1152/ajpregu.00231.2013 [DOI] [PubMed] [Google Scholar]
- 13.Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, et al. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. Journal of applied physiology. 2016;120(2):166–177. doi: 10.1152/japplphysiol.00488.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998;158(2):378–385. doi: 10.1164/ajrccm.158.2.9710084 [DOI] [PubMed] [Google Scholar]
- 15.Winslow C, Bode RK, Felton D, Chen D, Meyer PR. Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest. 2002;121(5):1548–1554. doi: 10.1378/chest.121.5.1548 [DOI] [PubMed] [Google Scholar]
- 16.Purro A, Appendini L, De Gaetano A, Gudjonsdottir M, Donner CF, Rossi A. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1115–1123. doi: 10.1164/ajrccm.161.4.9812160 [DOI] [PubMed] [Google Scholar]
- 17.Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–371. doi: 10.1164/rccm.201004-0670OC [DOI] [PubMed] [Google Scholar]
- 18.Smuder AJ, Sollanek KJ, Nelson WB, et al. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic Biol Med. 2018;115:179–190. doi: 10.1016/j.freeradbiomed.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loss SH, de Oliveira RP, Maccari JG, et al. The reality of patients requiring prolonged mechanical ventilation: a multicenter study. Rev Bras Ter Intensiva. 2015;27(1):26–35. doi: 10.5935/0103-507X.20150006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerwin AJ, Zuniga YD, Yorkgitis BK, et al. Diaphragm pacing improves respiratory mechanics in acute cervical spinal cord injury. J Trauma Acute Care Surg. 2020;89(3):423–428. doi: 10.1097/TA.0000000000002809 [DOI] [PubMed] [Google Scholar]
- 21.Onders RP, Khansarinia S, Weiser T, et al. Multicenter analysis of diaphragm pacing in tetraplegics with cardiac pacemakers: positive implications for ventilator weaning in intensive care units. Surgery. 2010;148(4):893–897; discussion 897–898. doi: 10.1016/j.surg.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 22.Onders RP, Elmo M, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23(7):1433–1440. doi: 10.1007/s00464-008-0223-3 [DOI] [PubMed] [Google Scholar]
- 23.Tedde ML, Vasconcelos Filho P, Hajjar LA, et al. Diaphragmatic pacing stimulation in spinal cord injury: anesthetic and perioperative management. Clinics. 2012;67(11):1265–1269. doi: 10.6061/clinics/2012(11)07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onders RP, Elmo M, Kaplan C, Schilz R, Katirji B, Tinkoff G. Long-term experience with diaphragm pacing for traumatic spinal cord injury: Early implantation should be considered. Surgery. 2018;164(4):705–711. doi: 10.1016/j.surg.2018.06.050 [DOI] [PubMed] [Google Scholar]
- 25.Posluszny JA, Onders R, Kerwin AJ, et al. Multicenter review of diaphragm pacing in spinal cord injury: successful not only in weaning from ventilators but also in bridging to independent respiration. J Trauma Acute Care Surg. 2014;76(2):303–309; discussion 309–310. doi: 10.1097/TA.0000000000000112 [DOI] [PubMed] [Google Scholar]
- 26.Crameri RM, Weston AR, Rutkowski S, Middleton JW, Davis GM, Sutton JR. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: a pilot study. Eur J Appl Physiol. 2000;83(4–5):409–415. doi: 10.1007/s004210000263 [DOI] [PubMed] [Google Scholar]
- 27.Dirks ML, Wall BT, Snijders T, Ottenbros CLP, Verdijk LB, van Loon LJC. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf). 2014;210(3):628–641. doi: 10.1111/apha.12200 [DOI] [PubMed] [Google Scholar]
- 28.Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJC. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci (Lond). 2015;128(6):357–365. doi: 10.1042/CS20140447 [DOI] [PubMed] [Google Scholar]
- 29.Cooper BA, Saarinen-Rahikka H. Interrelationships of theory, clinical models and research. Physiother Can. 1986;38(2):97–100. [PubMed] [Google Scholar]
- 30.Gundogdu I, Ozturk EA, Umay E, Karaahmet OZ, Unlu E, Cakci A. Implementation of a respiratory rehabilitation protocol: weaning from the ventilator and tracheostomy in difficult-to-wean patients with spinal cord injury. Disability and Rehabilitation. 2017;39(12):1162–1170. doi: 10.1080/09638288.2016.1189607 [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez CJ, Harrow J, Haines F. Using an evidence-based protocol to guide rehabilitation and weaning of ventilator-dependent cervical spinal cord injury patients. J Rehabil Res Dev. 2003;40(5 Suppl 2):99–110. doi: 10.1682/jrrd.2003.10.0099 [DOI] [PubMed] [Google Scholar]
- 32.Richard-Denis A, Feldman D, Thompson C, Albert M, Mac-Thiong J-M. The impact of a specialized spinal cord injury center as compared with non-specialized centers on the acute respiratory management of patients with complete tetraplegia: an observational study. Spinal Cord. 2018;56(2):142–150. doi: 10.1038/s41393-017-0003-9 [DOI] [PubMed] [Google Scholar]
- 33.Fox EJ, Cavka K, Freeborn P, et al. Diaphragm Stimulation Enhances Respiratory Function After Cervical Spinal Cord Injury. The FASEB Journal. 2019;33(1_supplement):843.5–843.5. doi: 10.1096/fasebj.2019.33.1_supplement.843.5 [DOI] [Google Scholar]
- 34.Smith BK, Fuller DD, Martin AD, et al. Diaphragm Pacing as a Rehabilitative Tool for Patients With Pompe Disease Who Are Ventilator-Dependent: Case Series. Phys Ther. 2016;96(5):696–703. doi: 10.2522/ptj.20150122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn B, Beaver T, Martin T, et al. Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery. Am J Respir Crit Care Med. 2014;190(7):837–839. doi: 10.1164/rccm.201405-0993LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin AD, Joseph AM, Beaver TM, et al. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med. 2014;42(2):e152–e156. doi: 10.1097/CCM.0b013e3182a63fdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair J, Streeter KA, Turner SMF, et al. Anatomy and physiology of phrenic afferent neurons. Journal of Neurophysiology. 2017;118(6):2975–2990. doi: 10.1152/jn.00484.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985). 2008;104(6):1818–1827. doi: 10.1152/japplphysiol.01192.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffell LD, Donaldson N de N. A Comparison of FES and SCS for Neuroplastic Recovery After SCI: Historical Perspectives and Future Directions. Frontiers in Neurology. 2020;11:607. doi: 10.3389/fneur.2020.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadowsky CL, Hammond ER, Strohl AB, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med. 2013;36(6):623–631. doi: 10.1179/2045772313Y.0000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ragnarsson KT. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord. 2008;46(4):255–274. doi: 10.1038/sj.sc.3102091 [DOI] [PubMed] [Google Scholar]
- 42.Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137(Pt 3):654–667. doi: 10.1093/brain/awt262 [DOI] [PubMed] [Google Scholar]
- 43.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil. 2001;82(6):818–824. doi: 10.1053/apmr.2001.23752 [DOI] [PubMed] [Google Scholar]
- 44.Harkema S, Gerasimenko Y, Hodes J, et al. Effect of Epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–1947. doi: 10.1016/S0140-6736(11)60547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma. 2006;23(3–4):560–570. doi: 10.1089/neu.2006.23.560 [DOI] [PubMed] [Google Scholar]
- 46.Kapadia NM, Zivanovic V, Furlan JC, Craven BC, McGillivray C, Popovic MR. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: randomized control trial. Artif Organs. 2011;35(3):212–216. doi: 10.1111/j.1525-1594.2011.01216.x [DOI] [PubMed] [Google Scholar]
- 47.Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91(1):48–60. doi: 10.2522/ptj.20090359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Possover M, Forman A. Recovery of supraspinal control of leg movement in a chronic complete flaccid paraplegic man after continuous low-frequency pelvic nerve stimulation and FES-assisted training. Spinal Cord Ser Cases. 2017;3:16034. doi: 10.1038/scsandc.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong S, Shem K, Crew J. Specialized Respiratory Management for Acute Cervical Spinal Cord Injury: A Retrospective Analysis. Topics in Spinal Cord Injury Rehabilitation. 2012;18(4):283–290. doi: 10.1310/sci1804-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spruit MA, Singh SJ, Garvey C, et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 51.Mazwi NL, Adeletti K, Hirschberg RE. Traumatic Spinal Cord Injury: Recovery, Rehabilitation, and Prognosis. Curr Trauma Rep. 2015;1(3):182–192. doi: 10.1007/s40719-015-0023-x [DOI] [Google Scholar]
- 52.National Spinal Cord Injury Statistical Center. COMPLETE PUBLIC VERSION OF THE 2019 ANNUAL STATISTICAL REPORT for the SPINAL CORD INJURY MODEL SYSTEMS. Published online 2019. [Google Scholar]
- 53.Carson SS. Outcomes of prolonged mechanical ventilation. Curr Opin Crit Care. 2006;12(5):405–411. doi: 10.1097/01.ccx.0000244118.08753.dc [DOI] [PubMed] [Google Scholar]
- 54.Charlifue S, Apple D, Burns SP, et al. Mechanical Ventilation, Health, and Quality of Life Following Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. 2011;92(3):457–463. doi: 10.1016/j.apmr.2010.07.237 [DOI] [PubMed] [Google Scholar]
- 55.Körner M. Interprofessional teamwork in medical rehabilitation: a comparison of multidisciplinary and interdisciplinary team approach. Clin Rehabil. 2010;24(8):745–755. doi: 10.1177/0269215510367538 [DOI] [PubMed] [Google Scholar]
- 56.Neumann V, Gutenbrunner C, Fialka-Moser V, et al. Interdisciplinary team working in physical and rehabilitation medicine. J Rehabil Med. 2010;42(1):4–8. doi: 10.2340/16501977-0483 [DOI] [PubMed] [Google Scholar]
- 57.Singh R, Küçükdeveci AA, Grabljevec K, Gray A. The Role of Interdisciplinary Teams in Physical and Rehabilitation Medicine. doi:info:doi/ 10.2340/16501977-2364 [DOI] [PubMed] [Google Scholar]
- 58.Onders RP, Elmo MJ, Ignagni AR. Diaphragm Pacing Stimulation System for Tetraplegia in Individuals Injured During Childhood or Adolescence. J Spinal Cord Med. 2007;30(Suppl 1):S25–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy P, Rogers BA. Anxiety and depression after spinal cord injury: A longitudinal analysis. Archives of Physical Medicine and Rehabilitation. 2000;81(7):932–937. doi: 10.1053/apmr.2000.5580 [DOI] [PubMed] [Google Scholar]
- 60.Migliorini C, Tonge B, Taleporos G. Spinal Cord Injury and Mental Health. Aust N Z J Psychiatry. 2008;42(4):309–314. doi: 10.1080/00048670801886080 [DOI] [PubMed] [Google Scholar]
- 61.Körner M, Bengel J. [Teamwork and team success in multi- and interdisciplinary teams in medical rehabilitation]. Rehabilitation (Stuttg). 2004;43(6):348–357. doi: 10.1055/s-2004-828533 [DOI] [PubMed] [Google Scholar]
- 62.Collins EG, Bauldoff G, Carlin B, et al. Clinical Competency Guidelines for Pulmonary Rehabilitation Professionals: POSITION STATEMENT OF THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATION. Journal of Cardiopulmonary Rehabilitation and Prevention. 2014;34(5):291–302. doi: 10.1097/HCR.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 63.Martin AD, Smith BK, Davenport PD, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Critical Care. 2011;15(2):R84. doi: 10.1186/cc10081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Postma K, Haisma JA, Hopman MTE, Bergen MP, Stam HJ, Bussmann JB. Resistive Inspiratory Muscle Training in People With Spinal Cord Injury During Inpatient Rehabilitation: A Randomized Controlled Trial. Phys Ther. 2014;94(12):1709–1719. doi: 10.2522/ptj.20140079 [DOI] [PubMed] [Google Scholar]
- 65.Roth EJ, Stenson KW, Powley S, et al. Expiratory Muscle Training in Spinal Cord Injury: A Randomized Controlled Trial. Archives of Physical Medicine and Rehabilitation. 2010;91(6):857–861. doi: 10.1016/j.apmr.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 66.Tamplin J, Berlowitz DJ. A systematic review and meta-analysis of the effects of respiratory muscle training on pulmonary function in tetraplegia. Spinal Cord. 2014;52(3):175–180. doi: 10.1038/sc.2013.162 [DOI] [PubMed] [Google Scholar]
- 67.Berlowitz DJ, Tamplin J. Respiratory muscle training for cervical spinal cord injury. Cochrane Database of Systematic Reviews. 2013;(7). doi: 10.1002/14651858.CD008507.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raab AM, Krebs J, Perret C, Pfister M, Hopman M, Mueller G. Evaluation of a clinical implementation of a respiratory muscle training group during spinal cord injury rehabilitation. Spinal Cord Ser Cases. 2018;4:40. doi: 10.1038/s41394-018-0069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raab AM, Krebs J, Perret C, Michel F, Hopman MT, Mueller G. Maximum Inspiratory Pressure is a Discriminator of Pneumonia in Individuals With Spinal-Cord Injury. Respir Care. 2016;61(12):1636–1643. doi: 10.4187/respcare.04818 [DOI] [PubMed] [Google Scholar]
- 70.Raab AM, Krebs J, Pfister M, Perret C, Hopman M, Mueller G. Respiratory muscle training in individuals with spinal cord injury: effect of training intensity and -volume on improvements in respiratory muscle strength. Spinal Cord. 2019;57(6):482–489. doi: 10.1038/s41393-019-0249-5 [DOI] [PubMed] [Google Scholar]
- 71.Welch JF, Sutor TW, Vose AK, Perim RR, Fox EJ, Mitchell GS. Synergy between Acute Intermittent Hypoxia and Task-Specific Training. Exerc Sport Sci Rev. 2020;48(3):125–132. doi: 10.1249/JES.0000000000000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zbrzeski A, Bornat Y, Hillen B, et al. Bio-Inspired Controller on an FPGA Applied to Closed-Loop Diaphragmatic Stimulation. Front Neurosci. 2016;10:275. doi: 10.3389/fnins.2016.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 74.Postma K, Vlemmix LY, Haisma JA, et al. Longitudinal association between respiratory muscle strength and cough capacity in persons with spinal cord injury: An explorative analysis of data from a randomized controlled trial. J Rehabil Med. 2015;47(8):722–726. doi: 10.2340/16501977-1986 [DOI] [PubMed] [Google Scholar]
- 75.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 76.Postma K, Bussmann JB, Haisma JA, van der Woude LH, Bergen MP, Stam HJ. Predicting respiratory infection one year after inpatient rehabilitation with pulmonary function measured at discharge in persons with spinal cord injury. J Rehabil Med. 2009;41(9):729–733. doi: 10.2340/16501977-0410 [DOI] [PubMed] [Google Scholar]
- 77.Kelley A, Garshick E, Gross ER, Lieberman SL, Tun CG, Brown R. Spirometry testing standards in spinal cord injury. Chest. 2003;123(3):725–730. doi: 10.1378/chest.123.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med. 2007;30(4):309–318. doi: 10.1080/10790268.2007.11753946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med. 2011;34(6):535–546. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beauregard L, Guindon A, Noreau L, Lefebvre H, Boucher N. Community Needs of People Living With Spinal Cord Injury and Their Family. Top Spinal Cord Inj Rehabil. 2012;18(2):122–125. doi: 10.1310/sci1802-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.